Improvement of the Tribological Characteristics of AISI 8620, 8640 and 52100 Steels through Thermo-Reactive Treatments
Abstract
1. Introduction
2. Materials and Methods
3. Results
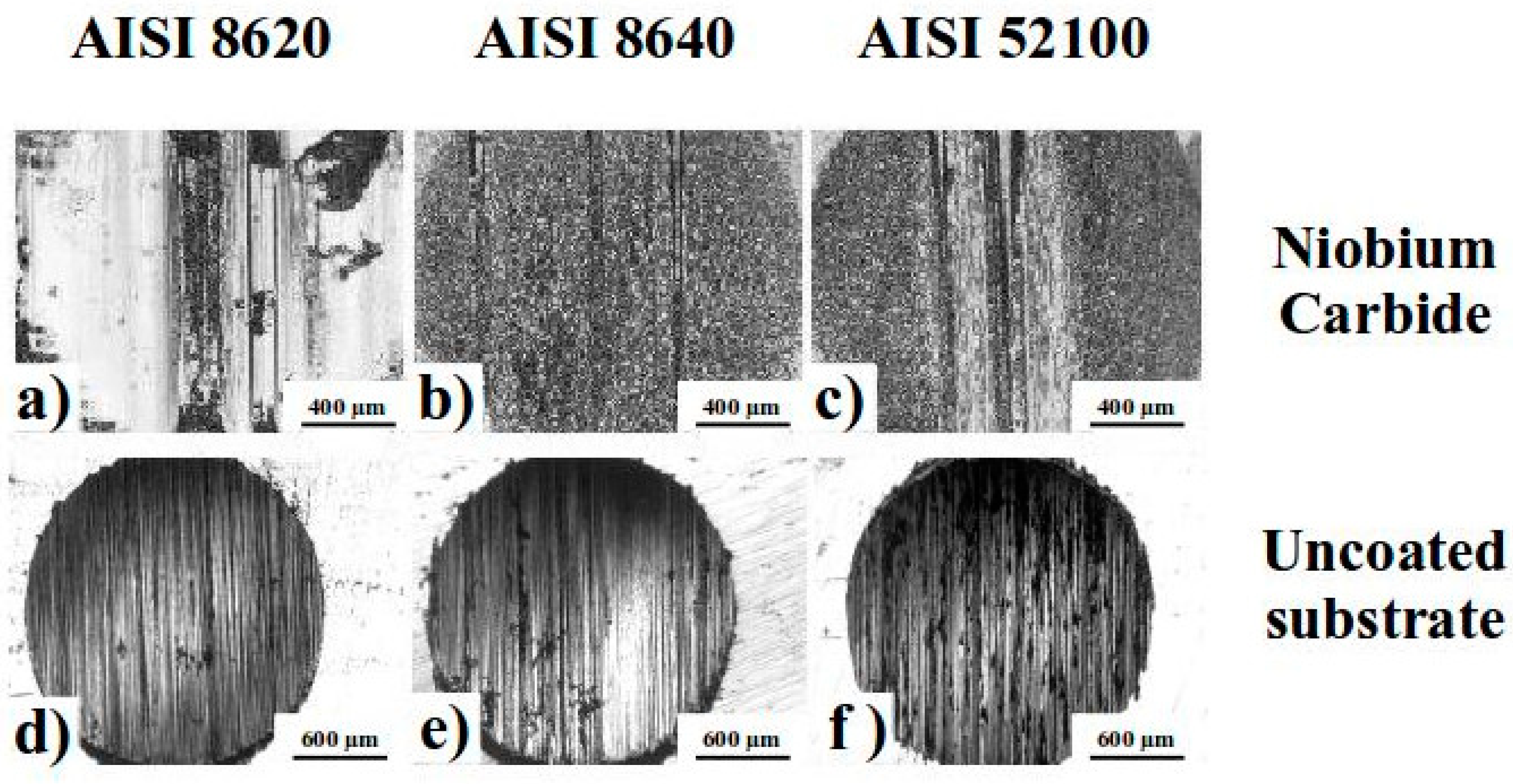
3.1. Metallography Analysis
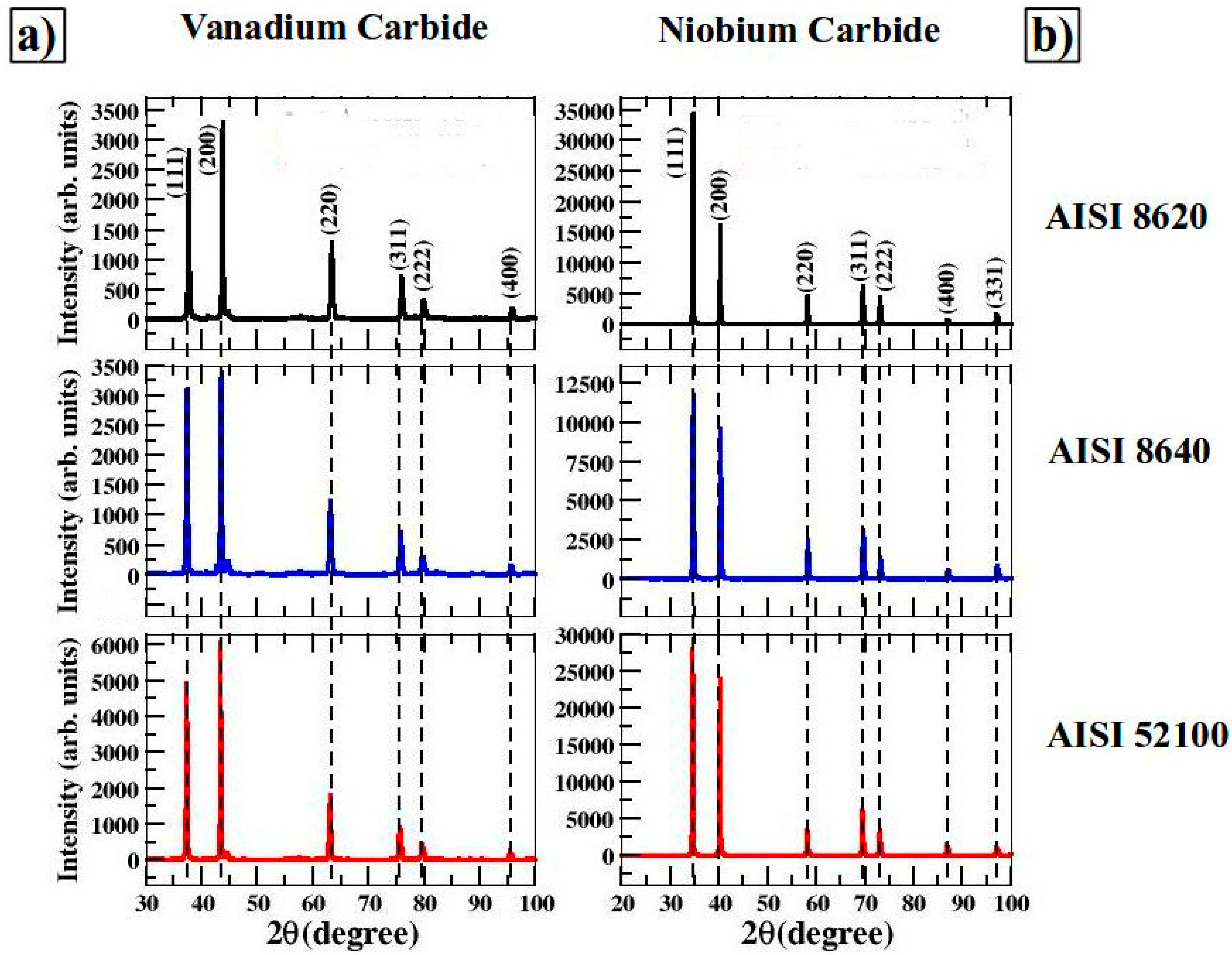
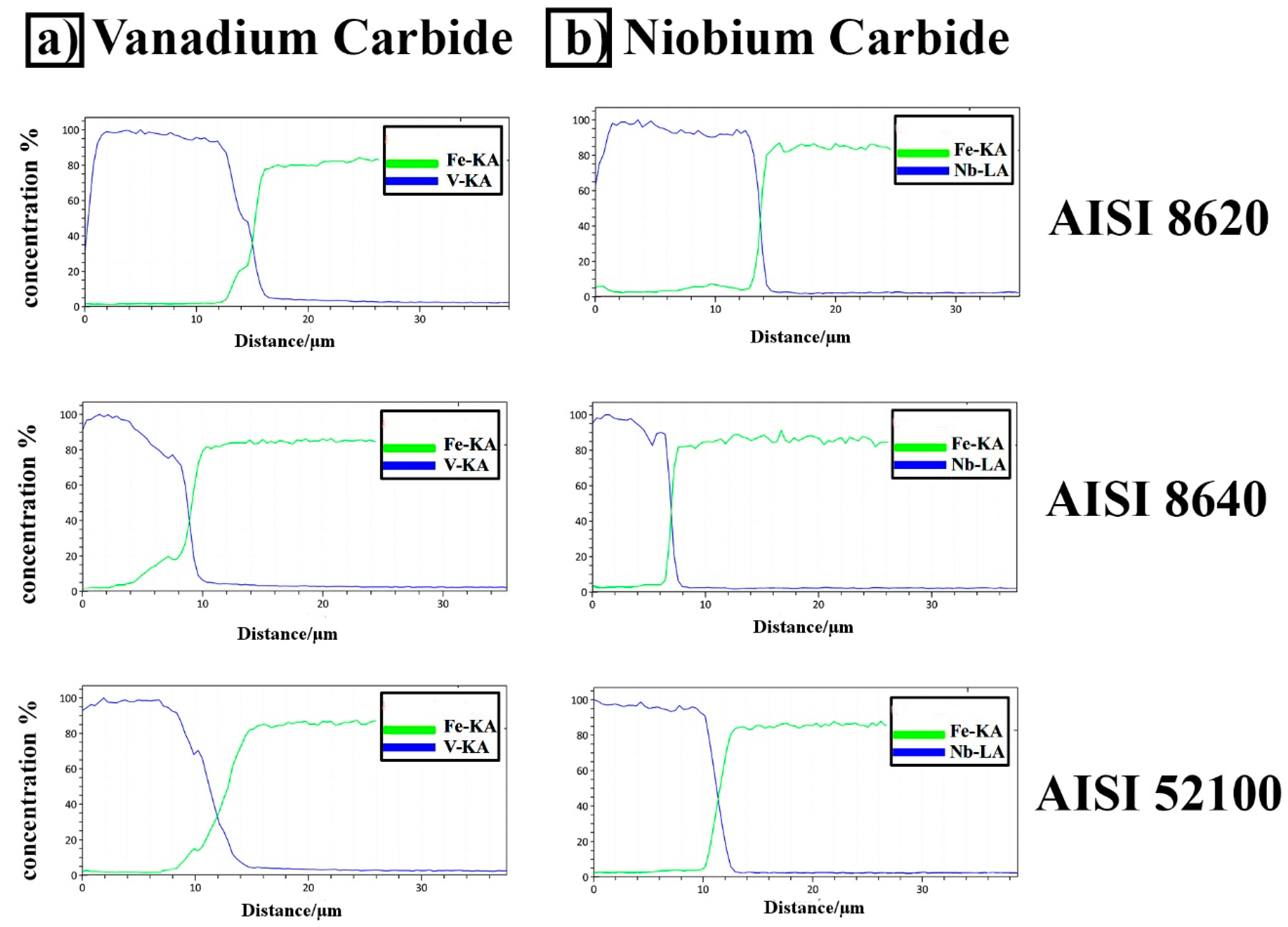
3.2. X-ray Diffraction and EDS Analysis
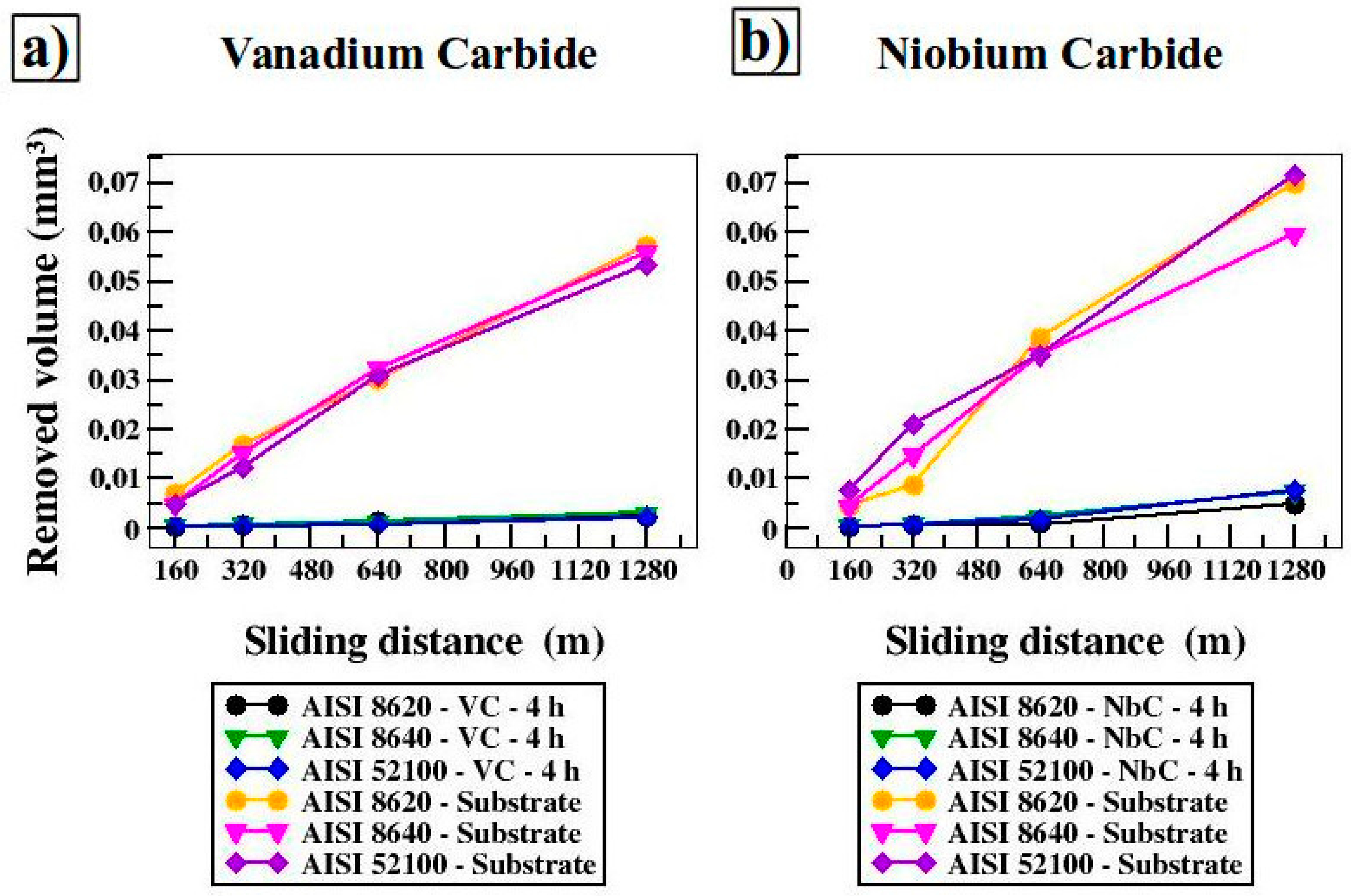
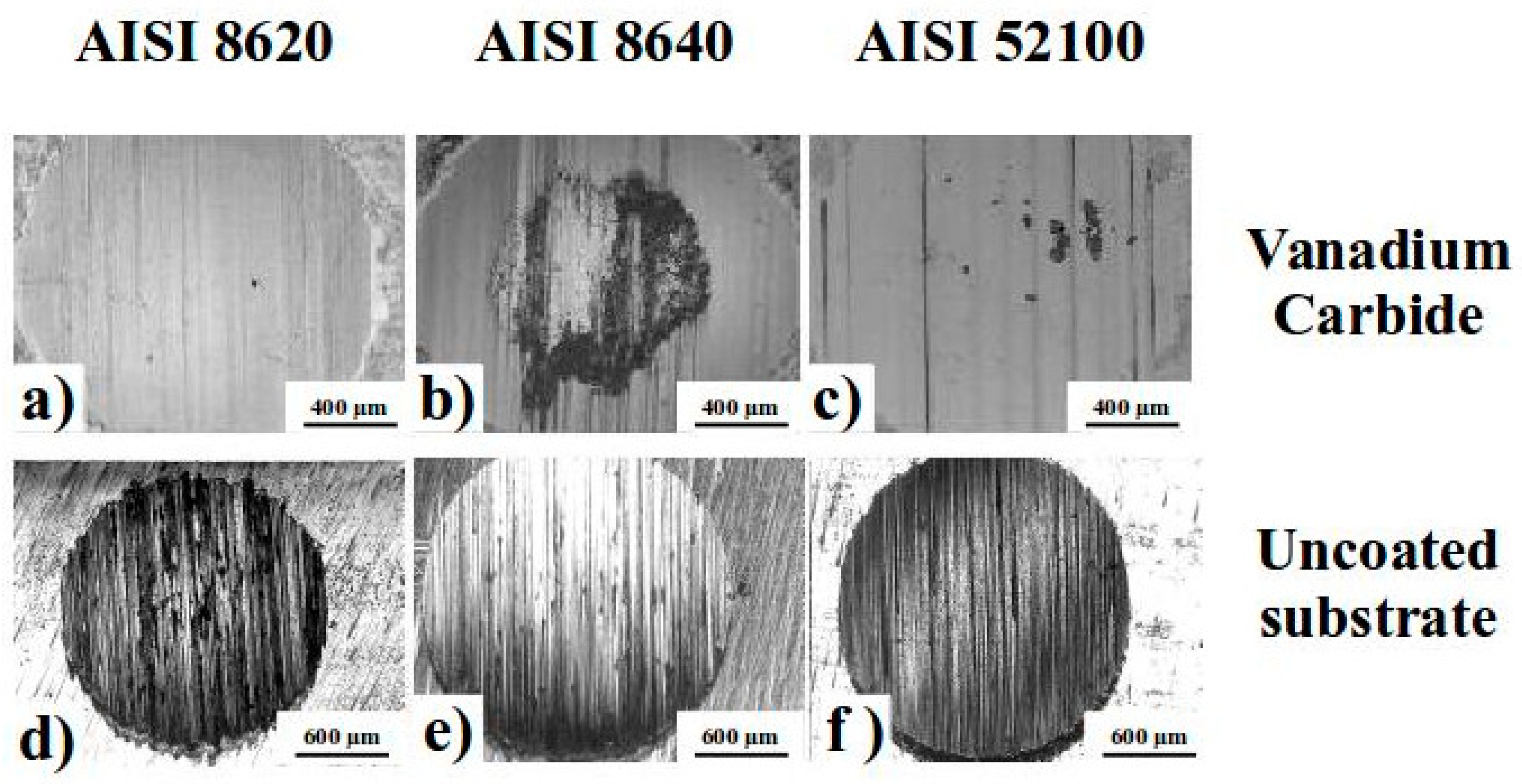
3.3. Microhardness and Wear Tests
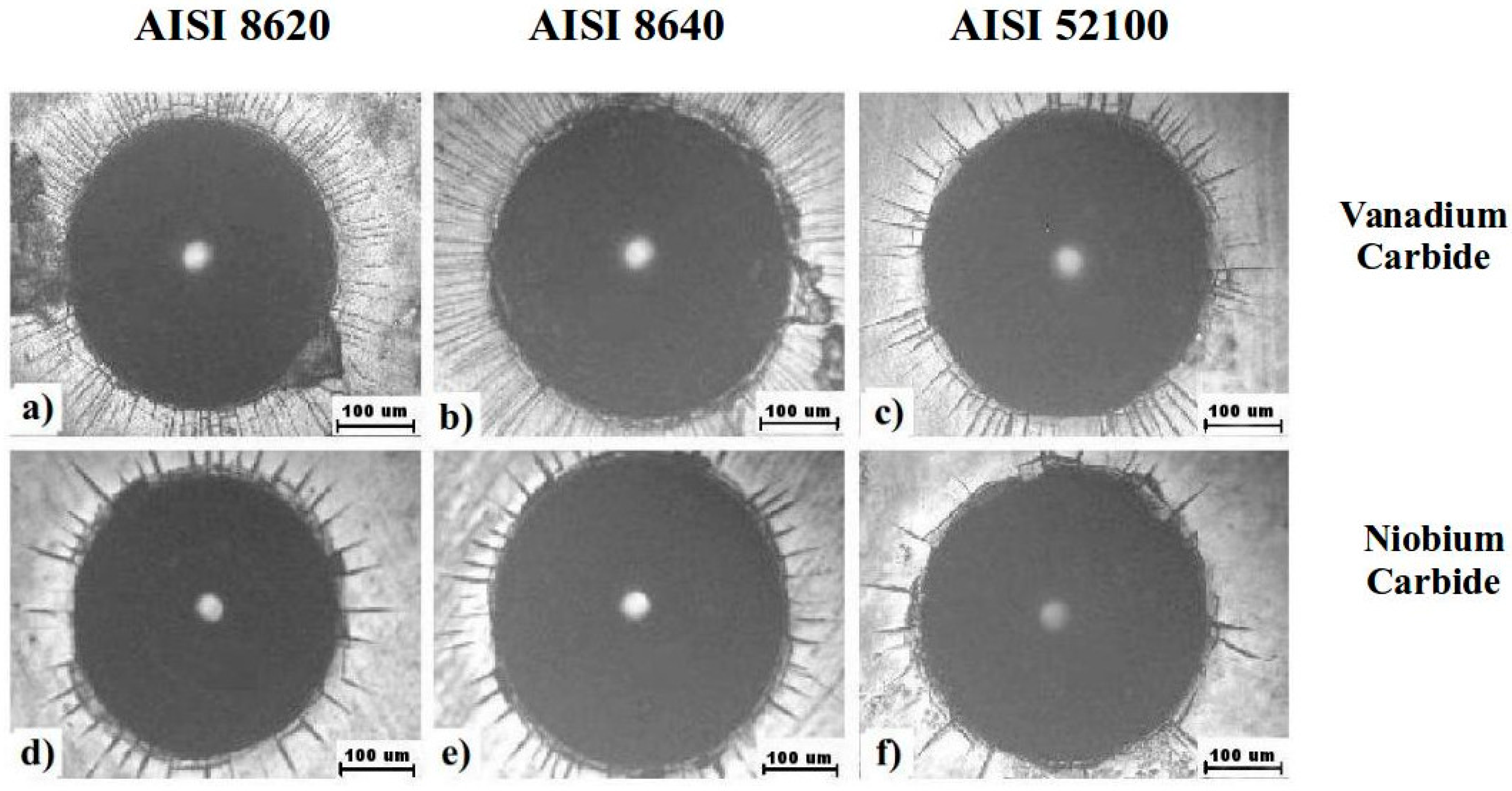
3.4. VDI 3198 Standard Adhesion Test
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- OrjuelaG, A.; Rincón, R.; Olaya, J.J. Corrosion resistance of niobium carbide coatings produced on AISI 1045 steel via thermo-reactive diffusion deposition. Surf. Coat. Technol. 2014, 259, 667–675. [Google Scholar] [CrossRef]
- Cuppari, M.G.D.V.; Santos, S.F. Physical Properties of the NbC Carbide. Metals 2016, 6, 250. [Google Scholar] [CrossRef]
- Johansson, L.I. Electronic and structural properties of transition-metal carbide and nitride surfaces. Surf. Sci. Rep. 1995, 21, 177–250. [Google Scholar] [CrossRef]
- Zhang, K.; Wen, M.; Cheng, G.; Li, X.; Meng, Q.; Lian, J.; Zheng, W. Reactive magnetron sputtering deposition and characterization of niobium carbide films. Vacuum 2014, 99, 233–241. [Google Scholar] [CrossRef]
- Aguzzoli, C.; Figueroa, C.; De Souza, F.; Spinelli, A.; Baumvol, I. Corrosion and nanomechanical properties of vanadium carbide thin film coatings of tool steel. Surf. Coat. Technol. 2012, 206, 2725–2731. [Google Scholar] [CrossRef]
- Wei, Y.; Zhang, D.W.; Wang, J.; Cai, H.Z.; Zhang, X.X.; Chen, L.; Guo, J.M.; Hu, C.Y. Microstructure and deposition kinetics of Nb prepared by chemical vapor deposition. Mod. Phys. Lett. B 2018, 32, 1–11. [Google Scholar] [CrossRef]
- Soltani, R.; Sohi, M.H.; Ansari, M.; Haghighi, A.; Ghasemi, H.M.; Haftlang, F. Evaluation of niobium carbide coatings produced on AISI L2 steel via thermo-reactive diffusion technique. Vacuum 2017, 146, 44–51. [Google Scholar] [CrossRef]
- Casteletti, L.C.; Fernandes, F.A.P.; Heck, S.C.; de Oliveira, C.K.N.; Neto-Lombardi, A.; Totten, G.E. Pack and salt bath diffusion treatments on steels. Heat Treat. Prog. 2009, 9, 49–52. [Google Scholar]
- Ghadi, A.; Saghafian, H.; Soltanieh, M.; Yang, Z.G. Diffusion mechanism in molten salt baths during the production of carbide coatings via thermal reactive diffusion. Int. J. Miner. Met. Mater. 2017, 24, 1448–1458. [Google Scholar] [CrossRef]
- Mittemeijer, E.J.; Somers, M.A. Thermochemical Surface Engineering of Steels; Woodhead Publishing: Cambridge, UK, 2015; Chapter 19; p. 703. [Google Scholar]
- Arai, T.; Fujita, H.; Sugimoto, Y.; Ohta, Y. Diffusion carbide layers formed in molten borax systems. J. Mater. Eng. 1987, 9, 183–189. [Google Scholar] [CrossRef]
- Arai, T.; Komatsu, N. Carbide Coating Process by Use of Salt Bath and its Application to Metal Forming Dies. In Proceedings of the 18th International Machine Tool Design and Research Conference, London, UK, 14 September 1977. [Google Scholar]
- Trezona, R.; Hutchings, I. Three-body abrasive wear testing of soft materials. Wear 1999, 233, 209–221. [Google Scholar] [CrossRef]
- Oliveira, C.; Riofano, R.M.; Casteletti, L. Micro-abrasive wear test of niobium carbide layers produced on AISI H13 and M2 steels. Surf. Coat. Technol. 2006, 200, 5140–5144. [Google Scholar] [CrossRef]
- Soares, C.; Mariani, F.E.; Casteletti, L.C.; Lombardi, A.N.; Totten, G.E. Characterization of Niobium Carbide Layers Produced in Ductile Cast Iron Using Thermo-Reactive Treatments. Mater. Perform. Charact. 2017, 6, 20160093. [Google Scholar] [CrossRef]
- Biesuz, M.; Sglavo, V.M. Chromium and vanadium carbide and nitride coatings obtained by TRD techniques on UNI 42CrMoS4 (AISI 4140) steel. Surf. Coat. Technol. 2016, 286, 319–326. [Google Scholar] [CrossRef]
- Antonio, R.R.M.; Jairo, O.F.J.; Jesús, T.A.V. Evaluación tribológica de recubrimientos de nbc y VC sobre acero AISI D2 producidos por la técnica deposición difusión termo-reactiva. Ing. Investig. Y Tecnol. 2015, 16, 287–294. [Google Scholar] [CrossRef]
- Günen, A.; Kurt, B.; Milner, P.; Gök, M.S. Properties and tribological performance of ceramic-base chromium and vanadium carbide composite coatings. Int. J. Refract. Met. Hard Mater. 2019, 81, 333–344. [Google Scholar] [CrossRef]
- Strahin, B.L.; Shreeram, D.D.; Doll, G.L. Properties and Tribological Performance of Vanadium Carbide Coatings on AISI 52100 Steel Deposited by Thermoreactive Diffusion. JOM 2017, 69, 1160–1164. [Google Scholar] [CrossRef]
- Petersen, D.; Link, R.; Rutherford, K.; Hutchings, I. Theory and Application of a Micro-Scale Abrasive Wear Test. J. Test. Eval. 1997, 25, 250. [Google Scholar] [CrossRef]
- Vidakis, N.; Antoniadis, A.; Bilalis, N. The VDI 3198 indentation test evaluation of a reliable qualitative control for layered compounds. J. Mater. Process. Technol. 2003, 143, 481–485. [Google Scholar] [CrossRef]
- Kayali, Y.; Yalçin, Y.; Taktak, S.; Yalcin, Y. Adhesion and wear properties of boro-tempered ductile iron. Mater. Des. 2011, 32, 4295–4303. [Google Scholar] [CrossRef]
- Güneş, I.; Kayali, Y. Investigation of mechanical properties of borided Nickel 201 alloy. Mater. Des. 2014, 53, 577–580. [Google Scholar] [CrossRef]
- Matijević, B. A Model of Vanadium Carbide Growth on Steel Surfaces Obtained by Thermo Reactive Deposition. JOM 2013, 65, 1395–1402. [Google Scholar] [CrossRef]
- Fan, X.S.; Yang, Z.G.; Zhang, C.; Zhang, Y.D.; Che, H.Q. Evaluation of vanadium carbide layers on AISI H13 obtained by thermo-reactive deposition/diffusion technique. Surf. Coat. Technol. 2010, 205, 641–646. [Google Scholar] [CrossRef]
- Lipatnikov, V.; Lengauer, W.; Ettmayer, P.; Keil, E.; Groboth, G.; Kny, E. Effects of vacancy ordering on structure and properties of vanadium carbide. J. Alloy. Compd. 1997, 261, 192–197. [Google Scholar] [CrossRef]
- Taktak, S.; Ulu, S. Wear behaviour of TRD carbide layers at elevated temperatures. Ind. Lubr. Tribol. 2010, 62, 37–45. [Google Scholar] [CrossRef]
- Sen, S.; Sen, U. Sliding wear behavior of niobium carbide coated AISI 1040 steel. Wear 2008, 264, 219–225. [Google Scholar] [CrossRef]
- Castillejo, F.; Marulanda, D.; Olaya, J.J.; Rodriguez, O. Electrical furnace for producing carbide layers using the Thermoreactive deposition/diffusion technique. Dyna 2011, 78, 192–197. [Google Scholar]
- Aghaie-Khafri, M.; Fazlalipour, F. Vanadium carbide coatings on die steel deposited by the thermo-reactive diffusion technique. J. Phys. Chem. Solids 2008, 69, 2465–2470. [Google Scholar] [CrossRef]
- Matijević, B.; Stupnišek, M. Novelty in Diffusion Coating Technology. Mater. Manuf. Process. 2009, 24, 887–893. [Google Scholar] [CrossRef]
- De Oliveira, P.G.B.; Aureliano, R.T.; Mariani, F.E.; Totten, G.E.; Casteletti, L.C. Boriding of AISI 440B Stainless Steel and Coating Characterization. In Proceedings of the 29th ASM Heat Treating Society Conference, Columbus, OH, USA, 24–26 October 2017. [Google Scholar]




| Coating | Substrate | Thickness (μm) | Hardness Variation | CoF Variation | Reference |
|---|---|---|---|---|---|
| NbC | Ductile cast iron | 31 | 4.5 times higher | - | [15] |
| VC | AISI 4140 | 16 | 2.7 times higher | - | [16] |
| NbC VC | AISI D2 | 16.3 15.8 | 2–4 times higher | 0.17 substrate COF | [17] |
| (V-Cr-C) | AISI D2 | 11.3–23.2 | 2.7–3.4 times higher | 0.95–0.47 substrate COF | [18] |
| VC | AISI 52100 | 4 | - | 0.72 substrate COF | [19] |
| Element (wt.%) | AISI 8620 | AISI 8640 | AISI 52100 |
|---|---|---|---|
| C | 0.24 | 0.45 | 0.85 |
| Mn | 0.85 | 0.78 | 0.32 |
| Ni | 0.43 | 0.42 | 0.03 |
| Cr | 0.43 | 0.46 | 0.42 |
| Si | 0.32 | 0.21 | 0.05 |
| Mo | 0.16 | 0.15 | - |
| P | 0.03 | 0.02 | 0.01 |
| S | 0.038 | 0.013 | 0.001 |
| Fe | Balance | Balance | Balance |
| Treatment Time | AISI 8620 (VC) | AISI 8620 (NbC) | AISI 8640 (VC) | AISI 8640 (NbC) | AISI 52100 (VC) | AISI 52100 (NbC) |
|---|---|---|---|---|---|---|
| 2 h | 8.57 μm | 8.28 μm | 4.29 μm | 4.14 μm | 8.6 μm | 8.31 μm |
| 4 h | 15.30 μm | 15.17 μm | 8.89 μm | 8.25 μm | 14.86 μm | 14.79 μm |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Triani, R.M.; Mariani, F.E.; Gomes, L.F.D.A.; De Oliveira, P.G.B.; Totten, G.E.; Casteletti, L.C. Improvement of the Tribological Characteristics of AISI 8620, 8640 and 52100 Steels through Thermo-Reactive Treatments. Lubricants 2019, 7, 63. https://doi.org/10.3390/lubricants7080063
Triani RM, Mariani FE, Gomes LFDA, De Oliveira PGB, Totten GE, Casteletti LC. Improvement of the Tribological Characteristics of AISI 8620, 8640 and 52100 Steels through Thermo-Reactive Treatments. Lubricants. 2019; 7(8):63. https://doi.org/10.3390/lubricants7080063
Chicago/Turabian StyleTriani, Rafael Magalhães, Fábio Edson Mariani, Lucas Fuscaldi De Assis Gomes, Pedro Gabriel Bonella De Oliveira, George Edward Totten, and Luiz Carlos Casteletti. 2019. "Improvement of the Tribological Characteristics of AISI 8620, 8640 and 52100 Steels through Thermo-Reactive Treatments" Lubricants 7, no. 8: 63. https://doi.org/10.3390/lubricants7080063
APA StyleTriani, R. M., Mariani, F. E., Gomes, L. F. D. A., De Oliveira, P. G. B., Totten, G. E., & Casteletti, L. C. (2019). Improvement of the Tribological Characteristics of AISI 8620, 8640 and 52100 Steels through Thermo-Reactive Treatments. Lubricants, 7(8), 63. https://doi.org/10.3390/lubricants7080063







