Abstract
In this work, anchoring of cinchona derivatives to trifunctional cores (hub approach) was demonstrated to obtain size-enlarged organocatalysts. By modifying the cinchona skeleton in different positions, we prepared four C3-symmetric size-enlarged cinchona derivatives (hub-cinchonas), which were tested as organocatalysts and their catalytic activities were compared with the parent cinchona (hydroquinine) catalyst. We showed that in the hydroxyalkylation reaction of indole, hydroquinine provides good enantioselectivities (up to 73% ee), while the four new size-enlarged derivatives resulted in significantly lower values (up to 29% ee) in this reaction. Anchoring cinchonas to trifunctional cores was found to facilitate nanofiltration-supported catalyst recovery using the PolarClean alternative solvent. The C3-symmetric size-enlarged organocatalysts were completely rejected by all the applied membranes, whereas the separation of hydroquinine was found to be insufficient when using organic solvent nanofiltration. Furthermore, the asymmetric catalysis was successfully demonstrated in the case of the Michael reaction of 1,3-diketones and trans-β-nitrostyrene using Hub3-cinchona (up to 96% ee) as a result of the positive effect of the C3-symmetric structure using a bulkier substrate. This equates to an increased selectivity of the catalyst in comparison to hydroquinine in the latter Michael reaction.
1. Introduction
Over the years, catalysis has been widely explored for the more economical and often more selective production of high-value products [1]. As the preparation of enantiopure organic compounds is of great interest, asymmetric catalysis has developed into a dynamic, rapidly evolving field [2]. Compounds with rotational symmetry have gained increased attention in asymmetric synthesis because they are believed to be able to improve enantioselectivity by decreasing the number of possible transition states during the reaction [3,4,5]. Due to their beneficial effect on enantioselectivity, C2- and C3-symmetric molecules have been the focus of extensive research and, as a result, C3-symmetric compounds have been successfully applied as catalysts, ligands, molecular receptors, supra- and macromolecular constructs, gelators, metal-organic materials (MOMs), etc. [6,7,8,9,10,11].
Organocatalysts containing three equal catalytic units in a C3-symmetrical fashion have also been studied. Following the application of tripodal phosphinamide ligands in the enantioselective BH3 reduction of ketones [12,13], Han et al. successfully applied tribenzyl- and triphenylphosphine oxide-based proline organocatalysts for aldol reactions [14]. Subsequently, Moorthy et al. applied C3-symmetrical organocatalysts by anchoring proline and pyrrolidine to trifunctional trialkylbenzene cores. These C3-symmetric organocatalysts provided the Michael adducts of carbonyl compounds and trans-β-nitrostyrene with high stereoselectivities [15]. In addition to proline and pyrrolidine, trisimidazoline derivatives were also successfully utilized as catalysts by Fujioka et al. in Michael addition reactions, α-amination of β-ketoesters, and bromolactonization of alkenoic acids [16,17,18]. Later, the application of C3-symmetric trisimidazoline organocatalysts was extended to an enantio- and diastereoselective Betti/aza-Michael sequence as well [19].
Cinchona-based C2- and C3-symmetric compounds have also been widely studied [20]. Jew et al. demonstrated the high enantioselectivity of a novel trimeric cinchona alkaloid ammonium salt as a phase-transfer catalyst (PTC) in the catalytic asymmetric alkylation of the N-(diphenylmethylene)glycine tert-butyl ester [21]. Later, the C3-symmetric cinchona PTC catalyzed asymmetric synthesis of α-amino acids and the highly enantioselective Michael reaction of chalcones and diethyl malonate were performed by Siva et al. [22,23,24,25]. Csámpai and co-workers examined the in vitro antitumor activity of acylated mono-, bis-, and tris-cinchona-based amines [26]. Other than high selectivity, Dong et al. also showed the good recyclability of cinchonine squaramide-based C3-symmetric catalysts in enantioselective Michael addition, in hydroxyalkylation of indoles with alkyl trifluoropyruvate, and in asymmetric chlorolactonization of carboxylic acids [27,28,29,30]. These catalysts were easily recovered by precipitation and used for four to six cycles without significant loss of productivity or selectivity.
Organic solvent nanofiltration (OSN), also called solvent-resistant nanofiltration (SRNF), is a pressure-driven sustainable separation technology applied in fine chemical and petrochemical purification, which can separate solutes between 50 and 2000 g mol−1 in a wide range of organic solvents [31]. OSN is a predictable and energy-efficient technology compared to other separation techniques such as distillation, chromatography, and extraction [32]. There is increasing interest in applying OSN for the purification of pharmaceutically relevant compounds [33,34,35,36], as well as for solvent recovery [37,38]. OSN has also been proposed for the recovery of enlarged metal catalysts [39], and organocatalysts [40]. As an alternative recycling method to precipitation, Livingston et al. demonstrated the OSN enabled recovery of C3-symmetric quinidine-based organocatalysts [41]. The immobilization of cinchonas on trifunctional cores was found to be an effective molecular weight enlargement (MWE) method to facilitate their retention by OSN without destroying the catalytic efficiency of the organocatalysts in Michael addition reactions. Immobilizing organocatalysts on multifunctional cores, the so-called “hub-approach”, is an MWE method, in which the number of catalytic motifs in the size-enlarged molecule is increased, while the extent of non-functional “spacers” is decreased in comparison to polymer- or dendrimer-based supports. By adjusting the length and type of the linkers between the core (hub) and the catalytic units, the rigidity of the resulting size-enlarged catalyst can be regulated, which has a direct effect on the selectivity experienced in the organocatalytic reactions.
Taking these results into account, we intended to further explore the organocatalytic opportunities of size-enlarged C3-symmetric cinchona-based organocatalysts. Thus, multiple hub-cinchona structures both with or without H-bond donor capabilities were designed and tested in the hydroxyalkylation of indole and Michael addition reactions, in which the structure–selectivity relationships are also discussed. Finally, the expected superior membrane rejection of the size-enlarged catalysts is presented.
2. Results
During our work, we prepared and explored two types of C3-symmetric cinchona organocatalysts with varied H-bond donor properties (Figure 1). Compounds belonging to Type A are structurally the simplest as they have no H-bond donor units due to the derivatization of the hydroxyl group (9-OH, see Figure 1c) of the cinchona skeleton during the immobilization process. On the contrary, the mono H-bond donor 9-OH has been reserved in the case of Type B compounds as the cinchona motif was anchored to the hub, either through the aromatic quinoline (Williamson ether formation) or through the quinuclidine unit using copper(I) catalyzed azide–alkyne cycloaddition (CuAAC). As a hub, we used 1,3,5-triethynylbenzene, 1,3,5-tris(bromomethyl)benzene, or tripropargylamine.
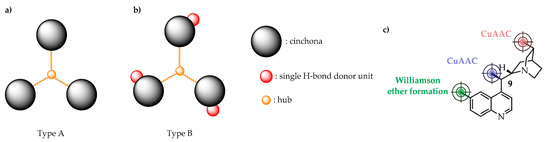
Figure 1.
We explored C3-symmetric hub-cinchona structures containing no (a), or single H-bond donor units (b). Cinchona skeleton was anchored through different positions (c). CuAAC: copper(I) catalyzed azide–alkyne cycloaddition.
2.1. Synthesis of New C3-Symmetric Hub-Cinchona Catalysts
First, we prepared Hub1,2-cinchona (Type A) organocatalysts using a common intermediate (3, Scheme 1). Cinchona azide 3 was obtained in two steps: mesylation of hydroquinine (1), followed by substitution with azide anion applying NaN3. Then, azide 3 was reacted with either 1,3,5-triethynylbenzene (4), or tripropargylamine (5) in a CuAAC reaction, which gave Hub1- and Hub2-cinchona, respectively, with moderate yields. Having been functionalized at the secondary hydroxyl group of the cinchona moiety, these compounds contain no H-bond donor units. However, other non-covalent interactions can still be formed through the protonated quinuclidine N-atom (ionic) or the aromatic quinoline ring (π–π stacking). Furthermore, the triazole-rings formed by the CuAAC reaction are good electron pair donors, which could interact with metallic species, combining the advantageous catalytic qualities of organocatalysts and transition metals to promote new chemical transformations [42,43].
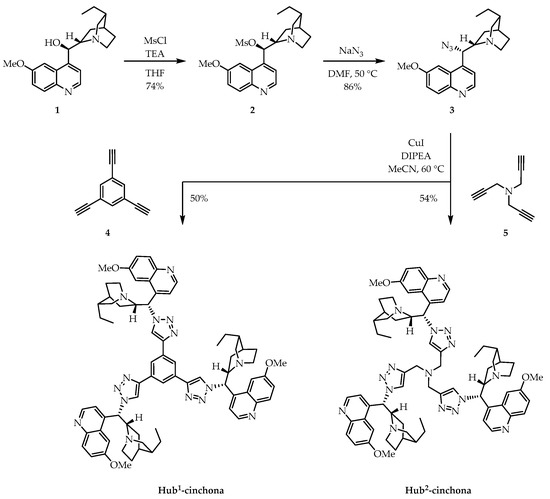
Scheme 1.
Syntheses of the size-enlarged C3-symmetric Hub1- and Hub2-cinchona organocatalysts (Type A) by CuAAC using a common cinchona azide intermediate (3) and trifunctional alkynes (4 or 5) with different chemical and structural properties. TEA: triethylamine; MsCl: methanesulfonyl chloride; DIPEA: N,N-diisopropylethylamine.
Next, cinchona derivatives with mono H-bond donor units (Type B) have been prepared. Hydroquinine (1) was demethylated using BBr3 (1M in DCM) to obtain dihydrocupreine 6 that bears a free phenolic hydroxyl group (Scheme 2). Then, using Cs2CO3, as a base, the phenolate of 6 was formed, which could react with 1,3,5-tris(bromomethyl)benzene (7) in a Williamson ether formation reaction to give the size-enlarged organocatalyst Hub3-cinchona with a good yield. Consequently, we connected the cinchona motif to the core through a stable ether bond, and the H-bond donor hydroxyl group remained intact.
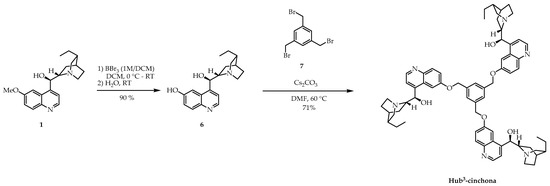
Scheme 2.
Synthesis of the size-enlarged C3-symmetric Hub3-cinchona organocatalyst by Williamson ether formation.
Organocatalyst Hub4-cinchona was prepared via convergent synthesis (Scheme 3), utilizing an alternative anchoring method. We converted the commercially available quinine (8) into didehydroquinine (9) using a Br2 addition–HBr elimination reaction. In a separate reaction, we reacted 1,3,5-tris(bromomethyl)benzene (7) with NaN3 in a mixture of water:acetone (1/100) to give the triazido-derivative 10. Finally, alkyne 9 and triazide 10 were subjected to a CuAAC reaction, which gave organocatalyst Hub4-cinchona with a moderate yield.
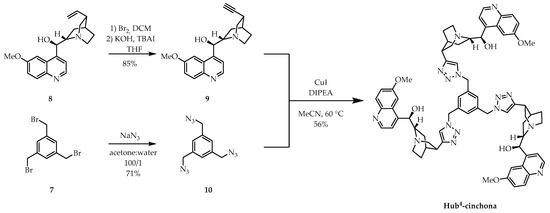
Scheme 3.
Convergent synthesis of size-enlarged C3-symmetric Hub4-cinchona organocatalyst by CuAAC using a triazide core unit (10). TBAI: tetra-n-butylammonium iodide; DIPEA: N,N-diisopropylethylamine.
2.2. Application of Hub-Cinchona Catalysts in Hydroxyalkylation of Indole and Michael Addition Reactions
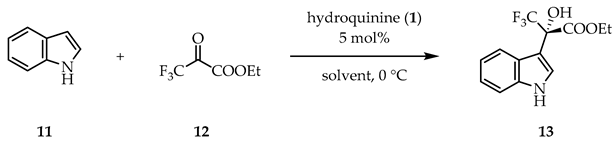
We started our organocatalytic reactions with the hydroxyalkylation of indole. First, the optimal solvent and reaction time were chosen using 5 mol% hydroquinine (1), the parent catalytic unit of the hub-cinchona derivatives. As solvents, 11 conventional and alternative agents were used (Table 1). In general, ether-type solvents showed better enantioselectivities in this reaction, while the protic ethanol gave a practically racemic product. An explanation for this solvent effect could be the formation of competing H-bonds between the solvent and the catalyst/substrates. Regarding the yield, in toluene and ethanol we achieved almost complete transformation, while the other solvents also gave good results (>67%). For the subsequent experiments, cyclopentyl methyl ether (CPME) was chosen, because this solvent provided the best enantioselectivity (73% ee) and the yield was still good after 24 h stirring at 0 °C (82%). Based on the 19F NMR spectra, the yield did not change significantly when the reaction time was reduced to 1 h.

Table 1.
Hydroquinine (1) catalyzed indole hydroxyalkylation reaction in different solvents 1.
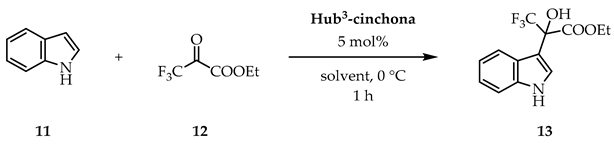
With the best solvent and reaction time in hand, we used our newly prepared hub-cinchona organocatalysts in the hydroxyalkylation reaction (Table 2). While the yield was only slightly lower (~10% difference), the size-enlarged catalysts showed significantly lower enantioselectivities in comparison to hydroquinine (1). The highest enantiomeric excess was achieved with Hub4-cinchona (29% ee), while Hub2-cinchona practically provided the hydroxyalkylated product as a racemic mixture (2% ee). Due to the tripropargylamine hub, the latter catalyst (Hub2-cinchona) contains a competitive basic unit with the quinuclidine N-atom, which can explain the lack of enantioselectivity. Comparing the structural features of the other catalysts, we can conclude that Hub1- and Hub4-cinchonas have more rigid structures, which can be attributed to the triazole rings that also serve as spacers between the hub and the catalytically active motifs. Therefore, the cinchona units in these cases are more separated from each other. Still, the formation of non-covalent interactions between the individual catalytic motifs within the hub-cinchonas can explain, in general, the significantly lower enantioselectivity and why Hub1,4-cinchonas gave better results than the structurally more flexible Hub3-cinchona. The Structures, NMR spectra, MS spectra and HPLC chromatograms of the prepared C3-symmetric hub-cinchonas (Hub1-4-cinchonas) are shown in Supplementary Materials.

Table 2.
Hydroquinine (1) and Hub1–4-cinchonas catalyzed indole hydroxyalkylation reaction 1.
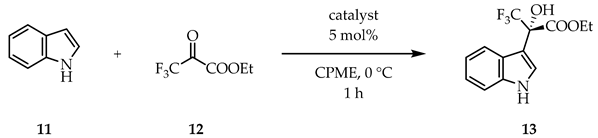
As the solvent can significantly alter the formation of non-covalent interactions, we performed the complete solvent screen with Hub3-cinchona (Table 3). While no higher enantioselectivity was achieved, the previously observed trend was still recognizable: ether-type solvents gave good results, but the protic ethanol promoted the formation of the racemic product. Interestingly, in some cases (toluene, DCM, and MeCN) the other antipode of 13 was found to be present in excess.

Table 3.
Hub3-cinchona catalyzed indole hydroxyalkylation reaction in different solvents 1.
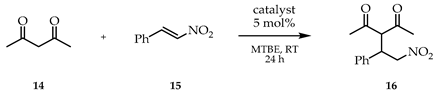
Additionally, the catalytic efficiencies of the size-enlarged hub-cinchonas and hydroquinine (1) were also compared in the Michael addition reaction of pentane-2,4-dione (14) and trans-β-nitrostyrene (15). The applied reaction conditions were chosen based on our previous work [44]. Based on the measured enantioselectivities (Table 4), hydroquinine (1) is not a suitable catalyst for this reaction (14% ee). While organocatalysts Hub1,2,4-cinchonas gave nearly racemic mixtures, Hub3-cinchona showed two times higher enantioselectivity than catalyst 1 (32% ee vs. 14% ee) with a preference to the mirror image stereoisomer.

Table 4.
Hydroquinine (1) and hub-cinchona catalyzed Michael addition reaction of pentane-2,4-dione (14) and trans-β-nitrostyrene (15) 1.
Considering that these two catalysts (1 and Hub3-cinchona) are structurally very similar in regard to the catalytical motif(s), the higher selectivity clearly suggests that the C3-symmetric structural feature of the size-enlarged Hub3-cinchona has a positive effect on the enantioselectivity. This advantageous outcome can be attributed either to the formation of a sterically more hindered space during the transition state or to an alternative catalyst–substrate interaction layout including two or more cinchona motifs.
Next, using Hub3-cinchona, the Michael addition reaction was also performed with a structurally bulkier and electronically more favorable Michael donor, 1,3-diphenylpropane-1,3-dione (17, Scheme 4). The applied reaction conditions were based on our previous work [45]. Although only 1 mol% of catalyst was used, the enantioselectivity observed was significantly higher regardless of the solvent (2 mL), e.g., DCM (53% ee), EtOAc (64% ee), MeCN (71% ee), or toluene (80% ee). The best result was achieved by using MTBE. In this case, the selectivity reached 93% ee and the yield was 69% after purification by preparative thin-layer chromatography (TLC). In comparison, the reaction catalyzed by hydroquinine (1) gave only 6% ee with an 84% preparative yield.
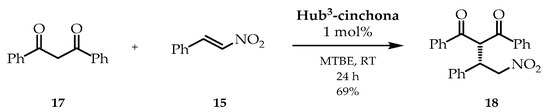
Scheme 4.
Michael addition of 1,3-diphenylpropane-1,3-dione (17) and trans-β-nitrostyrene (15) catalyzed by Hub3-cinchona organocatalyst.
To conclude, the Michael addition reaction showed increased selectivity for the size-enlarged Hub3-cinchona catalysts compared to its cinchona unit (hydroquinine, 1), which indicates the positive effect of the C3-symmetric structure. Furthermore, a Michael adduct prepared from the bulkier substrate was obtained with excellent enantioselectivities (up to 93% ee) with Hub3-cinchona size-enlarged organocatalyst.
2.3. Membrane Rejection of Hub-Cinchona Organocatalysts
Given the bulky nature of the size-enlarged catalysts, they were fully retained on all the tested membranes with rejection values of 100% (Figure 2a). It is important to achieve 100% rejection in order to avoid the loss of any valuable catalyst during the recovery process. The rejections of indole (11) and the hydroxyalkylated product 13 vary between 5% and 55% depending on both the membrane and the molecular weight (MW). For efficient catalyst recovery, the rejection gap between the catalyst and the other solutes needs to be as large as possible. Consequently, one can conclude that the best membrane for hub-cinchona organocatalyst recovery is DM900. This membrane exhibited substrate solute rejections below 30%, while still maintaining complete retention of the catalysts. Moreover, DM900 is the most open membrane with the highest flux of 6.7 ± 0.24 L m−2 h−1 (Figure 2b). It is important to maximize the flux in order to achieve an efficient catalyst recovery process. Comparing the membrane rejections of the hub-cinchonas with hydroquinine (1), the advantage of molecular size-enlargement can be clearly seen. Due to the similar rejection values of hydroquinine (1) and product 13, membrane recovery of 1 from the reaction mixture would be inadequate.
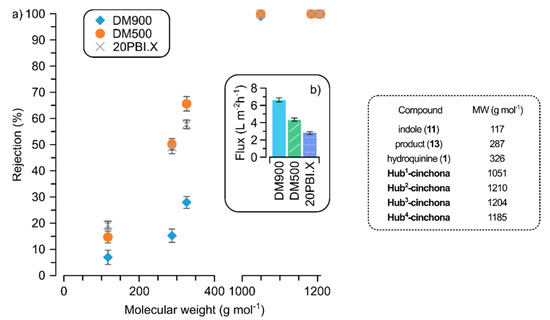
Figure 2.
Rejection (a) and flux (b) values for the three screened solvent-resistant membranes in PolarClean green solvent at 10 bar in crossflow mode.
3. Materials and Methods
3.1. General Information
Starting materials were purchased from commercially available sources (Sigma-Aldrich, Merck, and Alfa Aesar). Infrared spectra were recorded on a Bruker Alpha-T FT-IR spectrometer (Bruker, Ettlingen, Germany). Optical rotations were measured on a Perkin-Elmer 241 polarimeter (Perkin-Elmer, Waltham, MA, USA) that was calibrated by measuring the optical rotations of both enantiomers of menthol. Silica gel 60 F254 (Merck) and aluminum oxide 60 F254 neutral type E (Merck) plates were used for TLC. Aluminum oxide (neutral, activated, Brockman I) and silica gel 60 (70–230 mesh, Merck) were used for column chromatography. Ratios of solvents for the eluents are given in volumes (mL mL−1). Melting points were taken on a Boetius micro-melting point apparatus (VEB Dresden Analytik, Dresden, Germany) and they were uncorrected. N,N-Dimethylacetamide (DMAc) solution of polybenzimidazole (PBI, 26 wt%) was purchased from PBI Performance Products (USA). The previously reported 20PBI.X membrane was obtained based on Schaepertoens et al. [46]. PBI was selected as it is an emerging polymer for OSN [47,48]. DuraMem solvent-resistant membranes (DM500 and DM900) can be obtained from Evonik (Germany). PolarClean solvent is produced by Solvay (Italy). NMR spectra were recorded either on a Bruker DRX-500 Avance spectrometer (at 500 MHz for 1H, at 125 MHz for 13C, and at 376 MHz for 19F spectra) or on a Bruker 300 Avance spectrometer (at 300 MHz for 1H, at 75 MHz for 13C, and at 222.5 MHz for 19F spectra) or on a Bruker Avance III HD (at 600 MHz for 1H and at 150 MHz for 13C spectra). HPLC-MS was performed on an HPLC system using Agilent Technologies 1200 Series-Agilent Technologies 6130 Quadrupole; column: Phenomenex Kinetex C18 100A (2.6 μm, 50 × 3.00 mm); A eluent: water (1% HCOONH4); B eluent: MeCN (8% water, 1% HCOONH4); gradient: 20–100%. In case of indole hydroxyalkylation, HPLC-MS was performed using a Shimadzu LCMS-2020 (Shimadzu Corp., Kyoto, Japan) device, equipped with a Reprospher (Altmann Analytik Corp., München, Germany) 100 C18 (5 µm, 100 × 3 mm) column and a positive/negative double ion source (DUIS±) with a quadrupole MS analyzer in a range of 50–1000 m/z. The samples were eluted with gradient elution, using eluent A (0.1% formic acid in water) and eluent B (0.1% formic acid in MeCN). The flow rate was set to 1.5 mL min−1. The initial condition was 5% eluent B, followed by a linear gradient to 100% eluent B by 1.5 min; from 1.5 to 4.0 min, 100% eluent B was retained, and from 4 to 4.5 min, it went back by a linear gradient to 5% eluent B, which was retained from 4.5 to 5 min. The column temperature was kept at room temperature, and the injection volume was 1–10 µL. The purity of the compounds was assessed by HPLC with UV detection at 215 and 254 nm. High resolution mass measurements were performed on a Thermo Exactive plus EMR Orbitrap mass spectrometer, which was used with a Thermo Ultimate 3000 UHPLC with 100% methanol as the mobile phase, or on a Thermo Velos Pro Orbitrap Elite (Thermo Fisher Scientific) system. The ionization method was ESI operated in positive ion mode. The samples were dissolved in methanol. Data acquisition and analysis were accomplished with Xcalibur software version 4.0 (Thermo Fisher Scientific). The enantiomeric ratios of the samples were determined by chiral high-performance liquid chromatography (HPLC) measurements using either reversed-phase mode (Thermo Finnigan Surveyor LC System, Thermo Fisher Scientific, Waltham, MA, USA) or normal phase mode (PerkinElmer Series 200 LC System, PerkinElmer, Inc, Shelton, CT, USA), and the exact conditions are indicated in the correspondent asymmetric reaction in the Experimental Section.
3.2. Preparation of Compounds
(1R)-(–)-((2S,4S,5R)-5-Ethylquinuclidin-2-yl)(6-methoxyquinolin-4-yl)methyl methanesulfonate (2):
This compound was prepared based on the description in the literature [49]. A solution of hydroquinine (1, 3.00 g, 9.19 mmol, 1.0 eq) in dry tetrahydrofuran (THF, 55 mL) was stirred under Ar at 0 °C. Triethylamine (TEA, 6.2 mL, 44.1 mmol, 4.8 eq) was added to this solution, followed by dropwise addition of methanesulfonyl chloride (2.9 mL, 36.8 mmol, 4.0 eq). Next, the reaction mixture was allowed to warm up to room temperature, and it was stirred for 4 h. The solvent was evaporated under reduced pressure. The residue was dissolved in a mixture of DCM (50 mL) and sat. aqueous solution of NaHCO3 (50 mL). The aqueous phase was extracted with DCM (2 × 50 mL). The combined organic phase was dried over anhydrous MgSO4, filtered, and the solvent was removed under reduced pressure. The crude product was purified by column chromatography (silica gel, MeOH/toluene 1:6) to obtain hydroquinine mesylate (2, 2.20 g, 73%) as a yellow solid.
Rf: 0.37 (silica gel, MeOH/toluene 1:4); Mp: 102.9–104.3 °C (lit Mp: 105–108 °C) [48]; MS-ESI+ (m/z): [M + H]+ calculated for C21H29N2O4S: 405.2, found: 405.0. Spectroscopic data are fully consistent with those reported in the literature [50].
(2S,4S,5R)-(+)-2-((S)-Azido(6-methoxyquinolin-4-yl)methyl)-5-ethylquinuclidine (3):
Compound 3 was prepared based on the description in the literature [49]. A solution of mesylate 2 (2.00 g, 4.94 mmol, 1.0 eq) in dry dimethylformamide (DMF, 55 mL) was stirred under Ar atmosphere at room temperature. NaN3 was then added (1.45 g, 22.3 mmol, 4.5 eq) to this solution. Next, the reaction mixture was warmed up to 45 °C and it was stirred at this temperature until the reaction was completed. The solvent was evaporated under reduced pressure, then, water was added to the residue, and this aqueous mixture was extracted with Et2O (3 × 15 mL). The combined organic phase was evaporated to obtain azido-hydroquinine as a yellow oil (3, 1.44 g, 83%) and used without further purification.
Rf: 0.20 (silica gel, DCM/MeOH 10:1); MS-ESI+ (m/z): [M + H]+ calculated for C20H26N5O4: 352.2, found: 352.1. Spectroscopic data are fully consistent with those reported in the literature [44,51].
Dihydrocupreine (6):
Hydroquinine (1, 1.00 g, 3.06 mmol, 1.0 eq) was dissolved in DCM (90 mL) under an Ar atmosphere, then this solution was cooled to 0 °C. Next, BBr3 (1 M DCM solution, 26.0 mL, 26.0 mmol, 8.5 eq) was added dropwise. Next, the reaction mixture was left to slowly warm up to room temperature, and it was stirred overnight. After complete consumption of the starting material (TLC, silica gel, DCM/MeOH/NH4OH 5:1:0.01), a solution of 10% NaOH (aq., 40 mL) was added to the mixture. Following the separation of the two phases, the aqueous phase was washed with DCM (3 × 50 mL). Next, cc. HCl (aq.) was added to neutralize the aqueous phase, followed by extraction with DCM (3 × 50 mL). The combined organic phase was dried over anhydrous MgSO4, filtered, and the solvent was evaporated under reduced pressure to yield 6 (860 mg, 90%) as a brown solid.
Rf: 0.12 (silica gel; MeOH/toluene 1:4); : -180.9° (c 1.00, CHCl3); Mp. 224.4−225.9 °C (lit. Mp. 230 °C, [52]). Spectroscopic data are fully consistent with those reported in the literature [53].
(R)-[(2S,4S,5S)-5-Ethynylquinuclidine-2-yl)(6-methoxyquinoline-4-yl]methanol (9)
To a solution of quinine (8, 5.00 g, 15.4 mmol, 1.0 eq) in DCM (150 mL), a mixture of Br2 (1.70 mL, 30.9 mmol, 2.0 eq) and DCM (7 mL) was added at 0 °C. The reaction mixture was stirred at 0 °C for 1 h while a yellow solid precipitated. After stirring the reaction mixture for an additional hour at room temperature, hexane (300 mL) was added, stirred for 10 min, and filtered. The filtrate was washed with hexane and dried under infrared lamp for 1 h. The yellow solid was dissolved in THF (150 mL), and tetrabutylammonium iodide (TBAI, 550 mg, 1.71 mmol, 0.1 eq) was added to this solution. Then, finely powdered potassium hydroxide (KOH, 5.00 g, 89.1 mmol, 5.8 eq) was added to the mixture, and stirred at 45 °C for 1 h when an additional batch of KOH (5.00 g, 89.1 mmol, 5.8 eq) was added to it. The reaction mixture was stirred for 12 h at room temperature. After complete consumption of the starting materials (TLC, silica gel, MeOH/DCM/TEA 1:10:0.2), the mixture was filtered, dried over anhydrous MgSO4, and the solvent was removed under reduced pressure. The crude product was purified by dry column vacuum chromatography (silica gel, EtOAc/NH4OH 20:1, EtOAc/NH4OH/MeOH 95:5–45:55) to yield 9 (4.25 g, 85%) as a brown solid.
Rf: 0.50 (silica gel, MeOH/DCM/TEA 1:10:0.2). Mp. 167–170 °C; Spectroscopic data are fully consistent with those reported in the literature [54].
1,3,5-Tris(azidomethyl)benzene (10)
Tris(bromomethyl)benzene (7, 500 mg, 1.40 mmol, 1.0 eq) was dissolved in a mixture of acetone/H2O 1:0.01 (10 mL), then NaN3 (550 mg, 8.50 mmol, 6.1 eq) was added, and the resulting mixture was stirred at room temperature for 1 day. After consumption of the starting material (TLC, silica gel, EtOAc/hexane 1:2), the mixture was concentrated, and to the remaining aqueous mixture EtOAc (15 mL) and water (15 mL) were added. The separated organic phase was washed with water (2 × 15 mL), dried over anhydrous MgSO4, filtered, and the solvent was removed under reduced pressure to yield 10 (244 mg, 71%) as an oil. This product was used without further purification.
Rf: 0.65 (silica gel, EtOAc/hexane 1:2). Spectroscopic data are fully consistent with those reported in the literature [54]. Although the tris(azidomethyl)benzene is reported to be relatively insensitive to heat and shock, special care was taken during its synthesis and application to avoid accidents [55,56].
Hub1-cinchona
To a mixture of cinchona azide (3, 700 mg, 2.00 mmol, 6.0 eq), 1,3,5-triethynylbenzene (4, 50 mg, 0.33 mmol, 1.0 eq) and N,N-diisopropylethylamine (DIPEA, 1.22 mL, 7.00 mmol, 21.0 eq) a suspension of CuI (38 mg, 0.20 mmol, 0.6 eq) in MeCN (1 mL) was added. The mixture was stirred at 60 °C for 2 days. After complete consumption of the starting material (TLC, silica gel, MeOH/DCM/NH4OH 1:10:0.01), the solvent was evaporated under reduced pressure, and the crude product was purified by column chromatography (silica gel, MeOH/DCM/NH4OH 1:20:0.01–1:5:0.01 to yield Hub1-cinchona (200 mg, 50%) as light-yellow solid.
Rf: 0.40 (silica gel, MeOH/DCM/NH4OH 1:10:0.01); Mp. 195 °C; 1H NMR (600 MHz, DMSO-d6): δ 0.85 (m, 12H), 1.40 (m, 12H), 1.53 (overlapping, 3H), 1.63 (br, 6H), 1.73 (br, 3H), 2.41 (m, 3H), 2.96 (m, 3H), 3.43 (overlapping, 3H), 3.99 (s, 9H), 4.09 (overlapping, 3H), 6.64 (m, 3H), 7.46 (m, 3H), 7.85 (br, 3H), 7.88 (d, J = 6.0 Hz, 3H), 7.98 (d, J = 6.0 Hz, 3H), 8.11 (m, 3H), 8.86 (m, 6H); 13C NMR (125 MHz, DMSO-d6): δ 12.3, 25.4, 26.5, 27.3, 28.0, 37.0, 40.7, 56.0, 57.0, 57.1, 59.7, 102.5, 120.6, 121.1, 121.2, 121.7, 127.9, 131.7, 132.1, 140.0, 144.4, 145.6, 148.1, 158.0; HPLC-MS-ESI+ (m/z): [M + H]+ calculated for C72H82N15O3: 1204.66, found: 1204.44; triflate salt formed by the addition of Cu(CF3SO3)2: HRMS-ESI+ (m/z): [(M + 2H + Tf)/2]2+ calculated for C73H84O6N15F3S: 677.81954; found: 677.81628.
Hub2-cinchona
To a mixture of cinchona azide (3, 803 mg, 2.29 mmol, 6.0 eq), tripropargylamine (5, 50 mg, 0.38 mmol, 1.0 eq) and DIPEA (1.39 mL, 8.00 mmol, 21.0 eq) was added a suspension of CuI (44 mg, 0.23 mmol, 0.6 eq) in MeCN (1 mL). The mixture was stirred at 60 °C for 2 days. After complete consumption of the starting material (TLC, silica gel, MeOH/DCM/NH4OH 1:10:0.01), the solvent was evaporated under reduced pressure, and the crude product was purified by column chromatography (silica gel, MeOH/DCM/NH4OH 1:20:0.01–1:5:0.01 to yield Hub2-cinchona (243 mg, 54%) as light-yellow solid.
Rf: 0.40 (silica gel, MeOH/DCM/NH4OH 1:10:0.01); Mp. 162 °C; 1H NMR (600 MHz, DMSO-d6): δ 0.73 (br, 3H), 0.82 (m, 9H), 1.35 (overlapping, 12H), 1.60 (overlapping, 9H), 2.33 (br, 3H), 2.46 (br, 3H), 2.90 (br, 3H), 3.42 (overlapping, 9H), 3.89 (s, 9H), 3.98 (br, 3H), 6.52 (br, 3H), 7.43 (m, 3H), 7.74 (br, 3H), 7.82 (m, 3H), 7.96 (d, J = 9.0 Hz, 3H), 8.16 (overlapping, 3H), 8.78 (m, 3H); 13C NMR (125 MHz, DMSO-d6): δ 12.3, 25.3, 26.4, 27.1, 28.1, 37.0, 40.7, 46.8, 56.0, 57.0, 57.4, 59.3, 102.3, 120.8, 121.7, 123.9, 127.9, 131.7, 140.3, 142.5, 144.4, 147.9, 158.0; HPLC-MS-ESI+ (m/z): [M + H]+ calculated for C69H85N16O3: 1185.69, measured: 1185.55; HRMS-ESI+ (m/z): [M + H]+ calculated for C69H85N16O3: 1185.6985, found: 1185.6951.
Hub3-cinchona
To a solution of dihydrocupreine (6, 500 mg, 1.60 mmol, 6.0 eq) in dry DMF (50 mL), Cs2CO3 (772 mg, 2.39 mmol, 9.0 eq) was added, and the resulting mixture was stirred at 60 °C for 1 h. Next, 1,3,5-tris(bromomethyl)benzene (7, 95 mg, 0.27 mmol, 1.0 eq) was added to the mixture, and stirred for 3 h. Then, the solvent was evaporated, and the residue was taken up in a mixture of EtOAc (50 mL) and H2O (50 mL). The forming brown precipitate was filtered and washed with EtOAc (3 × 20 mL) to yield Hub3-cinchona (398 mg, 71%) as a brown solid.
Rf: 0.41 (aluminum oxide, MeOH/DCM/NH4OH 10:1:0.01); Mp. 166–175 °C; IR (film) νmax: 3267, 2929, 2871, 1618, 1590, 1507, 1457, 1378, 1359, 1325, 1238, 1217, 1131, 1115, 1085, 1052, 1024, 1004, 937, 880, 857, 820, 758, 693, 642, 620, 609, 568, 549, 530, 467, 435, 417, 401 cm−1; 1H NMR (600 MHz, DMSO-d6): δ): 0.76 (t, J = 7 Hz, 9H), 1.22 (m, 6H), 1.25 (m, 3H), 1.28 (m 3H), 1.57 (m, 3H), 1.61 (m, 3H), 1.64 (m, 3H), 1.65 (m, 3H), 2.06 (m, 3H), 2.31 (m, 3H), 2.75 (m, 3H), 2.98 (m, 3H), 3.10 (br, 3H), 5.20 (br, 3H), 5.32 (d, J = 12.6 Hz, 3H), 5.34 (d, J = 12.6 Hz, 3H), 5.67 (br, 3H), 7.47 (dd, J = 9.1 Hz, 2.7 Hz, 3H), 7.50 (d, J = 4.5 Hz, 3H), 7.64 (br d, J = 2.7 Hz, 3H), 7.66 (br, 3H), 7.94 (d, J = 9.1 Hz, 3H), 8.68 (d. J = 4.5 Hz, 3H); 13C NMR (125 MHz, DMSO-d6): δ12.3, 24.1, 25.3, 27.4, 28.4, 37.3, 42.0, 57.8, 60.8, 69.7, 71.1, 104.1, 119.4, 121.5, 126.4, 127.3, 131.5, 137.7, 144.2, 147.9, 149.7, 156.0; HPLC-MS-ESI+ (m/z): [M + H]+ calculated for C66H79N6O6: 1051.60, found: 1051.8.
Hub4-cinchona
To a mixture of triazide (10, 110 mg, 0.45 mmol, 1.0 eq), cinchona derivative 9 (875 mg, 2.71 mmol, 6.0 eq) and DIPEA (1.66 mL, 9.49 mmol, 21.0 eq)—a suspension of CuI (51.7 mg, 0.27 mmol, 0.6 eq) in MeCN (3 mL)—were added. The mixture was stirred at 60 °C for 2 days. After complete consumption of the starting material (TLC, silica gel, MeOH/DCM/NH4OH 1:5:0.01, or DCM/hexane 1:2), the solvent was evaporated under reduced pressure, and the crude product was purified by column chromatography (aluminum oxide, MeOH/DCM/NH4OH 1:20:0.01–1:5:0.01 to yield Hub4-cinchona (306 mg, 56%) as a light-brown solid.
Rf: 0.49 (aluminum oxide, MeOH/DCM/NH4OH 1:20:0.01); Mp. 179–180 °C; 1H NMR (600 MHz, DMSO-d6): δ 1.53 (m, 3H), 1.57 (m, 6H), 1.71 (m, 3H), 1.98 (m, 3H), 2.52 (m, 3H), 2.87 (m, 3H), 2.96 (m, 3H), 3.02 (m, 3H), 3.20 (m, 3H), 3.21 (m, 3H), 3.88 (s, 9H), 5.23 (m, 3H), 5.46 (s, 6H), 5.62 (d, J = 5.1 Hz, 3H), 7.08 (s, 3H), 7.38 (dd, J = 2.8; 9.2 Hz, 3H), 7.48 (d, J = 4.5 Hz, 3H), 7.51 (d, J = 4.5 Hz, 3H), 7.92 (d, J = 9.2 Hz, 3H), 7.93 (s, 3H), 8.86 (d, J = 4.5 Hz, 3H); 13C NMR (125 MHz, DMSO-d6): δ 24.5, 27.5, 27.7, 32.8, 42.1, 52.4, 55.5, 55.6, 60.6, 71.1, 102.7, 119.4, 121.2, 122.2, 126.9, 127.4, 131.3, 137.6, 144.1, 147.7, 149.5; 150.7; 156.9; HPLC-MS-ESI+ (m/z): [M + H]+ calculated for C69H76N15O6: 1210.60, measured: 1210.40; HRMS-ESI+ (m/z): [M + H]+ calculated for C69H76N15O6: 1210.60975, found: 1210.61203.
General procedure for the indole hydroxyalkylation reaction: ethyl (S)-(+)-3,3,3-trifluoro-2-hydroxy-2-(1H-indol-3-yl)propanoate (13)
To a solution of indole (11, 36 mg, 0.31 mmol, 1.0 eq) in the given solvent (1 mL), hydroquinine (1, 5 mg, 0.015 mmol, 5 mol%) was added and the resulting reaction mixture was stirred for 1 h at 0 °C. Next, ethyl trifluoropyruvate (12, 27 μL, 0.31 mmol, 1.0 eq) was added to it, and stirred further at 0 °C. After the corresponding reaction time (see Table 1, Table 2 and Table 3) the volatile components were removed under reduced pressure. The crude product was purified by preparative thin-layer chromatography (Rf: 0.45, silica gel, DCM) to give product 13 as a solid.
Rf: 0.45 (silica gel, DCM); : +9.1 (CHCl3, c 1.00, 87% ee, S config.) (lit. : +11.3, CHCl3, c 1.01, 74% ee, S config. [57]); Colorless crystals. Mp. 71–72 °C (lit. Mp: 70.5–71.8 °C, [58]); MS-ESI- (m/z): [M − H+]- 286; The yield was determined by 19F NMR. Enantiomeric excess was determined by reversed phase HPLC analysis using Phenomenex Lux Cellulose-1 (5 μm, 250 × 4.6 mm) column, eluent water (0.1% NH4OAc)/MeCN = 40/60, 0.8 mL min−1, UV detector 222 nm. Retention time for (R)-13 and (S)-13 are 6.5 min and 7.2 min, respectively. Spectroscopic data are fully consistent with those reported in the literature [58].
General procedure for the Michael addition reaction of pentane-2,4-dione (16):
To a solution of 1,3-dioxo compound 14 (78 μL, 0.77 mmol, 2.5 eq) and trans-β-nitrostyrene (15, 46 mg, 0.31 mmol, 1.0 eq) in the given solvent (1 mL), hydroquinine (1, 5 mg, 0.015 mmol, 5 mol%) was added. The resulting mixture was stirred at room temperature for 24 h. Then, the volatile components were removed under reduced pressure. The crude product was purified by thin-layer chromatography (hexane/EtOAc, 2:1) to give the Michael adduct as a solid.
(S)-(+)-3-(2-Nitro-1-phenylethyl)pentane-2,4-dione (16): Rf: 0.13 (silica gel, hexane/EtOAc, 4:1); : +195.2 (CHCl3, c 1.00, 99.1% ee, S config.) (lit. : +196.7, CHCl3, c 1.01, 88% ee, S config. [59]); White crystals. Mp. 125–128 °C (lit. Mp: 124–126 °C, [59]); MS-ESI+ (m/z): [M + NH4]+ 267.1; Enantiomeric excess was determined by normal phase HPLC analysis using Phenomonex Lux Cellulose-1 column (5 μm, 250 × 4.6 mm), eluent hexane/ethanol = 85/15, isocratic mode; 0.8 mL min−1; temperature 20 °C, UV detector 254 nm. Retention time for (S)-16: 16.1 min, for (R)-16: 17.6 min. Spectroscopic data are fully consistent with those reported in the literature [59].
General procedure for the Michael addition reaction of 1,3-diphenylpropan-1,3-dione (18):
To a solution of 1,3-dioxo compound 17 (107 mg, 0.48 mmol, 1.0 eq) and trans-β-nitrostyrene (15, 213 mg, 1.43 mmol, 3.0 eq) in the given solvent (2 mL), hydroquinine (1, 5 mg, 0.015 mmol, 5 mol%) was added. The resulting mixture was stirred at room temperature for 24 h. Then, the volatile components were removed under reduced pressure. The crude product was purified by thin-layer chromatography (hexane/EtOAc, 4:1) to give the Michael adduct as a solid.
(S)-(+)-2-(2-Nitro-1-phenylethyl)-1,3-diphenylpropane-1,3-dione (18): Rf: 0.35 (silica gel, hexane/EtOAc, 4:1); : +22.1 (DCM, c 1.0, 99.3% ee, S config.) (lit. : +21.3, DCM, c 1.0, 98% ee, S config. [60]); White crystals. Mp. 136.2 °C (lit. Mp: 135.6 °C [61]); MS-ESI+ (m/z): [M + H]+ 391.2. When Hub1–4-cinchonas were used as catalysts, enantiomeric excess was determined by reversed phase HPLC analysis using Phenomenex Lux Cellulose-1 column (5 μm, 250 × 4.6 mm), eluent water (0.1% NH4OAc)/MeCN = 30/70, isocratic mode; 0.8 mL∙min−1, temperature 20 °C, UV detector 222 nm. Retention time for (S)-18: 7.6 min, for (R)-18: 8.9 min. When hydroquinine (1) was used as a catalyst, enantiomeric excess value was determined by normal phase HPLC analysis with Kromasil® 5-Amycoat column (250 × 4.6 mm ID, 5 μm) using hexane/ethanol (85:15) eluent. Retention time for (R)-18: 19.2 min, for (S)-18: 26.1 min. Spectroscopic data are fully consistent with those reported in the literature [61].
3.3. Nanofiltration
Membrane separation was carried out in a crossflow nanofiltration rig with 53 cm2 effective area (A), as described in the literature [62]. PolarClean, as a green solvent [63], was used for the filtration, and the applied pressure was 10 bar. Two independent measurements were carried out, and the presented results are mean values. Equations (1) and (2) were used to calculate the rejection and permeance after 24 h recirculation in the rig, respectively.
where Cp and Cf are the permeate and feed concentrations of the solutes, respectively; V is the permeate volume, while t is the time of solvent permeation through the membrane with certain membrane area (A).
4. Conclusions
Four structurally different C3-symmetric cinchona organocatalysts were prepared, in which the catalytic units are covalently anchored to a trifunctional central core (hub). Depending on the immobilization site, we obtained compounds either containing or lacking mono H-bond donor groups on the cinchona skeleton.
The catalytic activities of these size-enlarged molecules were tested in the hydroxyalkylation of indole and Michael addition reaction. While the parent hydroquinine was found to be an efficient catalyst for the Friedel–Crafts reaction of indole (up to 73% ee), the hub-cinchona catalysts showed significantly lower enantioselectivities, regardless of the solvent applied. The structure–selectivity correlations revealed that catalysts with more rigid and extensive spacers performed better, suggesting a disadvantageous interaction of the individual cinchona units. On the contrary, in the Michael addition reaction, the hub-cinchona catalyst showed increased selectivity compared to hydroquinine, which indicates the positive effect of the C3-symmetric structure. Furthermore, Hub3-cinchona was also shown to provide enantioselectivities up to 96% ee, in the case of a bulkier substrate. Finally, membrane recovery of the size-enlarged organocatalysts using the PolarClean alternative solvent was found to be straightforward thanks to their ~four-fold increase in size compared to hydroquinine.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-8994/13/3/521/s1.
Author Contributions
Methodology, P.K. (Péter Kisszékelyi), Z.F., S.N. and J.K.; synthesis of compounds, P.K. (Petra Kozma), Z.F., S.N. and P.K. (Péter Kisszékelyi); determination of ee values by chiral HPLC measurements, P.B. and B.M.; performed NMR experiments, data analysis, Z.G.; performed MS measurements, data analysis, M.D. and B.M.; writing—original draft preparation, P.K. (Péter Kisszékelyi); writing—review and editing, J.K., Z.F., S.N., P.K. (Péter Kisszékelyi), P.B. and P.H.; project administration, J.K.; funding acquisition, P.H. and J.K.; resources, P.H. and J.K.; supervision, J.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the New National Excellence Program of the Ministry of Human Capacities, grant numbers ÚNKP-20-4-I-BME-320 (Péter Kisszékelyi), ÚNKP-20-3-II-BME-325 (S.N.) and ÚNKP-20-5-BME-322 (J.K.), and the János Bolyai Research Scholarship of the Hungarian Academy of Science (J.K.). It was also supported by the National Research, Development, and Innovation Office (grant number K128473), the Servier–Beregi PhD Research Fellowship (S.N.), and the Gedeon Richter’s Talentum Foundation (Péter Kisszékelyi and Z.F.). This work was also funded by the Cooperative Doctoral Program Doctoral Student Scholarship (KDP-2020-1007075) of the Ministry of Innovation and Technology (Z.G.).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data created and analyzed in this study can be found within the article or supplementary material. Therefore we would not select any Data Availability Statement from MDPI Research Data Policies.
Acknowledgments
The authors are grateful to András Dancsó from Egis Pharmaceuticals Plc., Directorate of Drug Substance Development, for his assistance with the NMR measurements.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kamer, P.; Vogt, D.; Thybaut, J.W. (Eds.) Contemporary Catalysis: Science, Technology, and Applications, Gld. ed.; The Royal Society of Chemistry: London, UK, 2017. [Google Scholar]
- Jacobsen, E.N.; Pfaltz, A.; Yamamoto, H. Comprehensive Asymmetric Catalysis I–III (Comprehensive Overviews in Chemistry), 1st ed.; Springer: Berlin/Heidelberg, Germany, 1999. [Google Scholar]
- Moberg, C. C3 Symmetry in Asymmetric Catalysis and Chiral Recognition. Angew. Chem. Int. Ed. 1998, 37, 248–268. [Google Scholar] [CrossRef]
- Moberg, C. The Role of Symmetry in Asymmetric Catalysis. Isr. J. Chem. 2012, 52, 653–662. [Google Scholar] [CrossRef]
- Gade, L.H.; Bellemin-Laponnaz, S. Exploiting Threefold Symmetry in Asymmetric Catalysis: The Case of Tris(oxazolinyl)ethanes (“Trisox”). Chem. A Eur. J. 2008, 14, 4142–4152. [Google Scholar] [CrossRef]
- Gibson, S.E.; Castaldi, M.P. Applications of chiral C3-symmetric molecules. Chem. Commun. 2006, 37, 3045–3062. [Google Scholar] [CrossRef]
- Rodríguez, L.-I.; Roth, T.; Fillol, J.L.; Wadepohl, H.; Gade, L.H. The More Gold-The More Enantioselective: Cyclohydroaminations of γ-Allenyl Sulfonamides with Mono-, Bis-, and Trisphospholane Gold(I) Catalysts. Chem. A Eur. J. 2012, 18, 3721–3728. [Google Scholar] [CrossRef]
- Yamanaka, M.; Nakagawa, T.; Aoyama, R.; Nakamura, T. Synthesis and estimation of gelation ability of C3-symmetry tris-urea compounds. Tetrahedron 2008, 64, 11558–11567. [Google Scholar] [CrossRef]
- Crişan, C.V.; Soran, A.; Bende, A.; Hӑdade, N.D.; Terec, A.; Grosu, I. Synthesis, Structure and Supramolecular Properties of a Novel C3 Cryptand with Pyridine Units in the Bridges. Molecules 2020, 25, 3789. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.-H.; Liu, X.-T.; Space, B.; Chang, Z.; Bu, X.-H. Metal-organic materials with triazine-based ligands: From structures to properties and applications. Co-ord. Chem. Rev. 2021, 427, 213518. [Google Scholar] [CrossRef]
- García, A.; Insuasty, B.; Herranz, M.A.; Martínez-Álvarez, R.; Martín, N. New Building Block forC3Symmetry Molecules: Synthesis ofs-Triazine-Based Redox Active Chromophores. Org. Lett. 2009, 11, 5398–5401. [Google Scholar] [CrossRef]
- Burns, B.; King, N.P.; Tye, H.; Studley, J.R.; Gamble, M.; Wills, M. Chiral phosphinamides: New catalysts for the asymmetric reduction of ketones by borane. J. Chem. Soc. Perkin Trans. 1998, 1, 1027–1038. [Google Scholar] [CrossRef]
- Du, D.-M.; Fang, T.; Xu, J.; Zhang, S.-W. Structurally Well-Defined, RecoverableC3-Symmetric Tris(β-hydroxy phosphoramide)-Catalyzed Enantioselective Borane Reduction of Ketones. Org. Lett. 2006, 8, 1327–1330. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, L.; Pan, Y.; Han, J.; Wu, H.; Teng, M.; Li, Z. Novel Tripod l-Prolinamide Catalysts Based on Tribenzyl- and Triphenyl-phosphine Oxide for the Direct Aldol Reaction. Synlett 2009, 2009, 933–936. [Google Scholar] [CrossRef]
- Moorthy, J.N.; Saha, S. C3-Symmetric Proline-Functionalized Organocatalysts: Enantioselective Michael Addition Reactions. Eur. J. Org. Chem. 2010, 2010, 6359–6365. [Google Scholar] [CrossRef]
- Murai, K.; Fukushima, S.; Hayashi, S.; Takahara, Y.; Fujioka, H. C3-Symmetric Chiral Trisimidazoline: Design and Application to Organocatalyst. Org. Lett. 2010, 12, 964–966. [Google Scholar] [CrossRef]
- Murai, K.; Fukushima, S.; Nakamura, A.; Shimura, M.; Fujioka, H. C3-Symmetric chiral trisimidazoline: The role of a third imidazoline and its application to the nitro Michael reaction and the α-amination of β-ketoesters. Tetrahedron 2011, 67, 4862–4868. [Google Scholar] [CrossRef]
- Murai, K.; Nakamura, A.; Matsushita, T.; Shimura, M.; Fujioka, H. C3-Symmetric Trisimidazoline-Catalyzed Enantioselective Bromolactonization of Internal Alkenoic Acids. Chem. A Eur. J. 2012, 18, 8448–8453. [Google Scholar] [CrossRef]
- Takizawa, S.; Sako, M.; Abozeid, M.A.; Kishi, K.; Wathsala, H.D.P.; Hirata, S.; Murai, K.; Fujioka, H.; Sasai, H. Enantio- and Diastereoselective Betti/aza-Michael Sequence: Single Operated Preparation of Chiral 1,3-Disubstituted Isoindolines. Org. Lett. 2017, 19, 5426–5429. [Google Scholar] [CrossRef]
- Boratyński, P.J. Dimeric Cinchona alkaloids. Mol. Divers. 2015, 19, 385–422. [Google Scholar] [CrossRef]
- Park, H.-G.; Jeong, B.-S.; Yoo, M.-S.; Park, M.-K.; Huh, H.; Jew, S.-S. Trimeric Cinchona alkaloid phase-transfer catalyst: α,α′,α′′-tris[O(9)-allylcinchonidinium]mesitylene tribromide. Tetrahedron Lett. 2001, 42, 4645–4648. [Google Scholar] [CrossRef]
- Siva, A.; Murugan, E. A New Trimeric Cinchona Alkaloid as a Chiral Phase-Transfer Catalyst for the Synthesis of Asymmetric α-Amino Acids. Synthesis 2005, 2005, 2927–2933. [Google Scholar] [CrossRef]
- Siva, A.; Murugan, E. New trimeric Cinchona alkaloid-based quaternary ammonium salts as efficient chiral phase transfer catalysts for enantioselective synthesis of α-amino acids. J. Mol. Catal. A Chem. 2006, 248, 1–9. [Google Scholar] [CrossRef]
- Siva, A.; Jayaraman, S.; Kumaraguru, D.; Arockiam, J.B.; Paulpandian, S.; Rajendiran, B. Highly Enantioselective Asymmetric Michael Addition Reactions with New Chiral Multisite Phase-Transfer Catalysts. Synlett 2014, 25, 1685–1691. [Google Scholar] [CrossRef]
- Beneto, A.J.; Sivamani, J.; AshokKumar, V.; Duraimurugan, K.; Balasaravanan, R.; Siva, A. Highly enantioselective Michael addition reactions with new trimeric chiral phase transfer catalysts. New J. Chem. 2015, 39, 3098–3104. [Google Scholar] [CrossRef]
- Károlyi, B.I.; Bősze, S.; Orbán, E.; Sohár, P.; Drahos, L.; Gál, E.; Csámpai, A. Acylated mono-, bis- and tris- Cinchona-Based Amines Containing Ferrocene or Organic Residues: Synthesis, Structure and in Vitro Antitumor Activity on Selected Human Cancer Cell Lines. Molecules 2012, 17, 2316–2329. [Google Scholar] [CrossRef]
- Min, C.; Han, X.; Liao, Z.; Wu, X.; Zhou, H.-B.; Dong, C. C3-Symmetrical Cinchonine-Squaramide as New Highly Efficient, and Recyclable Organocatalyst for Enantioselective Michael Addition. Adv. Synth. Catal. 2011, 353, 2715–2720. [Google Scholar] [CrossRef]
- Han, X.; Liu, B.; Zhou, H.-B.; Dong, C. Enhanced efficiency of recyclable C3-symmetric cinchonine-squaramides in the asymmetric Friedel–Crafts reaction of indoles with alkyl trifluoropyruvate. Tetrahedron Asymmetry 2012, 23, 1332–1337. [Google Scholar] [CrossRef]
- Han, X.; Dong, C.; Zhou, H.-B. C3-Symmetric Cinchonine-Squaramide-Catalyzed Asymmetric Chlorolactonization of Styrene-Type Carboxylic Acids with 1,3-Dichloro-5,5-dimethylhydantoin: An Efficient Method to Chiral Isochroman-1-ones. Adv. Synth. Catal. 2014, 356, 1275–1280. [Google Scholar] [CrossRef]
- Lv, W.; Guo, C.; Dong, Z.; Tang, S.; Liu, B.; Dong, C. C3-Symmetric cinchonine-squaramide as a recyclable efficient organocatalyst for tandem Michael addition–cyclisation of malononitrile and nitrovinylphenols. Tetrahedron Asymmetry 2016, 27, 670–674. [Google Scholar] [CrossRef]
- Le Phuong, H.A.; Blanford, C.F.; Szekely, G. Reporting the unreported: The reliability and comparability of the literature on organic solvent nanofiltration. Green Chem. 2020, 22, 3397–3409. [Google Scholar] [CrossRef]
- Hu, J.; Kim, C.; Halasz, P.; Kim, J.F.; Kim, J.; Szekely, G. Artificial intelligence for performance prediction of organic solvent nanofiltration membranes. J. Membr. Sci. 2021, 619, 118513. [Google Scholar] [CrossRef]
- Le Phuong, H.A.; Cseri, L.; Whitehead, G.F.S.; Garforth, A.; Budd, P.; Szekely, G. Environmentally benign and diastereoselective synthesis of 2,4,5-trisubstituted-2-imidazolines. RSC Adv. 2017, 7, 53278–53289. [Google Scholar] [CrossRef]
- Dong, R.; Liu, R.; Gaffney, P.R.J.; Schaepertoens, M.; Marchetti, P.; Williams, C.M.; Chen, R.; Livingston, A.G. Sequence-defined multifunctional polyethers via liquid-phase synthesis with molecular sieving. Nat. Chem. 2019, 11, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Székely, G.; Schaepertoens, M.; Gaffney, P.R.J.; Livingston, A.G. Beyond PEG2000: Synthesis and Functionalisation of Monodisperse PEGylated Homostars and Clickable Bivalent Polyethyleneglycols. Chem. A Eur. J. 2014, 20, 10038–10051. [Google Scholar] [CrossRef] [PubMed]
- Razali, M.; Didaskalou, C.; Kim, J.F.; Babaei, M.; Drioli, E.; Lee, Y.M.; Szekely, G. Exploring and Exploiting the Effect of Solvent Treatment in Membrane Separations. ACS Appl. Mater. Interfaces 2017, 9, 11279–11289. [Google Scholar] [CrossRef]
- Voros, V.; Drioli, E.; Fonte, C.; Szekely, G. Process Intensification via Continuous and Simultaneous Isolation of Antioxidants: An Upcycling Approach for Olive Leaf Waste. ACS Sustain. Chem. Eng. 2019, 7, 18444–18452. [Google Scholar] [CrossRef]
- Alammar, A.; Park, S.-H.; Williams, C.J.; Derby, B.; Szekely, G. Oil-in-water separation with graphene-based nanocomposite membranes for produced water treatment. J. Membr. Sci. 2020, 603, 118007. [Google Scholar] [CrossRef]
- Keraani, A.; Nasser, G.; Shahane, S.; Renouard, T.; Bruneau, C.; Rabiller-Baudry, M.; Fischmeister, C. Syntheses and characterization of molecular weight enlarged olefin metathesis pre-catalysts. Comptes Rendus Chim. 2017, 20, 717–723. [Google Scholar] [CrossRef]
- Kisszékelyi, P.; Nagy, S.; Fehér, Z.; Huszthy, P.; Kupai, J. Membrane-Supported Recovery of Homogeneous Organocatalysts: A Review. Chemestry 2020, 2, 742–758. [Google Scholar] [CrossRef]
- Siew, W.E.; Ates, C.; Merschaert, A.; Livingston, A.G. Efficient and productive asymmetric Michael addition: Development of a highly enantioselective quinidine-based organocatalyst for homogeneous recycling via nanofiltration. Green Chem. 2013, 15, 663–674. [Google Scholar] [CrossRef]
- Shao, Z.; Zhang, H. Combining transition metal catalysis and organocatalysis: A broad new concept for catalysis. Chem. Soc. Rev. 2009, 38, 2745–2755. [Google Scholar] [CrossRef]
- Zhong, C.; Shi, X. When Organocatalysis Meets Transition-Metal Catalysis. Eur. J. Org. Chem. 2010, 2010, 2999–3025. [Google Scholar] [CrossRef]
- Didaskalou, C.; Kupai, J.; Cseri, L.; Barabas, J.; Vass, E.; Holtzl, T.; Szekely, G. Membrane-Grafted Asymmetric Organocatalyst for an Integrated Synthesis–Separation Platform. ACS Catal. 2018, 8, 7430–7438. [Google Scholar] [CrossRef]
- Kisszekelyi, P.; Alammar, A.; Kupai, J.; Huszthy, P.; Barabas, J.; Holtzl, T.; Szente, L.; Bawn, C.; Adams, R.; Szekely, G. Asymmetric synthesis with cinchona-decorated cyclodextrin in a continuous-flow membrane reactor. J. Catal. 2019, 371, 255–261. [Google Scholar] [CrossRef]
- Schaepertoens, M.; Didaskalou, C.; Kim, J.F.; Livingston, A.G.; Szekely, G. Solvent recycle with imperfect membranes: A semi-continuous workaround for diafiltration. J. Membr. Sci. 2016, 514, 646–658. [Google Scholar] [CrossRef]
- Zhao, D.; Kim, J.F.; Ignacz, G.; Pogany, P.; Lee, Y.M.; Szekely, G. Bio-Inspired Robust Membranes Nanoengineered from Interpenetrating Polymer Networks of Polybenzimidazole/Polydopamine. ACS Nano 2019, 13, 125–133. [Google Scholar] [CrossRef]
- Ignacz, G.; Fei, F.; Szekely, G. Ion-Stabilized Membranes for Demanding Environments Fabricated from Polybenzimidazole and Its Blends with Polymers of Intrinsic Microporosity. ACS Appl. Nano Mater. 2018, 1, 6349–6356. [Google Scholar] [CrossRef]
- Cassani, C.; Martín-Rapún, R.; Arceo, E.; Bravo, F.; Melchiorre, P. Synthesis of 9-amino(9-deoxy)epi cinchona alkaloids, general chiral organocatalysts for the stereoselective functionalization of carbonyl compounds. Nat. Protoc. 2013, 8, 325–344. [Google Scholar] [CrossRef] [PubMed]
- Skarżewski, J.; Zielińska-Błajet, M.; Kucharska, M. Simple Enantiospecific Synthesis of Sulfides ofCinchonaAlkaloids. Synthesis 2006, 2006, 1176–1182. [Google Scholar] [CrossRef]
- Yaegashi, K.; Mikami, M. Preparation of 9-azido Cinchona Alkaloids, Their 9-amino Derivatives, and Their 9-(substituted thioureido) Derivatives. JP Patent 2010024173 (20100204), 4 February 2010. [Google Scholar]
- Heidelberger, M.; Jacobs, W.A. Syntheses in the cinchona series. I. The simpler cinchona alkaloids and their dihydro derivatives. J. Am. Chem. Soc. 1919, 41, 817–833. [Google Scholar] [CrossRef]
- Brandes, S.; Niess, B.; Bella, M.; Prieto, A.; Overgaard, J.; Jørgensen, K.A. Non-Biaryl Atropisomers in Organocatalysis. Chem. A Eur. J. 2006, 12, 6039–6052. [Google Scholar] [CrossRef]
- Braje, W.M.; Frackenpohl, J.; Schrake, O.; Wartchow, R.; Beil, W.; Hoffmann, H.M.R. Synthesis of 10,11-DidehydroCinchona Alkaloids and Key Derivatives. Helvetica Chim. Acta 2000, 83, 777–792. [Google Scholar] [CrossRef]
- Song, Y.; Kohlmeir, E.K.; Meade, T.J. Synthesis of Multimeric MR Contrast Agents for Cellular Imaging. J. Am. Chem. Soc. 2008, 130, 6662–6663. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, E.E.; Voreck, W.E. Evaluation of a new organic azide: Hexakis(azidomethyl)benzene (HAB). Propellants Explos. Pyrotech. 1989, 14, 19–23. [Google Scholar] [CrossRef]
- Lyle, M.P.A.; Draper, N.D.; Wilson, P.D. Enantioselective Friedel−Crafts Alkylation Reactions Catalyzed by a Chiral NonracemicC2-Symmetric 2,2‘-Bipyridyl Copper(II) Complex. Org. Lett. 2005, 7, 901–904. [Google Scholar] [CrossRef]
- Török, B.; Abid, M.; London, G.; Esquibel, J.; Török, M.; Mhadgut, S.C.; Yan, P.; Prakash, G.K.S. Highly Enantioselective Organocatalytic Hydroxyalkylation of Indoles with Ethyl Trifluoropyruvate. Angew. Chem. Int. Ed. 2005, 44, 3086–3089. [Google Scholar] [CrossRef]
- Evans, D.A.; Mito, A.S.; Seidel, D. Scope and Mechanism of Enantioselective Michael Additions of 1,3-Dicarbonyl Compounds to Nitroalkenes Catalyzed by Nickel(II)−Diamine Complexes. J. Am. Chem. Soc. 2007, 129, 11583–11592. [Google Scholar] [CrossRef]
- Tan, B.; Zhang, X.; Chua, P.J.; Zhong, G. Recyclable organocatalysis: Highly enantioselective Michael addition of1,3-diaryl-1,3-propanedioneto nitroolefins. Chem. Commun. 2009, 779–781. [Google Scholar] [CrossRef]
- Gonczi, K.; Kudar, V.; Jaszay, Z.; Bombicz, P.; Faigl, F.; Madarász, J. Solid state structural relation and binary melting phase diagram of (S-) and racemic 2-(2-nitro-1-phenylethyl)-1,3-diphenyl-propane-1,3-dione. Thermochim. Acta 2014, 580, 46–52. [Google Scholar] [CrossRef][Green Version]
- Fei, F.; Le Phuong, H.A.; Blanford, C.F.; Szekely, G. Tailoring the Performance of Organic Solvent Nanofiltration Membranes with Biophenol Coatings. ACS Appl. Polym. Mater. 2019, 1, 452–460. [Google Scholar] [CrossRef]
- Cseri, L.; Szekely, G. Towards cleaner PolarClean: Efficient synthesis and extended applications of the polar aprotic solvent methyl 5-(dimethylamino)-2-methyl-5-oxopentanoate. Green Chem. 2019, 21, 4178–4188. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).