Abstract
miRNAs, a class of small non-coding RNAs, play a role in post-transcriptional gene expression. Therefore, this study aimed to conduct a systematic review of miRNAs associated with GDM to build a panel of miRNAs. A bibliographic search was carried out in the PubMed/Medline, Virtual Health Library (VHL), Web of Science, and EMBASE databases, selecting observational studies in English without time restriction. The protocol was registered on the PROSPERO platform (number CRD42021291791). Fifty-five studies were included in this systematic review, and 82 altered miRNAs in GDM were identified. In addition, four miRNAs were most frequently dysregulated in GDM (mir-16-5p, mir-20a-5p, mir-222-3p, and mir-330-3p). The dysregulation of these miRNAs is associated with the mechanisms of cell cycle homeostasis, growth, and proliferation of pancreatic β cells, glucose uptake and metabolism, insulin secretion, and resistance. On the other hand, identifying miRNAs associated with GDM and elucidating its main mechanisms can assist in the characterization and definition of potential biomarkers for the diagnosis and treatment of GDM.
1. Introduction
Gestational Diabetes Mellitus (GDM) is defined as any degree of glucose intolerance first diagnosed during pregnancy [1]. Hyperglycemia during pregnancy can be transient or persist after birth, presenting itself as an independent risk factor for the future development of Type 2 Diabetes Mellitus (T2DM) [2]. According to the International Diabetes Federation, the global prevalence of GDM averages 14% [3], ranging from 1.8–31% depending on the population evaluated and the diagnostic criteria adopted between countries [4]. GDM is the most common metabolic disease during pregnancy, occurring in 3–25% of pregnancies, and its incidence in the population has increased along with T2DM and obesity [5].
There is no universally accepted standard for screening or diagnosing GDM. Guidelines from local medical organizations are followed. However, the test with better sensitivity and specificity is the oral glucose tolerance test (OGTT) with 75 g of glucose, considering values between 153 and 199 mg/dL for GDM [6]. GDM diagnosis can be performed throughout pregnancy from the beginning of prenatal care. However, it is usually performed in the second or third trimester of pregnancy (24–28 weeks), which can cause risks to the mother and the fetus [7]. Short-term adverse outcomes are observed, such as hypoglycemia, hypoxia, respiratory distress syndrome, higher rates of preeclampsia, and large for gestational age or macrosomic newborns, among others [2,5,8]. In addition, children born from mothers with GDM have an increased risk for metabolic and cerebrovascular diseases in adult age [5].
Pregnancy stresses the body and promotes physiological changes to ensure the proper growth of the embryo/fetus. Adaptations and/or dysregulations during pregnancy are performed by placental hormones and increased levels of cortisol and progesterone [9]. Additionally, it is reported that molecules such as placenta-derived microRNAs (miRNAs) may be involved in these adaptations. Variations in the expression of these miRNAs may indicate changes in the maternal metabolic adaptation mechanism [7,10].
miRNAs are a class of endogenous non-coding RNAs with approximately 22 nucleotides, which act as regulators of post-transcriptional gene expression, inhibiting the translation of messenger RNAs (mRNA) or degrading them [5,11]. miRNAs regulate more than one target mRNA, and studies previously described that occurs a control of the expression to an average of 30% of protein-coding genes [7,12]. The main well-known functions of miRNAs are the regulation of cell proliferation and differentiation, apoptosis, stress response, and transcriptional regulation [7].
Studies indicate that the human placenta expresses more than 500 miRNAs, and only some of these are also expressed in other tissues [13]. Therefore, the characterization of miRNAs during pregnancy is necessary to understand better the regulatory mechanisms of healthy and complicated pregnancy [14]. Currently, studies have sought to identify biomarkers for the diagnosis of GDM before 24–28 weeks of gestation, and these miRNAs have revealed great potential as biomarkers for GDM in the early trimester, mainly due to their high stability and accessibility in body fluids [15,16].
Additionally, investigating the regulation of placental and circulating miRNAs and their metabolic adaptation associated with GDM can improve diagnostic, therapeutic, and personalized prognosis [7]. Thus, understanding the functions of miRNAs can improve broadening the insight into the etiology and pathophysiology of GDM and identify possible biomarkers with clinical value to elaborate diagnostic strategies and prevent obstetric and maternal–fetal complications.
2. Materials and Methods
2.1. Registration, Data Source, and Search Strategy
Based on the guiding question, “Which microRNAs are associated with the pathophysiological mechanisms of Gestational Diabetes Mellitus (GDM)?”, the systematic review was carried out to identify the miRNAs associated with the pathophysiological mechanisms of GDM. We used the PEO (Population, Exposure, Outcome) framework: Population: pregnant woman with GDM; Exposure: microRNAs; Outcome: association of microRNAs as a risk factor for GDM.
This systematic review was carried out according to the Preferred Report Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [17]. To avoid duplication, this study had the protocol registered in the International Prospective Register of Systematic Reviews (PROSPERO) (number CRD42021291791) on 17 December 2021 (File S1).
The literature search was carried out in PubMed/Medline, Virtual Health Library (VHL), Web of Science, and EMBASE databases from 27 January 2022 to 15 February 2022 in the English language. Combined terms cataloged in Medical Subject Headings (MeSH) for the keywords (Gestational Diabetes OR Gestational Diabetes Mellitus OR Diabetes Induced by Pregnancy) AND (MicroRNA OR miRNA OR Circulating MicroRNA OR Cell-Free MicroRNA) were used to develop the search strategy, later adapted for each database (Table 1).

Table 1.
Search strategy applied in each database.
As criteria for inclusion of studies in this systematic review, we used: observational studies with original research in the area of human and medical genetics that identified miRNAs in pregnant women with GDM and controls (pregnant women without GDM); no age restrictions; published in English; without the restriction of the year of publication of the study. For exclusion, the following criteria were established: Studies that did not address the research topic; and with another study design.
2.2. Selection of Studies
Two reviewers (PHCMS and LS) independently selected and identified articles at all stages of the systematic review. The articles identified in the databases were imported into the Rayyan platform [18] to optimize the analysis. Initially, the title and abstract were read (phase I), then the articles selected in the first screening were read in full (phase II). In both phases, the articles were evaluated according to the pre-established inclusion and exclusion criteria.
2.3. Risk of Bias Assessment
The risk of methodological bias was assessed using The Joanna Briggs Institute (JBI) Critical Assessment Tool [19] for each study design. All articles selected for inclusion in the systematic review underwent rigorous evaluation. This tool is composed of questions with the possible answers: “Yes”, “No”, “Not applicable”, and “Unclear”.
A critical assessment tool was applied to each type of study: case-control studies, cohort studies, and cross-sectional studies. Those articles that had 100% of the answers “yes” were considered at low risk of bias; those who scored 70–99% “yes” were considered moderate risk; and those with less than 70% of “yes” answers were excluded from the study, being considered at high risk of methodological bias. Differences of opinion between reviewers were discussed and resolved by consensus.
2.4. Data Extraction and Synthesis
Studies evaluating miRNAs lack more robust statistical analyses; most describe only the type of regulation identified and whether there was a significant difference in this regulation between the evaluated groups (usually considering p < 0.05). Thus, this systematic review used a qualitative and descriptive approach for data analysis, extracting the following data: (1) authors and year of publication; (2) study design; (3) country or continent where the study was performed; (4) sample size; (5) mean age; (6) gestational time; (7) tissue analyzed; (8) microRNA identification technique; (9) microRNA; (10) regulation; (11) p-value. The extracted data were imported into a predefined Excel worksheet, and authors of studies with missing data were contacted. Data extraction was performed independently by two reviewers, and disagreements between reviewers were resolved by consensus.
3. Results
After the initial search, 1138 articles were identified in the databases, and 626 duplicates were excluded, resulting in 512 articles for phase I. Based on the reading of titles and abstracts (phase I), 413 articles were excluded because they did not meet the pre-established inclusion criteria. Therefore, phase II comprised the complete reading of the 99 selected articles. Forty-four were excluded, and 55 were included in this systematic review (Figure 1).
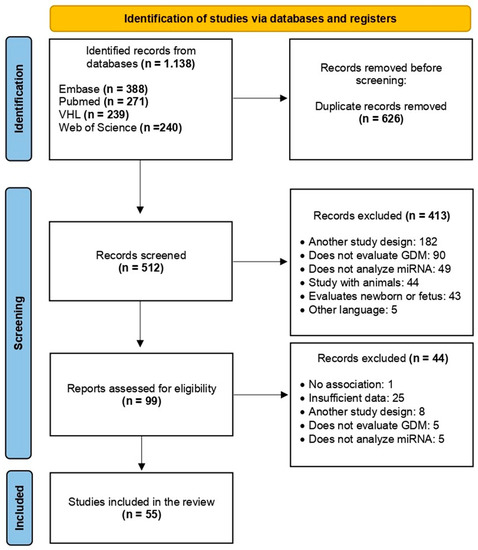
Figure 1.
PRISMA flow chart demonstrating the process of exclusion or inclusion of studies in this systematic review. Adapted from: [17].
Of the included studies, 48 were case-control studies, six were cohort studies, and one was a cross-sectional study. The selected studies were published between 2011 and 2022. The mean age of the case and control groups ranged between 20 and 40 years. A total of 2749 cases (pregnant women with GDM) and 2710 controls (pregnant women without GDM) were analyzed, totaling 5459 individuals. The samples collected and analyzed were blood, plasma, placenta, adipose tissue, and urine (urinary exosome) (Table 2).

Table 2.
Data extracted from studies included in the systematic review.
Additionally, the studies were homogeneous in terms of methodological quality assessment, individually reaching a minimum of 70% of positive responses, being considered at low or moderate risk of bias (Table 3). For case-control studies, 10 parameters were evaluated, cohorts 11, and cross-sectional studies 8 parameters, respectively. Most case-control studies reported that there was no identification of possible confounding factors (question 6—Q6), and, consequently, the non-applicability of question 7 (Q7) declared strategies to deal with confounding factors.

Table 3.
Summary of responses for each study included in the risk of bias assessment.
In this systematic review, 82 miRNAs were identified, highlighting the most cited in the literature due it was deregulated in GDM (mir-16-5p, mir-20a-5p, mir-222-3p, and mir-330-3p). According to the results, the identified miRNAs were down- and/or up-regulated in the GDM, considering p < 0.05 as significant. Studies evaluating miRNAs lack more robust statistical analyses, often describing whether there was a significant difference and the type of miRNA regulation between the evaluated groups.
Among those most frequently found in the literature, mir-16-5p [40,47,68,70] and mir-330-3p [28,39,50] were up-regulated, and mir-20a-5p and mir-222-3p were up- and down-regulated in different studies [40,51,62,68,70,72] (Figure 2). In general, the dysregulation of these miRNAs is associated with mechanisms of inflammation, growth, and proliferation of pancreatic β cells, glucose uptake and metabolism, insulin secretion, and resistance (Table 4).
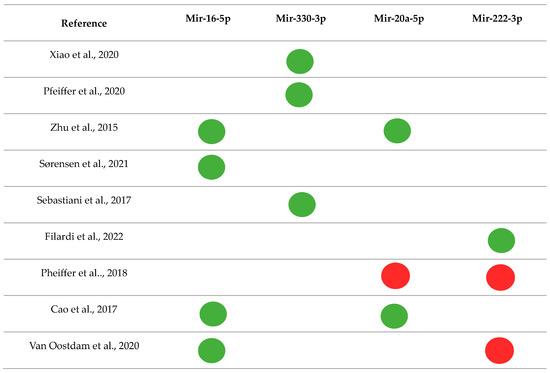

Figure 2.
Main miRNAs and their regulations were found in the systematic review [28,39,40,47,50,51,62,68,70,72].

Table 4.
Biological function and regulation of miRNAs found in the systematic review.
4. Discussion
miRNAs have been studied as potential biomarkers of GDM, and knowledge about their regulation and function, as well as metabolic adaptation associated with GDM, have been investigated and may help in the understanding of the pathogenesis of the disease [7]. In this systematic review, we identified 82 altered miRNAs in GDM. Of these, four were most cited, mir-16-5p and mir-330-3p were up-regulated, and mir-20a-5p and mir-222-3p were found to be up-regulated and down-regulated, respectively.
According to Gao and Zhao [75], mir-16-5p controls cell proliferation, migration, and invasion, affecting the cell cycle and promoting apoptosis. In addition, this miRNA modulates the PI3K/Akt signaling pathway, an important cell cycle regulator that includes genes such as Pi3Kr1, Pi3kr3, mTOR, and Mapk3, among others. Kwon et al. [76] observed that the up-regulation of mir-16-5p in the liver of Cmah-null mice, used as models for T2DM, can negatively regulate the insulin/PI3K-AKT signaling pathway in association with other genes. These results suggest that the impairment of insulin mechanisms may favor the development of metabolic disorders, such as chronic hyperglycemia.
Other targets of this miRNA are the genes Insulin Receptor Substrate 1 and 2 (IRS1/IRS2) and Insulin-Like Growth Factor 1 (IGF-1), which are closely related to insulin resistance, a characteristic condition of diabetes [68,76]. In addition, the up-regulation of this miRNA in GDM patients in the second trimester of gestation has demonstrated a correlation with the down-regulation of IRS1 and IRS2, leading to abnormal signaling of the Wnt/β-catenin pathway, important for embryonic development and adult tissue homeostasis [77].
Whereas mir-330-3p is associated with proliferation, differentiation, and insulin secretion and is highly expressed in GDM related to high glucose concentration. It also acts as a central regulator of cell cycle homeostasis. Thus, the up-regulation of this miRNA in patients with GDM may contribute to pancreatic β cell dysfunction, altering the proliferation and growth of these cells [39,50]. On the other hand, studies have revealed that the E2F Transcription Factor 1 (E2F1) and Cell Division Cycle 42 (CDC42) genes are targets of mir-330-3p. Both are involved in the growth and proliferation of β cells and the control of insulin secretion. Therefore, the low expression of these genes is caused by increased miRNA levels that can compromise β cell proliferation and insulin secretion [39,50].
Moreover, the Angiotensin II receptor Type 2 (AGTR2) gene was also identified as a target of this miRNA; this gene acts during the development of the pancreas in the embryonic stage and is a possible mediator of the regeneration of β cells in the adult pancreas. Thus, it is hypothesized that elevated levels of mir-330-3p may inhibit pancreatic neogenesis through the down-regulation of AGTR2, causing a defect in the regeneration of the endocrine pancreas under high metabolic demand [50].
Additionally, in this systematic review, Zhu et al. [40] and Cao et al. [68] identified up-regulated mir-20a-5p, while Pheiffer et al. [62] found it down-regulated in GDM. Mir-20a-5p belongs to the mir-17-92 cluster and is associated with angiogenesis [62]. In GDM, the expression of angiogenic proteins is increased, such as Vascular Endothelial Growth Factor-A (VEGFA), Hypoxia-inducible factor 1 subunit alpha (HIF1A) [78], Phosphatase and Tensin homolog (PTEN) [79], and BCL2 apoptosis regulator (BCL2) [80]. This fact corroborates the regulatory effect of the decreased expression of mir-20a-5p found by Pheiffer et al. [62].
Zhu et al. [40] also associated mir-20a-5p with the insulin, MAPK, TGF-β, and mTOR signaling pathways. The PI3K/Akt pathway that regulates the cell cycle, glucose homeostasis, and insulin signaling, as well as the FoxO protein, which also regulates the insulin/PI3K/Akt pathway, were considered targets of mir-20a-5p. Thus, this miRNA becomes a possible biomarker of GDM, and the alteration of its expression can interfere with these pathways, generating hyperglycemia observed in GDM patients [62].
MAPK signaling pathway plays a role in developing vascular lesions, such as diabetes [81], and its abnormal signaling has been identified in pregnancy complications [40]. In addition, the TGF-β signaling pathway has been associated with preeclampsia [82]. In contrast, the mTOR signaling pathway controls energy balance [83] and blocking these pathways may contribute to the development of GDM [40].
Finally, mir-222-3p was identified by Tagoma et al. [72] and Filardi et al. [51] found it up-regulated, while Oostdam et al. [70] and Pheiffer et al. [62] found it down-regulated in GDM. Mir-222-3p belongs to the mir-222 cluster, is abundant in plasma during 24–28 weeks of gestation, and is a placental miRNA that acts on the proliferation of endometrial stromal cells [51,84], regulating the expression of estrogen receptor-α (ER-α) in estrogen-induced insulin resistance in GDM [55,62], and is strongly linked to glucose metabolism in pregnancy, profoundly impacting in the weight birth [51].
Furthermore, high levels of this miRNA were found in the adipose tissue of GDM patients, negatively correlated with the levels of ER-α and glucose transporter type 4 (GLUT4) [55]. Women with GDM have higher levels of estradiol when compared to healthy women, and the estradiol and ER-α act on the GLUT4, becoming critical regulators of obesity and insulin resistance [51].
Due to the relation between miRNAs and the regulation of gene expression, information about the tissue origin of malignant cells could be generated. Since their initial discovery, it has been clear that miRNAs are expressed in a variety of cell types and that their expression patterns are tissue-specific and thus could have great diagnostic importance and prognostic value [85].
The placenta produces several miRNAs expressed specifically by placental cells. They can be dysregulated in the plasm and placenta of women with GDM, being also associated with pregnancy and birth-related outcomes. Detection of placental miRNAs in placental cells and circulation contributes to the understanding of the molecular pathways and intracellular signalization and their influences on GDM genetic background [86]. Therefore, identifying possible GDM biomarkers has a significant clinical value in developing diagnostic strategies and possibly preventing obstetric and maternal–fetal complications [7]. However, studies on early recognition, diagnostic criteria, possible biomarkers, and therapeutic targets for GDM show controversial results, mainly due to differences in ethnic, geographic, genetic, and environmental factors and diagnostic criteria [7,87,88].
5. Conclusions
A miRNA can regulate up to 200 mRNAs, indicating that miRNAs can individually regulate many different biological processes [52]. Thus, it is suggested that miRNAs be used as biomarkers in miRNA panels or risk assessment algorithms and not as individual biomarkers for diagnosing GDM [62]. In this systematic review, we found 55 articles that analyzed the alteration of miRNAs in GDM, identifying 82 altered miRNAs. The expression of these miRNAs varied depending on the type of sample used and the different gestational ages, which may explain the differences in the results. Additionally, these miRNAs were associated with several mechanisms, such as cell cycle homeostasis, growth, and proliferation of pancreatic β cells, glucose uptake and metabolism, insulin secretion, and resistance. Certainly, miRNAs are potential biomarkers for the early diagnosis of GDM. However, more research is needed to elucidate their diagnostic and predictive value and their pathogenic mechanisms in pregnancy.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jpm13071126/s1, File S1. Protocol registered in the International Prospective Register of Systematic Reviews (PROSPERO) on 16 November 2021, under number CRD42021291791.
Author Contributions
Conceptualization, R.d.S.S. and A.A.d.S.R.; Methodology, P.H.C.M.d.S., L.d.S., K.d.F.S., R.d.S.S. and A.A.d.S.R.; Software, K.d.F.S., R.d.S.S. and A.A.d.S.R.; Validation, R.d.S.S. and A.A.d.S.R.; Formal Analysis, P.H.C.M.d.S., L.d.S. and K.d.F.S.; Investigation, P.H.C.M.d.S., L.d.S., K.d.F.S., R.d.S.S. and A.A.d.S.R.; Resources, R.d.S.S. and A.A.d.S.R.; Data Curation, P.H.C.M.d.S., L.d.S. and K.d.F.S.; Writing—Original Draft Preparation, P.H.C.M.d.S., K.d.F.S., C.C.P.d.C., R.d.S.S. and A.A.d.S.R.; Writing—Review and Editing, P.H.C.M.d.S., K.d.F.S., C.C.P.d.C., R.d.S.S. and A.A.d.S.R.; Supervision, R.d.S.S. and A.A.d.S.R.; Project Administration, A.A.d.S.R.; Funding Acquisition, A.A.d.S.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing does not apply to this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Caughey, A.B.; Turrentine, M. ACOG practice bulletin No. 190 Summary: Gestational diabetes mellitus. Obs. Gynecol. 2018, 131, 406–408. [Google Scholar]
- Durnwald, C. Gestational Diabetes Mellitus: Screening, Diagnosis, and Prevention; Nathan, D.M., Werner, E.F., Eds.; UpToDate: Wellesley, MA, USA, 2022. [Google Scholar]
- Carracher, A.M.; Marathe, P.H.; Close, K.L. International Diabetes Federation 2017. J. Diabetes 2018, 10, 353–356. [Google Scholar] [CrossRef]
- McIntyre, H.D.; Catalano, P.; Zhang, C.; Desoye, G.; Mathiesen, E.R.; Damm, P. Gestational diabetes mellitus. Nat. Rev. Dis. Prim. 2019, 5, 48. [Google Scholar] [CrossRef]
- Yang, X.; Wu, N. MicroRNAs and Exosomal microRNAs May Be Possible Targets to Investigate in Gestational Diabetes Mellitus. Diabetes Metab. Syndr. Obes. 2022, 15, 321–330. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Diagnostic criteria and classification of hyperglycemia first detected in pregnancy: A World Health Organization guideline. Diabetes Res. Clin. Pract. 2014, 103, 341–363. [Google Scholar] [CrossRef]
- Liu, Z.-N.; Jiang, Y.; Liu, X.-Q.; Yang, M.M.; Chen, C.; Zhao, B.-H.; Huang, H.-F.; Luo, Q. MiRNAs in Gestational Diabetes Mellitus: Potential Mechanisms and Clinical Applications. J. Diabetes Res. 2021, 2021, 4632745. [Google Scholar] [CrossRef]
- Metzger, B.E.; Coustan, D.R.; Trimble, E.R. Hyperglycemia and adverse pregnancy outcomes. Clin. Chem. 2019, 65, 937–938. [Google Scholar] [CrossRef]
- Guarino, E.; Poggi, C.D.; Grieco, G.E.; Cenci, V.; Ceccarelli, E.; Crisci, I.; Sebastiani, G.; Dotta, F. Circulating MicroRNAs as Biomarkers of Gestational Diabetes Mellitus: Updates and perspectives. Int. J. Endocrinol. 2018, 2018, 6380463. [Google Scholar] [CrossRef]
- Cuffe, J.S.M.; Holland, O.; Salomon, C.; Rice, G.E.; Perkins, A.V. Review: Placental derived biomarkers of pregnancy disorders. Placenta 2017, 54, 104–110. [Google Scholar] [CrossRef]
- Jonas, S.; Izaurralde, E. Towards a molecular understanding of microRNA-mediated gene silencing. Nat. Rev. Genet. 2015, 16, 421–433. [Google Scholar] [CrossRef]
- Sun, B.K.; Tsao, H. Small RNAs in development and disease. J. Am. Acad. Derm. 2008, 59, 725–737. [Google Scholar] [CrossRef] [PubMed]
- Morales-Prieto, D.M.; Ospina-Prieto, S.; Schmidt, A.; Chaiwangyen, W.; Markert, U.R. Elsevier Trophoblast Research Award Lecture: Origin, evolution and future of placenta miRNAs. Placenta 2014, 35, S39–S45. [Google Scholar] [CrossRef]
- Cai, M.; Kolluru, G.K.; Ahmed, A. Small molecule, big prospects: Microrna in pregnancy and its complications. J. Pregnancy 2017, 2017, 6972732. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, S.; Bohl, M.; Gregersen, S.; Hermansen, K.; O’Driscoll, L. Blood-based biomarkers for metabolic syndrome. Trends Endocrinol. Metab. 2016, 27, 363–374. [Google Scholar] [CrossRef]
- Iljas, J.D.; Guanzon, D.; Elfeky, O.; Rice, G.E.; Salomon, C. Review: Bio-compartmentalization of microRNAs in exosomes during gestational diabetes mellitus. Placenta 2017, 54, 76–82. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef] [PubMed]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- Institute TJB. JBI Reviewer’s Manual. Joanna Briggs Inst. 32. The Joanna Briggs Institute 2022. Available online: https://reviewersmanual.joannabriggs.org/ (accessed on 30 September 2022).
- Ke, W.; Chen, Y.; Zheng, L.; Zhang, Y.; Wu, Y.; Li, L. miR-134-5p promotes inflammation and apoptosis of trophoblast cells via regulating FOXP2 transcription in gestational diabetes mellitus. Bioengineered 2022, 13, 319–330. [Google Scholar] [CrossRef]
- Wang, P.; Ma, Z.; Wang, Z.; Wang, X.; Zhao, G.; Wang, Z. MiR-6869-5p Induces M2 Polarization by Regulating PTPRO in Gestational Diabetes Mellitus. Mediat. Inflamm. 2021, 2021, 6696636. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, J.; Liu, Y. MicroRNA-1323 serves as a biomarker in gestational diabetes mellitus and aggravates high glucose-induced inhibition of trophoblast cell viability by suppressing TP53INP1. Exp. Ther. Med. 2021, 21, 230. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, T.; Sun, D.; Cheng, G.; Ren, H.; Hong, H.; Chen, L.; Jiao, X.; Du, Y.; Zou, Y.; et al. Diagnostic value of dysregulated microribonucleic acids in the placenta and circulating exosomes in gestational diabetes mellitus. J. Diabetes Investig. 2021, 12, 1490–1500. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhuang, J. miR-345-3p serves a protective role during gestational diabetes mellitus by targeting BAK1. Exp. Ther. Med. 2021, 21, 2. [Google Scholar] [CrossRef] [PubMed]
- Guan, C.-Y.; Tian, S.; Cao, J.-L.; Wang, X.-Q.; Ma, X.; Xia, H.-F. Down-Regulated miR-21 in Gestational Diabetes Mellitus Placenta Induces PPAR-α to Inhibit Cell Proliferation and Infiltration. Diabetes Metab. Syndr. Obes. 2020, 13, 3009–3034. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Huang, Y.; Li, L.; Chen, H.; Su, J. Serum miR-29a/b expression. in gestational diabetes mellitus and its influence on prognosis evaluation. J. Int. Med. Res. 2020, 48, 300060520954763. [Google Scholar] [CrossRef]
- Zhao, C.; Zhao, C.; Zhao, H. Defective insulin receptor signaling in patients with gestational diabetes is related to dysregulated miR-140 which can be improved by naringenin. Int. J. Biochem. Cell Biol. 2020, 128, 105824. [Google Scholar] [CrossRef]
- Xiao, Y.; Ding, J.; Shi, Y.; Lin, L.; Huang, W.; Shen, D.; Wang, W. MiR-330-3p contributes to INS-1 cell dysfunction by targeting glucokinase in gestational diabetes mellitus. J. Obs. Gynaecol. Res. 2020, 46, 864–875. [Google Scholar] [CrossRef]
- Tang, L.; Li, P.; Li, L. Whole transcriptome expression profiles in placenta samples from women with gestational diabetes mellitus. J. Diabetes Investig. 2020, 11, 1307–1317. [Google Scholar] [CrossRef]
- Hocaoglu, M.; Demirer, S.; Senturk, H.; Turgut, A.; Komurcu-Bayrak, E. Differential expression of candidate circulating microRNAs in maternal blood leukocytes of the patients with preeclampsia and gestational diabetes mellitus. Pregnancy Hypertens. 2019, 17, 5–11. [Google Scholar] [CrossRef]
- Stirm, L.; Huypens, P.; Sass, S.; Batra, R.; Fritsche, L.; Brucker, S.; Abele, H.; Hennige, A.M.; Theis, F.; Beckers, J.; et al. Maternal whole blood cell miRNA-340 is elevated in gestational diabetes and inversely regulated by glucose and insulin. Sci. Rep. 2018, 8, 1366. [Google Scholar] [CrossRef]
- Wander, P.L.; Boyko, E.J.; Hevner, K.; Parikh, V.J.; Tadesse, M.G.; Sorensen, T.K.; Williams, M.A.; Enquobahrie, D.A. Circulating early- and mid-pregnancy microRNAs and risk of gestational diabetes. Diabetes Res. Clin. Pract. 2017, 132, 1–9. [Google Scholar] [CrossRef]
- Hocaoglu, M.; Demirer, S.; Karaalp, I.L.; Kaynak, E.; Attar, E.; Turgut, A.; Karateke, A.; Komurcu-Bayrak, E. Identification of miR-16-5p and miR-155-5p microRNAs differentially expressed in circulating leukocytes of pregnant women with polycystic ovary syndrome and gestational diabetes. Gynecol. Endocrinol. 2020, 37, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Hua, Z.; Li, D.; Wu, A.; Cao, T.; Luo, S. miR-377 inhibition enhances the survival of trophoblast cells via upregulation of FNDC5 in gestational diabetes mellitus. Open Med. 2021, 16, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Sun, J.; Liu, J.; Wang, L.; Dong, L. miR-181d promotes pancreatic beta cell dysfunction by targeting IRS2 in gestational diabetes mellitus. Ginekol. Pol. 2021, 92, 563–570. [Google Scholar] [CrossRef]
- Balci, S.; Gorur, A.; Yildirim, D.D.; Cayan, F.; Tamer, L. Expression level of miRNAS in patients with gestational diabetes. Turk. J. Biochem. 2020, 45, 825–831. [Google Scholar] [CrossRef]
- Ji, Y.; Zhang, W.; Yang, J.; Li, C. MiR-193b inhibits autophagy and apoptosis by targeting IGFBP5 in high glucose-induced trophoblasts. Placenta 2020, 101, 185–193. [Google Scholar] [CrossRef]
- Tu, C.; Wang, L.; Tao, H.; Gu, L.; Zhu, S.; Chen, X. Expression of miR-409-5p in gestational diabetes mellitus and its relationship with insulin resistance. Exp. Med. 2020, 20, 3324–3329. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, S.; Sánchez-Lechuga, B.; Donovan, P.; Halang, L.; Prehn, J.H.M.; Campos-Caro, A.; Byrne, M.M.; López-Tinoco, C. Circulating miR-330-3p in Late Pregnancy is Associated with Pregnancy Outcomes Among Lean Women with GDM. Sci. Rep. 2020, 10, 908. [Google Scholar] [CrossRef]
- Zhu, Y.; Tian, F.; Li, H.; Zhou, Y.; Lu, J.; Ge, Q. Profiling maternal plasma microRNA expression in early pregnancy to predict gestational diabetes mellitus. Int. J. Gynaecol. Obstet. 2015, 130, 49–53. [Google Scholar] [CrossRef]
- Zhao, C.; Dong, J.; Jiang, T.; Shi, Z.; Yu, B.; Zhu, Y.; Chen, D.; Xu, J.; Huo, R.; Dai, J.; et al. Early Second-Trimester Serum MiRNA Profiling Predicts Gestational Diabetes Mellitus. PLoS ONE 2011, 6, e23925. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, X.; Zhou, H. Role of cell free microRNA-19a and microRNA-19b in gestational diabetes mellitus patients. 3 Biotech 2019, 9, 406. [Google Scholar] [CrossRef]
- Gillet, V.; Ouellet, A.; Stepanov, Y.; Rodosthenous, R.S.; Croft, E.K.; Brennan, K.; Abdelouahab, N.; Baccarelli, A.; Takser, L. miRNA Profiles in Extracellular Vesicles from Serum Early in Pregnancies Complicated by Gestational Diabetes Mellitus. J. Clin. Endocrinol. Metab. 2019, 104, 5157–5169. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wang, H.; Li, C.; Zhang, X.; Xiu, X.; Teng, P.; Wang, Z. Dysregulation of microRNA-657 influences inflammatory response via targeting interleukin-37 in gestational diabetes mellitus. J. Cell. Physiol. 2019, 234, 7141–7148. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wang, Z.; Liu, G.; Jin, C.; Zhang, Q.; Man, S.; Wang, Z. miR-657 Promotes Macrophage Polarization toward M1 by Targeting FAM46C in Gestational Diabetes Mellitus. Mediat. Inflamm. 2019, 2019, 4851214. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Song, L.; Zhou, L.; Wu, J.; Sheng, C.; Chen, H.; Liu, Y.; Gao, S.; Huang, W. A MicroRNA Signature in Gestational Diabetes Mellitus Associated with Risk of Macrosomia. Cell. Physiol. Biochem. 2015, 37, 243–252. [Google Scholar] [CrossRef]
- Sørensen, A.E.; van Poppel, M.N.M.; Desoye, G.; Damm, P.; Simmons, D.; Jensen, D.M.; Dalgaard, L.T.; DALI Core Investigator Group. The Predictive Value of miR-16, -29a and -134 for Early Identification of Gestational Diabetes: A Nested Analysis of the DALI Cohort. Cells 2021, 10, 170. [Google Scholar] [CrossRef]
- Xu, K.; Bian, D.; Hao, L.; Huang, F.; Xu, M.; Qin, J.; Liu, Y. microRNA-503 contribute to pancreatic β cell dysfunction by targeting the mTOR pathway in gestational diabetes mellitus. EXCLI J. 2017, 16, 1177–1187. [Google Scholar]
- He, Y.; Bai, J.; Liu, P.; Dong, J.; Tang, Y.; Zhou, J.; Han, P.; Xing, J.; Chen, Y.; Yu, X. miR-494 protects pancreatic β-cell function by targeting PTEN in gestational diabetes mellitus. EXCLI J. 2017, 16, 1297–1307. [Google Scholar]
- Sebastiani, G.; Guarino, E.; Grieco, G.E.; Formichi, C.; Poggi, C.D.; Ceccarelli, E.; Dotta, F. Circulating microRNA (miRNA) expression Profiling in Plasma of Patients with gestational Diabetes Mellitus reveals Upregulation of miRNA mir-330-3p. Front. Endocrinol. 2017, 8, 345. [Google Scholar] [CrossRef]
- Filardi, T.; Catanzaro, G.; Grieco, G.E.; Splendiani, E.; Trocchianesi, S.; Santangelo, C.; Brunelli, R.; Guarino, E.; Sebastiani, G.; Dotta, F.; et al. Identification and Validation of miR-222-3p and miR-409-3p as Plasma Biomarkers in Gestational Diabetes Mellitus Sharing Validated Target Genes Involved in Metabolic Homeostasis. Int. J. Mol. Sci. 2022, 23, 4276. [Google Scholar] [CrossRef]
- Yu, X.; Liu, Z.; Fang, J.; Qi, H. miR-96-5p: A potential diagnostic marker for gestational diabetes mellitus. Medicine 2021, 100, e25808. [Google Scholar] [CrossRef]
- Zhou, X.; Xiang, C.; Zheng, X. miR-132 serves as a diagnostic biomarker in gestational diabetes mellitus and its regulatory effect on trophoblast cell viability. Diagn. Pathol. 2019, 14, 119. [Google Scholar] [CrossRef]
- Abdeltawab, A.; Zaki, M.E.; Abdeldayem, Y.; Mohamed, A.A.; Zaied, S.M. Circulating Micro RNA-223 and Angiopoietin-Like Protein 8 as Biomarkers of Gestational Diabetes mellitus. Br. J. Biomed. Sci. 2020, 78, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Zhao, C.; Guo, X.; Ding, H.; Cui, Y.; Shen, R.; Liu, J. Differential Expression of MicroRNAs in Omental Adipose Tissue from Gestational Diabetes Mellitus Subjects Reveals miR-222 as a Regulator of ER Expression in Estrogen-Induced Insulin Resistance. Endocrinology 2014, 155, 1982–1990. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Jiang, Y.; Li, L.-F.; Liu, X. Upregulation of YWHAZ in placental tissues, blood, and amniotic fluid from patients with gestational diabetes mellitus related to downregulation of microRNA-214. Int. J. Clin. Exp. Med. 2019, 12, 9961–9968. [Google Scholar]
- Sun, D.-G.; Tian, S.; Zhang, L.; Hu, Y.; Guan, C.-Y.; Ma, X.; Xia, H.-F. The miRNA-29b Is Downregulated in Placenta During Gestational Diabetes Mellitus and May Alter Placenta Development by Regulating Trophoblast Migration and Invasion Through a HIF3A-Dependent Mechanism. Front. Endocrinol. 2020, 11, 169. [Google Scholar] [CrossRef] [PubMed]
- Yoffe, L.; Polsky, A.; Gilam, A.; Raff, C.; Mecacci, F.; Ognibene, A.; Crispi, F.; Gratacós, E.; Kanety, H.; Mazaki-Tovi, S.; et al. Early diagnosis of gestational diabetes mellitus using circulating microRNAs. Eur. J. Endocrinol. 2019, 181, 565–577. [Google Scholar] [CrossRef]
- Zhang, Y.-L.; Chen, X.-Q. Dysregulation of microRNA-770-5p influences pancreatic-β-cell function by targeting TP53 regulated inhibitor of apoptosis 1 in gestational diabetes mellitus. Eur. Rev. Med. Pharm. Sci. 2020, 24, 793–801. [Google Scholar]
- Wen, J.; Bai, X. miR-520h Inhibits cell survival by targeting mTOR in gestational diabetes mellitus. Acta Biochim. Pol. 2021, 68, 65–70. [Google Scholar] [CrossRef]
- Zhang, M.; Zhu, X. miR-9-5p plays an important role in gestational diabetes mellitus (GDM) progression by targeting HK-2. Int. J. Clin. Exp. Med. 2018, 11, 6694–6701. [Google Scholar]
- Pheiffer, C.; Dias, S.; Rheeder, P.; Adam, S. Decreased Expression of Circulating miR-20a-5p in South African Women with Gestational Diabetes Mellitus. Mol. Diagn. Ther. 2018, 22, 345–352. [Google Scholar] [CrossRef]
- Feng, Y.; Qu, X.; Chen, Y.; Feng, Q.; Zhang, Y.; Hu, J.; Li, X. MicroRNA-33a-5p sponges to inhibit pancreatic β-cell function in gestational diabetes mellitus LncRNA DANCR. Reprod. Biol. Endocrinol. 2020, 18, 61. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, L.; Chen, J.; Song, F.; Guo, Y. Differential Expression of miR-136 in Gestational Diabetes Mellitus Mediates the High-Glucose-Induced Trophoblast Cell Injury through Targeting E2F1. Int. J. Genom. 2020, 2020, 3645371. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, K.; Tian, S.; Wang, X.-Q.; Li, J.-H.; Dong, Y.-C.; Xia, H.-F.; Ma, X. Down-regulation of microRNA-30d-5p is associated with gestational diabetes mellitus by targeting RAB8A. J. Diabetes Complicat. 2021, 35, 107959. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Pan, Y.; Dai, F.; Wang, F.; Qiu, H.; Huang, X. Serum miR-195-5p is upregulated in gestational diabetes mellitus. J. Clin. Lab. Anal. 2020, 34, e23325. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.-L.; Zhang, L.; Li, J.; Tian, S.; Lv, X.-D.; Wang, X.-Q.; Su, X.; Li, Y.; Hu, Y.; Ma, X.; et al. Up-regulation of miR-98 and unraveling regulatory mechanisms in gestational diabetes mellitus. Sci. Rep. 2016, 6, 32268. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.-L.; Jia, Y.-J.; Xing, B.; Shi, D.; Dong, X. Plasma microRNA-16-5p, -17-5p and -20a-5p: Novel diagnostic biomarkers for gestational diabetes mellitus. J. Obs. Gynaecol. Res. 2017, 43, 974–981. [Google Scholar] [CrossRef]
- Wang, F.; Li, Z.; Zhao, M.; Ye, W.; Wu, H.; Liao, Q.; Bu, S.; Zhang, Y. Circulating miRNAs miR-574-5p and miR-3135b are potential metabolic regulators for serum lipids and blood glucose in gestational diabetes mellitus. Gynecol. Endocrinol. 2021, 37, 665–671. [Google Scholar] [CrossRef]
- Van Oostdam, A.S.H.; Toro-Ortíz, J.C.; López, J.A.; Noyola, D.E.; Garcia-López, D.A.; Durán-Figueroa, N.V.; Martínez-Martínez, E.; Portales-Pérez, D.P.; Salgado-Bustamante, M.; López-Hernández, Y. Placental exosomes isolated from urine of patients with gestational diabetes exhibit a differential profile expression of microRNAs across gestation. Int. J. Mol. Med. 2020, 46, 546–560. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.-Y.; Li, H.-P.; Li, M.-Q. High glucose induces dysfunction of human umbilical vein endothelial cells by upregulating miR-137 in gestational diabetes mellitus. Microvasc. Res. 2018, 118, 90–100. [Google Scholar] [CrossRef]
- Tagoma, A.; Alnek, K.; Kirss, A.; Uibo, R.; Haller-Kikkatalo, K. MicroRNA profiling of second trimester maternal plasma shows upregulation of miR-195-5p in patients with gestational diabetes. Gene 2018, 672, 137–142. [Google Scholar] [CrossRef]
- Monfared, Y.K.; Ghadimi, F.; Foroughi, F.; Honardoost, M.; Hashemipour, S.; Sefidi, F.; Sarookhani, M.R. Determination and comparison miR135a in the serum between women with GDM, non-pregnant type 2 diabetes, healthy pregnant and control group. Middle East J. Fam. Med. 2018, 16, 193–197. [Google Scholar] [CrossRef]
- Wang, S.; Wei, D.; Sun, X.; Li, Y.; Li, D.; Chen, B. MiR-190b impedes pancreatic β cell proliferation and insulin secretion by targeting NKX6-1 and may associate to gestational diabetes mellitus. J. Recept. Signal Transduct. Res. 2021, 41, 349–356. [Google Scholar] [CrossRef]
- Gao, X.; Zhao, S. miRNA-16-5p inhibits the apoptosis of high glucose-induced pancreatic β cells via targeting of CXCL10: Potential biomarkers in type 1 diabetes mellitus. Endokrynol. Pol. 2020, 71, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Kwon, D.N.; Chang, B.S.; Kim, J.H. MicroRNA dysregulation in liver and pancreas of CMP-Neu5Ac hydroxylase null mice disrupts insulin/PI3K-AKT signaling. Biomed. Res. Int. 2014, 2014, 236385. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.; Ju, Y.; Ren, F.; Qiu, Y.; Tomita, Y.; Tomoeda, M.; Kishida, M.; Wang, Y.; Jin, L.; Su, F.; et al. Insulin receptor substrate 1/2 (IRS1/2) regulates Wnt/β-catenin signaling through blocking autophagic degradation of dishevelled2. J. Biol. Chem. 2014, 289, 11230–11241. [Google Scholar] [CrossRef]
- Li, H.-P.; Chen, X.; Li, M.-Q. Gestational diabetes induces chronic hypoxia stress and excessive inflammatory response in murine placenta. Int. J. Clin. Exp. Pathol. 2013, 6, 650–659. [Google Scholar]
- Li, Y.; Xiao, R.; Li, C.-P.; Huangfu, J.; Mao, J.-F. Increased plasma levels of FABP4 and PTEN is associated with more severe insulin resistance in women with gestational diabetes mellitus. Med. Sci. Monit. 2015, 21, 426–431. [Google Scholar]
- Magee, T.R.; Ross, M.G.; Wedekind, L.; Desai, M.; Kjos, S.; Belkacemi, L. Gestational diabetes mellitus alters apoptotic and inflammatory gene expression of trophobasts from human term placenta. J. Diabetes Complicat. 2014, 28, 448–459. [Google Scholar] [CrossRef]
- Nishiyama, A.; Yoshizumi, M.; Rahman, M.; Kobori, H.; Seth, D.M.; Miyatake, A.; Zhang, G.-X.; Yao, L.; Hitomi, H.; Shokoji, T.; et al. Effects of AT1 receptor blockade on renal injury and mitogen-activated protein activity in Dahl salt-sensitive rats. Kidney Int. 2004, 65, 972–981. [Google Scholar] [CrossRef]
- Perucci, L.O.; Gomes, K.B.; Freitas, L.G.; Godoi, L.C.; Alpoim, P.N.; Pinheiro, M.B.; Miranda, A.S.; Teixeira, A.L.; Dusse, L.M.; Sousa, L.P. Soluble endoglin, transforming growth factor-Beta 1 and soluble tumor necrosis factor alpha receptors in different clinical manifestations of preeclampsia. PLoS ONE 2014, 9, e97632. [Google Scholar] [CrossRef]
- Kumar, A.; Lawrence Jr, J.C.; Jung, D.Y.; Ko, H.J.; Keller, S.R.; Kim, J.K.; Magnuson, M.A.; Harris, T.E. Fat cell-specific ablation of rictor in mice impairs insulin-regulated fat cell and whole-body glucose and lipid metabolism. Diabetes 2010, 59, 1397–1406. [Google Scholar] [CrossRef] [PubMed]
- Qian, K.; Hu, L.; Chen, H.; Li, H.; Liu, N.; Li, Y.; Ai, J.; Zhu, G.; Tang, Z.; Zhang, H. Hsa-miR-222 is involved in differentiation of endometrial stromal cells in vitro. Endocrinology 2009, 150, 4734–4743. [Google Scholar] [CrossRef]
- Margaritis, K.; Margioula-Siarkou, G.; Giza, S.; Kotanidou, E.P.; Tsinopoulou, V.R.; Christoforidis, A.; Galli-Tsinopoulou, A. Micro-RNA implications in type-1 diabetes mellitus: A review of literature. Int. J. Mol. Sci. 2021, 22, 12165. [Google Scholar] [CrossRef] [PubMed]
- Poirier, C.; Desgagné, V.; Guérin, R.; Bouchard, L. MicroRNAs in pregnancy and gestational diabetes mellitus: Emerging role in maternal metabolic regulation. Curr. Diab. Rep. 2017, 17, 1–10. [Google Scholar] [CrossRef]
- Long, H.; Cundy, T. Establishing consensus in the diagnosis of gestational diabetes following HAPO: Where do we stand? Curr. Diabetes Rep. 2013, 13, 43–50. [Google Scholar] [CrossRef]
- Yang, Y.; Li, Q.; Wang, Q.; Ma, X. Thyroid antibodies and gestational diabetes mellitus: A meta-analysis. Fertil. Steril. 2015, 104, 665–671. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).