Partitioning Pattern of Natural Products Based on Molecular Properties Descriptors Representing Drug-Likeness
Abstract
1. Introduction
2. Materials and Methods
2.1. Natural Molecules Dataset
2.2. Multivariate Statistical Methods
3. Results and Discussion
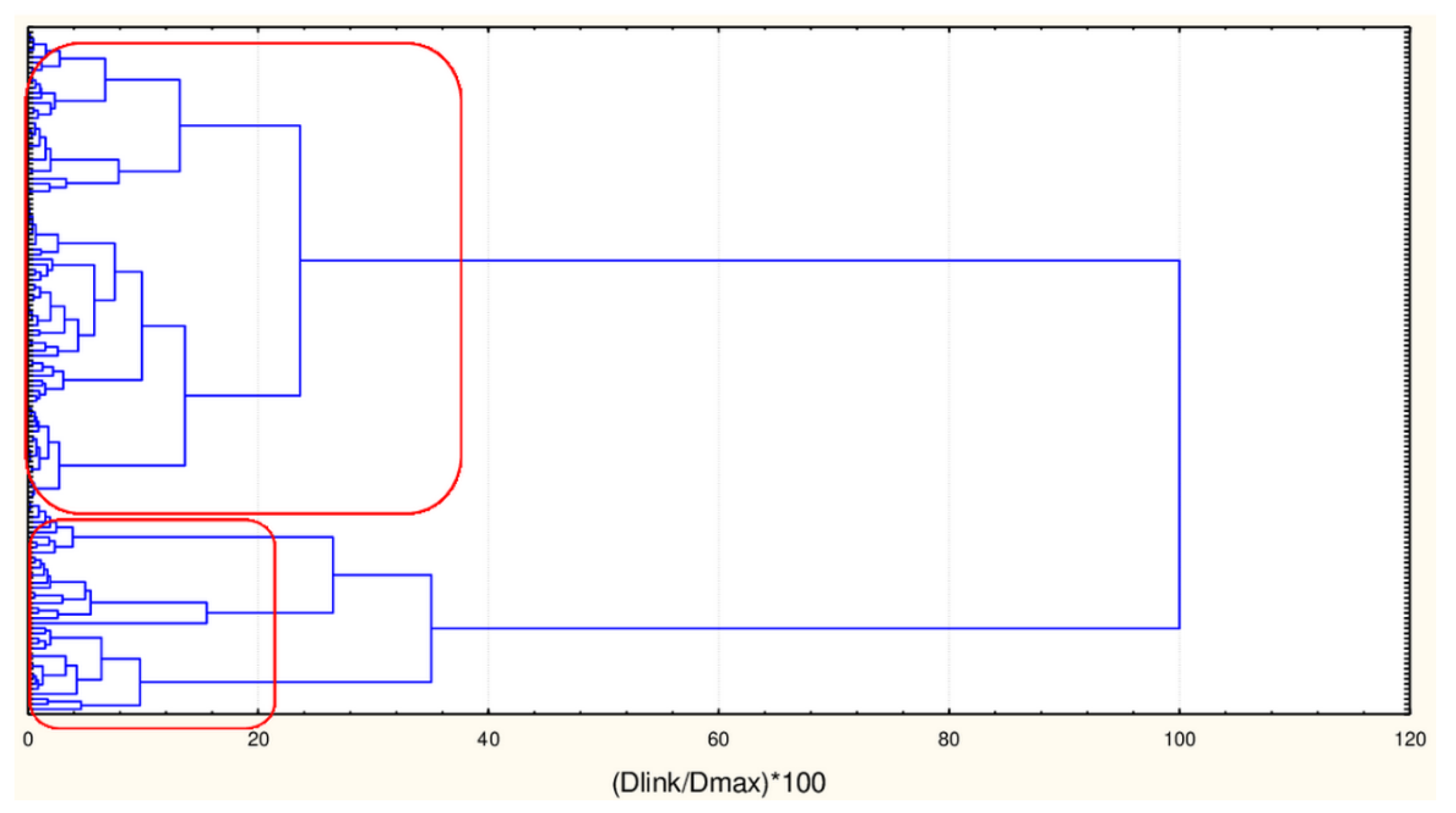
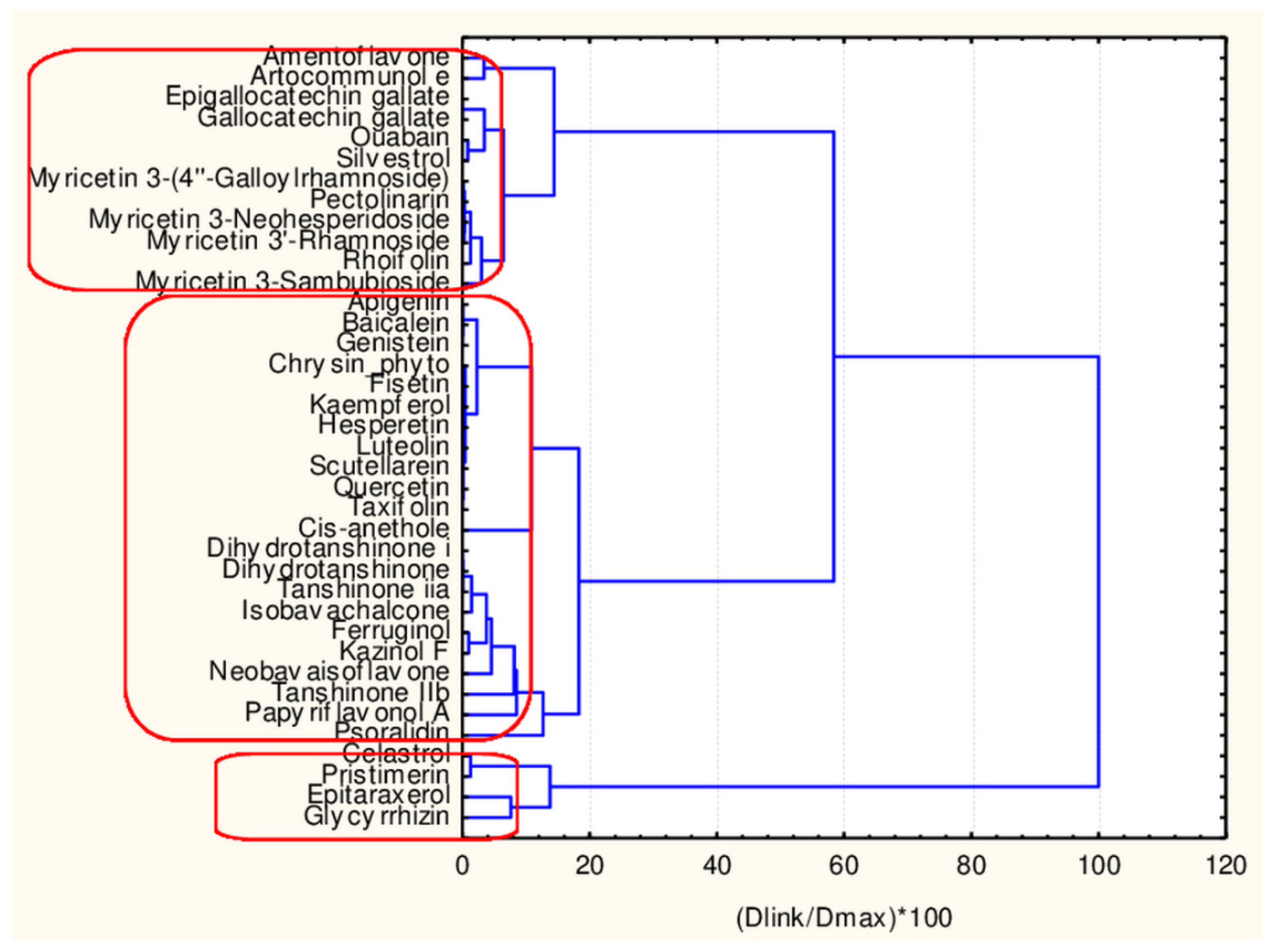
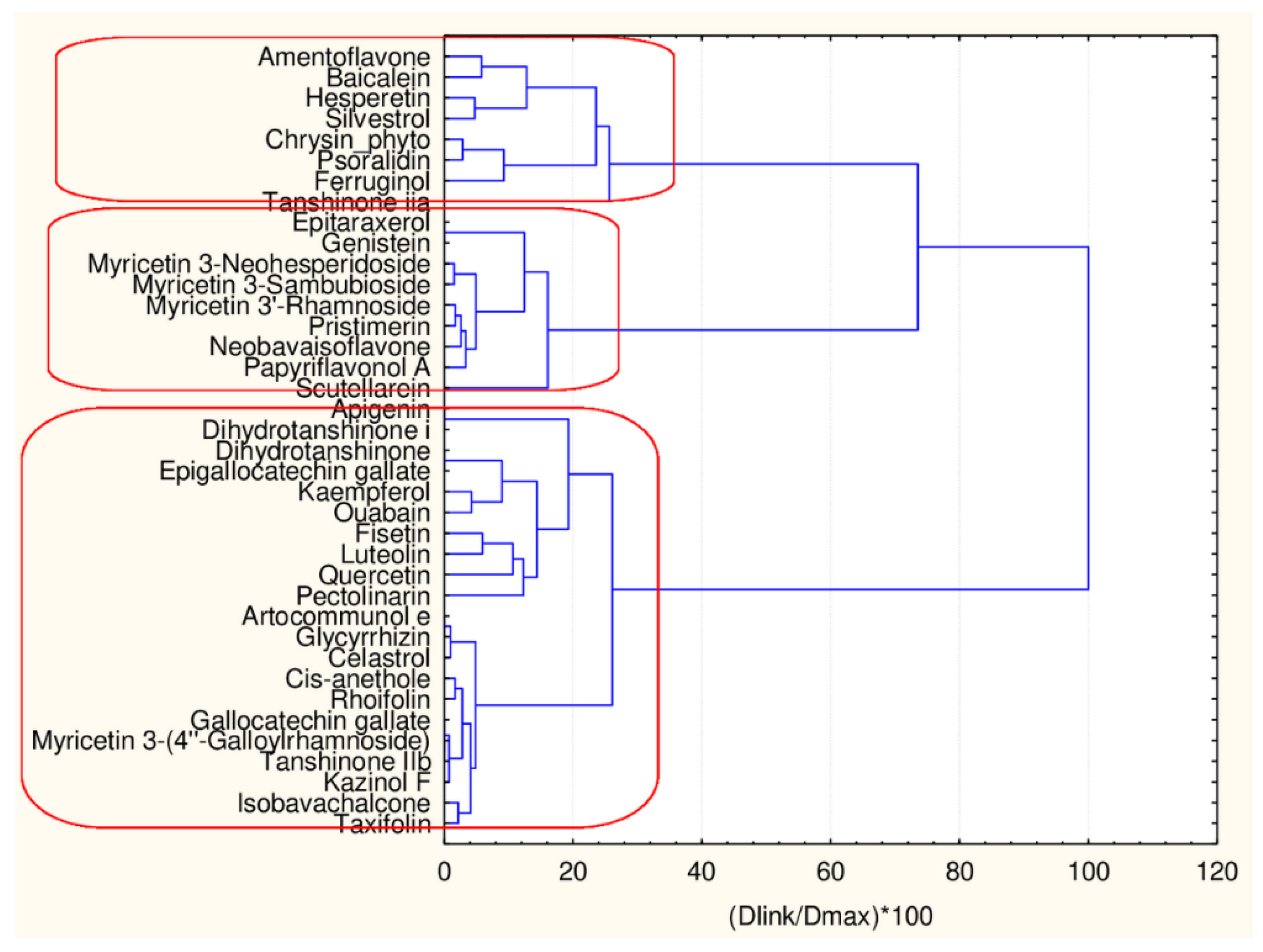
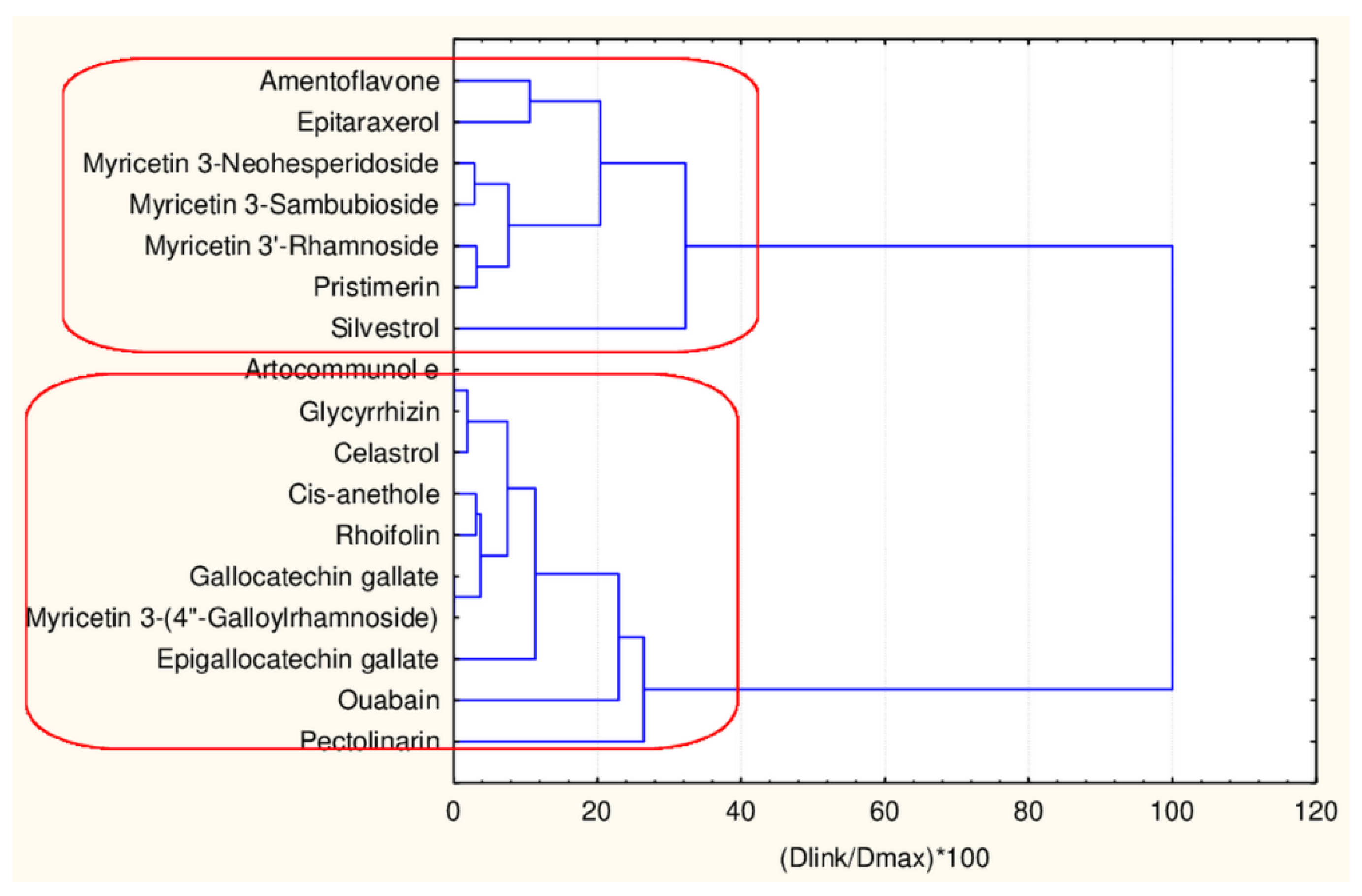
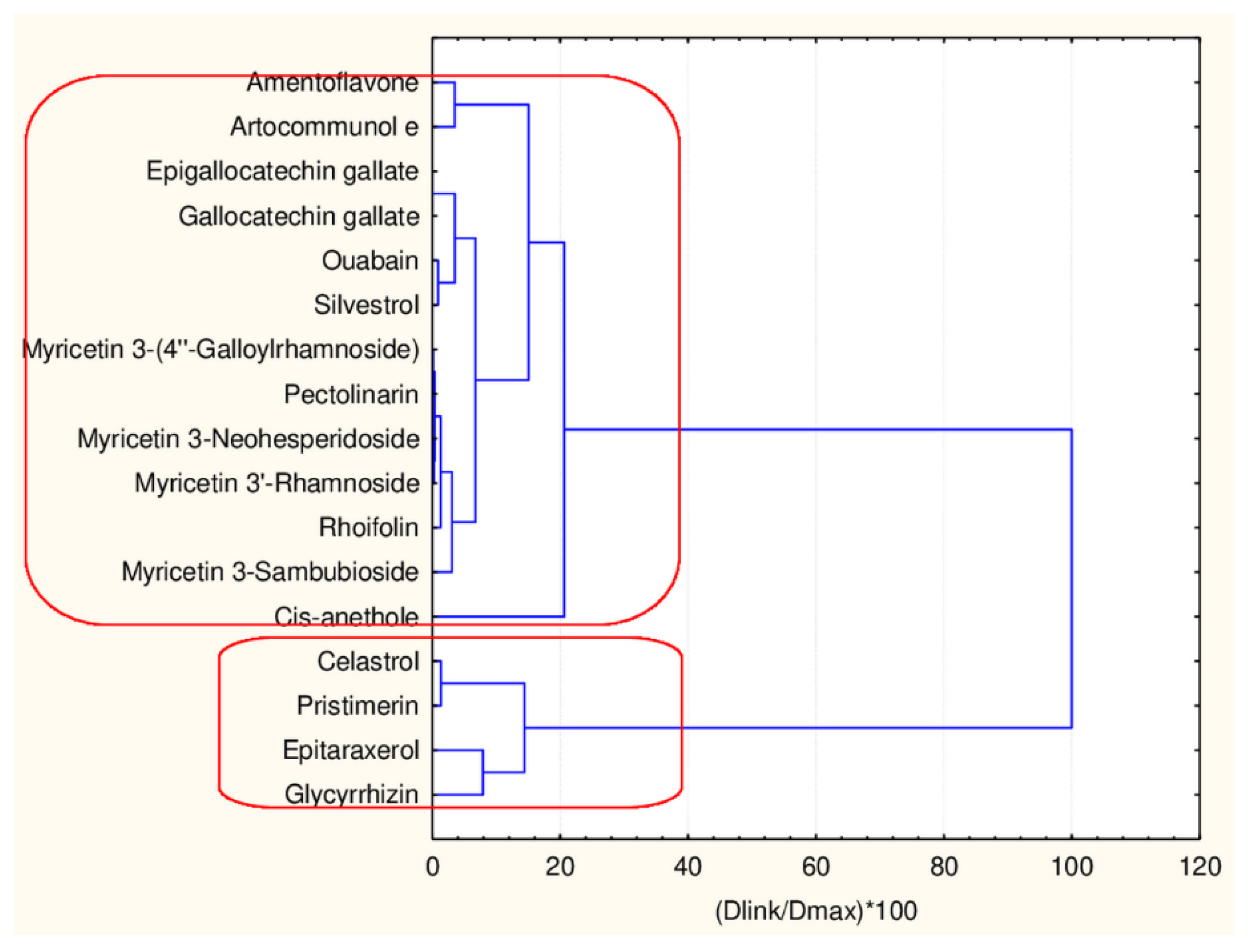
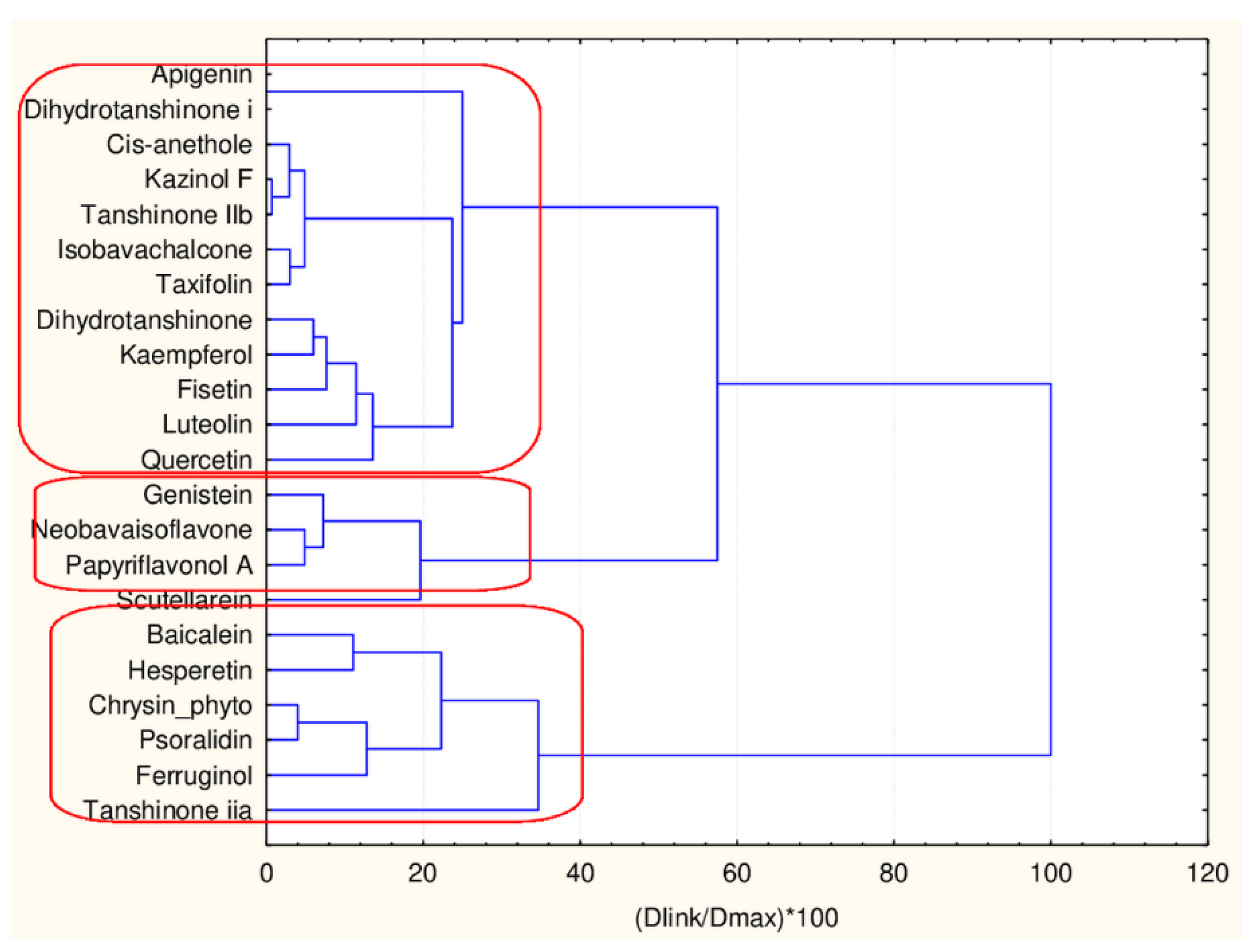
Hierarchical Cluster Analysis
- Flavonoids—41.4 %
- Triterpens—26.8%
- Saponins—21.2%
- Alkaloids—7.3%
- Tanin—2.4%
- Flavonoides—26.3%
- Sesquiterpenes—15.1%
- Monoterpenes—13.8%
- Curcuminoides—4.2%
- Alkaloids—2.0%
- Xanton—single case
- With—single case
- Chalkones—single case
- Diterpenes—single case
- Anthr—single case
- Stilben—single case
- Isoflavones—single case
- Lecitin—single case
- Vinil chlo—single case
- Leicyclo—single case
- Alkohol-ester—single case
- Pen acid—single case
- Prenolip—single case
- The application of larger number of descriptors gives more opportunities to explain the partitioning;
- The use of a smaller number of descriptors elucidates some more specific properties of some flavonoids.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Searls, D.B. Data integration: Challenges for drug discovery. Nat. Rev. Drug Discov. 2005, 4, 45–58. [Google Scholar] [CrossRef]
- Lo, Y.-C.; Rensi, S.E.; Torng, W.; Altman, R.B. Machine learning in chemoinformatics and drug discovery. Drug Discov. Today 2018, 23, 1538–1546. [Google Scholar] [CrossRef]
- Atanasov, A.G.; The International Natural Product Sciences Taskforce; Zotchev, S.B.; Dirsch, V.M.; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- Zin, P.P.K.; Williams, G.J.; Ekins, S. Cheminformatics Analysis and Modeling with MacrolactoneDB. Sci. Rep. 2020, 10, 6284. [Google Scholar] [CrossRef]
- Sandoval-Lira, J.; Mondragón-Solórzano, G.; Lugo-Fuentes, L.I.; Barroso-Flores, J. Accurate Estimation of pKb Values for Amino Groups from Surface Electrostatic Potential (VS,min) Calculations: The Isoelectric Points of Amino Acids as a Case Study. J. Chem. Inf. Model. 2020, 60, 1445–1452. [Google Scholar] [CrossRef]
- Caballero-García, G.; Mondragón-Solórzano, G.; Torres-Cadena, R.; Díaz-García, M.; Sandoval-Lira, J.; Barroso-Flores, J. Calculation of VS,max and Its Use as a Descriptor for the Theoretical Calculation of pKa Values for Carboxylic Acids. Molecules 2019, 24, 79. [Google Scholar] [CrossRef] [PubMed]
- Sabbah, D.A.; Haroon, R.A.; Bardaweel, S.K.; Hajjo, R.; Sweidan, K. N-phenyl-6-chloro-4-hydroxy-2-quinolone-3-carboxamides: Molecular Docking, Synthesis, and Biological Investigation as Anticancer Agents. Molecules 2021, 26, 73. [Google Scholar] [CrossRef] [PubMed]
- Ancuceanu, R.; Hovanet, M.V.; Anghel, A.I.; Furtunescu, F.; Neagu, M.; Constantin, C.; Dinu, M. Computational Models Using Multiple Machine Learning Algorithms for Predicting Drug Hepatotoxicity with the DILIrank Dataset. Int. J. Mol. Sci. 2020, 21, 2114. [Google Scholar] [CrossRef] [PubMed]
- Agoni, C.; Olotu, F.A.; Ramharack, P.; Soliman, M.E. Druggability and drug-likeness concepts in drug design: Are biomodelling and predictive tools having their say? J. Mol. Model. 2020, 26, 120. [Google Scholar] [CrossRef]
- Cheng, A.C.; Coleman, R.G.; Smyth, K.T.; Cao, Q.; Soulard, P.; Caffrey, D.R.; Salzberg, A.C.; Huang, E.S. Structure-based maximal affinity model predicts small-molecule druggability. Nat. Biotechnol. 2007, 25, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Barril, X. Druggability predictions: Methods, limitations, and applications. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2013, 3, 327–338. [Google Scholar] [CrossRef]
- Kozakov, D.; Hall, D.R.; Napoleon, R.L.; Yueh, C.; Whitty, A.; Vajda, S. New Frontiers in Druggability. J. Med. Chem. 2015, 58, 9063–9088. [Google Scholar] [CrossRef] [PubMed]
- Danishuddin; Khan, A.U. Descriptors and their selection methods in QSAR analysis: Paradigm for drug design. Drug Discov. Today 2016, 21, 1291–1302. [Google Scholar] [CrossRef] [PubMed]
- Nedyalkova, M.; Simeonov, V. Multivariate Chemometrics as a Strategy to Predict the Allergenic Nature of Food Proteins. Symmetry 2020, 12, 1616. [Google Scholar] [CrossRef]
- Szefler, B.; Czeleń, P. Docking of Platinum Compounds on Cube Rhombellane Functionalized Homeomorphs. Symmetry 2020, 12, 749. [Google Scholar] [CrossRef]
- Halder, A.K.; Cordeiro, M.N.D.S. Development of Multi-Target Chemometric Models for the Inhibition of Class I PI3K Enzyme Isoforms: A Case Study Using QSAR-Co Tool. Int. J. Mol. Sci. 2019, 20, 4191. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A. Lead and drug-like compounds: The rule-of-five revolution. Drug Discov. Today: Technol. 2004, 1, 337–341. [Google Scholar] [CrossRef]
- Mishra, B.B.; Tiwari, V.K. Natural products: An evolving role in future drug discovery. Eur. J. Med. Chem. 2011, 46, 4769–4807. [Google Scholar] [CrossRef] [PubMed]
- Cragg, G.M.; Newman, D.J. Natural products: A continuing source of novel drug leads. Biochim. Biophys. Acta (BBA) Gen. Subj. 2013, 1830, 3670–3695. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Calixto, J.B. The role of natural products in modern drug discovery. An. Acad. Bras. Ciências 2019, 91, e20190105. [Google Scholar] [CrossRef] [PubMed]
- Chiang, L.-C.; Ng, L.-T.; Cheng, P.-W.; Chiang, W.; Lin, C.-C. Antiviral activities of extracts and selected pure constituents of Ocimum basilicum. Clin. Exp. Pharmacol. Physiol. 2005, 32, 811–816. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.-W.; Ng, L.-T.; Chiang, L.-C.; Lin, C.-C. Antiviral effects of saikosaponins on human Coronavirus 229e In Vitro. Clin. Exp. Pharmacol. Physiol. 2006, 33, 612–616. [Google Scholar] [CrossRef]
- Li, S.-I.; Chen, G.; Zhang, H.-Q.; Guo, H.-Y.; Wang, H.; Wang, L.; Zhang, X.; Hua, S.-N.; Yu, J.; Xiao, P.-g.; et al. Identification of natural compounds with antiviral activities against SARS-associated coronavirus. Antivir. Res. 2005, 67, 18–23. [Google Scholar] [CrossRef]
- Rivero-Segura, N.A.; Gomez-Verjan, J.C. In Silico Screening of Natural Products Isolated from Mexican Herbal Medicines against COVID-19. Biomolecules 2021, 11, 216. [Google Scholar] [CrossRef]
- Gil, B.; Sanz, M.J.; Terencio, M.C.; Ferrandiz, M.L.; Paya, M.; Gunasegaran, R.; Alcaraz, M.J. Effects of flavonoids on Naja naja and human recombinant synovial phospholipase A2 and inflammatory responses in mice. Life Sci. 1994, 54, PL333–PL338. [Google Scholar] [CrossRef]
- Chang, H.; Baek, S.; Chung, K.; Son, K.; Kim, H.; Kang, S. Inactivation of Phospholipase A2 by Naturally Occurring Biflavonoid, Ochnaflavone. Biochem. Biophys. Res. Commun. 1994, 205, 843–849. [Google Scholar] [CrossRef]
- Cheon, B.S.; Kim, Y.H.; Son, K.S.; Chang, H.W.; Kang, S.S.; Kim, H.P. Effects of Prenylated Flavonoids and Biflavonoids on Lipopolysaccharide-Induced Nitric Oxide Production from the Mouse Macrophage Cell Line RAW 264.7. Planta Med. 2000, 66, 596–600. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.; Li, Z.; Liu, Q.; Gao, Y.; Dai, J.; Bao, B.; Zhang, L.; Ding, A. Cellular Metabolomics Revealed the Cytoprotection of Amentoflavone, a Natural Compound, in Lipopolysaccharide-Induced Injury of Human Umbilical Vein Endothelial Cells. Int. J. Mol. Sci. 2016, 17, 1514. [Google Scholar] [CrossRef]
- Mauri, A. alvaDesc: A tool to calculate and analyze molecular descriptors and fingerprints. In Ecotoxicological QSARs. Methods in Pharmacology and Toxicology; Roy, K., Ed.; Humana: New York, NY, USA, 2020. [Google Scholar]
- Elisabet, G.; Mestres, J. SHED: Shannon Entropy Descriptors from Topological Feature Distributions. J. Chem. Inf. Modeling 2006, 46, 1615–1622. [Google Scholar]
| Code | Decriptors Categories | |
|---|---|---|
| CATS2D_00_LL Var 1 | Pharmacophore descriptors—Chemically Advanced Template Search—Dono-Donor at lag 00 to lag 06 (number of H bond donor atoms) | |
| CATS2D_01_LL Var 2 | ||
| CATS2D_02_LL Var 3 | ||
| CATS2D_03_LL Var 4 | ||
| CATS2D_04_LL Var 5 | ||
| CATS2D_05_LL Var 6 | ||
| CATS2D_06_LL Var 7 | ||
| SHED_DL Var 8 | Pharmacophore descriptors—Shannon Entropy Descriptors DL (donor-lipophilic; AA—(acceptor-acceptor) AL (acceptor-lipophilic) LL (lipophiloc-lipophilic) | |
| SHED_AA Var 9 | ||
| SHED_AL Var 10 | ||
| SHED_LL Var 11 | ||
| Uc Var 12 | Unsaturation count; unsaturation index; hydrophiloic factor; Wildman-Grippen molar refractivity; molar refractivity (consensus); topological polar surface area using N,O polar contributions; topological polar surface area using N,O, S, N polar contributions; Moriguchi octanol-water partition coeff. (logP); Moriguchi octanol-water partition coeff. (logP2); Wildman–Gripen octanol-water partition coeff. (logP); octanol-water partition coeff. (logP consensus); estimated solubility(LogS) for aqueous solubility; total surface area from P_VCA like descriptors; surface area of acceptor atoms from P_VCA like descriptors; surface area of donor atoms from P_VCA like descriptors; McGowan volume; Van der Waals volume from McGowan volume; Van der Waals volume from Zhao–Abraham Zissimos equation; packing density index; Synthetic accessability score | Molecular properties |
| Ui Var 13 | ||
| Hy Var 14 | ||
| MR99 Var 15 | ||
| MRcons Var 16 | ||
| TPSA(NO) Var 17 | ||
| TPSA(Tot) Var 18 | ||
| MLOGP Var 19 | ||
| MLOGP2 Var 20 | ||
| LOGP99 Var 21 | ||
| LOGPcons Var22 | ||
| ESOL Var 23 | ||
| SAtot Var 24 | ||
| SAacc Var 25 | ||
| SAdon Var 26 | ||
| Vx Var 27 | ||
| VvdwMG Var 28 | ||
| VvdwZAZ Var 29 | ||
| PDI Var 30 | ||
| SAscore Var 31 | ||
| Ro5 Var 32 | Lipinski rule of 5; Complementary Lipinski alert index; modified drug-like score from Lipinski (4 rules); modified drug-like score from Oprea (6 rules); modified drug-like score from Walters(6 rules); modified drug-like score from Chen (4 rules); modified drug-like score from Zheng (2 rules); modified drug-like score from Rishton (6 rules); modified drug-like score from Veber (2 rules); DRAGON consensus drug-like score; modified lead-like score from Congreve et al.(6 rules); modified lead-like score from Mongue et al.(8 rules); Quantitative estimation of drug-likeness (unweighted); Quantitative estimation of drug-likeness | Drug-like indices |
| cRo5 Var 33 | ||
| DLS_01 Var 34 | ||
| DLS_02 Var35 | ||
| DLS_03 Var 36 | ||
| DLS_04 Var 37 | ||
| DLS_05 Var 38 | ||
| DLS_06 Var 39 | ||
| DLS_07 Var 40 | ||
| DLS_cons Var 41 | ||
| LLS_01 Var 42 | ||
| LLS_02 Var 43 | ||
| QEDu Var 44 | ||
| QED Var 45 |
| Clusters Modes | Descriptors with Highest Levels | Descriptors with Lowest Levels |
|---|---|---|
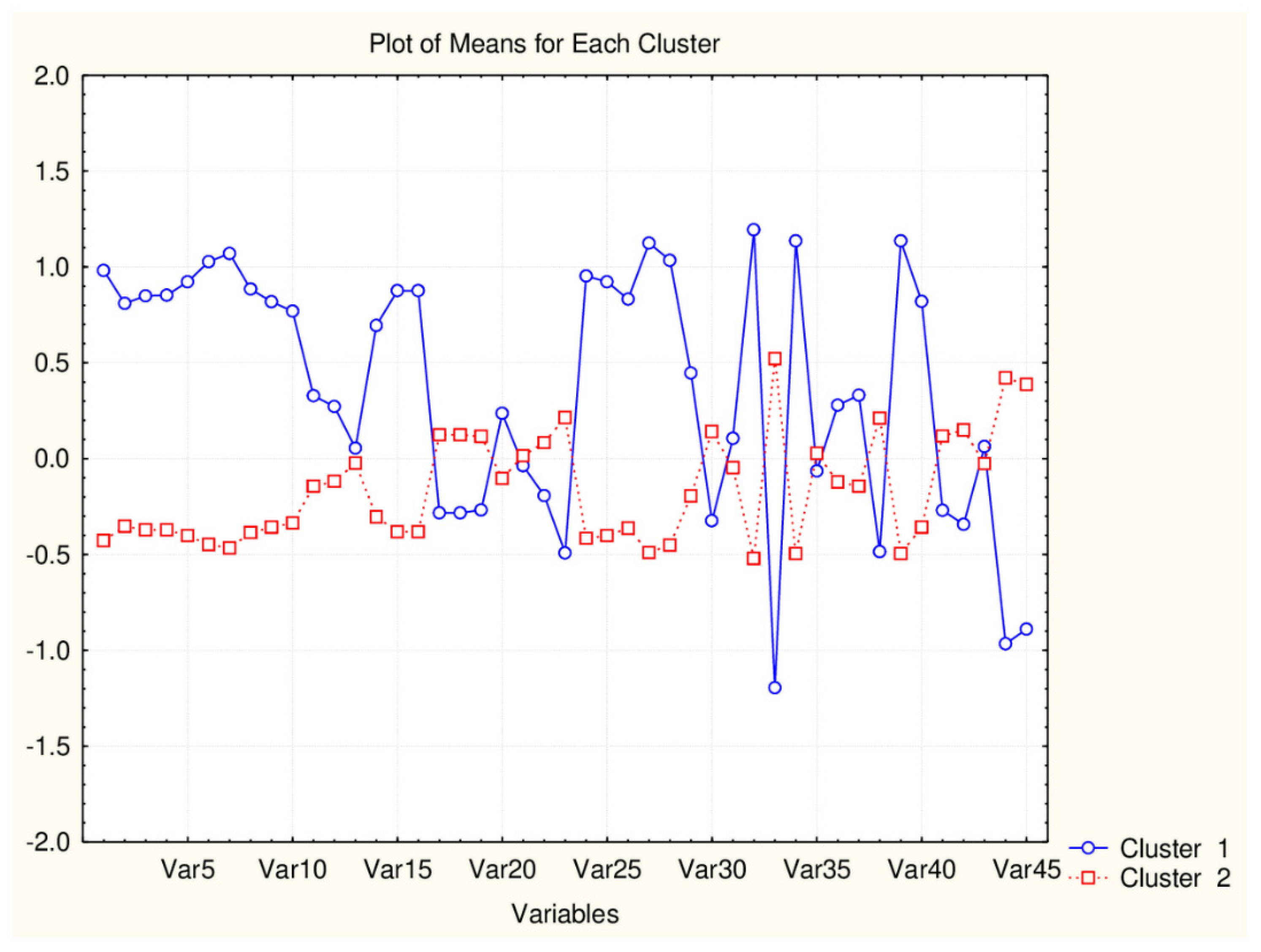
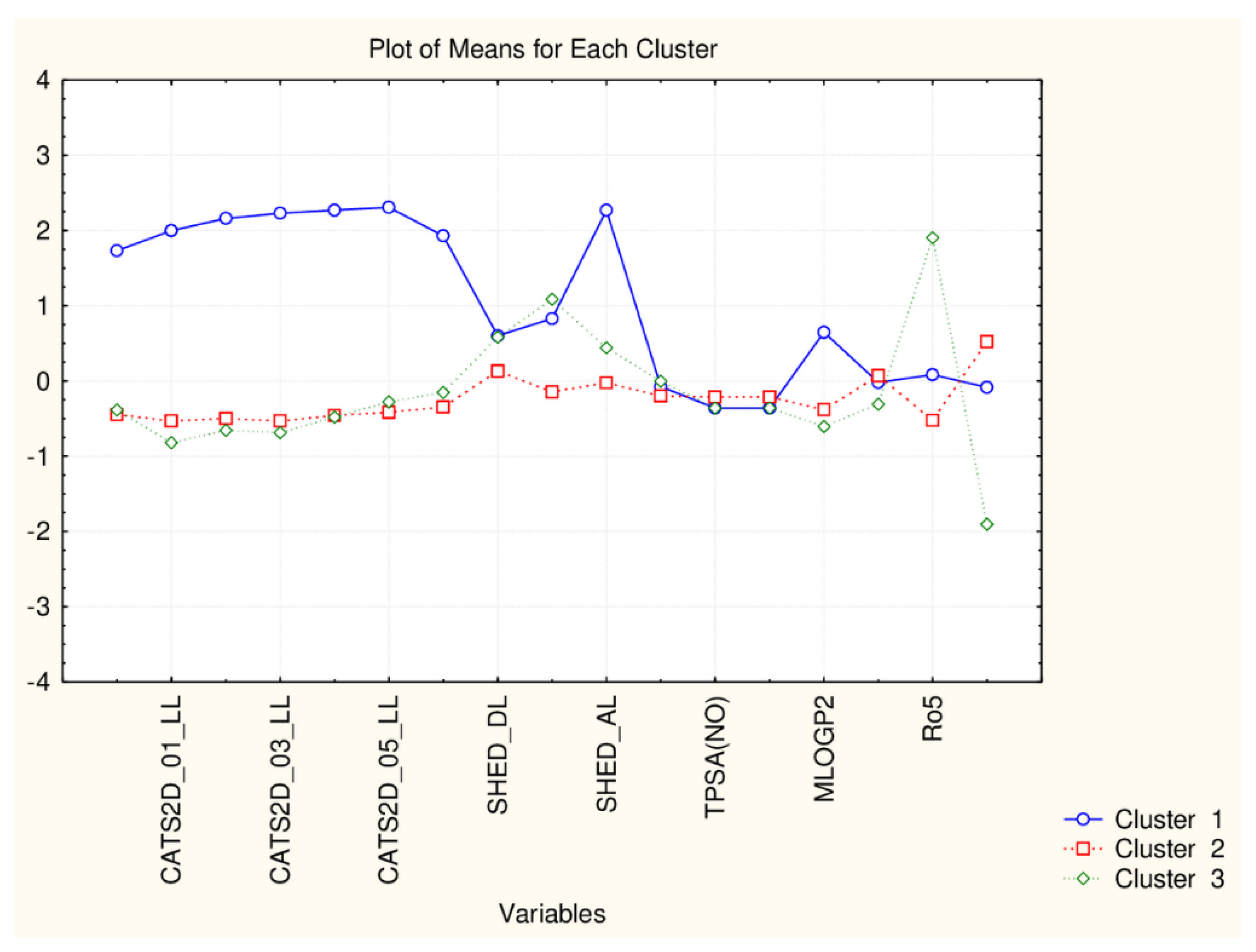
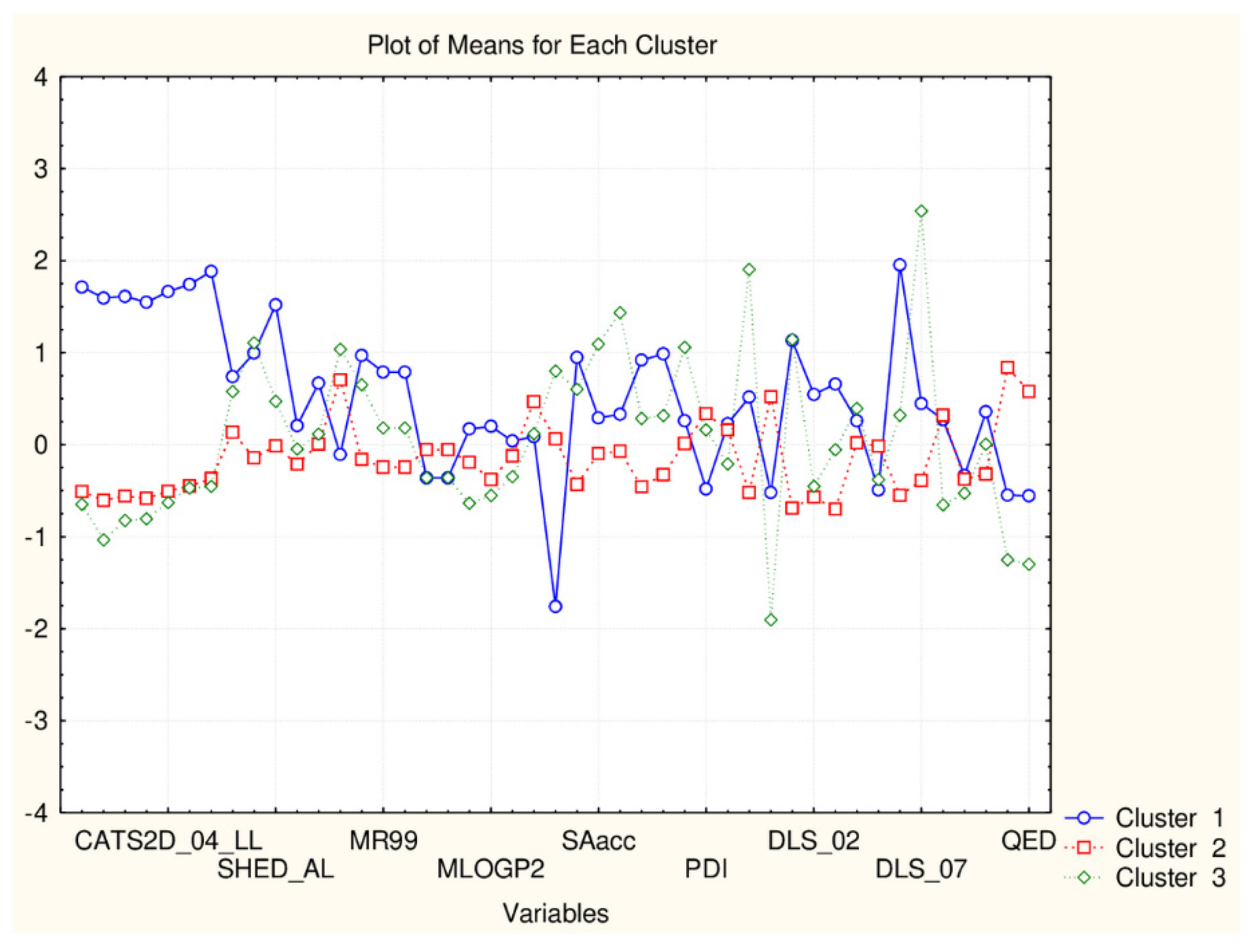
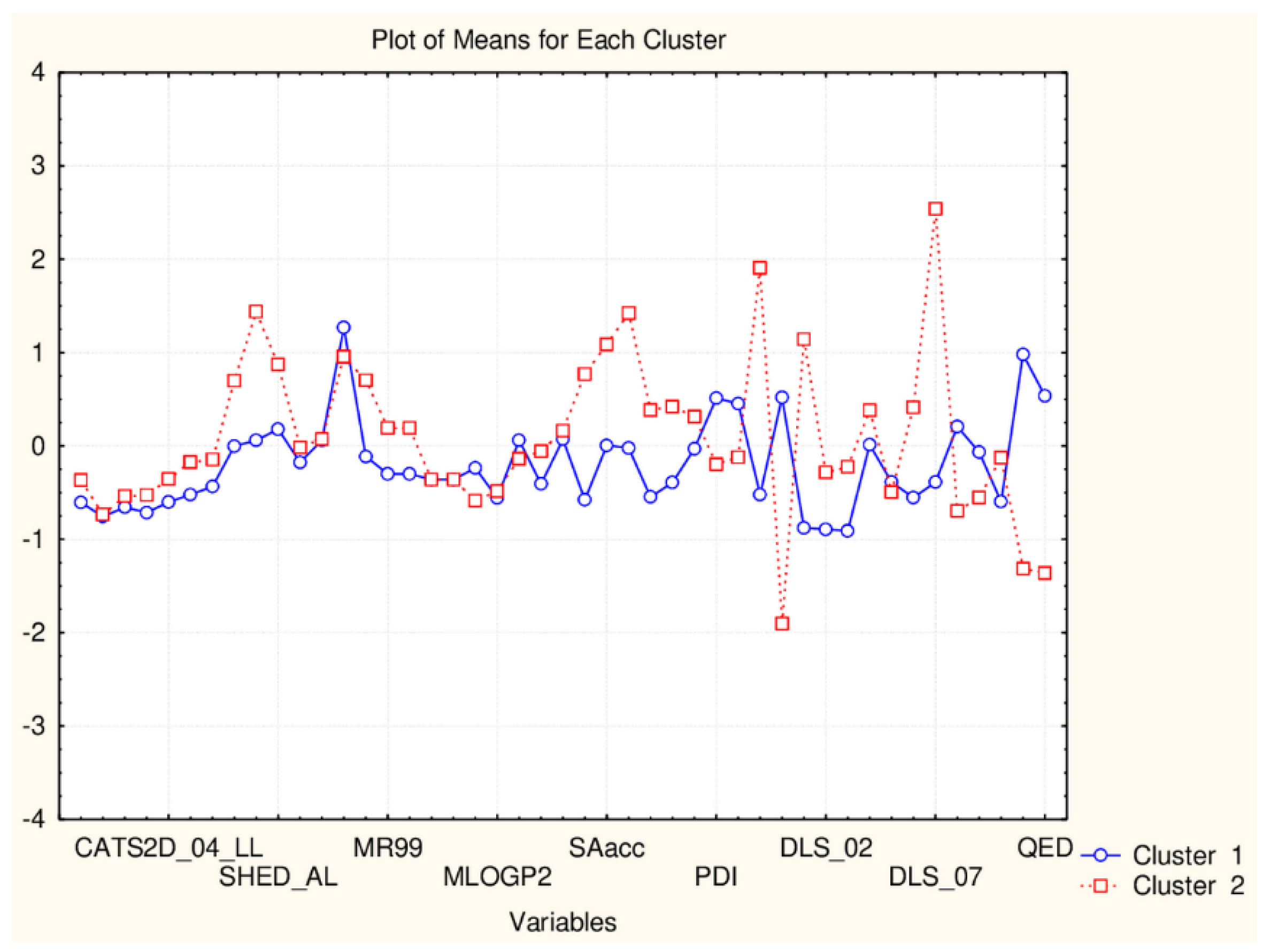
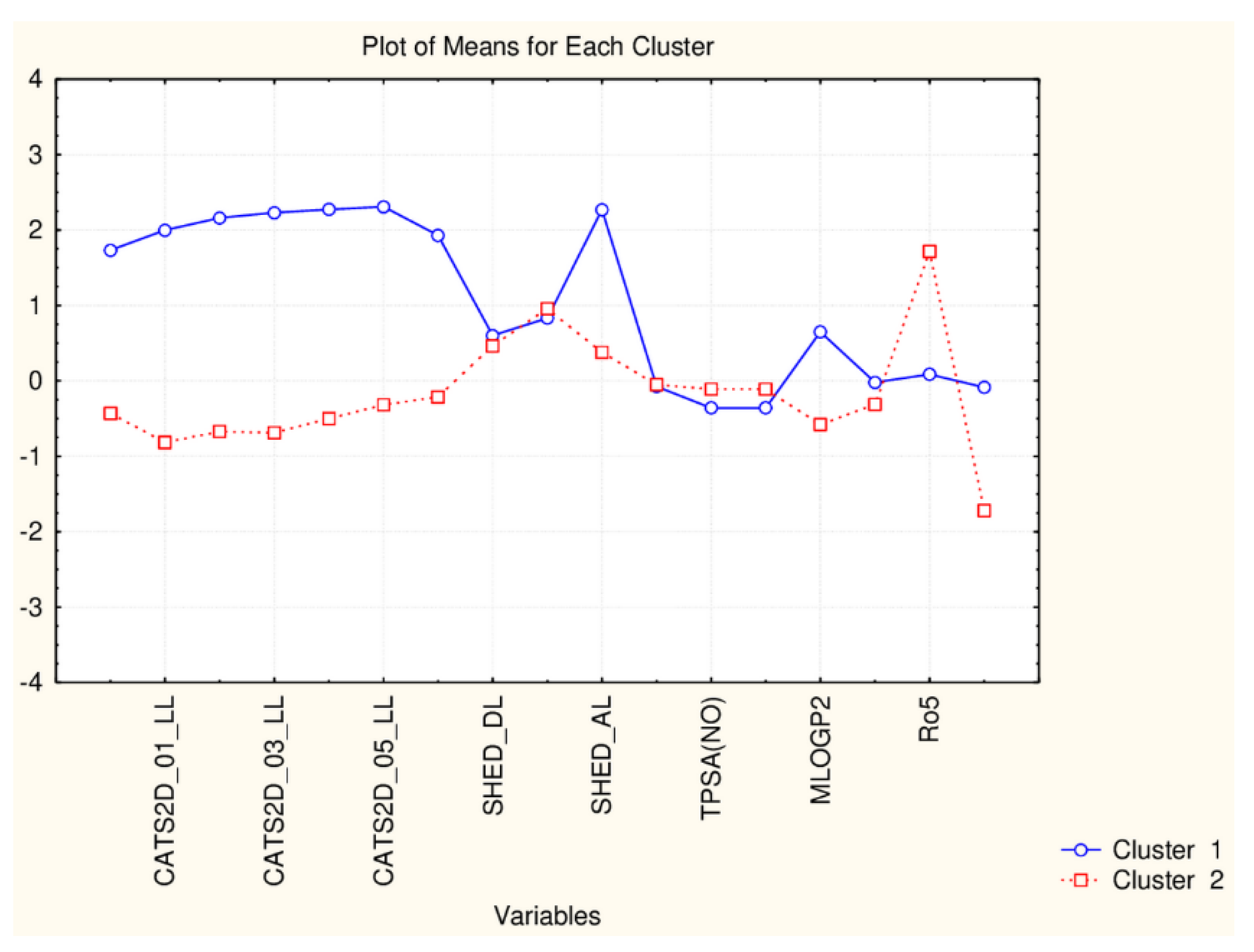
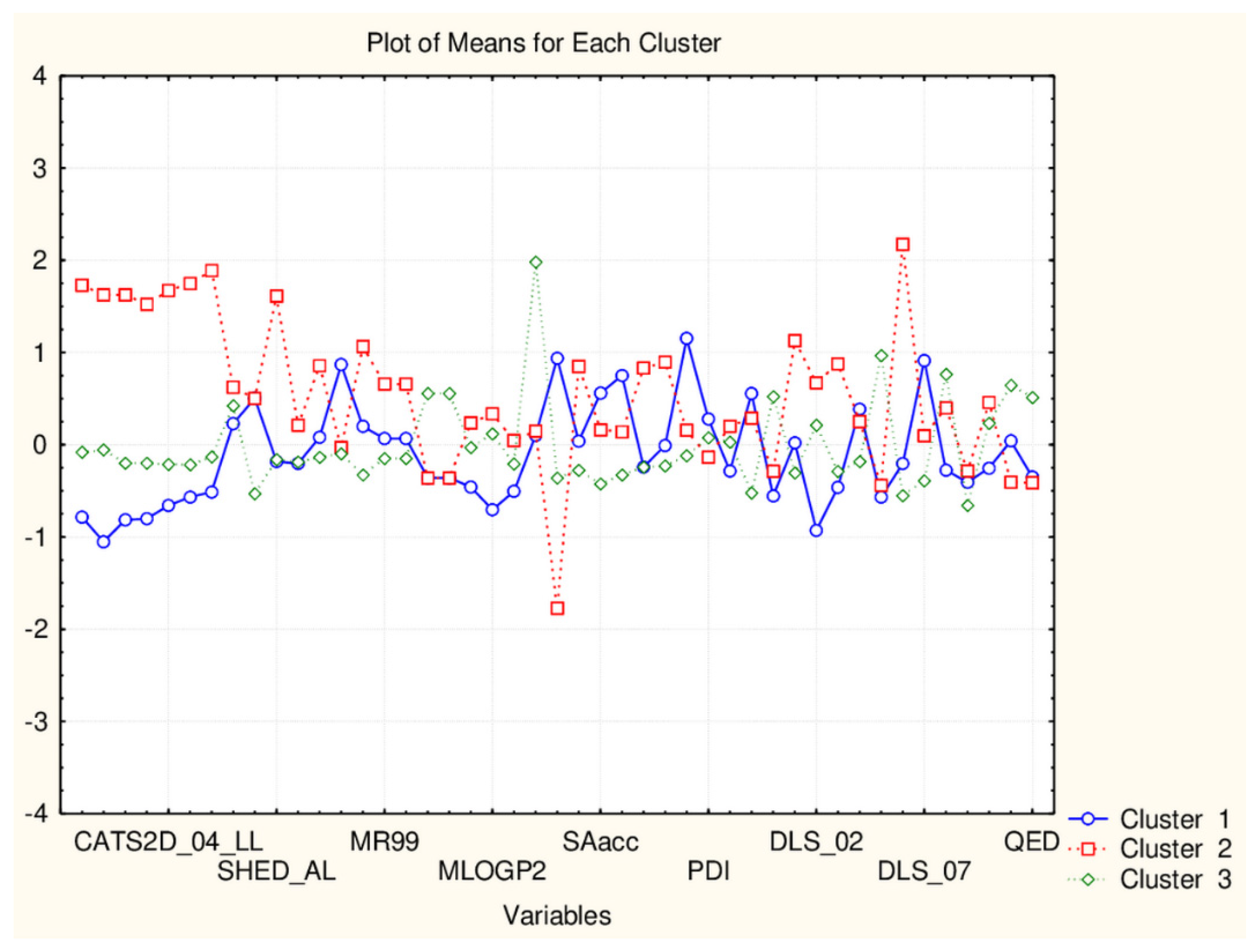
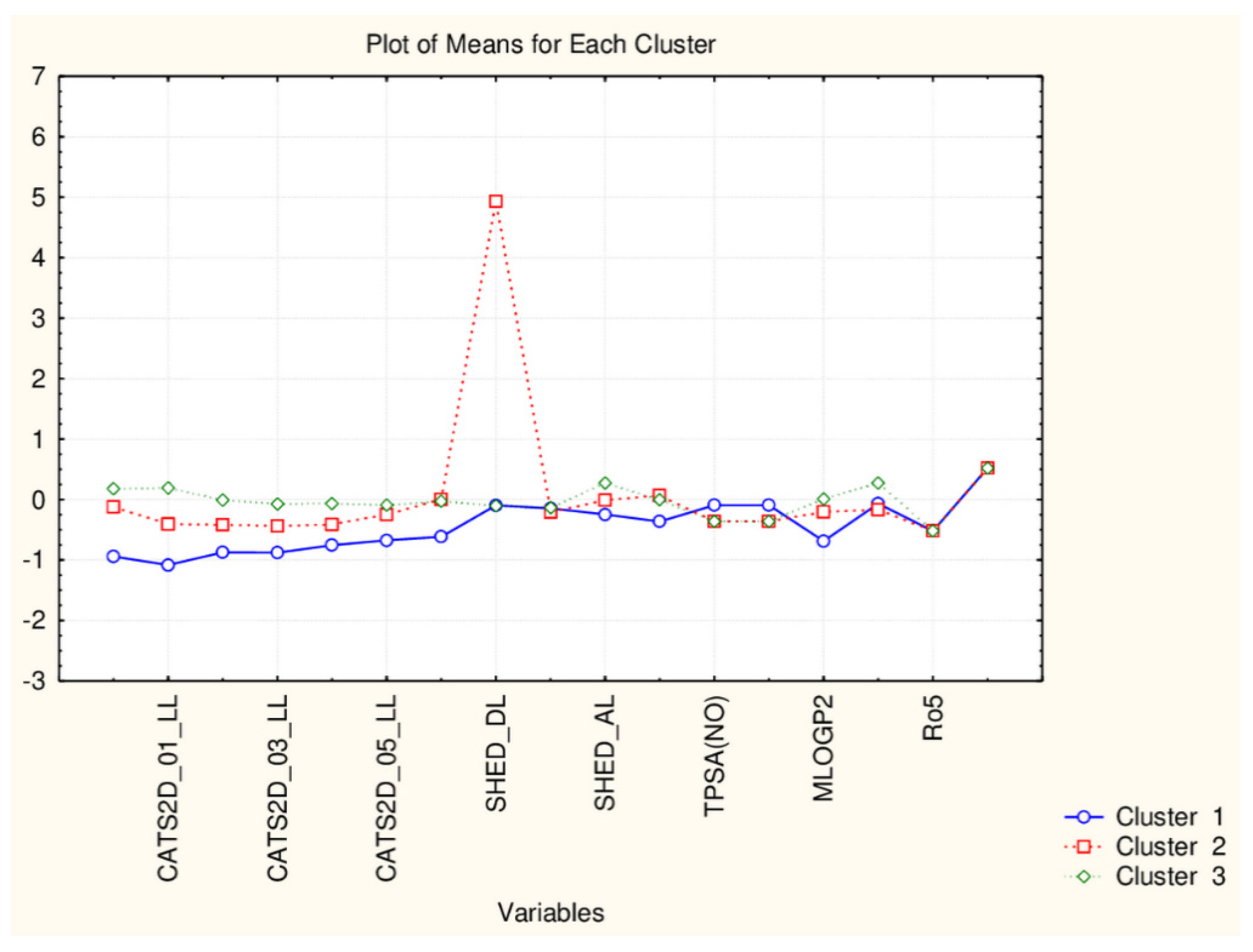
| C2_135_45 (94) | TPSA(NO), TPSA(Tot), MLOGP, MLOGP2, LOGP99, LOGPcons, ESOL, PDI), cRo5, DLS_02, DLS_05, DLS_cons, LLS_01, LLS_02, QEDu, QED | All CATS2D and SHED, Uc, Ui, Hy. MR99, MRcons, SAtot, SAacc, Vx, Vvdw, MG, VvdwZAZ, Sascore, Ro5, DLS_01), DLS_03, DLS_04, DLS_06, DLS_07. |
| Big cluster | ||
| Sesquiterpenes | ||
| Monoterpenes | ||
| Curcuminoids | ||
| Flavonoids | ||
| C1_135_45 (41) | All CATS2D and SHED, Uc, Ui, Hy. MR99, MRcons, SAtot, SAacc, Vx, Vvdw, MG, VvdwZAZ, Sascore, Ro5, DLS_01), DLS_03, DLS_04, DLS_06, DLS_07. | TPSA(NO), TPSA(Tot), MLOGP, MLOGP2, LOGP99, LOGPcons, ESOL, PDI, cRo5, DLS_02, DLS_05, DLS_cons, LLS_01, LLS_02, QEDu, QED |
| Small cluster | ||
| Flavonoids | ||
| Triterpenes | ||
| Saponines | ||
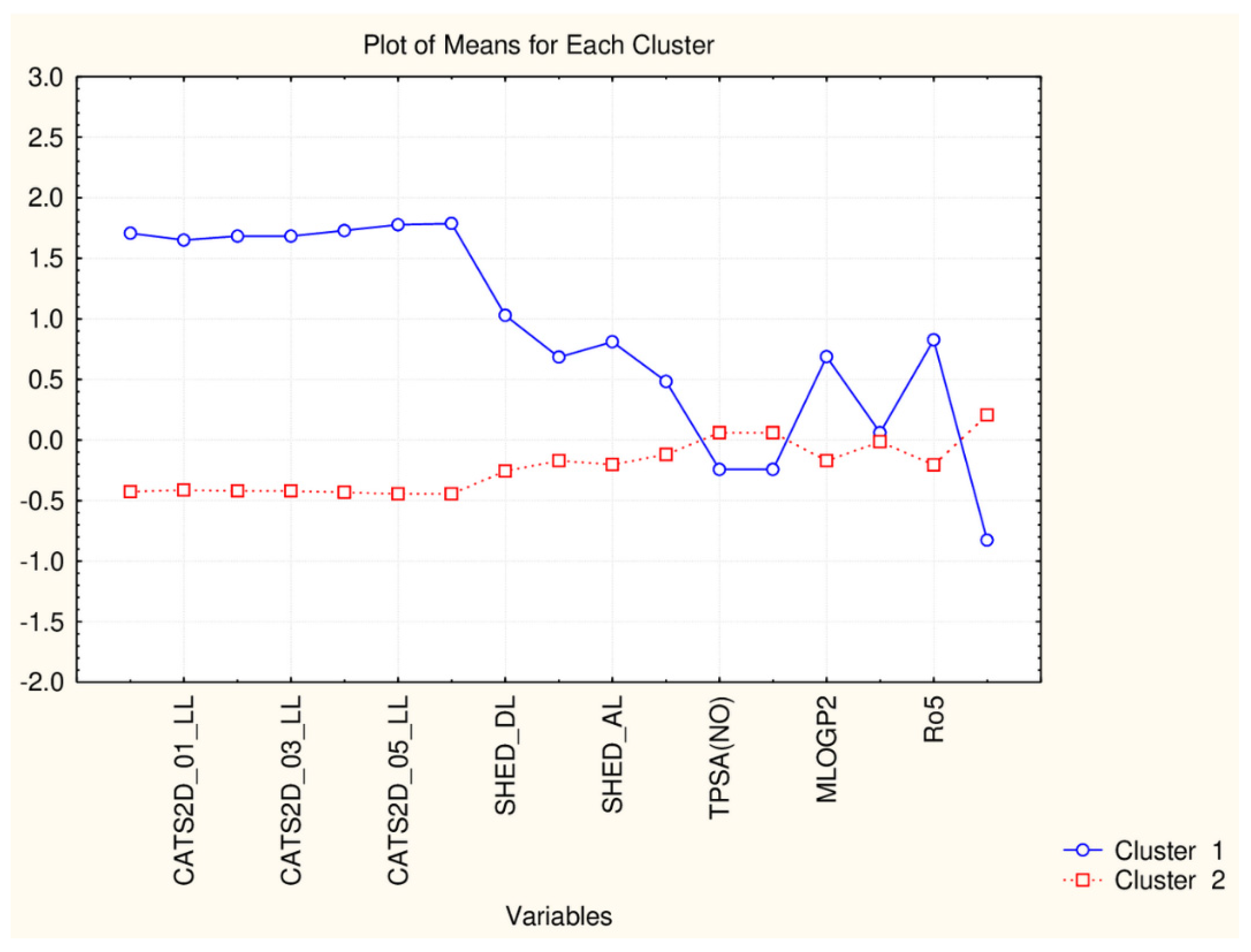
| C2_135_17 (94) | All TPSA (2), LOPGP99, cR05 | All CATS2D (7), all SHED (4), Ro5, MLOGP |
| Big cluster | ||
| Sesquiterpenes | ||
| Monoterpenes | ||
| Curcuminoids | ||
| Flavonoids | ||
| C1_135_17 (41) | All CATS2D (7), all SHED (4), Ro5, MLOGP | All TPSA (2), LOGPGP99, cR05 |
| Small cluster | ||
| Flavonoids | ||
| Triterpenes | ||
| Saponines |
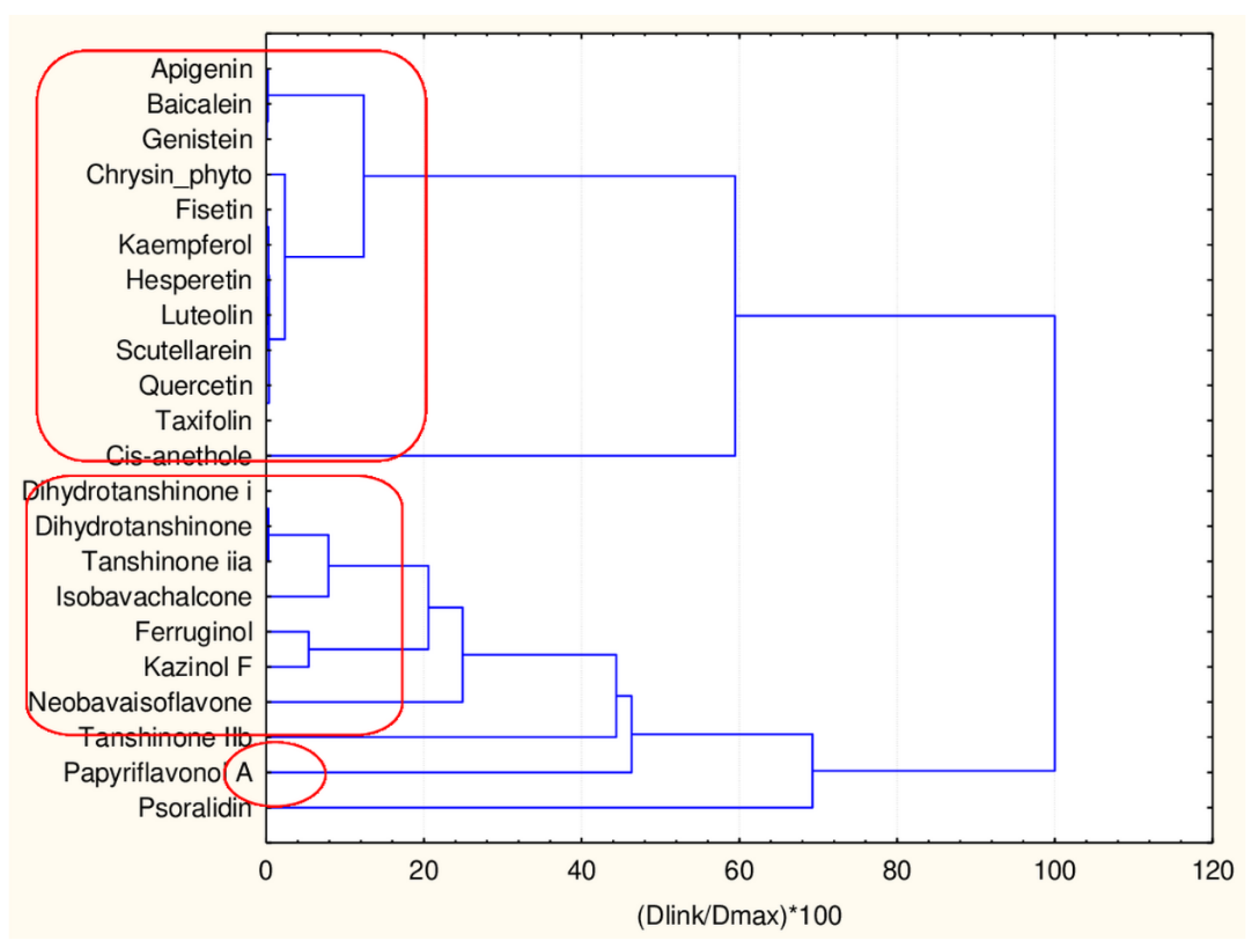
| Natural Compound | Classes | 45 Descriptors Clustering | 17 Descriptors Clustering |
|---|---|---|---|
| Smilagenin | saponin | 2 | 2 |
| 10′hydroxyusambarensine | alkaloid | 1 | 1 |
| Saikosaponin B2 | saponin | 2 | 2 |
| Celastrol | flavonoid | 2 | 2 |
| Amentoflavone | flavonoid | 2 | 2 |
| Epitaraxerol | flavonoid | 2 | 2 |
| Baicalein | flavonoid | 1 | 1 |
| Glycyrrhizin | flavonoid | 2 | 2 |
| Pristimerin | flavonoid | 2 | 2 |
| Chrysin | flavonoid | 1 | 1 |
| Myricetinetin_3′-Rhamnoside | flavonoid | 2 | 2 |
| Ardisia Saponin | saponin | 2 | 2 |
| Friedelin | triterpen | 2 | 2 |
| Rhoifolin | flavonoid | 2 | 2 |
| Psoralidin | flavones | 1 | 1 |
| Dihydrotanshinone | flavonoid | 1 | 1 |
| Epigallocatechin_gallate | flavonoid | 2 | 2 |
| Akebia_saponin_c | saponin | 2 | 2 |
| Gallocatechin | flavonoid | 2 | 2 |
| Myricetin_3-(4′′-Galloylrhamnoside) | flavonoid | 2 | 2 |
| Pectolinarin | flavonoid | 2 | 2 |
| Dihydrotanshinone i | flavonoid | 1 | 1 |
| Isobavachalcone | flavonoid | 1 | 1 |
| Pyranojacareubin | xanthone | 1 | 1 |
| Withanone | withanolides | 1 | 1 |
| Papyriflavonol A | flavonoid | 1 | 1 |
| Scutellarein | flavonoid | 1 | 1 |
| Quercetin | flavonoid | 1 | 1 |
| Ouabain | flavonoid | 2 | 2 |
| Ursane | Triterpenes | 2 | 2 |
| Demethoxycurcumin | curcuminoid | 1 | 1 |
| Luteolin | flavonoid | 1 | 1 |
| Monodemethylcurcumin | curcuminoid | 1 | 1 |
| Xanthoangelol | chalcone | 1 | 1 |
| Fisetin | flavonoid | 1 | 1 |
| Neobavaisoflavone | flavonoid | 1 | 1 |
| Biochanin a | phytoestrogen | 1 | 1 |
| Broussochalcone b | chalcone | 1 | 1 |
| Schimperinone | triterpene | 2 | 2 |
| Tanshinone i | diterpene | 1 | 1 |
| Tau-Cadinol | sesquiterpenoid | 1 | 1 |
| Quadrangularic acid | triterpenoid | 2 | 2 |
| 6-Oxoisoiguesterin | terpenoid | 2 | 2 |
| Isoliquiritigenin | chalcone | 1 | 1 |
| Beta-Sitosterol | phytosterol | 2 | 2 |
| Rhein | anthraquinone derivative | 1 | 1 |
| Nummularine B | alkaloid | 2 | 2 |
| Jubanine G | alkaloid | 2 | 2 |
| Genistein | flavonoid | 1 | 1 |
| Betulinic acid | triterpene | 2 | 2 |
| Tetrahydrocurcumin | curcuminoid | 1 | 1 |
| Hesperetin | flavonoid | 1 | 1 |
| Dehydroabieta-7-one | terpene | 1 | 1 |
| Iguesterin | triterpene | 2 | 2 |
| 7-Methoxycryptopleurine | alkaloid | 1 | 1 |
| Artocommunol e | flavonoids | 2 | 2 |
| Ferruginol | flavonoids | 1 | 1 |
| Tanshinone | flavonoids | 1 | 1 |
| Kaempferol | flavonoid | 1 | 1 |
| Apigenin | flavonoid | 1 | 1 |
| Tanshinone IIb | flavonoid | 1 | 1 |
| Myricetin—3- Sambubioside | flavonoid | 2 | 2 |
| Gamma-Gurjunene | terpenes | 1 | 1 |
| Ampelopsin | Stilbene | 1 | 1 |
| Allo-Aromadendrene | sesquiterpenoid | 1 | 1 |
| Sanggenol E | flavonoid | 2 | 2 |
| Ledene | sesquiterpenoid | 1 | 1 |
| Jubanine H | alkaloid | 2 | 2 |
| Formononetin | flavonoid | 1 | 1 |
| Camazulene | sesquiterpene | 1 | 1 |
| Germacrene b | sesquiterpenoid | 1 | 1 |
| Myricetin_3-Neohesperidoside | flavonoid | 2 | 2 |
| 6,7-dehydroroyleanone | terpenoid | 1 | 1 |
| Alpha-Cubebene | sesquiterpenoid | 1 | 1 |
| Kazinol F | flavonoid | 1 | 1 |
| Taxifolin | flavonoid | 1 | 1 |
| Silvestrol | flavonoid | 2 | 2 |
| Muurolene | sesquiterpenoid | 1 | 1 |
| Caryophyllene oxide | terpenoid | 1 | 1 |
| Methyl tanshinonate | tanshinone | 1 | 1 |
| Spathulenol | sesquiterpenoid | 1 | 1 |
| Guaiol | sesquiterpenoid | 1 | 1 |
| Emodin | hydroxyanthraquinone | 1 | 1 |
| Alpha-selinene | sesquiterpenoid | 1 | 1 |
| Alpha-Bisabolol | sesquiterpenoid | 1 | 1 |
| Terpinen-4-ol | monoterpenoid | 1 | 1 |
| 4-Terpinyl acetate | monoterpenoid | 1 | 1 |
| Curcumin | curcuminoid | 1 | 1 |
| Eugenol | methoxyphenol | 1 | 1 |
| Isoledene | sesquiterpene | 1 | 1 |
| APA | lectin | 1 | 1 |
| Carvacrol | monoterpene | 1 | 1 |
| Viridiflorol | sesquiterpenoid | 1 | 1 |
| Cryptojaponol | terpene | 1 | 1 |
| Blancoxanthone | pyranoxanthone | 1 | 1 |
| Limonene | monoterpene | 1 | 1 |
| Sappanchalcone | chalcones | 1 | 1 |
| Pinocarvone | prenol lipids | 1 | 1 |
| (E)-caryophyllene | terpenoids | 1 | 1 |
| Longifolen | sesquiterpene | 1 | 1 |
| 1-Cyclopentyl-2-propen-1-ol- | vinyl chloride | 1 | 1 |
| Beta-Thujone | monoterpene | 1 | 1 |
| Sabinene | monoterpene | 1 | 1 |
| Myrcene | monoterpene | 1 | 1 |
| Bicyclogermacrene | bicyclogermacrane | 1 | 1 |
| Citronellyl acetate | alcohol ester | 1 | 1 |
| Gallic acid | phenolic acid | 1 | 1 |
| Artemisia alcohol | carboxylic acid ester | 1 | 1 |
| Cis-anethole | flavonoid | 1 | 1 |
| Isopinocamphone | terpenoid | 1 | 1 |
| L-Thujone | terpene | 1 | 1 |
| Alpha-pinene | terpene | 1 | 1 |
| Anethole | flavonoid | 1 | 1 |
| (+)-artemisinic_alcohol | carboxylic acid ester | 1 | 1 |
| Artemisia_ketone | enone | 1 | 1 |
| Ascaridole | monoterpene | 1 | 1 |
| 1,8-Cineole | monoterpene | 1 | 1 |
| Piperitone | monoterpene | 1 | 1 |
| Thujene | monoterpene | 1 | 1 |
| Beta-pinene | monoterpene | 1 | 1 |
| 3-beta-Friedelanol | terpenes | 1 | 1 |
| Linalool | monoterpenoid | 1 | 1 |
| Trans-anethole | anisoles (essential oils) | 1 | 1 |
| Camphor | terpenoid ketones | 1 | 1 |
| Camphene | monoterpene | 1 | 1 |
| 12-Deoxy-6,7-Dehydroroyleanone | tricyclic diterpenoid | 1 | 1 |
| Cyclamine | saponin | 2 | 2 |
| Ginsenoside | triterpenoids | 2 | 2 |
| Ginsenoside | triterpenoids | 2 | 2 |
| Ginsenoside rb1 | triterpenoids | 2 | 2 |
| Mi-saponin A | saponin | 2 | 2 |
| Saikosaponin C | saponin | 2 | 2 |
| Saikosaponin D | saponin | 2 | 2 |
| Saponin Pk | saponin | 2 | 2 |
| Tannic acid | tannin | 2 | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nedyalkova, M.; Simeonov, V. Partitioning Pattern of Natural Products Based on Molecular Properties Descriptors Representing Drug-Likeness. Symmetry 2021, 13, 546. https://doi.org/10.3390/sym13040546
Nedyalkova M, Simeonov V. Partitioning Pattern of Natural Products Based on Molecular Properties Descriptors Representing Drug-Likeness. Symmetry. 2021; 13(4):546. https://doi.org/10.3390/sym13040546
Chicago/Turabian StyleNedyalkova, Miroslava, and Vasil Simeonov. 2021. "Partitioning Pattern of Natural Products Based on Molecular Properties Descriptors Representing Drug-Likeness" Symmetry 13, no. 4: 546. https://doi.org/10.3390/sym13040546
APA StyleNedyalkova, M., & Simeonov, V. (2021). Partitioning Pattern of Natural Products Based on Molecular Properties Descriptors Representing Drug-Likeness. Symmetry, 13(4), 546. https://doi.org/10.3390/sym13040546







