Abstract
A total of 385 species of diatoms were identified in the phytoplankton of 14 small Arctic tundra water bodies in the vicinity of Tiksi Bay. We found that the species composition of phytoplankton in each lake is strictly individual. The ecological preferences of diatom species in the studied water bodies were determined for more than 90% of the list. Indicator characteristics show a certain response of the species composition of phytoplankton to changes in salinity and organic pollution. Several regularities were revealed in the spatial distribution of diatom communities in the study area in connection with the physicochemical parameters of their habitat, the height of the lake, its remoteness from the seacoast, and belonging to a specific watershed. Statistical mapping of the data on the diversity of communities and the chemical properties of water revealed a strong reaction of the communities of water bodies to point one-time anthropogenic pollution, and also made it possible to assume the influence of summer, northeast winds on the species composition as a climatic factor. The results of the study are important for developing the foundations for monitoring the non-impact (background), ecologically sensitive territory of the Arctic. They are highly relevant for assessing the consequences of local anthropogenic impacts and climate change in the future. Spatial ecological mapping in conjunction with bioindication can be used as a new method for identifying natural and non-natural stress factors.
Keywords:
diatoms; lakes; phytoplankton; statistical mapping; bioindicators; Tiksi Bay coast; Yakutia; Arctic 1. Introduction
Diatoms are recognized as one of the most diverse groups that are used in the assessment of the ecological state of water bodies [1,2,3] under the European Framework Directive [4], because their great diversity and our extensive knowledge of the ecology [5] allow for the use of their bio-indicator properties [6,7]. The aquatic ecosystems of the Eurasian High Arctic are still insufficiently studied but have recently attracted more and more attention due to the development of Arctic resources. Diatoms were studied on the islands in the Arctic Ocean [8,9,10], Eurasian northern coast of the Arctic Ocean [11,12], Chukotka [13,14,15], and the continental part of Yakutia [16]. Our studies of aquatic communities in northern Yakutia were conducted to assess the impact of climatic and anthropogenic factors on them [17,18,19,20,21]. The study of the Arctic environment is important in connection with permafrost properties under the phenomenon of global climate change [22,23].
Particular attention in this regard is paid to communities of water bodies in areas adjacent to protected areas, but where there is not only a gradient of climatic but also anthropogenic factors. If reserves represent the background diversity of the territory where the reserve is located, then in nearby land areas subject to anthropogenic impacts, one can not only trace the change in species composition, but also assess the degree of vulnerability of biodiversity of the entire territory when compared with a protected area.
Water bodies around the coast of Laptev Sea in northern Eurasia attract the attention of researchers because it is an area that connects two major transport arteries, the Lena River and the Northern Sea Route, and, as a result experiences climatic and anthropogenic impacts. Part of the coast and the delta of the Lena River is preserved as the Lena Delta Wildlife Reserve. Both the reserve and adjacent territories are located beyond the Arctic Circle in a zone of that continuously exhibits permafrost soils. Algological studies were initiated almost one hundred years ago of this area, including the lower reaches of the Lena River, its delta, the vast water area of the Laptev Sea, tundra reservoirs of the mainland (spurs of the Kharaulakh Range), and the New Siberian Islands. The latest species list of the algal flora in the region, including the Lena Delta Wildlife Reserve and the adjusted area, was published as a database on the GBIF.org portal [19]. In accordance with this, the diatom flora in the region includes information on 413 taxa with a rank below the genus prior to our investigation.
Previously, it was shown that the species composition and diversity of algae in Arctic water bodies is influenced by several regional features, such as the area of the water body, the direction of prevailing winds, water temperature, salinity, and pH [11,24].
The aim of this study is to determine the species composition of diatoms and environmental variables in 14 small, fresh water bodies in the Tiksi region to identify indicator species and analyze their spatial distribution, to determine the environmental factors affecting the diversity of this group of hydrobionts in the studied water bodies, and to compare the two species lists: present and the Lena Delta Wildlife Reserve.
2. Materials and Methods
2.1. Description of Study Site
The study area is located north of the Arctic Circle, 50 km southeast of the border of the Lena Delta Wildlife Reserve. The territory is located on the northern slope of the Primorsky Ridge, which is the eastern spur of the Kharaulakh Range of the Verkhoyansk Mountain system and forms a section of the coast of the Laptev Sea in the Arctic Ocean (Tiksi Bay and Neelov Gulf). The maximum altitude of the Primorsky Ridge is 400 m above sea level. The northeastern slope of the ridge mainly consists of shales, sandstones, limestones, and partly effusive rocks [25], which, according to some data, were formed as a result of catastrophic outbursts of a glacier-dammed lake in the late Pleistocene–early Holocene [26,27]. The studied area belongs to the tundra and mountain tundra, natural zones. The climate is maritime polar, the average annual air temperature is −9–11 °C [28], and the average frost-free period is 45 days [29]. The depth of seasonal thawing of permafrost soils is 0.2–1.2 m [30]. The average annual precipitation reaches 212 mm, of which the bulk falls from June to August. The phenomena of a polar day in summer and a polar night in winter are characteristic of the area. Strong winds are frequent; however, July (the month when our observations were performed) is characterized by the lowest-average hourly wind speed of the year, which is 15.5 km/h in the southeast direction (from the sea to the mainland). Due to limited drainage due to the reduced thickness of the seasonally thawed permafrost layer, the territory is characterized by an abundance of small tundra water bodies [30]. Our work was conducted on 14 different water bodies, which were shallow tundra lakes, small water bodies, and a hollow in the swampy tundra (mochezina) that has never been studied before (Figure 1, Table 1).
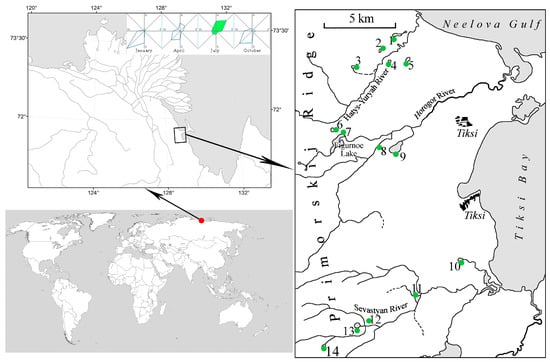
Figure 1.
Sampling points on the studied water bodies in July 2021 with a wind rose. 1–14 are numbers of sampling points according to the Table 1.

Table 1.
Sampling station geographical coordinates and parameters.
2.2. Sampling
Phytoplankton sampling was conducted between 3 and 7 July 2021. Phytoplankton samples were obtained with Apstein’s net SEFAR NITEX fabric, with a mesh diameter of 15 µm. One sample from lake 10 was obtained by washing off the biofilm from the surface of a submerged rock using a brush. Fixation with 4% neutral formaldehyde solution was performed immediately after collection. The temperature of the water and the morphometric parameters of each lake were determined during the collection of the phytoplankton. The coordinates and altitude of the sampling stations were defined by a Garmin eTrex GPS navigator (Table 1). Water samples of 1 L were collected from each lake for the chemical analysis. All samples were transported to perform determinations at the Institute for Biological Problems of Cryolithozone SB RAS, Yakutsk.
2.3. Water Chemistry Analysis
Chemical analyses of water samples were performed following standard methods [31]. Water color was determined using a photometric method. The pH was measured using a potentiometric method. Oxygen concentration was measured using a titration method with iodometric determination. Water salinity (TDS) was calculated as the sum of ions using the following methods: turbidimetry for sulfate anions; flame spectrophotometry for potassium and sodium cations; mercurimetric titration for chloride ions; and titration for calcium, magnesium, and bicarbonate ions. A photometric method was applied to determine nutrients’ concentrations. Nessler reagent, Griess reagent, salicylic acid, ammonium molybdate, and sulfosalicylic acid were used for the measurements of ammonium ion, nitrite ion, nitrate ion, phosphate ions, and total iron, respectively. A combined reagent composed of ammonium molybdate and ascorbic acid was used to determine the total phosphorus content. A titration method with iodometric determination was used to measure biological oxygen demand (BOD5). A photometric method was applied to determine the chemical oxygen demand (COD). The content of manganese and copper was determined by atomic absorption spectrometry with electrothermal vaporization. For the quality control of the analysis, the method provides repeatability limit coefficients (R) that correspond to the following values: R = 3 (pH, O2, HCO3), R = 4 (hardness, SO4), R = 8 (Ca, Mg, Cl), R = 12 (Na, P tot), R = 7 (K), R = 15 (NH4), R = 14 (NO3), R = 10 (color), R = 13 (BOD), R = 25 (COD), R = 27 (Fe tot), R = 31 (Mn), and R = 28 (Cu). The measurement (Xmean) was taken as the arithmetic mean of two parallel detections (X1, X2), for which the following condition was satisfied: for pH; for dissolved oxygen (O2), hardness, calcium (Ca), bicarbonates (HCO3), sulfates (SO4), ammonium (NH4), color, and BOD; for magnesium (Mg), sodium (Na), potassium (K), chlorides (Cl), nitrates (NO3), total phosphorus (P tot), COD, iron total (Fe tot), manganese (Mn), and copper (Cu).
2.4. Diatom Analysis
Diatom shells were freed from organic matter by burning with 30% hydrogen peroxide followed by a 6 h thermal treatment in a thermostat at 85 °C [32]. The preparations were examined in a JEOL JSM-6510 LV scanning electron microscope (JEOL Ltd.; Tokyo, Japan). Handbooks and individual articles were used for species determinations [15,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54]. The species names were unified according to the modern system using algaebase.org [55].
2.5. Bioindicators and Statistical Analysis
To determine the environmental factors affecting the diversity of diatoms in the studied water bodies, various approaches were used. Bioindicator analysis was performed according to [56], with species-specific ecological preferences of the revealed diatoms [57,58]. Statistical maps of the environmental variables and bioindicators were constructed as the network analysis in JASP (significant only) using the botnet package in statistic R of [59] to follow the comparison of their distribution. The statistical analysis of species and environmental variables’ relationships was performed with the CANOCO Program 4.5 [60].
3. Results
3.1. Water Chemistry
Chemical data were obtained for 11 of the 14 studied water bodies and are presented in Appendix A Table A1. The water temperature of most studied lakes varied from 13.3° to 16.7 °C, excluding lake 4, where the water warmed up to 20.4 °C. Water pH was neutral (pH 6.65–7.51). Suspended matter was low. Dissolved oxygen content varied insignificantly from 8.7 to 10.47 mg L−1. The oxygen regime was favorable. The water from the lakes was fresh, with low and mild salinity levels, in terms of hardness—soft or medium-hard. The water of the studied lakes was of the hydrocarbonate-class calcium group according to major ionic constituents, except for lakes 1 and 4, where the water was of the sulfate-class calcium group. A high concentration of total iron was detected in the lakes. Low nitrate nitrogen and total phosphorus content were defined in the water of the studied lakes; mineral phosphorus, nitrite nitrogen, and silica also had negligible concentrations. The color of the water for most of the lakes was reduced; an increase in this variable only occurred for lake 4. The easy-to-oxidized organic substances (as BOD) for lakes 1, 4, 7, 10, and 11 were characterized by high values, whereas this variable was low in the other studied lakes. The content of hard-to-oxidize organic substances, estimated (as COD) in lakes 1, 2, and 4, was low. For the rest of the lakes, an increased COD value was noted. Technogenic pollutants were characterized by their low content: the concentration of oil products and phenols was below the detection limit of the analysis and therefore was not included in Appendix A Table A1. Among the microelements, a high content of iron and a low content of copper and manganese were revealed.
3.2. Taxonomical and Ecological Analyses
For the first time, 14 studied lakes and water bodies in the Tiksi Bay region revealed 385 species with intraspecies of diatoms. Some species were defined up to the genus level and therefore, excluding this, the floristic list contains 356 taxa (337 species) in total. The species in the genera Pinnularia and Eunotia strongly prevailed with 42 and 40 species, respectively (Appendix A Table A2). One of the floristic parameters of diatoms in the studied water bodies was calculated as the response to the question about how the species list was created for the studied area. The square of the area where the studied water bodies were located was 180 km2; therefore, the calculated number of diatom species per area as index Sp./Area was 2.14 species in 1 km2.
The ecological preferences of the revealed species in 14 studied water bodies are presented in Appendix A Table A3. The percentile distribution of the indicators in ecological categories can be observed in Figure 2 and Figure 3. The data of the indicators were grouped in the histograms according to three catchment basins of the studied territory from down to up. It can be observed that planktonic and planktonic–benthic inhabitants increased with the lake’s altitude (Figure 2a). Temperate-temperature species strongly prevailed, excluding lake 4, where eurythermic indicators dominated (Figure 2b). Indicators of semi-oxygenated waters prevailed in each lake (Figure 2c) and the distribution shows decreasing oxygenation with the decrease in group str with an increasing altitude. Indicators of low salinity groups hb and i prevailed in the studied water bodies community and its distribution is difficult to compare to the altitude and distance from the seacoast (Figure 2d), but seems to partly increase with this distance.
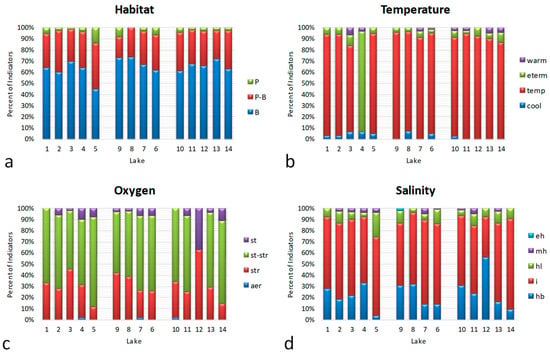
Figure 2.
Bioindicator distributions in the 14 studied water bodies: (a) habitat preferences (P—planktonic, P-B—plankto-benthic, B—benthic); (b) temperature (cool—cool water, temp—temperate, eterm—eurythermic, warm—warm water); (c) oxygen (st—standing water, str—streaming water, st-str—low streaming water, aer—aerophiles); (d) salinity (hb—oligohalobes–halophobes, i— oligohalobes–indifferents, hl—halophiles; mh—mesohalobes, eh—euhalobes). Ecological groups in each figure are placed in ascending order of the environmental parameter.
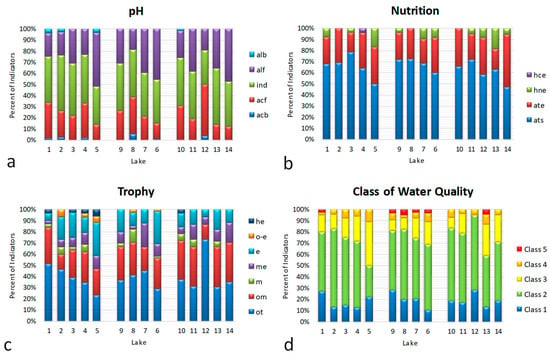
Figure 3.
Bioindicator distributions in the 14 studied water bodies: (a) pH (alb—alkalibiontes; alf—alkaliphiles, ind—indifferent; acf—acidophiles; acb—acidobiontes); (b) nutrition type (ats—nitrogen autotrophic taxa tolerating very small concentrations of organically bound nitrogen; ate—nitrogen autotrophic taxa tolerating elevated concentrations of organically bound nitrogen; hne—facultative nitrogen heterotrophic taxa needing periodically elevated concentrations of organically bound nitrogen; hce—obligate nitrogen heterotrophic taxa needing continuously elevated concentrations of organically bound nitrogen); (c) trophic state (ot—oligotraphentic; om—oligomesotraphentic; m—mesotraphentic; me—mesoeutraphentic; e—eutraphentic; he—hypereutraphentic; o–e—oligo-to-eutraphentic (hypereutraphentic)); (d); class of water quality: 1–5.
The indicators of water pH distribution show an increase in groups with a high pH corresponding to altitude (Figure 3a). The nutrition-type indicators’ distribution demonstrates an increase in mixotrophs with the water bodies’ altitude and distance from the seacoast (Figure 3b). Indicators of trophic state and water-quality class show an increase in trophicity and organic pollution with the water bodies’ altitude and distance from the seacoast (Figure 3c,d).
3.3. Comparative Analysis
The comparison of the indicator spectrum of the studied water bodies community together with the environmental variables was compared with calculations of similarity at the bottom of Appendix A Table A4 and Table A1. Figure 4 shows four groups of lakes’ indicator spectrums that are the most similar. Group 1 combines the community of lakes 6 and 7 of the greatest distance from the seacoast and with a similar chemical composition (Appendix A Table A1). These two communities were enriched by mixotrophic indicator species of eutrophic and alkaline waters. Group 2 included lakes 3 and 14, which represented the typical indicator distributions with the highest TDS and lowest O2. These lakes also had similar lake-surface areas and coastal lengths. Group 3, including lakes 9 and 10, were characterized by similar indicators spectra; however, the main factor of their similarity was that they had the highest lake altitude. The rest of the lakes could be designated to Group 4, where lakes 1 and 2 were the closest to the seacoast.
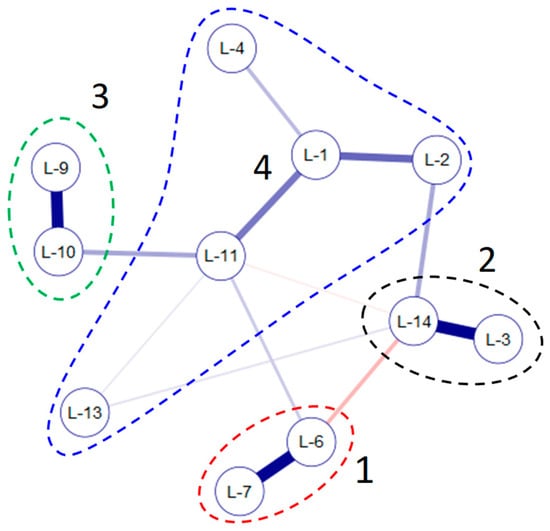
Figure 4.
JASP Network plot for the 11 studied small water bodies (p < 0.5), based on the bottom of Appendix A Table A4.
3.4. Species–Environment Relationship
The high individuality of the studied communities caused us to calculate the relationship of biological and environmental data for the 11 studied lakes. RDA triplot allowed us to identify three groups of environmental factors to which there was a definite response of biological variables (Figure 5). Cluster 1 increased the water temperature with a number of eurythermic indicators and species per lake. Only lake 4 combined these variables and they did not present an increase in water oxygenation and temperate species number. Cluster 2 included the number of benthic inhabitants, indicators of oxygen enrichment, and increase in species community. Only lake 6 represented this combination of factors. Cluster 3 included only one factor, water pH, which was opposed the direction of the factors in Cluster 2 but did not show a special reaction of the lake’s communities.

Figure 5.
RDA triplot for species indicators and environmental variables in the 11 studied lakes based on the data from Table 1 and Appendix A Table A1 and Table A4.
3.5. Statistical Mapping
Since all the previous analyses clearly showed the involvement of not only chemical factors, but also factors related to the location of the lake and its morphometry, for the species composition and ecological characteristics of the communities, we decided to conduct the statistical mapping of the environmental and biological data. During the first stage, the altitude of the lake located in the study area was mapped. As can be seen in Figure 6a, the altitude map of the location of the lakes coincides with their position in Google Maps. In this way, the adequacy of the subsequent mapping of our data was checked. As can be seen in the constructed maps, oxygen was the lowest in lakes 3, 11, and especially in lake 4 (Figure 6b). A similar distribution was observed for the pH of the water (Figure 6c). The highest TDS was in the lakes along the coast (Figure 6d) due to sulfates (Figure 6e), and along the entire southern drainage basin due to sulfates and chlorides (Figure 6f).

Figure 6.
Statistical maps of environmental variables in the 11 studied water bodies: (a) altitude; (b) oxygen; (c) pH; (d) TDS; (e) chlorides; (f) sulfates.
The distribution of temperature and water color values was similar to the maximum in lake 4 (Figure 7a,b). The distribution of BOD values showed a maximum in the vicinity of the Tiksi settlement (Figure 7c) and along the entire coast. Nitrate nitrogen and phosphates increased towards the north (Figure 7d,e). The concentration of iron, necessary for the development of algae, had a complex distribution, showing the highest values at higher elevations along the basin of the southern watercourse and in lake 4 (Figure 7f).

Figure 7.
Statistical maps of environmental variables in the 11 studied water bodies: (a) water temperature; (b) water color; (c) BOD5; (d) N-NO3; (e) total P; (f) total Fe.
Interestingly, the distribution of species richness (Table 1) coincided with the distribution of water pH and presented a negative relationship between these two parameters (Figure 6c and Figure 8a). At the same time, the highest value of the number of species per surface area of the lake was only calculated for lake 4 (Figure 8b).

Figure 8.
Statistical maps of species richness and species per area index in the 14 studied water bodies: (a) no. of species; (b) number species per area of the lake.
Statistical maps of the bio-indicator numbers in each studied water body could be divided into two sets. The first one combined indicators of water pH, temperature, salinity, organic pollution in two systems, and the diatoms nutrition type (Figure 9). These maps demonstrate the bilateral distribution of different groups of environmental indicators where its number was mostly on the two sides of the study area and lowest in the middle part. This type of distribution can be compared to the July wind rose (Figure 1), and we observed that they were both similar in northeast to southwest direction.

Figure 9.
Statistical maps of bioindicator distributions in the 14 studied water bodies: (a) acidophiles (acf); (b) cool-water indicators (cool); (c) oligohalobes-indifferents (i); (d) organic pollution indicators of Class 2’s water quality; (e) Watanabe indicators of low organic pollution, saproxenes (sx); (f) nitrogen autotrophic taxa tolerating very small concentrations of organically bound nitrogen (ats).
The second set of statistical distribution maps is presented in Figure 10. There are two maps showing high organic pollution indicator distributions—eutrophic and hypertrophic (Figure 10a,b). This number is low; however, the maps show its presence in the water bodies close to the seacoast and absent from the continental stations. Therefore, even a low species number but their presence in the communities allowed us to assume that the seacoast water bodies can be influenced by the sea, so that the trophic level of the lake can be higher if the sea’s influence increases. The diatom heterotrophic nutrition indicator map (hce) highlights lake 4 (Figure 10c) as an exclusive community that contains heterotrophic species that are not represented in any other studied lakes. This map, in comparison with the maps for nitrate concentration, water color, BOD, and iron (Figure 7a–f), and with a high index of species number per lake surface area (Figure 8b), can be combined with the set of variables that show a non-systemic anthropogenic influence. This influence led to the restructuring of the community with an unprecedented growth diversity of diatoms and the enrichment of heterotrophic species that reflect some toxic impact.

Figure 10.
Statistical maps of bioindicator distributions in the 14 studied water bodies: (a) hypertrophic (he); (b) from oligo-to-eutrophic (o–e); (c) obligate nitrogen heterotrophic taxa needing continuously elevated concentrations of organically bound nitrogen (hce).
4. Discussion
A total of 385 species of diatoms were identified in the phytoplankton of 14 small Arctic tundra water bodies in the vicinity of Tiksi Bay. The studied lakes and watered areas were sampled for the first time in this region. Information about the diatoms in the Lena Delta Wildlife Reserve and the previously studied adjusted area included 413 diatom species [19]. We compared the lists of published species in the reserve and the 14 water bodies studied in this paper, and observed that the diversity of both areas was rather unique. However, similar for both floristic lists were the prevailing species of the genera Pinnularia and Eunotia, which, in our studied area, contained 42 and 40 species, respectively (Appendix A Table A2). Enrichments of flora by species of Pinnularia and Eunotia was revealed in diverse Arctic aquatic flora that has been identified in some lakes in Svalbard [9], Canada [61], Greenland [62], Bolshezemel’skaya Tundra [12], Arctic Chukotka [13,14], and the northern Yakutia lakes [9].
We tried to find a relationship between the morphometry of the studied lakes and diatom species richness. As can be seen in Table 1, the studied lakes were small, shallow, and located at a narrow-range altitude close to the coastline of Tiksi Bay. The number of species in the studied lakes ranged from 33 to 137 (Table 1). The calculated of Sp./Area index for each lake ranged between 128 species per 1 km2 in lake 7 and 8891 species per 1 km2 in lake 4. This is comparable to the index value of lakes in the Kostyanoy Nos Reserve [11]. At the same time, the Sp./Area index for the total studied area of 180 km2 was 2.14 species in 1 km2 for the studied water bodies area of the vicinity of Tiksi Bay, which is comparable with that in the Kostyanoy Nos Reserve in the Bolshezemelskaya Tundra [11]. Table 1 compares the index value and species richness and shows that the species richness is the highest in lake 6; however, the Sp./Area index has the highest value for lake 4. Therefore, lake 4 differs from the usual diversity and morphometry variables in the Tiksi Bay coastal area.
The bio-indicators of the studied lakes on the Tiksi Bay coast demonstrates the predominance of water with the following characteristics: temperate temperature, moderate oxygen, low-to-moderate organic material-enriched, low alkalinity, circumneutral and low salinity. These results are similar to the previously studied bio-indicators in Pechora Bay coast [11] and the closely situated Lena Delta Wildlife Reserve [20,21]. Therefore, Arctic regions’ diversity patterns that are mostly studied with diatoms can advance with the bio-indicator properties and new indices [63]; however, the total mechanisms of its distribution are not enough for a detailed conclusion [64]. In this regard, we divided the lakes into three drainage basins and organized the groups of bio-indicators in a gradient increasing their altitude from low to high. Therefore, the indicators’ distributions not only show the full picture of their content but also the importance of the distance from the coastline. The ecological mapping of the diverse environmental and biological data of the studied lakes’ ecosystems helped to reveal the distribution of the many important variables from northeast to southwest that coincide with the wind direction in the period of July.
An interesting conclusion from the study is the powerful result of a burst of diversity in one lake that presented a one-time impact. Lake 4 stands out for its number of species, as well as its high Species/Area index relative to all other lakes. In addition, the presence of indicators of organic pollution, high trophicity, and heterotrophic nutrition characterize the ecosystem of this lake as having undergone an anthropogenic impact, but successfully coping with it. However, such a conclusion turned out to be possible only with the help of statistical maps of the distribution of various indicators, both environmental and bio-, which, as a method, have already proven their application in environmental analyses [20,21,65,66,67]. Thus, the monitoring of Arctic freshwater habitats is very important, as the world’s freshwater sources remain under threat [68] in the face of global warming and the trend of intensified development of the Arctic.
5. Conclusions
As a result of our study, it can be concluded that, in the 14 lakes in the coastal zone of Tiksi Bay, a high diversity of diatoms was revealed with 385 taxa under the genus rank, among which the genera Pinnularia and Eunotia were the most representative, as in the majority of the studied habitats in the High Arctic. The indicator properties of the identified diatom species allowed us to conclude that, in general, the waters of the studied lakes were fresh, had a neutral pH, and had low salinity and organic pollution levels, except for one of them, which was subjected to one-time anthropogenic pollution, which led to an increase in diversity. The influence of the climatic factor, the direction of the northeasterly winds on the distribution of diatoms, and the geographical factor, the distance from the coastline, was also highlighted. A new approach to the spatial statistical mapping of the diversity and indicator properties of the discovered diatoms and indicators of these habitats helped us to establish these factors. Therefore, spatial ecological mapping in combination with bio-indication is recommended for monitoring the anthropogenic impact on sensitive and threatened aquatic ecosystems in the High Arctic.
Author Contributions
Conceptualization, S.B. and V.G.; methodology, S.B. and V.G.; software, S.B.; validation, S.B., V.G. and S.G.; formal analysis, V.G., S.G. and O.G.; investigation, V.G. and O.G.; resources, V.G.; data curation, S.G. and V.G.; writing—original draft preparation, S.B. and V.G.; writing—review and editing, S.B., V.G., S.G. and O.G.; visualization, S.B.; supervision, V.G.; project administration, V.G.; funding acquisition, V.G. All authors have read and agreed to the published version of the manuscript.
Funding
The research was conducted within the state assignment of the Ministry of Science and Higher Education of the Russian Federation (theme No. FWRS-2021-0023, reg. No. AAAA-A21-121012190038-0; theme No. FWRS-2021-0026, reg. No. AAAA-A21-121012190036-6), (theme No. 121051100099-5).
Data Availability Statement
Data of this research is available with DOI of this paper.
Acknowledgments
We are grateful to the Israeli Ministry of Aliyah and Integration for partial support of this work.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
Averaged chemical variables with standard deviations of the 11 studied water bodies in the vicinity of Tiksi Bay, July 2021.
Table A1.
Averaged chemical variables with standard deviations of the 11 studied water bodies in the vicinity of Tiksi Bay, July 2021.
| Variable/Water Body | 1 | 2 | 3 | 4 | 6 | 7 | 9 | 10 | 11 | 13 | 14 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Altitude, m a.s.l. | 25.00 | 66.00 | 109.00 | 38.00 | 76.00 | 76.00 | 54.00 | 52.00 | 38.00 | 85.00 | 154.00 |
| Water temperature, °C | 16.70 | 15.10 | 15.00 | 20.40 | 16.10 | 14.50 | 15.10 | 13.30 | 14.00 | 14.60 | 14.70 |
| pH | 7.51 ± 0.04 | 7.42 ± 0.03 | 6.70 ± 0.04 | 6.74 ± 0.02 | 6.73 ± 0.04 | 7.30 ± 0.03 | 7.3 ± 0.00 | 6.89 ± 0.01 | 6.65 ± 0.00 | 7.22 ± 0.03 | 7.34 ± 0.00 |
| O2, mg L−1 | 9.87 ± 0.04 | 10.28 ± 0.03 | 9.40 ± 0.14 | 8.70 ± 0.14 | 10.10 ± 0.07 | 10.47 ± 0.04 | 9.98 ± 0.03 | 10.1 ± 0.00 | 9.60 ± 0.14 | 9.86 ± 0.06 | 9.77 ± 0.05 |
| TDS, mg L−1 | 267.5 ± 3.07 | 168.7 ± 2.56 | 225.6 ± 1.74 | 178.9 ± 2.63 | 177.4 ± 1.87 | 160.7 ± 2.33 | 181.6 ± 1.68 | 243.2 ± 2.42 | 234.1 ± 2.36 | 260.9 ± 2.49 | 259.8 ± 2.94 |
| Hardness, mmol. L−1 | 3.48 ± 0.04 | 2.28 ± 0.03 | 3.09 ± 0.01 | 2.29 ± 0.02 | 2.37 ± 0.03 | 2.13 ± 0.04 | 2.44 ± 0.02 | 3.34 ± 0.02 | 3.16 ± 0.01 | 3.61 ± 0.02 | 3.34 ± 0.01 |
| Ca, mg L−1 | 45.00 ± 0.14 | 22.44 ± 0.08 | 38.60 ± 0.07 | 32.00 ± 0.14 | 27.20 ± 0.14 | 28.60 ± 0.14 | 28.40 ± 0.14 | 35.20 ± 0.14 | 36.80 ± 0.14 | 32.40 ± 0.14 | 38.20 ± 0.14 |
| Mg, mg L−1 | 15.00 ± 0.71 | 14.09 ± 0.71 | 14.20 ± 0.57 | 8.40 ± 0.42 | 12.30 ± 0.57 | 8.60 ± 0.42 | 12.40 ± 0.57 | 19.20 ± 0.85 | 16.10 ± 0.85 | 24.20 ± 0.85 | 17.40 ± 0.71 |
| Na, mg L−1 | 5.87 ± 0.28 | 2.6 ± 0.21 | 1.14 ± 0.07 | 3.91 ± 0.28 | 1.12 ± 0.08 | 0.66 ± 0.04 | 1.43 ± 0.11 | 0.93 ± 0.07 | 1.28 ± 0.08 | 1.39 ± 0.11 | 6.03 ± 0.40 |
| K, mg L−1 | 1.46 ± 0.07 | 0.81 ± 0.03 | 0.49 ± 0.01 | 0.46 ± 0.01 | 0.57 ± 0.01 | 0.58 ± 0.03 | 0.57 ± 0.01 | 0.48 ± 0.01 | 0.37 ± 0.01 | 0.86 ± 0.04 | 1.6 ± 0.07 |
| HCO3, mg L−1 | 100 ± 0.42 | 67.12 ± 0.57 | 98.6 ± 0.71 | 79.50 ± 0.85 | 98.60 ± 0.57 | 89.40 ± 0.85 | 90.60 ± 0.57 | 120.4 ± 0.57 | 120.5 ± 0.42 | 130.4 ± 0.57 | 110.2 ± 0.85 |
| Cl, mg L−1 | 6.20 ± 0.03 | 3.55 ± 0.00 | 4.80 ± 0.03 | 4.25 ± 0.07 | 3.50 ± 0.07 | 3.40 ± 0.14 | 3.20 ± 0.00 | 3.50 ± 0.07 | 4.00 ± 0.14 | 8.20 ± 0.07 | 4.80 ± 0.07 |
| SO4, mg L−1 | 94.00 ± 1.41 | 58.12 ± 0.96 | 67.80 ± 0.28 | 50.40 ± 0.85 | 34.20 ± 0.42 | 29.50 ± 0.71 | 45.00 ± 0.28 | 63.50 ± 0.71 | 55.00 ± 0.71 | 63.50 ± 0.71 | 81.60 ± 0.71 |
| N-NH4, mg L-1 | 0.26 ± 0.01 | 0.20 ± 0.00 | 0.08 ± 0.00 | 0.19 ± 0.02 | 0.14 ± 0.01 | 0.15 ± 0.00 | 0.12 ± 0.00 | 0.19 ± 0.01 | 0.24 ± 0.02 | 0.09 ± 0.00 | 0.16 ± 0.01 |
| N-NO3, mg L−1 | 0.17 ± 0.01 | 0.12 ± 0.01 | 0.12 ± 0.01 | 0.19 ± 0.01 | 0.14 ± 0.01 | 0.09 ± 0.01 | 0.11 ± 0.01 | 0.12 ± 0.01 | 0.13 ± 0.01 | 0.14 ± 0.01 | 0.13 ± 0.01 |
| P tot, mg L−1 | 0.20 ± 0.01 | 0.08 ± 0.01 | 0.05 ± 0.00 | 0.08 ± 0.01 | 0.14 ± 0.01 | 0.13 ± 0.01 | 0.07 ± 0.00 | 0.09 ± 0.00 | 0.11 ± 0.00 | 0.10 ± 0.01 | 0.10 ± 0.00 |
| Color, Pt/Co grad. | 16.00 ± 0.71 | 13.00 ± 0.71 | 13.00 ± 0.71 | 25.00 ± 0.71 | 12.00 ± 0.71 | 19.00 ± 0.71 | 15.00 ± 0.71 | 11.00 ± 0.71 | 10.00 ± 0.71 | 18.00 ± 0.71 | 19.00 ± 0.71 |
| BOD, mg O L−1 | 2.48 ± 0.17 | 1.75 ± 0.14 | 1.46 ± 0.06 | 2.45 ± 0.08 | 1.01 ± 0.07 | 2.00 ± 0.07 | 1.61 ± 0.14 | 2.48 ± 0.14 | 2.39 ± 0.14 | 1.43 ± 0.11 | 0.83 ± 0.04 |
| COD, mg O L−1 | 14.20 ± 0.99 | 14.40 ± 0.85 | 17.60 ± 0.85 | 14.00 ± 0.71 | 18.20 ± 0.85 | 18.40 ± 0.99 | 17.80 ± 0.85 | 18.00 ± 0.99 | 16.20 ± 0.85 | 16.80 ± 0.85 | 16.40 ± 1.13 |
| Fe tot, mg L−1 | 0.41 ± 0.06 | 0.29 ± 0.04 | 0.40 ± 0.04 | 0.70 ± 0.06 | 0.55 ± 0.06 | 0.67 ± 0.07 | 0.29 ± 0.06 | 0.65 ± 0.07 | 0.68 ± 0.07 | 0.62 ± 0.06 | 0.50 ± 0.06 |
| Mn, µg L−1 | 7.00 ± 1.41 | 4.00 ± 0.42 | 2.00 ± 0.28 | 6.00 ± 0.57 | 3.00 ± 0.28 | 2.00 ± 0.42 | 4.00 ± 0.71 | 7.00 ± 1.41 | 7.00 ± 1.41 | 6.00 ± 0.71 | 7.00 ± 1.41 |
| Cu, µg L−1 | 5.00 ± 0.57 | 3.00 ± 0.57 | 3.00 ± 0.57 | 5.00 ± 0.71 | 3.00 ± 0.28 | 2.00 ± 0.28 | 3.00 ± 0.57 | 1.00 ± 0.14 | 4.00 ± 0.71 | 3.00 ± 0.35 | 4.00 ± 0.71 |

Table A2.
Diatom species richness in the 14 studied water bodies in the vicinity of Tiksi Bay, July 2021. “1”—present, “0”—absent.
Table A2.
Diatom species richness in the 14 studied water bodies in the vicinity of Tiksi Bay, July 2021. “1”—present, “0”—absent.
| Taxa | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Achnanthes adnata Bory | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Achnanthes ingratiformis Lange-Bertalot | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Achnanthidium minutissimum (Kützing) Czarnecki | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 |
| Achnanthidium nodosum (Cleve) Tseplik and Chudaev | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Achnanthidium petersenii (Hustedt) C. E. Wetzel, L. Ector, D. M. Williams, and I. Jüttner | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 |
| Achnanthidium saprophilum (H. Kobayashi and Mayama) Round and Bukhtiyarova | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 |
| Achnanthidium sp. | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Amphora copulata (Kützing) Schoeman and R. E. M. Archibald | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 1 |
| Amphora indistincta Levkov | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 |
| Amphora pseudosibirica Levkov and Pavlov | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 |
| Amphora sp. | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Aneumastus tusculus (Ehrenberg) D. G. Mann and A. J. Stickle | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Asterionella formosa Hassall | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Aulacoseira alpigena (Grunow) Krammer | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 |
| Aulacoseira ambigua (Grunow) Simonsen | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 |
| Aulacoseira granulata (Ehrenberg) Simonsen | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 |
| Aulacoseira islandica (O. Müller) Simonsen | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Aulacoseira lirata (Ehrenberg) R. Ross | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Aulacoseira perglabra (Østrup) E. Y. Haworth | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Aulacoseira pfaffiana (Reinsch) Krammer | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Aulacoseira pusilla (F. Meister) A. Tuji and A. Houki | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Aulacoseira scalaris (Grunow) Houk, Klee, and Passauer | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 |
| Aulacoseira subarctica (O. Müller) E. Y. Haworth | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Aulacoseira valida (Grunow) Krammer | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Boreozonacola hustedtii Lange-Bertalot, Kulikovskiy, and Witkowski | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 |
| Brachysira brebissonii R. Ross | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 0 |
| Brachysira calcicola Lange-Bertalot | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Brachysira neoexilis Lange-Bertalot | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 0 |
| Brachysira procera Lange-Bertalot and Gerd Moser | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 |
| Brachysira styriaca (Grunow) R. Ross | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Caloneis arctica (Krasske) Lange-Bertalot and S. I. Genkal | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Caloneis bacillum (Grunow) Cleve | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 0 |
| Caloneis holarctica Kulikovskiy, Lange-Bertalot, and A. Witkowski | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 0 |
| Caloneis silicula (Ehrenberg) Cleve var. silicula | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Caloneis silicula var. elliptica Mayer | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Campylodiscus hibernicus Ehrenberg | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Cavinula cocconeiformis (W. Gregory ex Greville) D. G. Mann and A. J. Stickle | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 |
| Cavinula jaernefeltii (Hustedt) D. G. Mann and A. J. Stickle | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 1 |
| Cavinula pseudoscutiformis (Hustedt) D. G. Mann and Stickle | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 |
| Cavinula sp. | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Chamaepinnularia begeri (Krasske) Lange-Bertalot | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Chamaepinnularia circumborealis Lange-Bertalot | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Chamaepinnularia krookiformis (Krammer) Lange-Bertalot and Krammer | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Chamaepinnularia sp. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Cocconeis lineata Ehrenberg | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cocconeis neodiminuta Krammer | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cocconeis placentula Ehrenberg var. placentula | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Cocconeis placentula var. euglypta (Ehrenberg) Cleve | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Cocconeis sp. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Craticula molestiformis (Hustedt) Mayama | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Cyclostephanos dubius (Hustedt) Round | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cyclostephanos makarovae (S. I. Genkal) K. Schultz | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Cyclotella atomus Hustedt | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Cyclotella distinguenda Hustedt | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 |
| Cyclotella meduanae H. Germain | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Cymatopleura elliptica (Brébisson) W. Smith | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cymbella arctica (Lagerstedt) A. W. F. Schmidt | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cymbella cleve-eulerae Krammer | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Cymbella cymbiformis C. Agardh | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cymbella hantzschiana Krammer | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cymbella krammeri Bahls | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 |
| Cymbella neogena (Grunow) Krammer | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Cymbella proxima Reimer | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cymbella subcistula Krammer | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cymbella sp. | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cymbopleura amphicephala (Nägeli ex Kützing) Krammer | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Cymbopleura anglica (Lagerstedt) Krammer | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 0 |
| Cymbopleura angustata var. spitsbergensis Krammer | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 |
| Cymbopleura designata (Krammer) Bahls | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cymbopleura elliptica Krammer | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Cymbopleura hybrida (Grunow ex Cleve) Krammer | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 |
| Cymbopleura incertiformis Krammer | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Cymbopleura naviculiformis (Auerswald ex Heiberg) Krammer | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cymbopleura oblongata var. stenoraphe Krammer | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Cymbopleura subanglica Krammer | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Cymbopleura subapiculata Krammer | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cymbopleura subcuspidata (Krammer) Krammer | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 |
| Cymbopleura truncata Krammer | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cymbopleura tynnii (Krammer) Krammer | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 |
| Cymbopleura sp. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Denticula tenuis Kützing | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Diatoma moniliformis (Kützing) D. M. Williams | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Diatoma vulgaris Bory | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Diploneis boldtiana Cleve | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 |
| Diploneis modica Hustedt | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 |
| Diploneis oblongella (Nägeli ex Kützing) A. Cleve | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 |
| Diploneis oculata (Brébisson) Cleve | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 0 |
| Diploneis ovalis (Hilse) Cleve | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Diploneis parma Cleve | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Diploneis subovalis Cleve | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 |
| Discostella pseudostelligera (Hustedt) Houk and Klee | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 |
| Discostella stelligera (Cleve and Grunow) Houk and Klee | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Encyonema auerswaldii Rabenhorst | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Encyonema elginense (Krammer) D. G. Mann | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Encyonema gaeumannii (F. Meister) Krammer | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Encyonema groenlandica (Foged) Kulikovskiy and Lange-Bertalot | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Encyonema latens (Krasske) D. G. Mann | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Encyonema lunatum (W. Smith) Van Heurck | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 |
| Encyonema minutum (Hilse) D. G. Mann var. minutum | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 |
| Encyonema neogracile Krammer | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
| Encyonema perpusillum (A. Cleve) D. G. Mann | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Encyonema reichardtii (Krammer) D. G. Mann | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Encyonema silesiacum (Bleisch) D. G. Mann | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 0 |
| Encyonema ventricosum (C. Agardh) Grunow | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 |
| Encyonema vulgare Krammer | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Encyonema sp. | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Encyonopsis cesatiformis Krammer | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Encyonopsis cesatii (Rabenhorst) Krammer | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 0 |
| Encyonopsis perborealis Krammer | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Entomoneis ornata (Bailey) Reimer | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Eucocconeis alpestris (Brun) Lange-Bertalot | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Eucocconeis depressa (Cleve) Lange-Bertalot | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Eucocconeis diluviana (Hustedt) Lange-Bertalot | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Eucocconeis flexella (Kützing) F. Meister | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Eucocconeis laevis (Østrup) Lange-Bertalot | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 1 |
| Eucocconeis leptostriata Lange-Bertalot apud H. Lange-Bertalot and S. I. Genkal | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Eucocconeis quadratarea (Østrup) Lange-Bertalot | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 1 |
| Eunotia ambivalens Lange-Bertalot and Tagliaventi | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Eunotia arcus Ehrenberg | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Eunotia bidens Ehrenberg | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Eunotia bigibboidea Lange-Bertalot and Witkowski | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Eunotia bilunaris (Ehrenberg) Schaarschmidt | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Eunotia boreoalpina Lange-Bertalot and Nörpel-Schempp | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Eunotia boreotenuis Nörpel-Schempp and Lange-Bertalot | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 0 |
| Eunotia botuliformis F. Wild, Nörpel, and Lange-Bertalot | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 |
| Eunotia cantonatii Lange-Bertalot and Tagliaventi | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Eunotia chelonia Nörpel-Schempp, Lange-Bertalot, and Metzeltin | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Eunotia curtagrunowii Nörpel-Schempp and Lange-Bertalot | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 |
| Eunotia elegans Østrup | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Eunotia eurycephala (Grunow) Nörpel-Schempp and Lange-Bertalot | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Eunotia ewa Lange-Bertalot and Witkowski | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Eunotia faba Ehrenberg | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 |
| Eunotia flexuosa (Brébisson ex Kützing) Kützing | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Eunotia fureyae Lange-Bertalot | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Eunotia genuflexa Nörpel-Schempp | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Eunotia groenlandica Nörpel-Schempp and Lange-Bertalot | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Eunotia incisa W. Smith ex W. Gregory | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 |
| Eunotia islandica Østrup | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Eunotia julma Lange-Bertalot | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 |
| Eunotia major (W. Smith) Rabenhorst | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Eunotia meisteri Hustedt | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Eunotia minor (Kützing) Grunow | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Eunotia monnieri Lange-Bertalot and Tagliaventi | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 |
| Eunotia mucophila (Lange-Bertalot, Nörpel-Schempp, and Alles) Lange-Bertalot | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Eunotia naegelii Migula | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 |
| Eunotia neocompacta var. vixcompacta Lange-Bertalot | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Eunotia paralleladubia Lange-Bertalot and S.Mayama | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 |
| Eunotia parapraerupta Lange-Bertalot and Metzeltin | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Eunotia pseudogroenlandica Lange-Bertalot and Tagliaventi | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Eunotia rhomboidea Hustedt | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Eunotia scandiorussica Kulikovskiy, Lange-Bertalot, Genkal, and Witkowski | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Eunotia semicircularis (Ehrenberg) Lange-Bertalot and Metzeltin | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Eunotia septentrionalis Østrup | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Eunotia subarcuatoides Alles, Nörpel, and Lange-Bertalot | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 |
| Eunotia subherkiniensis Lange-Bertalot | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Eunotia ursamaioris Lange-Bertalot and Nörpel-Schempp | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 |
| Eunotia sp. | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Fallacia crassicostata Lange-Bertalot and Werum | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Fallacia pygmaea (Kützing) Stickle and D. G. Mann | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Fallacia sp. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Fragilaria aquaplus Lange-Bertalot and S. Ulrich | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Fragilaria capucina Desmazières | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Fragilaria radians (Kützing) D. M. Williams and Round | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 |
| Fragilaria rumpens (Kützing) G. W. F. Carlson | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Fragilaria saxoplanctonica Lange-Bertalot and S. Ulrich | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Fragilaria vaucheriae (Kützing) J. B. Petersen | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 |
| Fragilaria sp. | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 |
| Fragilariforma bicapitata (A.Mayer) D. M. Williams and Round | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Fragilariforma constricta (Ehrenberg) D. M. Williams and Round | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 0 |
| Fragilariforma mesolepta (Rabenhorst) Kharitonov | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Fragilariforma virescens (Ralfs) D. M. Williams and Round | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Frustulia crassinervia (Brébisson ex W. Smith) Lange-Bertalot and Krammer | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 |
| Frustulia erifuga Lange-Bertalot and Krammer | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Frustulia krammeri Lange-Bertalot and Metzeltin | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Frustulia saxonica Rabenhorst | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 |
| Geissleria davydovae Genkal et Yaruschina | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Genkalia digituloides (Lange-Bertalot) Lange-Bertalot and Kulikovskiy | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 |
| Gololobovia obliqua (W. Gregory) Kulikovskiy, Glushchenko, and Kociolek | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Gomphonema acuminatum Ehrenberg | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Gomphonema angusticephalum E. Reichardt and Lange-Bertalot | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 |
| Gomphonema brebissonii Kützing | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Gomphonema capitatum Ehrenberg | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Gomphonema coronatum Ehrenberg | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Gomphonema gracile Ehrenberg | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 |
| Gomphonema hebridense W. Gregory | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Gomphonema italicum Kützing | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Gomphonema lagerheimii A. Cleve | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 0 |
| Gomphonema laticollum E. Reichardt | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Gomphonema microcapitatum Kulikovskiy, Kociolek, and Solak | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Gomphonema mihoi Levkov | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Gomphonema minutum f. pachypus Lange-Bertalot and E. Reichardt | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Gomphonema olivaceoides Hustedt | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Gomphonema parvulum (Kützing) Kützing | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 |
| Gomphonema pseudacuminatum Kulikovskiy, Kociolek, and Solak | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Gomphonema truncatum Ehrenberg | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Gomphonema sp. | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 0 |
| Gomphosphenia vallei Beauger, C. E. Wetzel, Allain, and Ector | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Gomphosphenia sp. | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Gyrosigma acuminatum (Kützing) Rabenhorst | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| Gyrosigma sp. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Halamphora hassiaca (Krammer and S.Strecker) Lange-Bertalot | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Handmannia antiqua (W.Smith) Kociolek et Khursevich | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Handmannia comta (Ehrenberg) Kociolek et Khursevich emend. Genkal | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Hantzschia sp. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Hippodonta capitata (Ehrenberg) Lange-Bertalot, Metzeltin, and Witkowski | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Hippodonta hungarica (Grunow) Lange-Bertalot, Metzeltin, and Witkowski | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Humidophila brekkaensis (J. B. Petersen) R. L. Lowe, Kociolek, J. R. Johansen, Van de Vijver, Lange-Bertalot, and Krammer et Kopalova | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Humidophila gallica (W. Smith) Lowe, Kociolek, Q. You, Q. Wang, and Stepanek | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 |
| Humidophila perpusilla (Grunow) R. L. Lowe, Kociolek, J. R. Johansen, Van de Vijver, Lange-Bertalot, and Kopalová | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Humidophila schmassmannii (Hustedt) Buczkó and Wojtal | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Humidophila sp. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Hygropetra balfouriana (Grunow ex Cleve) Krammer and Lange-Bertalot | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 |
| Iconella curvula (W. Smith) Ruck and Nakov | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Iconella linearis (W. Smith) Ruck and Nakov | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Iconella splendida (Ehrenberg) Ruck and Nakov | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Karayevia laterostrata (Hustedt) Bukhtiyarova | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 1 |
| Kobayasiella parasubtilissima (H. Kobayasi and T. Nagumo) Lange-Bertalot | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Kobayasiella subtilissima (Cleve) Lange-Bertalot | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Mayamaea disjuncta (Hustedt) J. Y. Li and Y. Z. Qi | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Melosira varians C. Agardh | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Navicula angusta Grunow | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Navicula chiarae Lange-Bertalot and Genkal | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Navicula cryptocephala Kützing | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 |
| Navicula cryptotenella Lange-Bertalot | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 |
| Navicula cryptotenelloides Lange-Bertalot | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Navicula mediocostata E. Reichardt | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Navicula notha J. H. Wallace | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Navicula phyllepta Kützing | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 |
| Navicula phylleptosoma Lange-Bertalot | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 |
| Navicula radiosa Kützing | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 |
| Navicula reinhardtii (Grunow) Grunow | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Navicula rostellata Kützing | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Navicula tripunctata (O. F. Müller) Bory | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Navicula trivialis Lange-Bertalot | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Navicula viridulacalcis Lange-Bertalot | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 |
| Navicula wygaschii Lange-Bertalot | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Navicula sp. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Naviculadicta sp. | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 1 |
| Navigeia paludosa (Hustedt) Bukhtiyarova | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Navigeia thingvallae (Østrup) Bukhtiyarova | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Neidiopsis wulffii (J. B. Petersen) Lange-Bertalot | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 |
| Neidium affine (Ehrenberg) Pfitzer | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Neidium ampliatum (Ehrenberg) Krammer | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 |
| Neidium bisulcatum (Lagerstedt) Cleve | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 0 |
| Neidium dubium (Ehenberg) Cleve | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Neidium hercynicum Ant. Mayer | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Neidium hitchcockii (Ehrenberg) Cleve | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Neidium iridis (Ehrenberg) Cleve | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 |
| Neidium sp. | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Nitzschia acicularis (Kützing) W. Smith | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Nitzschia acidoclinata Lange-Bertalot | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Nitzschia alpina Hustedt | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Nitzschia capitellata Hustedt | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Nitzschia commutatoides Lange-Bertalot | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 |
| Nitzschia dissipata (Kützing) Rabenhorst | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 |
| Nitzschia fonticola (Grunow) Grunow | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Nitzschia frustulum (Kützing) Grunow | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 |
| Nitzschia graciliformis Lange-Bertalot and Simonsen | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Nitzschia gracilis Hantzsch | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Nitzschia inconspicua Grunow | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 |
| Nitzschia intermedia Hantzsch ex Cleve and Grunow | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Nitzschia linearis W. Smith | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 |
| Nitzschia media Hantzsch | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 |
| Nitzschia perminuta Grunow | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 |
| Nitzschia rosenstockii Lange-Bertalot | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| Nupela impexiformis (Lange-Bertalot) Lange-Bertalot | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 |
| Nupela neogracillima Kulikovskiy and Lange-Bertalot | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Nupela silvahercynia (Lange-Bertalot) Lange-Bertalot | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Nupela tenuicephala (Hustedt) Lange-Bertalot | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pantocsekiella costei (J. C. Druart and F. Straub) K. T. Kiss and E. Ács | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Pinnularia acoricola Hustedt | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 |
| Pinnularia ammerensis Kulikovskiy, Lange-Bertalot, and Metzeltin | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Pinnularia anglica Krammer | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pinnularia angustarea Kulikovskiy, Lange-Bertalot, A. Witkovski, and N. I. Dorofeyuk | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Pinnularia brebissonii (Kützing) Rabenhorst | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Pinnularia bottnica Krammer | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pinnularia brandelii Cleve | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pinnularia canadensis Krammer | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 |
| Pinnularia cuneola E. Reichardt | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pinnularia decrescens (Grunow) Krammer | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Pinnularia divergens var. sublinearis Cleve | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Pinnularia eifeliana (Krammer) Krammer | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Pinnularia grunowii Krammer | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pinnularia halophila Krammer | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Pinnularia krammeri Metzeltin | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 |
| Pinnularia lagerstedtii (Cleve) A. Cleve | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pinnularia lailaensis Foged | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Pinnularia macilenta Ehrenberg | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 |
| Pinnularia microstauron var. rostrata Krammer | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pinnularia neohalophila Kulikovskiy, Genkal, and Mikheeva | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Pinnularia nodosa (Ehrenberg) W. Smith var. nodosa | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pinnularia nodosa var. percapitata Krammer | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pinnularia nodosa var. robusta (Foged) Krammer | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pinnularia notabilis Krammer | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 |
| Pinnularia oriunda Krammer | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Pinnularia oriundiformis Krammer | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pinnularia parvulissima Krammer | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Pinnularia permicrostauron Krammer and Metzeltin | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pinnularia pluvianiformis Krammer | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pinnularia rhombarea Krammer | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 |
| Pinnularia rupestris Hantzsch | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Pinnularia septentrionalis Krammer | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pinnularia similiformis Krammer | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pinnularia spitsbergensis Cleve | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Pinnularia stricta Hustedt | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 |
| Pinnularia subanglica Krammer | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pinnularia subrostrata (A. Cleve) A. Cleve | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 |
| Pinnularia subrupestris Krammer | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Pinnularia subundulata Østrup | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pinnularia undula (Schumann) Krammer | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pinnularia viridis (Nitzsch) Ehrenberg | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pinnularia sp. | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 |
| Placogeia similis (Krasske) Bukhtiyarova | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 0 |
| Placoneis amphibola (Cleve) E. J. Cox | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Placoneis clementioides (Hustedt) E. J. Cox | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Placoneis elginensis (W.Gregory) E. J. Cox | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Placoneis interglacialis (Hustedt) E. J. Cox | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Placoneis opportuna (Hustedt) Chudaev and Gololobova | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Placoneis sp. | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Planothidium straubianum C. E. Wetzel, Van de Vijver, and L.Ector | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Planothidium sp. | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 |
| Pleurosigma elongatum W. Smith | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Praestephanos triporus (Genkal and G. V. Kuzmin) A. Tuji and J.-S. Ki | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Psammothidium bioretii (H. Germain) Bukhtiyarova and Round | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 0 |
| Psammothidium chlidanos (M. H. Hohn and Hellerman) Lange-Bertalot | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 |
| Psammothidium daonense (Lange-Bertalot) Lange-Bertalot | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Psammothidium helveticum (Hustedt) Bukhtiyarova and Round | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Psammothidium kryophilum (J. B. Petersen) E. Reichardt | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Psammothidium levanderi (Hustedt) Bukhtiyarova and Round | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Psammothidium marginulatum (Grunow) Bukhtiyarova and Round | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 |
| Psammothidium rechtense (Leclercq) Lange-Bertalot | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 |
| Psammothidium rossii (Hustedt) Bukhtiyarova and Round | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 |
| Psammothidium scoticum (R. J. Flower and V. J. Jones) Bukhtiyarova and Round | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Psammothidium subatomoides (Hustedt) Bukhtiyarova and Round | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Psammothidium subsalsum (J. B. Petersen) Kulikowskiy, Witkowski, and Pliński | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Psammothidium ventrale (Krasske) Bukhtiyarova and Round | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 0 |
| Psammothidium sp. | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Pseudostaurosira brevistriata (Grunow) D. M. Williams and Round | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Pseudostaurosira parasitica (W. Smith) E. Morales | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
| Pulchellophycus obsitus (Hustedt) Edlund and M. J. Wynne | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Pulchellophycus sp. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Reimeria sinuata (W. Gregory) Kociolek and Stoermer | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 |
| Sellaphora bacillum (Ehrenberg) D. G. Mann | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 |
| Sellaphora difficillima (Hustedt) C. E. Wetzel, L. Ector, and D. G. Mann | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Sellaphora insolita (É. Manguin ex Kociolek and B. de Reviers) P. B. Hamilton and D. Antoniades | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 |
| Sellaphora laevissima (Kützing) D. G. Mann | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 0 |
| Sellaphora vitabunda (Hustedt) D. G. Mann | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 |
| Sellaphora sp. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
| Simonsenia delognei (Grunow) Lange-Bertalot | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Skabitschewskia oestrupii (A. Cleve) Kuliskovskiy and Lange-Bertalot | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 1 |
| Skabitschewskia peragalloi (Brun and Héribaud) Kuliskovskiy and Lange-Bertalot | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 1 |
| Stauroneis amphicephala Kützing | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Stauroneis anceps Ehrenberg | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Stauroneis gracilis Ehrenberg | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 |
| Stauroneis guslyakovii Genkal and Yarushina | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Stauroneis phoenicenteron (Nitzsch) Ehrenberg | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 |
| Stauroneis reichardtii Lange-Bertalot, Cavacini, Tagliaventi, and Alfinito | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 0 |
| Stauroneis schulzii Jousé | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Stauroneis smithii Grunow | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 |
| Stauroneis sp. | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Staurosira sviridae Kulikovskiy, Genkal, and Mikheeva | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Staurosirella lanceolata (Hustedt) E. A. Morales, C. Wetzel, and L. Ector | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Staurosirella pinnata (Ehrenberg) D. M. Williams and Round | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 1 |
| Stenopterobia heribaudii (Playfair) Playfair | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Stephanocyclus meneghinianus (Kützing) Kulikovskiy, Genkal, and Kociolek | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Stephanodiscus hantzschii Grunow | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 |
| Stephanodiscus hashiensis H. Tanaka | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Stephanodiscus minutulus (Kützing) Cleve and Möller | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Stephanodiscus neoastraea Håkansson and Hickel emend. Casper, Scheffler et Augsten | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Surirella angusta Kützing | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Surirella librile (Ehrenberg) Ehrenberg | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 |
| Surirella minuta Brébisson ex Kützing | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Surirella roba Leclercq | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Surirella sp. | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Tabellaria flocculosa (Roth) Kützing | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 |
| Tetracyclus glans (Ehrenberg) F. W. Mills | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Thalassiosira pseudonana Hasle and Heimdal | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Thalassiosira sp. | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Tryblionella angustata W. Smith | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Tryblionella calida (Grunow) D. G. Mann | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Tryblionella hungarica (Grunow) Frenguelli | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Tryblionella littoralis (Grunow) D. G. Mann | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Ulnaria acus (Kützing) Aboal | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| Ulnaria ulna (Nitzsch) Compère | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 |
| Ulnaria sp. | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |

Table A3.
Diatom species ecological preferences in 14 studied water bodies in the vicinity of Tiksi Bay, July 2021.
Table A3.
Diatom species ecological preferences in 14 studied water bodies in the vicinity of Tiksi Bay, July 2021.
| No | Taxa | HAB | T | OXY | HAL | pH | pH-ran | D | Index S | SAP | AUT-HET | TRO |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Achnanthes adnata Bory | B | - | - | mh | alf | - | - | 2.0 | b | - | me |
| 2 | Achnanthes ingratiformis Lange-Bertalot | B | - | - | - | - | 8.00 | - | - | - | - | - |
| 3 | Achnanthidium minutissimum (Kützing) Czarnecki | P-B | eterm | st-str | i | ind | 4.3–9.2 | es | 0.95 | b | ate | e |
| 4 | Achnanthidium nodosum (Cleve) Tseplik and Chudaev | B | - | - | hb | acf | - | - | 1.0 | o | - | ot |
| 5 | Achnanthidium petersenii (Hustedt) C. E. Wetzel, L. Ector, D. M. Williams, and I. Jüttner | B | - | str | hb | ind | 6.9 | - | 0.2 | o | ats | ot |
| 6 | Achnanthidium saprophilum (H. Kobayashi and Mayama) Round and Bukhtiyarova | B | temp | - | - | - | 7.3–7.8 | - | - | - | - | - |
| 7 | Achnanthidium sp. | - | - | - | - | - | - | - | - | - | - | - |
| 8 | Amphora copulata (Kützing) Schoeman and R. E. M. Archibald | B | temp | st-str | i | alf | 7.7 | es | 1.5 | o-b | ate | e |
| 9 | Amphora indistincta Levkov | - | - | - | - | - | - | - | - | - | - | - |
| 10 | Amphora pseudosibirica Levkov and Pavlov | - | - | - | - | - | - | - | - | - | - | - |
| 11 | Amphora sp. | - | - | - | - | - | - | - | - | - | - | - |
| 12 | Aneumastus tusculus (Ehrenberg) D. G. Mann and A. J. Stickle | P-B | - | st | i | alb | - | - | 0.9 | b | ate | o–e |
| 13 | Asterionella formosa Hassall | P | temp | st-str | i | alf | 6.4–7.99 | sx | 1.35 | b | ate | me |
| 14 | Aulacoseira alpigena (Grunow) Krammer | P-B | temp | st-str | i | alf | 4.8–8.4 | sp | 0.8 | x-b | ate | ot |
| 15 | Aulacoseira ambigua (Grunow) Simonsen | P | temp | st-str | i | alf | 6.0–8.5 | sp | 1.7 | b-o | ate | om |
| 16 | Aulacoseira granulata (Ehrenberg) Simonsen | P-B | temp | st-str | i | alf | 5.8–9.4 | es | 2.0 | b | ate | e |
| 17 | Aulacoseira islandica (O. Müller) Simonsen | P-B | cool | st-str | i | ind | 8.0 | es | 2.0 | b | ate | o–e |
| 18 | Aulacoseira lirata (Ehrenberg) R. Ross | P-B | temp | - | hb | acf | 4.6–7.3 | - | 0.5 | x-o | ate | - |
| 19 | Aulacoseira perglabra (Østrup) E. Y. Haworth | P-B | temp | - | hb | acf | 4.76 | - | 1.0 | o | ate | ot |
| 20 | Aulacoseira pfaffiana (Reinsch) Krammer | P-B | cool | str | hb | acf | - | - | 0.3 | x | ats | ot |
| 21 | Aulacoseira pusilla (F. Meister) A. Tuji and A. Houki | - | - | - | - | - | - | - | - | - | - | - |
| 22 | Aulacoseira scalaris (Grunow) Houk, Klee, and Passauer | - | - | - | - | - | - | - | - | - | - | - |
| 23 | Aulacoseira subarctica (O. Müller) E. Y. Haworth | P | temp | st-str | i | alf | 6.72–8.14 | - | 1.7 | b-o | ats | om |
| 24 | Aulacoseira valida (Grunow) Krammer | P-B | - | - | i | alf | - | es | 1.3 | o | ate | om |
| 25 | Boreozonacola hustedtii Lange-Bertalot, Kulikovskiy, and Witkowski | - | - | - | - | - | - | - | - | - | - | - |
| 26 | Brachysira brebissonii R. Ross | P-B | temp | st-str | hb | acf | 4.6–7.8 | sx | 0.4 | o | ats | ot |
| 27 | Brachysira calcicola Lange-Bertalot | B | - | - | - | - | - | - | 1.0 | o | - | - |
| 28 | Brachysira neoexilis Lange-Bertalot | B | - | - | - | acf | 7.8 | - | 0.5 | x-o | - | om |
| 29 | Brachysira procera Lange-Bertalot and Gerd Moser | B | - | - | - | acf | 6.3–6.5 | - | - | - | - | - |
| 30 | Brachysira styriaca (Grunow) R. Ross | B | temp | - | i | ind | 6.45–7.26 | es | 1.0 | o | - | ot |
| 31 | Caloneis arctica (Krasske) Lange-Bertalot and S. I. Genkal | - | - | - | - | - | - | - | - | - | - | - |
| 32 | Caloneis bacillum (Grunow) Cleve | B | temp | st-str | i | alf | 6.8–8.4 | es | 1.3 | b | ats | me |
| 33 | Caloneis holarctica Kulikovskiy, Lange-Bertalot, and A.Witkowski | - | - | - | - | - | - | - | - | - | - | - |
| 34 | Caloneis silicula (Ehrenberg) Cleve var. silicula | B | warm | st | i | ind | 6.3–9.0 | sp | 1.3 | o | ats | om |
| 35 | Caloneis silicula var. elliptica Mayer | - | - | - | - | - | - | - | - | - | - | - |
| 36 | Campylodiscus hibernicus Ehrenberg | B | - | st | i | ind | - | es | 2.0 | b | ats | ot |
| 37 | Cavinula cocconeiformis (W. Gregory ex Greville) D. G. Mann and A. J. Stickle | P-B | temp | st-str | i | ind | 6.57–7.5 | es | 0.4 | x-o | ats | om |
| 38 | Cavinula jaernefeltii (Hustedt) D. G. Mann and A. J. Stickle | B | temp | str | i | acf | 6.71–7.5 | - | 2.0 | b | ats | om |
| 39 | Cavinula pseudoscutiformis (Hustedt) D. G. Mann and Stickle | P-B | temp | st-str | i | ind | 6.2–8.4 | sx | 0.4 | b | ats | me |
| 40 | Cavinula sp. | - | - | - | - | - | - | - | - | - | - | - |
| 41 | Chamaepinnularia begeri (Krasske) Lange-Bertalot | B | temp | - | i | ind | 6.35 | - | 1.0 | o | ats | - |
| 42 | Chamaepinnularia circumborealis Lange-Bertalot | - | - | - | - | - | - | - | - | - | - | - |
| 43 | Chamaepinnularia krookiformis (Krammer) Lange-Bertalot and Krammer | B | - | - | hl | neu | - | - | 1.0 | o | - | - |
| 44 | Chamaepinnularia sp. | - | - | - | - | - | - | - | - | - | - | - |
| 45 | Cocconeis lineata Ehrenberg | P-B | temp | st-str | i | alf | 6.3–9.5 | sx | 1.2 | b | ate | e |
| 46 | Cocconeis neodiminuta Krammer | P-B | temp | st-str | i | alf | 7–9 | sx | 0.9 | b | ats | me |
| 47 | Cocconeis placentula Ehrenberg var. placentula | P-B | temp | st-str | i | alf | 5.5–9.0 | es | 1.35 | o | ate | me |
| 48 | Cocconeis placentula var. euglypta (Ehrenberg) Cleve | P-B | temp | st-str | i | alf | 5.5–9.0 | sx | 1.3 | b | ate | om |
| 49 | Cocconeis sp. | - | - | - | - | - | - | - | - | - | - | - |
| 50 | Craticula molestiformis (Hustedt) Mayama | B | temp | st | i | alf | 6.8–8.4 | - | 3.6 | a-p | hne | e |
| 51 | Cyclostephanos dubius (Hustedt) Round | P-B | temp | st-str | hl | alf | 5.7–9.5 | es | 2.0 | a | ate | e |
| 52 | Cyclostephanos makarovae (S. I. Genkal) K. Schultz | - | - | - | - | - | - | - | - | - | - | - |
| 53 | Cyclotella atomus Hustedt | P-B | - | st-str | hl | alf | 6.9–8.5 | sp | 2.5 | b-a | ate | e |
| 54 | Cyclotella distinguenda Hustedt | P | - | str | hl | alf | 8.2 | - | 1.3 | o | - | om |
| 55 | Cyclotella meduanae H. Germain | - | - | - | - | - | - | - | - | - | - | - |
| 56 | Cymatopleura elliptica (Brébisson) W. Smith | P-B | temp | st-str | i | alf | 5.7–8.5 | - | 1.4 | b | ate | e |
| 57 | Cymbella arctica (Lagerstedt) A. W. F. Schmidt | B | - | - | i | alf | 8.30 | - | 1.0 | o | - | ot |
| 58 | Cymbella cleve-eulerae Krammer | - | - | - | - | - | - | - | - | - | - | - |
| 59 | Cymbella cymbiformis C. Agardh | B | temp | st-str | i | alf | 6.2–9.0 | sx | 2.0 | b | ats | om |
| 60 | Cymbella hantzschiana Krammer | - | - | - | - | - | - | - | - | - | - | - |
| 61 | Cymbella krammeri Bahls | - | - | - | - | - | - | - | - | - | - | - |
| 62 | Cymbella neogena (Grunow) Krammer | - | - | - | - | - | - | - | - | - | - | - |
| 63 | Cymbella proxima Reimer | B | - | - | i | alf | - | es | 1.0 | o | - | m |
| 64 | Cymbella subcistula Krammer | B | - | - | - | - | - | - | 1.2 | o | - | - |
| 65 | Cymbella sp. | - | - | - | - | - | - | - | - | - | - | - |
| 66 | Cymbopleura amphicephala (Nägeli ex Kützing) Krammer | B | - | st-str | i | ind | 4.6–8.2 | - | - | - | - | - |
| 67 | Cymbopleura anglica (Lagerstedt) Krammer | B | - | - | hb | ind | - | sx | 1.2 | o | ats | om |
| 68 | Cymbopleura angustata var. spitsbergensis Krammer | B | - | str | i | ind | 4.9–8.20 | - | 1.0 | o | - | ot |
| 69 | Cymbopleura designata (Krammer) Bahls | B | temp | st-str | i | alf | 6.3–9.0 | - | 1.8 | o-a | ats | m |
| 70 | Cymbopleura elliptica Krammer | - | - | - | - | - | - | - | - | - | - | - |
| 71 | Cymbopleura hybrida (Grunow ex Cleve) Krammer | P-B | - | - | i | ind | 8.10 | - | 1.2 | o | ats | - |
| 72 | Cymbopleura incertiformis Krammer | - | - | - | - | - | - | - | - | - | - | - |
| 73 | Cymbopleura naviculiformis (Auerswald ex Heiberg) Krammer | B | temp | st-str | i | ind | 7.8–6.94 | - | - | - | - | - |
| 74 | Cymbopleura oblongata var. stenoraphe Krammer | - | - | - | - | - | - | - | - | - | - | - |
| 75 | Cymbopleura subanglica Krammer | - | - | - | - | - | - | - | - | - | - | - |
| 76 | Cymbopleura subapiculata Krammer | - | - | - | - | - | - | - | - | - | - | - |
| 77 | Cymbopleura subcuspidata (Krammer) Krammer | P-B | - | str | i | acf | - | sx | 1.0 | o | ats | om |
| 78 | Cymbopleura truncata Krammer | - | - | - | - | - | - | - | - | - | - | - |
| 79 | Cymbopleura tynnii (Krammer) Krammer | B | - | - | - | - | - | - | - | - | - | - |
| 80 | Cymbopleura sp. | - | - | - | - | - | - | - | - | - | - | - |
| 81 | Denticula tenuis Kützing | B | - | st-str | i | alf | 7.42–8.0 | - | - | - | - | - |
| 82 | Diatoma moniliformis (Kützing) D. M. Williams | P-B | temp | st-str | i | alf | 8.0–8.5 | - | 0.4 | x-o | - | - |
| 83 | Diatoma vulgaris Bory | P-B | temp | st-str | i | alf | 6.2–8.9 | - | 2.4 | b-a | - | - |
| 84 | Diploneis boldtiana Cleve | B | - | st-str | i | ind | - | - | - | - | - | - |
| 85 | Diploneis modica Hustedt | B | - | - | - | - | 6.58 | - | - | - | - | - |
| 86 | Diploneis oblongella (Nägeli ex Kützing) A. Cleve | B | - | st-str | i | ind | 6.9–8.0 | - | - | - | - | - |
| 87 | Diploneis oculata (Brébisson) Cleve | B | temp | st-str | i | alf | 7.4–8.2 | - | - | - | - | - |
| 88 | Diploneis ovalis (Hilse) Cleve | B | - | st-str | i | alf | 6.5–9.0 | - | 0.9 | x-b | ate | m |
| 89 | Diploneis parma Cleve | B | cool | - | i | alf | 6.6–8.6 | - | - | - | - | - |
| 90 | Diploneis subovalis Cleve | B | temp | st-str | hl | ind | - | - | - | - | - | - |
| 91 | Discostella pseudostelligera (Hustedt) Houk and Klee | P | temp | st-str | i | ind | 6.32–8.5 | - | 2.7 | a-o | - | - |
| 92 | Discostella stelligera (Cleve and Grunow) Houk and Klee | P-B | temp | st-str | i | ind | 5.1–9.0 | - | - | - | - | - |
| 93 | Encyonema auerswaldii Rabenhorst | B | - | - | i | ind | - | - | - | - | - | - |
| 94 | Encyonema elginense (Krammer) D. G. Mann | B | temp | st-str | hb | acf | 5.5–9.0 | - | - | - | - | - |
| 95 | Encyonema gaeumannii (F. Meister) Krammer | B | temp | str | hb | acf | 4.6–7.9 | - | - | - | - | - |
| 96 | Encyonema groenlandica (Foged) Kulikovskiy and Lange-Bertalot | - | - | - | - | - | - | - | - | - | - | - |
| 97 | Encyonema latens (Krasske) D. G. Mann | B | - | - | - | - | 7.8–8.0 | - | 1.0 | o | ats | ot |
| 98 | Encyonema lunatum (W. Smith) Van Heurck | B | temp | - | - | ind | 4.9–7.8 | es | 1.3 | o | ats | e |
| 99 | Encyonema minutum (Hilse) D. G. Mann var. minutum | B | temp | st-str | i | ind | 4.9–8.9 | sx | 1.5 | o-b | ats | - |
| 100 | Encyonema neogracile Krammer | P-B | - | - | - | ind | 6.4 | - | - | - | hne | - |
| 101 | Encyonema perpusillum (A. Cleve) D. G. Mann | P-B | temp | str | hb | ind | 6.1–6.16 | - | - | - | - | - |
| 102 | Encyonema reichardtii (Krammer) D. G. Mann | B | temp | str | i | ind | 7.6–7.8 | - | 1.0 | o | ats | ot |
| 103 | Encyonema silesiacum (Bleisch) D. G. Mann | B | temp | st-str | i | ind | 6.2–8.6 | - | - | - | - | - |
| 104 | Encyonema ventricosum (C. Agardh) Grunow | B | - | st-str | i | ind | 6.2–8.0 | - | - | - | ate | - |
| 105 | Encyonema vulgare Krammer | B | - | - | - | - | - | - | - | o | ats | me |
| 106 | Encyonema sp. | - | - | - | - | - | - | - | - | - | - | - |
| 107 | Encyonopsis cesatiformis Krammer | - | - | - | - | - | - | - | - | - | - | - |
| 108 | Encyonopsis cesatii (Rabenhorst) Krammer | B | temp | str | i | ind | 5.7–8.0 | - | 1.5 | o-b | - | - |
| 109 | Encyonopsis perborealis Krammer | - | - | - | - | - | - | - | - | - | - | - |
| 110 | Entomoneis ornata (Bailey) Reimer | B | - | st-str | i | alf | - | - | 2.0 | b | hne | - |
| 111 | Eucocconeis alpestris (Brun) Lange-Bertalot | B | temp | str | hb | ind | 6.35–7.09 | - | - | - | - | - |
| 112 | Eucocconeis depressa (Cleve) Lange-Bertalot | B | - | - | hb | acf | - | - | 1.0 | o | - | ot |
| 113 | Eucocconeis diluviana (Hustedt) Lange-Bertalot | - | - | - | - | - | - | - | - | - | - | - |
| 114 | Eucocconeis flexella (Kützing) F.Meister | B | temp | str | mh | ind | 7.13–8.10 | - | - | - | - | - |
| 115 | Eucocconeis laevis (Østrup) Lange-Bertalot | B | - | str | hb | neu | 7.90 | sx | 0.2 | x | ats | ot |
| 116 | Eucocconeis leptostriata Lange-Bertalot apud H. Lange-Bertalot and S. I. Genkal | B | - | - | i | neu | - | sx | - | - | ate | - |
| 117 | Eucocconeis quadratarea (Østrup) Lange-Bertalot | B | - | - | i | acf | - | - | 1.0 | o | - | - |
| 118 | Eunotia ambivalens Lange-Bertalot and Tagliaventi | B | - | - | i | acf | - | - | 3.9 | b-p | - | - |
| 119 | Eunotia arcus Ehrenberg | B | temp | st-str | i | acf | 5.8–6.95 | sx | 0.4 | x-o | ats | ot |
| 120 | Eunotia bidens Ehrenberg | P-B,aer | cool | st-str | hb | acf | - | - | 1.0 | o | - | ot |
| 121 | Eunotia bigibboidea Lange-Bertalot and Witkowski | - | - | - | - | - | - | - | - | - | - | - |
| 122 | Eunotia bilunaris (Ehrenberg) Schaarschmidt | B | temp | st-str | i | acf | 5.0–7.8 | - | - | - | - | - |
| 123 | Eunotia boreoalpina Lange-Bertalot and Nörpel-Schempp | B | - | - | hb | - | - | - | - | - | - | - |
| 124 | Eunotia boreotenuis Nörpel-Schempp and Lange-Bertalot | B | - | - | hb | - | - | - | 1.0 | o | - | ot |
| 125 | Eunotia botuliformis F. Wild, Nörpel and Lange-Bertalot | B | - | - | hb | acf | - | - | 1.0 | o | - | ot |
| 126 | Eunotia cantonatii Lange-Bertalot and Tagliaventi | - | - | - | - | - | - | - | - | - | - | - |
| 127 | Eunotia chelonia Nörpel-Schempp, Lange-Bertalot, and Metzeltin | B | - | - | i | acf | - | - | - | - | - | - |
| 128 | Eunotia curtagrunowii Nörpel-Schempp and Lange-Bertalot | P-B | - | - | hb | acf | - | - | 0.4 | x-o | ats | ot |
| 129 | Eunotia elegans Østrup | P-B | - | st-str | hb | acf | 6.3 | - | 0.2 | x | ats | ot |
| 130 | Eunotia eurycephala (Grunow) Nörpel-Schempp and Lange-Bertalot | B | - | - | i | - | - | - | - | - | - | - |
| 131 | Eunotia ewa Lange-Bertalot and Witkowski | - | - | - | - | - | - | - | - | - | - | - |
| 132 | Eunotia faba Ehrenberg | B | temp | st-str | - | alf | 5.38–7.0 | - | - | o | ats | ot |
| 133 | Eunotia flexuosa (Brébisson ex Kützing) Kützing | B | temp | st-str | i | acf | 4.5–7.8 | - | 0.4 | x-o | - | - |
| 134 | Eunotia fureyae Lange-Bertalot | - | - | - | - | - | - | - | - | - | - | - |
| 135 | Eunotia genuflexa Nörpel-Schempp | - | - | - | - | - | - | - | - | - | - | - |
| 136 | Eunotia groenlandica Nörpel-Schempp and Lange-Bertalot | B | - | st-str | hb | acf | 3.80 | - | 0.6 | o-x | - | - |
| 137 | Eunotia incisa W. Smith ex W. Gregory | P-B | temp | st-str | i | acf | 4.5–7.0 | - | - | o | ats | om |
| 138 | Eunotia islandica Østrup | B | - | - | i | acf | - | - | 0.4 | x-o | - | ot |
| 139 | Eunotia julma Lange-Bertalot | - | - | - | - | - | - | - | - | - | - | - |
| 140 | Eunotia major (W. Smith) Rabenhorst | B | - | st-str | hb | acf | 6.7 | - | 1.0 | o | - | - |
| 141 | Eunotia meisteri Hustedt | P-B | - | str | i | acf | 4.5–7.2 | - | 0.4 | x-o | ats | - |
| 142 | Eunotia minor (Kützing) Grunow | B | temp | st-str | hb | acf | 4.5–8.2 | - | - | - | - | - |
| 143 | Eunotia monnieri Lange-Bertalot and Tagliaventi | - | - | - | - | - | - | - | - | - | - | - |
| 144 | Eunotia mucophila (Lange-Bertalot, Nörpel-Schempp, and Alles) Lange-Bertalot | P-B | temp | st-str | hb | acf | 5.25–6.4 | - | - | - | - | - |
| 145 | Eunotia naegelii Migula | P-B | temp | str | hb | acf | 4.5–6.0 | sx | 0.5 | x-o | ate | ot |
| 146 | Eunotia neocompacta var. vixcompacta Lange-Bertalot | - | - | - | - | - | - | - | - | - | - | - |
| 147 | Eunotia paralleladubia Lange-Bertalot and S. Mayama | - | - | - | - | - | - | - | - | - | - | - |
| 148 | Eunotia parapraerupta Lange-Bertalot and Metzeltin | - | - | - | - | - | - | - | - | - | - | - |
| 149 | Eunotia pseudogroenlandica Lange-Bertalot and Tagliaventi | - | - | - | - | - | - | - | - | - | - | - |
| 150 | Eunotia rhomboidea Hustedt | B | temp | str | hb | acf | 4.84–6.4 | - | 1.0 | o | - | - |
| 151 | Eunotia scandiorussica Kulikovskiy, Lange-Bertalot, Genkal, and Witkowski | - | - | - | - | - | - | - | - | - | - | - |
| 152 | Eunotia semicircularis (Ehrenberg) Lange-Bertalot and Metzeltin | - | - | - | - | - | - | - | - | - | - | - |
| 153 | Eunotia septentrionalis Østrup | P-B | - | str | hb | acf | 4.5–7.5 | - | 1.0 | o | - | ot |
| 154 | Eunotia subarcuatoides Alles, Nörpel, and Lange-Bertalot | B | - | str | hb | acb | 6.7 | - | 0.4 | x-o | - | - |
| 155 | Eunotia subherkiniensis Lange-Bertalot | - | - | - | - | - | - | - | - | - | - | - |
| 156 | Eunotia ursamaioris Lange-Bertalot and Nörpel-Schempp | B | - | - | hb | - | - | - | 1.0 | o | - | ot |
| 157 | Eunotia sp. | - | - | - | - | - | - | - | - | - | - | - |
| 158 | Fallacia crassicostata Lange-Bertalot and Werum | - | - | - | - | - | - | - | - | - | - | - |
| 159 | Fallacia pygmaea (Kützing) Stickle and D. G. Mann | P-B | - | st-str | mh | alf | 7.4–9.1 | - | - | - | ats | - |
| 160 | Fallacia sp. | - | - | - | - | - | - | - | - | - | - | - |
| 161 | Fragilaria aquaplus Lange-Bertalot and S. Ulrich | - | - | - | - | - | - | - | - | - | - | - |
| 162 | Fragilaria capucina Desmazières | P-B | temp | st-str | i | ind | 6.4–8.9 | - | - | - | - | - |
| 163 | Fragilaria radians (Kützing) D. M. Williams and Round | P-B | warm | st-str | i | alf | 7.0–7.5 | - | - | - | - | - |
| 164 | Fragilaria rumpens (Kützing) G. W. F. Carlson | P-B | eterm | st-str | i | ind | 6.5–8.8 | - | 2.0 | b | ats | e |
| 165 | Fragilaria saxoplanctonica Lange-Bertalot and S. Ulrich | - | - | - | - | - | - | - | - | - | - | - |
| 166 | Fragilaria vaucheriae (Kützing) J. B. Petersen | P-B,Ep | temp | st-str | i | alf | 6.5–8.8 | - | - | - | - | - |
| 167 | Fragilaria sp. | - | - | - | i | - | 4.9–7.8 | es | - | - | hne | - |
| 168 | Fragilariforma bicapitata (A. Mayer) D. M. Williams and Round | P-B | - | st-str | hb | ind | - | - | - | - | - | - |
| 169 | Fragilariforma constricta (Ehrenberg) D. M. Williams and Round | B | - | str | hb | acf | 4.6–7.0 | - | 1.3 | o | ats | m |
| 170 | Fragilariforma mesolepta (Rabenhorst) Kharitonov | P-B | - | st-str | i | alf | 6.3–9.0 | - | 1.0 | o | - | ot |
| 171 | Fragilariforma virescens (Ralfs) D. M. Williams and Round | P-B | temp | st-str | hb | ind | 4.6–8.2 | - | 1.0 | o | - | ot |
| 172 | Frustulia crassinervia (Brébisson ex W. Smith) Lange-Bertalot and Krammer | B | - | str | hb | acf | 4.7–7.2 | sx | 0.5 | x-o | ats | ot |
| 173 | Frustulia erifuga Lange-Bertalot and Krammer | B | temp | str | hb | acf | 5.85–6.49 | - | - | o | ats | e |
| 174 | Frustulia krammeri Lange-Bertalot and Metzeltin | B | - | - | - | acf | - | - | - | - | - | e |
| 175 | Frustulia saxonica Rabenhorst | B | temp | st-str | hb | acf | 4.5–7.2 | - | - | - | ate | - |
| 176 | Geissleria davydovae Genkal et Yaruschina | - | - | - | - | - | - | - | - | - | - | - |
| 177 | Genkalia digituloides (Lange-Bertalot) Lange-Bertalot and Kulikovskiy | - | - | - | - | - | - | - | - | - | - | - |
| 178 | Gololobovia obliqua (W. Gregory) Kulikovskiy, Glushchenko, and Kociolek | - | - | - | - | - | - | - | - | - | - | - |
| 179 | Gomphonema acuminatum Ehrenberg | B | temp | st-str | i | ind | 6.3–9.5 | - | 0.8 | x-b | - | - |
| 180 | Gomphonema angusticephalum E. Reichardt and Lange-Bertalot | - | - | - | - | - | - | - | - | - | - | - |
| 181 | Gomphonema brebissonii Kützing | B | - | st | i | ind | - | - | - | - | - | m |
| 182 | Gomphonema capitatum Ehrenberg | B | temp | st | i | alf | 6.9–8.9 | - | 1.2 | o | - | om |
| 183 | Gomphonema coronatum Ehrenberg | B | - | st | i | ind | 7.33 | - | - | - | - | - |
| 184 | Gomphonema gracile Ehrenberg | B | temp | st-str | i | alf | 6.4–8.6 | - | - | - | - | - |
| 185 | Gomphonema hebridense W. Gregory | B | - | - | - | acf | 6.1 | - | 1.0 | o | - | - |
| 186 | Gomphonema italicum Kützing | - | - | - | - | - | - | - | - | - | - | - |
| 187 | Gomphonema lagerheimii A. Cleve | B | - | str | hb | acf | - | - | - | - | - | m |
| 188 | Gomphonema laticollum E. Reichardt | - | - | - | - | - | - | - | 1.0 | o | ats | ot |
| 189 | Gomphonema microcapitatum Kulikovskiy, Kociolek, and Solak | - | - | - | - | - | - | - | - | - | - | - |
| 190 | Gomphonema mihoi Levkov | - | - | - | - | - | - | - | - | - | - | - |
| 191 | Gomphonema minutum f. pachypus Lange-Bertalot and E. Reichardt | - | - | - | - | - | - | - | - | - | - | - |
| 192 | Gomphonema olivaceoides Hustedt | B | - | str | hb | ind | - | - | 1.0 | o | - | - |
| 193 | Gomphonema parvulum (Kützing) Kützing | B | temp | st-str | i | ind | 4.5–8.6 | - | 0.7 | o-x | ats | ot |
| 194 | Gomphonema pseudacuminatum Kulikovskiy, Kociolek, and Solak | - | - | - | - | - | - | - | - | - | - | - |
| 195 | Gomphonema truncatum Ehrenberg | B | temp | st-str | i | ind | 7.19 | - | 2.0 | b | - | - |
| 196 | Gomphonema sp. | B | - | - | i | - | 5.7–7.8 | sx | - | - | - | - |
| 197 | Gomphosphenia vallei Beauger, C. E. Wetzel, Allain, and Ector | - | - | - | - | - | - | - | - | - | - | - |
| 198 | Gomphosphenia sp. | B | - | - | - | - | - | - | - | - | - | - |
| 199 | Gyrosigma acuminatum (Kützing) Rabenhorst | B | temp | st-str | i | alf | 6.3–9.5 | - | - | - | - | - |
| 200 | Gyrosigma sp. | B | - | - | - | - | - | - | - | - | - | - |
| 201 | Halamphora hassiaca (Krammer and S. Strecker) Lange-Bertalot | - | - | - | - | - | - | - | - | - | - | - |
| 202 | Handmannia antiqua (W. Smith) Kociolek et Khursevich | P-B | temp | - | hb | acf | 7.25–7.27 | - | 1.2 | o | - | - |
| 203 | Handmannia comta (Ehrenberg) Kociolek et Khursevich emend. Genkal | P | temp | st | i | alf | 6.0–7.8 | - | - | - | - | - |
| 204 | Hantzschia sp. | B | - | - | - | - | - | - | - | - | - | - |
| 205 | Hippodonta capitata (Ehrenberg) Lange-Bertalot, Metzeltin, and Witkowski | B | temp | st-str | hl | alf | 6.6–9.5 | - | - | a-b | - | me |
| 206 | Hippodonta hungarica (Grunow) Lange-Bertalot, Metzeltin, and Witkowski | B | - | st-str | hl | alf | 6.9–8.6 | - | - | - | - | - |
| 207 | Humidophila brekkaensis (J. B. Petersen) R. L. Lowe, Kociolek, J. R. Johansen, Van de Vijver, Lange-Bertalot, and Krammer et Kopalova | B | - | aer | mh | alf | - | - | - | - | - | - |
| 208 | Humidophila gallica (W. Smith) Lowe, Kociolek, Q. You, Q. Wang, and Stepanek | B | - | st-str | i | ind | 7.60 | es | 0.7 | o-x | ate | om |
| 209 | Humidophila perpusilla (Grunow) R. L. Lowe, Kociolek, J. R. Johansen, Van de Vijver, Lange-Bertalot, and Kopalová | B | warm | st-str | i | ind | - | - | - | - | - | - |
| 210 | Humidophila schmassmannii (Hustedt) Buczkó and Wojtal | B | cool | - | - | acf | - | sp | 0.7 | o-x | ats | om |
| 211 | Humidophila sp. | - | - | - | - | - | - | - | - | - | - | - |
| 212 | Hygropetra balfouriana (Grunow ex Cleve) Krammer and Lange-Bertalot | B,aer | temp | - | i | ind | 6.89–7.60 | - | - | - | - | ot |
| 213 | Iconella curvula (W. Smith) Ruck and Nakov | B | - | str | hb | acf | - | - | 2.0 | b | - | me |
| 214 | Iconella linearis (W. Smith) Ruck and Nakov | P-B | - | st-str | i | ind | 4.6–9.0 | - | - | - | - | - |
| 215 | Iconella splendida (Ehrenberg) Ruck and Nakov | P-B | - | st-str | i | alf | - | - | - | - | - | - |
| 216 | Karayevia laterostrata (Hustedt) Bukhtiyarova | B | temp | st-str | hb | ind | 6.89–8.1 | - | - | - | - | - |
| 217 | Kobayasiella parasubtilissima (H. Kobayasi and T. Nagumo) Lange-Bertalot | B | temp | str | hb | acb | 5.41 | - | 1.5 | o-b | - | - |
| 218 | Kobayasiella subtilissima (Cleve) Lange-Bertalot | B | temp | st-str | i | acb | 4.6–7.0 | - | 1.6 | b-o | ats | me |
| 219 | Mayamaea disjuncta (Hustedt) J. Y. Li and Y. Z. Qi | B | - | str | i | ind | 7.5 | sp | 3.0 | a | ate | he |
| 220 | Melosira varians C. Agardh | P-B | temp | st-str | hl | ind | 5–9 | - | 2.4 | b-a | - | - |
| 221 | Navicula angusta Grunow | B | - | st-str | i | ind | 7.6–8.2 | - | 1.0 | o | - | - |
| 222 | Navicula chiarae Lange-Bertalot and Genkal | - | - | - | - | - | 8.30 | - | - | - | hce | - |
| 223 | Navicula cryptocephala Kützing | P-B | temp | st-str | i | ind | 6.5–8.4 | - | 2.4 | b-a | - | - |
| 224 | Navicula cryptotenella Lange-Bertalot | P-B | temp | st-str | i | ind | 6.5–8.7 | - | - | - | - | - |
| 225 | Navicula cryptotenelloides Lange-Bertalot | B | - | - | oh | alf | 7.9–8.19 | - | 1.0 | o | - | - |
| 226 | Navicula mediocostata E. Reichardt | B | - | - | oh | alf | - | es | 3.0 | a | ate | e |
| 227 | Navicula notha J. H. Wallace | B | - | str | i | acf | 6.3–7.5 | - | - | - | - | - |
| 228 | Navicula phyllepta Kützing | B | - | - | hl | - | - | - | - | - | - | - |
| 229 | Navicula phylleptosoma Lange-Bertalot | B | - | - | mh | alf | 7.7 | - | - | - | - | - |
| 230 | Navicula radiosa Kützing | B | temp | st-str | i | ind | 5–9 | sx | - | - | - | - |
| 231 | Navicula reinhardtii (Grunow) Grunow | - | - | - | - | - | - | - | - | - | - | - |
| 232 | Navicula rostellata Kützing | B | - | st-str | i | alf | 7.7–8.6 | - | 0.7 | o-x | ate | ot |
| 233 | Navicula tripunctata (O. F. Müller) Bory | P-B | temp | st-str | i | alf | 7.0–8.6 | es | - | - | - | e |
| 234 | Navicula trivialis Lange-Bertalot | B | temp | st-str | i | alf | 7.2–8.1 | es | - | - | - | - |
| 235 | Navicula viridulacalcis Lange-Bertalot | B | - | - | - | - | - | - | - | - | - | - |
| 236 | Navicula wygaschii Lange-Bertalot | - | - | - | - | - | - | - | - | - | - | - |
| 237 | Navicula sp. | - | - | - | - | - | - | - | - | - | - | - |
| 238 | Naviculadicta sp. | - | - | - | - | - | - | - | - | - | - | - |
| 239 | Navigeia paludosa (Hustedt) Bukhtiyarova | B | - | str | i | ind | 8.11 | sx | - | - | - | - |
| 240 | Navigeia thingvallae (Østrup) Bukhtiyarova | B | - | - | - | - | - | - | - | - | - | - |
| 241 | Neidiopsis wulffii (J. B. Petersen) Lange-Bertalot | - | - | - | - | - | 7.80 | - | - | - | ats | ot |
| 242 | Neidium affine (Ehrenberg) Pfitzer | B | temp | st-str | i | ind | 4.5–7.8 | - | - | - | - | - |
| 243 | Neidium ampliatum (Ehrenberg) Krammer | B | temp | st | i | ind | 5.2–8.6 | - | - | - | - | - |
| 244 | Neidium bisulcatum (Lagerstedt) Cleve | B | - | st-str | i | ind | 4.9–7.0 | - | 1.0 | o | - | - |
| 245 | Neidium dubium (Ehenberg) Cleve | B | - | str | i | alf | - | - | - | - | - | - |
| 246 | Neidium hercynicum Ant. Mayer | B | - | - | i | acf | - | - | - | - | - | - |
| 247 | Neidium hitchcockii (Ehrenberg) Cleve | P-B | - | st | I | ind | - | es | 0.6 | o-x | ats | ot |
| 248 | Neidium iridis (Ehrenberg) Cleve | B | temp | st-str | hb | ind | 5.1–8.9 | - | - | - | - | - |
| 249 | Neidium sp. | B | - | - | - | - | 4.6–6.9 | - | - | - | - | - |
| 250 | Nitzschia acicularis (Kützing) W. Smith | P-B | temp | st | i | alf | 6.8–8.1 | es | 1.4 | o-b | ats | om |
| 251 | Nitzschia acidoclinata Lange-Bertalot | B | temp | str | hb | ind | 6.5–8.0 | - | 3.6 | a-b | ate | e |
| 252 | Nitzschia alpina Hustedt | P-B | temp | str | i | acf | 7.39 | - | 1.0 | o | - | - |
| 253 | Nitzschia capitellata Hustedt | B | temp | - | i | ind | 6.9–8.6 | - | 3.6 | a-b | - | o–e |
| 254 | Nitzschia commutatoides Lange-Bertalot | - | - | - | hl | - | - | - | - | - | - | - |
| 255 | Nitzschia dissipata (Kützing) Rabenhorst | B | temp | st-str | i | alf | 6.5–8.5 | sx | 1.4 | o-b | - | - |
| 256 | Nitzschia fonticola (Grunow) Grunow | P-B | temp | st-str | i | alf | 6.0–8.9 | - | 3.6 | a-b | hne | - |
| 257 | Nitzschia frustulum (Kützing) Grunow | P-B | temp | st-str | hl | alf | 6.7–8.8 | es | 2.7 | a-o | - | - |
| 258 | Nitzschia graciliformis Lange-Bertalot and Simonsen | B | - | - | i | alf | - | es | 1.0 | o | - | - |
| 259 | Nitzschia gracilis Hantzsch | P-B | temp | st-str | i | ind | 5.51–8.25 | - | - | - | - | - |
| 260 | Nitzschia inconspicua Grunow | B | temp | st-str | hl | alf | 6.7–8.9 | - | - | - | - | - |
| 261 | Nitzschia intermedia Hantzsch ex Cleve and Grunow | P-B | temp | - | i | ind | 6.6–8.1 | - | - | - | - | - |
| 262 | Nitzschia linearis W. Smith | B | temp | - | i | alf | 7.1–8.1 | es | 1.7 | b-o | ate | me |
| 263 | Nitzschia media Hantzsch | - | - | - | - | - | - | - | - | - | - | - |
| 264 | Nitzschia perminuta Grunow | P-B | temp | str | hl | alf | 5.79–8.0 | - | - | - | - | - |
| 265 | Nitzschia rosenstockii Lange-Bertalot | B | - | - | hl | - | - | - | - | - | - | - |
| 266 | Nupela impexiformis (Lange-Bertalot) Lange-Bertalot | B | - | - | - | ind | 6.8–7.3 | sx | 0.5 | x-o | ats | ot |
| 267 | Nupela neogracillima Kulikovskiy and Lange-Bertalot | P-B | - | - | i | ind | - | - | - | - | - | ot |
| 268 | Nupela silvahercynia (Lange-Bertalot) Lange-Bertalot | B | - | - | i | - | - | - | - | - | - | - |
| 269 | Nupela tenuicephala (Hustedt) Lange-Bertalot | B | - | - | - | acf | - | es | - | - | - | - |
| 270 | Pantocsekiella costei (J. C. Druart and F. Straub) K. T. Kiss and E. Ács | - | - | - | - | - | - | - | - | - | - | - |
| 271 | Pinnularia acoricola Hustedt | B | - | st-str | i | acf | - | - | - | - | - | - |
| 272 | Pinnularia ammerensis Kulikovskiy, Lange-Bertalot, and Metzeltin | - | - | - | - | - | - | - | - | - | - | - |
| 273 | Pinnularia anglica Krammer | B | - | - | - | acf | - | es | 2.3 | b | - | e |
| 274 | Pinnularia angustarea Kulikovskiy, Lange-Bertalot, A. Witkovski, and N. I. Dorofeyuk | - | - | - | - | - | - | - | - | - | - | - |
| 275 | Pinnularia brebissonii (Kützing) Rabenhorst | B | temp | st-str | i | ind | - | - | 1.0 | o | - | - |
| 276 | Pinnularia bottnica Krammer | B | - | - | hl | - | - | - | - | - | - | - |
| 277 | Pinnularia brandelii Cleve | B | - | - | hb | acf | - | - | - | - | - | - |
| 278 | Pinnularia canadensis Krammer | - | - | - | - | - | - | - | - | - | - | - |
| 279 | Pinnularia cuneola E. Reichardt | - | - | - | - | - | - | - | - | - | - | - |
| 280 | Pinnularia decrescens (Grunow) Krammer | B | - | str | hb | ind | - | - | - | - | - | - |
| 281 | Pinnularia divergens var. sublinearis Cleve | - | - | - | - | - | - | - | - | - | - | - |
| 282 | Pinnularia eifeliana (Krammer) Krammer | B | - | - | - | - | - | - | 1.0 | o | - | - |
| 283 | Pinnularia grunowii Krammer | - | - | - | - | - | - | - | - | - | - | - |
| 284 | Pinnularia halophila Krammer | B | - | - | hl | - | - | - | 0.2 | x | ats | om |
| 285 | Pinnularia krammeri Metzeltin | - | - | - | - | - | - | - | - | - | - | - |
| 286 | Pinnularia lagerstedtii (Cleve) A. Cleve | B | - | aer | hb | ind | - | - | - | - | - | - |
| 287 | Pinnularia lailaensis Foged | - | - | - | - | - | - | - | - | - | - | - |
| 288 | Pinnularia macilenta Ehrenberg | B | - | - | - | - | - | - | 0.9 | x-b | - | - |
| 289 | Pinnularia microstauron var. rostrata Krammer | - | - | - | - | - | - | - | - | - | - | - |
| 290 | Pinnularia neohalophila Kulikovskiy, Genkal, and Mikheeva | - | - | - | - | - | - | - | - | - | - | - |
| 291 | Pinnularia nodosa (Ehrenberg) W. Smith var. nodosa | B | temp | str | i | ind | 6.79 | - | 0.4 | x-o | - | - |
| 292 | Pinnularia nodosa var. percapitata Krammer | - | - | - | - | - | - | - | - | - | - | - |
| 293 | Pinnularia nodosa var. robusta (Foged) Krammer | B | - | - | - | - | - | - | 0.4 | x-o | ats | ot |
| 294 | Pinnularia notabilis Krammer | B | - | - | - | - | - | - | 0.6 | o-x | - | - |
| 295 | Pinnularia oriunda Krammer | B | - | - | i | neu | - | - | 1.0 | o | ats | ot |
| 296 | Pinnularia oriundiformis Krammer | - | - | - | - | - | - | - | - | - | - | - |
| 297 | Pinnularia parvulissima Krammer | - | - | - | - | - | - | - | - | - | - | - |
| 298 | Pinnularia permicrostauron Krammer and Metzeltin | - | - | - | - | - | - | - | - | - | - | - |
| 299 | Pinnularia pluvianiformis Krammer | - | - | - | - | - | - | - | - | - | - | - |
| 300 | Pinnularia rhombarea Krammer | - | - | - | - | - | - | - | - | - | - | - |
| 301 | Pinnularia rupestris Hantzsch | B | temp | str | i | acf | 5.39 | - | - | - | - | - |
| 302 | Pinnularia septentrionalis Krammer | B | - | - | i | ind | - | - | 1.0 | o | - | om |
| 303 | Pinnularia similiformis Krammer | B | - | - | - | acf | - | - | 1.0 | o | - | ot |
| 304 | Pinnularia spitsbergensis Cleve | B | - | - | hb | ind | - | - | - | - | ats | ot |
| 305 | Pinnularia stricta Hustedt | - | - | - | - | - | - | - | - | - | - | - |
| 306 | Pinnularia subanglica Krammer | - | - | - | - | - | - | - | - | - | - | - |
| 307 | Pinnularia subrostrata (A. Cleve) A. Cleve | B | - | - | hb | acf | - | - | 1.0 | o | - | - |
| 308 | Pinnularia subrupestris Krammer | B | - | - | hb | acf | - | - | 0.4 | x-o | - | - |
| 309 | Pinnularia subundulata Østrup | B | - | - | - | acf | - | - | 0.3 | x | - | ot |
| 310 | Pinnularia undula (Schumann) Krammer | B | - | - | i | ind | - | - | 1.0 | o | - | - |
| 311 | Pinnularia viridis (Nitzsch) Ehrenberg | P-B | temp | st-str | i | ind | 5.24–7.1 | - | 0.9 | x-b | - | ot |
| 312 | Pinnularia sp. | - | - | - | - | - | 4.5–7.8 | - | - | - | - | - |
| 313 | Placogeia similis (Krasske) Bukhtiyarova | B | - | - | i | ind | - | - | - | - | - | e |
| 314 | Placoneis amphibola (Cleve) E. J. Cox | B | cool | st-str | i | ind | - | - | - | - | - | - |
| 315 | Placoneis clementioides (Hustedt) E. J. Cox | B | - | - | i | alf | - | - | - | - | - | - |
| 316 | Placoneis elginensis (W. Gregory) E. J. Cox | P-B | - | st-str | i | alf | 7.0–8.2 | - | - | - | - | - |
| 317 | Placoneis interglacialis (Hustedt) E. J. Cox | B | - | - | i | ind | - | - | 2.0 | b | - | - |
| 318 | Placoneis opportuna (Hustedt) Chudaev and Gololobova | B | - | - | - | - | - | es | - | - | - | - |
| 319 | Placoneis sp. | - | - | - | - | - | - | - | - | - | - | - |
| 320 | Planothidium straubianum C. E. Wetzel, Van de Vijver, and L. Ector | B | - | str | i | alf | 8.0 | - | - | a | ats | e |
| 321 | Planothidium sp. | - | - | - | - | - | - | - | - | - | - | - |
| 322 | Pleurosigma elongatum W. Smith | P-B | - | - | mh | alf | - | - | - | - | - | - |
| 323 | Praestephanos triporus (Genkal and G. V. Kuzmin) A. Tuji and J.-S. Ki | P | - | - | i | alf | - | - | - | - | - | - |
| 324 | Psammothidium bioretii (H. Germain) Bukhtiyarova and Round | B | - | str | i | ind | 6.08–7.9 | - | 0.7 | o-x | ats | ot |
| 325 | Psammothidium chlidanos (M. H. Hohn and Hellerman) Lange-Bertalot | B | - | - | hb | acf | 7.1–7.9 | - | 1.0 | o | - | ot |
| 326 | Psammothidium daonense (Lange-Bertalot) Lange-Bertalot | B | temp | str | hb | ind | 6.6–8.2 | - | - | o | ats | ot |
| 327 | Psammothidium helveticum (Hustedt) Bukhtiyarova and Round | B | temp | st-str | hb | alf | 6.0–7.4 | es | 2.4 | b-a | ate | m |
| 328 | Psammothidium kryophilum (J. B. Petersen) E. Reichardt | P-B | - | str | i | ind | 8.10 | sx | 0.5 | x-o | ats | ot |
| 329 | Psammothidium levanderi (Hustedt) Bukhtiyarova and Round | B | temp | str | i | ind | 6.6–8.4 | sx | 2.0 | b | ats | om |
| 330 | Psammothidium marginulatum (Grunow) Bukhtiyarova and Round | B | temp | st-str | hb | acf | 4.6–7.9 | sx | 0.2 | x | ats | ot |
| 331 | Psammothidium rechtense (Leclercq) Lange-Bertalot | B | - | str | hb | alf | - | - | 1.0 | o | ats | ot |
| 332 | Psammothidium rossii (Hustedt) Bukhtiyarova and Round | B | - | str | hb | ind | - | - | 1.0 | o | ats | ot |
| 333 | Psammothidium scoticum (R. J. Flower and V. J. Jones) Bukhtiyarova and Round | B | temp | - | - | - | 6.42 | - | - | - | - | - |
| 334 | Psammothidium subatomoides (Hustedt) Bukhtiyarova and Round | P-B | temp | str | hb | acf | 6.4–8.01 | sx | 2.0 | b | ats | me |
| 335 | Psammothidium subsalsum (J. B. Petersen) Kulikowskiy, Witkowski, and Pliński | B | - | - | - | - | - | - | - | - | - | - |
| 336 | Psammothidium ventrale (Krasske) Bukhtiyarova and Round | B | - | str | hb | acf | 7.45 | - | 2.0 | b | ats | om |
| 337 | Psammothidium sp. | - | - | - | - | - | - | - | - | - | - | - |
| 338 | Pseudostaurosira brevistriata (Grunow) D. M. Williams and Round | P-B | temp | st-str | i | alf | 5.2–8.4 | - | 2.0 | b | ate | - |
| 339 | Pseudostaurosira parasitica (W. Smith) E. Morales | P-B | temp | st-str | i | alf | 6.41–8.22 | - | 1.0 | o | ate | ot |
| 340 | Pulchellophycus obsitus (Hustedt) Edlund and M. J. Wynne | - | - | - | - | - | - | - | - | - | - | - |
| 341 | Pulchellophycus sp. | - | - | - | - | - | - | - | - | - | - | - |
| 342 | Reimeria sinuata (W. Gregory) Kociolek and Stoermer | P-B,aer | temp | st-str | i | ind | 6.6–8.9 | - | - | - | - | - |
| 343 | Sellaphora bacillum (Ehrenberg) D. G. Mann | B | - | st-str | i | alf | 7–9 | sx | 1.5 | o-b | ats | me |
| 344 | Sellaphora difficillima (Hustedt) C. E. Wetzel, L. Ector, and D. G. Mann | B | temp | str | hb | acf | 7.8 | - | 1.0 | o | ate | om |
| 345 | Sellaphora insolita (É. Manguin ex Kociolek and B. de Reviers) P. B. Hamilton, and D. Antoniades | - | - | - | - | - | - | - | - | - | - | - |
| 346 | Sellaphora laevissima (Kützing) D. G. Mann | B | - | st-str | i | ind | 5.7–8.1 | - | 2.0 | b | ats | om |
| 347 | Sellaphora vitabunda (Hustedt) D. G. Mann | B | - | - | i | alf | 8.06 | es | 1.0 | o | ats | om |
| 348 | Sellaphora sp. | B | - | - | - | - | - | - | - | - | - | - |
| 349 | Simonsenia delognei (Grunow) Lange-Bertalot | B | temp | str | oh | alf | 7.5–8.1 | - | 3.0 | a | hne | e |
| 350 | Skabitschewskia oestrupii (A. Cleve) Kuliskovskiy and Lange-Bertalot | B | - | str | i | ind | 7.6 | - | 1.0 | o | ats | om |
| 351 | Skabitschewskia peragalloi (Brun and Héribaud) Kuliskovskiy and Lange-Bertalot | B | - | str | i | ind | 8.20 | sx | 0.4 | x-o | ats | om |
| 352 | Stauroneis amphicephala Kützing | P-B | temp | st-str | i | ind | 4.8–8.2 | sx | 1.3 | o | ats | om |
| 353 | Stauroneis anceps Ehrenberg | P-B | temp | st-str | i | ind | 4.8–8.2 | sx | 1.3 | o | ats | om |
| 354 | Stauroneis gracilis Ehrenberg | B | - | - | I | ind | 5.3 | - | - | o | - | - |
| 355 | Stauroneis guslyakovii Genkal and Yarushina | - | - | - | - | - | - | - | - | - | - | - |
| 356 | Stauroneis phoenicenteron (Nitzsch) Ehrenberg | P-B | temp | st-str | i | ind | 6.01–8.5 | - | - | - | - | - |
| 357 | Stauroneis reichardtii Lange-Bertalot, Cavacini, Tagliaventi, and Alfinito | P-B | temp | st-str | i | ind | 4.8–8.2 | sx | 1.3 | o | ats | om |
| 358 | Stauroneis schulzii Jousé | B | - | - | i | alf | - | - | - | - | ats | - |
| 359 | Stauroneis smithii Grunow | P-B | - | st-str | i | alf | - | - | 1.0 | o | - | om |
| 360 | Stauroneis sp. | B | - | - | - | - | - | - | 1.5 | o-b | ate | o–e |
| 361 | Staurosira sviridae Kulikovskiy, Genkal, and Mikheeva | - | - | - | - | - | - | - | - | - | - | - |
| 362 | Staurosirella lanceolata (Hustedt) E. A. Morales, C. Wetzel, and L. Ector | - | - | - | - | - | - | - | - | - | - | - |
| 363 | Staurosirella pinnata (Ehrenberg) D. M. Williams and Round | P-B | temp | st-str | hl | alf | 6.2–9.3 | es | 1.1 | o | ats | om |
| 364 | Stenopterobia heribaudii (Playfair) Playfair | P-B | - | st | - | - | - | - | 0.4 | x-o | - | - |
| 365 | Stephanocyclus meneghinianus (Kützing) Kulikovskiy, Genkal, and Kociolek | P-B | temp | st-str | hl | alf | 5.5–9.0 | sp | 2.8 | a | hne | e |
| 366 | Stephanodiscus hantzschii Grunow | P | temp | - | i | - | 8.0–8.5 | - | - | - | - | - |
| 367 | Stephanodiscus hashiensis H. Tanaka | - | - | - | - | - | - | - | - | - | - | - |
| 368 | Stephanodiscus minutulus (Kützing) Cleve and Möller | P | temp | st-str | i | alb | 6.5–9.0 | es | 3.6 | a-o | hne | he |
| 369 | Stephanodiscus neoastraea Håkansson and Hickel emend. Casper, Scheffler et Augsten | P | temp | st-str | i | alb | 5.5–9.0 | es | - | - | - | - |
| 370 | Surirella angusta Kützing | P-B | temp | st-str | i | alf | 6.9–8.9 | - | - | - | - | - |
| 371 | Surirella librile (Ehrenberg) Ehrenberg | P-B | temp | st-str | i | alf | 8.0 | - | - | - | hne | - |
| 372 | Surirella minuta Brébisson ex Kützing | B | temp | st-str | i | alf | 6.9–8.6 | - | - | - | - | - |
| 373 | Surirella roba Leclercq | B | - | str | i | acf | - | - | - | - | - | - |
| 374 | Surirella sp. | B | - | - | mh | - | - | es | 1.85 | o-a | hne | - |
| 375 | Tabellaria flocculosa (Roth) Kützing | P-B | eterm | st-str | i | acf | 4.5–8.0 | - | 3.0 | a | - | - |
| 376 | Tetracyclus glans (Ehrenberg) F. W. Mills | P-B | temp | - | i | acf | 6.95 | - | 1.0 | x-o | - | ot |
| 377 | Thalassiosira pseudonana Hasle and Heimdal | P | temp | st-str | hl | alf | 7.4–8.0 | - | 2.4 | b-a | hne | he |
| 378 | Thalassiosira sp. | B | - | - | - | - | - | - | - | - | - | - |
| 379 | Tryblionella angustata W. Smith | P-B | temp | st | i | alf | 6.86–7.7 | sx | 1.5 | o-b | ats | e |
| 380 | Tryblionella calida (Grunow) D. G. Mann | P-B | - | - | hl | - | 7.8–8.2 | - | 2.6 | a-o | - | e |
| 381 | Tryblionella hungarica (Grunow) Frenguelli | P-B | - | st-str | mh | alf | 7.0–7.8 | sp | 2.9 | a | ate | e |
| 382 | Tryblionella littoralis (Grunow) D. G. Mann | B | - | st-str | eh | alf | - | es | 2.6 | a-o | ats | e |
| 383 | Ulnaria acus (Kützing) Aboal | P-B | warm | st-str | i | alf | 6.8–8.0 | es | 1.85 | o-a | ate | me |
| 384 | Ulnaria ulna (Nitzsch) Compère | P-B | temp | st-str | i | alf | 5.0–9.5 | es | 2.4 | b-a | ate | e |
| 385 | Ulnaria sp. | - | - | - | - | - | - | - | - | - | - | - |
Notes: habitat (P—planktonic, P-B—plankto-benthic, B—benthic); temperature preferences (cool—cool water, temp—temperate, eterm—eurythermic, warm—warm water); oxygenation and streaming (st—standing water, str—streaming water, st-str—low streaming water, aer—aerophiles); pH preference groups (pH) according to Hustedt (1957) [69] (alb—alkalibiontes; alf—alkaliphiles, ind—indifferent; acf—acidophiles; neu—neutrophiles as a part of pH-indifferent taxa); salinity ecological groups according to Hustedt (1938–1939) [70,71] (hb—oligohalobes–halophobes, i—oligohalobes-indifferents, hl—halophiles; mh—mesohalobes, eh—euhalobes); self-purification zone with index of saprobity (x/0.0—xenosaprobe; x-o/0.4—xeno-oligosaprobe; o-x/0.6—oligo-xenosaprobe; x-b/0.8—xeno-betamesosaprobe; o/1.0—oligosaprobe; o-b/1.4—oligo-betamesosaprobe; x-a/0.55—xeno-to-alphamesosaprobe; b-o/1.6—beta-oligosaprobe; o-a/1.8—oligo-alphamesosaprobe; b/2.0—betamesosaprobe; b-a/2.4—beta-alphamesosaprobe; a-o/2.6—alpha-oligosaprobe; b-p/2.8—beta-polysaprobe; a/3.0—alphamesosaprobe; a-p/3.4—alpha-polysaprobe; a-b/3.6—alpha-betamesosaprobe; p-a/4.0—poly-alphamesosaprobe; i/>4.0—i-eusaprobe); organic pollution indicators according Watanabe et al. (1986) [72]: sx—saproxenes; es—eurysaprobes; sp—saprophiles; nitrogen uptake metabolism (Aut-Het) [16]: ats—nitrogen autotrophic taxa tolerating very small concentrations of organically bound nitrogen; ate—nitrogen autotrophic taxa tolerating elevated concentrations of organically bound nitrogen; hne—facultative nitrogen heterotrophic taxa needing periodically elevated concentrations of organically bound nitrogen; hce—obligate nitrogen heterotrophic taxa needing continuously elevated concentrations of organically bound nitrogen; trophic-state indicators [16]: (ot—oligotraphentic; om—oligomesotraphentic; m—mesotraphentic; me—mesoeutraphentic; e—eutraphentic; he—hypereutraphentic; o–e—oligo-to-eutraphentic (hypereutraphentic)).

Table A4.
Bioindicator spectrum for diatom communities of 14 studied water bodies in the vicinity of Tiksi Bay, July 2021.
Table A4.
Bioindicator spectrum for diatom communities of 14 studied water bodies in the vicinity of Tiksi Bay, July 2021.
| Group of Indicators | Lake 1 | Lake 2 | Lake 3 | Lake 4 | Lake 5 | Lake 6 | Lake 7 | Lake 8 | Lake 9 | Lake 10 | Lake 11 | Lake 12 | Lake 13 | Lake 14 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Habitat | ||||||||||||||
| B | 36 | 24 | 44 | 36 | 13 | 66 | 42 | 42 | 35 | 40 | 58 | 21 | 54 | 24 |
| P-B | 17 | 15 | 18 | 18 | 12 | 34 | 19 | 15 | 9 | 22 | 26 | 10 | 19 | 13 |
| P | 3 | 1 | 1 | 2 | 4 | 7 | 2 | 0 | 4 | 3 | 2 | 1 | 2 | 1 |
| Temperature | ||||||||||||||
| cool | 1 | 1 | 2 | 2 | 1 | 3 | 0 | 2 | 0 | 1 | 0 | 0 | 0 | 0 |
| temp | 29 | 29 | 24 | 0 | 17 | 52 | 30 | 24 | 22 | 30 | 36 | 12 | 35 | 19 |
| eterm | 2 | 2 | 3 | 26 | 1 | 2 | 2 | 1 | 1 | 2 | 1 | 1 | 2 | 2 |
| warm | 0 | 0 | 2 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 2 | 1 |
| Oxygen | ||||||||||||||
| aer | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| str | 14 | 9 | 20 | 12 | 3 | 20 | 11 | 16 | 14 | 14 | 15 | 12 | 16 | 4 |
| st-str | 28 | 21 | 23 | 24 | 20 | 52 | 30 | 24 | 18 | 29 | 41 | 0 | 37 | 20 |
| st | 0 | 2 | 1 | 4 | 2 | 5 | 3 | 1 | 1 | 0 | 4 | 7 | 2 | 3 |
| Salinity | ||||||||||||||
| hb | 14 | 7 | 13 | 17 | 1 | 13 | 8 | 16 | 14 | 18 | 18 | 14 | 11 | 3 |
| i | 32 | 26 | 41 | 30 | 19 | 68 | 44 | 32 | 25 | 36 | 47 | 9 | 48 | 26 |
| hl | 4 | 4 | 4 | 2 | 6 | 12 | 3 | 2 | 5 | 3 | 8 | 2 | 7 | 3 |
| mh | 0 | 1 | 2 | 2 | 1 | 1 | 3 | 0 | 0 | 1 | 4 | 0 | 2 | 0 |
| eh | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| pH | ||||||||||||||
| acb | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 3 | 0 | 0 | 0 | 1 | 0 | 0 |
| acf | 17 | 9 | 12 | 15 | 4 | 13 | 12 | 18 | 11 | 18 | 14 | 12 | 9 | 4 |
| ind | 22 | 19 | 26 | 21 | 10 | 36 | 22 | 23 | 18 | 25 | 31 | 8 | 33 | 13 |
| alf | 11 | 8 | 17 | 11 | 14 | 42 | 22 | 10 | 13 | 14 | 28 | 5 | 23 | 15 |
| alb | 2 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Watanabe | ||||||||||||||
| sx | 11 | 6 | 11 | 9 | 3 | 15 | 13 | 7 | 8 | 8 | 15 | 5 | 10 | 6 |
| es | 7 | 6 | 6 | 3 | 5 | 16 | 10 | 6 | 9 | 10 | 13 | 1 | 9 | 7 |
| sp | 2 | 0 | 3 | 4 | 2 | 5 | 1 | 2 | 1 | 2 | 2 | 1 | 4 | 1 |
| Autotrophy-Heterotrophy | ||||||||||||||
| ats | 17 | 9 | 22 | 16 | 6 | 29 | 22 | 21 | 18 | 19 | 28 | 7 | 24 | 9 |
| ate | 6 | 4 | 5 | 8 | 4 | 15 | 7 | 8 | 6 | 10 | 9 | 4 | 7 | 9 |
| hne | 2 | 0 | 1 | 0 | 2 | 4 | 3 | 0 | 1 | 0 | 2 | 1 | 7 | 1 |
| hce | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Trophy | ||||||||||||||
| ot | 16 | 7 | 14 | 10 | 4 | 16 | 15 | 14 | 11 | 12 | 14 | 11 | 12 | 6 |
| om | 10 | 2 | 9 | 8 | 4 | 15 | 7 | 10 | 9 | 11 | 16 | 2 | 14 | 6 |
| m | 1 | 1 | 1 | 2 | 0 | 1 | 0 | 4 | 1 | 2 | 3 | 0 | 2 | 0 |
| me | 1 | 1 | 3 | 3 | 2 | 6 | 7 | 1 | 3 | 2 | 6 | 1 | 4 | 3 |
| e | 2 | 3 | 8 | 4 | 5 | 16 | 4 | 4 | 6 | 4 | 6 | 1 | 7 | 2 |
| o–e | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| he | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Class of Water Quality | ||||||||||||||
| Class 1 | 11 | 3 | 6 | 4 | 4 | 7 | 8 | 8 | 9 | 8 | 9 | 6 | 6 | 4 |
| Class 2 | 21 | 16 | 24 | 19 | 5 | 38 | 21 | 25 | 17 | 27 | 32 | 14 | 21 | 11 |
| Class 3 | 6 | 3 | 7 | 7 | 7 | 13 | 7 | 4 | 3 | 4 | 9 | 0 | 13 | 5 |
| Class 4 | 1 | 1 | 3 | 2 | 2 | 5 | 2 | 1 | 2 | 3 | 2 | 1 | 4 | 1 |
| Class 5 | 1 | 0 | 0 | 0 | 0 | 2 | 1 | 2 | 1 | 0 | 0 | 0 | 2 | 0 |
Note: 0, not found. Abbreviations: habitat (P—planktonic, P-B—plankto-benthic, B—benthic); temperature preferences (cool—cool water, temp—temperate, eterm—eurythermic, warm—warm water); oxygenation and streaming (st—standing water, str—streaming water, st-str—low streaming water, aer—aerophiles); pH preference groups (pH) according to Hustedt (1957) [69] (alb—alkalibiontes; alf—alkaliphiles, ind—indifferent; acf—acidophiles; neu—neutrophiles as a part of pH-indifferent taxa); salinity ecological groups according to Hustedt (1938–1939) [70,71] (hb—oligohalobes–halophobes, i— oligohalobes-indifferents, hl—halophiles; mh—mesohalobes, eh—euhalobes); self-purification zone with index of saprobity (x/0.0—xenosaprobe; x-o/0.4—xeno-oligosaprobe; o-x/0.6—oligo-xenosaprobe; x-b/0.8—xeno-betamesosaprobe; o/1.0—oligosaprobe; o-b/1.4—oligo-betamesosaprobe; x-a/0.55—xeno-to-alphamesosaprobe; b-o/1.6—beta-oligosaprobe; o-a/1.8—oligo-alphamesosaprobe; b/2.0—betamesosaprobe; b-a/2.4—beta-alphamesosaprobe; a-o/2.6—alpha-oligosaprobe; b-p/2.8—beta-polysaprobe; a/3.0—alphamesosaprobe; a-p/3.4—alpha-polysaprobe; a-b/3.6—alpha-betamesosaprobe; p-a/4.0—poly-alphamesosaprobe; i/>4.0—i-eusaprobe); organic pollution indicators according Watanabe et al. (1986) [72]: sx—saproxenes; es—eurysaprobes; sp—saprophiles; nitrogen uptake metabolism (Aut-Het) [16]: ats—nitrogen autotrophic taxa tolerating very small concentrations of organically bound nitrogen; ate—nitrogen-autotrophic taxa, tolerating elevated concentrations of organically bound nitrogen; hne—facultative nitrogen heterotrophic taxa needing periodically elevated concentrations of organically bound nitrogen; hce—obligate nitrogen heterotrophic taxa needing continuously elevated concentrations of organically bound nitrogen; trophic state indicators [16]: (ot—oligotraphentic; om—oligomesotraphentic; m—mesotraphentic; me—mesoeutraphentic; e—eutraphentic; he—hypereutraphentic; o–e—oligo-to-eutraphentic (hypereutraphentic)).
References
- Kelly, M.; Bennion, H.; Burgess, A.; Ellis, J.; Juggins, S.; Guthrie, R.; Jamieson, J.; Adriaenssens, V.; Yallop, M. Uncertainty in Ecological Status Assessments of Lakes and Rivers Using Diatoms. Hydrobiologia 2009, 633, 5–15. [Google Scholar] [CrossRef]
- Poikane, S.; Kelly, M.; Cantonati, M. Benthic Algal Assessment of Ecological Status in European Lakes and Rivers: Challenges and Opportunities. Sci. Total Env. 2016, 568, 603–613. [Google Scholar] [CrossRef]
- Bennion, H.; Sayer, C.D.; Tibby, J.; Carrick, H.J. Diatoms as Indicators of Environmental Change in Shallow Lakes. In The Diatoms: Applications for the Environmental and Earth Sciences; Cambridge University Press: Cambridge, UK, 2010; p. 686. [Google Scholar]
- Nõges, P.; Poikane, S.; Cardoso, A.C.; van de Bund, W. Water Framework Directive the Way to Water Ecosystems Sustainability in Europe. LakeLine 2006, 21, 36–43. [Google Scholar]
- Hofmann, G.; Werum, M.; Lange-Bertalot, H.; Lange-Bertalot, H. Diatomeen im Süßwasser-Benthos von Mitteleuropa: BestimmungsFlora Kieselalgen für die ökologische Praxis; Über 700 der Häufigsten Arten und Ihre Ökologie; Gantner: Ruggell, Germany, 2011; p. 908. (In German) [Google Scholar]
- Charles, D.F.; Kelly, M.G.; Stevenson, R.J.; Poikane, S.; Theroux, S.; Zgrundo, A.; Cantonati, M. Benthic Algae Assessments in the EU and the US: Striving for Consistency in the Face of Great Ecological Diversity. Ecol. Indic. 2021, 121, 107082. [Google Scholar] [CrossRef]
- Van Dam, H.; Mertens, A.; Sinkeldam, J. A coded checklist and ecological indicator values of freshwater diatoms from the Netherlands. Neth. J. Aquat. Ecol. 1994, 28, 117–133. [Google Scholar] [CrossRef]
- Pinseel, E.; Van de Vijver, B.; Kavan, J.; Verleyen, E.; Kopalová, K. Diversity, ecology and community structure of the freshwater littoral diatom flora from Petuniabukta (Spitsbergen). Polar Biol. 2017, 40, 533–551. [Google Scholar] [CrossRef]
- Picińska-Fałtynowicz, J. Freshwater benthic diatoms from the south-western part of the Hornsund fiord area, SW Spitsbergen. Polar Res. 1988, 6, 19–34. [Google Scholar] [CrossRef]
- Barinova, S.S. An assessment of the state of aquatic ecosystems of Novaya Zemlya Archipelago (Novozemel’skiy reserve, Russia). Int. J. Algae 2000, 2, 62–69. [Google Scholar] [CrossRef]
- Barinova, S.; Stenina, A. Diatom diversity and ecological variables in the Arctic lakes of the Kostyanoi Nos Cape (Nenetsky Natural Reserve, Russian North). Plant Biosyst.-Int. J. Deal. All Asp. Plant Biol. 2013, 147, 397–410. [Google Scholar] [CrossRef]
- Stenina, A.S. Diatoms (Bacillariophyta) in the Lakes of the East of the Bolshezemelskaya Tundra; Institute of Biology of Komi Scientific Centre of the Ural Branch of the Russian Academy of Sciences: Syktyvkar, Russia, 2009; p. 179. (In Russian) [Google Scholar]
- Kharitonov, V.G. Diatom algae of fresh water bodies. In Ecology of the Basin of the River Amguema; Institute of the Biological Problems of the North FEB RAS: Vladivostok, Russia, 1993; pp. 47–81. (In Russian) [Google Scholar]
- Kharitonov, V.G. Diatoms (Bacillariophyseae) in the Sediments of Three Mountain, Oligotrophic Lakes in the Amguema River Basin (Chukotka). Sib. Ecol. J. 2010, 4, 609–622. (In Russian) [Google Scholar]
- Kharitonov, V.G.; Genkal, S.I. Diatoms of Lake Elgygytgyn and Its Environs (Chukotka); SVNTs FEB RAS: Magadan, Russia, 2012; p. 402. (In Russian) [Google Scholar]
- Kopyrina, L.; Pshennikova, E.; Barinova, S. Diversity and ecological characteristic of algae and cyanobacteria of thermokarst lakes in Yakutia (Northeastern Russia). Oceanol. Hydrobiol. Stud. 2020, 49, 99–122. [Google Scholar] [CrossRef]
- Barinova, S.; Gabyshev, V.; Gabysheva, O. Climate impact of freshwater biodiversity: General patterns in extreme environments of North-Eastern Siberia (Russia). Br. J. Environ. Clim. Change 2014, 4, 423–443. [Google Scholar] [CrossRef]
- Barinova, S.; Gabyshev, V.; Boboev, M.; Kukhaleishvili, L.; Bilous, O. Algal Indication of Climatic Gradients. Am. J. Environ. Prot. 2015, 4, 72–77. [Google Scholar] [CrossRef]
- Gabyshev, V.; Ivanova, A.; Gabysheva, O. Long-Term Survey of Algae of the Lena Delta Wildlife Reserve including Data on Chemistry of Waters; Institute for Biological Problems of Cryolithozone, SB RAS: Yakutsk, Russia, 2021; Sampling event dataset Version 1.2. [Google Scholar] [CrossRef]
- Barinova, S.S.; Gabyshev, V.A.; Ivanova, A.P.; Gabysheva, O.I. Bioindication of the water salinity dynamics by the microalgae communities in the Lena River Delta, Laptev Sea, Russian Arctic. Mar. Biol. J. 2021, 6, 15–28. [Google Scholar] [CrossRef]
- Gabyshev, V.; Barinova, S.; Ivanova, A.; Gabysheva, O.; Curtean-Bănăduc, A. The Indicator Role of Algae in Assessing the Organic Pollution in the Lena River Delta, the Russian Arctic. Front. Environ. Sci. 2022, 10, 921819. [Google Scholar] [CrossRef]
- Gabysheva, O.I.; Gabyshev, V.A.; Barinova, S. Influence of the Active Layer Thickness of Permafrost in Eastern Siberia on the River Discharge of Nutrients into the Arctic Ocean. Water 2022, 14, 84. [Google Scholar] [CrossRef]
- Gabysheva, O.I.; Gabyshev, V.A.; Barinova, S.; Yakshina, I.A.; Pavlov, I.S. Influence of the Thickness of the Seasonally Thawed Layer of Permafrost in the Eastern Siberia Catchments on the Content of Organic Matter in River Waters. Hydrobiology 2022, 1, 243–251. [Google Scholar] [CrossRef]
- Bessudova, A.; Gabyshev, V.; Firsova, A.; Gabysheva, O.; Bukin, Y.; Likhoshway, Y. Diversity variation of silica-scaled chrysophytes related to differences in physicochemical variables in estuaries of rivers in an Arctic watershed. Sustainability 2021, 13, 13768. [Google Scholar] [CrossRef]
- Gvozdetsky, N.A.; Mikhailov, N.I. Physical Geography of the USSR (Asian Part), 3rd ed.; Mysl’: Moscow, Russia, 1978; p. 512. (In Russian) [Google Scholar]
- Grosswald, M.G.; Spektor, V.B. Glacial landforms of the Tiksi region (western coast of the Buor-Khaya Bay, Northern Yakutia). Geomorfologiya 1993, 1, 72–82. [Google Scholar]
- Spektor, V.B.; Shestakova, A.A.; Torgovkin, Y.I. New genetic types of landforms and deposits of the coastal part of the western sector of the Laptev Sea and the Lena River Delta. Arct. Subarct. Nat. Resour. 2021, 26, 81–93. [Google Scholar] [CrossRef]
- Konstantinov, P.Y.; Tregubov, O.D.; Zhang, M.; Li, G. Permafrost temperature dynamics over the last 30 years in certain regions of the Eastern sector of the Russian Arctic. Int. J. Appl. Sci. Technol. Integral 2022, 1, 260–267. (In Russian) [Google Scholar] [CrossRef]
- Reference Book on the Climate of the USSR. Issue. 24: Yakut ASSR. Part 2: Temperature of Air and Soil/Yakutsk Hydromete-Orological Observatory; Gidrometeoizdat: Leningrad, Russia, 1966; p. 398. (In Russian)
- Fedorov, A.N.; Botulu, T.A.; Varlamov, S.P.; Vasiliev, I.S.; Gribanova, S.P.; Dorofeev, I.V.; Klimovskiy, I.V.; Samsonova, V.V.; Soloviev, P.A. Permafrost Landscapes of Yakutia: Explanatory Note to the "Permafrost—Landscape Map of the Yakut ASSR" of Scale 1:2 500,000; GUGK: Novosibirsk, Russia, 1989; p. 176. (In Russian) [Google Scholar]
- Semenov, A.D. Guidance on the Chemical Analysis of Surface Waters of the Land; Gidrometeoizdat: Leningrad, Russia, 1977; p. 541. (In Russian) [Google Scholar]
- Balonov, I.M. Preparation of diatoms and golden algae for electron microscopy. In Methods for the Study of Biogeocenoses of Inland Waterbodies; Nauka: Moscow, Russia, 1975; pp. 87–89. (In Russian) [Google Scholar]
- Lange-Bertalot, H.; Moser, G. Brachysira: Monographie der Gattung; wichtige indicator-species für das Gewässer-Monitoring und Naviculadicta nov. gen.; ein Lösungsvorschlag zu dem Problem Navicula sensu lato onhe Navicula sensu stricto. Bibl. Diatomol. 1994, 29, 1–212. (In German) [Google Scholar]
- Krammer, K. Die cymbelloiden Diatomeen. Teil 1. Allgemeines und Encyonema part. Bibl. Diatomol. 1997, 36, 1–382. (In German) [Google Scholar]
- Krammer, K. Die cymbelloiden Diatomeen. Teil 2. Encyonema part. Encyonopsis und Cymbellopsis. Bibl. Diatomol. 1997, 7, 1–469. [Google Scholar]
- Krammer, K. Pinnularia. In Diatoms of Europe; Lange-Bertalot, H., Ed.; A.R.G. Gantner Verlag, K.G.: Ruggell, Liechtenstein, 2000; Volume 1, pp. 1–703. [Google Scholar]
- Krammer, K. Cymbella. In Diatoms of Europe; Lange-Bertalot, H., Ed.; A.R.G. Gantner Verlag, K.G.: Ruggell, Liechtenstein, 2002; Volume 3, pp. 1–584. [Google Scholar]
- Krammer, K. Cymbopleura, Delicata, Navicymbula, Gomphocymbellopsis, Afrocymbella. In Diatoms of Europe; Lange-Bertalot, H., Ed.; A.R.G. Gantner Verlag, K.G.: Ruggell, Liechtenstein, 2003; Volume 4, pp. 1–530. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae. Teil 1. Naviculaceae. In Die Süsswasserflora von Mitteleuropa; Spektrum Akademischer Verlag: Stuttgart, Germany, 1986; pp. 1–876. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae. Teil 2. Epithemiaceae, Bacillariaceae, Surirellaceae. In Die Süsswasserflora von Mitteleuropa; Spektrum Akademischer Verlag: Stuttgart, Germany, 1988; pp. 1–536. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae. Teil 3. Centrales, Fragilariaceae, Eunotiaceae. In Die Süsswasserflora von Mitteleuropa; Ettl, J.H., Gerloff, H., Eds.; Spektrum Akademischer Verlag: Stuttgart, Germany, 1991; bd. 2/3; pp. 1–576. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae. Teil. 4. Achnanthaceae, Kritische Erganzungen zu Navicula (Lineolatae) und Gomphonema. In Die Süsswasserflora von Mitteleuropa; Gustav Fisher Verlag: Stuttgart, Germany, 1991; pp. 1–437. [Google Scholar]
- Lange-Bertalot, H.; Genkal, S.I. Diatoms of Siberia. I. Iconogr. Diatomol. 1999, 6, 7–272. [Google Scholar]
- Reichardt, E. Zur revision der gattung Gomphonema. Iconogr. Diatomol. 1999, 8, 1–203. [Google Scholar]
- Lange-Bertalot, H. Navicula sensu stricto, 10 genera separated from Navicula sensu lato Frustulia. In Diatoms of Europe; Lange-Bertalot, H., Ed.; A.R.G. Gantner Verlag, K.G.: Ruggell, Liechtenstein, 2001; Volume 2, pp. 1–526. [Google Scholar]
- Werum, M.; Lange-Bertalot, H. Diatoms in springs from Central Europe and elsewhere under the influence of hydrogeology and anthropogenic impacts. Iconogr. Diatomol. 2004, 13, 1–417. [Google Scholar]
- Levkov, Z. Amphora sensu lato. In Diatoms of Europe; Lange-Bertalot, H., Ed.; A.R.G. Gantner Verlag, K.G.: Ruggell, Liechtenstein, 2009; Volume 5, pp. 1–916. [Google Scholar]
- Genkal, S.I.; Bondarenko, N.A.; Shchur, L.A. Diatoms of Lakes in the South and North of Eastern Siberia; OAO Rybinsk Press House: Rybinsk, Russia, 2011; p. 72. [Google Scholar]
- Lange-Bertalot, H.; Bak, M.; Witkowski, A. Eunotia and some related genera. In Diatoms of Europe; A.R.G. Gantner Verlag, K.G.: Ruggell, Liechtenstein, 2011; Volume 6, pp. 1–747. [Google Scholar]
- Levkov, Z.; Metzeltin, D.; Pavlov, A. Luticola, Luticolopsis. In Diatoms of Europe; Lange-Bertalot, H., Ed.; A.R.G. Gantner Verlag, K.G.: Ruggell, Liechtenstein, 2013; Volume 7, pp. 1–697. [Google Scholar]
- Levkov, Z.; Mitić-Kopanja, D.; Reichardt, E. The diatom genus Gomphonema from the Republik of Macedonia. In Diatoms of Europe; Lange-Bertalot, H., Ed.; A.R.G. Gantner Verlag, K.G.: Ruggell, Liechtenstein, 2016; Volume 8, pp. 1–552. [Google Scholar]
- Kulikovskiy, M.S.; Glushchtnko, A.M.; Genkal, S.I.; Kuznetsova, I.V. Identification Book of Diatoms from Russia; Filigran: Yaroslavl, Russia, 2016; p. 804. [Google Scholar]
- Lange-Bertalot, H.; Hofmann, G.; Werum, M.; Cantonati, M. Freshwater Benthic Diatoms of Central Europe; Koeltz Botanical Books: Schmitten-Oberreifenberg, Germany, 2017; p. 942. [Google Scholar]
- Beauger, A.; Allain, E.; Voldoire, O.; Blavignac, C.; Rossi, S.; Wetzel, C.; Ector, L. Gomphosphenia vallei (Bacillariophyta), a new diatom species from a stream in the “Réserve Naturelle Nationale de la Vallée de Chaudefour”, Massif Central (France). Phytotaxa 2022, 542, 167–179. [Google Scholar] [CrossRef]
- Guiry, M.D.; Guiry, G.M. AlgaeBase World-Wide Electronic Publication; National University of Ireland: Galway, Ireland. Available online: http://www.algaebase.org (accessed on 24 June 2019).
- Barinova, S. Essential and practical bioindication methods and systems for the water quality assessment. Int. J. Environ. Sci. Nat. Resour. 2017, 2, 555588. [Google Scholar] [CrossRef]
- Barinova, S.S.; Medvedeva, L.A.; Anissimova, O.V. Diversity of Algal Indicators in Environmental Assessment; Pilies Studio: Tel Aviv, Israel, 2006; p. 498. (In Russian) [Google Scholar]
- Barinova, S.S.; Bilous, O.P.; Tsarenko, P.M. Algal Indication of Water Bodies in Ukraine: Methods and Prospects; Publishing House of Haifa University: Haifa, Israel, 2019; p. 367. (In Russian) [Google Scholar]
- Love, J.; Selker, R.; Marsman, M.; Jamil, T.; Dropmann, D.; Verhagen, J.A.; Ly, A.; Gronau, F.Q.; Smira, M.; Epskamp, S.; et al. JASP: Graphical statistical software for common statistical designs. J. Stat. Softw. 2019, 88, 1–17. [Google Scholar] [CrossRef]
- Ter Braak, C.J.F.; Šmilauer, P. CANOCO Reference Manual and CanoDraw for Windows User’s Guide: Software for Canonical Community Ordination; Version 4.5; Microcomputer Power Press: Ithaca, NY, USA, 2002; p. 500. [Google Scholar]
- Michelutti, N.; Douglas, M.S.V.; Smol, J.P. Diatom response to recent climatic change in a high arctic lake (Char Lake, Cornwallis Island, Nunavut). Glob. Planet Change 2003, 38, 257–271. [Google Scholar] [CrossRef]
- Cremer, H.; Bennike, O.; Kansson, L.H.; Hultzsch, N.; Klug, M.; Kobabe, S.; Wagner, B. Hydrology and diatom phytoplankton of high arctic lakes and ponds on Store Koldewey, Northeast Greenland. Int. Rev. Hydrobiol. 2005, 90, 84–99. [Google Scholar] [CrossRef]
- Bielczyńska, A. Bioindication on the Basis of Benthic Diatoms: Advantages and Disadvantages of the Polish Phytobenthos Lake Assessment Method (IOJ—the Diatom Index for Lakes)/Bioindykacja Na Podstawie Okrzemek Bentosowych: Mocne i Słabe Strony Polskiej Metody Oceny Jezior Na Podstawie Fitobentosu (IOJ—Indeks Okrzemkowy Jezior). Ochr. Srodowiska I Zasobów Nat. 2015, 26, 48–55. [Google Scholar] [CrossRef]
- Kelly, M. Data Rich, Information Poor? Phytobenthos Assessment and the Water Framework Directive. Eur. J. Phycol 2013, 48, 437–450. [Google Scholar] [CrossRef]
- Skorobogatova, O.; Yumagulova, E.; Storchak, T.; Barinova, S. Bioindication of the Influence of Oil Production on Sphagnum Bogs in the Khanty-Mansiysk Autonomous Okrug–Yugra, Russia. Diversity 2019, 11, 207. [Google Scholar] [CrossRef]
- Snigirova, A.; Bogatova, Y.; Barinova, S. Assessment of River-Sea Interaction in the Danube Nearshore Area (Ukraine) by Bioindicators and Statistical Mapping. Land 2021, 10, 310. [Google Scholar] [CrossRef]
- Barinova, S.; Krupa, E.; Khitrova, Y. Phytoplankton Diversity and Bioindication of the Lakes in the Burabay National Natural Park, Northern Kazakhstan. Ecologies 2023, 4, 242–268. [Google Scholar] [CrossRef]
- Bănăduc, D.; Simić, V.; Cianfaglione, K.; Barinova, S.; Afanasyev, S.; Öktener, A.; McCall, G.; Simić, S.; Curtean-Bănăduc, A. Freshwater as a Sustainable Resource and Generator of Secondary Resources in the 21st Century: Stressors, Threats, Risks, Management and Protection Strategies, and Conservation Approaches. Int. J. Environ. Res. Public Health 2022, 19, 16570. [Google Scholar] [CrossRef]
- Hustedt, F. Die Diatomeenflora des Flußsystems der Weser im Gebiet der Hansestadt Bremen. Abh. Nat. Ver. Zu Brem. 1957, 34, 181–440. [Google Scholar]
- Hustedt, F. Systematisch und Ökologische Untersuchungen über die Diatomeenflora von Java, Bali und Sumatra; Archiv für Hydrobiologie, Suppl.; E. Schweizerbart’sche Verlagsbuchhandlung: Stuttgart, Germany, 1938; Volume 15, pp. 131–177, 393–506, 638–790. [Google Scholar]
- Hustedt, F. Systematisch und Ökologische Untersuchungen über die Diatomeenflora von Java, Bali und Sumatra; Archiv für Hydrobiologie, Suppl.; E. Schweizerbart’sche Verlagsbuchhandlung: Stuttgart, Germany, 1939; Volume 16, pp. 1–155, 274–394. [Google Scholar]
- Watanabe, T.; Asai, K.; Houki, A. Numerical estimation of organic pollution of flowing water by using the epilithic diatom assemblage—Diatom Assemblage Index (DAIpo). Sci. Total Environ. 1986, 55, 209–218. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).