Abstract
Cystic fibrosis (CF) is the most common life-limiting genetic disorder in European descent populations. It is caused by pathogenic variants in the CFTR gene, and inheritance is autosomal recessive. This study provides an up-to-date, comprehensive estimation of the distribution of CFTR pathogenic variants in Latvia and their phenotypic characteristics. It also reports the first results of the CF newborn screening programme following its implementation in 2019. We analysed the clinical and molecular data of CF patients treated at the only tertiary hospital in Latvia providing specialised healthcare for the disorder. Between 1997 and 2022, 66 CF patients from 62 families were diagnosed based on symptoms or a molecular confirmation (six patients were diagnosed through the CF newborn screening programme). F508del was identified in 70.5% of all CF chromosomes. Known variants were identified in more than one family: dele2,3, R1006H, L1335P, W57R, R553X, 2143delT and 3849+10kb C>T (legacy names used). Furthermore, two novel variants were identified, namely, c.503C>A p.(Ser168Ter) and c.(743+1_744-1)_(1584+1_1585-1)del p.(?). The available follow-up results indicated that Latvian CF patients demonstrated similar tendencies to CF patients worldwide. The oldest age at diagnosis prior to the implementation of the CF newborn screening programme was 14 years. We provide here, for the first time, a comprehensive description of Latvian CF patients. An improvement in the healthcare of CF patients over time, including access to diagnosis, is evident. Two novel CF-causing variants are reported, and F508del is the most frequently occurring variant in the population, thus suggesting that F508del screening should be followed by the testing of the full CFTR gene.
1. Introduction
Cystic fibrosis (CF; MIM #219700) has an autosomal recessive mode of inheritance and is the most common life-limiting genetic disorder in Caucasian populations [1]. In Europe, the incidence ranges from 1/1353 in Ireland to 1/25,000 in Finland and is, on average, 1/4500 in Western Europe and 1/6000 in Northern and Central Europe [2]. The Cystic Fibrosis Foundation reports that 60,000 to 70,000 people worldwide suffer from CF [3].
CF is caused by pathogenic variants/mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene and is characterised by a multiorgan pathology affecting the upper and lower airways, gastrointestinal and reproductive tracts and endocrine system [4].
To date, more than 2000 CFTR variants have been identified [5]. Central, Northern, Western and Northeastern Europe show a large degree of homogeneity among the CFTR mutations. On average, 10.2 mutations per country account for 78.9% of the total number of CF chromosomes in these regions. This is largely attributable to the high prevalence of the F508del variant in these regions [6]. This variant is also the most frequent one in the general Caucasian population, accounting for ∼66% of CF chromosomes. Only a few CFTR variants reach worldwide frequencies of >1%, fewer than 20 have frequencies between 0.1 and 1.0% and the majority are found only in certain geographical regions, populations or single families (termed ‘private’) [7].
Currently, six major classes of CFTR mutations are distinguished. Class I mutations result in severely reduced or absent CFTR expression. Class II mutations severely reduce the number of CFTR molecules that reach the cell surface. Class III mutations impair the regulation of the CFTR channel, resulting in abnormal gating characterised by a reduced open probability. Class IV mutations alter the channel conductance by impeding the ion conduction pore, leading to a reduced unitary conductance. Class V mutations do not change the conformation of the protein but alter its abundance by introducing promoter or splicing abnormalities. Class VI mutations destabilise the channel in post-endoplasmic reticulum compartments and/or at the plasma membrane [4]. As studies have shown that a genetic variant can have characteristics of multiple classes, understanding the functionality of each mutation class is crucial for the development of drug combination strategies to improve the available treatments for CF patients [4]. Mutation classification is important as specific treatments are created, and that is the reason why some authors are suggesting to export variants in which no mRNA is created from the Class I into the new Class VII and to rename the mutation class to Theratype [8,9,10]. As guidelines in reclassification are still missing, we used the existing classification.
Presently, nationwide CF newborn screening is offered in nearly all European countries, the United States of America, Canada, Australia and even Russia, Turkey and Brazil [11]. In Latvia, nationwide CF newborn screening was implemented in 2019, following a 2009 pilot study [12]. Prior to the CF newborn screening programme, diagnosis was made after clinical manifestation (confirmed with a positive sweat test (chloride concentration > 60 mmol/L)) or through family anamnesis.
This study provides an up-to-date comprehensive estimation of the distribution of CFTR pathogenic variants in Latvia and their phenotypic characteristics. It also reports the first results of the CF newborn screening programme following its implementation in 2019.
2. Materials and Methods
Written informed consent was obtained from all individuals involved in the study. The study was performed according to the Declaration of Helsinki, and the protocol was approved by the Central Medical Ethics Committee of Latvia.
A clinical diagnosis of CF was established in 62 unrelated symptomatic patients (4 siblings) and 6 pre-symptomatic patients (through newborn screening) according to clinical and laboratory diagnostic consensus criteria [13]—in total, 70 patients. They represented all the CF patients in Latvia during the 25-year period from 1997 to June 2022. Due to various reasons (e.g., death (n = 18), emigration), only 45 patients are currently under clinical follow-up.
Over the 25-year period, our method for the molecular analysis of CFTR (HGNC:1884, reference sequences: NG_016465.4, NM_000492.3, LRG_663) evolved as new technologies were developed. The initial mutation screen was performed by polymerase chain reaction (PCR) for c.1521_1523delCTT p.(Phe508del) (legacy name F508del variant) and 54-5940_273+10250del21kb p.(Ser18ArgfsX16) (legacy name CFTRdele2,3) using duplex PCR and allele-specific PCR adapted from previous studies. Subsequently, PCR with restriction fragment length polymorphism (1998–2005), single-stranded conformation polymorphism with Sanger sequencing (2005–2007), APEX (2007–2012) and Elucigene (2012–2015) were the technologies employed. Since 2015, we have sequenced the whole coding part of CFTR using ThermoFisher’s BigDye Terminator v.3.1 Cycle Sequencing Kit (according to their protocol) and previously published primers [14]. In addition to the coding part, our current analysis also includes two deep intronic variants (c.3718-2477C>T p.(?) (legacy name 3849+10kbC>T) and c.1680-877G>T p.(?) (legacy name 1811+1.6kbA>G)), followed by multiplex ligation-dependent probe amplification (MRC-Holland). If two CF-causing variants were identified, to confirm variant localisation in the trans position and thus CF diagnosis, the parents were tested accordingly.
The databases CFTR1, CFTR2 and ClinVar were used for clinical interpretation. For rare variants, the American College of Medical Genetics guidelines were used.
The CF newborn screening strategy was as follows: immunoreactive trypsinogen (IRT) measured by a fluorometric enzyme immunoassay and Fluoroskan Ascent microplate fluorometer. If >70 ng/mL, then a second IRT measurement was performed. If elevated again, then DNA analysis (method detailed above) and a sweat test were performed in parallel. The IRT cut-off was set at 50 ng/mL for the first three months, after which it was adjusted to 70 ng/mL.
3. Results
3.1. CF Screening Results
A total of 50,859 samples were subjected to IRT assessment. The number of children screened represented more than 99% of the children born in the period. In total, 772 (1.5%) were re-assessed for IRT, with 184 having an increased IRT for the second time. Consequently, these 184 children underwent DNA analysis and a sweat test. Seven infants with two pathogenic CFTR variants were reported for diagnostic follow-up, giving an incidence of 1:7265.
3.2. CFTR Genotype
A total of 21 pathogenic variants in the CFTR gene were found in 128 of 132 (97%) affected chromosomes in the Latvian population. Two of these variants, c.503C>A p.(Ser168Ter) in exon 5 and c.(743+1_744-1)_(1584+1_1585-1)del p.(?) covering exon 7 through to exon 11, have not been previously described. Table 1 shows the frequency and pathogenicity class of the 21 CFTR CF-causing variants found in our patients. In total, 13.1% of Latvian patients carried Class I mutations, and 75.1% carried at least one Class II mutation.

Table 1.
Frequency and pathogenicity class of CFTR CF-causing variants found in 66 unrelated Latvian patients.
Of 66 CF individuals, two pathogenic or likely pathogenic variants were found in 62. A second pathogenic variant could not be identified in four cases (3% of alleles). This was because our current extensive molecular analysis of CFTR was not implemented until 2015, the patients were already deceased and additional DNA material was not available (Table S1 summarises the genotype results). The frequencies of the variants identified in our Latvian patients were compared with those reported in our closest neighbours to establish the degree of similarity (Table 2).

Table 2.
Frequencies of CFTR variants detected in CF patients from Latvia and neighbouring countries.
In 13 of the 70 (18.6%) Latvian patients, we revealed rare CFTR genotypes; their compound heterozygous state was confirmed by parental testing. Some of these rare cases that were available for follow-up are briefly phenotypically presented below.
3.2.1. Case 1 with Genotype c.[1521_1523delCTT];[169T>C] p.[(Phe508del)];[(Trp57Arg)], Legacy Nomenclature dF508/W57R
He was a male patient. CF diagnosis was confirmed at 10 months of age in 1993 by a positive sweat test result of 200 mmol/L. Poor weight gain, fatty stools, wet cough and symptoms of gastro-oesophageal reflux disease (GORD) were present from the first months of life. From the age of 6 years, he had severe pulmonary exacerbations (PExs) at least four times a year, which were treated with intravenous antibiotics. He developed chronic rhinosinusitis at the age of 8. Chronic multiresistant Pseudomonas aeruginosa infection was recognised early at the age of 12. At the age of 14, his disease was complicated by renal amyloidosis and progressive renal failure. He died at the age of 15 due to progressive respiratory failure.
3.2.2. Case 2 with Genotype c.[3844T>C];[2012delT] p.[(Trp1282Arg)];[(Leu671Ter)], Legacy Nomenclature W1282R/2143delT
She was a female patient. CF diagnosis was assumed at 4 months of age due to the clinical presentation of pancreatic exocrine insufficiency and productive cough and was subsequently confirmed with a positive sweat test result of 126 mmol/L. Despite receiving regular pancreatic enzyme replacement therapy with pancreatin, she has problems with weight and height gain. Accordingly, since the age of 9 years, a percutaneous endoscopic gastrostomy probe has provided enteral feeding. From the age of 4, she has had the frequent recurrence of PExs; seven episodes in the last year required hospitalisation and intravenous antibiotics. The patient is currently 10 years old.
3.2.3. Cases 3 and 4 with Genotype c.[1521_1523delCTT];[3844T>C] p.[(Phe508del)];[(Trp1282Arg)], Legacy Nomenclature dF508/W1282R
These cases involve two siblings—female and male (shown in Figure 1). The CF diagnosis of II-1 was confirmed by a positive sweat test result of 136 mmol/L at the age of 3 years. Symptoms of malabsorption with poor weight gain, fatty stools from birth and early respiratory symptoms (persistent and productive cough) were observed. GORD symptoms were observed periodically since adolescence. She had CF-associated diabetes since the age of 24. She died at the age of 32, with PExs escalating to seven episodes requiring hospitalisation in her final year, the progression of respiratory failure and the development of secondary pulmonary hypertension.
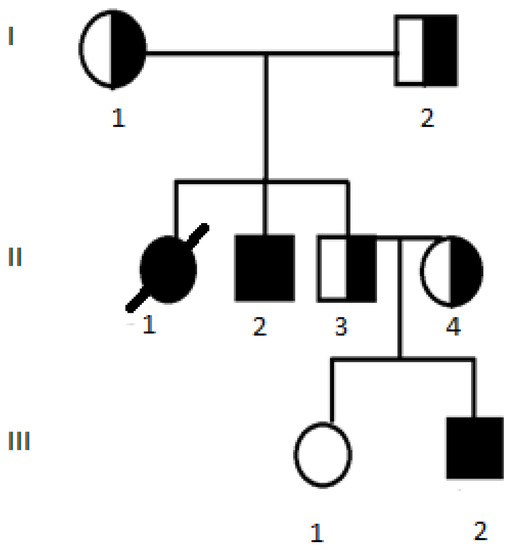
Figure 1.
Pedigree for Case 3 (II-1) and Case 4 (II-2). III-2 has genotype c.[1521_1523delCTT];[1521_1523delCTT] p.[(Phe508del)];[(Phe508del)], legacy nomenclature dF508/dF508.
Her brother, II-2, did not have his CF diagnosis confirmed until the age of 24 years. This was because his parents would not consent to him nor his healthy brother being assessed earlier. However, their attitude changed after the healthy brother’s second child was diagnosed with CF shortly after birth (III-2). Patient II-2 has mild pancreatic insufficiency and respiratory symptoms, despite chronic Pseudomonas aeruginosa colonisation, with mild PExs. The patient is currently 30 years old.
Patient III-2 is a male relative of the two previous cases. Due to the family history of CF, he was molecularly diagnosed with CF—homozygous for c.1521_1523delCTT p.(Phe508del)—shortly after birth. Concurrently, his diagnosis was clinically confirmed by a positive sweat test result of 122 mmol/L. He has had symptoms of pancreatic insufficiency since birth and a productive cough from 6 months of age. He also has the chronic colonisation of Staphylococcus aureus in his airways and mild PExs twice a year, which are treated with oral antibiotics. His episodic symptoms of GORD are treated with proton pump inhibitors. The patient is currently 6 years old. Interestingly, this pedigree shows that, in some families, a rare disease may occur more frequently than estimated.
3.2.4. Cases 5 and 6 with Genotype c.[1521_1523delCTT];[(743+1_744-1)_(1584+1_1585-1)dup] p.[(Phe508del)];[(?)], Legacy Nomenclature dF508/dup6b-10
These cases involve two male siblings. CF diagnosis of the index case was established at the age of 3 years. The diagnosis was confirmed by a positive sweat test result of 97 mmol/L. The disease at the time of diagnosis was manifested by respiratory tract (wet cough) and pancreatic insufficiency (poor weight gain, fatty stools) symptoms. GORD, oesophagitis and CF liver disease were diagnosed at the age of 12. Over a 25-year follow-up period, the patient has had mild PExs once or twice a year, which are most often treated with oral antibiotics; although, for the last two years, he has received one course of intravenous antibiotics each year. He has chronic Staphylococcus aureus colonisation in his airways; Haemophilus influenzae infection was detected once and successfully treated with one eradication course. The patient is currently 28 years old.
His sibling was clinically diagnosed with CF shortly after birth. The diagnosis was confirmed by a positive sweat test result of 100 mmol/L. At the time of diagnosis, fatty stools were observed, and, consequently, pancreatic enzyme replacement therapy was started. Respiratory symptoms appeared at the age of 1 year. The patient has mild PExs a couple times a year, which are treated with oral antibiotics. A diagnosis of CF liver disease was confirmed at the age of 14. He has chronic Staphylococcus aureus colonisation in his airways. The patient is currently 19 years old.
3.2.5. Case 7 with Genotype c.[503C>A];[4004T>C] p.[(Ser168Ter)];[(Leu1335Pro)], Legacy Nomenclature Ser168Ter/L1335P
She is a female patient. CF diagnosis was made at 3 weeks of age through the CF newborn screening programme (IRT 121.13 µg/L on day 8, IRT 83.16 µg/L on day 16) and was confirmed with two positive sweat tests (84 and 85 mmol/L). The patient is pancreatic sufficient; elastase 300 µg/kg. During her first year, she had symptoms of GORD (treated with proton pump inhibitors), recurrent episodes of wheezing bronchitis and the chronic colonisation of Staphylococcus aureus and Haemophilus influenzae. In her second year, she has had mild PExs twice (treated with oral antibiotics). The patient is currently 1 year and 11 months old.
3.3. Clinical and Laboratory Characteristics of CF Patients under Regular Follow-Up
From a total of 66 patients (62 index patients), regular follow-up data were available for only 45 cases (Table 3). During 2021, two patients died and nine ceased to be followed up.

Table 3.
Characterisation of CF patients currently under regular follow-up in 2022.
4. Discussion
This study presents a comprehensive overview of Latvian CF patients by investigating a representative cohort of 66 patients (62 unrelated index cases) over a 25-year period. Two-and-a-half years of newborn screening revealed a lower incidence of CF than previous calculations based on F508del carrier detection in 2001 and a CF newborn screening pilot study [12]. However, other studies have reported similar data indicating that epidemiological changes have occurred both in the incidence of CF, which appears to be decreasing in most countries, and in the survival of CF patients, which has greatly improved in recent decades [2]. For instance, in the Slovak population, the CF incidence was first estimated in 1979 at a frequency of 1:1800 newborns; however, three decades later, neonatal genetic screening between 2009 and 2015 revealed a lower incidence of 1:6315–7668 [25].
In Europe, countries such as Norway and the United Kingdom have offered newborn screening for CF for many years, whereas our neighbouring countries, Estonia and Lithuania, have yet to implement CF newborn screening [26]. Therefore, it is difficult at present to compare our data with those from geographically close populations. As we implemented our CF newborn screening programme only very recently (1 August 2019), it is still being optimised. One concern is the possibility of false negative cases as, fewer new cases than expected have been diagnosed since we started to screen newborns for CF. In Latvia, we have adopted the protocol of two successive elevated IRT measurements, followed by a sweat test and molecular analysis of the whole CFTR gene. However, in the near future, we plan to improve this protocol with earlier CFTR molecular analysis (i.e., IRT/DNA/IRT), similar to the procedures employed in Poland [27] and Switzerland [28]. After CF NBS implementation, six patients are discovered. Two had meconium ileus, which would lead to an early CF diagnosis anyway, but four cases, including a case with two rare mutations, were possible to diagnose so early only because CF NBS, as significant symptoms, were not present at the moment. Five out of six patients have the most common genotype identified: homozygoys F508del variant. Clinical symptoms comparison with patients diagnosed before and during the screening for the patients with the same genotype was not performed because the patients are still young, and long-term prognosis could only be predicted, but also has been changed used treatment.
A 2014 study reported that Latvia had a high frequency of CFTR Class II mutations (>90%) in comparison with other European countries; however, the study sample consisted of only 29 CF patients [29]. The present study, with a higher patient count, found that 75.1% of CF patients carried at least one CFTR Class II mutation. We identified very few patients with Class III mutations, which are interpreted differently by various researchers [4, 18]. In contrast, the frequency of Class III mutations has been reported to be 13.9% and 6.5% in Ireland and the United Kingdom, respectively [29]. We identified the F508del variant in 70.5% of all CF chromosomes. This is the highest frequency among the Baltic countries, with 52% and 51.7% being reported in Lithuania [16] and Estonia [20], respectively. However, it should be noted that these frequencies were reported over 15 years ago. Furthermore, in small populations, such as the one in Latvia, even just a few patients can change the data significantly. Molecular analysis of our patients’ genotypes revealed that 37 out of 45 were eligible for CFTR modulating therapy with elexacaftor/tezacaftor/ivacaftor and ivacaftor. This treatment is not yet available in Latvia but will hopefully be accessible soon. Four (6%) of our patients had a genotype for which CFTR modulating therapy is not suggested. It has recently been reported that approximately 10% of CF patients are not eligible for this type of therapy [30]. Hence, the development of effective therapies for these patients is a priority.
We identified two novel CFTR variants in our patient cohort. One patient (Case 7; pancreatic sufficient) carried a rare variant and a novel variant, c.503C>A p.(Ser168Ter). This patient was diagnosed through the CF newborn screening programme and exemplifies why it is necessary to conduct a full scan of CFTR after F508del analysis. Her novel point variant is interpreted as CF-causing based on the following American College of Medical Genetics criteria [31]: PVS1—the variant leads to a premature STOP codon, and protein function loss is a known mechanism for the development of the disease; PS1—the variant c.503C>T p.(Ser168Leu) has previously been described in the literature and identified in CF patients [32]; PM2—the variant is absent from the Genome Aggregation Database (gnomAD); PM3—the variant is in the trans position with a known CF-causing variant; PP4—CF newborn screening of the patient reveals two successive elevated IRT measurements and two positive sweat tests.
Interestingly, almost all of our molecular findings are shared with native Russians. For example, a very rare variant, c.3844T>C p.(Trp1282Arg) [23], that is not even reported in CFTR databases was detected at a higher incidence in our CF patients than in the Russian population (1.5% versus 0.76%). Researchers in Russia have classified it as a Class II or III variant, showing pancreatic insufficiency and a significant decrease in lung function. Indeed, both of our patients with this variant in the compound heterozygous state (F508del/W1282R and W1282R/2143delT) showed pancreatic insufficiency and a severe disease course. We were unable to find a mention of the W1282R/2143delT combination of CFTR variants in the literature, although Petrova et al. have reported the frequency of the 2143delT variant in Russia—2.71% [17]. Nevertheless, our patient with this CFTR variant combination displayed a classic CF phenotype with pancreatic insufficiency. To the best of our knowledge, both of our patients with the W1282R variant are of Latvian origin. Thus, this variant could be characteristic of Latvians as well; however, future studies focusing on native Latvians need to be conducted to confirm this. As mentioned above, we plan, in the near future, to change our newborn screening algorithm and perform DNA analysis directly after the measurement of the first elevated level of IRT. Our first-line DNA analysis will include W1282R as well as c.1545_1546delTA p.(Tyr515Ter) and c.169T>C p.(Trp57Arg), described in the Finnish population [22]. These variants are not included in the first-line panel of our neighbouring countries, such as Poland [27]. As it is evident that CFTR variants are highly heterogeneous among different populations, we believe that local studies and registries are very important, even in countries with relatively small numbers of CF patients.
Latvia has a relatively small population of approximately two million, consequently giving rise to a proportionally small number of CF patients. The available budget for rare disease treatment in Latvia is not as extensive as that in other European countries. For example, when dornase alfa was approved as a treatment for CF, only some of our patients received it due to financial constraints. Similarly, the only CFTR modulator available in Latvia is lumacaftor/ivacaftor (since the beginning of 2022). Furthermore, lung transplantation is not currently a realistic option, as Latvia is not yet a member of Scandiatransplant (http://www.scandiatransplant.org/ (accessed on 1 November 2022)). Therefore, transplantation would only be possible if the donor was Latvian and the patient’s clinical status allowed for it. Taking all these factors into consideration, the clinical data we have presented here reflect a good quality of care for CF patients in Latvia.
Regular follow-up is an important part of CF patient management. Over the last 10 years, death (in 2021, two patients died at the ages of 24 and 35 years) and emigration have resulted in patients not being followed up. Additionally, two patients attend follow-up irregularly, correlating with their adherence to treatment plans. It has been identified that a patient’s adherence to their treatment plan is stronger at an earlier age and decreases as patients grow up [33]. Parental depression has been proposed as a factor in treatment adherence based on the findings of a study conducted in Ireland [34]. In the present study, parental refusal to test offspring resulted in a delay in the molecular diagnosis of one of our patients described in the family of Cases 3 and 4.
Our patients showed a relatively low Pseudomonas aeruginosa infection rate (22.2%) in comparison to the colonisation rate reported by Petrova et al. in Bulgarian CF patients (60%) [21]. In Cypriot CF patients, 53.9% were found to be positive for the Pseudomonas aeruginosa pathogen, and 26.9% had chronic Pseudomonas aeruginosa colonisation [35].
A study of longitudinal changes in nutrition using BMI values over a 10-year period has reported that underweight CF patients decreased from 20.6% to 11.1%, while overweight/obese CF patients increased from 7% to 18.4% [36]. Most of our patients were found to be in the normal weight group, where the BMI is between 19 and 25, which is very important for better lung function.
5. Conclusions
The present study provides a comprehensive description of Latvian CF patients by documenting the genetic background of CFTR in the Latvian population and reporting two novel variants. Furthermore, the first results of the Latvian CF newborn screening programme since it was implemented in 2019 are presented. The challenges faced by countries like Latvia with relatively small populations, such as a modest healthcare budget and limited clinical experience, are also discussed. Lastly, due to the Latvian population’s unique ethnicity, several notable differences attributable to regional peculiarities are highlighted.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/diagnostics12112893/s1, Table S1: Genotypes of Latvian CF patients diagnosed between 1997 and 2022 and information about these genotypes held in the CFTR2 database (last updated 29 April 2022).
Author Contributions
Conceptualization, M.A. and E.A.; methodology, M.A. and L.G.; formal analysis, L.G., I.K. and G.T.; investigation, M.A., G.T., E.A., V.S., L.K., I.K. and L.G.; resources, L.G.; data curation, M.A., E.A. and L.G.; writing—original draft preparation, M.A., E.A. and L.G.; writing—review and editing, V.S., L.K., I.K. and G.T.; visualisation, M.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Central Medical Ethics Committee of Latvia.
Informed Consent Statement
Written informed consent was obtained from all individuals involved in the study.
Data Availability Statement
Not applicable.
Acknowledgments
We would like to gratefully acknowledge all the laboratory specialists who were involved in the molecular analysis of CFTR between 1997 and 2022. We also gratefully acknowledge all the medical doctors who recognised suspected CF patients prior to the implementation of the newborn screening programme in Latvia.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bareil, C.; Bergougnoux, A. CFTR Gene Variants, Epidemiology and Molecular Pathology. Arch. Pediatrie Organe Off. Soc. Fr. Pediatrie 2020, 27, eS8–eS12. [Google Scholar] [CrossRef]
- Scotet, V.; Gutierrez, H.; Farrell, P.M. Newborn Screening for CF across the Globe-Where Is It Worthwhile? Int. J. Neonatal Screen. 2020, 6, 18. [Google Scholar] [CrossRef] [PubMed]
- Banjar, H.H.; Tuleimat, L.; El Seoudi, A.A.A.; Mogarri, I.; Alhaider, S.; Nizami, I.Y.; Almaghamsi, T.; Alkaf, S.A.; Moghrabi, N. Genotype patterns for mutations of the cystic fibrosis transmembrane conductance regulator gene: A retrospective descriptive study from Saudi Arabia. Ann. Of. Saudi Med. 2020, 40, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Veit, G.; Avramescu, R.G.; Chiang, A.N.; Houck, S.A.; Cai, Z.; Peters, K.W.; Hong, J.S.; Pollard, H.B.; Guggino, W.B.; Balch, W.E.; et al. From CFTR Biology toward Combinatorial Pharmacotherapy: Expanded Classification of Cystic Fibrosis Mutations. Mol. Biol. Cell 2016, 27, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, M.; Matsvay, A.; Glazova, O.; Krasovskiy, S.; Usacheva, M.; Amelina, E.; Chernyak, A.; Ivanov, M.; Musienko, S.; Prodanov, T.; et al. Targeted Sequencing Reveals Complex, Phenotype-Correlated Genotypes in Cystic Fibrosis. BMC Medical Genomics 2018, 11 (Suppl. S1), 13. [Google Scholar] [CrossRef] [PubMed]
- Bobadilla, J.L.; Macek, M., Jr.; Fine, J.P.; Farrell, P.M. Cystic Fibrosis: A Worldwide Analysis of CFTR Mutations-Correlation with Incidence Data and Application to Screening. Hum. Mutat. 2002, 19, 575–606. [Google Scholar] [CrossRef] [PubMed]
- Ziętkiewicz, E.; Rutkiewicz, E.; Pogorzelski, A.; Klimek, B.; Voelkel, K.; Witt, M. CFTR Mutations Spectrum and the Efficiency of Molecular Diagnostics in Polish Cystic Fibrosis Patients. PLoS ONE 2014, 9, e89094. [Google Scholar] [CrossRef] [PubMed]
- Amaral, M.D. How to determine the mechanism of action of CFTR modulator compounds: A gateway to theranostics. Eur. J. Med. Chem. 2020, 210, 112989. [Google Scholar] [CrossRef] [PubMed]
- De Boeck, K.; Amaral, M.D. Progress in Therapies for Cystic Fibrosis. Lancet Respir. Med. 2016, 4, 662–674. [Google Scholar] [CrossRef]
- Marson, F.A.L.; Bertuzzo, C.S.; Ribeiro, J.D. Classification of CFTR Mutation Classes. Lancet Respir. Med. 2016, 4, e37–e38. [Google Scholar] [CrossRef]
- De Boeck, K. Cystic Fibrosis in the Year 2020: A Disease with a New Face. Acta Paediatr. 2020, 109, 893–899. [Google Scholar] [CrossRef] [PubMed]
- Lace, B.; Grīnblate, S.; Korņejeva, L.; Švābe, V.; Grauduma, I.; Vēvere, P.; Lugovska, R.; Krams, A.; Martinsons, A. Neonatal Cystic Fibrosis Screening in Latvia: A Pilot Project. Proc. Latv. Acad. Sci. Sect. 2009, 63, 147–150. [Google Scholar] [CrossRef]
- Farrell, P.M.; White, T.B.; Ren, C.L.; Hempstead, S.E.; Accurso, F.; Derichs, N.; Howenstine, M.; McColley, S.A.; Rock, M.; Rosenfeld, M.; et al. Diagnosis of Cystic Fibrosis: Consensus Guidelines from the Cystic Fibrosis Foundation. J. Pediatr. 2017, 181, S4–S15.e1. [Google Scholar] [CrossRef]
- Lucarelli, M.; Narzi, L.; Piergentili, R.; Ferraguti, G.; Grandoni, F.; Quattrucci, S.; Strom, R. A 96-Well Formatted Method for Exon and Exon/Intron Boundary Full Sequencing of the CFTR Gene. Anal. Biochem. 2006, 353, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Bell, S.C.; De Boeck, K.; Amaral, M.D. New Pharmacological Approaches for Cystic Fibrosis: Promises, Progress, Pitfalls. Pharmacol. Ther. 2015, 145, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Giannattasio, S.; Bobba, A.; Jurgelevičius, V.; Vacca, R.A.; Lattanzio, P.; Merafina, R.; Utkus, A.; Kučinskas, V.; Marra, E. Molecular Basis of Cystic Fibrosis in Lithuania: Incomplete CFTR Mutation Detection by PCR-Based Screening Protocols. Genet. Test. 2006, 10, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Petrova, N.V.; Kashirskaya, N.Y.; Vasilyeva, T.A.; Kondratyeva, E.I.; Zhekaite, E.K.; Voronkova, A.Y.; Sherman, V.D.; Galkina, V.A.; Ginter, E.K.; Kutsev, S.I.; et al. Analysis of CFTR Mutation Spectrum in Ethnic Russian Cystic Fibrosis Patients. Genes 2020, 11, 554. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Rab, A.; Pellicore, M.; Davis, E.F.; McCague, A.F.; Evans, T.A.; Joynt, A.T.; Lu, Z.; Cai, Z.; Raraigh, K.S.; et al. Residual Function of Cystic Fibrosis Mutants Predicts Response to Small Molecule CFTR Modulators. JCI Insight 2018, 3, e121159. [Google Scholar] [CrossRef]
- Raraigh, K.S.; Han, S.T.; Davis, E.; Evans, T.A.; Pellicore, M.J.; McCague, A.F.; Joynt, A.T.; Lu, Z.; Atalar, M.; Sharma, N.; et al. Functional Assays Are Essential for Interpretation of Missense Variants Associated with Variable Expressivity. Am. J. Hum. Genet. 2018, 102, 1062–1077. [Google Scholar] [CrossRef] [PubMed]
- Teder, M.; Klaassen, T.; Oitmaa, E.; Kaasik, K.; Metspalu, A. Distribution of CFTR Gene Mutations in Cystic Fibrosis Patients from Estonia. J. Med. Genet. 2000, 37, E16. [Google Scholar] [CrossRef] [PubMed]
- Petrova, G.; Yaneva, N.; Hrbková, J.; Libik, M.; Savov, A.; Macek, M. Identification of 99% of CFTR Gene Mutations in Bulgarian-, Bulgarian Turk-, and Roma Cystic Fibrosis Patients. Mol. Genet. Genom. Med. 2019, 7, e696. [Google Scholar] [CrossRef] [PubMed]
- Kinnunen, S.; Bonache, S.; Casals, T.; Monto, S.; Savilahti, E.; Kere, J.; Järvelä, I. Spectrum of Mutations in CFTR in Finland: 18 Years Follow-up Study and Identification of Two Novel Mutations. J. Cyst. Fibros. 2005, 4, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Petrova, N.; Balinova, N.; Marakhonov, A.; Vasilyeva, T.; Kashirskaya, N.; Galkina, V.; Ginter, E.; Kutsev, S.; Zinchenko, R. Ethnic Differences in the Frequency of CFTR Gene Mutations in Populations of the European and North Caucasian Part of the Russian Federation. Front. Genet. 2021, 12, 678374. [Google Scholar] [CrossRef] [PubMed]
- Quint, A.; Lerer, I.; Sagi, M.; Abeliovich, D. Mutation Spectrum in Jewish Cystic Fibrosis Patients in Israel: Implication to Carrier Screening. Am. J. Med. Genet. Part A 2005, 136A, 246–248. [Google Scholar] [CrossRef] [PubMed]
- Soltysova, A.; Tarova, E.T.; Ficek, A.; Baldovic, M.; Polakova, H.; Kayserova, H.; Kadasi, L. Comprehensive Genetic Study of Cystic Fibrosis in Slovak Patients in 25 Years of Genetic Diagnostics. Clin. Respir. J. 2017, 12, 1197–1206. [Google Scholar] [CrossRef] [PubMed]
- Loeber, J.; Platis, D.; Zetterström, R.; Almashanu, S.; Boemer, F.; Bonham, J.; Borde, P.; Brincat, I.; Cheillan, D.; Dekkers, E.; et al. Neonatal Screening in Europe Revisited: An ISNS Perspective on the Current State and Developments Since 2010. Int. J. Neonatal Screen. 2021, 7, 15. [Google Scholar] [CrossRef] [PubMed]
- Zybert, K.; Wozniacki, L.; Tomaszewska-Sobczyńska, A.; Wertheim-Tysarowska, K.; Czerska, K.; Ołtarzewski, M.; Sands, D. Clinical Characteristics of Rare CFTR Mutations Causing Cystic Fibrosis in Polish Population. Pediatric Pulmonol. 2020, 55, 2097–2107. [Google Scholar] [CrossRef] [PubMed]
- Torresani, T.; Fingerhut, R.; Rueegg, C.S.; Gallati, S.; Kuehni, C.E.; Baumgartner, M.R.; Barben, J. Newborn Screening for Cystic Fibrosis in Switzerland--Consequences after Analysis of a 4 Months Pilot Study. J. Cyst. Fibros. Off. J. Eur. Cyst. Fibros. Soc. 2013, 12, 667–674. [Google Scholar] [CrossRef][Green Version]
- De Boeck, K.; Zolin, A.; Cuppens, H.; Olesen, H.; Viviani, L. The Relative Frequency of CFTR Mutation Classes in European Patients with Cystic Fibrosis. J. Cyst. Fibros. 2014, 13, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Garratt, A.; Hill, A. Worldwide Rates of Diagnosis and Effective Treatment for Cystic Fibrosis. J. Cyst. Fibros. Off. J. Eur. Cyst. Fibros. Soc. 2022, 21, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and Guidelines for the Interpretation of Sequence Variants: A Joint Consensus Recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. Off. J. Am. Coll. Med. Genet. 2015, 17, 405–424. [Google Scholar] [CrossRef] [PubMed]
- Cambraia, A.; Junior, M.C.; Zembrzuski, V.M.; Junqueira, R.M.; Cabello, P.H.; de Cabello, G.M.K. Next-Generation Sequencing for Molecular Diagnosis of Cystic Fibrosis in a Brazilian Cohort. Dis. Markers 2021, 2021, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Amini, S.; Jafarrangraz, S.S.; Modaresi, M.; Gholami, K. Evaluation of adherence to medication treatment in pediatric patients with cystic fibrosis; a cross-sectional study. Eur. Respir. J. 2019, 54, pa4520. [Google Scholar] [CrossRef]
- A Goodfellow, N.; Hawwa, A.F.; Reid, A.J.; Horne, R.; Shields, M.D.; McElnay, J.C. Adherence to Treatment in Children and Adolescents with Cystic Fibrosis: A Cross-Sectional, Multi-Method Study Investigating the Influence of Beliefs about Treatment and Parental Depressive Symptoms. BMC Pulm. Med. 2015, 15, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Yiallouros, P.K.; Matthaiou, A.; Anagnostopoulou, P.; Kouis, P.; Libik, M.; Adamidi, T.; Eleftheriou, A.; Demetriou, A.; Ioannou, P.; Tanteles, G.A.; et al. Demographic Characteristics, Clinical and Laboratory Features, and the Distribution of Pathogenic Variants in the CFTR Gene in the Cypriot Cystic Fibrosis (CF) Population Demonstrate the Utility of a National CF Patient Registry. Orphanet J. Rare Dis. 2021, 16, 409. [Google Scholar] [CrossRef] [PubMed]
- Kutney, K.A.; Sandouk, Z.; Desimone, M.; Moheet, A. Obesity in Cystic Fibrosis. J. Clin. Transl. Endocrinol. 2021, 26, 100276. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).