Enhanced Microvascular Adaptation to Acute Physical Stress and Reduced Oxidative Stress in Male Athletes Who Consumed Chicken Eggs Enriched with n-3 Polyunsaturated Fatty Acids and Antioxidants—Randomized Clinical Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Production of n-3 PUFA-, Selenium-, Lutein- and Vitamin E-Enriched Chicken Eggs
2.3. Study Design
2.4. Basic Anthropometric, Cardiovascular, and Biochemical Measurements
2.5. Analysis of Fatty Acid Profile, Selenium, Vitamin E, and Lutein Levels in Serum
2.6. Evaluation of Peripheral Microvascular Reactivity
2.7. Protocol for Acute Strenuous Training Sessions
2.8. Measurement of Biomarkers of Oxidative Stress and Antioxidant Protection
2.9. Statistical Analysis
3. Results
3.1. Anthropometric, Cardiovascular, and Biochemical Parameters
3.2. Serum Fatty Acid Profile, Vitamin E, and Selenium Level Analysis
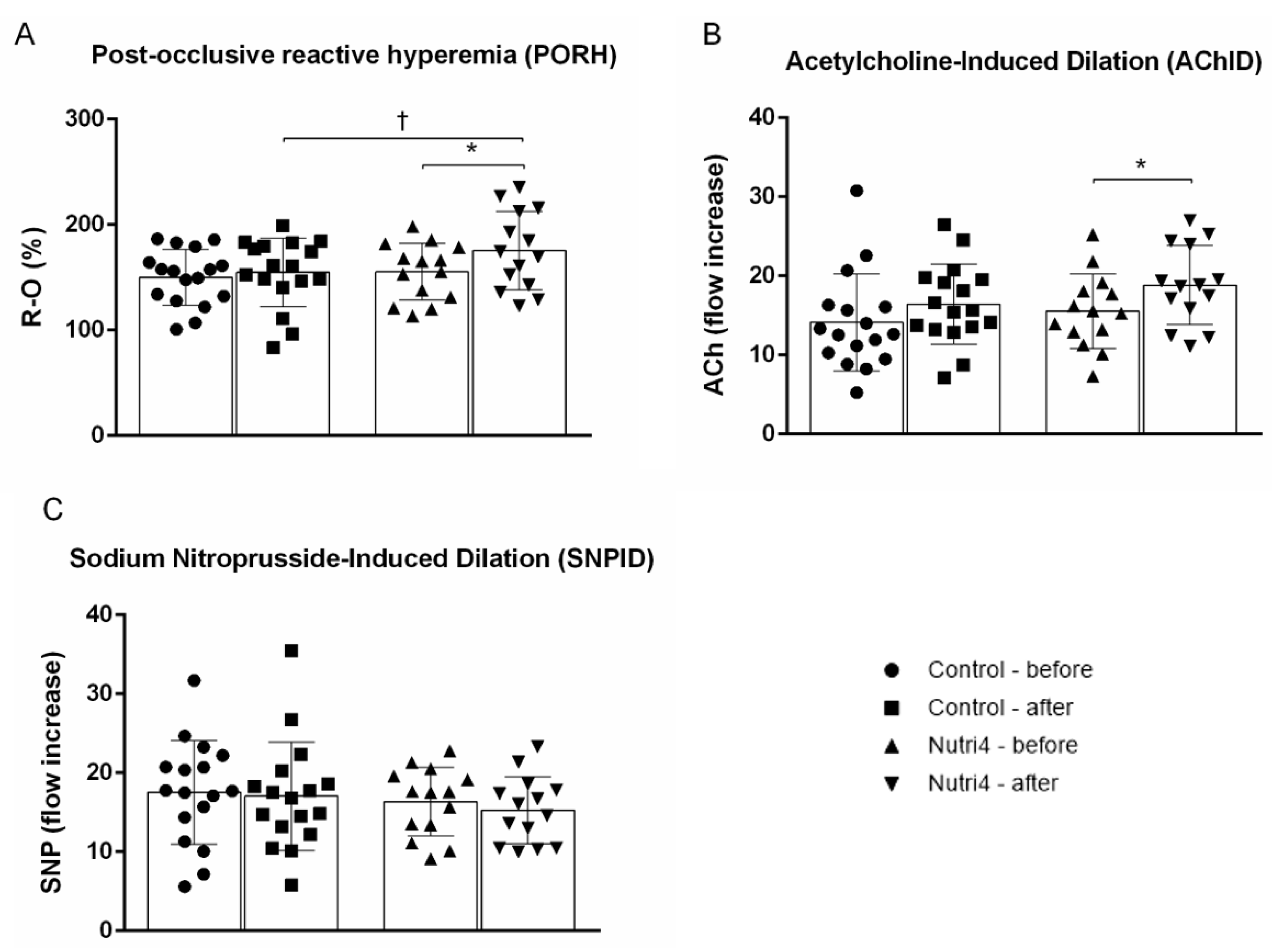
3.3. Endothelium-Dependent and Endothelium-Independent Vasodilation in Forearm Skin Microcirculation
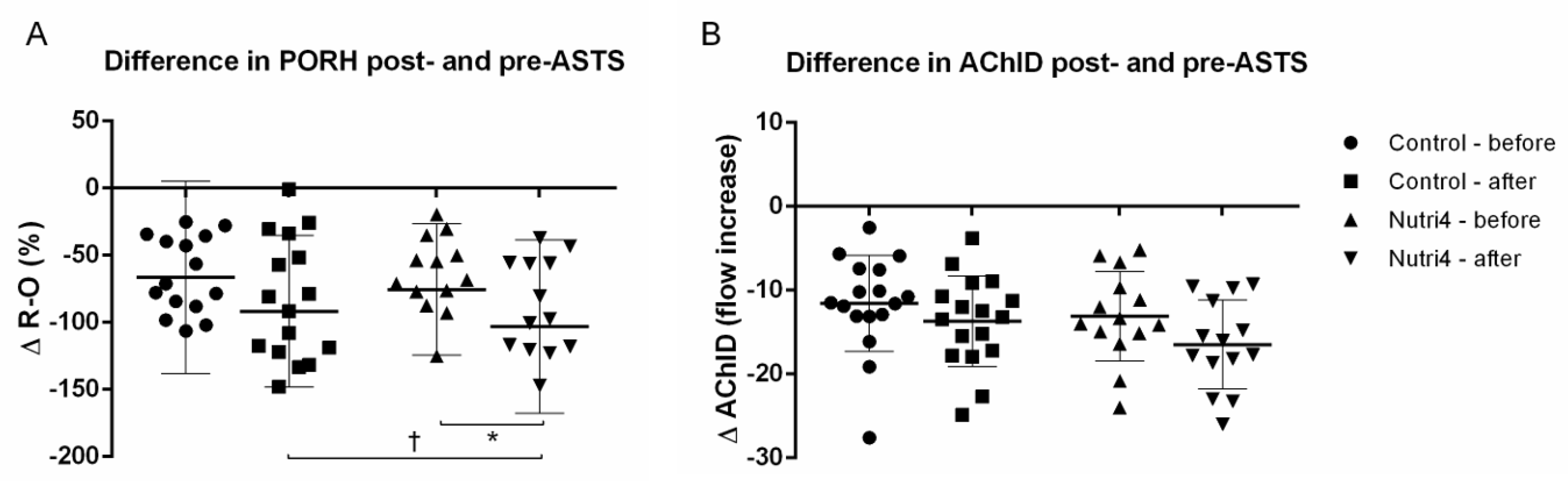
3.4. Acute Strenuous Training Session and Microvascular Responsiveness Range
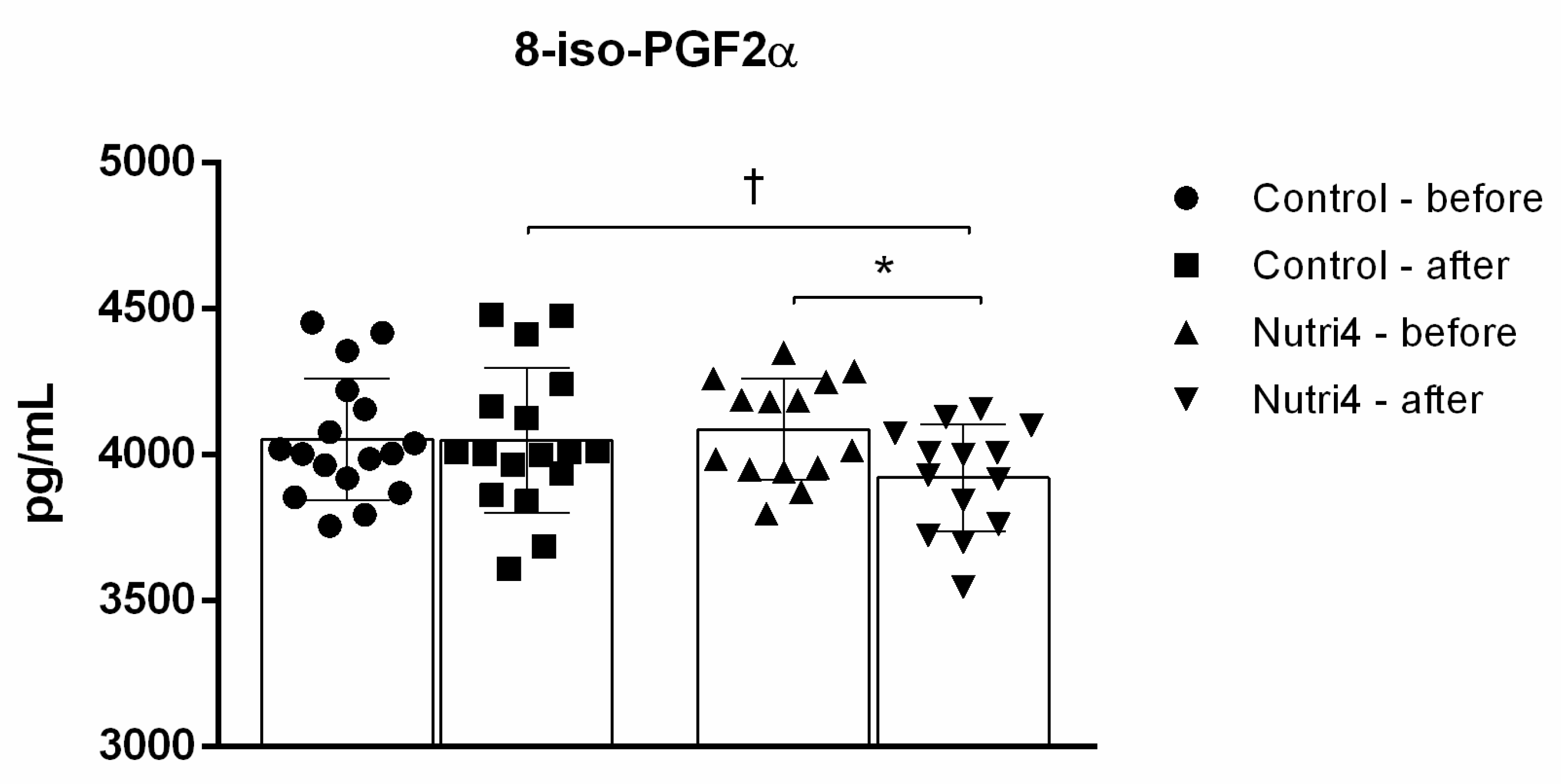
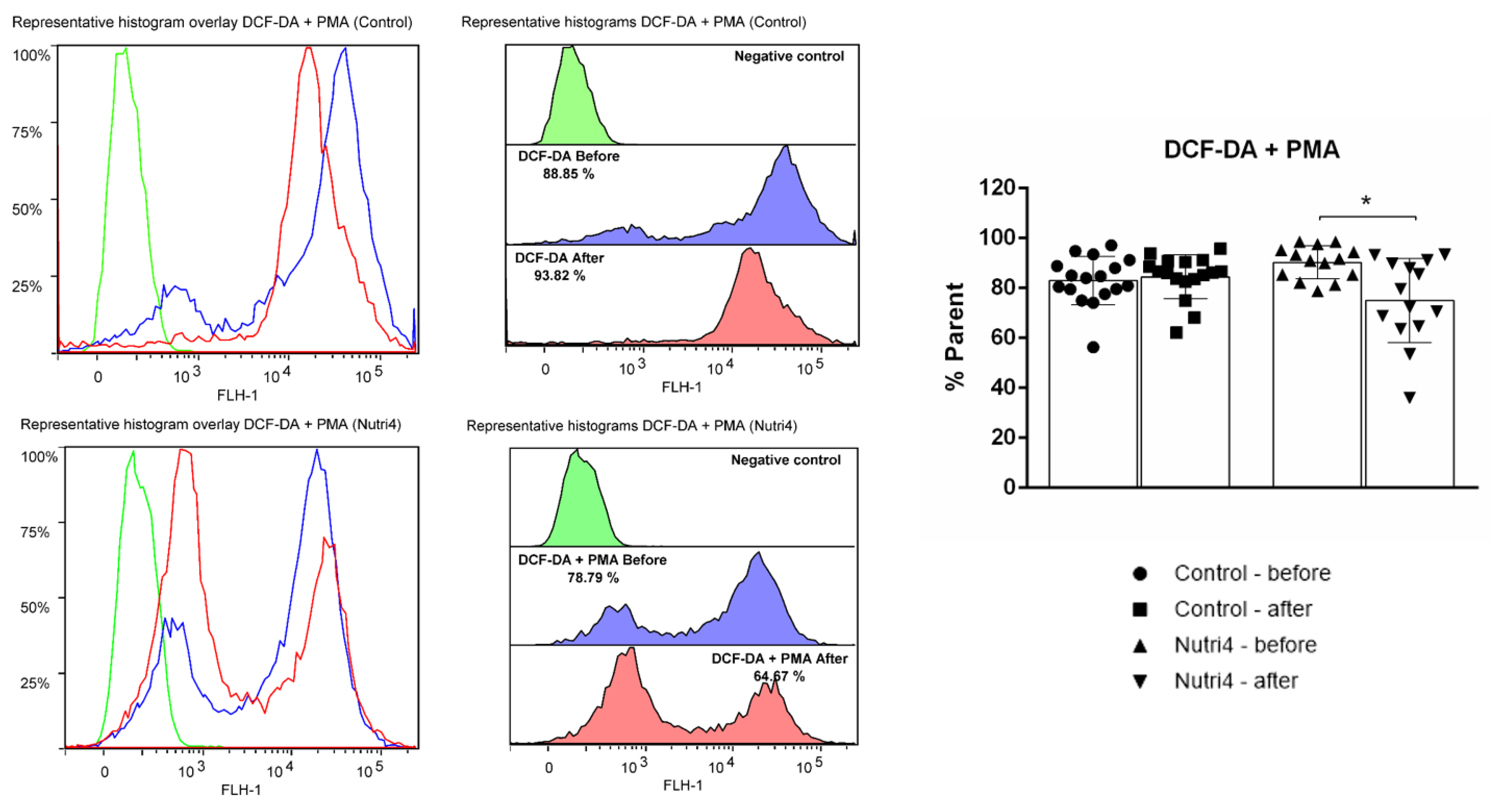
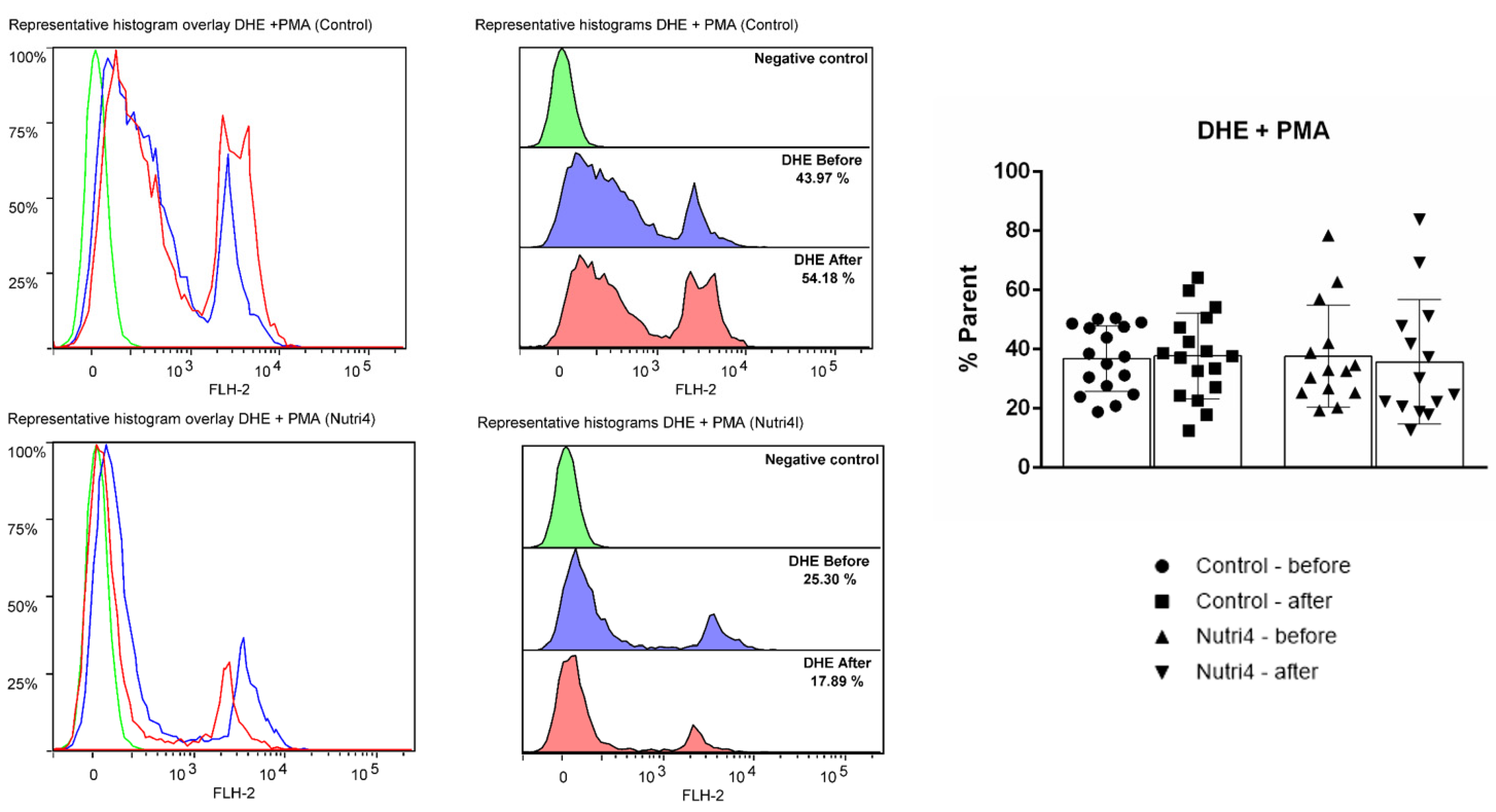
3.5. Biomarkers of Oxidative Stress and Antioxidant Defense
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Spriet, L.L. Performance Nutrition for Athletes. Sport. Med. 2019, 49, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Baranauskas, M.; Stukas, R.; Tubelis, L.; Žagminas, K.; Šurkienė, G.; Švedas, E.; Giedraitis, V.R.; Dobrovolskij, V.; Abaravičius, J.A. Nutritional habits among high-performance endurance athletes. Medicina (B. Aires). 2015, 51, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Ritz, P.P.; Rogers, M.B.; Zabinsky, J.S.; Hedrick, V.E.; Rockwell, J.A.; Rimer, E.G.; Kostelnik, S.B.; Hulver, M.W.; Rockwell, M.S. Dietary and Biological Assessment of the Omega-3 Status of Collegiate Athletes: A Cross-Sectional Analysis. PLoS ONE 2020, 15, e0228834. [Google Scholar] [CrossRef] [PubMed]
- Higgins, M.; Izadi, A.; Kaviani, M. Antioxidants and Exercise Performance: With a Focus on Vitamin E and C Supplementation. Int. J. Environ. Res. Public Health 2020, 17, 8452. [Google Scholar] [CrossRef] [PubMed]
- Stupin, A.; Mihalj, M.; Kolobarić, N.; Šušnjara, P.; Kolar, L.; Mihaljević, Z.; Matić, A.; Stupin, M.; Jukić, I.; Kralik, Z.; et al. Anti-Inflammatory Potential of n-3 Polyunsaturated Fatty Acids Enriched Hen Eggs Consumption in Improving Microvascular Endothelial Function of Healthy Individuals—Clinical Trial. Int. J. Mol. Sci. 2020, 21, 4149. [Google Scholar] [CrossRef] [PubMed]
- Devrim-Lanpir, A.; Bilgic, P.; Kocahan, T.; Deliceoğlu, G.; Rosemann, T.; Knechtle, B. Total Dietary Antioxidant Intake Including Polyphenol Content: Is It Capable to Fight against Increased Oxidants within the Body of Ultra-Endurance Athletes? Nutrients 2020, 12, 1877. [Google Scholar] [CrossRef]
- Busquets-Cortés, C.; Capó, X.; Martorell, M.; Tur, J.A.; Sureda, A.; Pons, A. Training Enhances Immune Cells Mitochondrial Biosynthesis, Fission, Fusion, and Their Antioxidant Capabilities Synergistically with Dietary Docosahexaenoic Supplementation. Oxid. Med. Cell. Longev. 2016, 2016, 1–10. [Google Scholar] [CrossRef]
- Rawson, E.S.; Miles, M.P.; Larson-Meyer, D.E. Dietary Supplements for Health, Adaptation, and Recovery in Athletes. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 188–199. [Google Scholar] [CrossRef]
- Oliver, J.M. Omega-3 Fatty Acids and Student-Athletes: Is It Time for Better Education and a Policy Change? J. Athl. Train. 2019, 54, 5–6. [Google Scholar] [CrossRef]
- Asgary, S.; Rastqar, A.; Keshvari, M. Functional Food and Cardiovascular Disease Prevention and Treatment: A Review. J. Am. Coll. Nutr. 2018, 37, 429–455. [Google Scholar] [CrossRef]
- Kralik, Z.; Kralik, G.; Košević, M.; Galović, O.; Samardžić, M. Natural Multi-Enriched Eggs with n-3 Polyunsaturated Fatty Acids, Selenium, Vitamin E, and Lutein. Animals 2023, 13, 321. [Google Scholar] [CrossRef]
- Oppedisano, F.; Macrì, R.; Gliozzi, M.; Musolino, V.; Carresi, C.; Maiuolo, J.; Bosco, F.; Nucera, S.; Caterina Zito, M.; Guarnieri, L.; et al. The Anti-Inflammatory and Antioxidant Properties of n-3 PUFAs: Their Role in Cardiovascular Protection. Biomedicines 2020, 8, 306. [Google Scholar] [CrossRef]
- Sakai, C.; Ishida, M.; Ohba, H.; Yamashita, H.; Uchida, H.; Yoshizumi, M.; Ishida, T. Fish oil omega-3 polyunsaturated fatty acids attenuate oxidative stress-induced DNA damage in vascular endothelial cells. PLoS ONE 2017, 12, e0187934. [Google Scholar] [CrossRef]
- Kyriakidou, Y.; Wood, C.; Ferrier, C.; Dolci, A.; Elliott, B. The effect of Omega-3 polyunsaturated fatty acid supplementation on exercise-induced muscle damage. J. Int. Soc. Sports Nutr. 2021, 18, 9. [Google Scholar] [CrossRef]
- Walser, B.; Giordano, R.M.; Stebbins, C.L. Supplementation with omega-3 polyunsaturated fatty acids augments brachial artery dilation and blood flow during forearm contraction. Eur. J. Appl. Physiol. 2006, 97, 347–354. [Google Scholar] [CrossRef]
- Kolar, L.; Stupin, M.; Stupin, A.; Šušnjara, P.; Mihaljević, Z.; Matić, A.; Jukić, I.; Kolobarić, N.; Drenjančević, I. Does the Endothelium of Competitive Athletes Benefit from Consumption of n-3 Polyunsaturated Fatty Acid-Enriched Hen Eggs? Prev. Nutr. Food Sci. 2021, 26, 388–399. [Google Scholar] [CrossRef]
- Da Boit, M.; Hunter, A.M.; Gray, S.R. Fit with good fat? The role of n-3 polyunsaturated fatty acids on exercise performance. Metabolism 2017, 66, 45–54. [Google Scholar] [CrossRef]
- Zehr, K.R.; Walker, M.K. Omega-3 polyunsaturated fatty acids improve endothelial function in humans at risk for atherosclerosis: A review. Prostaglandins Other Lipid Mediat. 2018, 134, 131–140. [Google Scholar] [CrossRef]
- Ziegler, M.; Wallert, M.; Lorkowski, S.; Peter, K. Cardiovascular and Metabolic Protection by Vitamin E: A Matter of Treatment Strategy? Antioxidants 2020, 9, 935. [Google Scholar] [CrossRef]
- Stupin, A.; Rasic, L.; Matic, A.; Stupin, M.; Kralik, Z.; Kralik, G.; Grcevic, M.; Drenjancevic, I. Omega-3 polyunsaturated fatty acids-enriched hen eggs consumption enhances microvascular reactivity in young healthy individuals. Appl. Physiol. Nutr. Metab. 2018, 43, 988–995. [Google Scholar] [CrossRef]
- Takanami, Y.; Iwane, H.; Kawai, Y.; Shimomitsu, T. Vitamin E Supplementation and Endurance Exercise. Sport. Med. 2000, 29, 73–83. [Google Scholar] [CrossRef]
- Serov, D.A.; Khabatova, V.V.; Vodeneev, V.; Li, R.; Gudkov, S.V. A Review of the Antibacterial, Fungicidal and Antiviral Properties of Selenium Nanoparticles. Materials 2023, 16, 5363. [Google Scholar] [CrossRef]
- Barchielli, G.; Capperucci, A.; Tanini, D. The Role of Selenium in Pathologies: An Updated Review. Antioxidants 2022, 11, 251. [Google Scholar] [CrossRef]
- Fernández-Lázaro, D.; Fernandez-Lazaro, C.I.; Mielgo-Ayuso, J.; Navascués, L.J.; Córdova Martínez, A.; Seco-Calvo, J. The Role of Selenium Mineral Trace Element in Exercise: Antioxidant Defense System, Muscle Performance, Hormone Response, and Athletic Performance. A Systematic Review. Nutrients 2020, 12, 1790. [Google Scholar] [CrossRef]
- Šušnjara, P.; Kolobarić, N.; Matić, A.; Mihaljević, Z.; Stupin, A.; Marczi, S.; Drenjančević, I. Consumption of Hen Eggs Enriched with n-3 Polyunsaturated Fatty Acids, Selenium, Vitamin E and Lutein Incites Anti-Inflammatory Conditions in Young, Healthy Participants—A Randomized Study. Front. Biosci. 2022, 27, 332. [Google Scholar] [CrossRef]
- Jargar, J.G.; Hattiwale, S.H.; Das, S.; Dhundasi, S.A.; Das, K.K. A modified simple method for determination of serum α-tocopherol (vitamin E). J. Basic Clin. Physiol. Pharmacol. 2012, 23, 45–48. [Google Scholar] [CrossRef]
- D’Ilio, S.; Violante, N.; Caimi, S.; Di Gregorio, M.; Petrucci, F.; Senofonte, O. Determination of trace elements in serum by dynamic reaction cell inductively coupled plasma mass spectrometry. Anal. Chim. Acta 2006, 573–574, 432–438. [Google Scholar] [CrossRef]
- Tzeng, M.-S.; Yang, F.-L.; Wang-Hsu, G.-S.; Chen, B.-H. Determination of major carotenoids in human serum by liquid chromatography. J. Food Drug Anal. 2020, 12, 13. [Google Scholar] [CrossRef]
- Stupin, M.; Stupin, A.; Rasic, L.; Cosic, A.; Kolar, L.; Seric, V.; Lenasi, H.; Izakovic, K.; Drenjancevic, I. Acute exhaustive rowing exercise reduces skin microvascular dilator function in young adult rowing athletes. Eur. J. Appl. Physiol. 2018, 118, 461–474. [Google Scholar] [CrossRef]
- Barić, L.; Drenjančević, I.; Mihalj, M.; Matić, A.; Stupin, M.; Kolar, L.; Mihaljević, Z.; Mrakovčić-Šutić, I.; Šerić, V.; Stupin, A. Enhanced Antioxidative Defense by Vitamins C and E Consumption Prevents 7-Day High-Salt Diet-Induced Microvascular Endothelial Function Impairment in Young Healthy Individuals. J. Clin. Med. 2020, 9, 843. [Google Scholar] [CrossRef]
- Hendrickse, P.; Degens, H. The role of the microcirculation in muscle function and plasticity. J. Muscle Res. Cell Motil. 2019, 40, 127–140. [Google Scholar] [CrossRef]
- Green, D.J.; Smith, K.J. Effects of Exercise on Vascular Function, Structure, and Health in Humans. Cold Spring Harb. Perspect. Med. 2018, 8, a029819. [Google Scholar] [CrossRef]
- Lavie, C.J.; Arena, R.; Swift, D.L.; Johannsen, N.M.; Sui, X.; Lee, D.; Earnest, C.P.; Church, T.S.; O’Keefe, J.H.; Milani, R.V.; et al. Exercise and the Cardiovascular System. Circ. Res. 2015, 117, 207–219. [Google Scholar] [CrossRef]
- Jurva, J.W.; Phillips, S.A.; Syed, A.Q.; Syed, A.Y.; Pitt, S.; Weaver, A.; Gutterman, D.D. The Effect of Exertional Hypertension Evoked by Weight Lifting on Vascular Endothelial Function. J. Am. Coll. Cardiol. 2006, 48, 588–589. [Google Scholar] [CrossRef]
- Phillips, S.A.; Das, E.; Wang, J.; Pritchard, K.; Gutterman, D.D. Resistance and aerobic exercise protects against acute endothelial impairment induced by a single exposure to hypertension during exertion. J. Appl. Physiol. 2011, 110, 1013–1020. [Google Scholar] [CrossRef]
- Birk, G.; Dawson, E.; Batterham, A.; Atkinson, G.; Cable, T.; Thijssen, D.H.; Green, D. Effects of Exercise Intensity on Flow Mediated Dilation in Healthy Humans. Int. J. Sports Med. 2012, 34, 409–414. [Google Scholar] [CrossRef]
- Atashak, S.; Sharafi, H.; Azarbayjani, M.A.; Stannard, S.R.; Goli, M.A.; Haghighi, M.M. Effect of omega-3 supplementation on the blood levels of oxidative stress, muscle damage and inflammation markers after acute resistance exercise in young athletes. Kinesiology 2013, 45, 22–29. [Google Scholar]
- Capó, X.; Martorell, M.; Sureda, A.; Llompart, I.; Tur, J.A.; Pons, A. Diet supplementation with DHA-enriched food in football players during training season enhances the mitochondrial antioxidant capabilities in blood mononuclear cells. Eur. J. Nutr. 2015, 54, 35–49. [Google Scholar] [CrossRef]
- de Oliveira, D.C.X.; Rosa, F.T.; Simões-Ambrósio, L.; Jordao, A.A.; Deminice, R. Antioxidant vitamin supplementation prevents oxidative stress but does not enhance performance in young football athletes. Nutrition 2019, 63–64, 29–35. [Google Scholar] [CrossRef]
- Mason, S.A.; Trewin, A.J.; Parker, L.; Wadley, G.D. Antioxidant supplements and endurance exercise: Current evidence and mechanistic insights. Redox Biol. 2020, 35, 101471. [Google Scholar] [CrossRef]
- Capó, X.; Martorell, M.; Busquets-Cortés, C.; Sureda, A.; Riera, J.; Drobnic, F.; Tur, J.A.; Pons, A. Effects of dietary almond- and olive oil-based docosahexaenoic acid- and vitamin E-enriched beverage supplementation on athletic performance and oxidative stress markers. Food Funct. 2016, 7, 4920–4934. [Google Scholar] [CrossRef] [PubMed]
- Drâgan, I.; Ploeşteanu, E.; Cristea, E.; Mohora, M.; Dinu, V.; Troescu, V.S. Studies on selenium in top athletes. Physiologie 1988, 25, 187–190. [Google Scholar]
- Elosua, R.; Molina, L.; Fito, M.; Arquer, A.; Sanchez-Quesada, J.; Covas, M.; Ordoñez-Llanos, J.; Marrugat, J. Response of oxidative stress biomarkers to a 16-week aerobic physical activity program, and to acute physical activity, in healthy young men and women. Atherosclerosis 2003, 167, 327–334. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Control | Nutri4 | ||
|---|---|---|---|---|
| Before | After | Before | After | |
| N | 17 | 14 | ||
| Age (years) | 22 ± 3 | 23 ± 4 | ||
| BMI (kg/m2) | 24.9 ± 4.0 | 24.7 ± 3.9 | 23.6 ± 2.4 | 23.5 ± 2.4 |
| WHR | 0.83 ± 0.09 | 0.84 ± 0.08 | 0.83 ± 0.04 | 0.82 ± 0.04 |
| SBP (mmHg) | 122.1 ± 12.2 | 119.4 ± 12.8 | 124.1 ± 14.6 | 118.4 ± 12.0 |
| DBP (mmHg) | 70.6 ± 9.2 | 70.8 ± 8.7 | 71.0 ± 11.2 | 70.4 ± 10.8 |
| MAP (mmHg) | 87.2 ± 9.4 | 87.4 ± 9.0 | 88.8 ± 10.7 | 85.0 ± 9.1 * |
| HR (beats per minute) | 72.2 ± 9.3 | 73.8 ± 11.6 | 70.1 ± 8.9 | 69.4 ± 11.7 |
| erythrocytes (×1012/L) | 5.0 ± 0.3 | 5.0 ± 0.4 | 5.1 ± 0.3 | 5.1 ± 0.3 |
| hemoglobin (g/L) | 149.1 ± 8.2 | 148.4 ± 10.6 | 147.4 ± 7.4 | 148.3 ± 9.0 |
| hematocrit (%) | 42.8 ± 2.4 | 42.6 ± 2.9 | 42.6 ± 2.0 | 43.2 ± 2.2 |
| leukocytes (×109/L) | 5.8 ± 1.0 | 5.7 ± 1.0 | 6.0 ± 1.1 | 6.0 ± 1.8 |
| thrombocytes (×109/L) | 214.1 ± 57.8 | 214.8 ± 43.4 | 233.8 ± 36.4 | 230.2 ± 61.6 † |
| urea (mmol/L) | 6.2 ± 1.2 | 6.8 ± 1.3 | 6.4 ± 0.9 | 6.3 ± 0.9 |
| creatinine (µmol/L) | 91.4 ± 9.9 | 91.8 ± 9.7 | 91.1 ± 10.0 | 90.4 ± 9.2 |
| sodium (mmol/L) | 139.4 ± 1.2 | 140.6 ± 1.9 * | 139.8 ± 1.4 | 139.8 ± 2.1 |
| potassium (mmol/L) | 4.2 ± 0.3 | 4.1 ± 0.2 | 4.3 ± 0.3 | 4.1 ± 0.3 * |
| calcium (mmol/L) | 2.6 ± 0.5 | 2.5 ± 0.4 | 2.4 ± 0.1 | 2.4 ± 0.1 |
| iron (μmol/L) | 17.0 ± 5.6 | 17.6 ± 4.6 | 19.2 ± 9.0 | 16.1 ± 7.0 |
| transferrin (g/L) | 2.5 ± 0.3 | 2.5 ± 0.3 | 2.6 ± 0.4 | 2.6 ± 0.3 |
| glucose (mmol/L) | 5.0 ± 0.5 | 4.8 ± 0.9 | 5.2 ± 1.0 | 5.2 ± 1.2 † |
| hsCRP (mg/L) | 0.9 ± 0.8 | 0.9 ± 1.3 | 0.7 ± 0.6 | 0.9 ± 0.8 |
| cholesterol (mmol/L) | 4.0 ± 0.7 | 4.2 ± 0.8 | 4.4 ± 1.1 | 4.6 ± 1.1 |
| triglycerides (mmol/L) | 1.0 ± 0.5 | 1.3 ± 1.1 | 0.9 ± 0.5 | 1.1 ± 0.7 |
| HDL cholesterol (mmol/L) | 1.3 ± 0.3 | 1.3 ± 0.3 | 1.5 ± 0.3 | 1.4 ± 0.3 |
| LDL cholesterol (mmol/L) | 2.4 ± 0.6 | 2.6 ± 0.6 | 2.6 ± 0.8 | 2.7 ± 0.8 |
| Parameter | Control | Nutri4 | |||
|---|---|---|---|---|---|
| Before | After | Before | After | ||
| SFA (μmol/L) | |||||
| C8:0 Caprylic acid | N/F | 96.9 | 29.5 | 63.8 ± 34.8 | |
| C12:0 Lauric acid | 27.2 ± 0.0 | 38.8 ± 8.6 | 36.7 ± 21.8 | 51.2 ± 34.0 | |
| C14:0 Myristic acid | 79.8 ± 33.1 | 106.6 ± 30.8 | 21.6 ± 23.7 | 70.7 ± 34.7 | |
| C15:0 Pentadecylic acid | 17.8 ± 6.3 | 21.0 ± 2.7 | 17.8 ± 2.2 | 16.7 ± 2.9 † | |
| C16:0 Palmitic Acid | 1701.5 ± 330.2 | 1872.8 ± 187.6 | 1811.1 ± 131.2 | 1634.4 ± 185.0 *† | |
| C17:0 Margaric acid | 21.5 ± 5.0 | 23.0 ± 1.9 | 23.0 ± 2.4 | 20.7 ± 3.6 | |
| C18:0 Stearic acid | 808.7 ± 116.6 | 802.3 ± 73.1 | 817.2 ± 94.0 | 772.1 ± 90.3 | |
| PUFA (μmol/L) | |||||
| n-5 | C14:1[cis-9] Myristoleic acid | <LOQ | 11.07 ± 0.0 | <LOQ | 11.26 ± 0.0 |
| n-7 | C16:1[cis-9] Palmitoleic acid | 87.8 ± 36.4 | 159.3 ± 106.1 | 84.7 ± 27.5 | 70.2 ± 16.9 |
| C17:1[cis-10] cis-10-Heptadecenoic acid | 23.0 ± 7.8 | 20.4 ± 6.4 | 22.5 ± 8.0 | 20.0 ± 2.9 | |
| n-9 | C18:1[trans-9] Elaidic acid | 1021.7 ± 307.6 | 1167.2 ± 0.0 | 941.2 ± 51.4 | 697.2 ± 0.0 |
| C18:1[cis-9] Oleic acid | 610.8 ± 595.7 | 1115.2 ± 694.1 | 494.6 ± 622.9 | 1047.4 ± 497.0 | |
| C20:1[cis-11] 11-Eicosenoic acid | 15.7 ± 3.3 | 16.5 ± 3.2 | 16.4 ± 2.0 | 14.7 ± 2.6 | |
| C24:1[cis-15] Nervonic acid | 6.5 ± 0.0 | 8.1 ± 1.0 | 6.9 ± 0.0 | <LOQ | |
| n-6 | C18:2[cis-9,12] Linoleic acid | 1696.2 ± 343.8 | 1921.4 ± 490.5 | 1881.5 ± 543.2 | 1969.4 ± 486.9 |
| C18:3[cis-6,9,12] gamma-Linolenic acid | 31.3 ± 5.9 | 46.7 ± 23.6 | 33.2 ± 16.5 | 28.4 ± 8.8 | |
| C21:2[cis-11,14] Eicosadienoic acid | 15.7 ± 3.3 | 16.5 ± 3.2 | 16.4 ± 2.0 | 14.7 ± 2.6 | |
| C20:3[cis-8,11,14] Dihomo-gamma-linolenic acid | 94.3 ± 37.1 | 136.9 ± 92.3 | 107.6 ± 30.4 | 90.5 ± 27.1 | |
| C20:4[cis-5,8,11,14] Arachidonic acid | 538.8 ± 67.5 | 600.1 ± 103.8 | 578.4 ± 53.9 | 538.3 ± 88.7 | |
| n-3 | C18:3[cis-9,12,15] alpha-Linolenic acid | 21.0 ± 9.9 | 24.1 ± 3.6 | 18.1 ± 4.2 | 23.0 ± 5.9 |
| C20:4[cis-5,8,11,14] Eicosa-5,8,11,14,17-pentaenoic acid | 22.6 ± 3.8 | 28.4 ± 2.4 | 20.4 ± 4.3 | 22.1 ± 5.1 | |
| C22:6[cis-4,7,10,13,16,19] cis-4,7,10,13,16,19-Docosahexaenoic acid | 111.1 ± 89.7 | 207.4 ± 81.5 | 108.0 ± 32.7 | 185.4 ± 86.6 * | |
| n6/n3 PUFAs | 25.2 ± 19.4 | 22.1 ± 22.3 | 19.5 ± 9.3 | 12.4 ± 3.2 *† | |
| Parameter | Control (N = 17) | Nutri4 (N = 14) | ||
|---|---|---|---|---|
| Before | After | Before | After | |
| Selenium (µg/L) | 74.5 ± 13.9 | 70.1 ± 8.2 | 76.2 ± 15.0 | 89.3 ± 9.3 *† |
| Vitamin E (μg/mL) | 7.0 ± 4.2 | 7.1 ± 4.3 | 7.6 ± 7.8 | 10.7 ± 5.3 *† |
| Lutein (μmol/L) | 0.089 ± 0.027 | 0.105 ± 0.061 | 0.110 ± 0.0409 | 0.088 ± 0.038 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kolar, L.; Šušnjara, P.; Stupin, M.; Stupin, A.; Jukić, I.; Mihaljević, Z.; Kolobarić, N.; Bebek, I.; Nejašmić, D.; Lovrić, M.; et al. Enhanced Microvascular Adaptation to Acute Physical Stress and Reduced Oxidative Stress in Male Athletes Who Consumed Chicken Eggs Enriched with n-3 Polyunsaturated Fatty Acids and Antioxidants—Randomized Clinical Trial. Life 2023, 13, 2140. https://doi.org/10.3390/life13112140
Kolar L, Šušnjara P, Stupin M, Stupin A, Jukić I, Mihaljević Z, Kolobarić N, Bebek I, Nejašmić D, Lovrić M, et al. Enhanced Microvascular Adaptation to Acute Physical Stress and Reduced Oxidative Stress in Male Athletes Who Consumed Chicken Eggs Enriched with n-3 Polyunsaturated Fatty Acids and Antioxidants—Randomized Clinical Trial. Life. 2023; 13(11):2140. https://doi.org/10.3390/life13112140
Chicago/Turabian StyleKolar, Luka, Petar Šušnjara, Marko Stupin, Ana Stupin, Ivana Jukić, Zrinka Mihaljević, Nikolina Kolobarić, Iva Bebek, Diana Nejašmić, Marija Lovrić, and et al. 2023. "Enhanced Microvascular Adaptation to Acute Physical Stress and Reduced Oxidative Stress in Male Athletes Who Consumed Chicken Eggs Enriched with n-3 Polyunsaturated Fatty Acids and Antioxidants—Randomized Clinical Trial" Life 13, no. 11: 2140. https://doi.org/10.3390/life13112140
APA StyleKolar, L., Šušnjara, P., Stupin, M., Stupin, A., Jukić, I., Mihaljević, Z., Kolobarić, N., Bebek, I., Nejašmić, D., Lovrić, M., & Drenjančević, I. (2023). Enhanced Microvascular Adaptation to Acute Physical Stress and Reduced Oxidative Stress in Male Athletes Who Consumed Chicken Eggs Enriched with n-3 Polyunsaturated Fatty Acids and Antioxidants—Randomized Clinical Trial. Life, 13(11), 2140. https://doi.org/10.3390/life13112140








