Chemical Evolution of Nb-Ta Oxides and Cassiterite in Phosphorus-Rich Albite-Spodumene Pegmatites in the Kangxiwa–Dahongliutan Pegmatite Field, Western Kunlun Orogen, China
Abstract
1. Introduction
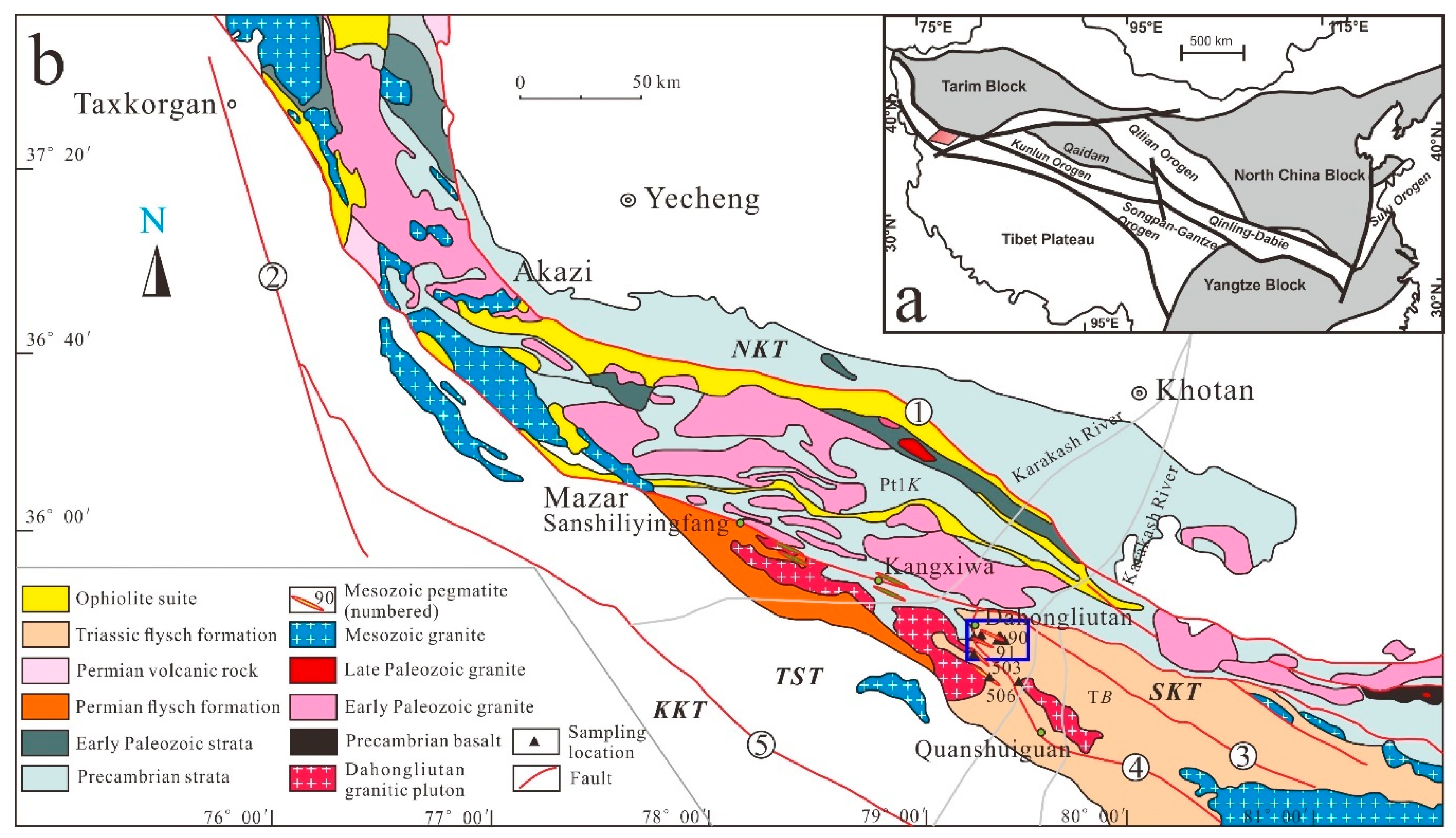
2. Geological Setting
3. Analytical Methods
4. Results
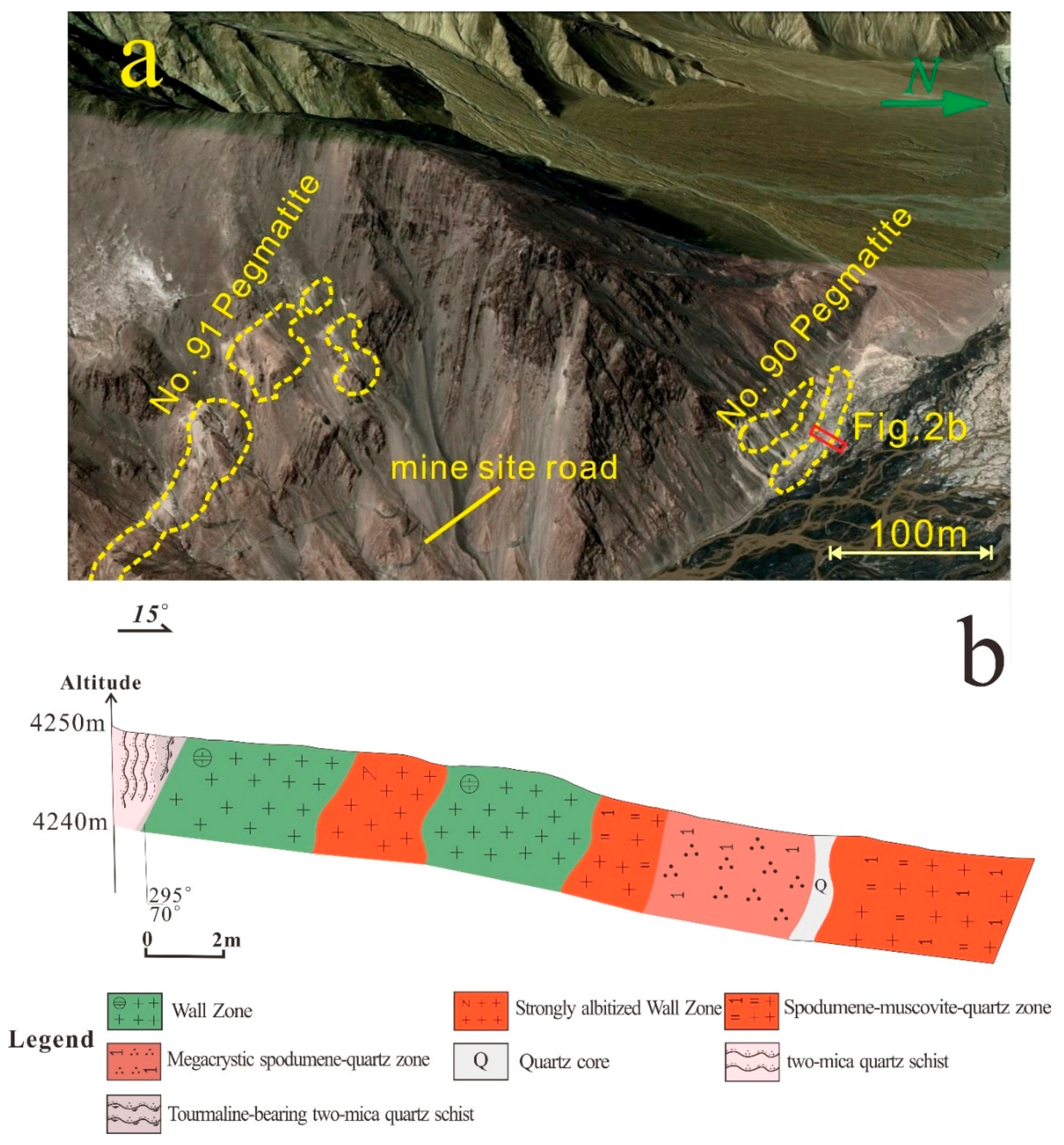
4.1. Field Observations
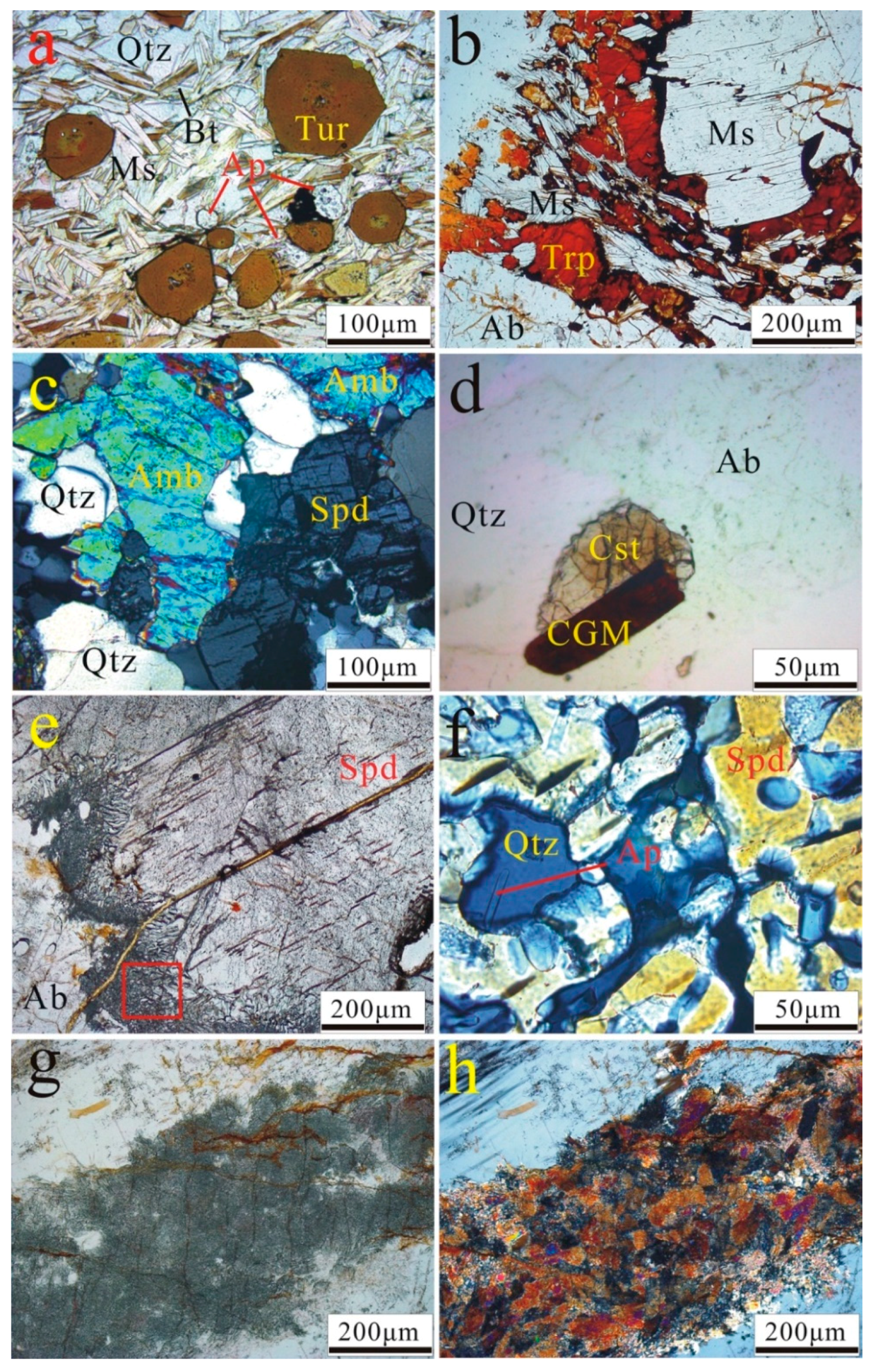
4.2. Petrographic Observations
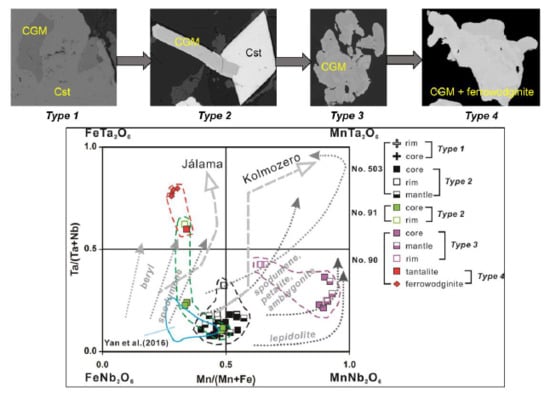
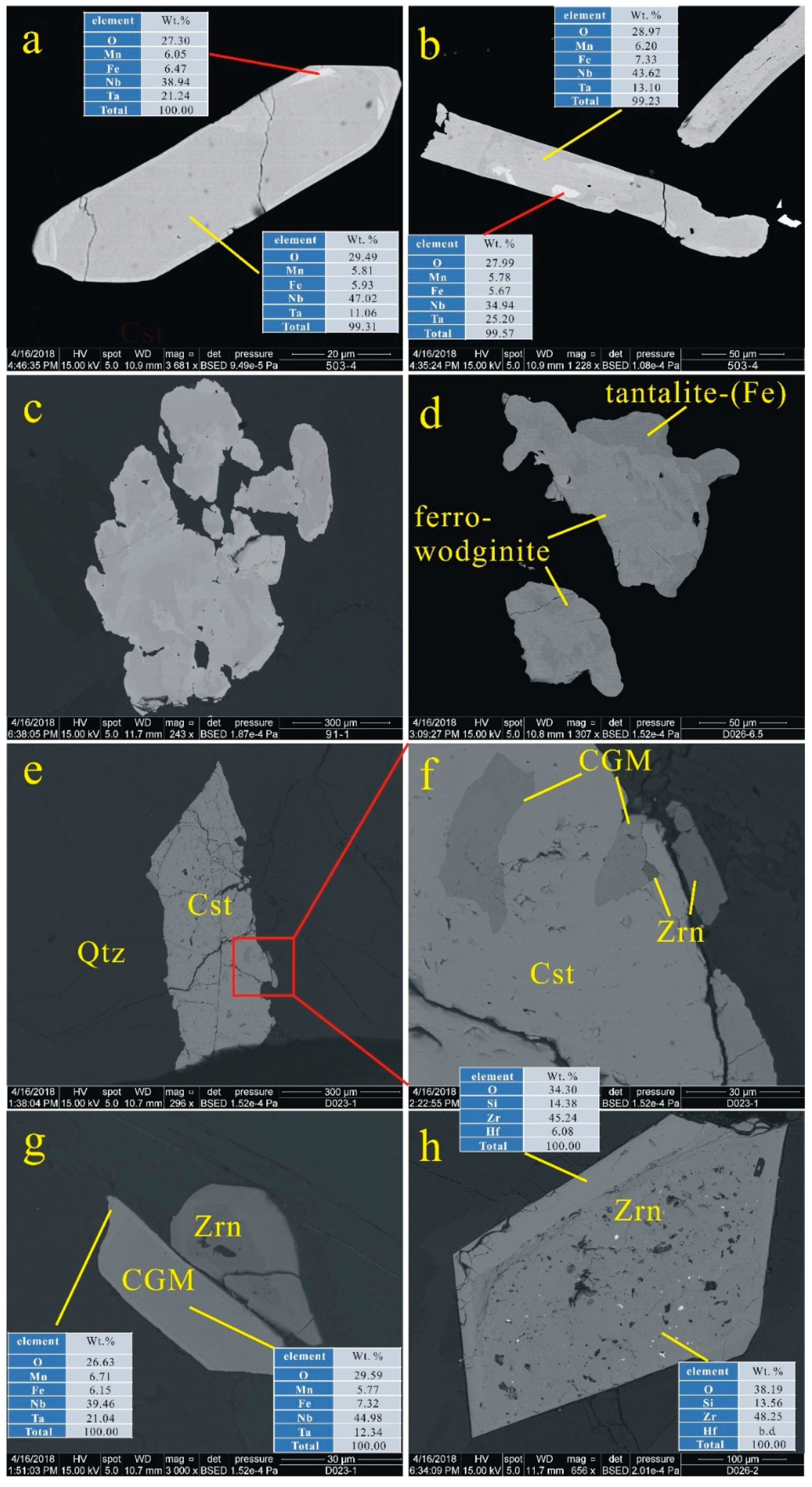
4.3. Important HFSE-Bearing Minerals and Classification of CGM
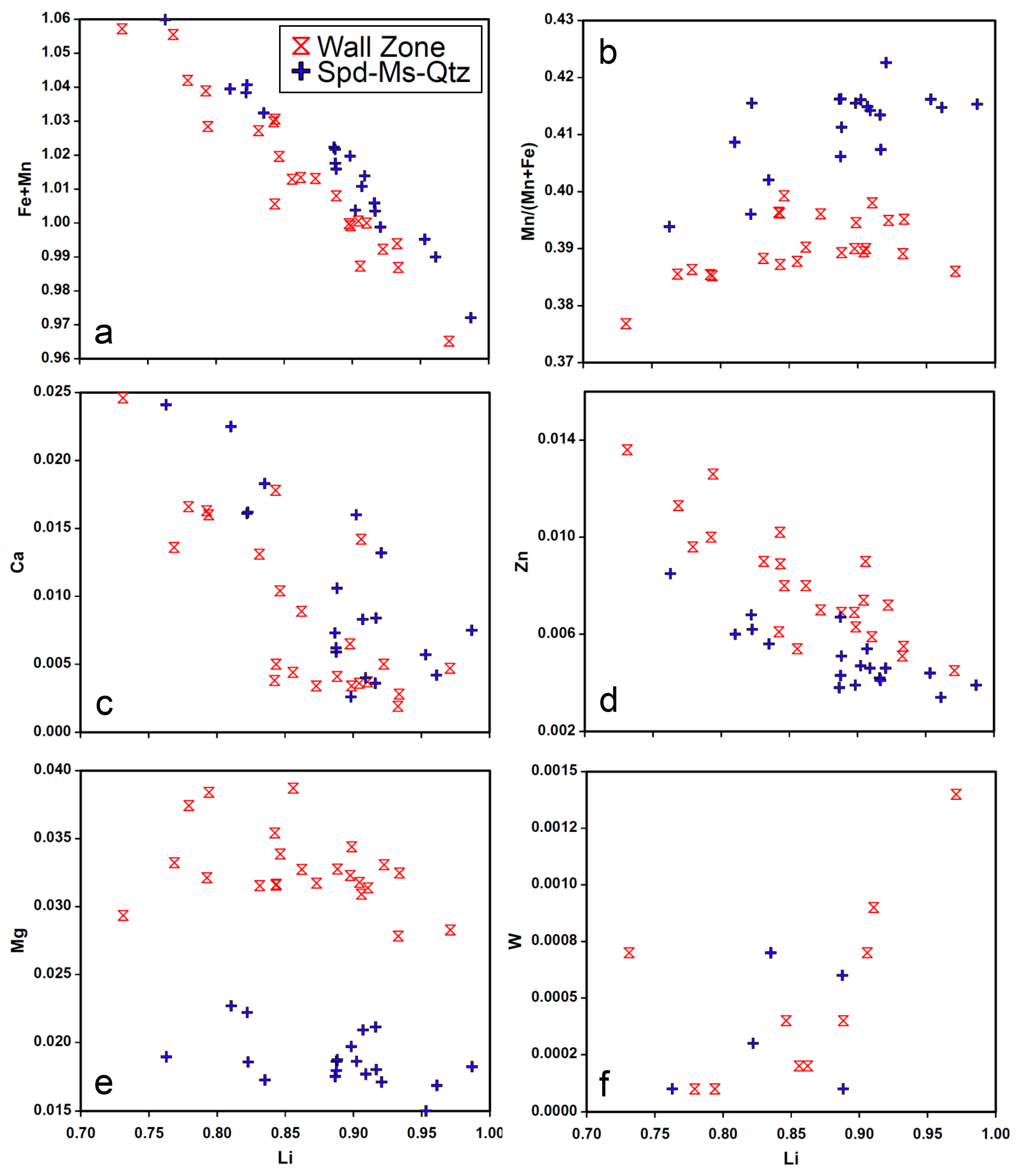
4.4. EPMA
4.4.1. CGM and Ferrowodginite
4.4.2. Cassiterite
4.4.3. Triphylite
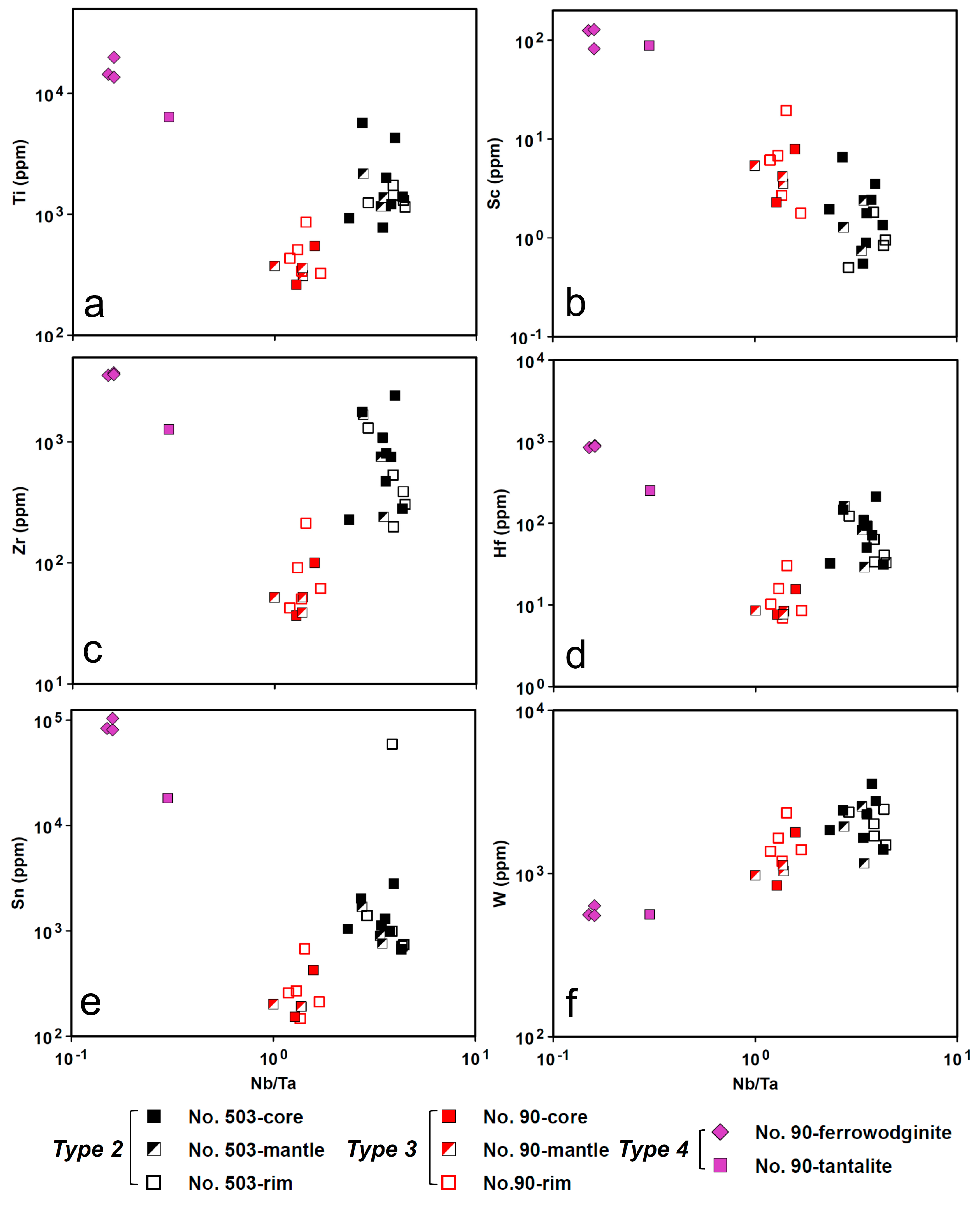
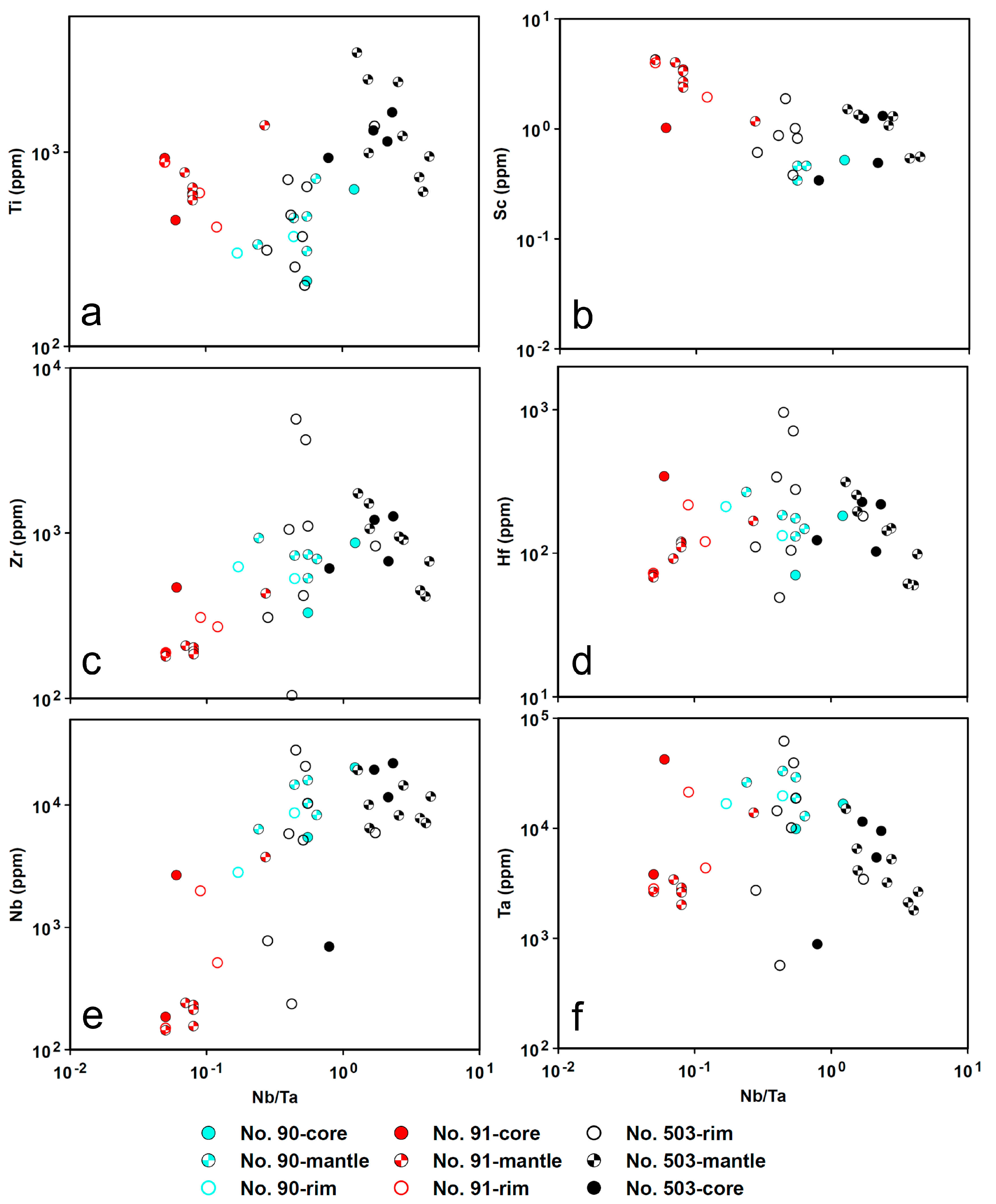
4.5. LA-ICP-MS
4.5.1. CGM and Ferrowodginite
4.5.2. Cassiterite
5. Discussion
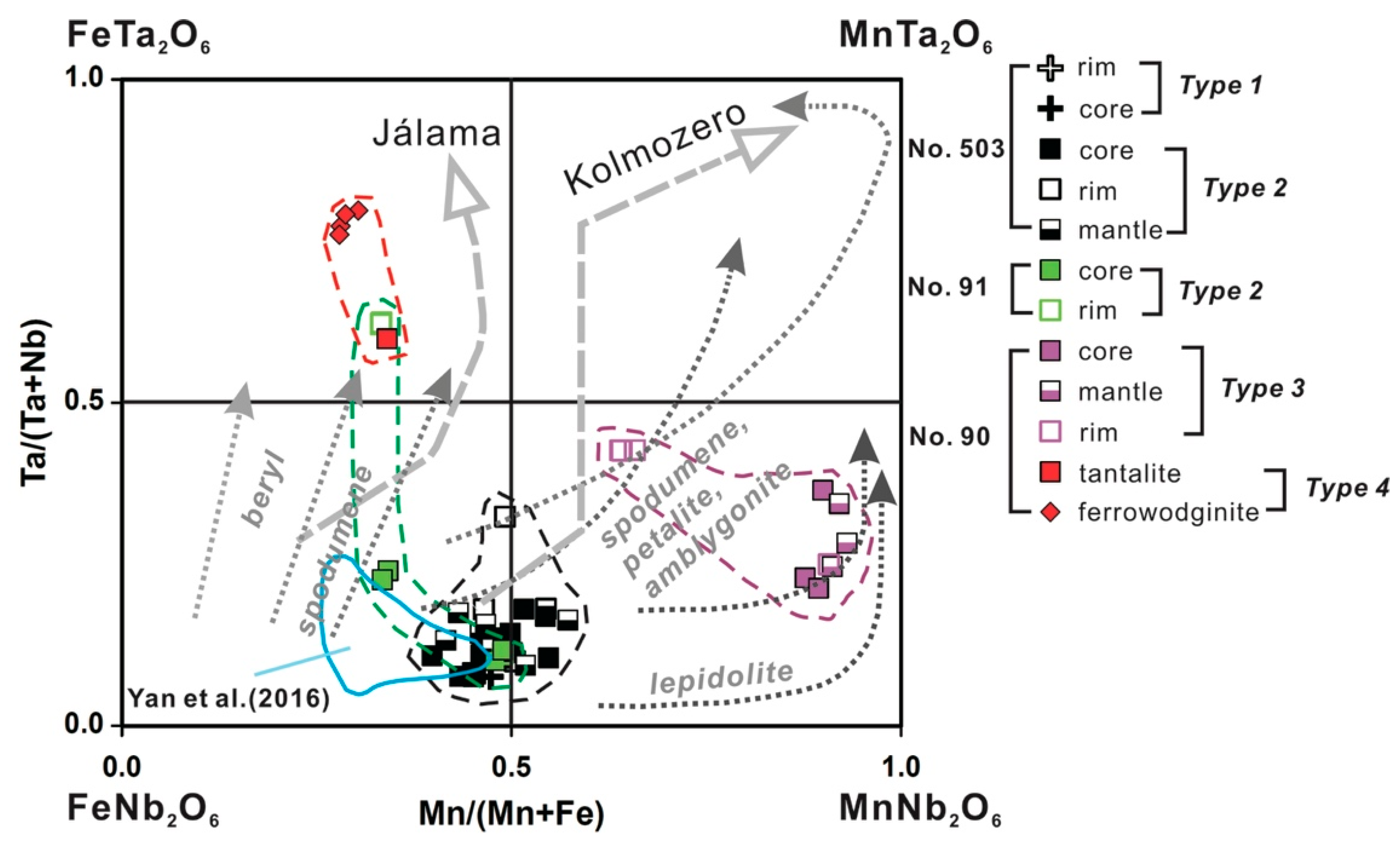
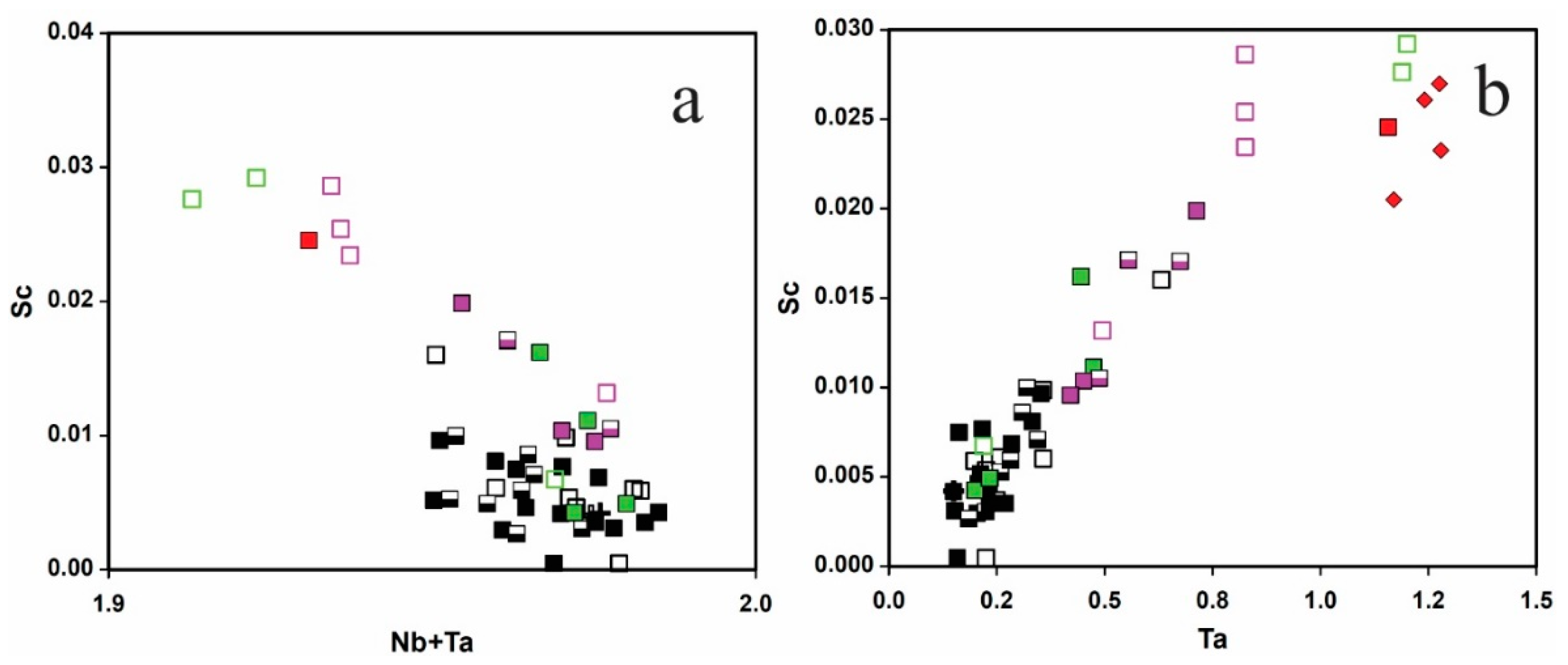
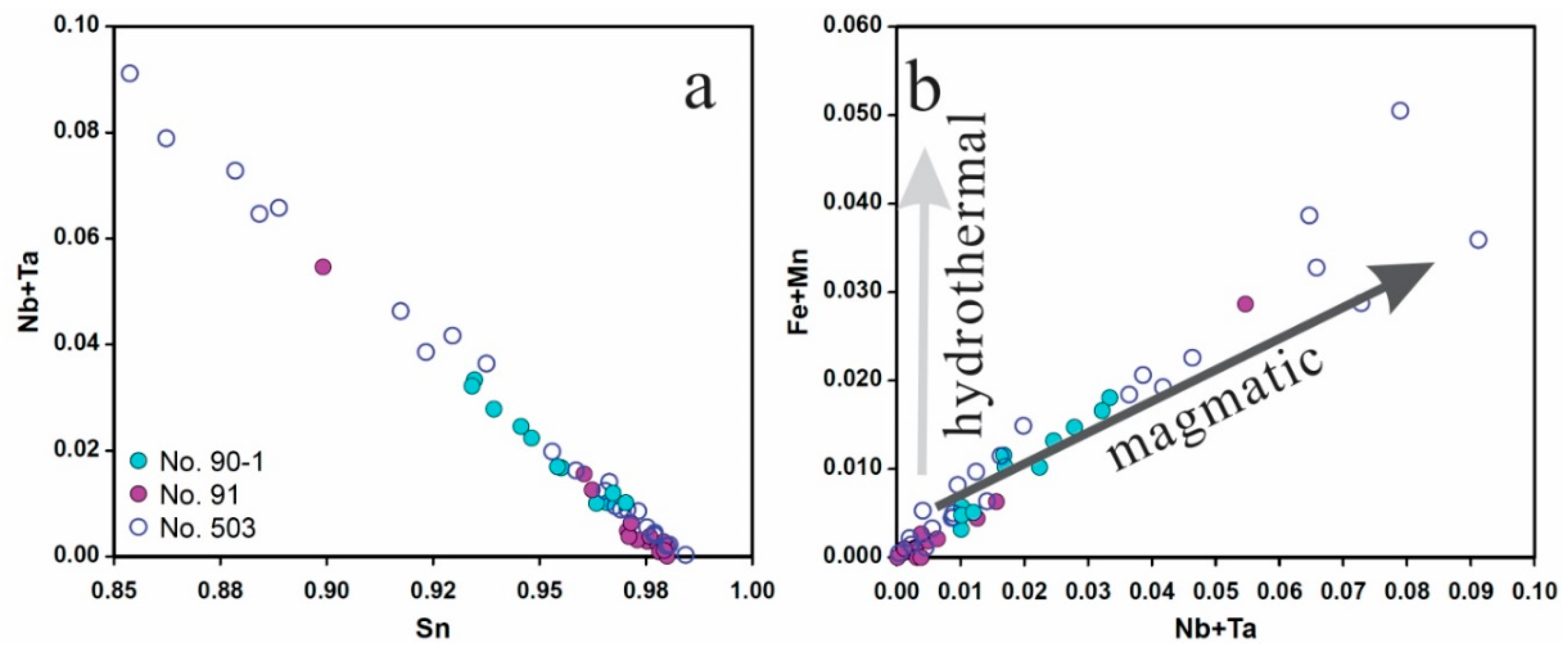
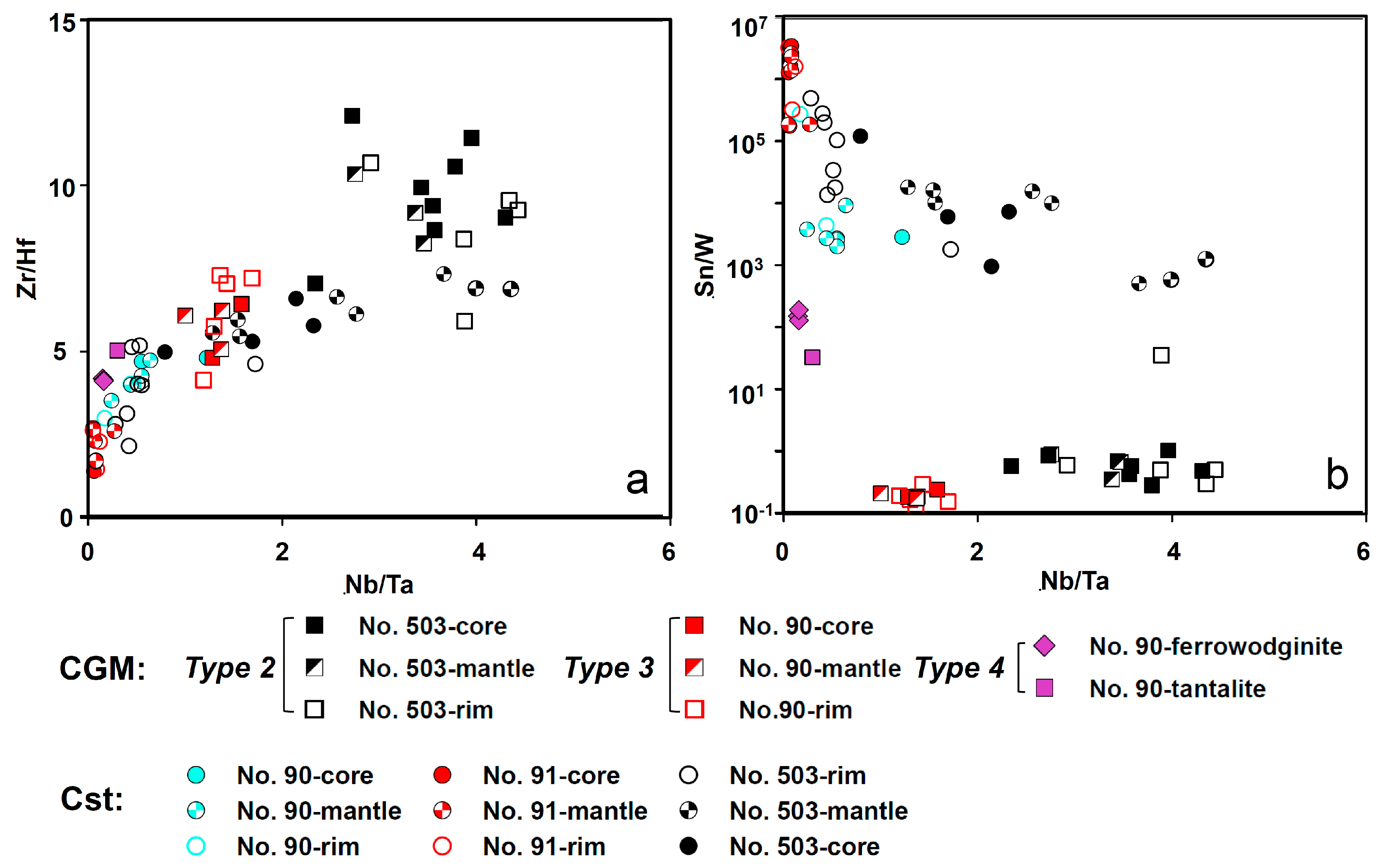
5.1. Origin of Cassiterite and CGM
5.2. Chemical Evolution of Cassiterite and CGM within a Single Pegmatite
5.2.1. No. 503 Pegmatite
5.2.2. No. 90 Pegmatite
5.2.3. No. 91 Pegmatite
5.3. Chemical Evolution of Cassiterite and CGM within the Pegmatite Group
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Linnen, R.L.; Van Lichtervelde, M.; Černý, P. Granitic pegmatites as sources of strategic metals. Elements 2012, 8, 275–280. [Google Scholar] [CrossRef]
- Linnen, R.L.; Samson, I.M.; Williams-Jones, A.E.; Chakhmouradian, A.R. Geochemistry of the rare-earth element, Nb, Ta, Hf, and Zr deposits. In Treatise on Geochemistry, 2nd ed.; Holland, H.D., Turekian, K.K., Eds.; Elsevier: Oxford, UK, 2014; Volume 13, pp. 543–564. [Google Scholar]
- Černý, P.; Ercit, T.S. Mineralogy of niobium and tantalum: Crystal chemical relationships, paragenetic aspects and their economic implications. In Lanthanides. Tantalum and Niobium; Möller, P., Černý, P., Saupé, F., Eds.; Springer: Berlin, Germany, 1989; pp. 27–79. [Google Scholar]
- Černý, P.; Blevin, P.L.; Cuney, M.; London, D. Granite-related ore deposits. Econ. Geol. Anniv. 2005, 337–370. [Google Scholar]
- Melcher, F.; Graupner, T.; Gäbler, H.-E.; Sitnikova, M.; Oberthür, T.; Gerdes, A.; Badanina, E.; Chudy, T. Mineralogical and chemical evolution of tantalum–(niobium–tin) mineralisation in pegmatites and granites. Part 2: Worldwide examples (excluding Africa) and an overview of global metallogenetic patterns. Ore Geol. Rev. 2017, 89, 946–987. [Google Scholar] [CrossRef]
- Fosso Tchunte, P.M.; Tchameni, R.; André-Mayer, A.S.; Dakoure, H.S.; Turlin, F.; Poujol, M.; Negue Nomo, E.; Saha Fouotsa, A.N.; Rouer, O. Evidence for Nb-Ta Occurrences in the Syn-Tectonic Pan-African Mayo Salah Leucogranite (Northern Cameroon): Constraints from Nb-Ta Oxide Mineralogy, Geochemistry and U-Pb LA-ICP-MS Geochronology on Columbite and Monazite. Minerals 2018, 8, 188. [Google Scholar] [CrossRef]
- Melcher, F.; Graupner, T.; Gäbler, H.-E.; Sitnikova, M.; Henjes-Kunst, F.; Oberthür, T.; Gerdes, A.; Dewaele, S. Tantalum–(niobium–tin) mineralisation in African pegmatites and rare metal granites: Constraints from Ta–Nb oxide mineralogy, geochemistry and U–Pb geochronology. Ore Geol. Rev. 2015, 64, 667–719. [Google Scholar] [CrossRef]
- Badanina, E.V.; Sitnikova, M.A.; Gordienko, V.V.; Melcher, F.; Gäbler, H.E.; Lodziak, J.; Syritso, L.F. Mineral chemistry of columbite–tantalite from spodumene pegmatites of Kolmozero, Kola Peninsula (Russia). Ore Geol. Rev. 2015, 64, 720–735. [Google Scholar] [CrossRef]
- Černý, P.; Ercit, T.S. Some recent advances in the mineralogy and geochemistry of Nb and Ta in rare-element granitic pegmatites. Bull. Minéral. 1985, 108, 499–532. [Google Scholar]
- Černý, P.; Goad, B.E.; Hawthorne, F.C.; Chapman, R. Fractionation trends of the Nb- and Ta-bearing oxide minerals in the Greer Lake pegmatitic granite and its pegmatite aureole, southeastern Manitoba. Am. Mineral. 1986, 71, 501–517. [Google Scholar]
- Tindle, A.G.; Breaks, F.W. Columbite–tantalite mineral chemistry from rare-element granitic pegmatites: Separation Lake area, N.W. Ontario, Canada. Mineral. Petrol. 2000, 70, 165–198. [Google Scholar] [CrossRef]
- Pal, D.C.; Mishra, B.; Bernhardt, H. Mineralogy and geochemistry of pegmatite-hosted Sn-, Ta–Nb-, and Zr–Hf-bearing minerals from the southeastern part of the Bastar-Malkangiri pegmatite belt, Central India. Ore Geol. Rev. 2007, 30, 30–55. [Google Scholar] [CrossRef]
- Breiter, K.; Skoda, R.; Uher, P. Nb–Ta–Ti–W–Sn-oxide minerals as indicators of a peraluminous P- and F-rich granitic system evolution: Podlesí, Czech Republic. Mineral. Petrol. 2007, 91, 225–248. [Google Scholar] [CrossRef]
- Llorens, T.; Moro, M.C. Oxide minerals in the granitic cupola of the Jálama Batholith, Salamanca, Spain. Part I: Accessory Sn, Nb, Ta and Ti minerals in leucogranites, aplites and pegmatites. J. Geosci. 2012, 57, 25–43. [Google Scholar] [CrossRef]
- Plimer, I.R.; Lu, J.; Kleeman, J.D. Trace and rare earth elements in cassiterite—Sources of components for the tin deposits of the Mole Granite, Australia. Miner. Depos. 1991, 26, 267–274. [Google Scholar] [CrossRef]
- Neiva, M.R. Geochemistry of cassiterite and its inclusion and exsolution products from tin and tungsten deposits in Portugal. Can. Mineral. 1996, 34, 745–768. [Google Scholar]
- Neiva, A.M.R. Geochemistry of cassiterite and wolframite from tin and tungsten quartz veins in Portugal. Ore Geol. Rev. 2008, 33, 221–238. [Google Scholar] [CrossRef]
- Xie, L.; Wang, R.-C.; Che, X.-D.; Huang, F.-F.; Erdmann, S.; Zhang, W.-L. Tracking magmatic and hydrothermal Nb–Ta–W–Sn fractionation using mineral textures and composition: A case study from the late Cretaceous Jiepailing ore district in the Nanling Range in South China. Ore Geol. Rev. 2016, 78, 300–321. [Google Scholar]
- Černý, P.; Ercit, T.S.; Smeds, S.-A.; Groat, L.A.; Chapman, R. Zirconium and hafnium in minerals of the columbite and wodginite groups from granitic pegmatites. Can. Mineral. 2007, 45, 185–202. [Google Scholar] [CrossRef]
- Zhou, B.; Sun, Y.X.; Kong, D.Y. Geological features and prospecting potential of rare metallic deposits in the Dahongliutan region, Xinjiang. Acta Geol. Sichuan 2011, 31, 288–292. (In Chinese) [Google Scholar]
- Yan, Q.-H.; Qiu, Z.-W.; Wang, H.; Wang, M.; Wei, X.-P.; Li, P.; Zhang, R.-Q.; Li, C.-Y.; Liu, J.P. Age of the Dahongliutan rare metal pegmatite deposit, West Kunlun, Xinjiang (NW China): Constraints from LA–ICP–MS U–Pb dating of columbite-(Fe) and cassiterite. Ore Geol. Rev. 2016. [Google Scholar] [CrossRef]
- Černý, P.; Ercit, T.S. The classification of granitic pegmatites revisited. Can. Mineral. 2005, 43, 2005–2026. [Google Scholar] [CrossRef]
- Xiao, W.J.; Han, F.L.; Windley, B.F.; Yuan, C.; Zhou, H.; Li, J.L. Multiple accretionary orogenesis and episodic growth of continents: Insights from the western Kunlun Range, Central Asia. Int. Geol. Rev. 2003, 45, 303–328. [Google Scholar] [CrossRef]
- Xiao, W.J.; Windley, B.F.; Liu, D.Y.; Jian, P.; Liu, C.Z.; Yuan, C.; Sun, M. Accretionary tectonics of the Western Kunlun orogen, China: A Paleozoic-Early Mesozoic, long lived active continental margin with implications for the growth of southern Eurasia. J. Geol. 2005, 113, 687–705. [Google Scholar] [CrossRef]
- Hu, J.; Wang, H.; Huang, C.Y.; Tong, L.X.; Mu, S.L.; Qiu, Z.W. Geological characteristics and age of the Dahongliutan Fe-ore deposit in the Western Kunlun orogenic belt, Xinjiang, northwestern China. J. Asian Earth Sci. 2015, 116, 1–25. [Google Scholar] [CrossRef]
- Deng, W.M. The geologic characteristics of the ophiolites in the Karakoram- West Kunlun region and their tectonic significance. Acta Petrol. Sin. 1995, 11, 98–111. [Google Scholar]
- Yin, J.X.; Bian, Q.T. Geological Map of the Karakoram-West Kunlun and Adjacent Regions; Science Press: Beijing, China, 1995. [Google Scholar]
- Pan, Y.S. Geological Evolution of the Karakorum and Kunlun Mountains; Seismological Press: Beijing, China, 1996. (In Chinese) [Google Scholar]
- Ding, D.G.; Wang, D.X.; Liu, W.X. The Western Kunlun Orogenic Belt and Basin; Geological Publishing House: Beijing, China, 1996; pp. 1–224. (In Chinese) [Google Scholar]
- Mattern, F.; Schneider, W. Suturing of the Proto- and Paleo-Tethys oceans in the Western Kunlun (Xijiang, China). J. Asian Earth Sci. 2000, 18, 637–650. [Google Scholar] [CrossRef]
- Huang, C.Y. Geological Characteristics and Genesis of the Iron Ore Deposit in the Bulunkuole Group, West Kunlun, Xinjiang. Ph.D. Thesis, Guangzhou Institute of Geochemistry Chinese Academy of Sciences, Guangdong, China, 2014. Unpublished (In Chinese). [Google Scholar]
- Jiang, Y.H.; Jia, R.Y.; Liu, Z.; Liao, S.Y.; Zhao, P.; Zhou, P. Origin of middle Triassic high-K calc-alkaline granitoids and their potassic microgranular enclaves from the Western Kunlun orogen, northwest China: A record of the closure of Paleo Tethys. Lithos 2013, 156, 13–30. [Google Scholar] [CrossRef]
- Xu, Z.; Qi, X.; Yang, J.; Ji, S.; Li, H.; Chen, F. Senses and timings of two kinds of shear in the Kangxiwar strike-slip shear zone, West Kunlun, and their tectonic significance. Geol. Bull. China 2007, 26, 1252–1261. (In Chinese) [Google Scholar]
- Qiao, G.B.; Zhang, H.D.; Wu, Y.Z.; Jin, M.S.; Du, W.; Zhao, X.J.; Chen, D.H. Petrogenesis of the Dahongliutan monzogranite in western Kunlun: Constraints from SHRIMP zircon U-Pb geochronology and geochemical characteristics. Acta Geol. Sin. 2015, 89, 1180–1194. (In Chinese) [Google Scholar]
- Wei, X.; Wang, H.; Hu, J.; Mu, S.; Qiu, Z.; Yan, Q.; Li, P. Geochemistry and geochronology of the Dahongliutan two-mica granite pluton in western Kunlun orogen: Geotectonic implications. Geochimica 2017, 46, 66–80. (In Chinese) [Google Scholar]
- Li, S.Z.; Jahn, B.; Zhao, S.J.; Dai, L.M.; Li, X.Y.; Suo, Y.H.; Guo, L.L.; Wang, Y.M.; Liu, X.C.; Lan, H.Y.; et al. Triassic southeastward subduction of North China Block to South China Block: Insights from new geological, geophysical and geochemical data. Earth-Sci. Rev. 2017. [Google Scholar] [CrossRef]
- London, D. Pegmatites; The Canadian Mineralogist; Special Publication: London, UK, 2008; p. 347. [Google Scholar]
- Fransolet, A.M.; Keller, P.; Fontan, F. The phoshate mineral associations of the Tsaobismund pegmatite, Namibia. Contrib. Mineral. Petrol. 1986, 92, 502–517. [Google Scholar] [CrossRef]
- Baijot, M.; Hatert, F.; Philippo, S. Mineralogy and geochemistry of phosphates and silicates in the Sapucaia pegmatite, Minas Gerais, Brazil: Genetic implications. Can. Mineral. 2012, 50, 1531–1554. [Google Scholar] [CrossRef]
- Vignola, P.; Diella, V.; Oppizzi, P.; Tiepolo, M.; Weiss, S. Phosphate assemblages from the Brissago granitic pegmatite, western Southern Alps, Switzerland. Can. Mineral. 2008, 46, 635–650. [Google Scholar] [CrossRef]
- Charoy, B.; Lhote, F.; Dusausoy, Y.; Noronha, F. The crystal chemistry of spodumene in some granitic aplite-pegmatite bodies of northern Portugal; a comparative review. Can. Mineral. 1992, 30, 639–651. [Google Scholar]
- Hensen, B.J. Mineralogy and petrography of some tin, lithium and beryllium bearing albite-pegmatites near Doade, Galicia, Spain. Leidse Geol. Mededel. 1967, 39, 249–259. [Google Scholar]
- Černý, P.; Ferguson, R.B. The Tanco pegmatite at Bernic Lake, Manitoba; IV, Petalite and spodumene relations. Can. Mineral. 1972, 11, 660–678. [Google Scholar]
- Geisler, T.; Schaltegger, U.; Tomaschek, F. Re-equilibration of Zircon in Aqueous Fluids and Melts. Elements 2007, 3, 43–50. [Google Scholar] [CrossRef]
- Černý, P. Exploration strategy and methods for pegmatite deposits of tantalum. In Lanthanides, Tantalum and Niobium; Springer: Berlin/Heidelberg, Germany, 1989; pp. 274–302. [Google Scholar]
- Černý, P.; Chapman, R.; Ferreira, K.; Smeds, S.-A. Geochemistry of oxide minerals of Nb, Ta, Sn, and Sb in the Varuträsk granitic pegmatite, Sweden: The case of an “anomalous” columbite–tantalite trend. Am. Mineral. 2004, 89, 505–518. [Google Scholar] [CrossRef]
- Tindle, A.G.; Breaks, F.W. Oxide minerals of the Separation Rapids rare-element granitic pegmatite group, northwestern Ontario. Can. Mineral. 1998, 36, 609–635. [Google Scholar]
- Hatert, F.; Roda-Robles, E.; Ottolini, L.; Schmid-Beurmann, P.; Baijot, M.; Dal Bo, F. Triphylite–Sarcopside Miscibility Gap in the FeO–MnO–Li2O–P2O5–H2O System: Experimental Investigation and Thermometric Application to Granitic Pegmatites. Can. Mineral. 2016, 54, 827–845. [Google Scholar] [CrossRef]
- Akoh, J.U.; Ogunleye, P.O.; Ibrahim, A.A. Geochemical evolution of micas and Sn-, Nb-, Ta-mineralization associated with the rare metal pegmatite in Angwan Doka, central Nigeria. J. Afr. Earth Sci. 2015, 112, 24–36. [Google Scholar] [CrossRef]
- Dewaele, S.; Hulsbosch, N.; Cryns, Y.; Boyce, A.; Burgess, R.; Muchez, P. Geological setting and timing of the world-class Sn, Nb–Ta and Li mineralization of Manono-Kitotolo (Katanga, Democratic Republic of Congo). Ore Geol. Rev. 2016, 72, 373–390. [Google Scholar] [CrossRef]
- Van Lichtervelde, M.; Salvi, S.; Beziat, D.; Linnen, R.L. Textural features and chemical evolution in tantalum oxides: Magmatic versus hydrothermal origins for Ta mineralization in the Tanco Lower pegmatite, Manitoba, Canada. Econ. Geol. 2007, 102, 257–276. [Google Scholar] [CrossRef]
- Tindle, A.G.; Breaks, F.W.; Webb, P.C. Wodginite-group minerals from the Separation Rapids rare-element granitic pegmatite group, northwestern Ontario. Can. Mineral. 1998, 36, 637–658. [Google Scholar]
- Linnen, R.; Cuney, M. Granite-related rare-element deposits and experimental constraints on Ta–Nb–W–Sn–Zr–Hf mineralization. In Rare-Element Geochemistry and Mineral Deposits; GAC Short Course Notes 17; Geological Association of Canada: Ottawa, ON, Canada, 2005; pp. 45–68. [Google Scholar]
- Van Lichtervelde, M.; Holtz, F.; Melcher, F. The effect of disequilibrium crystallization on Nb-Ta fractionation in pegmatites: Constraints from crystallization experiments of tantalite-tapiolite. Am. Mineral. J. Earth Planet. Mater. 2018, 103, 1401–1416. [Google Scholar] [CrossRef]
- Möller, P.; Dulski, P. Fractionation of Zr and Hf in cassiterite. Chem. Geol. 1983, 40, 1–12. [Google Scholar] [CrossRef]
- Möller, P.; Dulski, P.; Szacki, W.; Malow, G.; Riedel, E. Substitution of tin in cassiterite by tantalum, niobium, tungsten, iron and manganese. Geochim. Cosmochim. Acta 1988, 52, 1497–1503. [Google Scholar] [CrossRef]
- Galliski, M.A.; Marquez-Zavalía, M.F.; Cerný, P.; Martínez, V.A.; Chapman, R. The Ta–Nb–Sn–Ti oxide-mineral paragenesis from La Viquita, a spodumene-bearing rare-element granitic pegmatite, San Luis, Argentina. Can. Mineral. 2008, 46, 379–393. [Google Scholar] [CrossRef]
- Novak, M.; Černý, P. Niobium-tantalum oxide minerals from complex granitic pegmatites in the Moldanubicum, Czech Republic; primary versus secondary compositional trends. Can. Mineral. 1998, 36, 659–672. [Google Scholar]
- Černý, P.; Němec, D. Pristine vs. contaminated trends in Nb, Ta-oxide minerals of the Jihlava pegmatite district, Czech Republic. Mineral. Petrol. 1995, 55, 117–129. [Google Scholar]
- Wise, M.A.; Černý, P.; Falster, A.U. Scandium substitution in columbite-group minerals and ixiolite. Can. Mineral. 1998, 36, 673–680. [Google Scholar]
- Linnen, R.L. Discussion on change in Sn activity and formation of wodginite-group minerals during pegmatite evolution. Personal communication, 30 January 2019. [Google Scholar]
- Linnen, R.L. Depth of emplacement, fluid provenance and metallogeny in granitic terranes: A comparison of western Thailand with other tin belts. Miner. Depos. 1998, 33, 461–476. [Google Scholar] [CrossRef]

| Pegmatite | No. 503 | No. 503 | No. 503 | No. 503 | No. 503 | No. 503 | No. 91 | No. 91 | ||||||
| Type | 1 | 1 | 2 | 2 | 2 | 2 | 2 | 2 | ||||||
| mean | sd | mean | sd | mean | sd | mean | sd | mean | sd | mean | sd | |||
| n | 1 | 1 | 10 | 7 | 8 | 4 | 4 | 3 | ||||||
| Comments | CGM, c | CGM, r | CGM, c | CGM, m | CGM, r | unzoned CGM | CGM, c | CGM, r | ||||||
| FeOt | 10.67 | 10.83 | 10.25 | 0.89 | 10.35 | 0.90 | 10.17 | 0.65 | 11.25 | 0.09 | 11.58 | 1.40 | 10.91 | 0.50 |
| CaO | 0.03 | 0.02 | 0.02 | 0.01 | 0.01 | 0.01 | 0.01 | 0.00 | 0.02 | n.a. | 0.00 | 0.00 | b.d. | n.a |
| MnO | 9.32 | 9.61 | 9.80 | 0.90 | 9.33 | 1.07 | 9.70 | 0.55 | 8.99 | 0.28 | 8.03 | 1.84 | 6.92 | 2.45 |
| Al2O3 | 0.00 | 0.04 | 0.02 | 0.02 | 0.03 | 0.02 | 0.02 | 0.02 | 0.03 | 0.01 | 0.02 | n.a. | 0.03 | 0.03 |
| Sc2O3 | 0.08 | 0.08 | 0.10 | 0.04 | 0.12 | 0.05 | 0.12 | 0.08 | 0.07 | 0.06 | 0.17 | 0.10 | 0.33 | 0.18 |
| TiO2 | 0.03 | 0.10 | 0.18 | 0.17 | 0.36 | 0.24 | 0.12 | 0.05 | 0.27 | 0.05 | 0.20 | 0.01 | 0.14 | 0.06 |
| SiO2 | b.d. | b.d. | 0.02 | 0.01 | 0.00 | n.a. | 0.05 | 0.08 | b.d. | n.a. | 0.05 | 0.05 | 0.44 | 0.00 |
| SnO2 | 0.06 | 0.13 | 0.09 | 0.06 | 0.20 | 0.11 | 0.06 | 0.04 | 0.08 | 0.02 | 0.14 | 0.05 | 0.12 | 0.04 |
| Nb2O5 | 62.09 | 67.73 | 61.79 | 3.58 | 60.24 | 2.79 | 59.82 | 7.18 | 67.06 | 1.14 | 58.35 | 6.62 | 35.29 | 24.05 |
| Ta2O5 | 14.58 | 9.23 | 15.13 | 2.82 | 16.37 | 3.01 | 18.26 | 7.61 | 9.55 | 0.24 | 19.83 | 7.90 | 43.56 | 26.35 |
| WO3 | 0.27 | 0.15 | 0.28 | 0.17 | 0.37 | 0.11 | 0.35 | 0.13 | 0.41 | 0.07 | 0.18 | 0.04 | 0.31 | 0.11 |
| F | b.d. | b.d. | 0.03 | 0.01 | b.d. | n.a. | 0.05 | n.a. | b.d. | n.a. | 0.12 | 0.06 | b.d. | n.a |
| Total | 97.14 | 97.93 | 97.64 | 1.71 | 97.38 | 0.94 | 98.60 | 1.30 | 97.71 | 0.88 | 98.46 | 1.27 | 97.83 | 1.17 |
| a.p.f.u. | ||||||||||||||
| Fe2+ | 0.550 | 0.540 | 0.526 | 0.041 | 0.536 | 0.049 | 0.523 | 0.026 | 0.563 | 0.003 | 0.603 | 0.086 | 0.644 | 0.098 |
| Ca2+ | 0.002 | 0.002 | 0.001 | 0.001 | 0.000 | 0.001 | 0.000 | 0.000 | 0.000 | 0.001 | 0.000 | 0.000 | 0.000 | 0.000 |
| Mn2+ | 0.486 | 0.486 | 0.510 | 0.051 | 0.489 | 0.053 | 0.506 | 0.030 | 0.456 | 0.017 | 0.421 | 0.087 | 0.402 | 0.091 |
| Al3+ | 0.000 | 0.003 | 0.001 | 0.001 | 0.003 | 0.002 | 0.001 | 0.001 | 0.002 | 0.001 | 0.000 | 0.001 | 0.002 | 0.002 |
| Sc3+ | 0.004 | 0.004 | 0.006 | 0.002 | 0.006 | 0.003 | 0.007 | 0.005 | 0.004 | 0.003 | 0.009 | 0.006 | 0.021 | 0.013 |
| Ti4+ | 0.001 | 0.004 | 0.008 | 0.008 | 0.017 | 0.011 | 0.006 | 0.002 | 0.012 | 0.002 | 0.009 | 0.001 | 0.008 | 0.004 |
| Si4+ | 0.000 | 0.000 | 0.000 | 0.001 | 0.000 | 0.000 | 0.001 | 0.003 | 0.000 | 0.000 | 0.003 | 0.003 | 0.022 | 0.019 |
| Sn4+ | 0.002 | 0.003 | 0.002 | 0.002 | 0.005 | 0.003 | 0.002 | 0.001 | 0.002 | 0.000 | 0.003 | 0.001 | 0.003 | 0.001 |
| Nb5+ | 1.730 | 1.826 | 1.714 | 0.057 | 1.684 | 0.055 | 1.661 | 0.151 | 1.814 | 0.011 | 1.636 | 0.145 | 1.065 | 0.593 |
| Ta5+ | 0.244 | 0.150 | 0.254 | 0.054 | 0.276 | 0.054 | 0.310 | 0.144 | 0.156 | 0.006 | 0.338 | 0.142 | 0.869 | 0.563 |
| W6+ | 0.004 | 0.002 | 0.005 | 0.003 | 0.006 | 0.002 | 0.005 | 0.003 | 0.006 | 0.001 | 0.001 | 0.002 | 0.006 | 0.002 |
| F− | 0.000 | 0.000 | 0.001 | 0.002 | 0.000 | 0.000 | 0.001 | 0.004 | 0.000 | 0.000 | 0.012 | 0.016 | 0.000 | 0.000 |
| Mn/(Mn + Fe) | 0.47 | 0.47 | 0.49 | 0.04 | 0.48 | 0.05 | 0.49 | 0.03 | 0.45 | 0.01 | 0.41 | 0.08 | 0.38 | 0.09 |
| Ta/(Ta + Nb) | 0.12 | 0.08 | 0.13 | 0.03 | 0.14 | 0.03 | 0.16 | 0.07 | 0.08 | 0.00 | 0.17 | 0.07 | 0.45 | 0.30 |
| Pegmatite | No. 90 * | No. 90 | No. 90 | No. 90 | No. 90 | No. 90 | No. 90 | |||||||
| Type | 2 | 3 | 3 | 3 | 4 | 4 | 4 | |||||||
| mean | sd | mean | sd | mean | sd | mean | sd | mean | sd | mean | sd | |||
| n | 31 | 3 | 3 | 4 | 2 | 2 | 1 | |||||||
| Comments | CGM, c | CGM, m | CGM, r | fwdn, r | fwdn, c | unzoned CGM | ||||||||
| FeOt | 12.17 | 0.71 | 2.09 | 0.28 | 1.49 | 0.18 | 5.16 | 2.27 | 9.05 | 0.40 | 9.43 | 0.14 | 10.79 | |
| CaO | n.a. | 0.03 | 0.01 | 0.03 | 0.02 | 0.01 | 0.01 | 0.07 | 0.01 | 0.05 | 0.00 | 0.01 | ||
| MnO | 5.73 | 0.91 | 16.69 | 0.53 | 17.13 | 0.30 | 12.78 | 2.85 | 3.68 | 0.12 | 3.67 | 0.05 | 5.50 | |
| Al2O3 | n.a. | 0.02 | n.a. | 0.04 | 0.02 | 0.02 | 0.00 | 0.14 | 0.00 | 0.09 | 0.01 | b.d. | ||
| Sc2O3 | 0.10 | 0.03 | 0.23 | 0.09 | 0.26 | 0.06 | 0.38 | 0.10 | 0.36 | 0.03 | 0.35 | 0.06 | 0.38 | |
| TiO2 | 0.70 | 0.11 | 0.12 | 0.13 | 0.00 | n.a. | 0.38 | 0.11 | 1.63 | 0.49 | 3.27 | 2.47 | 0.37 | |
| SiO2 | n.a. | 0.13 | 0.08 | 0.11 | 0.03 | 0.19 | 0.09 | 0.44 | 0.01 | 0.42 | 0.01 | 0.41 | ||
| SnO2 | 0.09 | 0.05 | 0.02 | 0.01 | 0.03 | 0.02 | 0.09 | 0.08 | 12.71 | 1.14 | 11.48 | 1.46 | 0.11 | |
| Nb2O5 | 64.12 | 4.42 | 49.25 | 7.46 | 47.25 | 4.87 | 39.23 | 7.67 | 9.64 | 0.76 | 10.14 | 0.94 | 22.92 | |
| Ta2O5 | 15.65 | 4.67 | 29.76 | 7.89 | 32.07 | 4.34 | 39.82 | 7.82 | 58.60 | 1.34 | 58.27 | 1.99 | 56.91 | |
| WO3 | 1.10 | 0.23 | 0.24 | 0.10 | 0.23 | 0.01 | 0.26 | 0.05 | 0.28 | 0.17 | 0.24 | 0.21 | 0.27 | |
| F | n.a. | b.d. | n.a. | 0.11 | n.a. | 0.10 | 0.04 | b.d. | n.a. | b.d. | n.a. | 0.10 | ||
| Total | 99.66 | 0.87 | 98.47 | 0.34 | 98.67 | 0.75 | 98.29 | 1.10 | 96.58 | 1.13 | 97.41 | 0.21 | 97.75 | |
| a.p.f.u. | ||||||||||||||
| Fe2+ | 0.613 | 0.038 | 0.113 | 0.012 | 0.081 | 0.009 | 0.298 | 0.135 | 0.598 | 0.027 | 0.608 | 0.007 | 0.675 | |
| Ca2+ | n.a. | 0.002 | 0.001 | 0.002 | 0.001 | 0.001 | 0.001 | 0.006 | 0.000 | 0.004 | 0.000 | 0.001 | ||
| Mn2+ | 0.292 | 0.043 | 0.916 | 0.010 | 0.949 | 0.023 | 0.734 | 0.134 | 0.246 | 0.008 | 0.240 | 0.009 | 0.348 | |
| Al3+ | n.a. | 0.001 | 0.001 | 0.003 | 0.001 | 0.001 | 0.001 | 0.013 | 0.000 | 0.008 | 0.001 | 0.000 | ||
| Sc3+ | 0.005 | 0.002 | 0.013 | 0.006 | 0.015 | 0.004 | 0.023 | 0.007 | 0.025 | 0.002 | 0.024 | 0.005 | 0.025 | |
| Ti4+ | 0.032 | 0.005 | 0.004 | 0.006 | 0.000 | 0.000 | 0.015 | 0.011 | 0.097 | 0.030 | 0.188 | 0.138 | 0.021 | |
| Si4+ | n.a. | 0.006 | 0.006 | 0.007 | 0.002 | 0.013 | 0.006 | 0.035 | 0.001 | 0.032 | 0.002 | 0.030 | ||
| Sn4+ | 0.003 | 0.001 | 0.000 | 0.000 | 0.001 | 0.001 | 0.002 | 0.002 | 0.400 | 0.035 | 0.354 | 0.055 | 0.003 | |
| Nb5+ | 1.744 | 0.088 | 1.439 | 0.171 | 1.395 | 0.102 | 1.204 | 0.186 | 0.345 | 0.028 | 0.353 | 0.023 | 0.774 | |
| Ta5+ | 0.258 | 0.083 | 0.528 | 0.161 | 0.572 | 0.095 | 0.742 | 0.165 | 1.260 | 0.027 | 1.223 | 0.075 | 1.157 | |
| W6+ | 0.017 | 0.004 | 0.004 | 0.002 | 0.004 | 0.000 | 0.005 | 0.001 | 0.006 | 0.004 | 0.005 | 0.004 | 0.005 | |
| F− | n.a. | 0.000 | 0.000 | 0.007 | 0.013 | 0.015 | 0.012 | 0.000 | 0.000 | 0.000 | 0.000 | 0.023 | ||
| Mn/(Mn + Fe) | 0.32 | 0.04 | 0.89 | 0.01 | 0.92 | 0.01 | 0.71 | 0.13 | 0.29 | 0.02 | 0.28 | 0.01 | 0.34 | |
| Ta/(Ta + Nb) | 0.13 | 0.04 | 0.27 | 0.08 | 0.29 | 0.05 | 0.38 | 0.09 | 0.79 | 0.02 | 0.78 | 0.02 | 0.60 |
| Pegmatite | No. 91 | No. 91 | No.91 | No. 90 | No. 90 | |||||
| Mean | sd | Mean | sd | Mean | sd | Mean | sd | Mean | sd | |
| n | 2 | 4 | 5 | 4 | 6 | |||||
| Comments | c | m | r | c | m | |||||
| FeOt | 0.40 | 0.35 | 0.60 | 0.26 | 0.39 | 0.15 | 0.45 | 0.71 | 0.12 | 0.16 |
| CaO | 0.58 | 0.01 | 0.59 | 0.02 | 0.54 | 0.07 | 0.59 | 0.07 | 0.59 | 0.03 |
| MnO | 0.05 | n.a. | 0.02 | 0.00 | 0.08 | n.a. | 0.04 | 0.05 | 0.02 | 0.01 |
| Al2O3 | 0.14 | 0.07 | 0.16 | 0.09 | 0.10 | 0.05 | 0.09 | 0.04 | 0.05 | 0.04 |
| Sc2O3 | b.d. | n.a. | b.d. | n.a. | b.d. | n.a. | b.d. | n.a. | b.d. | n.a. |
| TiO2 | 0.05 | 0.01 | 0.03 | 0.04 | 0.05 | 0.04 | 0.06 | 0.01 | 0.04 | 0.03 |
| SiO2 | 0.50 | 0.11 | 0.38 | 0.03 | 0.38 | 0.06 | 0.47 | 0.10 | 0.45 | 0.10 |
| SnO2 | 94.61 | 2.93 | 93.89 | 1.77 | 94.31 | 1.05 | 95.69 | 3.86 | 96.03 | 0.92 |
| Nb2O5 | 0.83 | 0.06 | 1.02 | 0.50 | 0.69 | 0.50 | 0.18 | 0.27 | 0.09 | 0.09 |
| Ta2O5 | 1.37 | 1.70 | 1.66 | 0.86 | 1.35 | 0.16 | 2.11 | 3.40 | 0.60 | 0.79 |
| WO3 | b.d. | n.a. | 0.02 | 0.01 | b.d. | n.a. | b.d. | n.a. | b.d. | n.a. |
| F | b.d. | n.a. | 0.11 | n.a. | b.d. | n.a. | b.d. | n.a. | b.d. | n.a. |
| Total | 98.51 | 0.84 | 98.38 | 0.65 | 97.77 | 1.17 | 99.48 | 0.96 | 97.75 | 0.64 |
| based on 2 oxygen atoms | ||||||||||
| Fe2+ | 0.008 | 0.007 | 0.013 | 0.006 | 0.008 | 0.003 | 0.007 | 0.013 | 0.001 | 0.002 |
| Ca2+ | 0.016 | 0.000 | 0.016 | 0.001 | 0.015 | 0.002 | 0.016 | 0.002 | 0.016 | 0.001 |
| Mn2+ | 0.001 | 0.001 | 0.000 | 0.000 | 0.000 | 0.001 | 0.001 | 0.001 | 0.000 | 0.000 |
| Al3+ | 0.004 | 0.002 | 0.005 | 0.003 | 0.002 | 0.002 | 0.002 | 0.002 | 0.001 | 0.001 |
| Sc3+ | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Ti4+ | 0.001 | 0.000 | 0.000 | 0.001 | 0.000 | 0.001 | 0.001 | 0.000 | 0.001 | 0.001 |
| Si4+ | 0.013 | 0.003 | 0.009 | 0.001 | 0.010 | 0.001 | 0.012 | 0.002 | 0.012 | 0.003 |
| Sn4+ | 0.948 | 0.019 | 0.944 | 0.014 | 0.955 | 0.012 | 0.954 | 0.038 | 0.974 | 0.007 |
| Nb5+ | 0.009 | 0.001 | 0.012 | 0.006 | 0.008 | 0.006 | 0.002 | 0.003 | 0.001 | 0.001 |
| Ta5+ | 0.009 | 0.012 | 0.011 | 0.006 | 0.009 | 0.001 | 0.014 | 0.023 | 0.003 | 0.005 |
| W6+ | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| F− | 0.000 | 0.000 | 0.002 | 0.004 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Mn/(Mn + Fe) | 0.03 | 0.05 | 0.02 | 0.02 | 0.02 | 0.06 | 0.13 | 0.14 | 0.35 | 0.47 |
| Ta/(Ta + Nb) | 0.38 | 0.37 | 0.49 | 0.04 | 0.60 | 0.21 | 0.92 | 0.05 | 0.71 | 0.40 |
| Pegmatite | No. 90 | No. 503 | No. 503 | No. 503 | ||||||
| mean | sd | mean | sd | mean | sd | mean | sd | |||
| n | 5 | 8 | 7 | 9 | ||||||
| Comments | r | c | m | r | ||||||
| FeOt | 0.11 | 0.06 | 0.85 | 0.68 | 0.45 | 0.29 | 0.39 | 0.39 | ||
| CaO | 0.58 | 0.04 | 0.49 | 0.05 | 0.54 | 0.05 | 0.50 | 0.07 | ||
| MnO | b.d. | n.a. | 0.30 | 0.36 | 0.04 | 0.04 | 0.27 | 0.37 | ||
| Al2O3 | 0.16 | n.a. | 0.04 | 0.05 | 0.07 | 0.06 | 0.10 | 0.10 | ||
| Sc2O3 | b.d. | n.a. | b.d. | n.a. | b.d. | n.a. | b.d. | n.a. | ||
| TiO2 | 0.07 | 0.06 | 0.11 | 0.16 | 0.13 | 0.07 | 0.07 | 0.09 | ||
| SiO2 | 0.47 | 0.02 | 0.31 | 0.04 | 0.31 | 0.03 | 0.35 | 0.03 | ||
| SnO2 | 96.26 | 0.63 | 90.87 | 4.47 | 94.45 | 1.43 | 92.98 | 4.58 | ||
| Nb2O5 | 0.28 | 0.19 | 2.13 | 1.75 | 1.10 | 0.84 | 1.37 | 1.58 | ||
| Ta2O5 | 0.47 | 0.28 | 3.49 | 1.67 | 0.39 | 0.39 | 1.83 | 2.32 | ||
| WO3 | 0.07 | 0.05 | 0.15 | n.a. | 0.23 | n.a. | 0.03 | 0.01 | ||
| F | 0.16 | n.a. | 0.24 | 0.17 | b.d. | n.a. | 0.18 | n.a. | ||
| Total | 98.29 | 0.58 | 97.67 | 1.27 | 97.47 | 0.46 | 97.75 | 0.87 | ||
| based on 2 oxygen atoms | ||||||||||
| Fe2+ | 0.002 | 0.001 | 0.018 | 0.014 | 0.010 | 0.006 | 0.008 | 0.008 | ||
| Ca2+ | 0.016 | 0.001 | 0.013 | 0.001 | 0.015 | 0.001 | 0.014 | 0.002 | ||
| Mn2+ | 0.000 | 0.000 | 0.006 | 0.008 | 0.001 | 0.001 | 0.004 | 0.007 | ||
| Al3+ | 0.001 | 0.002 | 0.001 | 0.001 | 0.001 | 0.002 | 0.002 | 0.003 | ||
| Sc3+ | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | ||
| Ti4+ | 0.001 | 0.001 | 0.002 | 0.003 | 0.003 | 0.001 | 0.001 | 0.002 | ||
| Si4+ | 0.012 | 0.001 | 0.008 | 0.001 | 0.008 | 0.001 | 0.009 | 0.001 | ||
| Sn4+ | 0.970 | 0.008 | 0.921 | 0.048 | 0.957 | 0.019 | 0.941 | 0.049 | ||
| Nb5+ | 0.003 | 0.002 | 0.024 | 0.019 | 0.013 | 0.010 | 0.016 | 0.018 | ||
| Ta5+ | 0.003 | 0.002 | 0.017 | 0.015 | 0.003 | 0.003 | 0.013 | 0.016 | ||
| W6+ | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.001 | 0.000 | 0.000 | ||
| F− | 0.002 | 0.006 | 0.008 | 0.013 | 0.000 | 0.000 | 0.002 | 0.005 | ||
| Mn/(Mn + Fe) | 0.00 | 0.00 | 0.23 | 0.19 | 0.06 | 0.06 | 0.21 | 0.26 | ||
| Ta/(Ta + Nb) | 0.64 | 0.24 | 0.30 | 0.25 | 0.23 | 0.25 | 0.48 | 0.26 | ||
| Zones | Wall Zone | Spd-Ms-Qtz Zone | ||
|---|---|---|---|---|
| Mean | sd | Mean | sd | |
| n | 22 | 18 | ||
| MgO | 0.82 | 0.07 | 0.47 | 0.05 |
| MnO | 17.43 | 0.27 | 18.34 | 0.27 |
| P2O5 | 44.10 | 0.97 | 43.91 | 0.63 |
| Al2O3 | 0.02 | 0.01 | 0.01 | 0.01 |
| FeO | 27.57 | 0.31 | 26.57 | 0.60 |
| Nb2O5 | 0.10 | 0.06 | 0.09 | 0.05 |
| SiO2 | 0.06 | 0.04 | 0.06 | 0.03 |
| Ta2O5 | 0.05 | 0.05 | 0.04 | 0.03 |
| SnO2 | 0.01 | 0.01 | 0.01 | 0.02 |
| WO3 | 0.05 | 0.06 | 0.03 | 0.04 |
| CaO | 0.31 | 0.22 | 0.36 | 0.23 |
| ZnO | 0.40 | 0.12 | 0.25 | 0.06 |
| Sc2O3 | 0.01 | 0.01 | 0.02 | 0.01 |
| TiO2 | 0.005 | 0.006 | 0.006 | 0.007 |
| Total | 90.84 | 0.83 | 90.11 | 0.67 |
| * Li2O | 8.01 | 0.74 | 8.21 | 0.63 |
| a.p.f.u. based on P = 1 | ||||
| Mg2+ | 0.0327 | 0.0028 | 0.0187 | 0.0020 |
| Mn2+ | 0.3955 | 0.0082 | 0.4178 | 0.0070 |
| P5+ | 1.0000 | 0.0000 | 1.0000 | 0.0000 |
| Al3+ | 0.0004 | 0.0004 | 0.0003 | 0.0004 |
| Fe2+ | 0.6179 | 0.0176 | 0.5981 | 0.0187 |
| Nb5+ | 0.0009 | 0.0008 | 0.0010 | 0.0007 |
| Si4+ | 0.0016 | 0.0010 | 0.0015 | 0.0008 |
| Ta5+ | 0.0002 | 0.0003 | 0.0001 | 0.0002 |
| Sn4+ | 0.0000 | 0.0001 | 0.0001 | 0.0001 |
| W6+ | 0.0002 | 0.0004 | 0.0001 | 0.0002 |
| Ca2+ | 0.0089 | 0.0065 | 0.0106 | 0.0067 |
| Zn2+ | 0.0080 | 0.0025 | 0.0051 | 0.0013 |
| Sc3+ | 0.0001 | 0.0002 | 0.0003 | 0.0003 |
| Ti4+ | 0.0000 | 0.0001 | 0.0001 | 0.0001 |
| O2− | 3.5690 | 0.0308 | 3.5560 | 0.0288 |
| O difference | 0.4310 | 0.0308 | 0.4440 | 0.0288 |
| Li+ | 0.8621 | 0.0616 | 0.8881 | 0.0575 |
| Pegmatite | No. 90 | No. 90 | No. 90 | No. 90 | No. 90 | No. 90 | No. 90 | No. 90 | No. 90 | No. 90 | No. 90 | No. 90 | No. 90 | No. 90 | No. 503 |
| Analysis # | 90-1.17 | 90-1.24 | 90-1.18 | 90-1.19 | 90-1.25 | 90-1.20 | 90-1.21 | 90-1.22 | 90-1.23 | 90-1.26 | D026.1 | D026.2 | D026.3 | D026.4 | 503-4.1 |
| Types | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 4 | 4 | 4 | 4 | 2 |
| Comments | clb1. c | clb3. c | clb1. m | clb1. m | clb3. m | clb1. r | clb2. r | clb2. r | clb2. r | clb3. r | Fwdn2. c | Fwdn1. r | Fwdn1. r | ttl1 | clb7. c |
| in ppm | |||||||||||||||
| Li | 30.8 | 10.1 | 12.2 | 5.5 | 9.5 | 18.4 | 12.6 | 19.6 | 39.6 | 9.7 | 76.4 | 75.4 | 63.6 | 14.5 | 170.2 |
| Be | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | 0.6 | 0.7 | 0.1 | 0.1 | 1.0 |
| Na | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | 9.7 | b.d. | 70.9 |
| Mg | 55.7 | 23.1 | 31.4 | 17.3 | 38.6 | 46.3 | 39.3 | 34.2 | 70.4 | 32.4 | 349.5 | 392.8 | 385.4 | 505.7 | 19.5 |
| Al | 14 | 4 | 5 | b.d. | 5 | 7 | 6 | 6 | 10 | b.d. | 728 | 480 | 563 | 127 | 9495 |
| Si | b.d. | b.d. | b.d. | b.d. | b.d. | 2294 | b.d. | b.d. | b.d. | b.d. | 1511 | b.d. | b.d. | b.d. | 10668 |
| P | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | 110 | 81 | 59 | b.d. | b.d. | 74 |
| Ca | b.d. | 153 | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | 501 | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. |
| Sc | 7.9 | 2.3 | 3.5 | 5.4 | 4.2 | 6.8 | 2.7 | 1.8 | 19.5 | 6.1 | 128.1 | 125.4 | 82.1 | 88.4 | 1.8 |
| Ti | 547 | 262 | 311 | 375 | 361 | 511 | 340 | 326 | 863 | 433 | 19956 | 14455 | 13649 | 6380 | 2005 |
| V | b.d. | b.d. | 0.15 | b.d. | b.d. | 0.08 | 0.11 | 0.17 | 0.80 | 0.35 | 50.31 | 40.94 | 32.77 | 15.30 | 0.09 |
| Mn | 209,711 | 114,588 | 138,484 | 152,602 | 148,390 | 195,211 | 139,000 | 141,416 | 212,970 | 198,165 | 30,772 | 29,879 | 31,479 | 41,052 | 95,515 |
| Fe | 20,397 | 7261 | 11,569 | 6390 | 9880 | 18,616 | 13,707 | 17,922 | 27,640 | 11,270 | 70,410 | 69,081 | 68,962 | 77,241 | 85,527 |
| Ga | 0.5 | 0.2 | 0.2 | 0.3 | b.d. | 0.2 | 0.1 | 0.3 | 0.4 | 0.4 | 11.9 | 9.4 | 9.4 | 4.6 | 11.0 |
| Rb | 0.2 | b.d. | b.d. | 0.0 | 0.0 | b.d. | 0.2 | b.d. | b.d. | b.d. | 0.2 | b.d. | 1.4 | b.d. | 280.9 |
| Sr | 0.0 | 0.0 | b.d. | 0.1 | 0.0 | b.d. | 0.3 | b.d. | b.d. | b.d. | 0.3 | b.d. | 0.0 | 0.1 | b.d. |
| Y | 1.0 | 0.4 | 0.5 | 0.7 | 0.7 | 1.2 | 0.6 | 0.5 | 1.6 | 0.8 | 0.87 | 0.80 | 0.30 | 0.27 | 1.26 |
| Zr | 99.9 | 36.6 | 52.0 | 51.8 | 38.9 | 91.2 | 50.2 | 61.3 | 212.5 | 42.3 | 3613.8 | 3556.4 | 3727.2 | 1267.0 | 806.1 |
| Nb | 501,129 | 256,503 | 319,236 | 301,711 | 349,605 | 457,895 | 312,615 | 359,817 | 516,526 | 428,286 | 78,627 | 70,719 | 78,657 | 140,593 | 459,525 |
| Sn | 425 | 153 | 191 | 201 | 192 | 269 | 147 | 212 | 675 | 258 | 104055 | 83628 | 81062 | 18248 | 1308 |
| Sb | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | 0.4 | 0.2 | b.d. | b.d. | 0.4 | 0.4 | b.d. | 0.1 | 0.2 |
| Cs | 1.5 | b.d. | 0.2 | 0.2 | 0.3 | 0.4 | 0.5 | 0.3 | 0.6 | 0.3 | 0.3 | 0.1 | 0.5 | 0.2 | 11.7 |
| Ba | b.d. | b.d. | b.d. | b.d. | 0.1 | 0.1 | b.d. | b.d. | b.d. | b.d. | 1.4 | 0.4 | 0.7 | 0.1 | 0.3 |
| La | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | 0.01 | b.d. | b.d. | 0.01 | 0.01 | 0.02 | b.d. | b.d. |
| Ce | 0.01 | b.d. | 0.01 | 0.01 | b.d. | b.d. | b.d. | 0.01 | b.d. | 0.04 | 0.01 | b.d. | b.d. | b.d. | 0.03 |
| Pr | b.d. | b.d. | b.d. | 0.01 | b.d. | b.d. | b.d. | b.d. | 0.01 | 0.03 | 0.01 | 0.02 | 0.02 | 0.02 | b.d. |
| Nd | b.d. | 0.07 | b.d. | b.d. | 0.04 | b.d. | b.d. | 0.03 | b.d. | b.d. | b.d. | b.d. | b.d. | 0.05 | b.d. |
| Sm | 0.05 | b.d. | 0.12 | 0.13 | 0.05 | b.d. | b.d. | b.d. | b.d. | 0.10 | 0.03 | b.d. | b.d. | b.d. | b.d. |
| Eu | 0.01 | b.d. | 0.01 | b.d. | b.d. | 0.02 | b.d. | b.d. | 0.07 | 0.04 | b.d. | b.d. | b.d. | 0.03 | b.d. |
| Gd | 0.05 | b.d. | b.d. | 0.17 | b.d. | b.d. | 0.14 | b.d. | 0.18 | b.d. | 0.05 | b.d. | 0.03 | 0.06 | b.d. |
| Tb | 0.03 | 0.01 | 0.01 | 0.02 | 0.01 | 0.02 | 0.02 | 0.01 | 0.03 | 0.04 | b.d. | 0.03 | b.d. | b.d. | b.d. |
| Dy | 0.15 | 0.05 | 0.19 | 0.15 | 0.10 | 0.04 | b.d. | b.d. | 0.21 | 0.09 | 0.13 | 0.04 | 0.03 | 0.03 | 0.09 |
| Ho | 0.08 | 0.03 | b.d. | 0.02 | 0.02 | b.d. | 0.04 | 0.01 | 0.22 | 0.04 | 0.05 | b.d. | 0.02 | 0.00 | 0.05 |
| Er | 0.21 | 0.10 | 0.21 | 0.19 | b.d. | 0.30 | b.d. | 0.08 | 0.38 | 0.35 | 0.16 | 0.12 | 0.01 | 0.08 | 0.10 |
| Tm | 0.09 | 0.03 | 0.01 | 0.08 | 0.02 | 0.10 | 0.06 | 0.01 | 0.09 | 0.03 | 0.07 | 0.08 | 0.02 | 0.03 | 0.01 |
| Yb | 0.85 | 0.20 | 0.21 | 0.26 | 0.51 | 0.92 | 0.55 | b.d. | 0.65 | b.d. | 0.59 | 0.98 | b.d. | 0.25 | 0.35 |
| Lu | 0.11 | 0.02 | 0.06 | 0.12 | 0.07 | 0.13 | 0.06 | 0.01 | 0.22 | 0.10 | 0.19 | 0.17 | 0.06 | 0.11 | 0.07 |
| Hf | 15.5 | 7.6 | 8.3 | 8.5 | 7.7 | 15.8 | 6.9 | 8.5 | 30.2 | 10.2 | 879.8 | 847.8 | 898.4 | 251.8 | 93.2 |
| Ta | 318,036 | 200,654 | 231,005 | 301,075 | 255,958 | 352,612 | 230,227 | 212,414 | 361,285 | 360,368 | 488,731.9 | 472,115.2 | 487,675.4 | 466,054.9 | 128,658 |
| W | 1791.5 | 845.8 | 1037.9 | 974.9 | 1125.2 | 1652.8 | 1190.9 | 1400.2 | 2358.0 | 1368.2 | 552.9 | 558.8 | 634.7 | 562.5 | 2310.5 |
| Tl | 0.1 | 0.1 | 0.1 | 0.1 | 0.0 | b.d. | b.d. | 0.1 | b.d. | b.d. | 0.15 | 0.08 | 0.18 | b.d. | 3.3 |
| Pb | 26.4 | 7.0 | 10.4 | 12.4 | 9.5 | 15.7 | 7.3 | 9.0 | 42.2 | 12.2 | 24.3 | 16.6 | 23.3 | 53.9 | 42.3 |
| Bi | b.d. | b.d. | 0.11 | b.d. | 0.06 | 0.11 | 0.05 | 0.10 | b.d. | b.d. | 0.23 | 0.07 | 0.06 | b.d. | 0.13 |
| Th | 0.7 | 0.2 | 0.3 | 0.4 | 0.2 | 0.7 | 0.4 | 0.3 | 1.0 | 0.4 | 0.7 | 0.2 | 0.4 | 1.3 | 1.4 |
| U | 105.2 | 50.2 | 59.3 | 73.7 | 59.0 | 108.7 | 54.3 | 55.2 | 202.7 | 78.6 | 139.0 | 126.8 | 154.4 | 333.3 | 307.7 |
| ΣREE | 1.66 | 0.51 | 0.83 | 1.15 | 0.81 | 1.53 | 0.88 | 0.17 | 2.05 | 0.86 | 1.29 | 1.47 | 0.20 | 0.65 | 0.70 |
| Nb/Ta | 1.58 | 1.28 | 1.38 | 1.00 | 1.37 | 1.30 | 1.36 | 1.69 | 1.43 | 1.19 | 0.16 | 0.15 | 0.16 | 0.30 | 3.57 |
| Zr/Hf | 6.43 | 4.81 | 6.23 | 6.08 | 5.07 | 5.76 | 7.29 | 7.21 | 7.04 | 4.14 | 4.11 | 4.19 | 4.15 | 5.03 | 8.65 |
| Sn/W | 0.24 | 0.18 | 0.18 | 0.21 | 0.17 | 0.16 | 0.12 | 0.15 | 0.29 | 0.19 | 188.20 | 149.65 | 127.72 | 32.44 | 0.57 |
| Pegmatite | No. 503 | No. 503 | No. 503 | No. 503 | No. 503 | No. 503 | No. 503 | No. 503 | No. 503 | No. 503 | No. 503 | No. 503 | No. 503 | No. 503 | No. 503 |
| Analysis # | 503-4.2 | 503-4.3 | 503-4.4 | 503-4.5 | 503-4.6 | 503-4.7 | 503-4.8 | D023-1 | 503-4.8 | 503-4.9 | 503-4.10 | 503-4.11 | D023.1 | D023.2 | D023.3 |
| Types | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Comments | clb6. c | clb4. c | clb1. c | clb2. c | clb3. c | clb5. m | clb4. m | clb1. m | clb5. r | clb5. r | clb6. r | clb4. r | clb1. c | clb2. c | clb1. r |
| in ppm | |||||||||||||||
| Li | 112.4 | 36.6 | 61.5 | 95.5 | 78.0 | 6.0 | 49.0 | 79.8 | 6.8 | b.d. | 19.1 | 22.3 | 62.6 | 9.8 | 92.7 |
| Be | b.d. | 0.3 | b.d. | 0.3 | 6.4 | 0.4 | 116.4 | b.d. | b.d. | 2.3 | 0.3 | 0.2 | 0.5 | b.d. | 0.3 |
| Na | 883.5 | 31.1 | b.d. | 12.0 | 48.9 | 5515.3 | 46.8 | 13.7 | 347.7 | 3951.2 | 608.7 | 17.2 | 29.9 | b.d. | 30.7 |
| Mg | 22.6 | 13.2 | 5.9 | 16.8 | 11.9 | 11.2 | 8.3 | 19.9 | 9.2 | 31.1 | 10.6 | 21.8 | 819.1 | 12.4 | 14.3 |
| Al | 2804 | 317 | 25 | 626 | 1746 | 7718 | 401 | 34 | 658 | b.d. | 1435 | 336 | 44 | 33 | 44 |
| Si | 8512 | b.d. | 1214 | 1908 | 11,161 | 24,224 | 3010 | b.d. | 3931 | b.d. | 5444 | 1661 | b.d. | 906 | 769 |
| P | 122 | 257 | 40 | 843 | 202 | b.d. | b.d. | b.d. | b.d. | b.d. | 57 | b.d. | b.d. | b.d. | b.d. |
| Ca | 666 | 485 | 150 | 1340 | b.d. | b.d. | b.d. | b.d. | 250 | b.d. | b.d. | b.d. | b.d. | 119 | b.d. |
| Sc | 2.4 | 0.9 | 0.5 | 1.4 | 2.0 | 2.4 | 0.7 | 1.3 | 1.8 | b.d. | 0.9 | 0.8 | 3.5 | 6.6 | 0.5 |
| Ti | 1218 | 1168 | 777 | 1404 | 931 | 1377 | 1168 | 2172 | 1741 | 1443 | 1153 | 1304 | 4292 | 5716 | 1249 |
| V | 0.41 | b.d. | 0.12 | 0.40 | b.d. | 0.02 | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | 0.06 | 0.05 | b.d. |
| Mn | 104,709 | 84,983 | 83,320 | 85,186 | 88,741 | 80,000 | 92,045 | 76,141 | 92,070 | 88,322 | 79,636 | 83,900 | 69,900 | 80,644 | 74,618 |
| Fe | 72,601 | 70,831 | 83,251 | 77,891 | 112,605 | 82,811 | 74,902 | 85,222 | 84,915 | 81,037 | 72,604 | 81,980 | 94,834 | 75,496 | 80,977 |
| Ga | 2.2 | 0.5 | 0.2 | 1.2 | 6.5 | 4.9 | 0.6 | 0.7 | 0.8 | 24.8 | 1.1 | 0.7 | 1.1 | 1.6 | 1.0 |
| Rb | 2.5 | 1.6 | 0.7 | 6.0 | 81.1 | 0.1 | 0.6 | 0.4 | 0.1 | b.d. | 0.2 | 0.0 | 0.3 | b.d. | 0.8 |
| Sr | 8.3 | 20.4 | 0.0 | 5.9 | b.d. | 0.4 | b.d. | 1.7 | 0.6 | b.d. | 0.2 | 0.7 | 1.5 | b.d. | 0.5 |
| Y | 1.85 | 1.32 | 4.57 | 0.96 | 1.86 | 0.88 | 1.69 | 6.6 | 1.01 | 0.72 | 1.08 | 1.21 | 11.7 | 2.2 | 5.2 |
| Zr | 752.5 | 473.2 | 1084.8 | 280.6 | 227.7 | 239.8 | 755.7 | 1679.1 | 531.5 | 198.5 | 304.5 | 388.3 | 2422.1 | 1769.3 | 1302.5 |
| Nb | 450,878 | 421,721 | 434,038 | 447,760 | 410,809 | 439,337 | 446,369 | 394,689 | 446,593 | 465,600 | 421,735 | 445,481 | 438,009 | 388,776 | 409,879 |
| Sn | 984 | 992 | 1128 | 668 | 1047 | 760 | 897 | 1695 | 994 | 59377 | 741 | 718 | 2813 | 2037 | 1395 |
| Sb | 0.5 | 1.4 | b.d. | 1.7 | 1.1 | b.d. | 0.6 | 1.2 | 0.2 | 2.1 | b.d. | 0.4 | 1.1 | 0.4 | 0.8 |
| Cs | 2.3 | 4.3 | 1.1 | 0.9 | 3.4 | 0.6 | 21.9 | 1.1 | 0.4 | 25.5 | 0.4 | 0.7 | 1.0 | 0.4 | 1.2 |
| Ba | b.d. | 2.9 | b.d. | 1.0 | b.d. | 0.2 | b.d. | 2.4 | 0.1 | 8.3 | 0.1 | 0.8 | 8.0 | 0.8 | 3.1 |
| La | b.d. | b.d. | b.d. | 0.02 | b.d. | 0.02 | b.d. | 0.01 | b.d. | 0.10 | b.d. | b.d. | b.d. | b.d. | b.d. |
| Ce | b.d. | b.d. | b.d. | 0.03 | b.d. | 0.34 | b.d. | b.d. | b.d. | 0.04 | 0.02 | b.d. | b.d. | b.d. | b.d. |
| Pr | b.d. | b.d. | 0.01 | b.d. | b.d. | 0.01 | b.d. | 0.01 | b.d. | 0.04 | b.d. | b.d. | b.d. | b.d. | b.d. |
| Nd | 1.02 | b.d. | 0.07 | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | 0.43 | b.d. | b.d. | b.d. | b.d. | b.d. |
| Sm | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | 0.13 | b.d. | b.d. | b.d. | 0.32 | b.d. | b.d. |
| Eu | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | 0.01 | 0.03 | b.d. | b.d. | 0.02 | b.d. | b.d. | b.d. | b.d. |
| Gd | 0.55 | b.d. | b.d. | b.d. | b.d. | 0.10 | b.d. | 0.64 | 0.06 | b.d. | 0.09 | b.d. | 0.20 | b.d. | b.d. |
| Tb | b.d. | 0.02 | 0.10 | 0.03 | b.d. | b.d. | 0.02 | 0.14 | 0.01 | b.d. | 0.01 | b.d. | 0.30 | b.d. | 0.09 |
| Dy | 0.98 | 0.18 | 0.66 | 0.18 | b.d. | b.d. | 0.30 | 1.69 | b.d. | b.d. | b.d. | 0.26 | 2.50 | 0.43 | 0.57 |
| Ho | 0.40 | 0.02 | 0.16 | 0.02 | b.d. | b.d. | 0.08 | 0.25 | b.d. | b.d. | 0.07 | b.d. | 0.37 | b.d. | 0.13 |
| Er | b.d. | 0.10 | 0.47 | 0.05 | b.d. | 0.14 | 0.15 | 0.72 | 0.13 | b.d. | 0.15 | 0.22 | 0.94 | b.d. | 0.25 |
| Tm | 0.07 | 0.02 | 0.10 | 0.05 | b.d. | 0.01 | 0.05 | 0.15 | 0.02 | 0.03 | 0.01 | 0.08 | 0.25 | 0.09 | 0.04 |
| Yb | b.d. | 0.27 | 1.42 | 0.38 | 2.20 | 0.46 | 0.49 | 1.46 | 0.31 | b.d. | 0.28 | 0.28 | 1.87 | 0.08 | 0.70 |
| Lu | 0.08 | 0.04 | 0.23 | 0.10 | b.d. | 0.04 | 0.06 | 0.17 | 0.05 | b.d. | 0.04 | 0.04 | 0.16 | b.d. | 0.05 |
| Hf | 71.2 | 50.4 | 109.1 | 31.1 | 32.3 | 29.0 | 82.3 | 162.3 | 63.4 | 33.6 | 32.9 | 40.7 | 211.9 | 146.3 | 121.9 |
| Ta | 119,276 | 118,941 | 126,589 | 104,152 | 175,362 | 127,149 | 132,439 | 143,376 | 115,463 | 119,983 | 95,265 | 102,763 | 110,822 | 142,906 | 141,028 |
| W | 3549.0 | 2355.9 | 1655.1 | 1405.2 | 1849.4 | 1157.0 | 2586.0 | 1944.7 | 2018.9 | 1699.4 | 1498.9 | 2477.3 | 2786.0 | 2437.9 | 2380.8 |
| Tl | b.d. | 0.3 | 0.1 | b.d. | b.d. | 0.1 | b.d. | 0.32 | 0.1 | 3.7 | 0.0 | 0.1 | 0.05 | b.d. | 0.56 |
| Pb | 41.6 | 51.0 | 93.4 | 29.4 | 18.3 | 19.0 | 39.5 | 111.7 | 33.5 | 26.3 | 25.2 | 34.8 | 185.1 | 50.7 | 63.2 |
| Bi | 0.18 | 0.35 | 0.22 | 3.91 | 0.60 | 0.09 | 0.22 | 0.10 | 0.12 | 0.39 | 0.11 | 0.44 | 0.15 | 0.12 | 0.22 |
| Th | 1.8 | 1.4 | 7.7 | 1.2 | b.d. | 0.3 | 1.0 | 1.8 | 0.9 | 1.0 | 0.5 | 0.8 | 4.4 | 1.1 | 1.1 |
| U | 325.1 | 286.1 | 699.9 | 163.0 | 77.9 | 117.9 | 313.6 | 733.3 | 213.9 | 110.4 | 147.3 | 193.2 | 1355.7 | 469.9 | 464.2 |
| ΣREE | 3.12 | 0.65 | 3.23 | 0.86 | 2.20 | 1.11 | 1.16 | 5.26 | 0.70 | 0.64 | 0.67 | 0.89 | 6.90 | 0.60 | 1.83 |
| Nb/Ta | 3.78 | 3.55 | 3.43 | 4.30 | 2.34 | 3.46 | 3.37 | 2.75 | 3.87 | 3.88 | 4.43 | 4.34 | 3.95 | 2.72 | 2.91 |
| Zr/Hf | 10.57 | 9.39 | 9.94 | 9.03 | 7.05 | 8.26 | 9.18 | 10.34 | 8.38 | 5.91 | 9.26 | 9.55 | 11.43 | 12.10 | 10.68 |
| Sn/W | 0.28 | 0.42 | 0.68 | 0.48 | 0.57 | 0.66 | 0.35 | 0.87 | 0.49 | 34.94 | 0.49 | 0.29 | 1.01 | 0.84 | 0.59 |
| Pegmatite | No. 90 | No. 90 | No. 90 | No. 90 | No. 90 | No. 90 | No. 90 | No. 90 | No. 90 | No. 90 | No. 90 | No. 90 | No. 91 | No. 91 |
| Analysis # | 90-1.6 | 90-1.7 | 90-1.8 | 90-1.9 | 90-1.10 | 90-1.12 | 90-1.13 | 90-1.14 | 90-1.15 | D026-13.4-2 | D026-13.4-3 | D026-13.4-4 | 90-1-3 | 90-1-3 |
| Sample ID | 90-1 | 90-1 | 90-1 | 90-1 | 90-1 | 90-1 | 90-1 | 90-1 | 90-1 | 26-13.4 | 26-13.4 | 26-13.4 | 1-3.11 | 1-3.11-1 |
| Comments | cst1, c | cst1, m | cst1, m | cst1, r | cst2, c | cst2, m | cst2, m | cst2, m | cst2, r | cst1, c | cst1, m | cst1, r | cst1, c | cst1, m |
| in ppm | ||||||||||||||
| Li | 0.6 | b.d | b.d | b.d | b.d | b.d. | b.d. | b.d. | b.d. | 1.0 | b.d | 0.5 | 6.9 | 5.7 |
| Be | b.d | b.d | b.d | b.d | b.d | b.d. | b.d. | b.d. | b.d. | b.d | 0.1 | b.d | 3.4 | 17.3 |
| Na | b.d | b.d | b.d | b.d | b.d | b.d. | b.d. | b.d. | b.d. | b.d | b.d | b.d | 7.3 | 8.7 |
| Mg | 0.5 | 0.7 | 2.7 | 0.5 | 0.6 | 0.6 | 0.6 | 0.5 | b.d. | 0.7 | 0.8 | 0.6 | 2.0 | 1.2 |
| Al | 34 | 45 | 33 | 32 | 45 | 49 | 40 | 35 | 54 | 214 | 136 | 130 | 632 | 679 |
| Si | 1544 | 1608 | b.d | 1074 | 1417 | 1321 | 1618 | 1228 | b.d | b.d | 1289 | 960 | 1024 | 1169 |
| P | b.d | b.d | b.d | 67 | b.d | b.d | b.d | b.d | b.d | b.d | b.d | b.d | 37 | b.d. |
| Ca | b.d | b.d | b.d | b.d | b.d | b.d | 165 | b.d | b.d | b.d | b.d | b.d | b.d. | b.d. |
| Sc | 4.3 | 4.0 | 4.2 | 4.0 | 3.5 | 3.3 | 2.7 | 2.4 | 1.9 | 1.0 | 1.2 | b.d. | 0.5 | 0.5 |
| Ti | 932 | 785 | 889 | 886 | 614 | 656 | 599 | 565 | 411 | 447 | 1373 | 616 | 643 | 466 |
| V | 10.9 | 10.7 | 9.9 | 9.6 | 4.9 | 5.0 | 4.3 | 3.2 | 2.5 | 0.1 | b.d. | b.d. | 0.11 | 0.04 |
| Mn | 79 | 75 | 55 | 53 | 54 | 59 | 52 | 36 | 92 | 455 | 104 | 226 | 311 | 275 |
| Fe | 327 | 290 | 231 | 212 | 244 | 245 | 231 | 152 | 468 | 6795 | 2676 | 3352 | 7943 | 8872 |
| Ga | 1.9 | 1.8 | 1.1 | 1.9 | 2.0 | 1.8 | 1.8 | 1.5 | 2.2 | 7.7 | 4.1 | 4.2 | 19.4 | 21.7 |
| Rb | b.d. | b.d. | 0.0 | b.d. | 0.0 | 0.0 | b.d. | 0.0 | 0.0 | b.d. | 0.0 | 0.0 | 0.1 | b.d. |
| Sr | b.d. | b.d. | 0.1 | 0.0 | b.d. | b.d. | 0.0 | 0.0 | 0.0 | b.d. | b.d. | b.d. | 0.0 | 0.0 |
| Y | b.d. | b.d. | b.d. | b.d. | 0.0 | b.d. | 0.0 | b.d. | b.d. | b.d. | b.d. | b.d. | 0.0 | 0.0 |
| Zr | 189 | 208 | 179 | 188 | 199 | 203 | 192 | 184 | 270 | 468 | 431 | 308 | 873 | 742 |
| Nb | 185 | 241 | 144 | 150 | 218 | 232 | 212 | 156 | 514 | 2673 | 3753 | 1989 | 20,256 | 15,988 |
| Sn | 761,261 | 754,062 | 752,376 | 752,605 | 769,209 | 763,388 | 759,308 | 764,073 | 759,363 | 745,263 | 753,487 | 753,487 | 761,568 | 761,568 |
| Sb | 1.0 | 0.4 | 0.6 | 0.8 | b.d. | 0.5 | 0.7 | 1.7 | 0.5 | 0.7 | 0.8 | b.d. | 0.8 | 0.6 |
| Cs | 0.4 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.3 | 0.4 | 0.2 | 0.2 | 0.3 | 0.2 | 1.1 | 0.7 |
| Ba | 4.2 | 3.4 | 3.4 | 4.2 | 4.9 | 4.3 | 3.5 | 4.2 | 4.2 | 4.2 | 4.5 | 4.4 | 6.4 | 7.9 |
| La | 0.09 | 0.08 | 0.08 | 0.12 | 0.05 | 0.11 | 0.07 | 0.10 | 0.08 | 0.08 | 0.08 | 0.05 | 0.16 | 0.14 |
| Ce | 0.00 | 0.02 | 0.01 | 0.05 | 0.02 | 0.02 | 0.03 | 0.02 | 0.01 | b.d. | 0.03 | 0.02 | 0.04 | 0.02 |
| Pr | 0.05 | b.d. | 0.06 | 0.07 | 0.08 | 0.09 | 0.05 | 0.09 | 0.09 | 0.09 | 0.07 | 0.06 | b.d. | 0.13 |
| Nd | b.d. | b.d. | 0.02 | 0.04 | 0.02 | b.d. | b.d. | 0.04 | 0.02 | 0.04 | b.d. | b.d. | b.d. | b.d. |
| Sm | b.d. | b.d. | b.d. | b.d. | 0.02 | b.d. | b.d. | 0.04 | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. |
| Eu | b.d. | 0.01 | b.d. | 0.01 | b.d. | 0.01 | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. |
| Gd | 0.18 | 0.12 | 0.09 | 0.16 | 0.14 | b.d. | 0.10 | 0.27 | 0.21 | 0.25 | 0.29 | 0.20 | 0.07 | 0.15 |
| Tb | 0.02 | 0.01 | 0.03 | 0.01 | 0.01 | b.d. | 0.04 | 0.03 | 0.02 | 0.01 | 0.05 | 0.01 | 0.03 | 0.02 |
| Dy | b.d. | 0.03 | b.d. | b.d. | 0.02 | b.d. | b.d. | 0.01 | 0.01 | 0.02 | b.d. | 0.04 | b.d. | 0.04 |
| Ho | b.d. | b.d. | b.d. | b.d. | b.d. | 0.01 | b.d. | 0.01 | b.d. | b.d. | b.d. | b.d. | b.d. | 0.00 |
| Er | b.d. | b.d. | 0.02 | b.d. | b.d. | b.d. | 0.01 | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. |
| Tm | b.d. | b.d. | 0.01 | 0.01 | b.d. | b.d. | b.d. | 0.01 | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. |
| Yb | b.d. | b.d. | b.d. | 0.01 | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. |
| Lu | 0.01 | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | 0.00 | 0.00 | 0.00 | b.d. | b.d. | b.d. | 0.00 |
| Hf | 71 | 92 | 68 | 72 | 118 | 120 | 117 | 110 | 120 | 344 | 168 | 217 | 182 | 175 |
| Ta | 3811 | 3420 | 2642 | 2821 | 2755 | 2874 | 2613 | 2018 | 4359 | 42,261 | 13,835 | 21,336 | 16,646 | 29,071 |
| W | 0.6 | 0.5 | 4.2 | 0.2 | 0.2 | 0.3 | 0.3 | 0.6 | 0.5 | 4.3 | 4.1 | 2.4 | 269.5 | 380.1 |
| Tl | b.d. | 0.0 | 0.1 | b.d. | 0.1 | b.d. | 0.0 | 0.0 | b.d. | 0.02 | b.d. | 0.07 | b.d. | 0.1 |
| Pb | b.d. | 0.1 | 0.3 | 0.1 | b.d. | 0.0 | 0.0 | b.d. | 0.1 | 0.5 | b.d. | 0.2 | 2.5 | 2.3 |
| Bi | 0.01 | 0.00 | 0.02 | b.d. | 0.01 | 0.01 | 0.01 | b.d. | 0.02 | b.d. | 0.01 | 0.01 | b.d. | 0.04 |
| Th | b.d. | b.d. | 0.0 | 0.0 | b.d. | b.d. | 0.0 | b.d. | 0.0 | 0.0 | b.d. | b.d. | 0.01 | b.d. |
| U | 0.1 | b.d. | 0.1 | 0.1 | 0.0 | b.d. | 0.1 | 0.0 | 0.1 | 1.6 | 1.7 | 1.3 | 17.0 | 20.8 |
| Mn/(Mn + Fe) | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.1 | 0.0 | 0.1 | 0.0 | 0.0 |
| ΣREE | 0.36 | 0.27 | 0.32 | 0.49 | 0.37 | 0.24 | 0.29 | 0.62 | 0.43 | 0.51 | 0.52 | 0.39 | 0.30 | 0.50 |
| Nb/Ta | 0.05 | 0.07 | 0.05 | 0.05 | 0.08 | 0.08 | 0.08 | 0.08 | 0.12 | 0.06 | 0.27 | 0.09 | 1.22 | 0.55 |
| Zr/Hf | 2.67 | 2.27 | 2.63 | 2.59 | 1.69 | 1.69 | 1.64 | 1.67 | 2.25 | 1.36 | 2.57 | 1.42 | 4.79 | 4.24 |
| Sn/W | 1,239,414 | 1,575,795 | 180,947 | 3,091,815 | 3,327,149 | 2,548,274 | 2,216,522 | 1,341,883 | 1,562,796 | 171,594 | 181,988 | 315,745 | 2826 | 2004 |
| Pegmatite | No. 91 | No. 91 | No. 91 | No. 91 | No. 91 | No. 91 | No. 91 | No. 503 | No. 503 | No. 503 | No. 503 | No. 503 | No. 503 | No. 503 |
| Analysis # | 91-1-3 | 91-1-3 | 91-1-3 | 91-1-3 | 91-1-3 | 91-1-3 | 91-1-3 | D023-1 | D023-1 | D023-1 | D023-1 | D023-1 | D023-1 | D023-1 |
| Sample ID | 1-3.12 | 1-3.13 | 1-3.14 | 1-3.2 | 1-3.3 | 1-3.4 | 1-3.5 | 23-1.1 | 23-1.2 | 23-1.3 | 23-1.4 | 23-1.14 | 23-1.15 | 23-1.16 |
| Comments | cst1, m | cst1, m | cst1, r | cst2, c | cst2, m | cst2, m | cst2, r | cst1, c | cst1, m | cst1, m | cst1, r | cst3, c | cst3, m | cst3, r |
| in ppm | ||||||||||||||
| Li | 6.7 | 5.8 | 6.7 | 2.8 | 13.6 | 4.9 | 16.1 | 5.7 | 15.8 | 10.0 | 3.4 | 6.2 | 2.0 | |
| Be | 157.0 | 29.6 | 0.1 | 47.6 | 12.2 | 11.5 | 53.3 | 0.6 | 0.7 | 31.6 | 2.0 | 4.4 | 39.8 | 0.3 |
| Na | b.d. | b.d. | b.d. | b.d. | b.d. | 9.9 | b.d. | b.d. | b.d. | b.d. | 7.8 | b.d. | b.d. | b.d. |
| Mg | 0.8 | 1.3 | 1.8 | b.d. | 1.0 | 1.7 | 1.2 | 1.6 | 0.9 | 0.9 | b.d. | b.d. | 0.8 | 1.9 |
| Al | 1981 | 762 | 127 | 745 | 546 | 382 | 804 | 157 | 148 | 645 | 185 | 63 | 801 | 13 |
| Si | 1111 | b.d. | b.d. | b.d. | 1166 | b.d. | b.d. | 529 | 1099 | b.d. | 1329 | 1696 | 1339 | 1028 |
| P | 36 | b.d. | b.d. | b.d. | b.d. | 162 | b.d. | b.d. | b.d. | b.d. | 60 | b.d. | b.d. | b.d. |
| Ca | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. |
| Sc | 0.5 | b.d. | b.d. | b.d. | 0.3 | b.d. | b.d. | 1.2 | b.d. | 1.3 | b.d. | 0.3 | 1.1 | b.d. |
| Ti | 730 | 458 | 302 | 217 | 309 | 335 | 367 | 1293 | 990 | 2366 | 1362 | 934 | 2297 | 474 |
| V | b.d. | b.d. | 0.29 | b.d. | b.d. | b.d. | b.d. | b.d. | 0.06 | b.d. | 0.06 | b.d. | b.d. | 0.07 |
| Mn | 98 | 287 | 92 | 87 | 163 | 410 | 163 | 935 | 165 | 195 | 104 | 12 | 85 | 7 |
| Fe | 5269 | 9130 | 2912 | 3026 | 5548 | 5569 | 5361 | 7062 | 2319 | 4462 | 2235 | 323 | 3538 | 145 |
| Ga | 61.3 | 23.1 | 3.8 | 17.5 | 13.8 | 13.2 | 20.0 | 8.5 | 9.2 | 25.5 | 5.8 | 2.3 | 30.1 | 0.8 |
| Rb | b.d. | b.d. | 1.1 | 0.5 | b.d. | 5.3 | 0.0 | 0.3 | 0.3 | 0.2 | 0.2 | 0.3 | 0.2 | 0.2 |
| Sr | b.d. | 0.0 | 0.0 | b.d. | b.d. | b.d. | 0.0 | b.d. | 0.1 | 0.0 | 0.2 | 0.1 | b.d. | 0.0 |
| Y | 0.0 | 0.0 | b.d. | 0.0 | b.d. | b.d. | 0.0 | b.d. | 0.0 | 0.0 | b.d. | b.d. | b.d. | b.d. |
| Zr | 697 | 731 | 625 | 329 | 532 | 933 | 530 | 1202 | 1062 | 1513 | 834 | 611 | 951 | 104 |
| Nb | 8295 | 14,688 | 2816 | 5451 | 10,411 | 6352 | 8632 | 19,455 | 6480 | 10061 | 5933 | 696 | 8229 | 237 |
| Sn | 734,984 | 749,470 | 751,447 | 728,927 | 721,901 | 751,777 | 729,463 | 690,512 | 744,200 | 760,568 | 753,762 | 763,246 | 746,547 | 746,831 |
| Sb | 0.7 | 0.8 | 0.6 | 0.9 | 0.9 | 0.9 | 0.7 | 3.3 | 2.2 | 2.0 | 2.7 | 3.0 | 1.9 | 2.3 |
| Cs | 0.4 | 0.4 | 0.7 | 2.1 | 0.7 | 48.1 | 0.4 | 1.2 | 0.9 | 0.8 | 1.1 | 1.0 | 0.8 | 0.9 |
| Ba | 8.2 | 6.2 | 7.8 | 5.7 | 7.3 | 6.3 | 6.7 | 8.3 | 7.3 | 7.1 | 7.7 | 8.0 | 6.3 | 6.7 |
| La | 0.10 | 0.12 | 0.11 | 0.11 | 0.12 | 0.14 | b.d. | 0.11 | 0.15 | 0.12 | 0.13 | 0.13 | 0.13 | 0.11 |
| Ce | 0.03 | 0.02 | 0.03 | 0.05 | 0.01 | 0.04 | 0.02 | 0.05 | 0.03 | 0.03 | 0.03 | 0.03 | 0.02 | 0.03 |
| Pr | 0.16 | 0.15 | 0.09 | 0.10 | 0.14 | b.d. | 0.14 | 0.11 | 0.13 | 0.16 | 0.14 | 0.10 | 0.16 | 0.15 |
| Nd | b.d. | 0.02 | b.d. | b.d. | b.d. | b.d. | 0.01 | 0.07 | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. |
| Sm | b.d. | 0.04 | b.d. | 0.03 | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. |
| Eu | b.d. | 0.01 | 0.01 | 0.03 | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | 0.01 |
| Gd | 0.22 | 0.23 | 0.15 | 0.09 | b.d. | b.d. | 0.31 | b.d. | 0.18 | 0.12 | b.d. | 0.17 | 0.28 | b.d. |
| Tb | 0.02 | 0.04 | 0.03 | 0.04 | 0.03 | 0.04 | 0.02 | 0.04 | 0.02 | 0.03 | 0.06 | 0.02 | 0.01 | 0.02 |
| Dy | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | 0.03 | b.d. | 0.03 | b.d. | b.d. | b.d. | 0.02 | 0.01 |
| Ho | b.d. | b.d. | 0.01 | b.d. | b.d. | 0.01 | b.d. | 0.01 | b.d. | 0.00 | 0.01 | 0.00 | 0.01 | b.d. |
| Er | b.d. | b.d. | b.d. | b.d. | 0.01 | b.d. | b.d. | 0.03 | b.d. | b.d. | 0.03 | b.d. | b.d. | 0.02 |
| Tm | b.d. | 0.01 | 0.00 | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | 0.00 | b.d. |
| Yb | b.d. | 0.01 | b.d. | 0.04 | b.d. | b.d. | 0.02 | b.d. | 0.02 | b.d. | b.d. | b.d. | 0.04 | b.d. |
| Lu | b.d. | b.d. | 0.01 | b.d. | 0.00 | b.d. | b.d. | 0.01 | b.d. | 0.00 | 0.01 | b.d. | 0.00 | b.d. |
| Hf | 148 | 184 | 211 | 70 | 131 | 267 | 132 | 228 | 195 | 255 | 181 | 123 | 143 | 49 |
| Ta | 12,901 | 33,120 | 16,747 | 9871 | 18,964 | 26,138 | 19,714 | 11,490 | 4145 | 6530 | 3443 | 885 | 3211 | 567 |
| W | 80.5 | 276.8 | 2.8 | 270.9 | 282.1 | 200.0 | 167.3 | 115 | 74 | 48 | 421 | 6 | 48 | 4 |
| Tl | 0.1 | b.d. | 0.0 | b.d. | b.d. | b.d. | 0.0 | b.d. | 0.23 | b.d. | b.d. | 0.05 | b.d. | b.d. |
| Pb | 2.8 | 2.6 | 0.6 | 2.0 | 1.9 | 1.3 | 1.7 | 2.4 | 1.8 | 1.9 | 1.9 | b.d. | 1.9 | 0.6 |
| Bi | 0.01 | 0.01 | b.d. | 0.02 | b.d. | b.d. | b.d. | 0.08 | 0.05 | 0.05 | b.d. | b.d. | 0.01 | b.d. |
| Th | 0.00 | b.d. | b.d. | b.d. | b.d. | 0.03 | 0.00 | 0.0 | 0.0 | b.d. | 0.0 | 0.0 | 0.0 | 0.0 |
| U | 26.9 | 21.1 | 1.1 | 17.7 | 14.1 | 10.0 | 12.2 | 13.0 | 4.7 | 10.0 | 8.4 | 0.5 | 7.6 | 0.3 |
| Mn/(Mn + Fe) | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.1 | 0.0 | 0.1 | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| ΣREE | 0.53 | 0.63 | 0.44 | 0.49 | 0.32 | 0.23 | 0.57 | 0.42 | 0.55 | 0.46 | 0.41 | 0.46 | 0.68 | 0.34 |
| Nb/Ta | 0.64 | 0.44 | 0.17 | 0.55 | 0.55 | 0.24 | 0.44 | 1.69 | 1.56 | 1.54 | 1.72 | 0.79 | 2.56 | 0.42 |
| Zr/Hf | 4.71 | 3.97 | 2.96 | 4.68 | 4.07 | 3.49 | 4.00 | 5.28 | 5.44 | 5.94 | 4.60 | 4.96 | 6.63 | 2.12 |
| Sn/W | 9128 | 2708 | 267,663 | 2691 | 2559 | 3760 | 4360 | 5988 | 10,091 | 16,001 | 1790 | 117,983 | 15,412 | 196,541 |
| Pegmatite | No. 503 | No. 503 | No. 503 | No. 503 | No. 503 | No. 503 | No. 503 | No. 503 | No. 503 | No. 503 | No. 503 | No. 503 | No. 503 | |
| Analysis # | D023-1 | D023-1 | D023-1 | D023-1 | D023-1 | D023-1 | D023-1 | 503-4 | 503-4 | 503-4 | 503-4 | 503-4 | 503-4 | |
| Sample ID | 23-1.20 | 23-1.21 | 23-1.22 | 23-1.23 | 23-1.24 | 23-1.25 | 23-1.26 | 503-4.12 | 503-4.13 | 503-4.14 | 503-4.17 | 503-4.18 | 503-4.19 | |
| Comments | cst2, c | cst2, m | cst2, m | cst2, m | cst4, c | cst4, m | cst4, m | cst1, r | cst1, r | cst1, r | cst2, r | cst3, r | cst3, r | |
| in ppm | ||||||||||||||
| Li | 5.2 | 6.1 | 7.2 | 12.4 | 17.5 | 7.4 | 6.5 | 23.4 | 26.3 | b.d. | 0.4 | 5.1 | 25.3 | |
| Be | 1.6 | b.d. | 49.6 | 1.1 | 13.7 | 0.2 | b.d. | b.d. | b.d. | b.d. | b.d. | 0.3 | 0.7 | |
| Na | b.d. | b.d. | 8.9 | 9.9 | 5.5 | b.d. | b.d. | b.d. | b.d. | 7.9 | b.d. | 33.8 | 25.3 | |
| Mg | 2.4 | 0.7 | 2.1 | 3.4 | 0.7 | 0.9 | 0.5 | b.d. | b.d. | 0.3 | b.d. | 0.8 | 19.4 | |
| Al | 306 | 272 | 549 | 376 | 362 | 136 | 113 | 1102 | 1326 | 109 | 226 | 112 | 672 | |
| Si | b.d. | b.d. | b.d. | 1936 | 1109 | 1721 | 1493 | 958 | b.d. | 1026 | b.d. | 1904 | 1454 | |
| P | b.d. | b.d. | b.d. | b.d. | 40 | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | 79 | 307 | |
| Ca | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | 299 | 909 | |
| Sc | 1.3 | 1.3 | 1.5 | 0.6 | 0.5 | 0.5 | b.d. | 1.0 | 1.9 | 0.9 | 0.8 | 0.6 | 0.4 | |
| Ti | 1603 | 1210 | 3255 | 951 | 1135 | 744 | 628 | 206 | 256 | 722 | 664 | 313 | 367 | |
| V | b.d. | b.d. | b.d. | b.d. | 0.05 | b.d. | 0.02 | b.d. | 0 | 0 | 0 | b.d. | 1 | |
| Mn | 266 | 150 | 1569 | 320 | 827 | 144 | 96 | 5313 | 7258 | 195 | 283 | 24 | 537 | |
| Fe | 8221 | 5021 | 6820 | 3144 | 3786 | 2190 | 1816 | 4602 | 8276 | 3530 | 5469 | 536 | 3287 | |
| Ga | 11.3 | 11.8 | 20.5 | 9.0 | 12.5 | 5.4 | 5.1 | 43.1 | 57.6 | 4.6 | 9.6 | 1.4 | 5.2 | |
| Rb | 0.1 | 0.3 | 0.2 | 7.8 | 0.5 | 0.0 | 0.4 | 0.1 | b.d. | 0.0 | b.d. | 0.0 | 0.9 | |
| Sr | 0.1 | b.d. | b.d. | 0.2 | 0.1 | b.d. | 0.0 | b.d. | 0.0 | 0.0 | b.d. | 1.1 | 19.4 | |
| Y | 0.0 | b.d. | 0.0 | b.d. | 0.0 | 0.0 | 0.0 | 0.02 | 0.05 | 0.01 | 0.02 | 0.01 | b.d. | |
| Zr | 1262 | 911 | 1739 | 667 | 675 | 448 | 417 | 3674 | 4903 | 1052 | 1101 | 308 | 418 | |
| Nb | 21,988 | 14,473 | 19,321 | 11,493 | 11,591 | 7808 | 7147 | 20,751 | 28,119 | 5830 | 10,313 | 776 | 5169 | |
| Sn | 727,509 | 740,671 | 723,547 | 751,360 | 756,220 | 741,483 | 751,525 | 675,011 | 667,252 | 727,903 | 753,203 | 764,309 | 678,705 | |
| Sb | 1.3 | 2.9 | 1.9 | 4.2 | 3.1 | 1.5 | 3.9 | 0.4 | 0.5 | 0.6 | 0.5 | 2.3 | 8.8 | |
| Cs | 0.8 | 0.9 | 0.7 | 4.3 | 1.2 | 0.7 | 1.0 | 0.3 | 0.2 | 0.3 | 0.3 | 0.4 | 1.0 | |
| Ba | 7.3 | 7.9 | 7.0 | 6.4 | 6.5 | 6.6 | 7.9 | 6.0 | 4.7 | 4.6 | 5.9 | 4.6 | 5.3 | |
| La | 0.14 | 0.13 | 0.17 | 0.15 | 0.10 | 0.17 | 0.08 | 0.12 | 0.09 | 0.11 | 0.14 | 0.09 | 0.07 | |
| Ce | 0.03 | 0.02 | 0.02 | 0.05 | 0.02 | b.d. | 0.03 | 0.04 | 0.03 | 0.04 | b.d. | 0.01 | 0.02 | |
| Pr | 0.14 | 0.13 | b.d. | 0.14 | 0.17 | 0.17 | 0.10 | b.d. | 0.11 | 0.11 | 0.06 | 0.08 | 0.03 | |
| Nd | 0.03 | 0.09 | b.d. | 0.06 | 0.05 | b.d. | b.d. | b.d. | b.d. | 0.07 | b.d. | 0.02 | 0.03 | |
| Sm | b.d. | b.d. | b.d. | b.d. | 0.06 | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | |
| Eu | b.d. | 0.01 | 0.01 | b.d. | b.d. | b.d. | 0.01 | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | |
| Gd | 0.26 | 0.25 | 0.29 | 0.27 | 0.45 | 0.16 | 0.31 | 0.66 | 0.24 | 0.05 | b.d. | 0.29 | 0.32 | |
| Tb | 0.02 | 0.03 | 0.05 | 0.02 | b.d. | b.d. | 0.04 | 0.02 | 0.03 | b.d. | 0.03 | 0.02 | 0.02 | |
| Dy | 0.04 | 0.01 | 0.03 | b.d. | b.d. | 0.03 | b.d. | b.d. | b.d. | b.d. | 0.06 | b.d. | 0.04 | |
| Ho | b.d. | 0.00 | b.d. | 0.00 | b.d. | 0.02 | 0.02 | b.d. | 0.01 | 0.01 | 0.01 | 0.00 | b.d. | |
| Er | b.d. | b.d. | b.d. | b.d. | 0.01 | 0.05 | 0.01 | b.d. | b.d. | b.d. | 0.01 | b.d. | b.d. | |
| Tm | 0.01 | b.d. | b.d. | b.d. | 0.01 | b.d. | 0.01 | b.d. | 0.00 | 0.00 | b.d. | 0.01 | 0.00 | |
| Yb | b.d. | 0.05 | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | 0.07 | 0.02 | b.d. | b.d. | 0.05 | |
| Lu | b.d. | 0.00 | 0.01 | b.d. | b.d. | b.d. | b.d. | b.d. | 0.01 | 0.00 | b.d. | 0.01 | b.d. | |
| Hf | 219 | 149 | 314 | 97 | 103 | 61 | 60 | 712 | 959 | 340 | 278 | 111 | 105 | |
| Ta | 9460 | 5239 | 15,054 | 2639 | 5426 | 2135 | 1790 | 39,312 | 61,951 | 14,418 | 18,725 | 2729 | 10,112 | |
| W | 101 | 75 | 41 | 603 | 794 | 1457 | 1291 | 38 | 49 | 3 | 7 | 2 | 20 | |
| Tl | b.d. | b.d. | b.d. | 0.26 | 0.14 | b.d. | 0.06 | b.d. | 0.0 | b.d. | 0.1 | 0.0 | b.d. | |
| Pb | 2.2 | 2.7 | 2.3 | 2.5 | 5.3 | 2.9 | 2.5 | 1.1 | 2.1 | 0.6 | 0.5 | 1.1 | 20.1 | |
| Bi | b.d. | 0.02 | 0.10 | b.d. | b.d. | 0.02 | 0.10 | 0.01 | b.d. | b.d. | b.d. | 0.50 | 6.50 | |
| Th | 0.0 | 0.0 | 0.0 | 0.1 | 0.1 | 0.0 | b.d. | b.d. | b.d. | b.d. | 0.0 | b.d. | 0.3 | |
| U | 13.4 | 8.4 | 10.4 | 14.1 | 22.4 | 19.6 | 11.9 | 8.4 | 15.5 | 3.0 | 3.3 | 0.9 | 7.2 | |
| Mn/(Mn + Fe) | 0.0 | 0.0 | 0.2 | 0.1 | 0.2 | 0.1 | 0.1 | 0.5 | 0.5 | 0.1 | 0.1 | 0.0 | 0.1 | |
| ΣREE | 0.67 | 0.73 | 0.59 | 0.69 | 0.88 | 0.60 | 0.61 | 0.84 | 0.59 | 0.42 | 0.31 | 0.52 | 0.60 | |
| Nb/Ta | 2.32 | 2.76 | 1.28 | 4.35 | 2.14 | 3.66 | 3.99 | 0.53 | 0.45 | 0.40 | 0.55 | 0.28 | 0.51 | |
| Zr/Hf | 5.76 | 6.11 | 5.54 | 6.87 | 6.58 | 7.32 | 6.89 | 5.16 | 5.11 | 3.10 | 3.96 | 2.78 | 4.00 | |
| Sn/W | 7226 | 9900 | 17,836 | 1245 | 952 | 509 | 582 | 17,647 | 13,515 | 273,523 | 102,069 | 480,328 | 33,604 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, Y.; Liang, T.; Yang, X.; Zhang, Z.; Wang, Y. Chemical Evolution of Nb-Ta Oxides and Cassiterite in Phosphorus-Rich Albite-Spodumene Pegmatites in the Kangxiwa–Dahongliutan Pegmatite Field, Western Kunlun Orogen, China. Minerals 2019, 9, 166. https://doi.org/10.3390/min9030166
Feng Y, Liang T, Yang X, Zhang Z, Wang Y. Chemical Evolution of Nb-Ta Oxides and Cassiterite in Phosphorus-Rich Albite-Spodumene Pegmatites in the Kangxiwa–Dahongliutan Pegmatite Field, Western Kunlun Orogen, China. Minerals. 2019; 9(3):166. https://doi.org/10.3390/min9030166
Chicago/Turabian StyleFeng, Yonggang, Ting Liang, Xiuqing Yang, Ze Zhang, and Yiqian Wang. 2019. "Chemical Evolution of Nb-Ta Oxides and Cassiterite in Phosphorus-Rich Albite-Spodumene Pegmatites in the Kangxiwa–Dahongliutan Pegmatite Field, Western Kunlun Orogen, China" Minerals 9, no. 3: 166. https://doi.org/10.3390/min9030166
APA StyleFeng, Y., Liang, T., Yang, X., Zhang, Z., & Wang, Y. (2019). Chemical Evolution of Nb-Ta Oxides and Cassiterite in Phosphorus-Rich Albite-Spodumene Pegmatites in the Kangxiwa–Dahongliutan Pegmatite Field, Western Kunlun Orogen, China. Minerals, 9(3), 166. https://doi.org/10.3390/min9030166