Abstract
Selenium pollution in water is a worldwide issue. Se(IV) and Se(VI) are mainly found in contaminated water due to their high solubility and mobility; their presence poses a serious risk as they can severely harm human health. Although iron oxide and hydroxide nanoparticles can be efficient candidates for the removal of selenium oxyanions due to their high adsorption capacity, the role of each iron species has not been fully elucidated. Furthermore, iron species are often found to be less effective for Se(VI) than Se(IV). The challenge and novelty of this study was to develop a carbon material impregnated with different iron phases, including oxides (magnetite/hematite) and hydroxides (goethite/lepidocrocite) capable of removing both Se(IV) and Se(VI). Since the phase and morphology of the iron nanoparticles play a significant role in Se adsorption, the study evaluated both characteristics by modifying the impregnation method, which is based on an oxidative hydrolysis with FeSO4 7H2O and CH3COONa, and the type of carbonaceous support (activated carbon or sucrose-based carbon foam). Impregnated activated carbons provide better removal efficiencies (70–80%) than carbon foams (<40%), due to their high surface areas and point zero charges. These results show that the adsorption of Se(VI) is more favorable on magnetic oxides (78%) and hydroxides (71%) than in hematite (<40%). In addition, the activated carbon decorated with magnetite showed a high adsorption capacity for both selenium species, even in alkaline conditions, when the sorbent surface is negatively charged. A mechanism based on the adsorption of inner-sphere complexes was suggested for Se(IV) immobilization, whereas Se(VI) removal occurred through the formation of outer-sphere complexes and redox processes.
1. Introduction
Selenium is an essential micronutrient for living creatures. However, it is toxic at high concentrations, causing significant health and environmental concerns [1,2]. The dual effect of Se (positive or toxic) depends on Se speciation and varies between different species and organisms [3,4,5]. The presence of high concentrations of Se in the aquatic environment is of particular concern due to its tendency to bioaccumulate through the food chain [6,7].
The main anthropogenic sources of Se in the environment are metal smelting industries, mine drainages, selenium-containing pesticides and fungicides, and agricultural irrigation runoffs [8,9,10,11]. Selenium is a metalloid that can exist in four oxidation or ionic states in the environment: selenate (SeO42−), selenite (SeO32−), selenide (Se2−), and elemental selenium (Se0). The more mobile and toxic species, Se(VI) and Se(IV), are mostly found in contaminated water, with Se(VI) being the species of major concern due to its higher solubility and bioavailability.
The speciation of Se in water is controlled by redox conditions, pH, the accessibility of absorbent surfaces, and biological processes that occur in the water to be treated [2]. In water and wastewater treatment, speciation is a key factor, since the efficiency of the method usually depends on the oxidation state.
Technologies used in the treatment of wastewater with selenium include membrane filtration, ion exchange, coagulation–flocculation precipitation, electrocoagulation, and adsorption [5,12,13,14]. These methods have disadvantages such as high cost, operational complexity, and large volumes of Se-containing sludge resulting from post-treatment [12]. Alternatively, adsorption is currently receiving great attention because it is considered a promising technique for industrial application in terms of its high efficiency, low cost of implementation, and easy operational design [15,16].
Numerous adsorbents have been synthesized and evaluated for Se adsorption from aqueous media [17]. The adsorbents can be classified into different groups depending on the material used for their preparation [17]. The preparation of adsorbents from industrial by-products/wastes, agricultural/food residues and biochars has recently undergone significant growth [18,19,20]. However, these materials can present problems such as low adsorption capacity and coloration in water if they are not previously treated/modified. Another group of materials widely used in wastewater treatment is that of the carbon-based adsorbents, due to their high surface areas and low cost. Graphene oxide composites have been found to be effective for Se adsorption from aqueous media, due to the large number of oxidized functional groups that they can possess [21,22]. On the other hand, activated carbons are undoubtedly the most used adsorbents for water purification. However, they may present low efficiencies for some inorganic species such as Se(VI) and high costs for production and regeneration [12]. The capacity for Se(VI) and Se(IV) removal may be enhanced when activated carbons are supported with Fe species [23,24,25]. Despite the adsorbents mentioned above, and others such as metal-organic frameworks, zeolites, and amine-based adsorbents [16,26,27,28,29], it should be emphasized that more than 50% of the adsorbents developed for Se adsorption are Fe-based adsorbents [17], and that the affinity of Fe oxides/hydroxides for Se oxyanions is well known [25,30,31]. However, these materials may present some drawbacks such as low surface areas and incomplete availability of the active centers. Iron oxides/hydroxides such as magnetite (Fe3O4), hematite (α-Fe2O3), maghemite (γ-Fe2O3) and goethite (α-FeOOH) are the most extensively studied [32,33,34,35,36]. The interaction between iron oxides/hydroxides and Se oxyanions is attributed to the formation of Lewis acid–base complexes and, hence, is pH-dependent. In most cases, the sorption process responds to pseudo-second order kinetics and Langmuir isotherms [17]. In general, iron oxides have been found to be more effective for the removal of Se(IV) than of Se(VI), due to the formation of inner-sphere complexes [12,23,32,37]. Iron species play an essential role in Se(VI) adsorption. The formation of inner-sphere complexes was identified as the main mechanism of Se(VI) adsorption on hematite, whereas both outer- and inner-sphere complexes may be formed on goethite and hydrous ferric oxides depending on pH [30,31,32,37]. Although Se(VI) has been reported to be adsorbed more strongly onto iron oxy-hydroxides, such as ferrihydrite and goethite [31], than iron oxides, such as hematite [23], the mechanism of Se’s adsorption by different iron species is not yet fully understood.
In summary, the treatment of water and wastewater for Se adsorption poses a notable challenge for the scientific community. Although iron-based adsorbents represent a promising method for Se removal, there are still problems to be solved such as the poor efficiency of Se(VI) adsorption, the role of iron composition, and their use in continuous flow, which urgently call for a cost-effective, technologically viable, and environmentally sustainable method. The challenge of this study is to combine the advantages of carbon materials and iron oxides to give rise to a new generation of materials capable of effectively retaining Se.
Because surface complexation and Se(VI) and Se(IV) adsorption vary depending on iron composition, the objective of this study was to quantify and compare the adsorption capacity of Se(VI) and Se(IV) in two carbon supports, an activated carbon and a sucrose-based carbon foam impregnated with different iron species which has been little studied until now. This study provides new insights into the critical role of different iron species supported on carbon materials for Se adsorption in aqueous media. The impact of Se concentration, dose, and pH were evaluated, and the results of this study will be used in particular to determine the applicability of Fe oxides/hydroxides-based composites to attenuating the most mobile Se species (Se(VI)) under different conditions. Furthermore, the present study is intended to further our scientific knowledge by clarifying adsorption mechanisms.
2. Materials and Methods
2.1. Materials
Two materials were selected as supports for iron oxides/hydroxides: an activated carbon (AC) and a sucrose-based carbon foam (SF). The AC was the commercial activated carbon Norit RB3. The SF was elaborated using commercial sucrose as a precursor and Fe(NO3)3 · 9H2O (0.3 wt% Fe) as a foaming enhancer and activating agent. The preparation of SF involves the preparation of caramel by heating sucrose with an additive up to 170 °C, foaming the caramel at 150 °C (2.5 h) and 250 °C (3 h), and carbonizing the green foam under Ar flow at 800 °C for 2 h. SF was then subjected to an oxidative treatment by means of a 2 M solution of (NH4)2S2O8 in H2SO4 (1 M) during 5 h under reflux. All the chemicals used in this study were of analytical grade. The resultant foam (SFox) was collected via filtration and washed with abundant Milli-Q water. Both the sucrose foam and the activated carbon were ground to a size of 0.2–0.5 mm.
Finally, AC and SFox were impregnated with 10 wt% Fe, using a solution of iron sulfate heptahydrate (FeSO4 · 7H2O) and sodium acetate (CH3COONa) in MilliQ water under reflux for 2 h. With the aim of obtaining different phases and morphologies, several thermal treatments were performed over the reaction mixture. For methods 1 and 2, the impregnation step was carried out at 100 and 175 °C, respectively. In method 3, the sample obtained using method 1 was subjected to an additional thermal treatment at 300 °C in a muffle, for 2 h. The samples prepared were designated XFe-y, where X is the carbon support (AC or SFox) and y refers to the impregnation method (1, 2 or 3).
2.2. Characterization of the Carbon Materials
The specific surface area of the carbon supports was determined by the standard BET method, using N2 adsorption data. The total pore volume, Vt, was obtained from the amount of N2 adsorbed at a relative pressure of 0.975, and the micropore volume, Vmicro, was determined by fitting the Dubinin–Radushkevich (DR) equation to the N2 adsorption isotherm. The mesopore volume, Vmeso, was calculated as the difference between Vt and Vmicro. The samples were outgassed at 120 °C overnight before analysis. The macroporous texture of the carbon foams was determined via Hg intrusion analysis operating at a maximum pressure of 227 MPa.
Elemental analysis was carried out using a LECO CHN-2000 for C, H and N, and a LECO VTF-900 for direct oxygen determination.
The surface chemistry of the samples was studied by temperature programmed desorption (TPD) experiments, performed on a chemisorption analyzer equipped with a mass spectrometer. The samples were heated up to 1000 °C with a heating rate of 10 °C min−1 under Ar flow (50 mL min−1), recording the amounts of CO and CO2 released during the thermal analysis.
The pH was measured using a Seven Multi pHmeter (Mettler Toledo). The point of zero charge (pHpzc) of the adsorbents was determined following the pH drift test [38].
The distribution and morphology of the iron nanoparticles was studied via scanning electron microscopy (SEM), whereas the iron species were identified using X-ray diffraction (XRD). The lattice parameter ao was calculated from XRD pattern using EVA software (http://www.evasoftwaresolutions.com/) in the (311) reflection.
2.3. Batch Adsorption Experiments
Selenate and selenite stock solutions were prepared separately via dilution of 1000 mg L−1 of Na2SeO4 and Na2SeO3, respectively, to obtain working solutions of various concentrations. In this study, an initial Se concentration of 25 mg L−1 has been chosen to evaluate the effect of adsorbent dose and pH [39]. It must be taken into account that the concentration of Se in water can reach 100 mg L−1 in some industrial activities due to concentration processes. The effect of the dose on adsorption of Se(VI) and Se(IV) was evaluated with 1, 5 and 10 g L−1, whereas the effect of pH was evaluated at 4, 7 and 9. The initial pH of the solution was adjusted by adding HCl or NaOH (0.1 M). The concentrations of Se evaluated were 5, 10 and 25 mg L−1. All the experiments were conducted using glass bottles of 50 mL capacity. Mixing was maintained using a temperature-controlled orbital shaker at ambient temperature and a constant shaking speed of 100 rpm. The collected samples were filtered using 0.45 µm nylon syringe filter and further stored at 4 °C prior to analysis by inductively coupled plasm mass spectrometry (ICP-MS, Agilent 7700x, Santa Clara, CA, USA). All the experiments and analysis were conducted by duplicate. The overall error, expressed as relative standard deviation, for each observation was lower than 5%.
2.4. Adsorption Kinetics and Isotherms
The kinetics of the adsorption of Se(IV) and Se(VI) by Fe-loaded carbon materials were evaluated by fitting the experimental data to pseudo-first- and pseudo-second-order kinetic models that are characterized by Equations (1) and (2) [40]. The selection of these models was based on previous results reported for selenium remediation with metal oxides [15,30,41].
Pseudo-first-order equation:
Pseudo-second-order equation:
where, qt is the amount of adsorbate adsorbed at time t, qe is the amount adsorbed at equilibrium, and k1 and k2 are the adsorption constants of the pseudo-first and pseudo-second order, respectively.
Langmuir and Freundlich adsorption isotherm models were considered in this work to study the relationship between the adsorption capacity of Se(IV) and Se(VI) on Fe-loaded carbon materials and the equilibrium concentration. The models are defined by the following equations [40]:
Langmuir equation:
Freundlich equation:
where Qm is the maximum adsorption capacity, Ce is the concentration at equilibrium, KL is the Langmuir constant representing the energy of adsorption, and KF and 1/n are the Freundlich constants representing the adsorption capacity and the intensity of adsorption, respectively.
3. Results and Discussion
3.1. Characteristics of the Carbon Supports
The values of surface area (SBET), total pore volume (Vt), micropore volume (Vmicro) and mesopor volume (Vmeso) of the raw activated carbon and the sucrose foam are presented in Table 1. AC is a typical microporous material with a high BET surface area (1183 m2 g−1). Unlike activated carbons, carbon foams are 3D materials that are essentially microporous (Figure S1). Therefore, the potential use of carbon foams in monolithic form can be beneficial for fixed-bed applications. As can be seen in Figure S2, the morphology of support SFox consists of a network made of open spherical cells connected by macropores with sizes between 30–500 µm. The total volume of macropores determined in the ground fraction was 0.6 cm3 g−1, with pore sizes between 25–5 µm. In addition, the use of iron nitrate during foaming promoted the development of a small proportion of meso and micropores, reaching a SBET of 214 m2 g−1 (Table 1).

Table 1.
Textural and chemical properties of carbon supports AC and SFOX.
The presence of oxygen functionalities on the surface of the carbon supports can enhance the anchoring of iron oxides and hence promotes the interactions between the adsorbent and Se. Both samples present a moderate oxygen content, 14.2% for AC and 10.3% for SFox (Table 1). However, their CO and CO2 desorption profiles obtained by TPD present certain differences that are worth noting (Figure 1). For AC, the main release of CO took place at around 800 °C, which corresponds to basic compounds such as carbonyls and/or quinone-like structures [42], whereas for SFox, CO evolved at lower temperatures (<800 °C), which suggests the presence of phenols. In addition, SFox shows a sharp peak at 650 °C in both CO and CO2 profiles, which corresponds to mineral matter decomposition and may come from the iron compounds incorporated into the foam structure, as a consequence of the iron nitrate used as foaming agent [43]. On the other hand, CO2 profiles are quite similar for both samples. The release below 400 °C can be attributed to carboxylic acids and above 400 °C to carboxylic anhydrides and lactones.
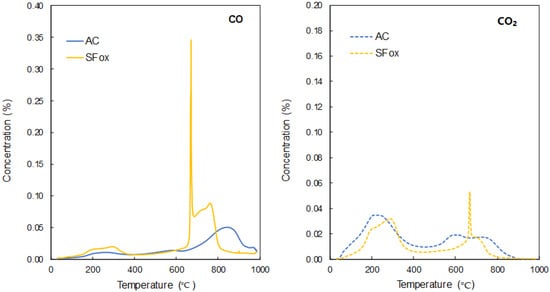
Figure 1.
Variation of the concentration of the evolved gases (CO and CO2) during the TPD analysis of AC and SFox supports.
It is worth mentioning that impregnation with iron oxides/hydroxides does not produce significant changes in the textural parameters of the supports. A reduction of around 10% in BET surface area and micro/mesopore volumes was observed compared to the parent supports. Furthermore, the macroporosity of the carbon foam was not affected by the impregnation treatment. However, its surface chemistry changed drastically after impregnation. Thus, for AC, the pHPZC shifted from a basic value of 9.8 to 6.3, while for SFox, it varied from 6.1 to an acid value (4.3).
As can be seen in Figure 2, the iron deposited in sample ACFe-1 presents heterogeneous morphologies (nanosheets horizontally aligned, nanorods, and octahedral particles), which agree with the different phases found by XRD (Figure 3). The main XRD peaks are identified as iron oxides with a spinel structure (magnetite (Fe3O4)/maghemite (γ-Fe2O3)). Due to its similar crystal structure, magnetite and maghemite have similar XRD patterns, although their lattice parameters are slightly different. The lattice constant of γ-Fe2O3 (0.8346 nm) is slightly smaller than that of Fe3O4 (0.8396 nm), so this parameter can be used to discern the nature of the iron oxide present in the sample. The lattice parameter calculated for ACFe-1 was 0.340 nm, which indicates that the iron oxide phase is magnetite. A mixture of iron hydroxides (goethite (α-FeOOH)/lepidocrocite (γ-FeOOH)) are also found, but in a minor proportion (Figure 3). When the same impregnation method was performed over sample SFox, the iron phase changed significantly. For sample, in SFoxFe-1, the iron phase identified by XRD corresponds to hematite (α-Fe2O3), along with a small amount of goethite (Figure 3). Moreover, SEM images show a main morphology consisting of spherical particles made up of stacked nanosheets and a secondary morphology of thin nanorods (Figure 2d). These findings suggest that the chemical structure of the carbon support may play a significant role in the phase and morphology of the iron deposited. Thus, phenols groups seem to promote hematite deposition, whereas more basic groups lead to magnetite. On the other hand, for the same AC sorbent, the iron compound deposited can be modified through the impregnation method: ACFe-2 presents thin nanorod structures on its surface, corresponding to goethite/lepidocrocite (Figure 2b). Therefore, a decrease in the impregnation temperature favors the formation of iron hydroxides instead of oxides. In addition, further heating of sample ACFe-1 in an air atmosphere at 300 °C transforms the iron compounds into hematite (Figure 3). SEM images also show a rearrangement of the nanorods on sample ACFe-3, leading to flower-like structures (Figure 2c).
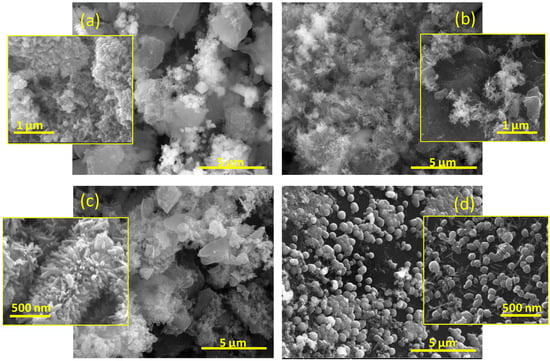
Figure 2.
SEM images of iron-impregnated carbons: (a) ACFe-1, (b) ACFe-2, (c) ACFe-3 and (d) SFoxFe-1.
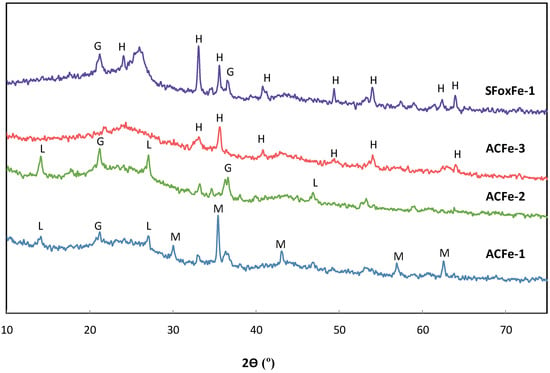
Figure 3.
XRD patterns of iron-impregnated carbon materials: ACFe-1, ACFe-2, ACFe-3 and SfoxFe-1 (H: hematite; G: goethite; L: lepidocrocite; M: magnetite).
3.2. Effect of Iron Composition
As pH is one of the main factors affecting Se speciation in water, and therefore, the chemistry of the solution and the redox potential [44], batch experiments were carried out at a particular pH value (Figure 4) to evaluate the role of iron composition.
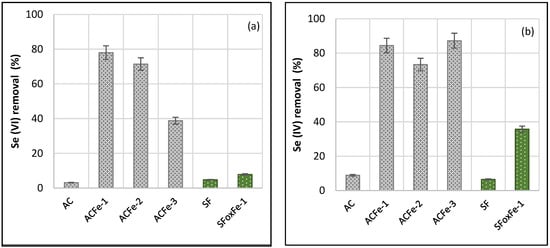
Figure 4.
Effect of iron composition on the removal of (a) Se(VI) and (b) Se(IV), using AC, ACFe-1 (goethite, lepidocrocite and magnetite), ACFe-2 (goethite, lepidocrocite), ACFe-3 (hematite), SF and SFoxFe-1 (goethite, hematite) (initial concentration, 25 mg L−1; pH, 4; dose, 10 g L−1 at 25 °C).
As can be deduced from Figure 4, impregnated AC supports showed better performance for both Se(VI) and Se(IV). This fact can be attributed to their better textural parameters and its pHPZC. AC showed a surface area of 1183 m2 g−1 and a micropore volume of 0.45 cm3 g−1, whereas SF showed 214 m2 g−1 and 0.09 cm3 g−1 (Table 1). The pHPZC of a material is the pH value at which the net charge on its surface is zero, in such a way that the surface will be positively charged at pH values below pHPZC and negatively charged above pHPZC [38]. Several studies have demonstrated that the pHPZC values of carbon materials decorated with iron oxides may vary depending on the synthesis method and the size distribution [45]. The value of pHPZC for sample ACFe-1 is approximately 5.3, which means that at pH 4 the surface will be positively charged, promoting the uptake of anions via electrostatic interactions. By contrast, sample SFoxFe-1 has a lower pHPZC (4.3). This value is very close to the pH, so a decrease in the electrostatic interactions is expected, as is, therefore, a lower Se adsorption (Figure 4). The higher Se adsorption capacity obtained with ACFe as compared with SFoxFe also suggests that magnetic oxides (Figure 3) favor Se removal as a consequence of the presence of carbonyls and/or quinone-like structures in AC supports (Figure 1). On the contrary, the presence of phenolic groups in SFox favored the formation of hematite, so the lower adsorption capacity of SFoxFe-1 for Se(VI) can also be attributed to this fact (Figure 4).
Comparing the different iron compounds present in support AC, it can be seen in Figure 4a that samples ACFe-1 and ACFe-2 are the most effective adsorbents for Se(VI) removal, achieving removal percentages of 78 and 71%, respectively. For sample ACFe-3, efficiency drops to values below 40%. These results suggest that the adsorption of Se(VI) is more favorable on magnetic oxides (ACFe-1) and hydroxides (ACFe-2) than in hematite (ACFe-3) (Figure 2 and Figure 3), i.e., with a distribution in the form of horizontally aligned nanosheets, nanorods, and octahedral particles (Figure 2a).
In the case of Se(IV), similar removal percentages were obtained for all the adsorbents derived from AC, being higher than 80% for samples with iron oxides (ACFe-1 and ACFe-3) and around 70% for the sample with iron hydroxides (ACFe 2).
Considering the results obtained, subsequent experiments have been carried out with ACFe-1 supports, which showed the highest removal efficiency for both Se(VI) and Se(IV). Furthermore, the magnetic properties of the iron oxides deposited on ACFe-1 will facilitate its recovery (Figure S3).
3.3. Effect of Adsorbent Dosage, pH and Initial Concentration
The experiments were performed with an initial concentration of 25 mg L−1 over a period of 72 h. The effect of dosage was studied at pH 4, 7 and 9 (Figure 5). The removal percentage of both Se(VI) and Se(IV) increases with the increasing ACFe-1 dose, which means a greater surface area and a greater number of available active adsorption sites. For an ACFe-1 dose of 10 g L−1, removal percentages of 66–74% of Se(VI) were achieved, depending on pH, whereas for Se(IV), a constant removal percentage of 85% was achieved. The adsorption of both Se(VI) and Se(IV) drops significantly at a dose of 1 g L−1.
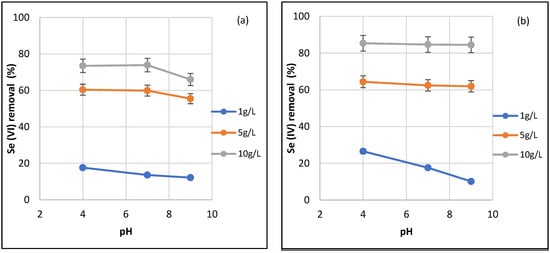
Figure 5.
Effect of adsorbent dosage at pH 4, 7 and 9 on the removal of (a) Se(VI) and (b) Se(IV) using ACFe-1 (initial concentration: 25 mg L−1 at 25 °C).
It is well known that the adsorption of inorganic Se species onto Fe oxides/hydroxides occurs mainly through electrostatic interaction between the negatively charged Se oxyanions and the partially protonated groups of the Fe oxides/hydroxides depending on pH [15]. In general, the adsorption of Se is favored by acidic pH due to an increase in the positive charge density of the adsorbent surface at lower pH [30]. From the results obtained (Figure 5), alkaline conditions seem to be less favorable for the adsorption of Se(VI) than for Se(IV). However, it should be mentioned that the efficiency of Se(IV) adsorption on ACFe-1 was unaffected by the change in pH, except for the dose of 1 g L−1 (Figure 5b). The immobilization of Se(IV) in iron oxides and hydroxides is attributed to the formation of inner-sphere complexes, and recent works have demonstrated that Se(IV) can be immobilized by magnetite/maghemite through the formation of inner-sphere complexes, even in alkaline conditions (pH > 10), wherein the iron oxide particle is negatively charged [46]. Therefore, the presence of these inner-sphere complexes between the solid surface and Se(IV) could explain the removal efficiency of sorbent ACFe-1 over the entire pH range. In addition, Se(IV) adsorption at acidic pH could also occur via dissolution of the magnetite, leading to the formation of Se–Fe aqueous species and further complexation of these species [47]. In the case of Se(VI), removal efficiencies of 60 and 75% were obtained at pH 4 and 7 with 5 and 10 g L−1, respectively (Figure 5a). Several studies have reported a significant decrease in Se(VI) adsorption onto goethite and/or hematite at pH > 4 [30,37], but magnetite and maghemite can be effective up to neutral pH [46]. The Se(VI) adsorption on ACFe-1 could occur through the following mechanisms: (1) the formation of outer-sphere complexes, which takes place up to pH 7, and (2) a redox process at pH ≥ 9, which involves the reduction of Se(VI) to Se(IV) and the subsequent formation of inner-sphere complexes, or the adsorption of a more reduced species (Se(0)) [23,48,49]. This mechanism based on reduction–adsorption processes has been confirmed under basic conditions (pH 8–9) for Se(VI) immobilization using green rust sulphate, a mixed Fe(II)–Fe(III) hydroxide compound [50].
The percentage of removal for various initial concentrations of Se(VI) and Se(IV) with a constant dose of ACFe-1 of 10 g L−1 and pH 4 is shown in Figure 6. The capacity of Se(VI) and Se(IV) adsorption increased from 75 to 87% and from 85 to 96%, respectively, when the initial concentration of Se decreased from 25 to 5 mg L−1. Therefore, shorter times are expected to achieve steady-state equilibrium concentration for an initial concentration of Se < 25 mg L−1.
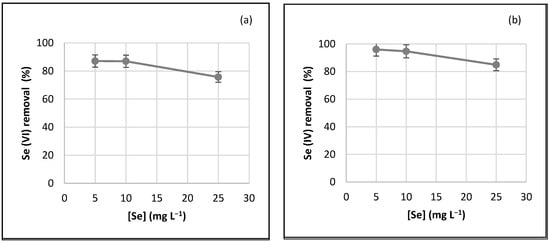
Figure 6.
Effect of the initial concentration on the removal of (a) Se(VI) and (b) Se(IV) using ACFe-1 (adsorbent dosage, 10 g L−1; pH 4 at 25 °C).
3.4. Kinetics of Adsorption
Figure 7 shows the kinetic results of Se(VI) and Se(IV) removal using ACFe-1 with an initial concentration of 25 mg L−1 and 10 g L−1 at pH 4, 7 and 9 over a period of 80–96 h. ACFe-1 attained an equilibrium within a time period of 32 h, with a removal percentage ranging from 70–85% for both Se(VI) and Se(IV). No significant reduction in Se adsorption was observed after prolonged residence time or increasing pH.
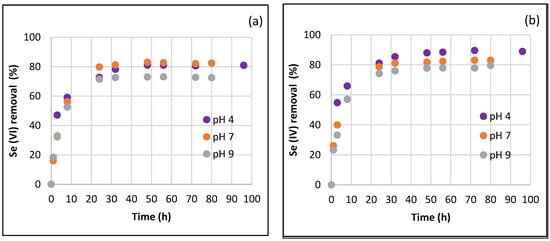
Figure 7.
Kinetics of (a) Se(VI) and (b) Se(IV) removal using ACFe-1 (initial concentration, 25 mg L−1; 10 g L−1; pH 4, 7 and 9 at 25 °C).
The graphical correlations of these results with pseudo-first-order and pseudo-second-order kinetic models are displayed in Figures S4–S9. In general, pseudo-second-order kinetics characterized Se(VI) and Se(IV) removal kinetics for ACFe-1 in all the pHs studied. This observation is corroborated by Table 2, which lists the values of the kinetic constants and the correlation parameters obtained with the two kinetic models considered. The values of the correlation parameter (R2) corresponding to the pseudo-second-order model are sensibly better, indicating that the adsorption rate is not determined by the concentration of adsorbate in the solution and that the rate-limiting step is the capacity of chemisorption on the surface of the adsorbent material [40].

Table 2.
Kinetic constants and correlation parameters of the adsorption of Se(IV) and Se(VI) by ACFe-1 at different pH conditions, corresponding to pseudo-first and pseudo-second order models.
3.5. Adsorption Isotherms
The correlations of the adsorption data corresponding to the experiments carried out for Se(VI) and Se(IV) using ACFe-1 at pH 7 with the Langmuir and Freundlich models are displayed in Figure 8. Similar correlations can be observed with the two models for both Se species. To gain a more accurate insight, the values of the model constants and correlation parameters are compared in Table 3. The Langmuir model provides the best values of the correlation parameter (R2), suggesting the homogenous distribution of energetically equivalent adsorption sites in the surface of ACFe-1 for both Se(VI) and Se(IV), and with each site adsorbing only one molecule of adsorbate [15,40]. The theoretical maximum adsorption capacity Qm is higher in the case of Se(VI). On the other hand, the less than 1 value of 1/n obtained using the Freundlich isotherm model suggests a non-cooperative sorption process [41].
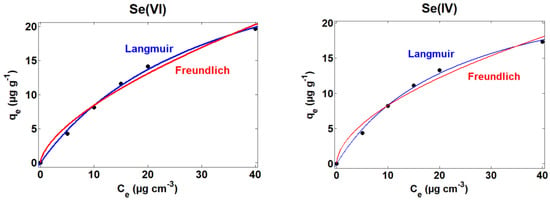
Figure 8.
Correlation of the adsorption data corresponding to Se(VI) and Se(IV), using ACFe-1 at pH 7 with the Langmuir and Freundlich isotherm models.

Table 3.
Isotherm constants and correlation parameters of the adsorption of Se(IV) and Se(VI) by ACFe-1 at pH 7, corresponding to the Langmuir and Freundlich isotherm models.
4. Conclusions
The adsorption of Se(VI) and Se(IV) varied depending on the Fe species deposited on the carbon material used as a support. Therefore, the challenge of this work was to better understand the role of Fe species via deposition of different iron oxides/hydroxides on two types of carbonaceous supports. Special emphasis was placed on Se(VI), the most mobile and difficult to retain in water. The morphology and composition of the Fe nanoparticles deposited on the supports depended on the chemical characteristics of the carbon material and the experimental conditions of the impregnation method, mainly the thermal treatment. The basic oxygen functionalities present in the activated carbon led to magnetite deposition, whereas the acidic groups of the carbon foam promoted hematite formation. In addition, the chemical and textural properties, the high surface area, and the micropore volume of the activated carbon and its pHPZC were more favorable to Se adsorption than those of the carbon foam (<40%). Efficiencies higher than 70% were obtained with a dose of 10 g L−1, using the activated carbon as the support and magnetite as the iron phase, with the highest number of active centers. The deposition of this phase was effective for both Se(VI) and Se(IV). Adsorption of Se(VI) and Se(IV) by ACFe-1 was characterized by a pseudo-second-order kinetic model, with better correlations obtained with the latter, suggesting the importance of chemisorption as the rate-limiting step. The Langmuir isotherm model provided the best correlations for the adsorption of both selenium species at pH 7, suggesting the homogenous distribution of energetically equivalent adsorption sites on ACFe-1. The adsorption of Se(IV) could involve the formation of inner-sphere complexes with magnetite particles, whereas Se(VI) adsorption involved different processes depending on pH, i.e., adsorption of outer-sphere complexes in contact with magnetite or redox reaction. The electrostatic attraction between Se species and the developed material did not decrease under neutral pH conditions, which would allow its implementation within a wider pH range. Although it would be premature to perform a comparative cost analysis between adsorbents at the level of development of this study, it is worth highlighting that the carbon materials developed in this study were prepared using both foaming processes and low-cost precursors. Based on the results obtained in this preliminary study carried out on synthetic waters, further research is focused on the applicability of Fe-based carbon materials for wastewater treatments, the use of surface complexation models to predict and corroborate the adsorption mechanisms, and the influence of other anions present in the water.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/w15193499/s1, Figure S1: Structure of AC and SF, highlighting the microporous and macroporous structure, respectively. Figure S2: SEM images of SF; Figure S3: The magnetic property of ACFe-1.; Figure S4: Removal of Se(IV) using ACFe-1 (initial concentration, 25 mg L−1; 10 g L−1; pH 4, 25 °C). Correlation with pseudo-first-order and pseudo-second-order kinetic models; Figure S5: Removal of Se(IV) using ACFe-1 (initial concentration, 25 mg L−1; 10 g L−1; pH 7, 25 °C). Correlation with pseudo-first-order and pseudo-second-order kinetic models; Figure S6: Removal of Se(IV) using ACFe-1 (initial concentration, 25 mg L−1; 10 g L−1; pH 9, 25 °C). Correlation with pseudo-first-order and pseudo-second-order kinetic models; Figure S7: Removal of Se(VI) using ACFe-1 (initial concentration, 25 mg L−1; 10 g L−1; pH 4, 25 °C). Correlation with pseudo-first-order and pseudo-second-order kinetic models; Figure S8: Removal of Se(VI) using ACFe-1 (initial concentration, 25 mg L−1; 10 g L−1; pH 7, 25 °C). Correlation with pseudo-first-order and pseudo-second-order kinetic models; Figure S9: Removal of Se(VI) using ACFe-1 (initial concentration, 25 mg L−1; 10 g L−1; pH 9, 25 °C). Correlation with pseudo-first-order and pseudo-second-order kinetic models.
Author Contributions
Conceptualization, Investigation, L.L.-T.; Investigation, Supervision, Writing—Review and Editing, E.R.; Formal analysis, Writing—Review and Editing, R.G.; Resources, Funding acquisition, M.R.M.-T.; Writing—Original Draft, Supervision. Project administration, Funding acquisition, M.A.L.-A. All authors have read and agreed to the published version of the manuscript.
Funding
The authors acknowledge the funding support from Ministerio de Ciencia e Innovación del Gobierno de España through the project PID2020-113558RB-C43 (MCIN/AEI/10.13039/501100011033) and Gobierno del Principado de Asturias through the project IDI/2021/000031. The authors are grateful for the funding received for the hiring of Lucía López-Toyos through Programa Investigo, Plan de Recuperación, Transformación y Resiliencia, Ministry of Labor and Social Economy (Government of Spain).
Data Availability Statement
Data will be made available on request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lenz, M.; Lens, P.N.L. The Essential Toxin: The Changing Perception of Selenium in Environmental Sciences. Sci. Total Environ. 2009, 407, 3620–3633. [Google Scholar] [CrossRef] [PubMed]
- Santos, S.; Ungureanu, G.; Boaventura, R.; Botelho, C. Selenium Contaminated Waters: An Overview of Analytical Methods, Treatment Options and Recent Advances in Sorption Methods. Sci. Total Environ. 2015, 521–522, 246–260. [Google Scholar] [CrossRef] [PubMed]
- Hawrylak-Nowak, B.; Matraszek, R.; Pogorzelec, M. The Dual Effects of Two Inorganic Selenium Forms on the Growth, Selected Physiological Parameters and Macronutrients Accumulation in Cucumber Plants. Acta Physiol. Plant. 2015, 37, 41. [Google Scholar] [CrossRef]
- Wu, L. Review of 15 Years of Research on Ecotoxicology and Remediation of Land Contaminated by Agricultural Drainage Sediment Rich in Selenium. Ecotoxicol. Environ. Saf. 2004, 57, 257–269. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Xiang, Y.; Zhou, Y.; Yang, Y.; Zhang, J.; Huang, H.; Shang, C.; Luo, L.; Gao, J.; Tang, L. Selenium Contamination, Consequences and Remediation Techniques in Water and Soils: A Review. Environ. Res. 2018, 164, 288–301. [Google Scholar] [CrossRef]
- Lemly, A.D. Aquatic Selenium Pollution Is a Global Environmental Safety Issue. Ecotoxicol. Environ. Saf. 2004, 59, 44–56. [Google Scholar] [CrossRef]
- Fordyce, F.M. Selenium Deficiency and Toxicity in the Environment. In Essentials of Medical Geology: Revised Edition; Springer: Dordrecht, The Netherlands, 2013; pp. 375–416. [Google Scholar] [CrossRef]
- Muscatello, J.R.; Janz, D.M. Selenium Accumulation in Aquatic Biota Downstream of a Uranium Mining and Milling Operation. Sci. Total Environ. 2009, 407, 1318–1325. [Google Scholar] [CrossRef]
- Griffith, M.B.; Norton, S.B.; Alexander, L.C.; Pollard, A.I.; LeDuc, S.D. The Effects of Mountaintop Mines and Valley Fills on the Physicochemical Quality of Stream Ecosystems in the Central Appalachians: A Review. Sci. Total Environ. 2012, 417–418, 1–12. [Google Scholar] [CrossRef]
- Wellen, C.C.; Shatilla, N.J.; Carey, S.K. Regional Scale Selenium Loading Associated with Surface Coal Mining, Elk Valley, British Columbia, Canada. Sci. Total Environ. 2015, 532, 791–802. [Google Scholar] [CrossRef]
- Lichtfouse, E.; Morin-Crini, N.; Bradu, C.; Boussouga, Y.-A.; Aliaskari, M.; Schäfer, A.I.; Das, S.; Wilson, L.D.; Ike, M.; Inoue, D.; et al. Technologies to Remove Selenium from Water and Wastewater. In Emerging Contaminants Vol. 2: Remediation; Springer International Publishing: Cham, Switzerland, 2021; Volume 2, pp. 207–304. [Google Scholar] [CrossRef]
- Okonji, S.O.; Achari, G.; Pernitsky, D. Environmental Impacts of Selenium Contamination: A Review on Current-Issues and Remediation Strategies in an Aqueous System. Water 2021, 13, 1473. [Google Scholar] [CrossRef]
- Stefaniak, J.; Dutta, A.; Verbinnen, B.; Shakya, M.; Rene, E.R. Selenium Removal from Mining and Process Wastewater: A Systematic Review of Available Technologies. J. Water Supply Res. Technol. 2018, 67, 903–918. [Google Scholar] [CrossRef]
- Lichtfouse, E.; Morin-Crini, N.; Bradu, C.; Boussouga, Y.A.; Aliaskari, M.; Schäfer, A.I.; Das, S.; Wilson, L.D.; Ike, M.; Inoue, D.; et al. Methods for Selenium Removal from Contaminated Waters: A Review. Environ. Chem. Lett. 2022, 20, 2019–2041. [Google Scholar] [CrossRef]
- Okonji, S.O.; Dominic, J.A.; Pernitsky, D.; Achari, G. Removal and Recovery of Selenium Species from Wastewater: Adsorption Kinetics and Co-Precipitation Mechanisms. J. Water Process Eng. 2020, 38, 101666. [Google Scholar] [CrossRef]
- Howarth, A.J.; Katz, M.J.; Wang, T.C.; Platero-Prats, A.E.; Chapman, K.W.; Hupp, J.T.; Farha, O.K. High Efficiency Adsorption and Removal of Selenate and Selenite from Water Using Metal-Organic Frameworks. J. Am. Chem. Soc. 2015, 137, 7488–7494. [Google Scholar] [CrossRef] [PubMed]
- Zoroufchi Benis, K.; McPhedran, K.N.; Soltan, J. Selenium Removal from Water Using Adsorbents: A Critical Review. J. Hazard. Mater. 2022, 424, 127603. [Google Scholar] [CrossRef]
- Hanada, K.; Watanabe, S.; Inagawa, A.; Uehara, N. Sulfated Steelmaking Slags as Se(IV) Adsorbents: Effects of Preparation Conditions on Adsorption Performance. ISIJ Int. 2021, 61, 506–512. [Google Scholar] [CrossRef]
- Rodríguez-Martínez, C.E.; González-Acevedo, Z.I.; Olguín, M.T.; Frías-Palos, H. Adsorption and Desorption of Selenium by Two Non-Living Biomasses of Aquatic Weeds at Dynamic Conditions. Clean Technol. Environ. Policy 2016, 18, 33–44. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, S.C.; Guan, Y. Efficient Removal of Selenate in Water by Cationic Poly(Allyltrimethylammonium) Grafted Chitosan and Biochar Composite. Environ. Res. 2021, 194, 110667. [Google Scholar] [CrossRef]
- Fu, Y.; Wang, J.; Liu, Q.; Zeng, H. Water-Dispersible Magnetic Nanoparticle–Graphene Oxide Composites for Selenium Removal. Carbon N. Y. 2014, 77, 710–721. [Google Scholar] [CrossRef]
- Bandara, P.C.; Perez, J.V.D.; Nadres, E.T.; Nannapaneni, R.G.; Krakowiak, K.J.; Rodrigues, D.F. Graphene Oxide Nanocomposite Hydrogel Beads for Removal of Selenium in Contaminated Water. ACS Appl. Polym. Mater. 2019, 1, 2668–2679. [Google Scholar] [CrossRef]
- Zhang, N.; Gang, D.D.; McDonald, L.; Lin, L.S. Background Electrolytes and PH Effects on Selenate Adsorption Using Iron-Impregnated Granular Activated Carbon and Surface Binding Mechanisms. Chemosphere 2018, 195, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Lin, L.S.; Gang, D. Adsorptive Selenite Removal from Water Using Iron-Coated GAC Adsorbents. Water Res. 2008, 42, 3809–3816. [Google Scholar] [CrossRef] [PubMed]
- Ying, A.; Evans, S.F.; Tsouris, C.; Parans Paranthaman, M. Magnetic Sorbent for the Removal of Selenium(IV) from Simulated Industrial Wastewaters: Determination of Column Kinetic Parameters. Water 2020, 12, 1234. [Google Scholar] [CrossRef]
- Halalsheh, N.; Alshboul, O.; Shehadeh, A.; Al Mamlook, R.E.; Al-Othman, A.; Tawalbeh, M.; Saeed Almuflih, A.; Papelis, C. Breakthrough Curves Prediction of Selenite Adsorption on Chemically Modified Zeolite Using Boosted Decision Tree Algorithms for Water Treatment Applications. Water 2022, 14, 2519. [Google Scholar] [CrossRef]
- Wang, S.; Xiao, K.; Mo, Y.; Yang, B.; Vincent, T.; Faur, C.; Guibal, E. Selenium(VI) and Copper(II) Adsorption Using Polyethyleneimine-Based Resins: Effect of Glutaraldehyde Crosslinking and Storage Condition. J. Hazard. Mater. 2020, 386, 121637. [Google Scholar] [CrossRef]
- Wei, J.; Shen, B.; Ye, G.; Wen, X.; Song, Y.; Wang, J.; Meng, X. Selenium and Arsenic Removal from Water Using Amine Sorbent, Competitive Adsorption and Regeneration. Environ. Pollut. 2021, 274, 115866. [Google Scholar] [CrossRef] [PubMed]
- Dobrzyńska, J. Amine- and Thiol-Functionalized SBA-15: Potential Materials for As(V), Cr(VI) and Se(VI) Removal from Water. Comparative Study. J. Water Process Eng. 2021, 40, 101942. [Google Scholar] [CrossRef]
- Rovira, M.; Giménez, J.; Martínez, M.; Martínez-Lladó, X.; de Pablo, J.; Martí, V.; Duro, L. Sorption of Selenium(IV) and Selenium(VI) onto Natural Iron Oxides: Goethite and Hematite. J. Hazard. Mater. 2008, 150, 279–284. [Google Scholar] [CrossRef]
- Das, S.; Jim Hendry, M.; Essilfie-Dughan, J. Adsorption of Selenate onto Ferrihydrite, Goethite, and Lepidocrocite under Neutral PH Conditions. Appl. Geochem. 2013, 28, 185–193. [Google Scholar] [CrossRef]
- Peak, D.; Sparks, D.L. Mechanisms of Selenate Adsorption on Iron Oxides and Hydroxides. Environ. Sci. Technol. 2002, 36, 1460–1466. [Google Scholar] [CrossRef]
- Wei, X.; Bhojappa, S.; Lin, L.S.; Viadero, R.C. Performance of Nano-Magnetite for Removal of Selenium from Aqueous Solutions. Environ. Eng. Sci. 2012, 29, 526–532. [Google Scholar] [CrossRef]
- Jordan, N.; Marmier, N.; Lomenech, C.; Giffaut, E.; Ehrhardt, J.J. Competition between Selenium (IV) and Silicic Acid on the Hematite Surface. Chemosphere 2009, 75, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Jordan, N.; Ritter, A.; Foerstendorf, H.; Scheinost, A.C.; Weiß, S.; Heim, K.; Grenzer, J.; Mücklich, A.; Reuther, H. Adsorption Mechanism of Selenium(VI) onto Maghemite. Geochim. Cosmochim. Acta 2013, 103, 63–75. [Google Scholar] [CrossRef]
- Martínez, M.; Giménez, J.; De Pablo, J.; Rovira, M.; Duro, L. Sorption of Selenium(IV) and Selenium(VI) onto Magnetite. Appl. Surf. Sci. 2006, 252, 3767–3773. [Google Scholar] [CrossRef]
- Su, C.; Suarez, D.L. Selenate and Selenite Sorption on Iron Oxides An Infrared and Electrophoretic Study. Soil Sci. Soc. Am. J. 2000, 64, 101–111. [Google Scholar] [CrossRef]
- Fernández-Pérez, A.; Rodríguez-Casado, V.; Valdés-Solís, T.; Marbán, G. A New Continuous Flow-through Structured Reactor for the Photodegradation of Aqueous Contaminants. J. Environ. Chem. Eng. 2018, 6, 4070–4077. [Google Scholar] [CrossRef]
- Witczak, A.; Pokorska-Niewiada, K.; Tomza-Marciniak, A.; Witczak, G.; Cybulski, J.; Aftyka, A. The Problem of Selenium for Human Health—Removal of Selenium from Water and Wastewater. Water 2023, 15, 2230. [Google Scholar] [CrossRef]
- Sahoo, T.R.; Prelot, B. Adsorption Processes for the Removal of Contaminants from Wastewater: The Perspective Role of Nanomaterials and Nanotechnology. In Nanomaterials for the Detection and Removal of Wastewater Pollutants; Elsevier: Amsterdam, The Netherlands, 2020; pp. 161–222. [Google Scholar] [CrossRef]
- Jadhav, A.S.; Ramteke, P.; Singh, S.K.; Labhasetwar, N.K. Sustainable Selenium Remediation from Water Using Aluminium–Iron Mixed Oxide: Batch and Column Adsorption Studies. J. Water Process Eng. 2022, 48, 102824. [Google Scholar] [CrossRef]
- Hotová, G.; Slovák, V.; Soares, O.S.G.P.; Figueiredo, J.L.; Pereira, M.F.R. Oxygen Surface Groups Analysis of Carbonaceous Samples Pyrolysed at Low Temperature. Carbon N. Y. 2018, 134, 255–263. [Google Scholar] [CrossRef]
- García, R.; Rodríguez, E.; Díez, M.A.; Arenillas, A.; Villanueva, S.F.; Rey-Raap, N.; Cuesta, C.; López-Antón, M.A.; Martínez-Tarazona, M.R. Synthesis of Micro- and Mesoporous Carbon Foams with Nanodispersed Metals for Adsorption and Catalysis Applications. Materials 2023, 16, 1336. [Google Scholar] [CrossRef]
- Sandy, T. Review of Available Technologies for the Removal of Selenium from Water. Final. Rep. Prep. North Am. Met. Counc. 2010, 2010, 48–60. [Google Scholar]
- Pashai Gatabi, M.; Milani Moghaddam, H.; Ghorbani, M. Point of Zero Charge of Maghemite Decorated Multiwalled Carbon Nanotubes Fabricated by Chemical Precipitation Method. J. Mol. Liq. 2016, 216, 117–125. [Google Scholar] [CrossRef]
- Börsig, N.; Scheinost, A.C.; Schild, D.; Neumann, T. Mechanisms of Selenium Removal by Partially Oxidized Magnetite Nanoparticles for Wastewater Remediation. Appl. Geochem. 2021, 132, 105062. [Google Scholar] [CrossRef]
- Missana, T.; Alonso, U.; Scheinost, A.C.; Granizo, N.; García-Gutiérrez, M. Selenite Retention by Nanocrystalline Magnetite: Role of Adsorption, Reduction and Dissolution/Co-Precipitation Processes. Geochim. Cosmochim. Acta 2009, 73, 6205–6217. [Google Scholar] [CrossRef]
- Hayashi, H.; Kanie, K.; Shinoda, K.; Muramatsu, A.; Suzuki, S.; Sasaki, H. PH-Dependence of Selenate Removal from Liquid Phase by Reductive Fe(II)–Fe(III) Hydroxysulfate Compound, Green Rust. Chemosphere 2009, 76, 638–643. [Google Scholar] [CrossRef]
- Reait, P.; Simon, L.; Génin, J.M.R. Reduction of SeO42- Anions and Anoxic Formation of Iron(II)–Iron(III) Hydroxy-Selenate Green Rust. Environ. Sci. Technol. 2000, 34, 819–825. [Google Scholar] [CrossRef]
- Onoguchi, A.; Granata, G.; Haraguchi, D.; Hayashi, H.; Tokoro, C. Kinetics and Mechanism of Selenate and Selenite Removal in Solution by Green Rust-Sulfate. R. Soc. Open Sci. 2019, 6, 182147. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).