Eruption Timing and Sequence of Primary Teeth in a Sample of Romanian Children
Abstract
:1. Introduction
2. Materials and Methods
2.1. Instruments Used in the Study and Variables Recorded
2.2. Parents Instruction and Procedure Calibration
2.3. Study Protocol
2.4. Sample Description
2.5. Data Analysis
3. Results
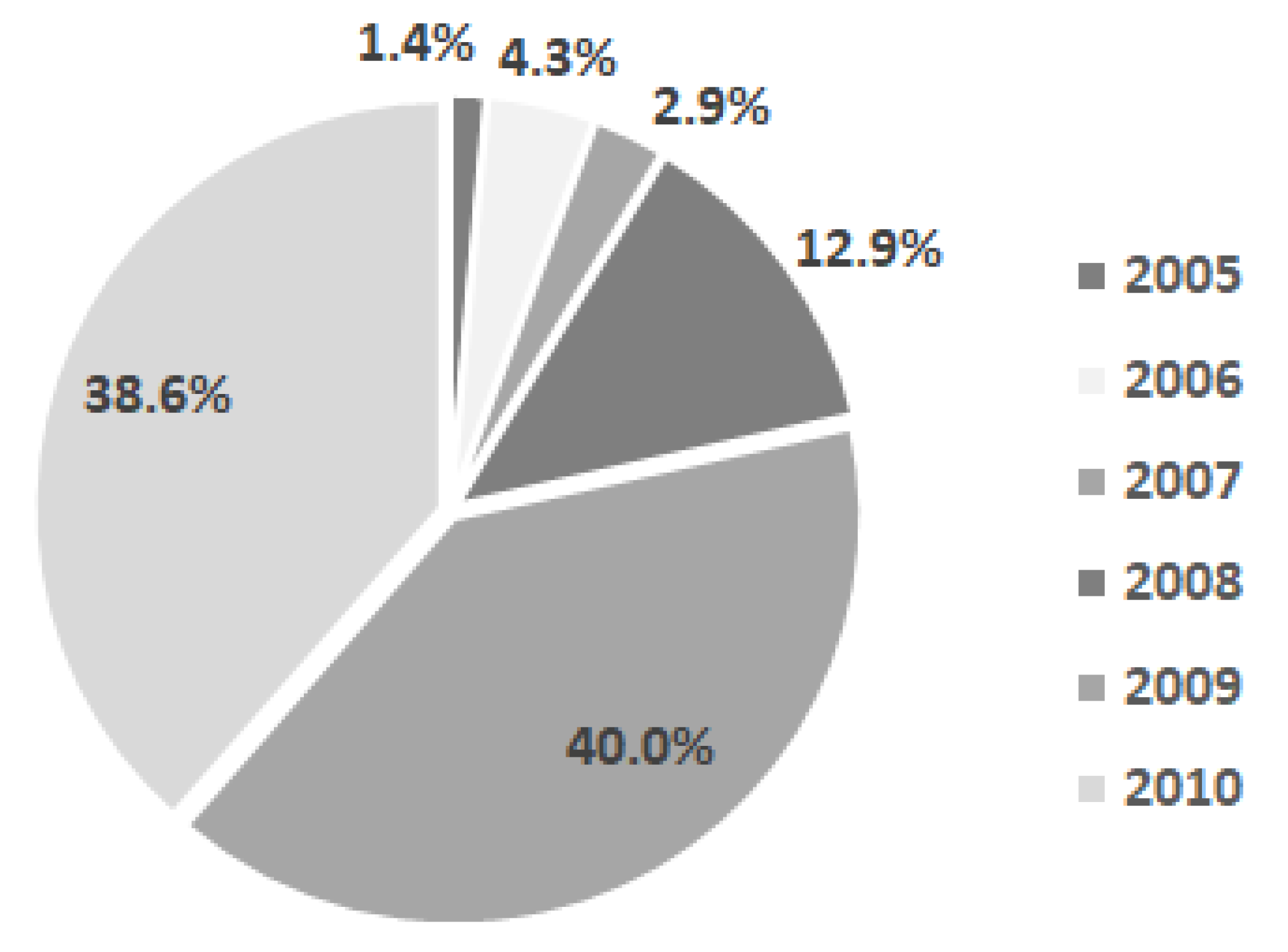
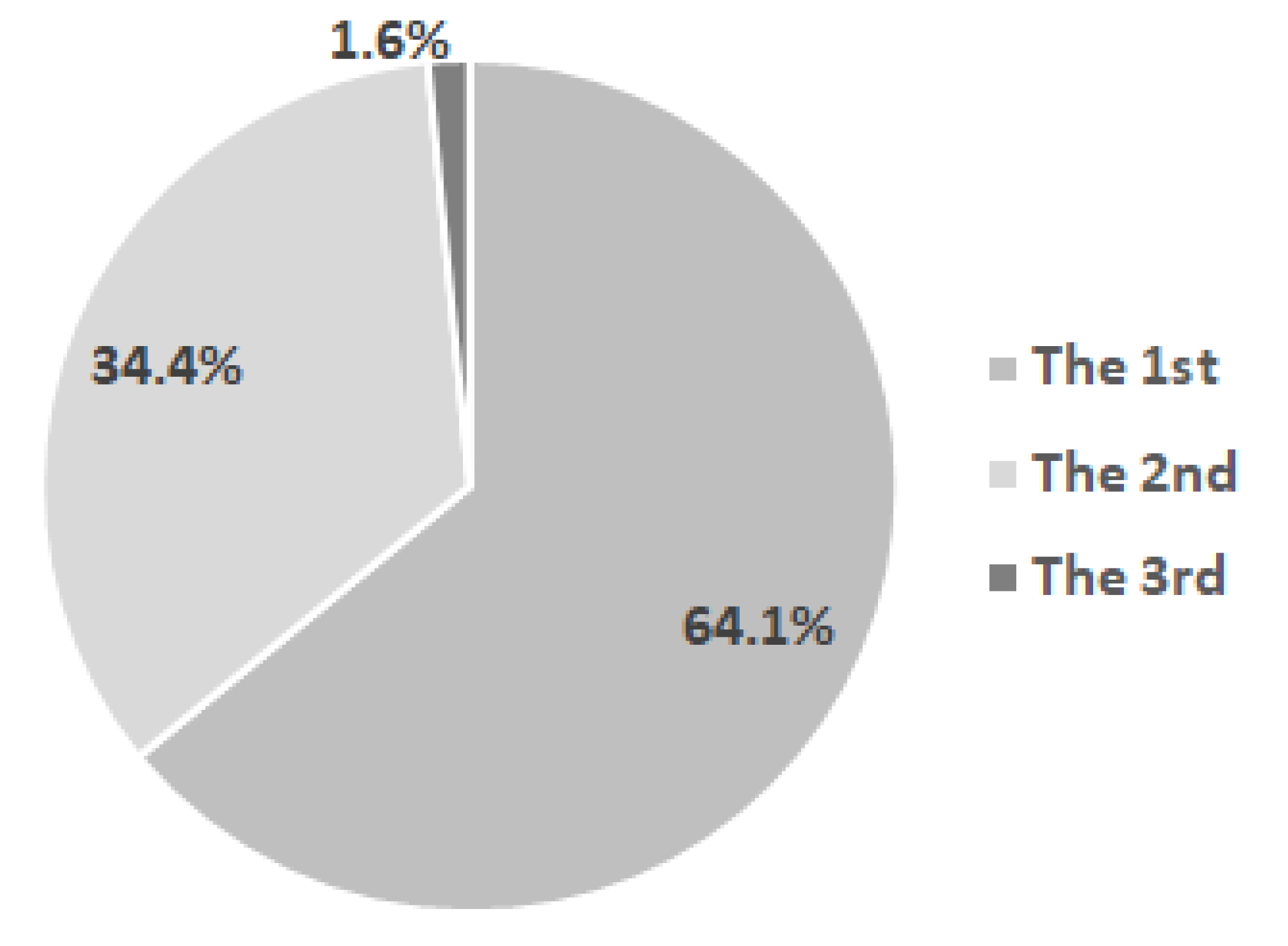
- General data related to the birth, general development, and feeding of children from the examined group: distribution of children in the group by year of birth, percentage of children born from primiparous and multiparous mothers, Apgar score, average age of the children’s mothers at birth, and distribution of children by the mother’s age at birth, height, and weight at birth and at different moments; food-related data: natural, artificial or mixed diet;
- Specific data for primary teeth eruption: mean age of eruption for each tooth and for pairs of homologous primary teeth for both sexes, mean sequence of eruption, comparison of mean ages of eruption between sexes and between the two jaws, and, on the same jaw, between the two hemiarches; average number of teeth present at different ages; average age of eruption of the first primary tooth and different correlations between this mean age and the general parameters.
3.1. General Data for the Current Study Sample
3.2. Specific Data for Primary Teeth Eruption
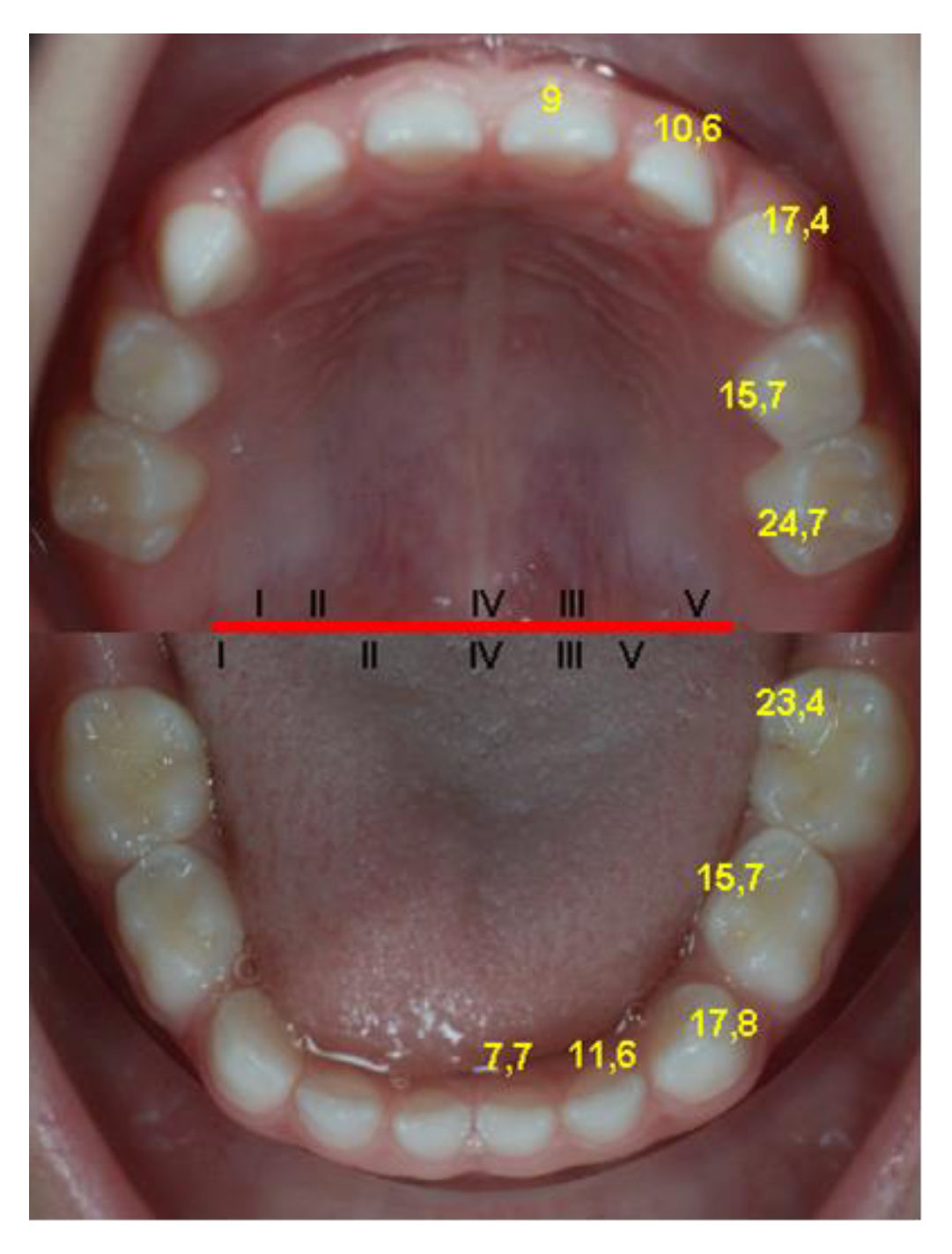
- the average age of eruption of each temporary tooth (Table 2); the teeth are ordered upwards by the age of eruption, which leads to the determination of the average sequence of deciduous teeth eruption, represented by the same table;
- the average age for the eruption of pairs of homologous deciduous teeth, determined on the total number of dental units of the same type (cumulated from the left and right sides) (Table 3); the groups of teeth are in ascending order, depending on the age of eruption, thus resulting in the average sequence of eruption of deciduous teeth;
- the average age for the eruption of pairs of homologous deciduous teeth, determined separately for the two sexes (Table 4);
- The average eruption age for most deciduous teeth is slightly decreased for girls compared to boys, with the exception of the upper central incisors and lower lateral incisors. The differences between the sexes are insignificant for most of the groups of teeth compared (the value p = 0.757), except for the maxillary canines (p = 0.047) and mandibular canines (p = 0.018), and for the lower deciduous second molars (p < 0.001), which are significantly increased in boys compared to girls.
- 4.
- the comparisons between the average eruption ages of the deciduous teeth by sex were made using the unpaired t-test, and those between the two hemiarches, respectively between the two arches (maxilla and mandible) were made using the paired t-test;
- The differences between the two hemiarches (right and left) are insignificant (p = 0.197), globally and also for every pair of teeth (p > 0.05).
- The differences between the maxilla and the mandible are significantly (p = 0.019 value) increased in the maxilla. The application of the same test on groups of teeth identified the earlier eruption of the central mandibular incisors and of the second mandibular molars, as well as of the maxillary lateral incisors.
- 5.
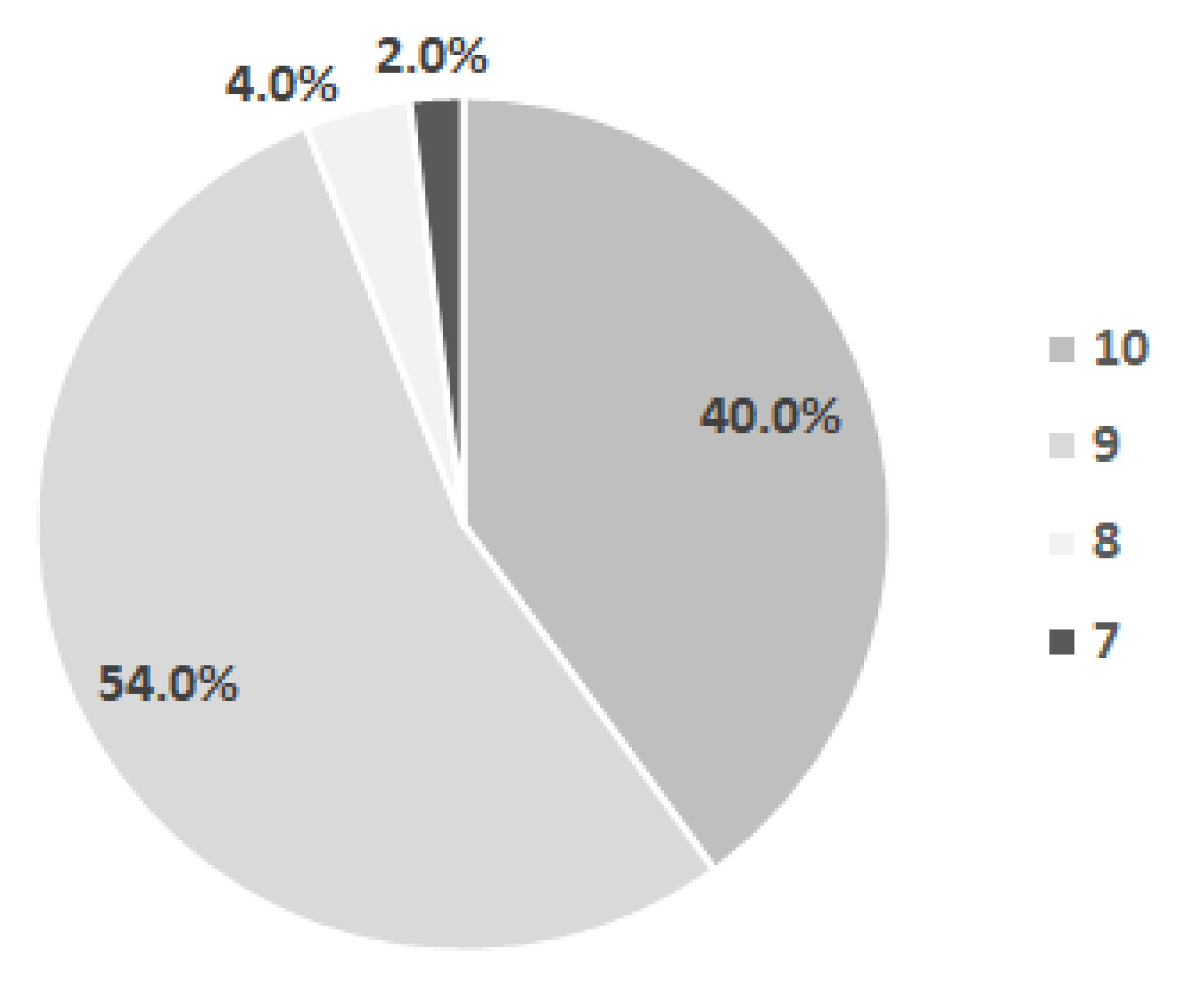
- the number of primary teeth present in the child’s mouth at different ages was also determined: 2.38 ± 1.39 at 6 months, 6.82 ± 3.10 at 12 months, 12.90 ± 2.74 at 18 months, and 16.85 ± 3.05 at 24 months.
- 6.
- the mean eruption age of the first primary tooth, independently of the exact type of tooth which erupted first, is 7.07 ± 1.99 months (Min 3 Months, Max 13 months).
- 7.
- correlations between the eruption timing of the first primary tooth and the weight and height at birth and at six months, the mother’s age at birth and the total number of breastfeeding months are direct, weak and insignificant, and the correlation between the number of teeth erupted at one year and the height at one year is weak, inverse and significant for the studied sample (Table 5).
- 8.
- a comparison between the timing of eruption of the first primary tooth according to the number of previous births (7.0 ± 1.70 for the first birth vs. 7.2 ± 2.49 for the second), and a comparison according to the type of diet have been made (7.22 ± 1.98 for natural diet vs. 7.20 ± 2.28 for artificial diet); the unpaired t-test was used and the differences were insignificant for both comparisons made (p = 0.665 for the first one and p = 0.983 for the second one).
4. Discussion
4.1. Updating the Reference Values
4.2. Study Limitation
4.3. Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A


References
- Wu, H.; Chen, T.; Ma, Q.; Xu, X.; Xie, K.; Chen, Y. Associations of maternal, perinatal and postnatal factors with the eruption timing of the first primary tooth. Sci. Rep. 2019, 9, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Koussoulakou, D.S.; Margaritis, L.H.; Koussoulakos, S.L. A curriculum vitae of teeth: Evolution, generation, regeneration. J. Int J. Biol. Sci. 2009, 5, 226–243. [Google Scholar] [CrossRef] [Green Version]
- Luca, R. Pedodonţie vol I, 2nd ed.; Editura Cerma: Bucuresti, Romania, 2007; pp. 15–20. [Google Scholar]
- Bratu, E.; Grivu, O.; Voinea, C. Erupţia Dentară Normală şi Patologică, 1st ed.; Editura Helicon: Timişoara, Romania, 1996; pp. 42–58, 123–131, 132–146. [Google Scholar]
- Al-Batayneh, O.B.; Shaweesh, A. Clinical duration of eruption of deciduous teeth in Jordanian children: A cross-sectional study. Arch. Oral Biol. 2018, 90, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Kariya, P.; Tandon, S.; Singh, S.; Tewari, N. Polymorphism in emergence of deciduous dentition: A cross-sectional study of Indian children. J. Investig. Clin. Dent. 2017, 9, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Savara, B.S.; Steen, J.C. Timing and Sequence of Eruption of Permanent Teeth in a Longitudinal Sample of Children from Oregon. JADA 1978, 99, 209–214. [Google Scholar] [CrossRef]
- Gupta, A.; Hiremath, S.S.; Singh, S.K.; Poudyal, S.; Niraula, S.R.; Baral, D.D.; Singh, R.K. Emergence of Primary Teeth in Children of Sunsari District of Eastern Nepal. McGill J. Med. 2007, 10, 11–15. [Google Scholar] [CrossRef]
- Folayan, M.; Owotade, F.; Adejuyigbe, E.; Sen, S.; Lawal, B.; Ndukwe, K. The Timing of Eruption of the Primary Dentition in Nigerian Children. Am. J. Phys. Anthropol. 2007, 134, 443–448. [Google Scholar] [CrossRef]
- Smith, B.H.; Crummett, T.L.; Brandt, K.L. Ages of eruption of primate teeth: A compendium for aging individuals and comparing histories. Year Phys. Anthropol. 1994, 37, 177–203. [Google Scholar] [CrossRef] [Green Version]
- Poureslami, H.; Aminabadi, N.A.; Deljavan, A.S.; Erfanparast, L.; Sohrabi, A.; Jamali, Z.; Oskouei, S.G.; Hazem, K.; Shirazi, S. Does Timing of Eruption in First Primary Tooth Correlate with that of First Permanent Tooth? A 9-years Cohort Study. J. Dent. Rent. Res. Dent. Clin. Dent. Prospect 2015, 9, 79–85. [Google Scholar] [CrossRef]
- Dawson, D.V.; Blanchette, D.R.; Douglass, J.M.; Tinanoff, N.; Kramer, K.W.O.; Warren, J.J.; Phipps, K.R.; Starr, D.E.; Marshall, T.A.; Mabry, T.R.; et al. Evidence of Early Emergence of the Primary Dentition in a Northern Plains American Indian Population. JDR Clin. Transl. Res. 2018, 3, 161–169. [Google Scholar] [CrossRef]
- Warren, J.J.; Fontana, M.; Blanchette, D.R.; Dawson, D.V.; Drake, D.R.; Levy, S.M.; Kolker, J.L.; Phipps, K.R. Timing of primary tooth emergence among U.S. racial and ethnic groups. Am. Assoc. Public Health Dent. 2016, 76, 1–4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pavičin, I.S.; Dumančić, J.; Badel, T.; Vodanović, M. Timing of emergence of the first primary tooth in preterm and full-term infants. Ann. Anat. 2015, 203, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Al-Batayneh, O.B.; Shaweesh, A.I.; Alsoreeky, E.S. Timing and sequence of emergence of deciduous teeth in Jordanian children. Arch. Oral Biol. 2015, 60, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Hughes, T.; Bockmann, M.; Seow, K.; Gotjamanos, T.; Gully, N.; Richards, L.; Townsend, G. Strong genetic control of emergence of human primary incisors. J. Dent. Res. 2007, 86, 1160–1165. [Google Scholar] [CrossRef] [Green Version]
- Pillas, D.; Hoggart, C.J.; Evans, D.M.; O’Reilly, P.F.; Sipilä, K.; Lähdesmäki, R.; Millwood, I.Y.; Kaakinen, M.; Netuveli, G.; Blane, D.; et al. Genome-Wide Association Study Reveals Multiple Loci Associated with Primary Tooth Development during Infancy. PLoS Genet. 2010, 6, e1000856. [Google Scholar] [CrossRef]
- Pstoter, W.J.; Morse, D.E.; Pendrys, D.G.; Zhang, H.; Mayne, S.T. Median ages of eruptions of the primary teeth in white and Hispanic children from Arizona. J. Pediatr Dent. 2003, 25, 257–261. [Google Scholar]
- Holman, D.J.; Jones, R.E. Longitudinal analysis of deciduous tooth emergence: III. Sexual dimorphism in Bangladeshi, Guatemalan, Japanese, and Javanese Children. Am. J. Phys. Anthropol. 2003, 122, 269–278. [Google Scholar] [CrossRef]
- Rantakallio, P.; Makinen, H. The effect of maternal smoking on the timing of deciduous tooth eruption. J. Growth 1983, 47, 122–128. [Google Scholar]
- Zadzinska, E.; Sitek, A.; Rosset, I. Relationship between pre- natal factors, the perinatal environment, motor development in the first year of life and the timing of first deciduous tooth emergence. J. Ann. Hum. Biol. 2015, 43, 25–33. [Google Scholar] [CrossRef]
- Un Lam, C.; Hsu, C.Y.S.; Yee, R.; Koh, D.; Lee, Y.S.; Chong, M.F.F.; Cai, M.; Kwek, K.; Saw, S.M.; Godfrey, K.; et al. Influence of metabolic-linked early life factors on the eruption timing of the first primary tooth. Clin. Oral Investig. 2016, 20, 1871–1879. [Google Scholar] [CrossRef]
- Ramos, S.R.P.; Gugisch, R.C.; Fraiz, F.C. The influence of gestational age and birth weight of the newborn on tooth eruption. J. Appl. Oral Sci. 2006, 14, 228–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aktoren, O.; Tuna, E.B.; Guven, Y.; Gokcay, G. A study of neonatal factors and eruption time of primary teeth. J. Community Dent. Health 2010, 27, 52–56. [Google Scholar] [CrossRef]
- Sajjadian, N.; Jahadi, R.; Barakat, M.G.; Sajjadian, A. Relationship between birth weight and time of first deciduous tooth eruption in 143 consecutively born infants. Pediatr Neonatol. 2010, 51, 235–237. [Google Scholar] [CrossRef] [Green Version]
- Bastos, L.J.; Peres, A.M.; Peres, G.K.; Barros, J.D.A. Infant growth, development and tooth emergence patterns: A longitudinal study from birth to 6 years of age. Arch. Oral Biol. 2007, 52, 598–606. [Google Scholar] [CrossRef] [PubMed]
- Alnemer, K.A.; Pani, S.C.; Althubaiti, A.M.; Bawazeer, M. Impact of birth characteristics, breast feeding and vital statistics on the eruption of primary teeth among healthy infants in Saudi Arabia: An observational study. BMJ Open 2017, 7, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kohli, M.V.; Patil, G.B.; Kulkarni, N.B.; Bagalkot, K.; Purohit, Z.; Dave, N.; Sagari, S.G.; Malaghan, M. A changing trend in eruption age and pattern of first deciduous tooth: Correlation to Feeding Pattern. J. Clin. Diagn. Res. 2014, 8, 199–201. [Google Scholar] [CrossRef]
- Ozieghe, E.O.; Adekoya-Sofowora, C.; Folayan, M.O.; Esan, T.A.; Owotabe, F.J. Relationship between socio-demographic and anthropometric variables and number of erupted primary teeth in suburban Nigerian children. J. Matern. Child. Nutr. 2009, 5, 86–92. [Google Scholar] [CrossRef]
- Pahkala, R.; Pahkala, A.; Laine, T. Eruption pattern of permanent teeth in a rural community in Northeastern Finland. Acta Odont. Scand. 1992, 49, 341–349. [Google Scholar] [CrossRef]
- Lunt, R.C.; Law, D.B. A review of the chronology of eruption of deciduous teeth. J. Am. Dent. Assoc. 1974, 89, 872–879. [Google Scholar] [CrossRef]
- Mugonzibwa, E.A.; Kuijpers-Jagtman, A.M.; Laine-Alawa, M.T.; Van’t Hof, M.A. Emergence of permanent teeth in Tanzanian children. Community Dent. Oral Epidemol. 2002, 30, 455–462. [Google Scholar] [CrossRef]
- Al- Jasser, N.M.; Bello, L.L. Time of eruption of Primary Dentition in Saudi Children. J. Contemp. Dent. Pract. 2003, 15, 65–75. [Google Scholar] [CrossRef]
- Hitchcock, N.E.; Gilmour, A.I.; Gracey, M.; Kailis, D.G. Australian Longitudinal study of time and order of eruption of primary teeth. Community Dent. Oral Epidemiol. 1984, 12, 260–263. [Google Scholar] [CrossRef] [PubMed]
- Robinow, M. The eruption of deciduous teeth (Factors involved in timing). J. Trop. Pediatr Environ. Child Health 1973, 18, 95–107. [Google Scholar]
- Singh, K.; Gorea, R.K.; Bharti, V. Age Estimation from Eruption of Temporary Teeth. JIAFM 2004, 26, 107–109. [Google Scholar]
- Bratu, E. Aspecte Clinice şi Experimentale Ale Erupţiei Dentare. Ph.D. Thesis, Institutul de Medicină Timişoara, Timişoara, Romania, 1982. [Google Scholar]
- English, J.D.; Peltomaeki, T.; Pham-Litschel, K. Mosby’s Orthodontic Review, 1st ed.; Mosby Elsevier: St. Louis, MO, USA, 2009; pp. 1–10, 13–21, 45–51. [Google Scholar]
- Proffit, W.R.; Fields, H.W.; Sarver, D.M. Contemporary Orthodontics, 4th ed.; Mosby Elsevier: St Louis, MO, USA, 2007; 86p. [Google Scholar]
- Guna Shekhar, M.; Tenny, J. Longitudinal study of age and order of eruption of primary teeth in Indian children. J. Clin. Exp. Dent. 2010, 3, e113–e116. [Google Scholar] [CrossRef]
- van Waes, H.J.M.; Stoeckli, P.W. Kinderzahnmedizin, 2nd ed.; Georg Thieme Verlag: Stuttgart, Germany, 2001; Volume 1, pp. 8–9. [Google Scholar]
- Woodroffe, S.; Mihailidis, S.; Hughes, T.; Bockmann, M.; Seow, W.K.; Gotjamanos, T.; Townsend, G. Primary Tooth emergence in Australian children: Timing, sequence and patterns of asymmetry. Aust. Dent. J. Sept. 2010, 55, 245–251. [Google Scholar] [CrossRef]
- Ramirez, O.; Planells, P.; Barberia, E. Age and order of eruption of primary teeth in Spanish children. Community Dent. Oral Epidemiol. 1994, 22, 56–59. [Google Scholar] [CrossRef]
| Variable | N | Mean ± Std. Deviation | Minimum | Maximum |
|---|---|---|---|---|
| The age of the mother at birth | ||||
| No. of mothers | 60 | 30.2 ± 4.03 | 22 | 42 |
| Weight and height at birth | ||||
| Weight(W) (kg) | 64 | 3321.7 ± 401.53 | 2500 | 4200 |
| Height(H) (cm) | 63 | 50.7 ± 2.02 | 45 | 56 |
| Weight (kg) at eight different times (months) | ||||
| W1 | 45 | 4045.7 ± 527.1 | 2700 | 5100 |
| W2 | 48 | 4965.8 ± 701.56 | 3200 | 6800 |
| W3 | 45 | 5659.7 ± 780.44 | 3500 | 7630 |
| W6 | 49 | 7486.0 ± 1041.99 | 3900 | 9400 |
| W12 | 46 | 9740.0 ± 1332.88 | 5930 | 12,500 |
| W18 | 26 | 11,498.4 ± 1998.3 | 6300 | 15,500 |
| W24 | 19 | 12,833.6 ± 1621.89 | 9010 | 16,200 |
| W30 | 6 | 14,800.0 ± 1649.24 | 13,000 | 17,800 |
| Height (cm) at eight different times (months) | ||||
| H1 | 34 | 54.0 ± 2.39 | 48 | 58 |
| H2 | 38 | 57.3 ± 3.02 | 50 | 64 |
| H3 | 36 | 60.4 ± 4.29 | 50 | 72 |
| H6 | 39 | 67.2 ± 5.2 | 52 | 86 |
| H12 | 37 | 75.6 ± 5.69 | 60 | 92 |
| H18 | 22 | 81.4 ± 7.06 | 62 | 94 |
| H24 | 17 | 89.2 ± 5.43 | 80 | 100 |
| H30 | 5 | 99.4 ± 8.35 | 90 | 110 |
| Tooth | N | Age (Months) | ||
|---|---|---|---|---|
| Mean ± Std. Deviation | Minimum | Maximum | ||
| 8.1 | 65 | 7.7 ± 2.28 | 3 | 14 |
| 7.1 | 66 | 7.7 ± 2.33 | 3 | 13 |
| 6.1 | 62 | 9.0 ± 2.36 | 3 | 17 |
| 5.1 | 61 | 9.0 ± 2.25 | 4 | 18 |
| 5.2 | 50 | 10.5 ± 2.53 | 6 | 16 |
| 6.2 | 56 | 10.8 ± 3.31 | 6 | 26 |
| 8.2 | 52 | 11.5 ± 3.84 | 6 | 28 |
| 7.2 | 53 | 11.7 ± 3.97 | 6 | 29 |
| 6.4 | 42 | 15.6 ± 2.69 | 11 | 21 |
| 8.4 | 39 | 15.7 ± 3.24 | 10 | 23 |
| 5.4 | 40 | 15.7 ± 3.01 | 11 | 23 |
| 7.4 | 44 | 15.7 ± 2.95 | 9 | 23 |
| 6.3 | 29 | 17.3 ± 2.99 | 10 | 22 |
| 5.3 | 29 | 17.4 ± 2.96 | 10 | 22 |
| 7.3 | 26 | 17.7 ± 3.19 | 10 | 24 |
| 8.3 | 27 | 18.0 ± 3.37 | 10 | 24 |
| 7.5 | 14 | 23.4 ± 2.24 | 20 | 29 |
| 8.5 | 14 | 23.5 ± 2.71 | 19 | 29 |
| 6.5 | 11 | 24.5 ± 2.12 | 21 | 29 |
| 5.5 | 10 | 25.0 ± 2.67 | 21 | 30 |
| Teeth | N | Age (Months) | ||
|---|---|---|---|---|
| Mean ± Std. Deviation | Minimum | Maximum | ||
| 7.1–8.1 | 131 | 7.7 ± 2.31 | 3 | 14 |
| 5.1–6.1 | 123 | 9.0 ± 2.29 | 3 | 18 |
| 5.2–6.2 | 106 | 10.6 ± 2.96 | 6 | 26 |
| 7.2–8.2 | 105 | 11.6 ± 3.87 | 6 | 29 |
| 5.4–6.4 | 82 | 15.7 ± 2.83 | 11 | 23 |
| 7.4–8.4 | 83 | 15.7 ± 3.07 | 9 | 23 |
| 5.3–6.3 | 58 | 17.4 ± 2.95 | 10 | 22 |
| 7.3–8.3 | 53 | 17.8 ± 3.25 | 10 | 24 |
| 7.5–8.5 | 28 | 23.4 ± 2.44 | 19 | 29 |
| 5.5–6.5 | 21 | 24.7 ± 2.35 | 21 | 30 |
| Group of Teeth | Female | Male | ||||||
|---|---|---|---|---|---|---|---|---|
| N | Mean ± Std. Deviation | Minimum | Maximum | N | Mean ± Std. Deviation | Minimum | Maximum | |
| 5.1–6.1 | 61 | 9.0 ± 2.814 | 3 | 18 | 62 | 9.0 ± 1.66 | 5 | 13 |
| 5.2–6.2 | 55 | 10.6 ± 3.56 | 6 | 26 | 51 | 10.7 ± 2.16 | 7 | 15 |
| 5.3–6.3 | 34 | 16.7 ± 3.35 | 10 | 22 | 24 | 18.3 ± 1.99 | 15 | 22 |
| 5.4–6.4 | 47 | 15.3 ± 3.04 | 11 | 23 | 35 | 16.1 ± 2.51 | 13 | 22 |
| 5.5–6.5 | 13 | 24.4 ± 1.66 | 22 | 28 | 8 | 25.3 ± 3.24 | 21 | 30 |
| 7.1–8.1 | 67 | 7.5 ± 2.6 | 3 | 14 | 64 | 8.0 ± 1.97 | 5 | 13 |
| 7.2–8.2 | 55 | 12.0 ± 4.63 | 7 | 29 | 50 | 11.2 ± 2.79 | 6 | 16 |
| 7.3–8.3 | 31 | 16.9 ± 3.69 | 10 | 23 | 22 | 19.1 ± 1.99 | 16 | 24 |
| 7.4–8.4 | 47 | 15.3 ± 3.33 | 9 | 23 | 36 | 16.1 ± 2.71 | 12 | 23 |
| 7.5–8.5 | 20 | 22.5 ± 1.91 | 19 | 26 | 8 | 25.8 ± 2.12 | 24 | 29 |
| Variable | Pearson Coefficient (r) | psignificance |
|---|---|---|
| Correlation between the eruption age of the first primary tooth and… | ||
| Weight at birth | 0.174 | 0.193 is |
| Height at birth | 0.157 | 0.239 is |
| Weight at 6 months | 0.198 | 0.183 is |
| Height at 6 months | 0.179 | 0.290 is |
| Mother’s age | 0.053 | 0.706 is |
| Natural diet | 0.19 | 0.190 is |
| Correlation between the number of teeth erupted at 1 year and… | ||
| Weight at 12 months | −0.048 | 0.768 is |
| Height at 12 months | −0.394 | 0.023 s |
| Teeth | Mean Age (Months) of Primary Teeth Eruption | ||||||
|---|---|---|---|---|---|---|---|
| Current Study | Peltomäki 2009 [38] | Proffit 2007 [39] | Stöckli 2001 [41] | Australia 2003 [42] | Australia 2010 [42] | India 2009 [40] | |
| 7.1–8.1 | 7.73 | 7 | 8 | 7 | 7.2 | 8.6 | 10.72 |
| 5.1–6.1 | 8.98 | 10 | 10 | 10 | 9 | 10.8 | 12.03 |
| 5.2–6.2 | 10.62 | 12 | 11 | 12 | 10.4 | 12.2 | 13.46 |
| 7.2–8.2 | 11.59 | 12 | 13 | 12 | 12.8 | 14.1 | 12.61 |
| 5.4–6.4 | 15.66 | 16 | 16 | 16 | 15.3 | 15.9 | 17.26 |
| 7.4–8.4 | 15.68 | 16 | 16 | 16 | 16 | 16.6 | 19.02 |
| 5.3–6.3 | 17.38 | 20 | 19 | 20 | 18.2 | 19.3 | 21.18 |
| 7.3–8.3 | 17.81 | 20 | 20 | 20 | 18.6 | 19.8 | 22.10 |
| 7.5–8.5 | 23.43 | 28 | 27 | 30 | 26.1 | 26.9 | 27.18 |
| 5.5–6.5 | 24.71 | 28 | 29 | 30 | 26.7 | 27.8 | 28.68 |
| Teeth | Mean Age of Primary Teeth Eruption | ||||||
|---|---|---|---|---|---|---|---|
| Current Study | Bratu Sample A 1979 [4] | Bratu Sample B 1978 [4] | Japan 1970 [42] | Australia1984 [42] | Canada 1984 [42] | Spain 1994 [42] | |
| 7.1–8.1 | 7.73 | 7.7 | 8.65 | 9.38 | 7.1 | 7.18 | 7.20 |
| 5.1–6.1 | 8.98 | 7 | 9.56 | 10.89 | 8.9 | 9.03 | 9.42 |
| 5.2–6.2 | 10.62 | 10.9 | 11.82 | 12.70 | 10.2 | 10.19 | 10.66 |
| 7.2–8.2 | 11.59 | 11.6 | 13.23 | 13.87 | 11.8 | 12.13 | 12.26 |
| 5.4–6.4 | 15.66 | 15 | 16.95 | 17.30 | 15 | 15.13 | 15.28 |
| 7.4–8.4 | 15.68 | 16.5 | 17.45 | 17.91 | 15.2 | 15.01 | 15.70 |
| 5.3–6.3 | 17.38 | 20.4 | 20.9 | 18.11 | 18.3 | 18.04 | 18.70 |
| 7.3–8.3 | 17.81 | 21.9 | 21.12 | 19.74 | 18.8 | 18.34 | 19.03 |
| 7.5–8.5 | 23.43 | 24.6 | 24.75 | 27.19 | 26 | 26.40 | 25.47 |
| 5.5–6.5 | 24.71 | 25.7 | 25.75 | 28.63 | 26.6 | 27.48 | 26.77 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ogodescu, E.; Popa, M.; Isac, C.; Pinosanu, R.; Olaru, D.; Cismas, A.; Tudor, A.; Miron, M. Eruption Timing and Sequence of Primary Teeth in a Sample of Romanian Children. Diagnostics 2022, 12, 606. https://doi.org/10.3390/diagnostics12030606
Ogodescu E, Popa M, Isac C, Pinosanu R, Olaru D, Cismas A, Tudor A, Miron M. Eruption Timing and Sequence of Primary Teeth in a Sample of Romanian Children. Diagnostics. 2022; 12(3):606. https://doi.org/10.3390/diagnostics12030606
Chicago/Turabian StyleOgodescu, Emilia, Malina Popa, Claudia Isac, Raluca Pinosanu, Diana Olaru, Anca Cismas, Anca Tudor, and Mariana Miron. 2022. "Eruption Timing and Sequence of Primary Teeth in a Sample of Romanian Children" Diagnostics 12, no. 3: 606. https://doi.org/10.3390/diagnostics12030606
APA StyleOgodescu, E., Popa, M., Isac, C., Pinosanu, R., Olaru, D., Cismas, A., Tudor, A., & Miron, M. (2022). Eruption Timing and Sequence of Primary Teeth in a Sample of Romanian Children. Diagnostics, 12(3), 606. https://doi.org/10.3390/diagnostics12030606






