Abstract
A 40-year-old woman who had obstetric history of one vaginal delivery and two surgical abortions to terminate early pregnancy received regular prenatal care without any systemic maternal diseases. During the detailed second trimester ultrasound, a homogenous adhesion-induced pseudocystic lesion of 8.6 × 7.4 cm was found between the inlet of the endocervix and the uterine cavity in the lower segment of the uterus. There was a clear septum with an inlet of about 2.6 cm near the right lower segment of the uterus. Transvaginal sonography showed a cervical length of 3.29 cm without dilatation. No gross fetal anomalies were found. Sometimes, the fetal head or limbs moved into this cystic space. At 36 3/7 weeks of gestation, a cesarean section was arranged for fetal breech presentation and pre-labor rupture of the membrane. After the delivery of the baby and its placenta, there was no obvious septum in the uterine cavity but only a very short fibrous tissue from the posterior wall of uterus, which could be destroyed when the baby was delivered. No adverse outcomes for the mother or the neonate were observed.
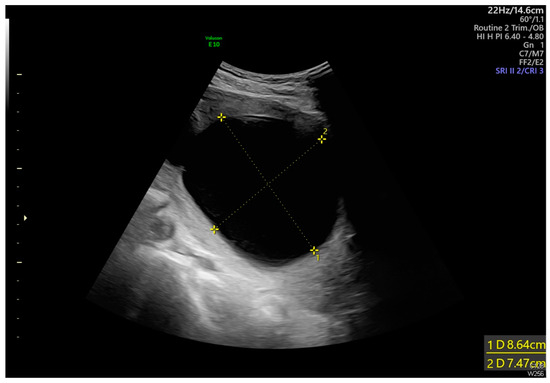
Figure 1.
Transabdominal ultrasound revealed an adhesion-induced pseudocyst measuring 8.6 × 7.4 cm located between the endocervical inlet and uterine cavity in the lower uterine segment (As shown by the dotted line distance). The inlet was identified at the 10 o’clock position of the pseudocyst. The longest inlet of the adhesion-induced pseudocyst measured 2.6 cm (Figure 2). Uterine septa were present on anterior and posterior walls, resulting from intrauterine adhesions (Figure 3 and Figure 4). Transvaginal ultrasound showed cervical length of 3.29 cm without dilation, excluding cervical incompetence (CI) (Figure 5). Detailed second-trimester ultrasound detected no gross fetal anomalies or bulging uterine diverticulum. Fetal limbs and head moved freely into and out of the adhesion-induced pseudocavity without hyperechoic band-like lesions, making amniotic band syndrome unlikely. At 36 3/7 weeks’ gestation, cesarean delivery was performed due to pre-labor rupture of membranes and fetal breech presentation. Post-placental delivery examination revealed only a short fibrous tissue extending from the posterior uterine wall (Figure 6). The adhesion-induced septa may have been disrupted during fetal delivery. No adverse maternal or neonatal outcomes were observed.
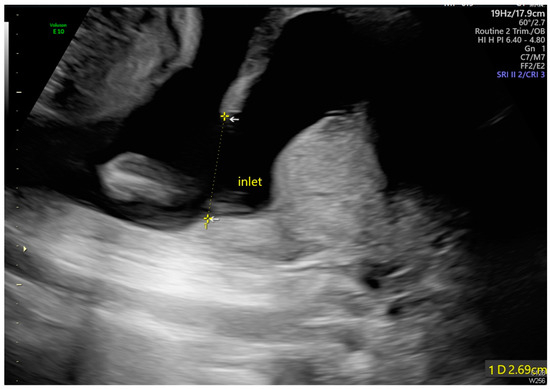
Figure 2.
The maximal diameter of the pseudocyst’s opening measured 2.69 cm (As shown by the dotted line distance).
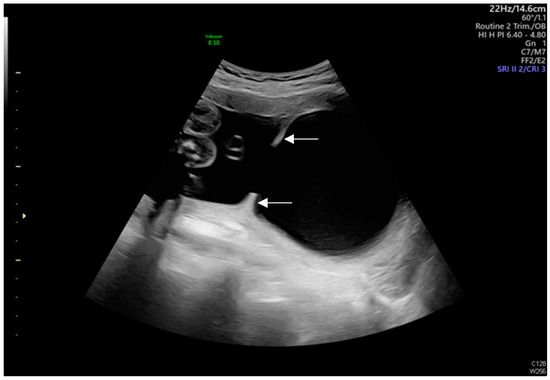
Figure 3.
Uterine septa were present on anterior and posterior walls, resulting from intrauterine adhesions (As shown by the white arrows).
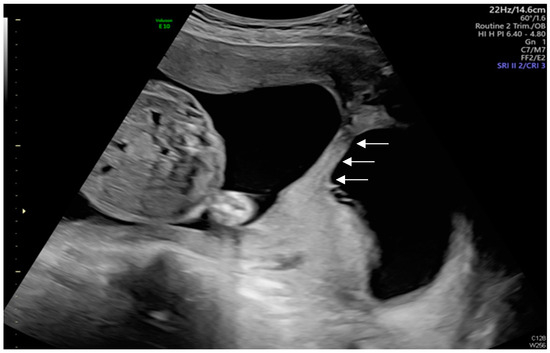
Figure 4.
When the ultrasound probe was moved to the right side of the uterus, the adhesion-induced septa were visualized perpendicular to the anterior and posterior uterine walls, without inlet (As shown by the white arrows).
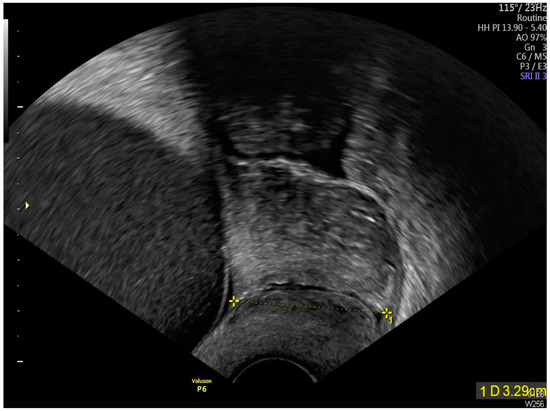
Figure 5.
Transvaginal sonographic assessment demonstrated a cervical length measurement of 3.29 cm (As shown by the dotted line distance), with no evidence of cervical dilation.
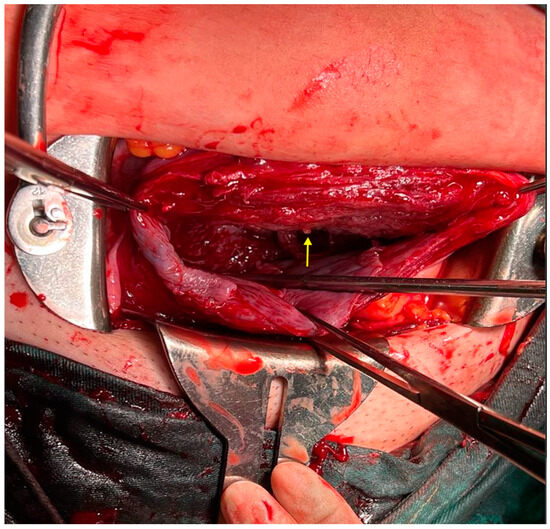
Figure 6.
Examination after placental delivery revealed only a small strand of fibrous tissue extending from the posterior uterine wall (As shown by the yellow arrow). Dilation and curettage (D&C) is a common surgical procedure for managing pathogenic gestational tissue or elective pregnancy termination. In non-pregnant patients, it also serves as a therapeutic and diagnostic tool for abnormal uterine bleeding [1,2,3]. Although D&C is generally considered a safe procedure, it carries potential complications including cervical or uterine bleeding, infection, uterine perforation, and intrauterine adhesions [4,5,6]. Notably, injury to the decidua basalis leading to endometrial fibrous scarring and fusion of opposing surfaces is believed to be a possible mechanism for intrauterine adhesion formation [7]. Studies have also shown that recurrent pregnancy loss is associated with intrauterine adhesions, with evidence suggesting that hysteroscopic surgery may improve pregnancy outcomes [8].This series of images warrants discussion regarding the distinction among intrauterine adhesions, uterine diverticulum (UD), and CI during pregnancy. UD is an uncommon condition that may be either secondary to intervention/trauma or primarily developed [9]. It is usually discovered incidentally on sonography, appearing as a cystic lesion adjacent to or arising from the uterus, with walls comprising myometrium and a cavity communicating with the uterine lumen [10]. Primary UD is an extremely rare anomaly resulting from the failed midline fusion of the Müllerian duct during final uterine development. Weak points in the uterine wall may dilate during pregnancy and labor, forming a diverticulum [11]. Primary UD typically presents with symptoms including abnormal uterine bleeding and dysmenorrhea. Secondary UD is an iatrogenic condition that develops after uterine intervention or trauma. It may lead to abnormal placental attachment disorders, such as placenta accreta spectrum or ectopic pregnancy [12]. Ultrasound screening for the thinning of the uterine segment is crucial; however, this sign was not present in our case. UD can be misdiagnosed as degenerating uterine myoma, adenomyotic cyst, uterine malformations (such as unicornuate uterus, bicornuate uterus with single cervix, and incomplete septate uterus), or adnexal cyst based on sonographic appearance [13,14,15,16]. Another differential diagnosis is CI, defined as cervical length < 25 mm before 24 weeks’ gestation on transvaginal ultrasound, which provides an accurate measurement of the maximum closed cervical canal length [17]. Cervical funneling, characterized by a dilated endocervical canal with protruding fetal membranes, fetal parts, or umbilical cord, is a more reliable indicator [18]. Fundal pressure during transvaginal sonography may aid early CI detection in symptomatic women [19].
Author Contributions
Conceptualization: K.-L.H., writing and review: J.-T.H. and K.-L.H., visualization: Y.-M.C., C.-C.T. and H.-H.C., data and image collection: Y.-J.L. and P.-F.L., supervision: T.-Y.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board (number: 202400016B0) of Kaohsiung Chang Gung Memorial hospital (date of approval: 8 January 2024).
Informed Consent Statement
Written informed consent has been obtained from this patient to publish this paper.
Acknowledgments
Grammar correction was performed using the Claude AI platform.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Chau, O.S.Y.; Law, T.S.M.; Ng, K.; Li, T.C.; Chung, J.P.W. Five-year retrospective review of ultrasound-guided manual vacuum aspiration for first-trimester miscarriage. Hong Kong Med. J. 2023, 29, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Schäffler, H.; Dimpfl, M.; Schochter, F.; Janni, W.; de Gregorio, N. Curative Polyendocrine Therapy in a 21-year-Old Patient with Endometrial Carcinoma: Case Report and Review of the Literature. Oncol. Res. Treat. 2023, 46, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.T.; Chang, W.H.; Chao, W.T.; Lai, T.J.; Lin, W.L.; Lim, H.C.; Liu, C.H.; Wang, P.H. The timing of intravenous oxytocin administration is crucial to minimize perioperative blood loss during first-trimester suction curettage for missed abortion. J. Chin. Med. Assoc. 2022, 85, 1061–1067. [Google Scholar] [CrossRef] [PubMed]
- Ben-Baruch, G.; Menczer, J.; Shalev, J.; Romem, Y.; Serr, D.M. Uterine perforation during curettage: Perforation rates and postperforation management. Isr. J. Med. Sci. 1980, 16, 821–824. [Google Scholar]
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 195: Prevention of Infection After Gynecologic Procedures. Obstet. Gynecol. 2018, 131, e172–e189. [Google Scholar] [CrossRef] [PubMed]
- March, C.M. Intrauterine adhesions. Obstet. Gynecol. Clin. N. Am. 1995, 22, 491–505. [Google Scholar] [CrossRef]
- Wang, P.H.; Yang, S.T.; Chang, W.H.; Liu, C.H.; Liu, H.H.; Lee, W.L. Intrauterine adhesion. Taiwan J. Obstet. Gynecol. 2024, 63, 312–319. [Google Scholar] [CrossRef]
- Qiao, X.; Liu, D.; Liu, C.; Pei, T.; Ouyang, Y. Reproductive outcomes after hysteroscopic adhesiolysis in patients experiencing recurrent pregnancy loss and intrauterine adhesions. J. Minim. Invasive Gynecol. 2024, S1553-4650(24)00415-1, Advance online publication. [Google Scholar] [CrossRef] [PubMed]
- Rajiah, P.; Eastwood, K.L.; Gunn, M.L.D.; Dighe, M. Uterine diverticulum. Obstet. Gynecol. 2009, 113, 525–527. [Google Scholar] [CrossRef]
- Nishiuchi, K.; Uotani, K.; Kobayashi, D.; Ono, Y.; Yamasaki, Y.; Kashima, Y.; Nishijima, M.; Ueno, Y.; Imaoka, I.; Murakami, T. Uterine diverticulum mimicking endometriotic cyst of the ovary. Radiol. Case Rep. 2024, 19, 934–938. [Google Scholar] [CrossRef]
- Akashi, E.; Ishiguro, T.; Nonaka, T.; Kobayashi, A.; Takakuwa, K.; Enomoto, T. Enlarged uterine fibroid forming uterine diverticulum during pregnancy: A case report. BMC Pregnancy Childbirth 2021, 21, 34. [Google Scholar] [CrossRef]
- Kulshrestha, V.; Agarwal, N.; Kachhawa, G. Post-caesarean Niche (Isthmocele) in Uterine Scar: An Update. J. Obstet. Gynaecol. India 2020, 70, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Chufal, S.; Thapliyal, N.; Gupta, M.; Pangtey, N. Huge uterine-cervical diverticulum mimicking as a cyst. Indian J. Pathol. Microbiol. 2012, 55, 372–374. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, M.; Kitajima, M.; Abe, S.; Murakami, N.; Kitajima, Y.; Miura, K.; Masuzaki, H. Huge uterine fibroid arising from primary uterine cervical diverticulum: A case report and review of the literatures. J. Obstet. Gynaecol. 2019, 39, 1186–1187. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Xue, M.; Xiao, S.; Wan, Y.; Wang, B. Uterine diverticulum complicating pregnancy diagnosed by ultrasound and uteroscopy. Int. J. Gynaecol. Obstet. 2010, 109, 247–248. [Google Scholar] [CrossRef]
- Umezaki, I.; Takagi, K.; Aiba, M.; Ohta, H. Uterine cervical diverticulum resembling a degenerated leiomyoma. Obstet. Gynecol. 2004, 103, 1130–1133. [Google Scholar] [CrossRef]
- Macdonald, R.; Smith, P.; Vyas, S. Cervical incompetence: The use of transvaginal sonography to provide an objective diagnosis. Ultrasound Obstet. Gynecol. 2001, 18, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Stolz, L.A.; Amini, R.; Situ-LaCasse, E.H.; Shareef, F.; Reed, H.A.; Adhikari, S. Cervical Funneling: Potential Pitfall of Point-of-Care Pelvic Ultrasound. Cureus 2017, 9, e1649. [Google Scholar] [CrossRef] [PubMed]
- Rozenberg, P.; Gillet, A.; Ville, Y. Transvaginal sonographic examination of the cervix in asymptomatic pregnant women: Review of the literature. Ultrasound Obstet. Gynecol. 2002, 19, 302–311. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).