Abstract
Donor safety remains an important concern. We introduced preoperative bioelectrical impedance analysis (BIA) in living donor hepatectomy, as it is a practical method for volemia assessment with the advantages of noninvasiveness, rapid processing, easy handling, and it is relatively inexpensive. We analyzed 51 living donors who underwent right hemihepatectomy between July 2015 and May 2022. The ratio of extracellular water:total body water (ECW/TBW; an index of volemic status) was measured. ECT/TBW < 0.378 was correlated to central venous pressure (CVP) < 5 mm Hg in a previous study and we used this value for personalized preoperative management. In the BIA group (n = 21), donors with ECW/TBW ≥ 0.378 (n = 12) required whole-day nothing by mouth (NPO), whereas those with ECW/TBW < 0.378 (n = 9) required midnight NPO, similar to the control group (n = 30). In comparison with the control group, the BIA group had a significantly lower central venous pressure (p < 0.001) from the start of surgery to the end of surgery, leading to a better surgical field grade (p = 0.045) and decreased operative duration (240.5 ± 45.6 vs. 276.5 ± 54.0 min, p = 0.016). A cleaner surgical field (surgical field grade 1) was significantly associated with decreased operative duration (p = 0.001) and estimated blood loss (p < 0.001). Preoperative BIA was the only significant predictor of a cleaner surgical field (odds ratio, 6.914; 95% confidence interval, 1.6985–28.191, p = 0.007). In conclusion, preoperative volemia assessment using BIA can improve operative outcomes by creating a favorable surgical environment in living donor hepatectomy.
1. Introduction
As the demand for liver transplantation (LT) is increasing, living donor LT (LDLT) has emerged as a valuable alternative option to deceased donor LT (DDLT) [1]. Currently, LDLT is performed at a greater frequency than that of DDLT due to the limited availability of deceased donor organs, especially in Asian populations [2]. LDLT is a preferred treatment for selected hepatocellular carcinoma (HCC) patients because it can treat both the tumor and underlying liver diseases including hepatitis B viral infection, which increases the risk of tumor recurrence after hepatic resection [3,4]. In LDLT, donor safety remains a major concern because living donors are typically healthy adults who do not derive any medical benefit from the procedure. It is important to make consistent efforts to minimize the potential risks to the living donors to justify such an operation.
Excessive blood loss during hepatic resection has been reported to be significantly associated with postoperative morbidity and mortality [5]. Bleeding from the hepatic vein, which drains directly into the inferior vena cava (IVC), can be severe and cannot be controlled by portal triad clamping [6]. A low central venous pressure (CVP) during hepatic resection has been a widely used approach to reduce surgical blood loss and improve clinical outcomes [7]. Hepatic blood congestion, induced by elevated CVP, leads to an incremental increase in the transmural pressure and the distension of the hepatic vein, which is consequently torn easily, promoting blood loss at the time of parenchymal transection [8]. Preoperative fluid restriction is a widely used method for lowering the CVP; however, it is sometimes not sufficient, which makes hepatic resection difficult [9].
Bioelectrical impedance analysis (BIA) is a practical method for volemia assessment, which has the advantages of noninvasiveness, rapid processing, easy handling, and it is relatively inexpensive [10,11]. This method evaluates the human body composition, and the ratio of extracellular water to total body water (ECW/TBW) can be calculated as an index of volemic status [12]. A previous study showed that ECW/TBW and CVP were significantly correlated, which can be used preoperatively to significantly reduce the intraoperative bleeding during hepatic resection [13]. However, no previous study has focused on the clinical application of BIA for the preoperative volemia assessment of living donors. Here, we introduced BIA in living donor hepatectomy.
This study aimed to investigate the usefulness of preoperative BIA for maintaining a low CVP during hepatic resection, to improve operative outcomes in living donor hepatectomy.
2. Patients and Methods
2.1. Patients
The study population consisted of 51 consecutive living donors who underwent right hemihepatectomy for LDLT between March 2015 and May 2022 at our hospital. The donors were selected according to a standardized protocol described previously in [14]. The first 30 donors were enrolled in the control group, and the next 21 donors who underwent BIA for preoperative volemia assessment were enrolled in the BIA group. In the BIA group, the time of nothing by mouth (NPO) was decided based on the value of ECW/TBW, measured 1 day before surgery after admission; ECW/TBW < 0.378 was correlated to CVP < 5 mm Hg in a previous study [13]. Donors with ECW/TBW ≥ 0.378 (n = 12) required whole-day NPO, whereas those with ECW/TBW < 0.378 (n = 9) required midnight NPO, similar to the control group (Figure 1). Demographics and intraoperative and postoperative outcomes were compared between the groups.
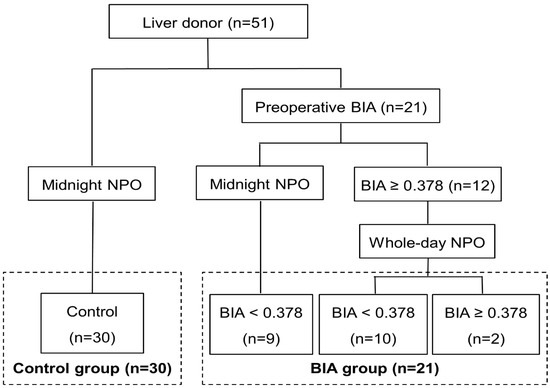
Figure 1.
Flow diagram of the study. Among 51 living donors, the first 30 consecutive donors were enrolled in the control group, and the next 21 donors with preoperative BIA were enrolled in the BIA group. Donors with ECW/TBW ≥ 0.378 had whole-day NPO, whereas those with ECW/TBW < 0.378 had midnight NPO, similar to the control group. BIA was selectively performed again on the operative day for donors with ECW/TBW ≥ 0.378 to analyze the changes in the value after whole-day NPO. BIA, bioelectrical impedance analysis; NPO, nothing by mouth.
2.2. Data Collection
Age, sex, presence of diabetes mellitus or hypertension, body mass index (BMI), type of graft, degree of hepatic steatosis, postoperative remnant liver volume, baseline liver function [total bilirubin (TB), international normalized ratio (INR), and albumin], and baseline renal function (creatinine) at admission were collected for all living donors. Operative details, such as operative duration, fluids administered including crystalloid and colloid, urine output, estimated blood loss, requirement for blood transfusion, inotropic use, and changes in CVP during hepatic resection were collected. The surgical field was assessed using a previously reported 4-point scale [15]: grade 1 = very lax IVC and minimal bleeding in the resection plane; grade 2 = lax IVC and a little bleeding in the resection plane; grade 3 = tense IVC and appreciable bleeding in the resection plane; grade 4 = very tense IVC, profuse bleeding in the resection plane, and very difficult to operate. Perioperative laboratory results, including TB, INR, albumin, and creatinine were analyzed. In addition, all available intake records, such as oral and parenteral fluids and output data including urine, gastrointestinal losses, and drains from the operative day to postoperative day 7 were collected. Acute kidney injury (AKI) was defined in accordance with the 2012 Kidney Disease Improving Global Outcomes guidelines, which have higher predictability compared with other criteria for assessing prognosis [16]; the guidelines were as follows: increase in serum creatinine by ≥0.3 mg/dL within 48 h, increase in serum creatinine to ≥1.5 times baseline within 7 days before surgery, or urine volume < 0.5 mL/kg/h for 6 h. Postoperative liver insufficiency was defined as a peak postoperative TB level > 7 mg/dL and/or the presence of ascites > 500 mL/day based on a previous study [17].
2.3. BIA
Body composition was assessed using a segmental and multifrequency BIA device (InBody S10; Biospace, Seoul, Korea) 1 day before surgery, after admission in the BIA group. Eight electrodes were placed on the surface of the thumb, fingers of the hand, and ball of the foot and heel with the patients in the supine position. We calculated the ECW/TBW ratio to estimate the volemic status of the patients. Three repeated measurements of BIA were performed, and the median value was chosen in order to avoid an incorrect measurement.
2.4. Anesthetic and Surgical Technique
Anesthetic management was performed using a standard protocol in our hospital. General anesthesia was induced with intravenous 100 μg of fentanyl and 1.2 mg/kg of propofol, followed by intravenous 1 mg/kg of rocuronium to facilitate endotracheal tube placement. General anesthesia was maintained with sevoflurane (2 to 3 volume%), nitrous oxide (1.8 L/min), and O2 (1.2 L/min). The CVP was continuously monitored using right jugular vein catheterization. Electrocardiogram, pulse oximetry, end-tidal carbon dioxide, invasive radial arterial pressure, and urine output were also monitored. Fluid was not administered preoperatively and was restrictively infused after the start of anesthesia, maintaining CVP less than 5 mm Hg until the hepatic parenchymal transection was complete. Thereafter, the crystalloid fluid was rapidly infused at 10 to 12 mL/kg/h to replace the surgical blood loss or fluid deficit, including insensible loss during the operation, and a colloid solution, hydroxyethyl-starch (HES), was used considering the volume status in the operating room. We used vasopressor drugs when the MAP decreased below 60 mm Hg. Mostly, 5 mg bolus of ephedrine was administered, but if an elevated heart rate was present, 50 mcg bolus of phenylephrine were injected. Red blood cells were transfused if the hemoglobin concentration decreased to <7 g/dL during the operation and in the perioperative period.
The detailed surgical techniques for mobilization of the liver, dissection of the hilum and parenchyma, and graft retrieval have been described previously in [18]. After the completion of hemostasis, a closed suction drain was inserted into the right subphrenic space. The abdominal wall was closed layer by layer. After subepidermal suturing, a sterile strip was applied to the skin incision.
2.5. Statistical Analysis
Clinico-demographic characteristics of living donors, changes in CVP during the operation and intraoperative and perioperative outcomes of patients were compared using the Student’s t-test for distributed data, presented as means ± standard deviations and the χ2 test for descriptive data. The surgical field grades were compared using the Mann–Whitney U test. Univariate and multivariate analysis of risk factors for a cleaner surgical field was performed using an ordinary logistic regression model. Differences in operative outcomes according to the surgical field grade were compared using the Student’s t-test. p values < 0.05 were considered to indicate statistical significance. Statistical analysis was conducted using the statistical package for the social sciences (SPSS) version 19.0 (IBM Corp., Armonk, NY, USA).
3. Results
The clinico-demographic characteristics of the living donors are summarized in Table 1. There were no significant differences in age, sex, prevalence of diabetes mellitus or hypertension, body mass index, hepatic steatosis, remnant liver volume, baseline liver function including TB, albumin, and INR, and baseline renal function (creatinine) between the two groups. The expanded criteria donors (age ≥ 50, BMI ≥ 25, hepatic macrosteatosis ≥ 10%, and remnant liver volume < 30%) also showed no significant differences between the two groups.

Table 1.
Clinico-demographic characteristics of living donors.
BIA was selectively performed again on the operative day for donors with ECW/TBW ≥ 0.378 to analyze the changes in the value after whole-day NPO. Overall, 10 of 12 donors (83.3%) showed a decrease in the ECW/TBW value to below 0.378, whereas 2 of 12 donors (16.7%) still had an ECW/TBW value ≥ 0.378.
The CVP was significantly lower in the BIA group than in the control group from the start of surgery to the end of surgery (p < 0.001). In both groups, the CVP showed a decreasing trend after the start of surgery until the completion of hepatic parenchymal transection and it was increased after fluid challenge (Figure 2).
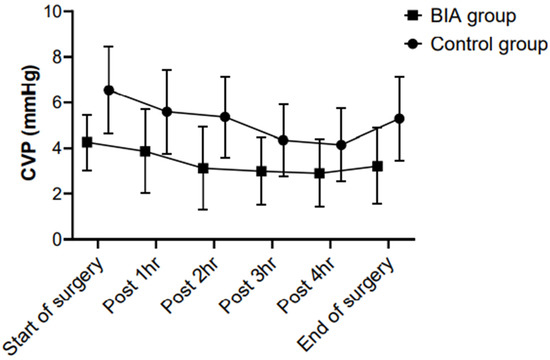
Figure 2.
Between-group comparisons of the CVP during hepatic resection. The CVP was significantly lower in the BIA group than in the control group from the start of surgery to the end of surgery (p < 0.001). In both groups, the CVP showed a decreasing trend after the start of surgery until the completion of hepatic parenchymal transection and was increased after fluid challenge. CVP, central venous pressure; BIA, bioelectrical impedance analysis.
The mean operative duration (240.5 ± 45.6 vs. 276.5 ± 54.0 min, p = 0.016) and hepatectomy time were significantly shorter in the BIA group than in the control group (2167.5 ± 32.5 vs. 203.9 ± 42.6 min, p = 0.002). Estimated blood loss was decreased in the BIA group compared with the control group; however, the difference was not significant (325 ± 212 vs. 382 ± 210 mL, p = 0.349). There were no significant between-group differences in the intraoperative fluid administration of crystalloid and colloid, amount of urine output, and inotropic use. There was no requirement for blood transfusion in both groups. With respect to postoperative complications, there were no significant differences in the incidence rates of pleural effusion, AKI, and postoperative liver insufficiency between the two groups. There was no major postoperative complication in both groups. The duration of postoperative hospital stay was slightly decreased in the BIA group compared with the control group, without statistical significance (10.4 ± 2.2 vs. 10.8 ± 3.6 days, p = 0.679; Table 2).

Table 2.
Operative outcomes of patients.
The surgical field grade showed a significant difference between the groups. In the BIA group, grade 1 (71.4%) was the most common, followed by grade 2 (28.6%), whereas in the control group, grade 2 (60.0%) was the most common, followed by grade 1 (36.7%) and there was one case of grade 3 (3.6%) (p = 0.045; Figure 3).
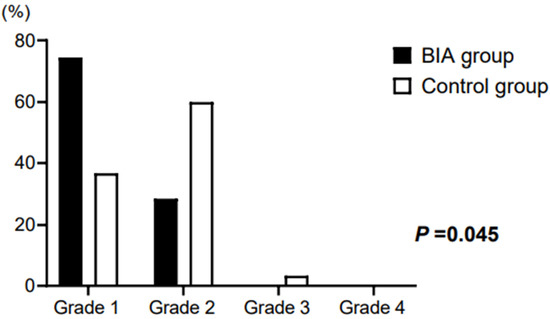
Figure 3.
Surgical field grade of patients. In the BIA group, grade 1 (71.4%) was the most common, followed by grade 2 (28.6%). In the control group, grade 2 (60.0%) was the most common, followed by grade 1 (36.7%) and grade 3 (3.6%) (p = 0.045). BIA, bioelectrical impedance analysis.
Preoperative BIA was the only significant predictor for a cleaner surgical field (surgical field grade 1) in multivariate analysis (odds ratio, 6.914; 95% confidence interval, 1.699–28.191, p = 0.007; Table 3).

Table 3.
Analysis of risk factors for cleaner surgical field.
Differences in the operative duration, estimated blood loss, and postoperative hospital stay of donors according to the surgical field grade were analyzed. Operative duration (238.1 ± 55.3 vs. 286.2 ± 38.8 min, p = 0.001) and estimated blood loss (196 ± 57 vs. 528 ± 174 mL, p < 0.001) were significantly decreased among donors with surgical field grade 1 compared with those with surgical field grade ≥ 2; however, there was no significant difference in postoperative hospital stay between the two groups (10.7 ± 3.6 vs. 10.6 ± 2.5 days, p = 0.988; Figure 4).
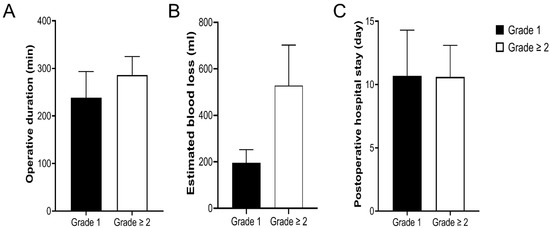
Figure 4.
Differences in operative duration (A), estimated blood loss (B), and postoperative hospital stay (C) according to the surgical field grade. A significant decrease in operative duration (p = 0.001) and estimated blood loss (p < 0.001) was observed among donors with surgical field grade 1 compared with those with surgical field grade ≥ 2. However, there was no significant difference in postoperative hospital stay between the two groups (p = 0.988).
4. Discussion
We investigated whether BIA is useful for preoperative volemia assessment in living donor hepatectomy. Donors with preoperative BIA had a significantly lower CVP during hepatic resection, which led to a better surgical field grade compared with those in the control group. Surgical field grade was significantly associated with decreased estimated blood loss and operative duration. Our results demonstrated that preoperative BIA can help improve the operative outcomes of living donors.
Maintaining a low CVP during hepatic resection significantly reduced intraoperative bleeding during hepatic resection in a previous study [8]. A low CVP is associated with low pressure in the hepatic vein and liver sinusoids, which reduces blood loss and allows easier control of bleeding in hepatic venous injury [19]. Although living donors with preoperative BIA had a significantly lower CVP with a better surgical field grade during hepatic resection compared with those in the control group, there was no significant difference in estimated blood loss between the two groups in this study. There might be several reasons for such discrepancies. First, some of the living donors maintained a low CVP only after midnight NPO; 11 of 30 donors (36.7%) in the control group had a CVP of <5 mm Hg in this study. Second, whole-day NPO was not sufficient for reducing the CVP; 2 of 12 donors (16.7%) in the BIA group with an ECW/TBW value of ≥0.378 at admission still had an elevated value on the operative day with a CVP of 6–7 mm Hg. Finally, the living donors had a normal healthy liver, which make hepatic parenchymal dissection and bleeding control easier than in other patients.
A study reported that BMI and hepatic steatosis were significant determinants of estimated blood loss in living donor hepatectomy [20]. Previously, obese donors were not considered for LDLT in many centers because some of them have moderate to severe hepatic steatosis, which may be associated with operative morbidity and mortality [21]. However, the use of expanded donor criteria has become more common owing to a considerable increase in waitlisted recipients and organ shortages in specific regions [2]. This study also included donors with BMI ≥ 25 (33.3%), the World Health Organization (WHO) definition of obesity for Asians [22]. We routinely performed magnetic resonance (MR) spectroscopy to screen for hepatic steatosis, and a weight reduction program was introduced to donors with ≥10% hepatic steatosis. They were given a scheduled protein-rich diet and performed exercises, and after several weeks of body weight reduction, follow-up MR spectroscopy was performed to evaluate the improvement in hepatic steatosis before surgery. Donors with a high BMI have increased ventilation pressure requirements, which can impair hepatic venous outflow and promote bleeding at the time of hepatic parenchymal transection [23]. Hepatic steatosis makes the liver tissue more frangible, which increases blood loss during hepatic resection [24]. Preoperative BIA for reducing the CVP during hepatic resection would be more important for these patients. In this study, preoperative BIA but not BMI and hepatic steatosis was the only significant predictor for surgical field grade ≥ 2.
BIA has many advantages for preoperative volemia assessment in living donor hepatectomy. First, it is a safe and noninvasive method that may replace CVP monitoring. It leads to a dry and cleaner surgical field, which is associated with a decrease in estimated blood loss; therefore, the placement of a central venous catheter is not required. Therefore, it can prevent catheter-related complications including arterial puncture, hematoma, pneumothorax, catheter-related infection, and thrombosis [25]. Second, it can quantify the volemic status of donors; thus, a personalized strategy for maintaining a low CVP suitable for hepatic resection may be possible. An exceedingly low CVP can increase the risk of the potential hypoperfusion of abdominal organs, causing AKI or air embolism of the lungs through hepatic venous injury during hepatic resection [26,27]. Third, it is easily and rapidly carried out with a portable instrument; thus, repetitive measurements to evaluate changes in the volemic status according to the duration of NPO are convenient.
There are some limitations in this study. This study was retrospective in nature, and the accuracy of the data analyzed relies on the completeness of medical records. Living donors in the control group received the operation before those in BIA group, so their experience of the operation might have influenced the operative outcomes. In addition, the study population was relatively small; thus, further large-scale prospective studies are warranted to confirm whether preoperative BIA can circumvent CVP monitoring without any increase in operative morbidity and mortality.
5. Conclusions
Living donors with preoperative BIA had a significantly lower CVP during hepatic resection, which led to a better surgical field grade and a decrease in the operative duration compared with those in the control group. A cleaner surgical field was significantly associated with decreased operative duration and estimated blood loss. Preoperative BIA was the only significant predictor a cleaner surgical field. Therefore, preoperative volemia assessment by BIA can help improve operative outcomes by creating a favorable surgical environment in living donor hepatectomy.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was approved by the Institutional Review Board of Chung-Ang University Hospital (IRB No. 2010-006-19335).
Informed Consent Statement
This study was exempt from the requirement to obtain informed consent because patient records were analyzed and no patient identification data were used.
Data Availability Statement
Data available from the authors upon request.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
BIA: bioelectrical impedance analysis; ECW/TBW, extracellular water:total body water; NPO, nothing by mouth; LT, liver transplantation; LDLT, living donor liver transplantation; DDLT, deceased donor liver transplantation; CVP, central venous pressure; BMI, body mass index; TB, total bilirubin; INR, international normalized ratio; IVC, inferior vena cava; AKI, acute kidney injury
References
- Humar, A.; Ganesh, S.; Jorgensen, D.; Tevar, A.; Ganoza, A.; Molinari, M.; Hughes, C. Adult living donor versus deceased donor liver transplant (LDLT versus DDLT) at a single center: Time to change our paradigm for liver transplant. Ann. Surg. 2019, 270, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Tran, L.; Humar, A. Current status of adult liver transplantation: Utilization of living donor versus deceased donor graft. Curr. Opin. Organ. Transplant. 2021, 26, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.; Shindoh, J.; Margonis, G.A.; Nishioka, Y.; Andreatos, N.; Sekine, A.; Hashimoto, M.; Pawlik, T.M. Effect of background liver cirrhosis on outcomes of hepatectomy for hepatocellular carcinoma. JAMA Surg. 2017, 152, e165059. [Google Scholar] [CrossRef]
- Menahem, B.; Lubrano, J.; Duvoux, C.; Mulliri, A.; Alves, A.; Costentin, C.; Mallat, A.; Launoy, G.; Laurent, A. Liver transplantation versus liver resection for hepatocellular carcinoma in intention to treat: An attempt to perform an ideal meta-analysis. Liver Transpl. 2017, 23, 836–844. [Google Scholar] [CrossRef] [PubMed]
- Niemann, C.U.; Feiner, J.; Behrends, M.; Eilers, H.; Ascher, N.L.; Roberts, J.P. Central venous pressure monitoring during living right donor hepatectomy. Liver Transpl. 2007, 13, 266–271. [Google Scholar] [CrossRef]
- Topaloglu, S.; Yesilcicek, C.K.; Calik, A.; Aydin, C.; Kocyigit, S.; Yaman, H.; Kutanis, D.; Karabulut, E.; Dohman, D.; Orem, A.; et al. Efficacy and safety of hepatectomy performed with intermittent portal triad clamping with low central venous pressure. Biomed Res. Int. 2013, 2013, 297971. [Google Scholar] [CrossRef] [PubMed]
- Hughes, M.J.; Ventham, N.T.; Harrison, E.M.; Wigmore, S.J. Central venous pressure and liver resection: A systematic review and meta-analysis. HPB 2015, 17, 863–871. [Google Scholar] [CrossRef]
- Zhang, X.L.; Wang, W.J.; Wang, W.J.; Cao, N. Effectiveness and safety of controlled venous pressure in liver surgery: A systematic review and network meta-analysis. BioMed Res Int. 2015, 2015, 290234. [Google Scholar] [CrossRef][Green Version]
- Yu, L.; Sun, H.; Jin, H.; Tan, H. The effect of low central venous pressure on hepatic surgical field bleeding and serum lactate in patients undergoing partial hepatectomy: A prospective randomized controlled trial. BMC Surg. 2020, 20, 25. [Google Scholar] [CrossRef]
- Davies, S.J.; Davenport, A. The role of bioimpedance and biomarkers in helping to aid clinical decision-making of volume assessments in dialysis patients. Kidney Int. 2014, 86, 489–496. [Google Scholar] [CrossRef]
- Zhu, F.; Levin, N.W. Estimation of body composition and normal fluid status using a calf bioimpedance technique. Blood Purif. 2015, 39, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Lopot, F.; Nejedly, B.; Novotna, H.; Mackova, M.; Sulkova, S. Age-related extracellular to total body water volume ratio (Ecv/TBW)--can it be used for “dry weight” determination in dialysis patients? Application of multifrequency bioimpedance measurement. Int. J. Artif. Organs 2002, 25, 762–769. [Google Scholar] [CrossRef] [PubMed]
- Suh, S.W.; Park, H.J.; Choi, Y.S. Preoperative volume assessment using bioelectrical impedance analysis for minimizing blood loss during hepatic resection. HPB 2022, 24, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Suh, K.S.; Suh, S.W.; Lee, J.M.; Choi, Y.; Yi, N.J.; Lee, K.W. Recent advancements in and views on the donor operation in living donor liver transplantation: A single-center study of 886 patients over 13 years. Liver Transpl. 2015, 21, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Ryu, H.G.; Nahm, F.S.; Sohn, H.M.; Jeong, E.J.; Jung, C.W. Low central venous pressure with milrinone during living donor hepatectomy. Am. J. Transplant. 2010, 10, 877–882. [Google Scholar] [CrossRef] [PubMed]
- Khwaja, A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin. Pract. 2012, 120, c179–c184. [Google Scholar] [CrossRef]
- Mullen, J.T.; Ribero, D.; Reddy, S.K.; Donadon, M.; Zorzi, D.; Gautam, S.; Abdalla, E.K.; Curley, S.A.; Capussotti, L.; Clary, B.M.; et al. Hepatic insufficiency and mortality in 1059 noncirrhotic patients undergoing major hepatectomy. J. Am. Coll. Surg. 2007, 204, 854–864. [Google Scholar] [CrossRef]
- Yi, N.J.; Suh, K.S.; Cho, J.Y.; Lee, H.W.; Cho, E.H.; Yang, S.H.; Cho, Y.B.; Lee, K.U. Three-quarters of right liver donors experienced postoperative complications. Liver Transpl. 2007, 13, 797–806. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Sun, Y.M.; Wu, F.X.; Yang, L.Q.; Lu, Z.J.; Yu, W.F. Controlled low central venous pressure reduces blood loss and transfusion requirements in hepatectomy. World J. Gastroenterol. 2014, 20, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.K.; Chin, J.H.; Kang, S.J.; Jun, I.G.; Song, J.G.; Jeong, S.M.; Park, J.Y.; Hwang, G.S. Association between central venous pressure and blood loss during hepatic resection in 984 living donors. Acta Anaesthesiol. Scand. 2009, 53, 601–606. [Google Scholar] [CrossRef]
- Shin, Y.H.; Ko, J.S.; Kim, G.S.; Gwak, M.S.; Sim, W.S.; Lee, A.R.; Yi, H.W.; Joh, J.W. Impact of hepatic macrovesicular and microvesicular steatosis on the postoperative liver functions after right hepatectomy in living donors. Transplant. Proc. 2012, 44, 512–515. [Google Scholar] [CrossRef] [PubMed]
- Low, S.; Chin, M.C.; Ma, S.; Heng, D.; Deurenberg-Yap, M. Rationale for redefining obesity in Asians. Ann. Acad. Med. Singap. 2009, 38, 66–69. [Google Scholar] [PubMed]
- Saunders, J.K.; Rosman, A.S.; Neihaus, D.; Gouge, T.H.; Melis, M. Safety of hepatic resections in obese veterans. Arch. Surg. 2012, 147, 331–337. [Google Scholar] [PubMed]
- McCormack, L.; Petrowsky, H.; Jochum, W.; Furrer, K.; Clavien, P.A. Hepatic steatosis is a risk factor for postoperative complications after major hepatectomy: A matched case-control study. Ann. Surg. 2007, 245, 923–930. [Google Scholar] [CrossRef]
- Dunki-Jacobs, E.M.; Philips, P.; Scoggins, C.R.; McMasters, K.M.; Martin, R.C.G., II. Stroke volume variation in hepatic resection: A replacement for standard central venous pressure monitoring. Ann. Surg. Oncol. 2014, 21, 473–478. [Google Scholar] [CrossRef]
- Shih, T.H.; Tsou, Y.H.; Huang, C.J.; Chen, C.L.; Cheng, K.W.; Wu, S.C.; Yang, S.S.; Juang, S.E.; Huang, C.E.; Lee, Y.E.; et al. The correlation between CVP and SVV and intraoperative minimal blood loss in living donor hepatectomy. Transplant. Proc. 2018, 50, 2661–2663. [Google Scholar] [CrossRef]
- Correa-Gallego, C.; Berman, A.; Denis, S.C.; Langdon-Embry, L.; O’Connor, D.; Arslan-Carlon, V.; Kingham, T.P.; D’Angelica, M.I.; Allen, P.J.; Fong, Y.; et al. Renal function after low central venous pressure-assisted liver resection: Assessment of 2116 cases. HPB 2015, 17, 258–264. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).