From Radical Resection to Precision Surgery: Integrating Diagnostic Biomarkers, Radiomics-Based Predictive Models, and Perioperative Systemic Therapy in Head and Neck Oncology †
Abstract
1. Introduction
2. Historical Evolution and Paradigm Shifts
2.1. The Era of Radical Surgery (19th Century–1970s)
2.2. The Organ Preservation Movement (1980s–2000s)
2.3. The Era of Minimally Invasive Precision Surgery and Diagnostic Integration (2000s–Present)
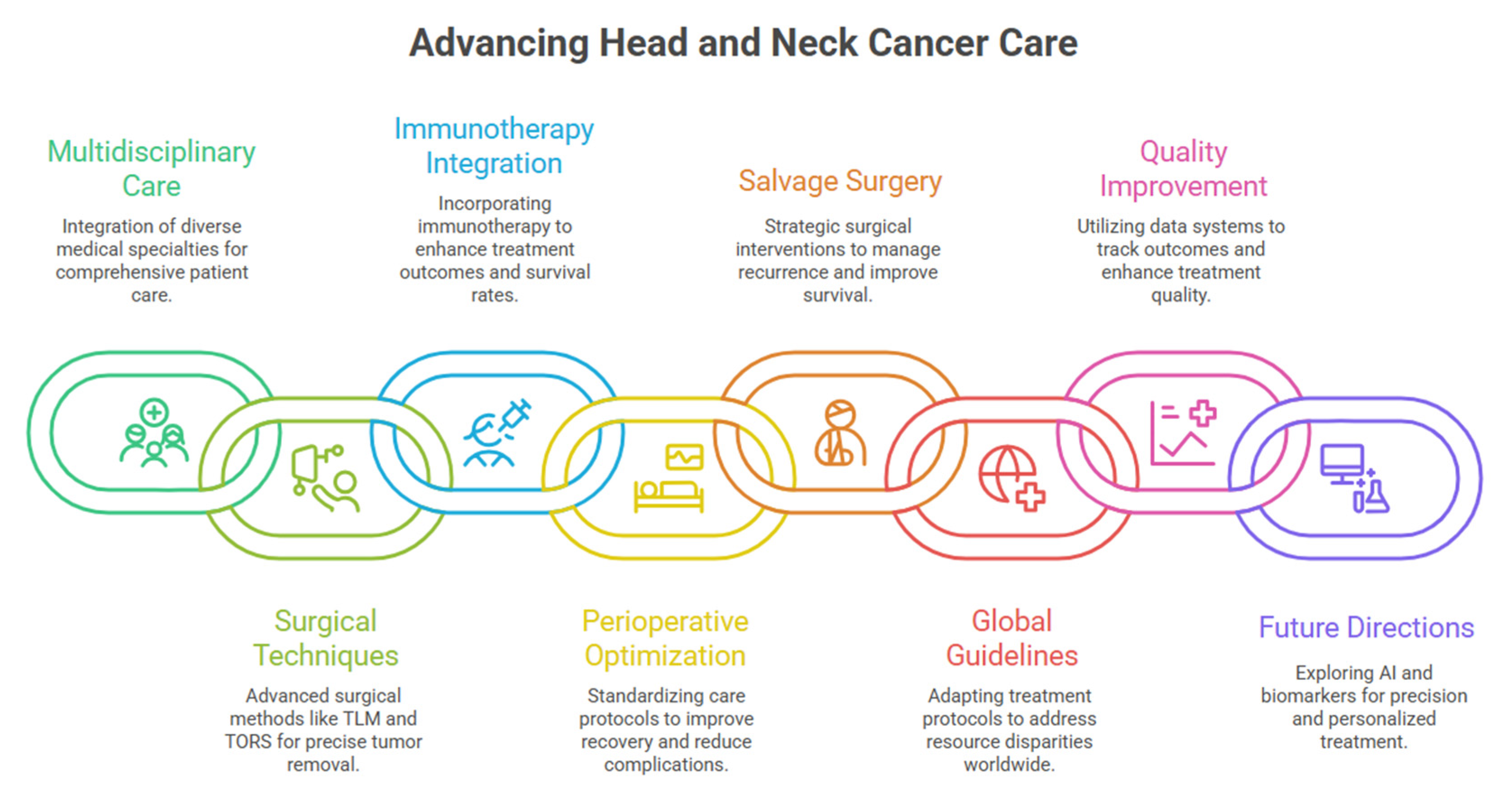
3. Multidisciplinary Care as the Foundation of Contemporary Practice
3.1. Evidence-Based Benefits of Integrated Care Models
3.2. Contemporary Treatment Team Structure
4. Contemporary Surgical Techniques and Evidence-Based Implementation
4.1. Transoral Laser Microsurgery: Precision Through Technology
4.2. Transoral Robotic Surgery: Evidence-Based Guidelines and Expanding Applications
4.3. Radical Surgical Approaches: Contemporary Indications
5. Evidence-Based Patient Selection Criteria
6. Diagnostic Biomarker Integration and Perioperative Systemic Therapy
Validated Predictive Biomarkers for Immunotherapy Selection
7. Comprehensive Neoadjuvant Strategies: Chemotherapy, Chemoradiation, and Targeted Approaches
7.1. Neoadjuvant Chemotherapy Plus Surgery
7.2. Neoadjuvant Chemoradiation
7.3. Neoadjuvant Targeted Therapies in Thyroid Cancer
8. Perioperative Care Coordination
9. Precision Medicine Integration: Molecular Profiling and Advanced Diagnostic Imaging
9.1. Molecular Pathology: From Single Biomarkers to Comprehensive Genomic Landscapes
9.2. Immune Microenvironment Characterization as Predictive Biomarker
9.3. Imaging Innovations: Radiomics-Based Predictive Models and Diagnostic Validation
9.4. Habitat Imaging for Neoadjuvant Response Prediction
9.5. Peptide Receptor Radionuclide Therapy in Advanced Thyroid Cancers
9.6. Fibroblast Activation Protein Radioligand Therapy: Emerging Approach
10. Impact on Neoadjuvant-to-Surgery Pathways
11. Salvage Surgery: Contemporary Stratification and Outcomes
Advanced Risk Stratification for Recurrent Disease
12. Global Perspectives: Resource-Adapted Treatment Strategies
Consensus Guidelines
13. Quality Improvement and Outcome Measurement
Development of Specialty-Specific Metrics
14. Emerging Technologies and Future Directions
Artificial Intelligence and Machine Learning Applications
15. Precision Medicine Integration: Future Directions for Diagnostic Technologies
16. Current Challenges and Future Integration
17. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jalisi, S. Head and neck tumors: Viewpoint—Surgery. In Head and Neck Tumors; Springer: New York, NY, USA, 2015. [Google Scholar] [CrossRef]
- Contrera, K.J.; Reddy, P.D.; Helou, V.; Myers, J.N.; Skinner, H.D.; Ferrarotto, R.; Myers, E.N. The evolution of multidisciplinary head and neck cancer treatment. Laryngoscope 2025, 135, 2255–2260. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shah, J.P. The changing role of surgery in the multidisciplinary management of tumors of the head and neck. Acad. Oncol. 2025, 2. [Google Scholar] [CrossRef]
- Department of Veterans Affairs Laryngeal Cancer Study Group; Wolf, G.T.; Fisher, S.G.; Hong, W.K.; Hillman, R.; Spaulding, M.; Laramore, G.E.; Endicott, J.W.; McClatchey, K.; Henderson, W.G. Induction chemotherapy plus radiation compared with surgery plus radiation in patients with advanced laryngeal cancer. N. Engl. J. Med. 1991, 324, 1685–1690. [Google Scholar] [CrossRef] [PubMed]
- Forastiere, A.A.; Goepfert, H.; Maor, M.; Pajak, T.F.; Weber, R.; Morrison, W.; Glisson, B.; Trotti, A.; Ridge, J.A.; Chao, C.; et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N. Engl. J. Med. 2003, 349, 2091–2098. [Google Scholar] [CrossRef] [PubMed]
- Andry, G.; Hamoir, M.; Leemans, C.R. The evolving role of surgery in the management of head and neck tumors. Curr. Opin. Oncol. 2005, 17, 241–248. [Google Scholar] [CrossRef]
- Ferlito, A.; Johnson, J.T.; Rinaldo, A.; Pratt, L.W.; Fagan, J.J.; Weir, N.; Suárez, C.; Folz, B.J.; Bień, S.; Towpik, E.; et al. European surgeons were the first to perform neck dissection. Laryngoscope 2007, 117, 797–802. [Google Scholar] [CrossRef] [PubMed]
- Sanabria, A.; Domenge, C.; D’Cruz, A.; Kowalski, L.P. Organ preservation protocols in developing countries. Curr. Opin. Otolaryngol. Head Neck Surg. 2010, 18, 83–88. [Google Scholar] [CrossRef]
- Holsinger, F.C.; Ferris, R.L. Transoral endoscopic head and neck surgery and its role within the multidisciplinary treatment paradigm of oropharynx cancer: Robotics, lasers, and clinical trials. J. Clin. Oncol. 2015, 33, 3285–3292. [Google Scholar] [CrossRef]
- Sadeghi, N.; Mascarella, M.A.; Khalife, S.; Ramanakumar, A.V.; Richardson, K.; Joshi, A.S.; Taheri, R.; Fuson, A.; Bouganim, N.; Siegel, R. Neoadjuvant chemotherapy followed by surgery for HPV-associated locoregionally advanced oropharynx cancer. Head Neck 2020, 42, 2145–2154. [Google Scholar] [CrossRef] [PubMed]
- Uppaluri, R.; Haddad, R.I.; Tao, Y.; Le Tourneau, C.; Lee, N.Y.; Westra, W.; Chernock, R.; Tahara, M.; Harrington, K.J.; Klochikhin, A.L.; et al. Neoadjuvant and adjuvant pembrolizumab in locally advanced head and neck cancer. N. Engl. J. Med. 2025, 393, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Ang, K.K.; Harris, J.; Wheeler, R.; Weber, R.; Rosenthal, D.I.; Nguyen-Tân, P.F.; Westra, W.H.; Chung, C.H.; Jordan, R.C.; Lu, C.; et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N. Engl. J. Med. 2010, 363, 24–35. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, S.W.; Li, S.H.; Shi, D.B.; Jiang, W.M.; Song, M.; Yang, A.K.; Li, Y.D.; Bei, J.X.; Chen, W.K.; Zhang, Q. Expression of PD-1/PD-L1 in head and neck squamous cell carcinoma and its clinical significance. Int. J. Biol. Markers 2019, 34, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Jawdyński, F. A case of the primary cancer of the neck, so-called Volkmann’s branchiogenic cancer. Resection together with internal jugular vein and common carotid artery. Cure. Gaz Lek. 1888, 8, 530–537. [Google Scholar]
- Liu, J.C.; Kaplon, A.; Blackman, E.; Miyamoto, C.; Savior, D.; Ragin, C. The impact of the multidisciplinary tumor board on head and neck cancer outcomes. Laryngoscope 2020, 130, 946–950. [Google Scholar] [CrossRef] [PubMed]
- Burkhardt, V.; El-Shabrawi, K.; Riemann, S.; Voss, P.; Becker, C. Multidisciplinary tumor boards in oral cavity cancer: Survival effect due to balancing guideline adherence and treatment delays. Front. Oral Health 2024, 5, 1493319. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schmitt, J.; Klinkhammer-Schalke, M.; Bierbaum, V.; Gerken, M.; Bobeth, C.; Rößler, M.; Dröge, P.; Ruhnke, T.; Günster, C.; Kleihues-van Tol, K.; et al. Initial cancer treatment in certified versus non-certified hospitals. Dtsch. Arztebl. Int. 2023, 120, 647–654. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Holsinger, F.C.; Ismaila, N.; Adkins, D.R.; Barber, B.R.; Burnette, G.; Fakhry, C.; Galloway, T.J.; Goepfert, R.P.; Miles, B.A.; Paleri, V.; et al. Transoral robotic surgery in the multidisciplinary care of patients with oropharyngeal squamous cell carcinoma: ASCO guideline. J. Clin. Oncol. 2025, 43, 1369–1392. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.J.; Shah, J.P. The role of the head and neck surgeon in contemporary multidisciplinary treatment programs for advanced head and neck cancer. Curr. Opin. Otolaryngol. Head Neck Surg. 2010, 18, 79–82. [Google Scholar] [CrossRef]
- Piazza, C.; Del Bon, F.; Nicolai, P.; Peretti, G. Nuove frontiere della chirurgia conservativa: Chirurgia laser e robot-assistita. In Chirurgia Conservativa del Distretto Cervico-Cefalico; Springer: Milano, Italy, 2011; pp. 159–174. [Google Scholar] [CrossRef]
- Ambrosch, P.; Meuret, S.; Dietz, A.; Fazel, A.; Fietkau, R.; Tostmann, R.; Schroeder, U.; Lammert, A.; Künzel, J.; Jäckel, M.C.; et al. Transoral laser microsurgery for supraglottic carcinomas: Results of a prospective multicenter trial (SUPRATOL). Front. Oncol. 2024, 14, 1440024. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Steiner, W.; Ambrosch, P. Endoscopic Laser Surgery of the Upper Aerodigestive Tract; Georg Thieme Verlag: Stuttgart, Germany, 2000. [Google Scholar]
- Ferris, R.L.; Flamand, Y.; Weinstein, G.S.; Li, S.; Quon, H.; Mehra, R.; Garcia, J.J.; Chung, C.H.; Gillison, M.L.; Duvvuri, U.; et al. Phase II randomized trial of transoral surgery and low-dose intensity modulated radiation therapy in resectable p16+ locally advanced oropharynx cancer: An ECOG-ACRIN Cancer Research Group Trial (E3311). J. Clin. Oncol. 2022, 40, 138–149. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rao, K.N.; Gangiti, K.K. Transoral robotic surgery. Indian J. Surg. Oncol. 2021, 12, 847–853. [Google Scholar] [CrossRef]
- Holsinger, F.C.; Magnuson, J.S.; Weinstein, G.S.; Chan, J.Y.K.; Starmer, H.M.; Tsang, R.K.Y.; Wong, E.W.Y.; Rassekh, C.H.; Bedi, N.; Hong, S.S.Y.; et al. A next-generation single-port robotic surgical system for transoral robotic surgery: Results from prospective nonrandomized clinical trials. JAMA Otolaryngol. Head Neck Surg. 2019, 145, 1027–1034. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ronen, O.; Robbins, K.T.; de Bree, R.; Guntinas-Lichius, O.; Hartl, D.M.; Homma, A.; Khafif, A.; Kowalski, L.P.; López, F.; Mäkitie, A.A.; et al. Standardization for oncologic head and neck surgery. Eur. Arch. Otorhinolaryngol. 2021, 278, 4663–4669. [Google Scholar] [CrossRef] [PubMed]
- Hartl, D.M.; Brasnu, D.F.; Shah, J.P.; Hinni, M.L.; Takes, R.P.; Olsen, K.D.; Kowalski, L.P.; Rodrigo, J.P.; Strojan, P.; Wolf, G.T.; et al. Is open surgery for head and neck cancers truly declining? Eur. Arch. Otorhinolaryngol. 2013, 270, 2793–2802. [Google Scholar] [CrossRef] [PubMed]
- Kaki, P.C.; Lam, D.; Sangal, N.R.; Rajasekaran, K.; Chalian, A.C.; Brody, R.M.; Weinstein, G.S.; Cannady, S.B. Transoral robotic surgery with free flap reconstruction: Functional outcomes of 241 patients at a single institution. Head Neck 2024, 46, 1601–1613. [Google Scholar] [CrossRef] [PubMed]
- Suárez, C.; Rodrigo, J.P. Transoral microsurgery for treatment of laryngeal and pharyngeal cancers. Curr. Oncol. Rep. 2013, 15, 134–141. [Google Scholar] [CrossRef]
- Hirsch, F.R.; McElhinny, A.; Stanforth, D.; Ranger-Moore, J.; Jansson, M.; Kulangara, K.; Richardson, W.; Towne, P.; Hanks, D.; Vennapusa, B.; et al. PD-L1 Immunohistochemistry Assays for Lung Cancer: Results from Phase 1 of the Blueprint PD-L1 IHC Assay Comparison Project. J. Thorac. Oncol. 2017, 12, 208–222. [Google Scholar] [CrossRef] [PubMed]
- Imamura, Y.; Kanno, M.; Fujieda, S. Comparative review of KEYNOTE-689 and NIVOPOSTOP trials and their impact on perioperative immunotherapy in locally advanced head and neck cancer. Cancer Treat Rev. 2025, 140, 103018. [Google Scholar] [CrossRef]
- Bourhis, J.; Auperin, A.; Borel, C.; Lefebvre, G.; Racadot, S.; Geoffrois, L.; Sun, X.S.; Saada, E.; Cirauqui, B.; Rutkowski, T.; et al. NIVOPOSTOP (GORTEC 2018-01): A phase III randomized trial of adjuvant nivolumab added to radio-chemotherapy in patients with resected head and neck squamous cell carcinoma at high risk of relapse. J. Clin. Oncol. 2025, 43, LBA2. [Google Scholar] [CrossRef]
- Huang, S.H.; Perez-Ordonez, B.; Weinreb, I.; Hope, A.; Massey, C.; Waldron, J.N.; Kim, J.; Bayley, A.J.; Cummings, B.; Cho, B.C.; et al. Natural course of distant metastases following radiotherapy or chemoradiotherapy in HPV-related oropharyngeal cancer. Oral Oncol. 2013, 49, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Pedroso, C.M.; de Pauli Paglioni, M.; Normando, A.G.C.; Chaves, A.L.F.; Kowalski, L.P.; Júnior, G.d.C.; Matos, L.L.; Junior, W.N.W.; de Oliveira, T.B.; de Marchi, P.; et al. Preoperative neoadjuvant chemotherapy or immunotherapy in head and neck cancer: A systematic review and meta-analysis of surgical risk and pathologic response. Crit. Rev. Oncol. Hematol. 2025, 212, 104742. [Google Scholar] [CrossRef]
- Chaukar, D.; Prabash, K.; Rane, P.; Patil, V.M.; Thiagarajan, S.; Ghosh-Laskar, S.; Sharma, S.; Pai, P.S.; Chaturvedi, P.; Pantvaidya, G.; et al. Prospective phase II open-label randomized controlled trial to compare mandibular preservation in upfront surgery with neoadjuvant chemotherapy followed by surgery in operable oral cavity cancer. J. Clin. Oncol. 2022, 40, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.; Kumar, P.; Pai, V.R.; Parikh, P.M. Neoadjuvant chemotherapy or chemoradiotherapy in head and neck cancer. Indian J. Cancer 2008, 45, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Riml, S.; Böhler, F.; Larcher, L.; de Vries, A.; Elsässer, W.; Kompatscher, P. Neoadjuvant radiotherapy of head and neck carcinoma: An obstacle for plastic reconstruction? Wien. Klin. Wochenschr. 2012, 124, 599–604. [Google Scholar] [CrossRef] [PubMed]
- Farlow, J.L.; McCrary, H.C.; Sipos, J.A.; Phay, J.E.; Konda, B.; Agrawal, A. Neoadjuvant dabrafenib and trametinib for functional organ preservation in recurrent BRAF V600E-mutated papillary thyroid cancer. Oral Oncol. 2023, 147, 106625. [Google Scholar] [CrossRef] [PubMed]
- Cabanillas, M.E.; Ferrarotto, R.; Garden, A.S.; Ahmed, S.; Busaidy, N.L.; Dadu, R.; Williams, M.D.; Skinner, H.; Gunn, G.B.; Grosu, H.; et al. Neoadjuvant BRAF- and Immune-Directed Therapy for Anaplastic Thyroid Carcinoma. Thyroid 2018, 28, 945–951. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jozaghi, Y.; Zafereo, M.; Williams, M.D.; Gule-Monroe, M.K.; Wang, J.; Grubbs, E.G.; Vaporciyan, A.; Hu, M.I.; Busaidy, N.; Dadu, R.; et al. Neoadjuvant selpercatinib for advanced medullary thyroid cancer. Head Neck 2021, 43, E7–E12. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Subbiah, V.; Kreitman, R.J.; Wainberg, Z.A.; Cho, J.Y.; Schellens, J.H.M.; Soria, J.C.; Wen, P.Y.; Zielinski, C.; Cabanillas, M.E.; Urbanowitz, G.; et al. Dabrafenib and Trametinib Treatment in Patients With Locally Advanced or Metastatic BRAF V600-Mutant Anaplastic Thyroid Cancer. J. Clin. Oncol. 2018, 36, 7–13. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dort, J.C.; Farwell, D.G.; Findlay, M.; Huber, G.F.; Kerr, P.; Shea-Budgell, M.A.; Simon, C.; Uppington, J.; Zygun, D.; Ljungqvist, O.; et al. Optimal perioperative care in major head and neck cancer surgery with free flap reconstruction: A consensus review and recommendations from the Enhanced Recovery After Surgery Society. JAMA Otolaryngol. Head Neck Surg. 2017, 143, 292–303. [Google Scholar] [CrossRef] [PubMed]
- Asarkar, A.A.; Vasudevan, S.S.; Fernandez-Alvarez, V.; Vermorken, J.B.; Álvarez, F.L.; Rao, K.N.; Saba, N.F.; de Bree, R.; Suárez, C.; Eisbruch, A.; et al. Global incidence and mortality of myocardial infarction in multi-modality head and neck cancer treatment: A systematic review and meta-analysis. Adv. Ther. 2025, 42, 4768–4796. [Google Scholar] [CrossRef]
- List, M.A.; Knackstedt, M.; Liu, L.; Kasabali, A.; Mansour, J.; Pang, J.; Asarkar, A.A.; Nathan, C.A. Enhanced recovery after surgery, current, and future considerations in head and neck cancer. Laryngoscope Investig. Otolaryngol. 2023, 8, 1240–1256. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schuman, A.D.; Bindal, M.; Amadio, G.; Turney, A.M.; Hernandez, D.J.; Sandulache, V.C.; Liou, N.E.; Wang, R.; Huang, A.T. Safety of An Enhanced Recovery After Surgery Protocol After Head and Neck Free Tissue Transfer. Laryngoscope 2024, 134, 4527–4534. [Google Scholar] [CrossRef] [PubMed]
- Van Hoe, S.; Hermans, R. Post-treatment surveillance imaging in head and neck cancer: A systematic review. Insights Imaging 2024, 15, 32. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Moubayed, S.P.; Mourad, M.; Urken, M.L. What Are the Optimal Monitoring Techniques in Head and Neck Microvascular Reconstruction? ORL J. Otorhinolaryngol. Relat. Spec. 2016, 78, 241–244. [Google Scholar] [CrossRef] [PubMed]
- Rothrie, S.; Fitzgerald, E.; Brady, G.C.; Roe, J.W.G. The role of the speech and language therapist in the rehabilitation of speech, swallowing, voice and trismus in people diagnosed with head and neck cancer. Br. Dent. J. 2022, 233, 801–805. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Li, H.; He, Y.; Yang, Z.; Yang, X.; Wei, J. Next-generation immunotherapies for head and neck squamous cell carcinoma: Targeting novel immune checkpoints. Ann. Med. 2025, 57, 2561223. [Google Scholar] [CrossRef]
- Isaic, A.; Motofelea, N.; Hoinoiu, T.; Motofelea, A.C.; Leancu, I.C.; Stan, E.; Gheorghe, S.R.; Dutu, A.G.; Crintea, A. Next-generation sequencing: A review of its transformative impact on cancer diagnosis, treatment, and resistance management. Diagnostics 2025, 15, 2425. [Google Scholar] [CrossRef]
- Tomlins, S.A.; Hovelson, D.H.; Suga, J.M.; Anderson, D.M.; Koh, H.A.; Dees, E.C.; McNulty, B.; Burkard, M.E.; Guarino, M.; Khatri, J.; et al. Real-world performance of a comprehensive genomic profiling test optimized for small tumor samples. JCO Precis. Oncol. 2021, 5, PO.20.00472. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mosele, F.; Remon, J.; Mateo, J.; Westphalen, C.B.; Barlesi, F.; Lolkema, M.P.; Normanno, N.; Scarpa, A.; Robson, M.; Meric-Bernstam, F.; et al. Recommendations for the use of next-generation sequencing (NGS) for patients with metastatic cancers: A report from the ESMO Precision Medicine Working Group. Ann. Oncol. 2020, 31, 1491–1505. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Cohen, M.; Hatzglou, V.; Zhang, Z.; Riaz, N.; Shamseddine, A.A.; Morris, L.G.T.; McBride, S.M.; Gelblum, D.Y.; Zakeri, K.; et al. Postoperative Human Papilloma Virus Circulating Tumor DNA Guided Adjuvant Therapy for Human Papilloma Virus-Related Oropharyngeal Carcinoma (PATH Study). Int. J. Radiat. Oncol. Biol. Phys. 2025, ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Lan, T.; Wu, F.; Chen, S.; Jiang, X.; Huo, C.; Li, Z.; Xie, S.; Wu, D.; Wang, R.; et al. Intratumoral CD103+CD8+ T cells predict response to neoadjuvant chemoimmunotherapy in advanced head and neck squamous cell carcinoma. Cancer Commun. 2023, 43, 1143–1163. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Taube, J.M.; Akturk, G.; Angelo, M.; Engle, E.L.; Gnjatic, S.; Greenbaum, S.; Greenwald, N.F.; Hedvat, C.V.; Hollmann, T.J.; Juco, J.; et al. The Society for Immunotherapy of Cancer statement on best practices for multiplex immunohistochemistry (IHC) and immunofluorescence (IF) staining and validation. J. Immunother. Cancer 2020, 8, e000155. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rojas, F.; Hernandez, S.; Lazcano, R.; Laberiano-Fernandez, C.; Parra, E.R. Multiplex immunofluorescence and the digital image analysis workflow for evaluation of the tumor immune environment in translational research. Front. Oncol. 2022, 12, 889886. [Google Scholar] [CrossRef] [PubMed]
- Song, S.S.; Lee, Z.H.; Yu, J.Z. Transoral robotic surgery (TORS) in head and neck reconstruction. J. Clin. Med. 2025, 14, 5775. [Google Scholar] [CrossRef]
- Zwanenburg, A.; Vallières, M.; Abdalah, M.A.; Aerts, H.J.W.L.; Andrearczyk, V.; Apte, A.; Ashrafinia, S.; Bakas, S.; Beukinga, R.J.; Boellaard, R.; et al. The Image Biomarker Standardization Initiative: Standardized Quantitative Radiomics for High-Throughput Image-Based Phenotyping. Radiology 2020, 295, 328–338. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lambin, P.; Leijenaar, R.T.H.; Deist, T.M.; Peerlings, J.; de Jong, E.E.C.; van Timmeren, J.; Sanduleanu, S.; Larue, R.T.H.M.; Even, A.J.G.; Jochems, A.; et al. Radiomics: The bridge between medical imaging and personalized medicine. Nat. Rev. Clin. Oncol. 2017, 14, 749–762. [Google Scholar] [CrossRef] [PubMed]
- Whybra, P.; Zwanenburg, A.; Andrearczyk, V.; Schaer, R.; Apte, A.P.; Ayotte, A.; Baheti, B.; Bakas, S.; Bettinelli, A.; Boellaard, R.; et al. The Image Biomarker Standardization Initiative: Standardized Convolutional Filters for Reproducible Radiomics and Enhanced Clinical Insights. Radiology 2024, 310, e231319. [Google Scholar] [CrossRef]
- Gillies, R.J.; Kinahan, P.E.; Hricak, H. Radiomics: Images are more than pictures, they are data. Radiology 2016, 278, 563–577. [Google Scholar] [CrossRef]
- Moslemi, A.; Osapoetra, L.O.; Safakish, A.; Sannachi, L.; Alberico, D.; Czarnota, G.J. Radiation therapy response prediction for head and neck cancer using multimodal imaging and multiview dynamic graph autoencoder feature selection. Med. Phys. 2025, 52, e70026. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yuan, Z.; Ai, S.; He, Q.; Wu, K.; Yang, M.; Zheng, K.; He, Y.; Tang, X.; Liu, Y.; Wu, Z.; et al. Intratumoral and peritumoral habitat radiomics of MRI predicts pathologic complete response to neoadjuvant chemoimmunotherapy in oral squamous cell carcinoma. Int. J. Surg. 2025, 111, 6232–6244. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, S.; Dai, Y.; Chen, J.; Yan, F.; Yang, Y. MRI-based habitat imaging in cancer treatment: Current technology, applications, and challenges. Cancer Imaging 2024, 24, 107. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dextraze, K.; Saha, A.; Kim, D.; Narang, S.; Lehrer, M.; Rao, A.; Narang, S.; Rao, D.; Ahmed, S.; Madhugiri, V.; et al. Spatial habitats from multiparametric MR imaging are associated with signaling pathway activities and survival in glioblastoma. Oncotarget 2017, 8, 112992–113003. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Juan-Albarracín, J.; Fuster-Garcia, E.; Manjón, J.V.; Robles, M.; Aparici, F.; Martí-Bonmatí, L.; García-Gómez, J.M. Automated glioblastoma segmentation based on a multiparametric structured unsupervised classification. PLoS ONE 2015, 10, e0125143. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wu, J.; Cao, G.; Sun, X.; Lee, J.; Rubin, D.L.; Napel, S.; Kurian, A.W.; Daniel, B.L.; Li, R. Intratumoral spatial heterogeneity at perfusion MR imaging predicts recurrence-free survival in locally advanced breast cancer treated with neoadjuvant chemotherapy. Radiology 2018, 288, 26–35. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shang, Y.; Zeng, Y.; Luo, S.; Wang, Y.; Yao, J.; Li, M.; Li, X.; Kui, X.; Wu, H.; Fan, K.; et al. Habitat imaging with tumoral and peritumoral radiomics for prediction of lung adenocarcinoma invasiveness on preoperative chest CT: A multicenter study. AJR Am. J. Roentgenol. 2024, 223, e2431675. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Li, Y.; Hu, W.; Cao, Y.; Lv, X.; Jia, X.; Shen, S.; Zhao, J.; Xu, C. Radiomics nomogram combined with clinical factors for predicting pathological complete response in resectable esophageal squamous cell carcinoma. Front. Oncol. 2024, 14, 1347650. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lin, P.; Xie, W.; Li, Y.; Zhang, C.; Wu, H.; Wan, H.; Gao, M.; Liang, F.; Han, P.; Chen, R.; et al. Intratumoral and peritumoral radiomics of MRIs predicts pathologic complete response to neoadjuvant chemoimmunotherapy in patients with head and neck squamous cell carcinoma. J. Immunother. Cancer 2024, 12, e009616. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Da-Ano, R.; Visvikis, D.; Hatt, M. Harmonization strategies for multicenter radiomics investigations. Phys. Med. Biol. 2020, 65, 24TR02. [Google Scholar] [CrossRef]
- Orlhac, F.; Eertink, J.J.; Cottereau, A.S.; Zijlstra, J.M.; Thieblemont, C.; Meignan, M.; Boellaard, R.; Buvat, I. A guide to ComBat harmonization of imaging biomarkers in multicenter studies. J. Nucl. Med. 2022, 63, 172–179. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Park, J.E.; Kim, D.; Kim, H.S.; Park, S.Y.; Kim, J.Y.; Cho, S.J.; Shin, J.H.; Kim, J.H. Quality of science and reporting of radiomics in oncologic studies: Room for improvement according to radiomics quality score and TRIPOD statement. Eur. Radiol. 2020, 30, 523–536. [Google Scholar] [CrossRef] [PubMed]
- Vickers, A.J.; Elkin, E.B. Decision curve analysis: A novel method for evaluating prediction models. Med. Decis. Making 2006, 26, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Moons, K.G.; Altman, D.G.; Reitsma, J.B.; Ioannidis, J.P.; Macaskill, P.; Steyerberg, E.W.; Vickers, A.J.; Ransohoff, D.F.; Collins, G.S. Transparent Reporting of a multivariable prediction model for Individual Prognosis Or Diagnosis (TRIPOD): Explanation and elaboration. Ann. Intern. Med. 2015, 162, W1–W73. [Google Scholar] [CrossRef] [PubMed]
- Maghsoomi, Z.; Emami, Z.; Malboosbaf, R.; Malek, M.; Khamseh, M.E. Efficacy and safety of peptide receptor radionuclide therapy in advanced radioiodine-refractory differentiated thyroid cancer and metastatic medullary thyroid cancer: A systematic review. BMC Cancer 2021, 21, 579. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Budiawan, H.; Salavati, A.; Kulkarni, H.R.; Baum, R.P. Peptide receptor radionuclide therapy of treatment-refractory metastatic thyroid cancer using (90)Yttrium and (177)Lutetium labeled somatostatin analogs: Toxicity, response and survival analysis. Am. J. Nucl. Med. Mol. Imaging 2013, 4, 39–52. [Google Scholar] [PubMed] [PubMed Central]
- Privé, B.M.; Boussihmad, M.A.; Timmermans, B.; van Gemert, W.A.; Peters, S.M.B.; Derks, Y.H.W.; van Lith, S.A.M.; Mehra, N.; Nagarajah, J.; Heskamp, S.; et al. Fibroblast activation protein-targeted radionuclide therapy: Background, opportunities, and challenges of first (pre)clinical studies. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 1906–1918. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Syed, M.; Flechsig, P.; Liermann, J.; Windisch, P.; Staudinger, F.; Akbaba, S.; Koerber, S.A.; Freudlsperger, C.; Plinkert, P.K.; Debus, J.; et al. Fibroblast activation protein inhibitor (FAPI) PET for diagnostics and advanced targeted radiotherapy in head and neck cancers. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 2836–2845. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ora, M.; Soni, N.; Nazar, A.H.; Dixit, M.; Singh, R.; Puri, S.; Graham, M.M.; Gambhir, S. Fibroblast Activation Protein Inhibitor-Based Radionuclide Therapies: Current Status and Future Directions. J. Nucl. Med. 2023, 64, 1001–1008. [Google Scholar] [CrossRef] [PubMed]
- Lindeman, S.D.; Mukkamala, R.; Horner, A.; Tudi, P.; Booth, O.C.; Huff, R.; Hinsey, J.; Hovstadius, A.; Martone, P.; Zhang, F.; et al. Fibroblast Activation Protein-Targeted Radioligand Therapy for Treatment of Solid Tumors. J. Nucl. Med. 2023, 64, 759–766. [Google Scholar] [CrossRef] [PubMed]
- Sampieri, C.; Cioccoloni, E.; Costantino, A.; Kim, D.; Lee, K.; Meccariello, G.; Cammaroto, G.; Vicini, C.; Kim, S.H. Neoadjuvant chemotherapy followed by transoral robotic surgery versus upfront surgery for locoregionally advanced oropharyngeal carcinoma: A propensity score matched analysis. Head Neck 2025, 47, 175–188. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.K.; Giger, R.; Auperin, A.; Bourhis, J.; Janot, F.; Temam, S. Salvage surgery after concomitant chemoradiation in head and neck squamous cell carcinomas—Stratification for postsalvage survival. Head Neck 2010, 32, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Hamoir, M.; Holvoet, E.; Ambroise, J.; Lengelé, B.; Schmitz, S. Salvage surgery in recurrent head and neck squamous cell carcinoma: Oncologic outcome and predictors of disease free survival. Oral Oncol. 2017, 67, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Quer, M.; León, X.; Casasayas, M.; Sansa, A.; López, M.; García Lorenzo, J. Salvage surgery in head and neck cancer: External validation of predictors of disease-specific survival. Oral Oncol. 2020, 109, 104876. [Google Scholar] [CrossRef] [PubMed]
- Hartl, D.M.; Guerlain, J.; Gorphe, P.; Kapre, M.; Kapre Gupta, N.; Saba, N.F.; Robbins, K.T.; Ronen, O.; Rodrigo, J.P.; Strojan, P.; et al. Review of outcomes after salvage surgery for recurrent squamous cell carcinoma of the head and neck. Cancers 2023, 15, 4692. [Google Scholar] [CrossRef] [PubMed]
- Goel, S.; Gunasekera, D.; Krishnan, G.; Krishnan, S.; Hodge, J.-C.; Lizarondo, L.; Foreman, A. Is transoral robotic surgery useful as a salvage technique in head and neck cancers: A systematic review and meta-analysis. Head Neck 2025, 47, 1018–1036. [Google Scholar] [CrossRef]
- Matos, L.L.; Kowalski, L.P.; Chaves, A.L.F.; de Oliveira, T.B.; Marta, G.N.; Curado, M.P.; de Castro Junior, G.; Farias, T.P.; Bardales, G.S.; Cabrera, M.A.; et al. Latin American consensus on the treatment of head and neck cancer. JCO Glob. Oncol. 2024, 10, e2300343. [Google Scholar] [CrossRef] [PubMed]
- Lewis, C.M.; Aloia, T.A.; Shi, W.; Martin, I.; Lai, S.Y.; Selber, J.C.; Hessel, A.C.; Hanasono, M.M.; Hutcheson, K.A.; Robb, G.L.; et al. Development and feasibility of a specialty-specific National Surgical Quality Improvement Program (NSQIP): The Head and Neck-Reconstructive Surgery NSQIP. JAMA Otolaryngol. Head Neck Surg. 2016, 142, 321–327. [Google Scholar] [CrossRef]
- Vinay, V.; Jodalli, P.; Chavan, M.S.; Buddhikot, C.S.; Luke, A.M.; Ingafou, M.S.H.; Reda, R.; Pawar, A.M.; Testarelli, L. Artificial intelligence in oral cancer: A comprehensive scoping review of diagnostic and prognostic applications. Diagnostics 2025, 15, 280. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Song, B.; Yadav, I.; Tsai, J.C.; Madabhushi, A.; Kann, B.H. Artificial intelligence for head and neck squamous cell carcinoma: From diagnosis to treatment. Am. Soc. Clin. Oncol. Educ. Book 2025, 45, e472464. [Google Scholar] [CrossRef]
- Rao, K.N.; Fernandez-Alvarez, V.; Guntinas-Lichius, O.; Sreeram, M.P.; de Bree, R.; Kowalski, L.P.; Forastiere, A.; Pace-Asciak, P.; Rodrigo, J.P.; Saba, N.F.; et al. The limitations of artificial intelligence in head and neck oncology. Adv. Ther. 2025, 42, 2559–2568. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chera, B.S.; Kumar, S.; Shen, C.; Amdur, R.; Dagan, R.; Green, R.; Goldman, E.; Weiss, J.; Grilley-Olson, J.; Patel, S.; et al. Plasma circulating tumor HPV DNA for the surveillance of cancer recurrence in HPV-associated oropharyngeal cancer. J. Clin. Oncol. 2020, 38, 1050–1058, Erratum in: J Clin Oncol. 2020, 38, 3579. https://doi.org/10.1200/JCO.20.02655. Erratum in: J Clin Oncol. 2023, 41, 4449. https://doi.org/10.1200/JCO.23.01228. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Galot, R.; van Marcke, C.; Helaers, R.; Mendola, A.; Goebbels, R.M.; Caignet, X.; Ambroise, J.; Wittouck, K.; Vikkula, M.; Limaye, N.; et al. Liquid biopsy for mutational profiling of locoregional recurrent and/or metastatic head and neck squamous cell carcinoma. Oral Oncol. 2020, 104, 104631. [Google Scholar] [CrossRef] [PubMed]
- Mes, S.W.; van Velden, F.H.P.; Peltenburg, B.; Peeters, C.F.W.; Te Beest, D.E.; van de Wiel, M.A.; Mekke, J.; Mulder, D.C.; Martens, R.M.; Castelijns, J.A.; et al. Outcome prediction of head and neck squamous cell carcinoma by MRI radiomic signatures. Eur. Radiol. 2020, 30, 6311–6321. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wu, W.; Wang, M.; Zhang, J.; Guo, C.; Han, G.; Wang, L. Integrated peripheral blood multi-omics profiling identifies immune signatures predictive of neoadjuvant PD-1 blockade efficacy in head and neck squamous cell carcinoma. J. Transl. Med. 2025, 23, 540. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Havel, J.J.; Chowell, D.; Chan, T.A. The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nat. Rev. Cancer 2019, 19, 133–150. [Google Scholar] [CrossRef]
- Subramanian, I.; Verma, S.; Kumar, S.; Jere, A.; Anamika, K. Multi-omics data integration, interpretation, and its application. Bioinform. Biol. Insights 2020, 14, 1177932219899051. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Hosen, M.I.; Ahmed, M.; Shekhar, H.U. Onco-Multi-OMICS approach: A new frontier in cancer research. Biomed Res. Int. 2018, 2018, 9836256. [Google Scholar] [CrossRef]
- Howard, F.M.; Kochanny, S.; Koshy, M.; Spiotto, M.; Pearson, A.T. Machine learning-guided adjuvant treatment of head and neck cancer. JAMA Netw. Open 2020, 3, e2025881. [Google Scholar] [CrossRef]
- Alabi, R.O.; Elmusrati, M.; Sawazaki-Calone, I.; Kowalski, L.P.; Haglund, C.; Coletta, R.D.; Mäkitie, A.A.; Salo, T.; Leivo, I.; Al-mangush, A. Machine learning application for prediction of locoregional recurrences in early oral tongue cancer: A Web-Based prognostic tool. Virchows Arch. 2019, 475, 489–497. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bur, A.M.; Holcomb, A.; Goodwin, S.; Woodroof, J.; Karadaghy, O.; Shnayder, Y.; Kakarala, K.; Brant, J.; Shew, M. Machine learning to predict occult nodal metastasis in early oral squamous cell carcinoma. Oral Oncol. 2019, 92, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, J.C.; Wang, H.M.; Wu, M.H.; Chang, K.P.; Chang, P.H.; Liao, C.T.; Liau, C.T. Review of emerging biomarkers in head and neck squamous cell carcinoma in the era of immunotherapy and targeted therapy. Head Neck 2019, 41, 19–45. [Google Scholar] [CrossRef]
- Cheung, A.T.M.; Palapattu, E.L.; Pompa, I.R.; Aldrighetti, C.M.; Niemierko, A.; Willers, H.; Huang, F.; Vapiwala, N.; Van Allen, E.; Kamran, S.C. Racial and ethnic disparities in a real-world precision oncology data registry. NPJ Precis Oncol. 2023, 7, 9. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhang, C.; Zhang, W.; Zhang, S.; Liu, Q.; Guo, Y. Applications and challenges of biomarker-based predictive models in proactive health management. Front. Public Health 2025, 13, 1633487. [Google Scholar] [CrossRef]
- Hsiao, S.J.; Sireci, A.N.; Pendrick, D.; Freeman, C.; Fernandes, H.; Schwartz, G.K.; Henick, B.S.; Mansukhani, M.M.; Roth, K.A.; Carvajal, R.D.; et al. Clinical Utilization, Utility, and Reimbursement for Expanded Genomic Panel Testing in Adult Oncology. JCO Precis. Oncol. 2020, 4, 1038–1048. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Head and Neck Cancers, Version 5.2025; NCCN: Plymouth Meeting, PA, USA, 2025. Available online: https://www.nccn.org/guidelines/category_1 (accessed on 14 October 2025).

| Team Member | Primary Responsibilities |
|---|---|
| Head and Neck Surgical Oncologist | Surgical candidacy determination Timing coordination Tumor resection and neck dissection Reconstruction planning (with plastic surgeon) Surgical complication management |
| Radiation Oncologist | Radiation therapy design and delivery Dose determination Adjuvant/definitive protocols Radiation toxicity management |
| Medical Oncologist | Systemic chemotherapy, target therapy and immunotherapy Perioperative therapy Clinical trial enrollment Systemic treatment toxicity management |
| Pathologist | Diagnosis including molecular analysis Margin and lymph node assessment Biomarker testing |
| Radiologist | Diagnostic imaging interpretation Radiomics analysis Resectability assessment Response evaluation |
| Speech-Language Pathologist | Swallowing and speech assessment and management Rehabilitation therapy |
| Nutritionist | Nutritional status evaluation; Feeding support coordination; Nutritional treatment-related complication management |
| Palliative Care Specialist< | Pain and symptom control; Psychosocial support; Goals-of-care discussions |
| Social Worker | Care coordination Psychosocial barrier management Resource connection |
| Nurse Navigator | Primary contact Patient/family education; Appointment coordination Treatment monitoring |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Kowalski, L.P.; Bradford, C.R.; Beitler, J.J.; Rodrigo, J.P.; Guntinas-Lichius, O.; Ambrosch, P.; Forastiere, A.A.; Rao, K.N.; Hamoir, M.; Saba, N.F.; et al. From Radical Resection to Precision Surgery: Integrating Diagnostic Biomarkers, Radiomics-Based Predictive Models, and Perioperative Systemic Therapy in Head and Neck Oncology. Diagnostics 2026, 16, 49. https://doi.org/10.3390/diagnostics16010049
Kowalski LP, Bradford CR, Beitler JJ, Rodrigo JP, Guntinas-Lichius O, Ambrosch P, Forastiere AA, Rao KN, Hamoir M, Saba NF, et al. From Radical Resection to Precision Surgery: Integrating Diagnostic Biomarkers, Radiomics-Based Predictive Models, and Perioperative Systemic Therapy in Head and Neck Oncology. Diagnostics. 2026; 16(1):49. https://doi.org/10.3390/diagnostics16010049
Chicago/Turabian StyleKowalski, Luiz P., Carol R. Bradford, Jonathan J. Beitler, Juan Pablo Rodrigo, Orlando Guntinas-Lichius, Petra Ambrosch, Arlene A. Forastiere, Karthik N. Rao, Marc Hamoir, Nabil F. Saba, and et al. 2026. "From Radical Resection to Precision Surgery: Integrating Diagnostic Biomarkers, Radiomics-Based Predictive Models, and Perioperative Systemic Therapy in Head and Neck Oncology" Diagnostics 16, no. 1: 49. https://doi.org/10.3390/diagnostics16010049
APA StyleKowalski, L. P., Bradford, C. R., Beitler, J. J., Rodrigo, J. P., Guntinas-Lichius, O., Ambrosch, P., Forastiere, A. A., Rao, K. N., Hamoir, M., Saba, N. F., Sanabria, A., Strojan, P., Robbins, K. T., & Ferlito, A. (2026). From Radical Resection to Precision Surgery: Integrating Diagnostic Biomarkers, Radiomics-Based Predictive Models, and Perioperative Systemic Therapy in Head and Neck Oncology. Diagnostics, 16(1), 49. https://doi.org/10.3390/diagnostics16010049












