Detection of High-Grade Cervical Intraepithelial Neoplasia by Electrical Impedance Spectroscopy in Women Diagnosed with Low-Grade Cervical Intraepithelial Neoplasia in Cytology
Abstract
:1. Introduction
2. Materials and Methods
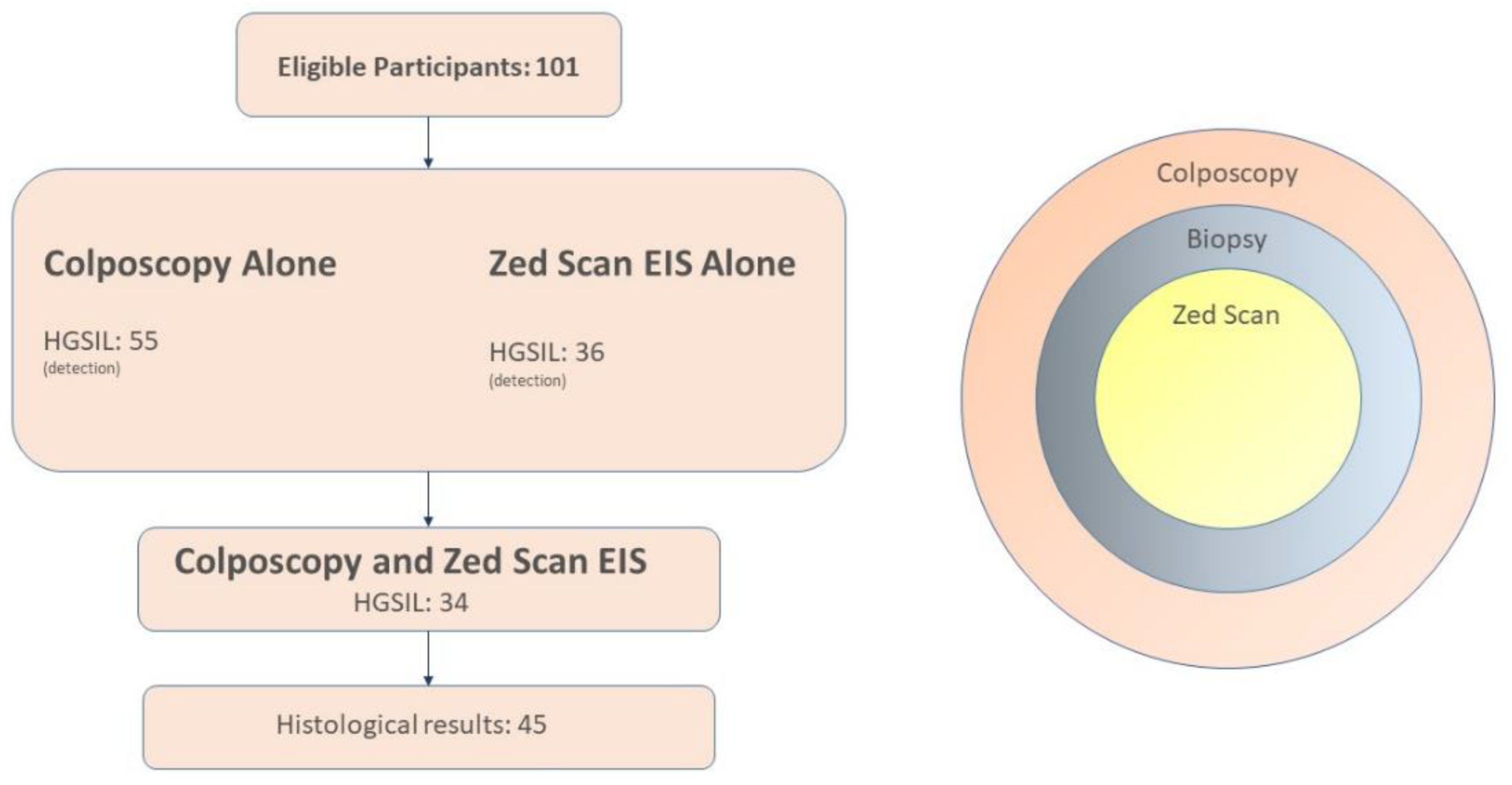
2.1. Design
- A colposcopy examination, with biopsies taken from suspicious cervical lesions;
- ZedScan spectroscopy, with extra biopsies of areas that had been identified as high risk for HGSIL;
- Histopathological analysis of tissues with suspected damage.
2.2. Participants
2.3. Inclusion–Exclusion Criteria
2.4. Intervention
2.5. Statistical Analysis
3. Results
3.1. Demographics
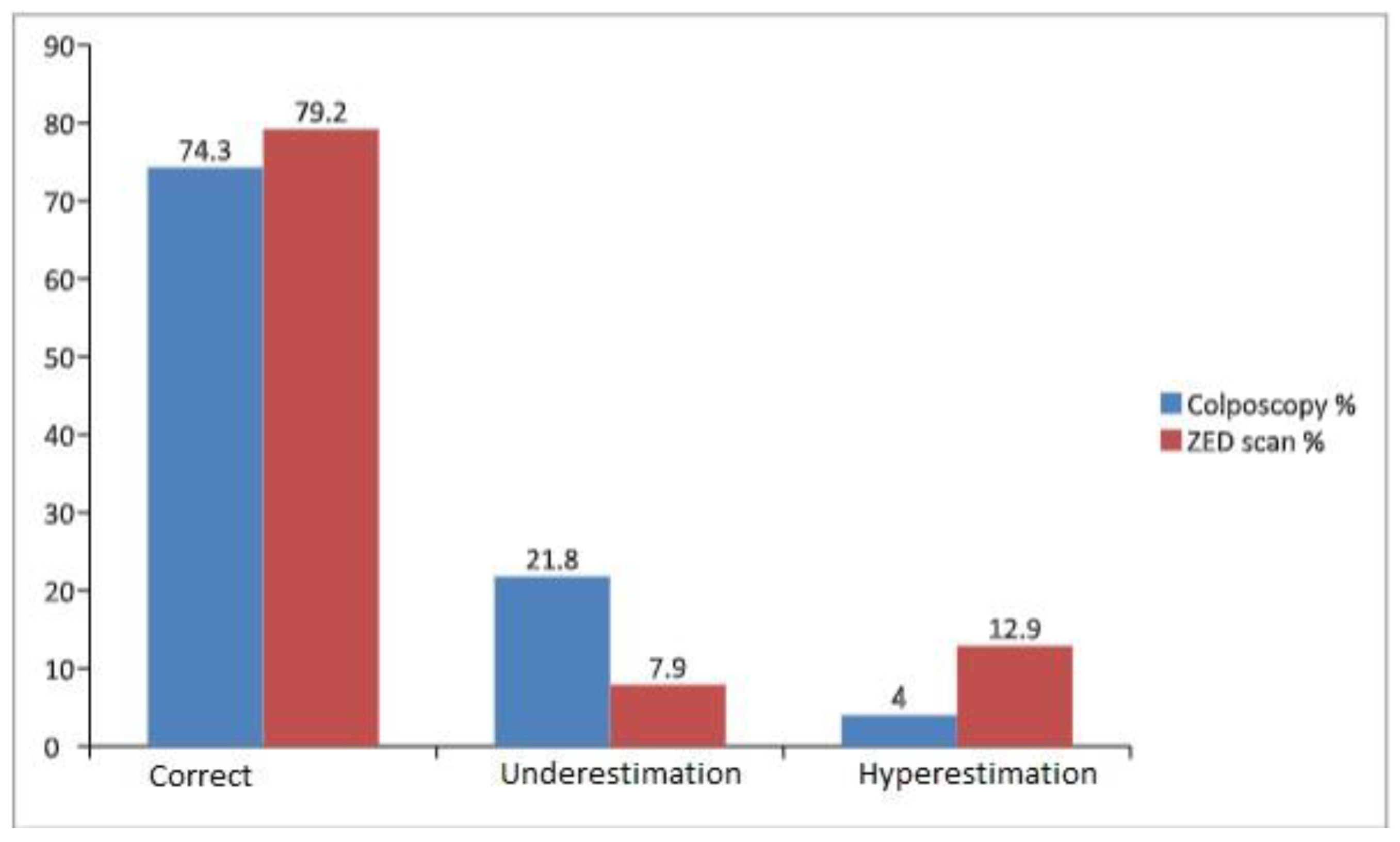
3.2. Cytological-Colposcopical and Histopathological Findings
4. Discussion
5. Conclusions
6. Ethical Approval
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Mattiuzzi, C.; Lippi, G. Cancer statistics: A comparison between World Health Organization (WHO) and Global Burden of Disease (GBD). Eur. J. Public Health 2020, 30, 1026–1027. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.who.int/news-room/fact-sheets/detail/cervical-cancer (accessed on 5 June 2023).
- zur Hausen, H. Papillomaviruses in anogenital cancer as a model to understand the role of viruses in human cancers. Cancer Res. 1989, 49, 4677–4681. [Google Scholar] [PubMed]
- Walboomers, J.M.; Jacobs, M.V.; Manos, M.M.; Bosch, F.X.; Kummer, J.A.; Shah, K.V.; Snijders, P.J.; Peto, J.; Meijer, C.J.; Muñoz, N. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J. Pathol. 1999, 189, 12–19. [Google Scholar] [CrossRef]
- Herfs, M.; Yamamoto, Y.; Laury, A.; Wang, X.; Nucci, M.R.; McLaughlin-Drubin, M.E.; Münger, K.; Feldman, S.; McKeon, F.D.; Xian, W.; et al. A discrete population of squamocolumnar junction cells implicated in the pathogenesis of cervical cancer. Proc. Natl. Acad. Sci. USA 2012, 109, 10516–10521. [Google Scholar] [CrossRef]
- Marina, O.C.; Sanders, C.K.; Mourant, J.R. Effects of acetic acid on light scattering from cells. J. Biomed. Opt. 2012, 17, 085002. [Google Scholar] [CrossRef]
- Yim, E.K.; Park, J.S. The role of HPV E6 and E7 oncoproteins in HPV-associated cervical carcinogenesis. Cancer Res. Treat. 2005, 37, 319–324. [Google Scholar] [CrossRef]
- Wentzensen, N.; Walker, J.L.; Gold, M.A.; Smith, K.M.; Zuna, R.E.; Mathews, C.; Dunn, S.T.; Zhang, R.; Moxley, K.; Bishop, E.; et al. Multiple biopsies and detection of cervical cancer precursors at colposcopy. J. Clin. Oncol. 2015, 33, 83–89. [Google Scholar] [CrossRef]
- Pretorius, R.G.; Zhang, W.-H.; Belinson, J.L.; Huang, M.-N.; Wu, L.-Y.; Zhang, X.; Qiao, Y.-L. Colposcopically directed biopsy, random cervical biopsy, and endocervical curettage in the diagnosis of cervical intraepithelial neoplasia II or worse. Am. J. Obstet. Gynecol. 2004, 191, 430–434. [Google Scholar] [CrossRef]
- van der Marel, J.; Rodriguez, A.; del Pino, M.; van Baars, R.; Jenkins, D.; van de Sandt, M.M.; Torné, A.; Ordi, J.; ter Harmsel, B.; Verheijen, R.H.; et al. The value of endocervical curettage in addition to biopsies in women referred to colposcopy. J. Low. Genit. Tract Dis. 2015, 19, 282–287. [Google Scholar] [CrossRef]
- Huh, W.K.; Ault, K.A.; Chelmow, D.; Davey, D.D.; Goulart, R.A.; Garcia, F.A.R.; Kinney, W.K.; Massad, L.S.; Mayeaux, E.J.; Saslow, D.; et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: Interim clinical guidance. Obstet. Gynecol. 2015, 125, 330–337. [Google Scholar] [CrossRef]
- Porras, C.; Wentzensen, N.; Rodríguez, A.C.; Morales, J.; Burk, R.D.; Alfaro, M.; Hutchinson, M.; Herrero, R.; Hildesheim, A.; Sherman, M.E.; et al. Switch from cytology-based to HPV-based cervical screening: Implications for colposcopy. Int. J. Cancer 2012, 130, 1879–1887. [Google Scholar] [CrossRef] [PubMed]
- Nayar, R.; Wilbur, D.C. The Pap Test and Bethesda 2014. The reports of my demise have been greatly exaggerated. (after a quotation from Mark Twain). Acta Cytol. 2015, 59, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Brown, B.H.; Tidy, J.A.; Boston, K.; Blackett, A.D.; Smallwood, R.H.; Sharp, F. Relation between tissue structure and imposed electrical current flow in cervical neoplasia. Lancet 2000, 355, 892–895. [Google Scholar] [CrossRef] [PubMed]
- Sauvaget, C.; Fayette, J.M.; Muwonge, R.; Wesley, R.; Sankaranarayanan, R. Accuracy of visual inspection with acetic acid for cervical cancer screening. Int. J. Gynaecol. Obstet. 2011, 113, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Sankaranarayanan, R.; Nene, B.M.; Shastri, S.S.; Jayant, K.; Muwonge, R.; Budukh, A.M.; Hingmire, S.; Malvi, S.G.; Thorat, R.; Kothari, A.; et al. HPV screening for cervical cancer in rural India. N. Engl. J. Med. 2009, 360, 1385–1394. [Google Scholar] [CrossRef]
- Muszynski, C.; Dupont, E.; Vaysse, B.; Lanta, S.; Tidy, J.; Sergent, F.; Gondry, J. The impact of using electrical impedance spectroscopy (ZedScan) on the performance of colposcopy in diagnosing high grade squamous lesions of the cervix. J. Gynecol. Obstet. Hum. Reprod. 2017, 46, 669–673. [Google Scholar] [CrossRef]
- Tidy, J.A.; Brown, B.H.; Healey, T.J.; Daayana, S.; Martin, M.; Prendiville, W.; Kitchener, H. Accuracy of detection of high-grade cervical intraepithelial neoplasia using electrical impedance spectroscopy with colposcopy. BJOG 2013, 120, 400–411. [Google Scholar] [CrossRef]
- Pathiraja, A.; Weerakkody, R.A.; von Roon, A.C.; Ziprin, P.; Bayford, R. The clinical application of electrical impedance technology in the detection of malignant neoplasms: A systematic review. J. Transl. Med. 2020, 18, 227. [Google Scholar] [CrossRef]
- Mitchell, M.F.; Schottenfeld, D.; Tortolero-Luna, G.; Cantor, S.B.; Richards-Kortum, R. Colposcopy for the diagnosis of squamous intraepithelial lesions: A meta-analysis. Obstet. Gynecol. 1998, 91, 626–631. [Google Scholar] [CrossRef]
- Braun, R.P.; Mangana, J.; Goldinger, S.; French, L.; Dummer, R.; Marghoob, A.A. Electrical Impedance Spectroscopy in Skin Cancer Diagnosis. Dermatol. Clin. 2017, 35, 489–493. [Google Scholar] [CrossRef]
- Balasubramani, L.; Brown, B.H.; Healey, J.; Tidy, J.A. The detection of cervical intraepithelial neoplasia by electrical impedance spectroscopy: The effects of acetic acid and tissue homogeneity. Gynecol. Oncol. 2009, 115, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.W.; Yun, J.; Lee, J.Z.; Shin, D.G.; Lee, J.H. Evaluation of Electrical Impedance Spectroscopy-on-a-Needle as a Novel Tool to Determine Optimal Surgical Margin in Partial Nephrectomy. Adv. Healthc. Mater. 2017, 6, 1700356. [Google Scholar] [CrossRef] [PubMed]
- Brown, B.H.; Milnes, P.; Abdul, S.; Tidy, J.A. Detection of cervical intraepithelial neoplasia using impedance spectroscopy: A prospective study. BJOG 2005, 112, 802–806. [Google Scholar] [CrossRef] [PubMed]
- Abdul, S.; Brown, B.H.; Milnes, P.; Tidy, J.A. The use of electrical impedance spectroscopy in the detection of cervical intraepithelial neoplasia. Int. J. Gynecol. Cancer 2006, 16, 1823–1832. [Google Scholar] [CrossRef] [PubMed]
- Traige Study (ALTS) ASCUS-LSIL Group. A randomized trial on the management of low-grade squamous intraepithelial lesion cytology interpretations. Am. J. Obstet. Gynecol. 2003, 188, 1393–1400. [Google Scholar] [CrossRef]
- Polo, T.C.F.; Miot, H.A. Use of ROC curves in clinical and experimental studies. J. Vasc. Bras. 2020, 19, e20200186. [Google Scholar] [CrossRef]
- Bekkers, R.L.; van de Nieuwenhof, H.P.; Neesham, D.E.; Hendriks, J.H.; Tan, J.; Quinn, M.A. Does experience in colposcopy improve identification of high-grade abnor-malities? Eur. J. Obstet. Gynecol. Reprod. Biol. 2008, 141, 75–78. [Google Scholar] [CrossRef]
- Cantor, S.B.; Cárdenas-Turanzas, M.; Cox, D.D.; Atkinson, E.N.; Nogueras-Gonzalez, G.M.; Beck, J.R.; Follen, M.; Benedet, J.L. Accuracy of colposcopy in the diagnostic setting compared with the screening setting. Obstet. Gynecol. 2008, 111, 7–14. [Google Scholar] [CrossRef]
- Sheshadri, V.; O’Connor, D. The agreement of colposcopic grading as compared to directed biopsy results. J. Low. Genit. Tract Dis. 1999, 3, 150–154. [Google Scholar] [CrossRef]
- Gage, J.C.; Hanson, V.W.; Abbey, K.; Dippery, S.; Gardner, S.; Kubota, J.; Schiffman, M.; Solomon, D.; Jeronimo, J. Number of cervical biopsies and sensitivity of colposcopy. Obstet. Gynecol. 2006, 108, 264–272. [Google Scholar] [CrossRef]
- Massad, L.S.; Collins, Y.C. Strength of correlations between colposcopic impression and biopsy histology. Gynecol. Oncol. 2003, 89, 424–428. [Google Scholar] [CrossRef]
- Wei, B.; Zhang, B.; Xue, P.; Seery, S.; Wang, J.; Li, Q.; Jiang, Y.; Qiao, Y. Improving colposcopic accuracy for cervical precancer detection: A retrospective multicenter study in China. BMC Cancer. 2022, 22, 388. [Google Scholar] [CrossRef] [PubMed]
- Tidy, J.A.; Brown, B.H. Increased detection of high grade CIN, when using electrical impedance spectroscopy as an adjunct to routine colposcopy, is maintained when used across international boundaries: Prospective data from nine European countries. Eur. J. Obstet. Gynecol. Reprod. Biol. 2022, 275, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Tsampazis, N.; Vavoulidis, E.; Siarkou, C.M.; Siarkou, G.M.; Pratilas, G.C.; Symeonidou, M.; Intzes, S.; Petousis, S.; Papanikolaou, A.; Dinas, K. Diagnostic comparison of electrical impedance spectroscopy with colposcopy and HPV mRNA-testing in the prediction of CIN2+ women in Greece. J. Obstet. Gynaecol. Res. 2023, 49, 1222–1229. [Google Scholar] [CrossRef] [PubMed]
- Homola, W.; Fuchs, T.; Baranski, P.; Zimmer, A.; Zimmer, M.; Pomorski, M. Use of electrical impedance spectroscopy as an adjunct to colposcopy in a pathway of cervical intraepithelial neoplasia diagnostics. Ginekol. Pol. 2019, 90, 628–632. [Google Scholar] [CrossRef]
- Macdonald, M.C.; Brown, B.H.; Lyon, R.E.; Healey, T.J.; Palmer, J.E.; Tidy, J.A. Influence of high risk HPV genotype on colposcopic performance: A large prospective study demonstrates improved detection of disease with ZedScan I, particularly in non-HPV 16 patients. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 211, 194–198. [Google Scholar] [CrossRef]
- Liu, Z.C.; Liu, W.D.; Liu, Y.H.; Ye, X.H.; Chen, S.D. Multiple Sexual Partners as a Potential Independent Risk Factor for Cervical Cancer: A Meta-analysis of Epidemiological Studies. Asian Pac. J. Cancer Prev. 2015, 16, 3893–3900. [Google Scholar] [CrossRef]

| Demographic Characteristics | |
|---|---|
| Age (years) | |
| 20–35 | 26 |
| 36–50 | 58 |
| 51–64 | 17 |
| Race | |
| Caucasian | 96 |
| Other | 5 |
| Number of sexual partners | |
| ≤3 | 58 |
| >3 | 43 |
| Systematic condom use | |
| yes | 79 |
| no | 22 |
| BMI | |
| <25 | 62 |
| >25 | 39 |
| Smoking | |
| Yes | 62 |
| No | 39 |
| Comorbidities | |
| Systematic Lupus Erythematosus | 9 |
| Diabetes Melitus | 21 |
| Ulcerative Colitis | 7 |
| Hypertension | 11 |
| HPV vaccination | |
| Yes | 14 |
| No | 87 |
| N | % | |
|---|---|---|
| ZED SCAN | ||
| Normal | 29 | 28.7 |
| LGSIL | 17 | 16.8 |
| HGSIL | 55 | 54.5 |
| Colposcopy | ||
| Normal | 34 | 33.7 |
| LGSIL | 31 | 30.7 |
| HGSIL | 36 | 35.6 |
| Biopsy | ||
| Normal | 25 | 24.8 |
| CIN 1 | 31 | 30.7 |
| CIN 2-CIN 3 | 45 | 44.6 |
| Biopsy | Results | |||||
|---|---|---|---|---|---|---|
| Normal | CIN 1 | CIN 2-CIN 3 | ||||
| N | % | N | % | N | % | |
| ZED SCAN | ||||||
| Normal | 21 | 84.0 | 6 | 19.4 | 2 | 4.4 |
| LGSIL | 1 | 4.0 | 16 | 51.6 | 0 | 0.0 |
| HGSIL | 3 | 12.0 | 9 | 29.0 | 43 | 95.6 |
| Colposcopy | ||||||
| Normal | 23 | 92.0 | 11 | 35.5 | 0 | 0.0 |
| LGSIL | 2 | 8.0 | 18 | 58.1 | 11 | 24.4 |
| HGSIL | 0 | 0.0 | 2 | 6.5 | 34 | 75.6 |
| Colposcopy | ||||||
|---|---|---|---|---|---|---|
| Normal | LGSIL | HGSIL | ||||
| N | % | N | % | N | % | |
| ZED SCAN | ||||||
| Normal | 23 | 67.6 | 4 | 12.9 | 2 | 5.6 |
| LGSIL | 8 | 23.5 | 9 | 29 | 0 | 0 |
| HGSIL | 3 | 8.8 | 18 | 58.1 | 34 | 94.4 |
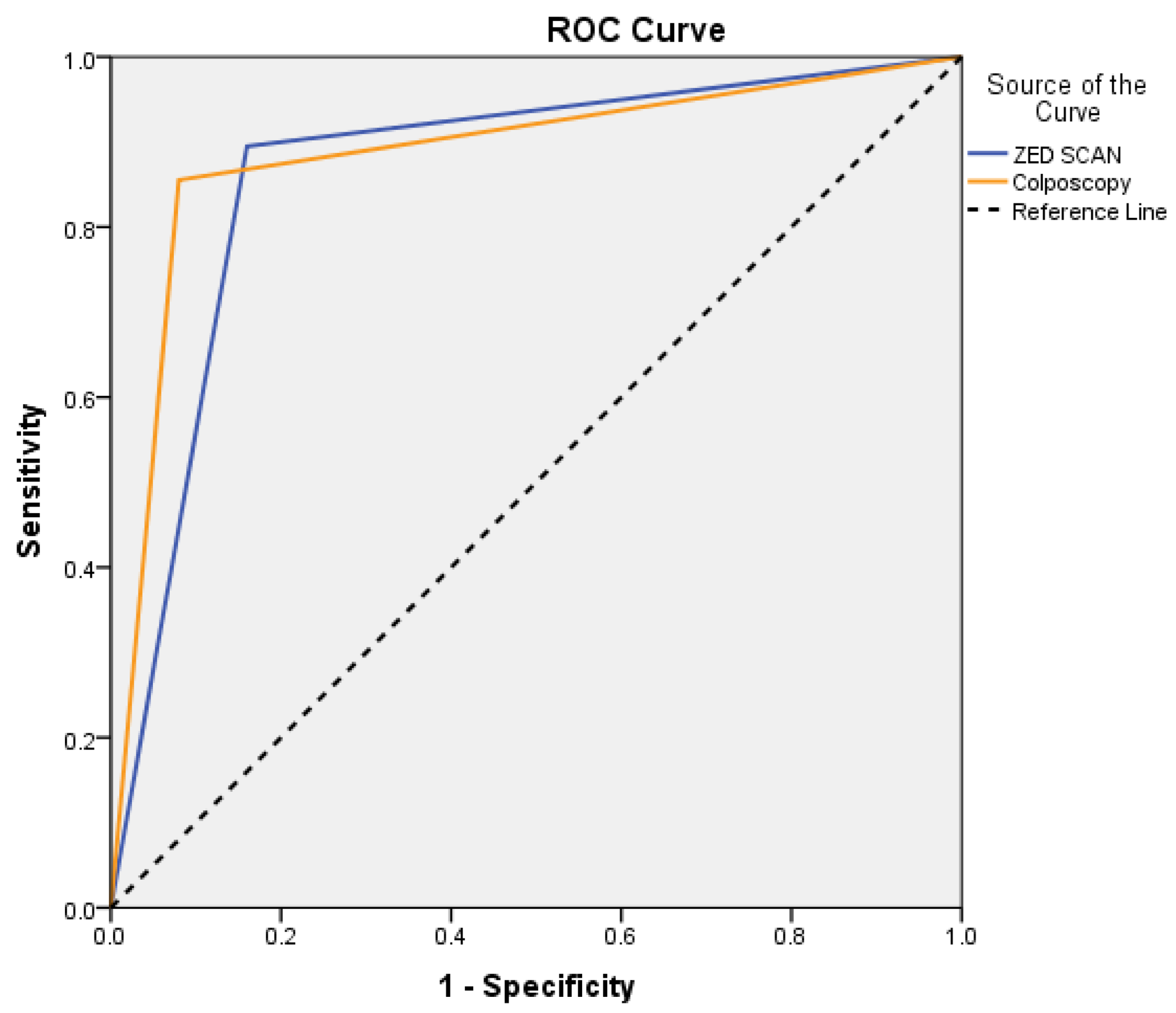
| Sensitivity % | Specificity % | PPV * % | NPV * % | AUC (95% CI) | p | |
|---|---|---|---|---|---|---|
| ZED SCAN | 89.5 | 84.0 | 94.4 | 72.4 | 0.87 (0.78–0.96) | <0.001 |
| Colposcopy | 85.5 | 92.0 | 97.0 | 67.6 | 0.89 (0.81–0.97) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panagakis, G.; Papapanagiotou, I.K.; Theofanakis, C.; Tsetsa, P.; Kontogeorgi, A.; Thomakos, N.; Rodolakis, A.; Haidopoulos, D. Detection of High-Grade Cervical Intraepithelial Neoplasia by Electrical Impedance Spectroscopy in Women Diagnosed with Low-Grade Cervical Intraepithelial Neoplasia in Cytology. Life 2023, 13, 2139. https://doi.org/10.3390/life13112139
Panagakis G, Papapanagiotou IK, Theofanakis C, Tsetsa P, Kontogeorgi A, Thomakos N, Rodolakis A, Haidopoulos D. Detection of High-Grade Cervical Intraepithelial Neoplasia by Electrical Impedance Spectroscopy in Women Diagnosed with Low-Grade Cervical Intraepithelial Neoplasia in Cytology. Life. 2023; 13(11):2139. https://doi.org/10.3390/life13112139
Chicago/Turabian StylePanagakis, Georgios, Ioannis K. Papapanagiotou, Charalampos Theofanakis, Paraskevi Tsetsa, Adamantia Kontogeorgi, Nikolaos Thomakos, Alexandros Rodolakis, and Dimitrios Haidopoulos. 2023. "Detection of High-Grade Cervical Intraepithelial Neoplasia by Electrical Impedance Spectroscopy in Women Diagnosed with Low-Grade Cervical Intraepithelial Neoplasia in Cytology" Life 13, no. 11: 2139. https://doi.org/10.3390/life13112139
APA StylePanagakis, G., Papapanagiotou, I. K., Theofanakis, C., Tsetsa, P., Kontogeorgi, A., Thomakos, N., Rodolakis, A., & Haidopoulos, D. (2023). Detection of High-Grade Cervical Intraepithelial Neoplasia by Electrical Impedance Spectroscopy in Women Diagnosed with Low-Grade Cervical Intraepithelial Neoplasia in Cytology. Life, 13(11), 2139. https://doi.org/10.3390/life13112139







