RETRACTED: Intraosteal Behavior of Porous Scaffolds: The mCT Raw-Data Analysis as a Tool for Better Understanding
Abstract
:1. Introduction
2. Materials and Methods
2.1. Porous Scaffold Implants Composition and Fabrication
2.2. Animal Selection and Conditioning
2.3. Scaffold Implantation: Anesthetic and Surgical Method
2.4. Euthanasia and Samples Collection
2.5. mCT Imaging Protocol and Descriptive Analysis
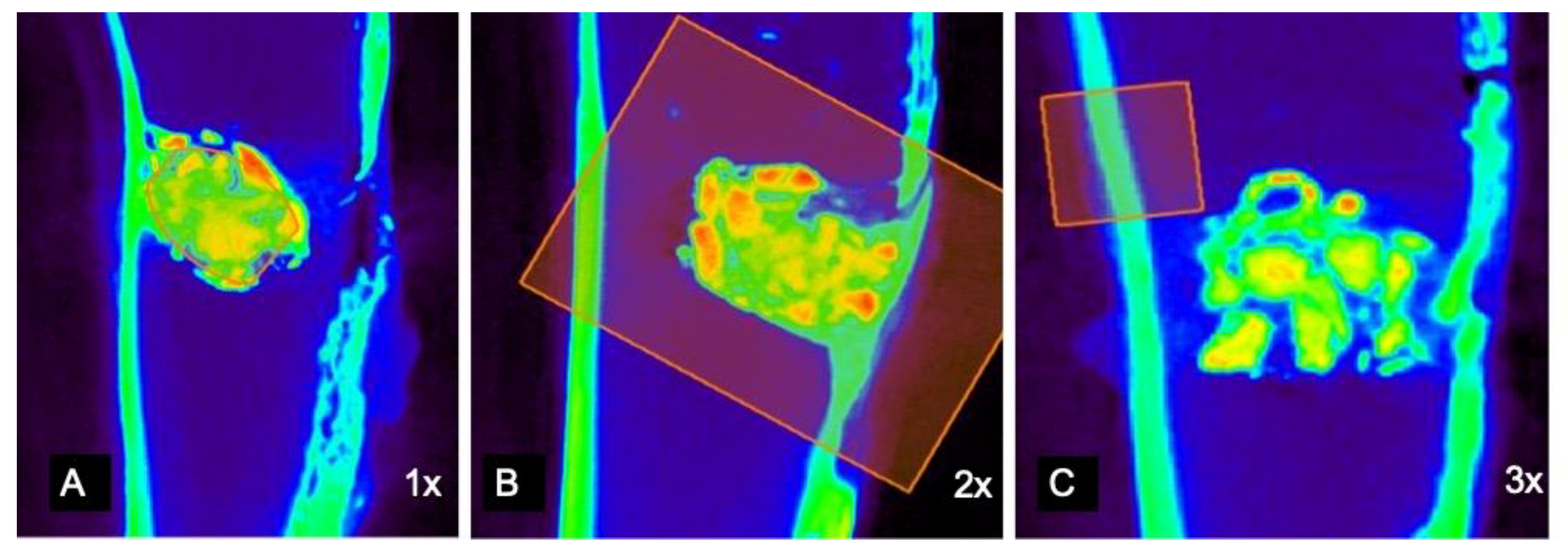
2.6. Mathematical Processing of mCT Raw Data
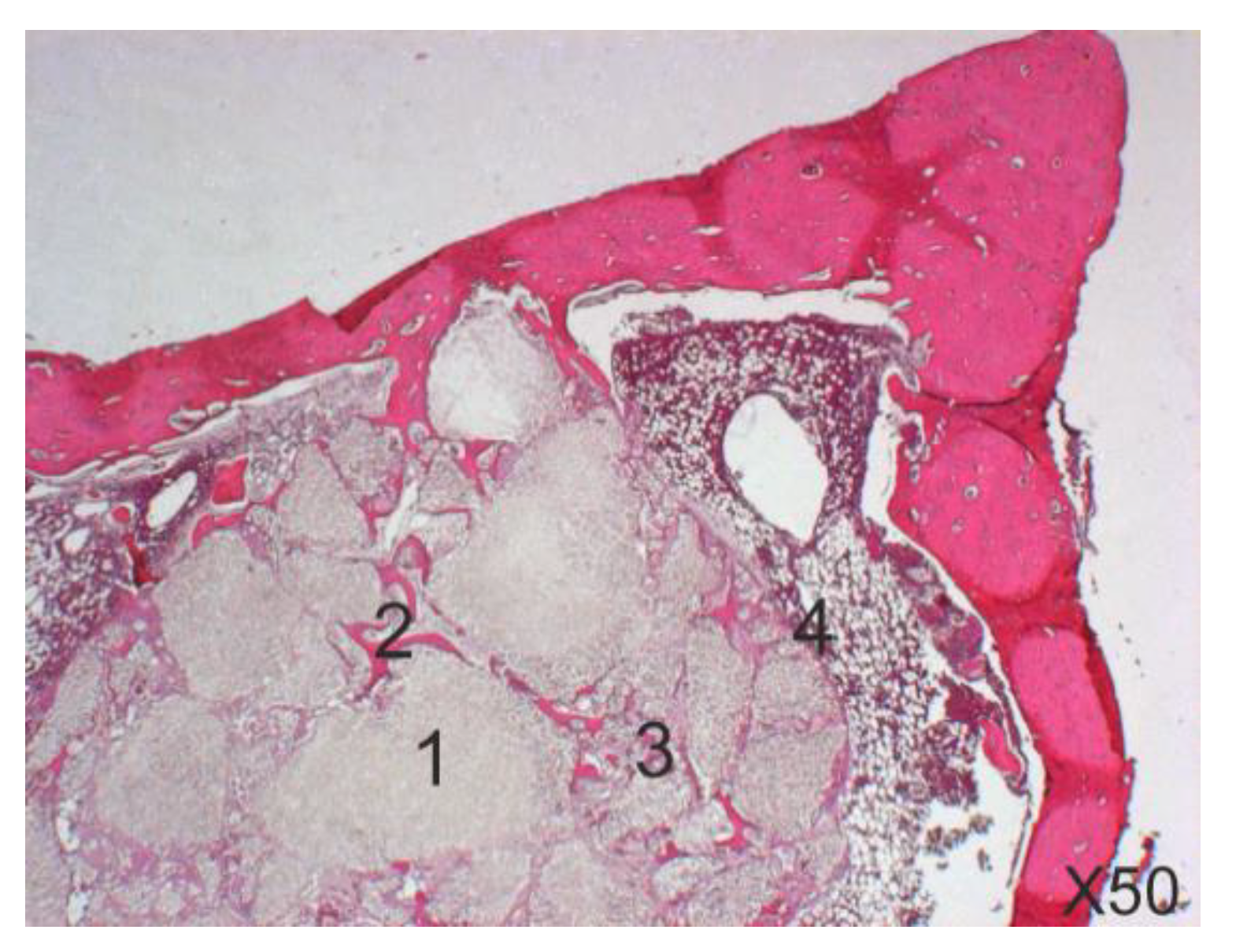
2.7. Histological and Histomorphometric Study
2.8. Statistical Analysis
3. Results
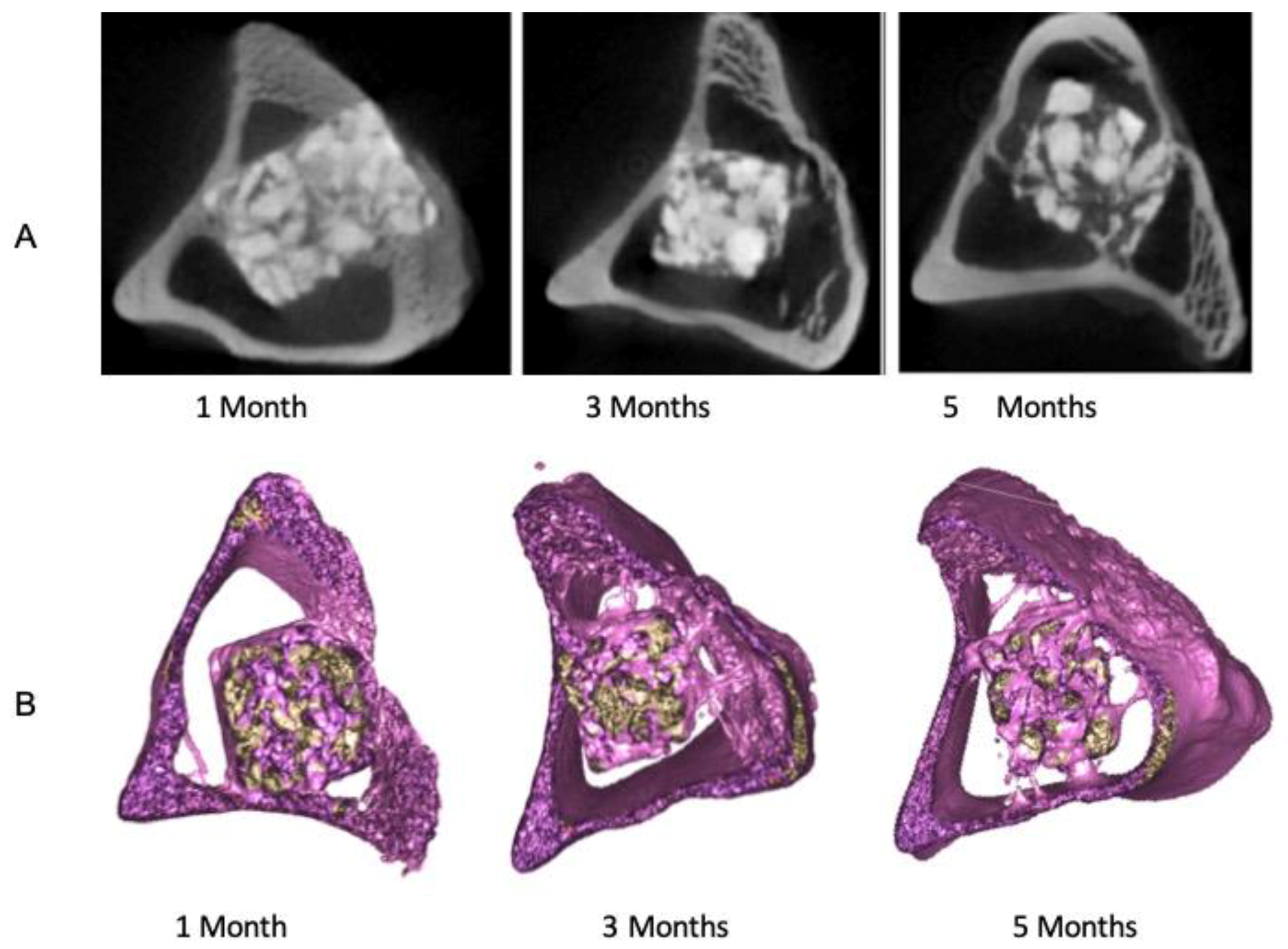
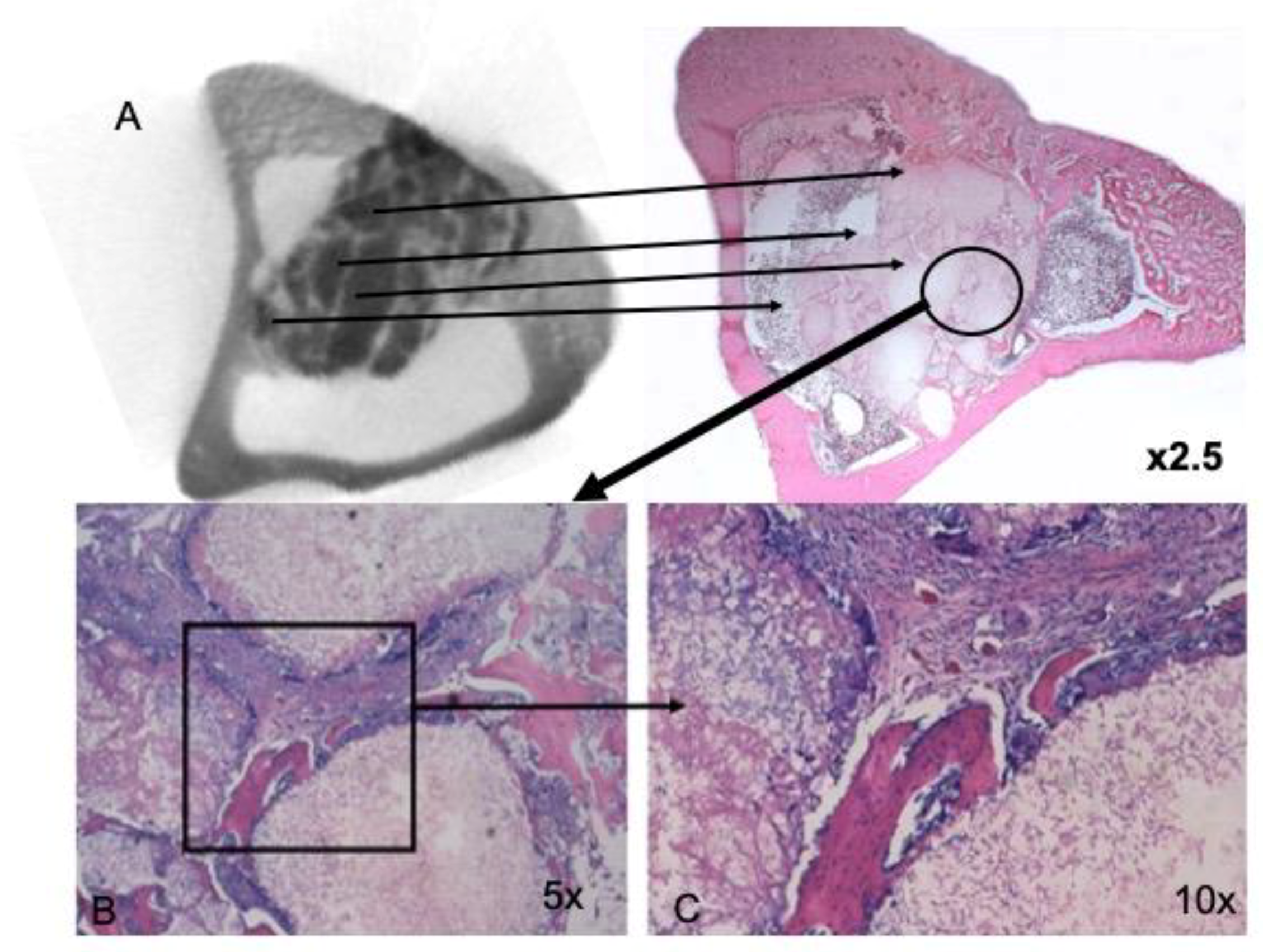
3.1. Descriptive Analysis of mCT Images
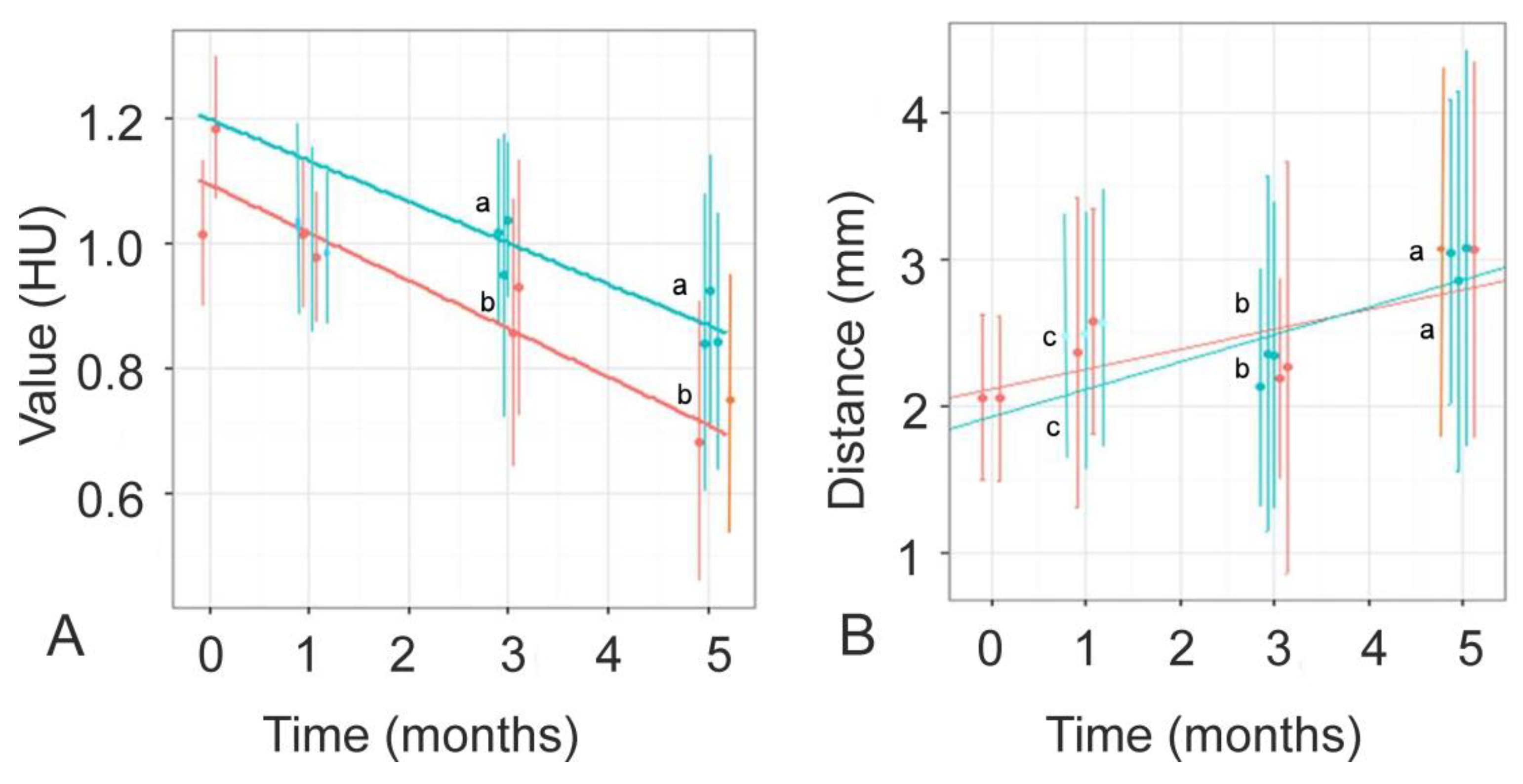
3.2. mCT Raw-Data Analysis
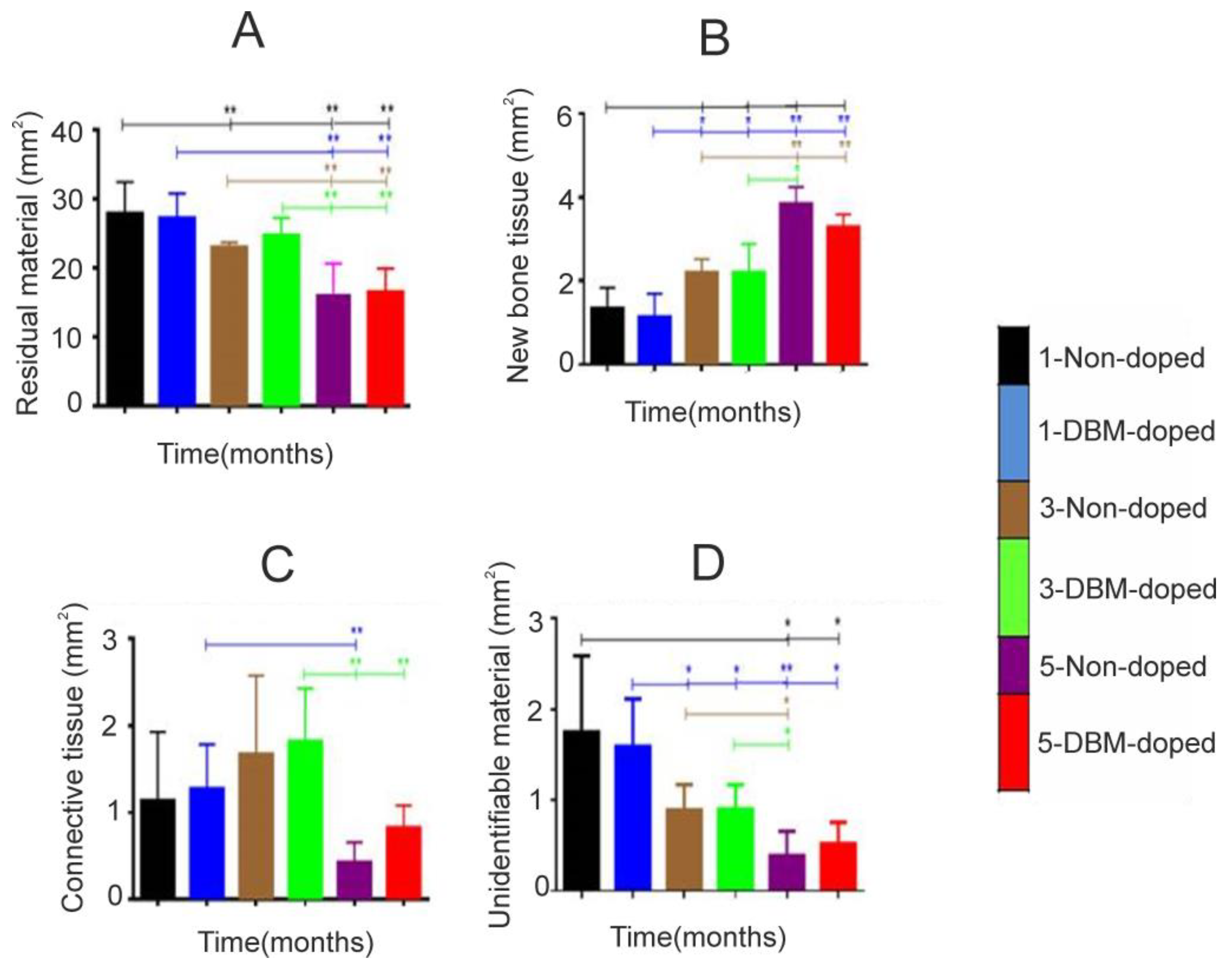
3.3. Histological and Histomorphometrical Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lehmann, T.M.; Meinzer, H.P.; Tolxdorff, T. Advances in biomedical image analysis ast present and future challenges. Methods Inf. Med. 2004, 43, 308–314. [Google Scholar]
- Lehmann, T.M.; Handels, H.; Maier-Hein ne Fritzsche, K.H.; Mersmann, S.; Palm, C.; Tolxdorff, T.; Wagenknecht, G.; Wittenberg, T. Viewpoints on medical image processing: From science to application. Curr. Med. Imaging Rev. 2013, 9, 79–88. [Google Scholar] [CrossRef]
- Bouxsein, M.L.; Boyd, S.K.; Christiansen, B.A.; Guldberg, R.E.; Jepsen, K.J.; Müller, R. Guidelines for assessment of bone microstructure in rodents using microcomputed tomography. J. Bone Miner. Res. 2010, 25, 1468–1486. [Google Scholar] [CrossRef]
- Höhne, K.H.; Bomans, M.; Pommert, A.; Riemer, M.; Schiers, C.; Tiede, U. 3D visualization of tomographic volume data using the generalized voxel model. Vis. Comput. 1990, 6, 28–36. [Google Scholar] [CrossRef]
- Beister, M.; Kolditz, D.; Kalender, W.A. Iterative reconstruction methods in X-ray CT. Phys. Med. 2012, 28, 94–108. [Google Scholar] [CrossRef]
- Garland, L.H. On the scientific evaluation of diagnostic procedures: Presidential address thirty-fourth annual meeting of the Radiological Society of North America. Radiology 1949, 52, 309–328. [Google Scholar] [CrossRef]
- Berlin, L. Radiologic errors, past, present and future. Diagnosis 2014, 1, 79–84. [Google Scholar] [CrossRef]
- Bruno, M.A.; Walker, E.A.; Abujudeh, H.H. Understanding and confronting our mistakes: The epidemiology of error in radiology and strategies for error reduction. RadioGraphics 2015, 35, 1668–1676. [Google Scholar] [CrossRef]
- Waite, S.; Scott, J.; Gale, B.; Fuchs, T.; Kolla, S.; Reede, D. Interpretive error in radiology. Am. J. Roentgenol. 2017, 208, 739–749. [Google Scholar] [CrossRef]
- Castellino, R.A. Computer aided detection (CAD): An overview. Cancer Imaging 2005, 5, 17–19. [Google Scholar] [CrossRef]
- Doi, K. Computer-aided diagnosis in medical imaging: Historical review, current status and future potential. Comput. Med. Imaging Graph. 2007, 31, 198–211. [Google Scholar] [CrossRef]
- Parrilla-Almansa, A.; García-Carrillo, N.; Ros-Tárraga, P.; Martínez, C.M.; Martínez-Martínez, F.; Meseguer-Olmo, L.; de Aza, P.N. Demineralized bone matrix coating Si-Ca-P ceramic does not improve the osseointegration of the scaffold. Materials 2018, 11, 1580. [Google Scholar] [CrossRef]
- Martinez, I.M.; Velasquez, P.A.; de Aza, P.N. Synthesis and stability of α-tricalcium phosphate doped with dicalcium silicate in the system Ca3(PO4)2–Ca2SiO4. Mater. Charact. 2010, 61, 761–767. [Google Scholar] [CrossRef]
- Kim, S.Y.; Kim, Y.K.; Park, Y.H.; Park, J.C.; Ku, J.K.; Um, I.W.; Kim, J.Y. Evaluation of the healing potential of demineralized dentin matrix fixed with recombinant human bone. Morphogenetic protein-2 in bone grafts. Materials 2017, 10, 1049. [Google Scholar] [CrossRef]
- Schmidt, R.; Muller, L.; Kress, A.; Hirschfelder, H.; Aplas, A.; Pitto, R.P. A computed tomography assessment of femoral and acetabular bone changes after total hip arthroplasty. Int. Orthop. 2002, 26, 299–302. [Google Scholar] [CrossRef]
- Mate-Sanchez de Val, J.E.; Calvo-Guirado, J.L.; Delgado-Ruiz, R.A.; Ramirez-Fernandez, M.P.; Negri, B.; Abboud, M.; Martinez, I.M.; de Aza, P.N. Physical properties, mechanical behavior, and electron microscopy study of a new α-TCP block graft with silicon in an animal model. J. Biomed. Mater. Res. A 2012, 100, 3446–3454. [Google Scholar] [CrossRef]
- Calvo-Guirado, J.L.; Ramirez-Fernandez, M.P.; Delgado-Ruiz, R.A.; Mate-Sanchez de Val, J.E.; Velasquez, P.; de Aza, P.N. Influence of biphasic β-TCP with and without the use of collagen membranes on bone healing of surgically critical size defects. A radiological, histological and histomorphometric study. Clin. Oral Implant Res. 2014, 25, 1228–1238. [Google Scholar] [CrossRef]
- Ros-Tarraga, P.; Mazon, P.; Rodriguez, M.A.; Meseguer-Olmo, L.; de Aza, P.N. Novel resorbable and osteoconductive calcium silicophosphate scaffold induced bone formation. Materials 2016, 9, 785. [Google Scholar] [CrossRef]
- Marsell, R.; Einhorn, T.A. The biology of fracture healing. Injury 2011, 42, 551–555. [Google Scholar] [CrossRef]
- Ramirez-Fernandez, M.P.; Mazon, P.; Gehrke, S.A.; Calvo-Guirado, J.L.; de Aza, P.N. Comparison of two xenograft materials used in sinus lift procedures: Material characterization and in vivo behavior. Materials 2017, 10, 623. [Google Scholar] [CrossRef]
- Bohner, M.; Baroud, G.; Bernstein, A.; Dobelin, N.; Galea, L.; Hesse, B.; Heuberger, R.; Meille, S.; Miche, P.; von Rechenberg, B.; et al. Characterization and distribution of mechanically competent mineralized tissue in micropores of β-tricalcium phosphate bone substitutes. Mater. Today 2017, 20, 106–115. [Google Scholar] [CrossRef]
- Sweedy, A.; Bohner, M.; Baroud, G. Multimodal analysis of in vivo resorbable CaP bone substitutes by combining histology, SEM, and microcomputed tomography data. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 106, 1567–1577. [Google Scholar] [CrossRef]
- Mate-Sanchez de Val, J.E.; Mazón, P.; Piattelli, A.; Calvo-Guirado, J.L.; Bueno, J.M.; de Aza, P.N. Comparison among the physical properties of calcium phosphate-based bone substitutes of natural or synthetic origin. Int. J. Appl. Ceram. Technol. 2018, 15, 930–937. [Google Scholar] [CrossRef]
- Calvo-Guirado, J.L.; Ballester-Montilla, A.M.; de Aza, P.N.; Fernández-Domínguez, M.; Gehrke, S.A.; Cegarra-Del Pino, P.; Mahesh, L.; Pelegrine, A.A.; Aragoneses, J.M.; Maté-Sánchez de Val, J.E. Particulate extracted human teeth characterization by SEM-EDX evaluation as a biomaterial for socket preservatio: An in vitro study. Materials 2019, 12, 380. [Google Scholar] [CrossRef]
- Mate Sanchez de Val, J.E.; Calvo Guirado, J.L.; Delgado Ruiz, R.A.; Gomez Moreno, G.; Ramírez Fernández, M.P.; Romanos, G.E. Bone neo-formation and mineral degradation of 4Bone.® Part I: Material characterization and SEM study in critical size defects in rabbits. Clin. Oral. Implants Res. 2015, 26, 116–1169. [Google Scholar]
- Barone, A.; Toti, P.; Quaranta, A.; Alfonsi, F.; Cucchi, A.; Calvo-Guirado, J.L.; Negri, B.; Di Felice, R.; Covani, U. Volumetric analysis of remodelling pattern after ridge preservation comparing use of two types of xenografts. A multicentre randomized clinical trial. Clin Oral Implants Res. 2016, 27, e105–e115. [Google Scholar] [CrossRef]
- Dozza, B.; Lesci, I.G.; Duchi, S.; Della Bella, E.; Martini, L.; Salamanna, F.; Falconi, M.; Cinotti, S.; Fini, M.; Lucarelli, E.; et al. When size matters: Differences in demineralized bone matrix particles affect collagen structure, mesenchymal stem cell behavior, and osteogenic potential. J. Biomed. Mater. Res. Part A 2017, 105, 1019–1033. [Google Scholar] [CrossRef]
- de Aza, P.N.; Rodríguez, M.A.; Gehrke, S.A.; Mate-Sanchez de Val, J.E.; Calvo-Guirado, J.L. A Si-αTCP scaffold for biomedical applications: An experimental study using the rabbit tibia model. Appl. Sci. 2017, 7, 706. [Google Scholar] [CrossRef]
- Velasquez, P.; Luklinska, Z.B.; Meseguer-Olmo, L.; Mate-Sanchez de Val, J.E.; Delgado-Ruiz, R.A.; Calvo-Guirado, J.L.; Ramirez-Fernandez, M.P.; de Aza, P.N. αTCP ceramic doped with dicalcium silicate for bone regeneration applications prepared by powder metallurgy method: In vitro and in vivo studies. J. Biomed. Mater. Res. Part A 2013, 101, 1943–1954. [Google Scholar] [CrossRef]
- Lugo, G.J.; Mazón, P.; de Aza, P.N. Phase transitions in single phase Si-Ca-P-based ceramic under thermal treatment. J. Eur. Ceram. Soc. 2015, 35, 3693–3700. [Google Scholar] [CrossRef]
- Lugo, G.J.; Mazón, P.; de Aza, P.N. Material processing of a new calcium silicophosphate ceramic. Ceram. Int. 2016, 42, 673–680. [Google Scholar] [CrossRef]
- Rubio, V.; de la Casa-Lillo, M.A.; de Aza, S.; de Aza, P.N. The system Ca3(PO4)2–Ca2SiO4: The sub-system Ca2SiO4-7CaOP2O52SiO2. J. Am. Ceram. Soc. 2011, 94, 4459–4462. [Google Scholar] [CrossRef]
- Shalash, M.A.; Rahman, H.A.; Azim, A.A.; Neemat, A.H.; Hawary, H.E.; Nasry, S.A. Evaluation of horizontal ridge augmentation using beta tricalcium phosphate and demineralized bone matrix: A comparative study. J. Clin. Exp. Dent. 2013, 5, e253–e259. [Google Scholar] [CrossRef]
- Andersen, T.L.; Del Carmen Ovejero, M.; Kirkegaard, T.; Lenhard, T.; Foged, N.T.; Delaissé, J.M. A scrutiny of matrix metalloproteinases in osteoclasts: Evidence for heterogeneity and for the presence of MMPs synthesized by other cells. Bone 2004, 35, 1107–1119. [Google Scholar] [CrossRef]
- Benedek, I.; Chitu, M.; Kovacs, I.; Balazs, B.; Benedek, T. Incremental value of preprocedural coronary computed tomographic angiography to classical coronary angiography for prediction of PCI complexity in left main stenosis. World J. Cardiovasc. Dis. 2013, 3, 573–580. [Google Scholar] [CrossRef]
- Chow, B.J.; Hoffmann, U.; Nieman, K. Computed tomographic coronary angiography: An alternative to invasive coronary angiography. Can. J. Cardiol. 2005, 21, 933–940. [Google Scholar]
- Beckmann, N.; Ledermann, B. Noninvasive small rodent imaging: Significance for the 3R principles. In Small Animal Imaging; Kiessling, F., Pichler, B., Hauff, P., Eds.; Springer: Berlin, Germary, 2017. [Google Scholar]
- Oryan, A.; Eslaminejad, M.B.; Kamali, A.; Hosseini, S.; Moshiri, A.; Baharvand, H. Mesenchymal stem cells seeded onto tissue-engineered osteoinductive scaffolds enhance the healing process of critical-sized radial bone defects in rat. Cell Tissue Res. 2018, 374, 63–81. [Google Scholar] [CrossRef]
- González-Gil, A.B.; Lamo-Espinosa, J.M.; Muiños-López, E.; Ripalda-Cemboráin, P.; Abizanda, G.; Valdés-Fernández, J.; López-Martínez, T.; Flandes-Iparraguirre, M.; Andreu, I.; Elizalde, M.R.; et al. Periosteum-derived mesenchymal progenitor cells in engineered implants promote fracture healing in a critical-size defect rat model. J. Tissue Eng. Regen. Med. 2019. [Google Scholar] [CrossRef]
- Develos Godoy, D.J.; Banlunara, W.; Jaroenporn, S.; Sangvanich, P.; Thunyakitpisal, P. Collagen and mPCL-TCP scaffolds induced differential bone regeneration in ovary-intact and ovariectomized rats. Bio-Med. Mater. Eng. 2018, 29, 389–399. [Google Scholar] [CrossRef]
- Francois, E.L.; Yaszemski, M.J. Preclinical bone repair models in regenerative medicine. Princ. Regen. Med. 2019, 43, 761–767. [Google Scholar]
- Ruehe, B.; Niehues, S.; Heberer, S.; Nelson, K. Miniature pigs as an animal model for implant research: Bone regeneration in critical-size defects. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2009, 108, 699–706. [Google Scholar] [CrossRef]
- Budán, F.; Szigeti, K.; Weszl, M.; Horváth, I.; Balogh, E.; Kanaan, R.; Berényi, K.; Lacza, Z.; Máthé, D.; Gyöngyi, Z. Novel radiomics evaluation of bone formation utilizing multimodal (SPECT/X-ray CT) in vivo imaging. PLoS ONE 2018, 13, e0204423. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parrilla-Almansa, A.; González-Bermúdez, C.A.; Sánchez-Sánchez, S.; Meseguer-Olmo, L.; Martínez-Cáceres, C.M.; Martínez-Martínez, F.; Calvo-Guirado, J.L.; Piñero de Armas, J.J.; Aragoneses, J.M.; García-Carrillo, N.; et al. RETRACTED: Intraosteal Behavior of Porous Scaffolds: The mCT Raw-Data Analysis as a Tool for Better Understanding. Symmetry 2019, 11, 532. https://doi.org/10.3390/sym11040532
Parrilla-Almansa A, González-Bermúdez CA, Sánchez-Sánchez S, Meseguer-Olmo L, Martínez-Cáceres CM, Martínez-Martínez F, Calvo-Guirado JL, Piñero de Armas JJ, Aragoneses JM, García-Carrillo N, et al. RETRACTED: Intraosteal Behavior of Porous Scaffolds: The mCT Raw-Data Analysis as a Tool for Better Understanding. Symmetry. 2019; 11(4):532. https://doi.org/10.3390/sym11040532
Chicago/Turabian StyleParrilla-Almansa, Andrés, Carlos Alberto González-Bermúdez, Silvia Sánchez-Sánchez, Luis Meseguer-Olmo, Carlos Manuel Martínez-Cáceres, Francisco Martínez-Martínez, José Luis Calvo-Guirado, Juan José Piñero de Armas, Juan Manuel Aragoneses, Nuria García-Carrillo, and et al. 2019. "RETRACTED: Intraosteal Behavior of Porous Scaffolds: The mCT Raw-Data Analysis as a Tool for Better Understanding" Symmetry 11, no. 4: 532. https://doi.org/10.3390/sym11040532
APA StyleParrilla-Almansa, A., González-Bermúdez, C. A., Sánchez-Sánchez, S., Meseguer-Olmo, L., Martínez-Cáceres, C. M., Martínez-Martínez, F., Calvo-Guirado, J. L., Piñero de Armas, J. J., Aragoneses, J. M., García-Carrillo, N., & De Aza, P. N. (2019). RETRACTED: Intraosteal Behavior of Porous Scaffolds: The mCT Raw-Data Analysis as a Tool for Better Understanding. Symmetry, 11(4), 532. https://doi.org/10.3390/sym11040532







