Abstract
The aim of this systematic review is to guide the physician in defining the pharmacologic and rehabilitative therapeutic approaches for adopting the best strategies described in the current literature. The search was conducted in PubMed, EMBASE, Cochrane Library and Web of Science to identify the treatment of small fiber neuropathies. Two reviewers independently reviewed and came to a consensus on which articles met inclusion/exclusion criteria. The authors excluded the duplicates, animal studies and included the English articles in which the treatment of patients with small fiber neuropathies was described. The search identified a total of 975 articles with the keywords “small fiber neuropathy” AND “rehabilitation” OR “therapy” OR “treatment”. Seventy-eight selected full-text were analyzed by the reviewers. Forty-two publications met the inclusion criteria and were included in the systematic review to describe the rehabilitative and pharmacologic treatment of small fiber neuropathies. Despite the range of different protocols of treatment for small fiber neuropathy, other robust trials are needed. In addition, always different therapeutic approaches are used; a unique protocol could be important for the clinicians. More research is needed to build evidence for the best strategy and to delineate a definitive therapeutic protocol.
1. Introduction
Small Fiber Neuropathy
Small fiber neuropathy (SFN) is caused by the impairment of unmyelinated C and thinly myelinated Aδ fibers. The symptoms are characterized by sensory symptoms, pain and autonomic symptoms, such as palpitations, gastrointestinal disturbances, and orthostatic dizziness. Neuropathic symptoms have a negative impact on the quality of life [1]. The symptoms and signs can be present as spontaneous (e.g., burning, deep, itching and paroxysmal) or evoked (e.g., thermal allodynia, light tough allodynia and hyperalgesia) pain.
Our systematic review defined the different rehabilitative and pharmacological approaches for SFN and to guide the physician to delineate a therapeutic protocol adopting the best strategies described in the current literature. In addition, we analyzed all the therapeutic approaches we found in the current literature to realize a guide to provide a common language to the multidisciplinary team such as physiatrists, neurologists, physiotherapists, nurses and neuropsychologists that must treat this disorder. The current literature did not describe a unique therapeutic approach, use arbitrarily different therapies. A therapeutic protocol should make more objective, reproducible, repeatable the outcomes and could help the multidisciplinary team to manage the patients.
2. Methods
2.1. Search Strategy
The search was carried out on the following medical electronic databases: PubMed, EMBASE, Cochrane Library and Scopus, Web of Science. The reference list of the related articles was also used to search for other suitable documents. The review was conducted from 22 May 2020 to 1 July 2020.
2.2. Selection Criteria and Data Extraction
Studies considered for this review must include the therapeutic methods in patients with SFN. We included English original articles about the rehabilitation and the pharmacological approaches for the SFN. We excluded animal studies, participants with other neuropathies. We also excluded all of the remaining duplicates.
Two reviewers (C.R. and V.M.) independently screened the titles and abstracts from the initial search to identify relevant records and to identify eligible studies based on title and abstract. Selected full texts were then reviewed and included in the systematic review, following the PRISMA protocol [2] and in accordance with the PICOS (population, intervention, comparison, outcome, and study design) criteria [3] shown in Table 1: Participants were all patients affected by SFN; intervention was based on rehabilitation therapy or pharmacological approaches; the comparator was any comparator; the outcomes included clinical assessments, diagnostic scales, electromyography and nerve conduction, biopsy; and study design was randomized clinical trials (RCTs), case series and case report, retrospective studies.

Table 1.
Treatment of SFN. Characteristics and outcomes of studies included in the systematic review.
3. Results
3.1. Description of the Studies
From 1984 to 2019, the database search of 975 articles with the following MeSH terms, words and combinations of words “small fiber neuropathy” AND “rehabilitation” OR “therapy” OR “treatment”, whose titles and abstracts were screened by the reviewers. The papers that remained for full-text screening were 78, and the eligibility of the study inclusion was assessed independently. Forty-two publications met the inclusion criteria and were included in the systematic review. Thirty-six were excluded for the following reasons: 18 involved individuals with different disorders from SFN, 6 examined different topics from our aim, 12 did not present any therapeutic procedure (Figure 1).
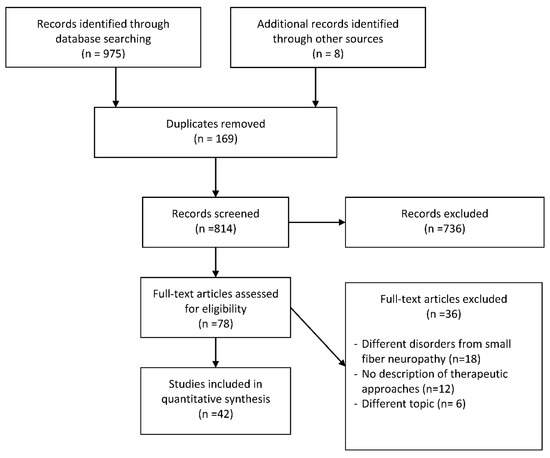
Figure 1.
Flowchart of the process of literature search and extraction of studies meeting the inclusion criteria.
The qualitative information synthesis for each parameter was attributed to the following evidence levels according to the recommendations of the Oxford Center for Evidence-Based Medicine: evidence from a systematic review of randomized controlled trials (1a), controlled clinical studies (2a), case–control-studies (3a) and from non-systematic reviews (4) (Table 1).
3.2. Variations of Experimental Conditions across the Studies
The selected 42 articles were described on the basis of the several therapeutic methods used in each study for the treatment of SFN. Characteristics of the studies are shown in Table 1.
All study groups were not homogeneous for relevant general clinical features as clinical presentation, duration of disease and of the symptoms, kinds of diagnostic measures, the severity of symptoms, rehabilitation and pharmacological therapy, time of starting therapy, duration of treatment, the follow-up period at the end of the therapy (Table 1).
3.3. Pharmacologic and Rehabilitation Therapy
Many different treatments were experienced. Opioid analgesics [8,21,24] or nonopioid analgesic [22,26], corticosteroids [8,10,33,34,37,44], intravenous immunoglobulin (IVIG) alone [8,12,15,23,27,31,37,39,46,47] or in combination with other specific drugs, such as azathioprine [29], anti-epileptic drugs [4,11,13,16,18,28,32], immunotherapy [14,19,37], hormone therapy [7,43]. Less used are the following therapeutic strategies, in used for specific disorders, such as ARA290, an erythropoietin derivate for sarcoidosis SFN [45], recombinant human nerve growth factor for diabetic SFN [5], propranolol for SFN related to aquagenic pruritus [9], plasma exchange therapy for complex regional pain syndrome [6], enzyme replacement therapy for Fabry related SFN [17,35], botulinum toxin type A for keloid [38].
Furthermore, two specific surgery strategies were described: the stellate ganglion blockade for SFN causing burning mouth syndrome [41] and the dorsal root ganglion stimulation for neuropathic pain of feet [25].
Motor exercises and a rehabilitative program could be part of the treatment strategy [18,20,21,30,36,40,42].
4. Discussion
Our systematic review focused on the several pharmacological and rehabilitative therapies used for SFN. We realized a comprehensive overview to give a guide to ease the collaboration of a multidisciplinary team.
4.1. Comparing Studies: Therapeutic Strategies
To choose the correct therapeutic approach, the first step is to confirm the diagnosis. Then it is essential to search for associated conditions because these could be treatable [48].
Several causes of SFN are potentially treatable [49], such as metabolic syndrome [50,51], and type 2 diabetes [52] associated with SFN. If the condition is not preventable, pharmacologic treatment and rehabilitation could improve the impairment and the quality of life. The treatment of symptoms is mandatory, and the possibility to add exercises and rehabilitation programs could permit to avoid disability and to maintain an adequate quality of life [53].
4.2. The Pharmacological Approaches
The management of neuropathic pain has been a challenging task for physicians [24]. There is limited evidence on the effectiveness of specific medications for the treatment of pain associated with SFN, and the most commonly used medications include antidepressants, anticonvulsants, mexiletine, topical agents, opiates and neuromodulation [54,55]. The guidelines for the pharmacologic management of neuropathic pain and diabetic painful polyneuropathies of the American Academy of Neurology (AAN) and the European Federation of Neurological Societies (EFNS) [56,57] could be adopted for the treatment of SFN. The opioid analgesics may contribute to a centrally-sensitized pain state, which may be refractory to other symptomatic approaches [58], with the activation of microglial cells [58] and of the central glutaminergic system [59]. About 45% of sarcoidosis-related SFN was treated with opioid analgesic therapy as the first approach [8]. In the case report of Mishra et al. [26], flupirtine, a nonopioid analgesic with muscle-relaxing properties, reduced neuropathic pain. Keohane et al. [22] proposed tafamidis, a non-NSAID highly specific transthyretin stabilizer, to delay the neurologic disease progression in the early-stage of transthyretin V30M familial amyloid polyneuropathy. A neuropathic pain related to SFN secondary to a keloid was treated successfully with botulinum toxin type A [38].
Immunotherapy with infliximab [19,37] or adalimumab [14] could play a crucial role in modifying the pathogenesis of SFN in immune-mediated inflammatory diseases [19].
The use of corticosteroids, immunosuppressive and anti-epileptic drugs showed discordant results. No improvements were reported in neuropathic symptoms and pain intensity after corticosteroid treatment in Sjogren, sarcoidosis and Guillain–Barré related SFN [8,37,44], or marked clinical improvement, according to other studies [10,33,34]. No clinical improvements were noted with methotrexate [37], but positive results with mycophenolate mofetil [33]. Nevoret et al. [29] added azathioprine to IVIG therapy, with consequent improvement in neuropathic symptomatology.
The benefits of intravenous immunoglobulin (IVIG) were reported for neuropathic pain in Sjogren [8,27]. In 8 studies, the efficacy and safety of IVIg are evaluated in patients with different features of SFN [12,15,23,27,31,37,39,46,47]. In contrast, IVIG had disappointing results, according to Pereira et al. [33] and Yuki et al. [44].
Gonzalez-Duarte et al. [16] showed improvements in prediabetic neuropathic pain after pregabalin treatment. De Greef et al. [11,13], Namer et al. [28] and Brouwer et al. [60] assessed the efficacy, safety, and tolerability of lacosamide, an anticonvulsant, in patients with SCN-associated small fiber neuropathy. Carbamazepine is useful to reduce SFN-related neuropathy [32] too. Gabapentin and naproxen [4] or duloxetine [18] were used for SFN associated with hantavirus infection [4] or in the absence of results with other therapies [4].
Enzyme replacement therapy (ERT) with recombinant human α-galactosidase significantly improved the function of C-, AΔ-, and Aß- nerve fibers and intradermal vibration receptors in Fabry neuropathy [17]. But according to Schiffmann et al. [35] epidermal nerve fiber regeneration did not occur after ERT. Van Velzen et al. [45] described the long-lasting beneficial effects of ARA290, an erythropoietin derivate, in symptoms of sarcoidosis-related SFN in patients. According to Apfel et al. [5], recombinant human nerve growth factors had significant beneficial effects on diabetic polyneuropathy. In the case report of Cao et al. [9], SFN related to aquagenic pruritus was treated with propranolol with significative benefit after a month of therapy. Plasma exchange therapy is effective in patients with severe long-standing complex regional pain syndrome [6].
4.3. Surgical Approaches
Stellate ganglion blockade [41] for recalcitrant pain in burning mouth syndrome and dorsal root ganglion stimulation [25] induced paraesthesia covering the entire pain area could be an effective therapy in SFN.
4.4. Rehabilitative Program
Another important field of therapy in SFN is the rehabilitation that could be added at a pharmacologic treatment [21,36] or used in the absence of pharmacologic results [18] and could be the first step of a therapy protocol (Table 2).

Table 2.
Rehabilitation programs.
4.5. Implication in Rehabilitation
Early recognition of SFN is important to start an appropriate and prompt treatment.
The aim of therapy is to relieve the neuropathic symptoms. The reduction of pain and the improvement in quality of life and in the ability to participate in activities is the purpose of rehabilitation approaches and could be the best complementary treatment to pharmacologic strategies. Specific exercises with proprioceptive and superficial sensibility stimulation could enhance recovery. Exercise may positively influence the pathological factors associated with neuropathy by promoting microvascular dilation, reducing oxidative stress, and increasing neurotrophic factors [61,62].
5. Limitations
A lack of uniformity among the papers (measured parameters and assessment scale) may affect the outcomes of considered articles. The absence of information about some clinical characteristics that could influence the symptomatology represents another limitation, such as comorbidities, the use of other specific drugs, psychologic traits. Furthermore, in some articles, the sample was very small. Several studies did not assess participants’ educational status; it could be a confounding factor and could influence the results.
6. Conclusions
The treatment of SFN is indispensable for the improvement of quality of life of individuals with neuropathic symptoms. SFN has a negative psychosocial impact on the lives of the patients and of their families.
We showed all the therapeutic approaches described in the current literature for SFN. On the basis of the different treatments, the physicians could obtain a guide and a common protocol for a multidisciplinary team. Despite the range of therapies for SFN, robust trials miss and always different therapeutic approaches are used. A comprehensive overview could give a guide to the physicians, and a complete protocol could ease the therapeutic and diagnostic approach to small fiber neuropathies. More research is needed to build evidence for the best therapy and to delineate a definitive therapeutic protocol.
Author Contributions
M.V., R.C., M.R., P.P., G.M., and G.L.M. conceived of the presented idea and verified the methods. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bakkers, M.; Faber, C.G.; Hoeijmakes, J.G.J.; Lauria, G.; Merkies, I.S.J. Small Fibers, Large Impact: Quality of Life in Small-Fiber Neuropathy. Muscle Nerve 2014, 49, 329–336. [Google Scholar] [CrossRef]
- Shamseer, L.; Moher, D.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: Elaboration and explanation. BMJ 2015, 350, 7647. [Google Scholar] [CrossRef]
- Higgins, J.P.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. Br. Med. J. 2011, 343, 889–893. [Google Scholar] [CrossRef]
- Anderson, D.; Beecher, G.; Power, C.; Bridgland, L.; Zochodne, D.W. A Neuropathic Pain Syndrome Associated With Hantavirus Infection. J. Neurovirol. 2017, 23, 919–921. [Google Scholar] [CrossRef]
- Apfel, S.C.; Schwartz, S.; Adornato, B.T.; Freeman, R.; Biton, V.; Rendell, M.; Vinik, A.; Giuliani, M.; Stevens, J.C.; Barbano, R.; et al. Efficacy and Safety of Recombinant Human Nerve Growth Factor in Patients With Diabetic Polyneuropathy: A Randomized Controlled Trial. rhNGF Clinical Investigator Group. JAMA 2000, 284, 2215–2221. [Google Scholar] [CrossRef]
- Aradillas, E.; Schwartzman, R.J.; Grothusen, J.R.; Goebel, A.; Alexandder, G.M. Plasma Exchange Therapy in Patients with Complex Regional Pain Syndrome. Pain Physician 2015, 18, 383–394. [Google Scholar]
- Azmi, S.; Ferdousi, M.; Petropoulos, I.N.; Ponirakis, G.; Fadavi, H.; Tavakoli, M.; Alam, U.; Jones, W.; Marshall, A.; Jeziorska, M.; et al. Corneal Confocal Microscopy Shows an Improvement in Small-Fiber Neuropathy in Subjects With Type 1 Diabetes on Continuous Subcutaneous Insulin Infusion Compared With Multiple Daily Injection. Diabetes Care 2015, 38, e3–e4. [Google Scholar] [CrossRef]
- Birnbaum, J.; Lalji, A.; Saed, A.; Baer, A.N. Biopsy-Proven Small-Fiber Neuropathy in Primary Sjögren’s Syndrome: Neuropathic Pain Characteristics, Autoantibody Findings, and Histopathologic Features. Arthritis Care Res. 2019, 71, 936–948. [Google Scholar] [CrossRef]
- Cao, T.; Yong, A.A.; Tan, K.B.; Tey, H.L. Idiopathic Aquagenic Pruritus: Pathogenesis and Effective Treatment with Atenolol. Dermatol. Ther. 2015, 28, 118–121. [Google Scholar] [CrossRef]
- Dabby, R.; Gilad, R.; Sadeh, M.; Lampl, Y.; Waterìmberg, N. Acute steroid responsive small-fiber sensory neuropathy: A new entity. J. Peripher. Nerv. Syst. 2006, 11, 47–52. [Google Scholar] [CrossRef]
- de Greef, B.; Merkies, I.S.J.; Geerts, M.; Faber, C.G.; Hoeijmakers, J.G.J. Efficacy, safety, and tolerability of lacosamide in patients with gain-of-function Nav1.7 mutation-related small fiber neuropathy: Study protocol of a randomized controlled trial-the LENSS study. Trials 2016, 17. [Google Scholar] [CrossRef]
- de Greef, B.T.; Geerts, M.; Hoeijmakers, J.G.; Faber, C.G.; Merkies, S.J. Intravenous immunoglobulin therapy for small fiber neuropathy: Study protocol for a randomized controlled trial. Trials 2016, 17, 330. [Google Scholar] [CrossRef]
- de Greef, B.T.; Hoeijmakers, J.G.; Geerts, M.; Oakes, M.; Church, T.J.; Waxman, S.G.; Dib-Hajj, S.D.; Faber, C.G.; Merkies, I.S. Lacosamide in patients with Nav1.7 mutations-related small fibre neuropathy: A randomized controlled trial. Brain 2019, 142, 263–275. [Google Scholar] [CrossRef]
- Favon, V.; Liguoi, R.; Incensi, A.; Fileccia, E.; Donadio, V. The Incidental Finding of Elevated Anti GQ1B Antibodies in a Patient With Selective Small Fiber Neuropathy. J. Neurol. Sci. 2018, 388, 192–194. [Google Scholar] [CrossRef] [PubMed]
- Gaillet, A.; Champion, K.; Lefaucheur, J.P.; Trout, H.; Bergmann, J.F.; Sene, D. Intravenous Immunoglobulin Efficacy for Primary Sjögren’s Syndrome Associated Small Fiber Neuropathy. Autoimmun. Rev. 2019, 18, 102387. [Google Scholar] [CrossRef]
- González-Duarte, A.; Lem, M.; Díaz-Díaz, E.; Castillo, C.; Cárdenas-Soto, K. The Efficacy of Pregabalin in the Treatment of Prediabetic Neuropathic Pain. Clin. J. Pain 2016, 32, 927–932. [Google Scholar] [CrossRef]
- Hilz, M.J.; Brys, M.; Marthol, H.; Stemper, B.; Dutsc, M. Enzyme Replacement Therapy for Fabry Disease. Neurology 2004, 62, 1066–1072. [Google Scholar] [CrossRef]
- Hoeijmakers, J.G.J.; Faber, C.G.; Miedema, C.J.; Merkies, I.S.J.; Vles, J.S.H. Small Fiber Neuropathy in Children: Two Case Reports Illustrating the Importance of Recognition. Pediatrics 2016, 138, e20161215. [Google Scholar] [CrossRef]
- Hoitsma, E.; Faber, C.G.; van Santen-Hoeufft, M.; De Vries, J.; Reulen, J.P.H.; Drent, M. Improvement of small fiber neuropathy in a sarcoidosis patient after treatment. Sarcoidosis Vasc. Diffuse Lung Dis. 2006, 23, 73–77. [Google Scholar]
- Hong, J.; Barnes, M.; Kessler, N. Case study: Use of vibration therapy in the treatment of diabetic peripheral small fiber neuropathy. J. Bodyw. Mov. Ther. 2013, 17, 235–238. [Google Scholar] [CrossRef]
- Kluding, P.M.; Pasnoor, M.; Singh, R.; Jernigan, S.; Farmer, K.; Rucker, J.; Sharma, N.K.; Wright, D.E. The Effect of Exercise on Neuropathic Symptoms, Nerve Function, and Cutaneous Innervation in People With Diabetic Peripheral Neuropathy. J. Diabetes Complicat. 2012, 26, 424–429. [Google Scholar] [CrossRef]
- Keohane, D.; Scwartz, J.; Gundapaneni, B.; Stewart, M.; Amass, L. Tafamidis delays disease progression in patients with early stage transthyretin familial amyloid polyneuropathy: Additional supportive analyses from the pivotal trial. Amyloid 2017, 24, 30–36. [Google Scholar] [CrossRef]
- Liu, X.; Treister, R.; Lang, M.; Oaklander, A.L. IVIg for Apparently Autoimmune Small-Fiber Polyneuropathy: First Analysis of Efficacy and Safety. Ther. Adv. Neurol. Disord. 2018, 11, 1756285617744484. [Google Scholar] [CrossRef]
- MacDonald, S.; Sharma, T.L.; Li, J.; Polston, D.; Li, Y. Longitudinal Follow-Up of Biopsy-Proven Small Fiber Neuropathy. Muscle Nerve 2019, 60, 376–381. [Google Scholar] [CrossRef]
- Maino, P.; Koetsier, E.; Kaelin-Lamg, A.; Gobbi, C.; Perez, R. Efficacious Dorsal Root Ganglion Stimulation for Painful Small Fiber Neuropathy: A Case Report. Pain Physician 2017, 20, E459–E463. [Google Scholar]
- Mishra, S.; Choudhary, P.; Joshi, S.; Bhatnagar, S. Successful Use of Flupirtine in Refractory Neuropathic Pain Due to Small Fiber Neuropathy. Am. J. Hosp. Palliat. Care 2013, 30, 91–93. [Google Scholar] [CrossRef]
- Morozumi, S.; Kawagashira, Y.; Lijima, M.; Koike, H.; Hattori, N.; Katsuno, M.; Tanaka, F.; Sobue, G. Intravenous Immunoglobulin Treatment for Painful Sensory Neuropathy Associated With Sjögren’s Syndrome. J. Neurol. Sci. 2009, 279, 57–61. [Google Scholar] [CrossRef]
- Namer, B.; Schmidt, D.; Eberhardt, E.; Maroni, M.; Dorfmeister, E.; Kleggetveit, I.P.; Kaluza, L.; Meents, J.; Gerlach, A.; Lin, Z.; et al. Pain Relief in a Neuropathy Patient by Lacosamide: Proof of Principle of Clinical Translation From Patient-Specific iPS Cell-Derived Nociceptors. EBioMedicine 2019, 39, 401–408. [Google Scholar] [CrossRef]
- Nevoret, M.; Vinik, A.I. CIDP Variants in Diabetes: Measuring Treatment Response with a Small Nerve Fiber Test. J. Diabetes Complicat. 2015, 29, 313–317. [Google Scholar] [CrossRef]
- Otis, J.D.; Sanderson, K.; Hardway, C.; Pincus, M.; Soumekh, S. A Randomized Controlled Pilot Study of a Cognitive-Behavioral therapy approach for painful diabetic peripheral neuropathy. J. Pain 2013, 14, 475–482. [Google Scholar] [CrossRef]
- Parambil, J.G.; Tavee, J.O.; Zhou, L.; Pearson, K.S.; Culver, D.A. Efficacy of intravenous immunoglobulin for small fiber neuropathy associated with sarcoidosis. Respir. Med. 2011, 105, 101–105. [Google Scholar] [CrossRef]
- Patel, P.; Zhang, Y.; Unikel, L.H.; Edwards, C. A case of sporadic erythromelalgia presenting with small fibre neuropathy. BMJ Case Rep. 2019, 12, e230549. [Google Scholar] [CrossRef]
- Pereira, P.R.; Viala, K.; Maisonobe, T.; Haroche, J.; Mathian, A.; Hiè, M.; Amoura, Z.; Aubart, F.C. Sjögren Sensory Neuronopathy (Sjögren Ganglionopathy): Long-Term Outcome and Treatment Response in a Series of 13 Cases. Medicine 2016, 95, e3632. [Google Scholar] [CrossRef]
- Saito, H.; Yamaguchi, T.; Adachi, Y.; Yamashita, T.; Wakai, Y.; Saito, K.; Shinora, Y.; Suzuki, K.; Yagihasshi, S.; Terada, J.; et al. Neurological Symptoms of Sarcoidosis-induced Small Fiber Neuropathy Effectively Relieved With High-dose Steroid Pulse Therapy. Intern. Med. 2015, 54, 1281–1286. [Google Scholar] [CrossRef]
- Schiffann, R.; Hauer, P.; Freeman, B.; Ries, M.; Scott, L.J.; Polydefkis, M.; Brady, R.O.; McArthur, J.C.; Wagner, K. Enzyme Replacement Therapy and Intraepidermal Innervation Density in Fabry Disease. Muscle Nerve 2006, 34, 53–56. [Google Scholar] [CrossRef]
- Smith, G.; Russell, J.; Feldman, E.L.; Goldstein, J.; Peltier, A.; Smith, S.; Hamwi, J.; Pollari, D.; Bixby, B.; Howard, J.; et al. Lifestyle Intervention for Pre-Diabetic Neuropathy. Diabetes Care 2006, 29, 1294–1299. [Google Scholar] [CrossRef]
- Tavee, J.O.; Karwa, K.; AAhmed, Z.; Thompson, N.; Parambil, J.; Culver, D.A. Sarcoidosis-associated Small Fiber Neuropathy in a Large Cohort: Clinical Aspects and Response to IVIG and anti-TNF Alpha Treatment. Respir. Med. 2017, 126, 135–138. [Google Scholar] [CrossRef]
- Uyesugi, B.; Lippincott, B.; Dave, S. Treatment of a Painful Keloid With Botulinum Toxin Type, A. Am. J. Phys. Med. Rehabil. 2010, 89, 153–155. [Google Scholar] [CrossRef]
- Wakasugi, D.; Kato, T.; Gono, T.; Ito, E.; Nodera, H.; Kawaguchi, Y.; Yamanaka, H.; Hara, M. Extreme Efficacy of Intravenous Immunoglobulin Therapy for Severe Burning Pain in a Patient With Small Fiber Neuropathy Associated With Primary Sjögren’s Syndrome. Mod. Rheumatol. 2009, 19, 437–440. [Google Scholar] [CrossRef] [PubMed]
- Waldinger, M.D.; Venema, P.L.; van Gils, A.P.; de Lint, G.J.; Schweitzer, D.H. Stronger evidence for small fiber sensory neuropathy in restless genital syndrome: Two case reports in males. J. Sex. Med. 2011, 8, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Walega, D.R.; Smith, C.; Epstein, J.B. Oral Pain From Burning Mouth Syndrome: A Case Report. J. Oral Facial Pain Headache Spring 2014, 28, 171–175. [Google Scholar] [CrossRef]
- Weintraub, M.I.; Herrmann, D.N.; Smith, A.G.; Backonja, M.M.; Cole, S.P. Pulsed Electromagnetic Fields to Reduce Diabetic Neuropathic Pain and Stimulate Neuronal Repair: A Randomized Controlled Trial. Arch. Phys. Med. Rehabil. 2009, 90, 1102–1109. [Google Scholar] [CrossRef]
- Windebank, A.J.; Sorenson, E.J.; Civil, R.; O’Brien, P.C. Role of Insulin-Like Growth factor-I in the Treatment of Painful Small Fiber Predominant Neuropathy. J. Peripher. Nerv. Syst. 2004, 9, 183–189. [Google Scholar] [CrossRef]
- Yuki, N.; Chan, A.C.; Wong, A.H.Y.; Inoue, T.; Yokai, M.; Kurihara, T.; Devaux, J.J.; Wider-Smith, E. Acute Painful Autoimmune Neuropathy: A Variant of Guillain-Barré Syndrome. Muscle Nerve 2018, 57, 320–324. [Google Scholar] [CrossRef]
- van Velzen, M.; Heij, L.; Niesters, M.; Cerami, A.; Dunne, A.; Dahan, A.; Brines, M. ARA 290 for Treatment of Small Fiber Neuropathy in Sarcoidosis. Expert Opin. Investig. Drugs 2014, 23, 541–550. [Google Scholar] [CrossRef]
- Oaklander, A.L. Immunotherapy Prospects for Painful Small-fiber Sensory Neuropathies and Ganglionopathies. Neurotherapeutics 2016, 13, 108–117. [Google Scholar] [CrossRef]
- Kizawa, M.; Mori, K.; Iijima, M.; Koike, H.; Hattori, N.; Sobue, G. Intravenous immunoglobulin treatment in painful sensory neuropathy without sensory ataxia associated with Sjögren’s syndrome. J. Neurol. Neurosurg. Psychiatry 2006, 77, 967–969. [Google Scholar] [CrossRef]
- Chiaramonte, R.; Romano, M.; Vecchio, M. A Systematic Review of the Diagnostic Methods of Small Fiber Neuropathies in Rehabilitation. Diagnostics 2020, 10, 613. [Google Scholar] [CrossRef]
- Themistocleous, A.C.; Ramirez, J.D.; Serra, J.; Bennett, D.L.H. The Clinical Approach to Small Fibre Neuropathy and Painful Channelopathy. Pract. Neurol. 2014, 14, 368–379. [Google Scholar] [CrossRef]
- Zhou, L.; Li, J.; Ontaneda, D.; Sperling, J. Metabolic Syndrome in Small Fiber Sensory Neuropathy. J. Clin. Neuromuscul. Dis. 2011, 12, 235–243. [Google Scholar] [CrossRef]
- Lagerburg, V.; Bakkers, M.; Bouwhuis, A.; Hoeijmakers, J.G.J.; Smit, M.; Van Den Berg, S.J.M.; Hordijk-De Boer, I.; Brouwer-Van Der Lee, M.D.G.; Kranendonk, D.; Reulen, J.P.H.; et al. Contact Heat Evoked Potentials: Normal Values and Use in Small-Fiber Neuropathy. Muscle Nerve 2015, 51, 743–749. [Google Scholar] [CrossRef]
- Callaghan, B.C.; Little, A.A.; Feldman, E.L.; Hughes, R.A.C. Enhanced Glucose Control for Preventing and Treating Diabetic Neuropathy. Cochrane Database Syst. Rev. 2012, 6, CD007543. [Google Scholar] [CrossRef]
- Castelnuovo, G.; Giusti, E.M.; Manzoni, G.M.; Saviola, D.; Gatti, A.; Gabrielli, S.; Lacerenza, M.; Pietrabissa, G.; Cattivelli, R.; Spatola, C.A.; et al. Psychological Treatments and Psychotherapies in the Neurorehabilitation of Pain: Evidences and Recommendations from the Italian Consensus Conference on Pain in Neurorehabilitation. Front. Psychol. 2016, 7, 115. [Google Scholar] [CrossRef]
- Chan, A.C.Y.; Wilder-Smith, E.P.W. Small Fiber Neuropathy: Getting Bigger! Muscle Nerve 2016, 53, 671–682. [Google Scholar] [CrossRef]
- Tavee, J.O. Office Approach to Small Fiber Neuropathy. Cleve Clin. J. Med. 2018, 85, 801–812. [Google Scholar] [CrossRef]
- Attal, N.; Cruccu, G.; Baron, R.; Haanpaa, M.; Hanssom, P.; Jensen, T.S.; Nurmikko, T. EFNS Guidelines on the Pharmacological Treatment of Neuropathic Pain: 2010 Revision. Eur. J. Neurol. 2010, 17, 1113-e88. [Google Scholar] [CrossRef]
- Bril, V.; England, J.; Franklin, G.M.; Backonja, M.; Cohen, J.; Del Toro, D.; Feldman, R. Evidence-based Guideline: Treatment of Painful Diabetic Neuropathy: Report of the American Academy of Neurology, the American Association of Neuromuscular and Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. Neurology 2011, 76, 1758–1765. [Google Scholar] [CrossRef]
- Miller, R.J.; Jung, H.; Bhangoo, S.K.; White, F.A. Cytokine and Chemokine Regulation of Sensory Neuron Function. Handb. Exp. Pharmacol. 2009, 417–449. [Google Scholar] [CrossRef]
- Mao, J.; Price, D.D.; Mayer, D.J. Mechanisms of Hyperalgesia and Morphine Tolerance: A Current View of Their Possible Interactions. Pain 1995, 62, 259–274. [Google Scholar] [CrossRef]
- Brouwer, B.; Merkies, I.J.; Gerrits, M.M.; Waxman, S.G.; Hoejmakers, J.G.J.; Faber, C.G. Painful Neuropathies: The Emerging Role of Sodium Channelopathies. J. Peripher. Nerv. Syst. 2014, 19, 53–65. [Google Scholar] [CrossRef]
- Kadoglou, N.P.E.; Iliadis, F.; Angelopoulou, N.; Perrea, D.; Ampatzidis, G.; Liapis, C.D.; Alevizos, M. The Anti-Inflammatory Effects of Exercise Training in Patients with Type 2 Diabetes Mellitus. Eur. J. Cardiovasc. Prev. Rehabil. 2007, 14, 837–843. [Google Scholar] [CrossRef]
- Maiorana, A.; O’Driscoll, G.; Cheetham, C.; Dembo, L.; Stanton, K.; Goodman, C.; Taylor, R.; Green, D. The Effect of Combined Aerobic and Resistance Exercise Training on Vascular Function in Type 2 Diabetes. J. Am. Coll. Cardiol. 2001, 38, 860–866. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).