Variability in Population Traits of a Sentinel Iberian Fish in a Highly Modified Mediterranean-Type River
Abstract
1. Introduction
2. Materials and Methods
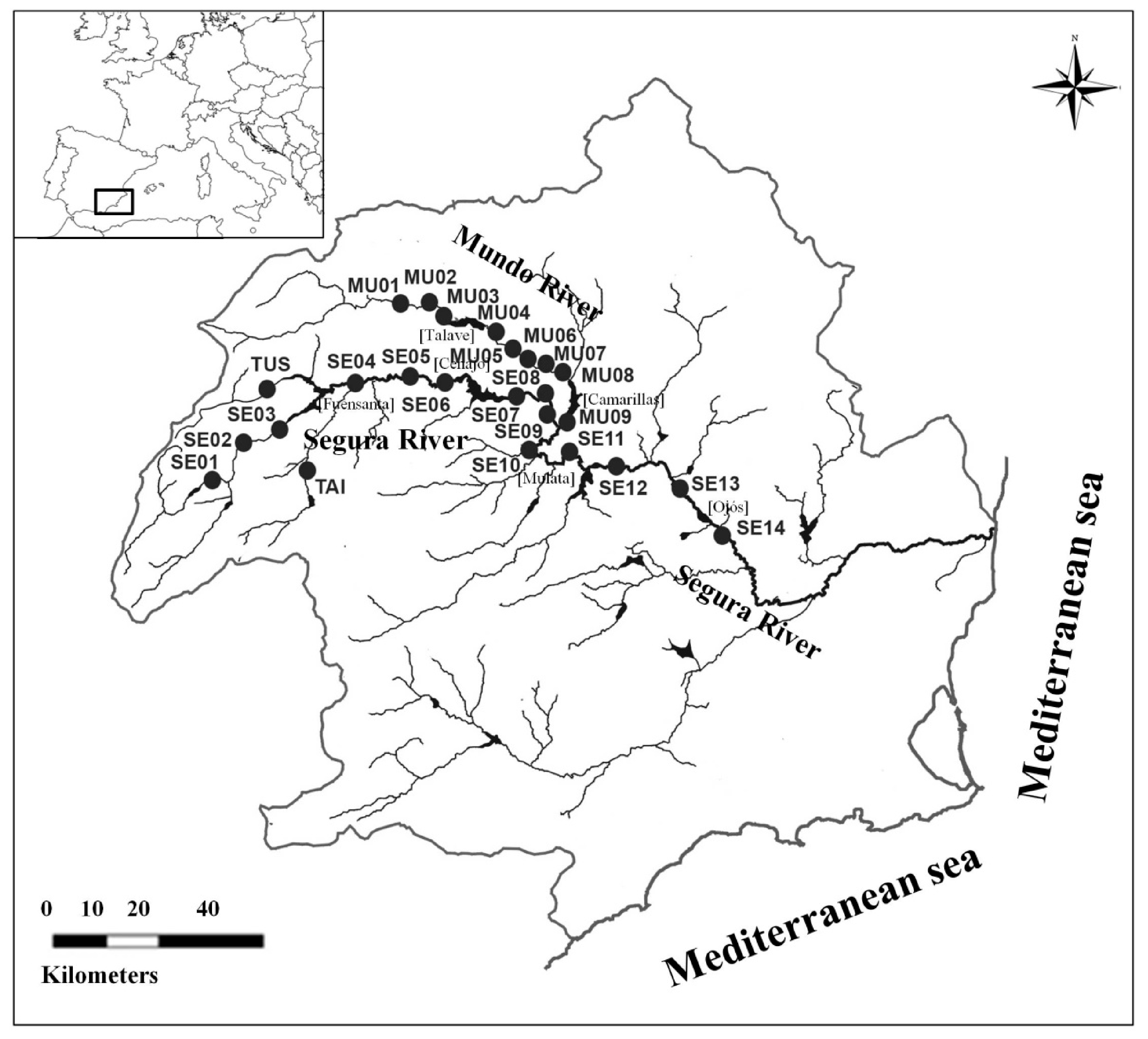
2.1. Study Area and Sampling Design
2.2. Environmental Variables
2.3. Fish Sampling and Population Traits
2.4. Effect of Environmental Stress Factors on Population Traits
3. Results
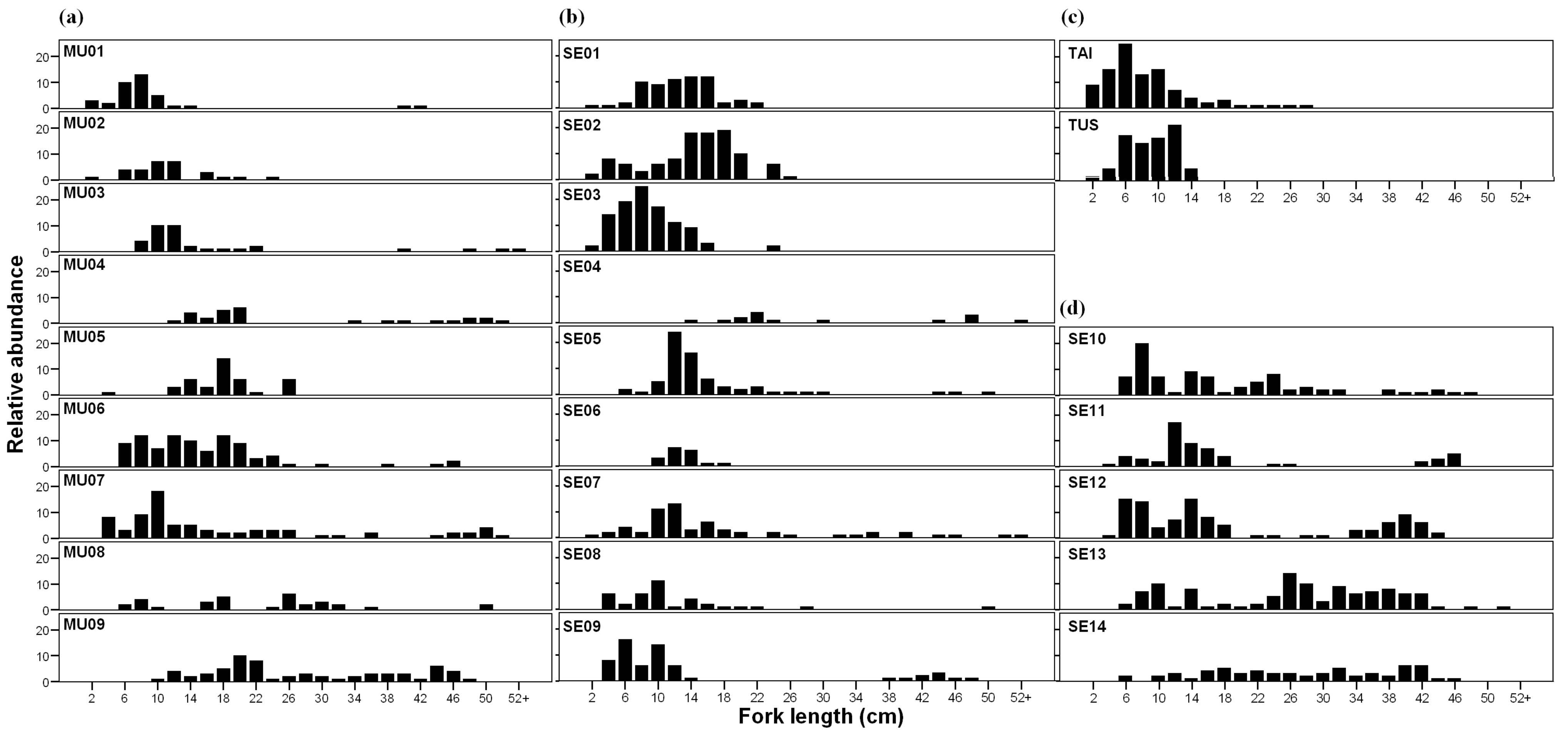
3.1. Relative Abundance and Population Traits
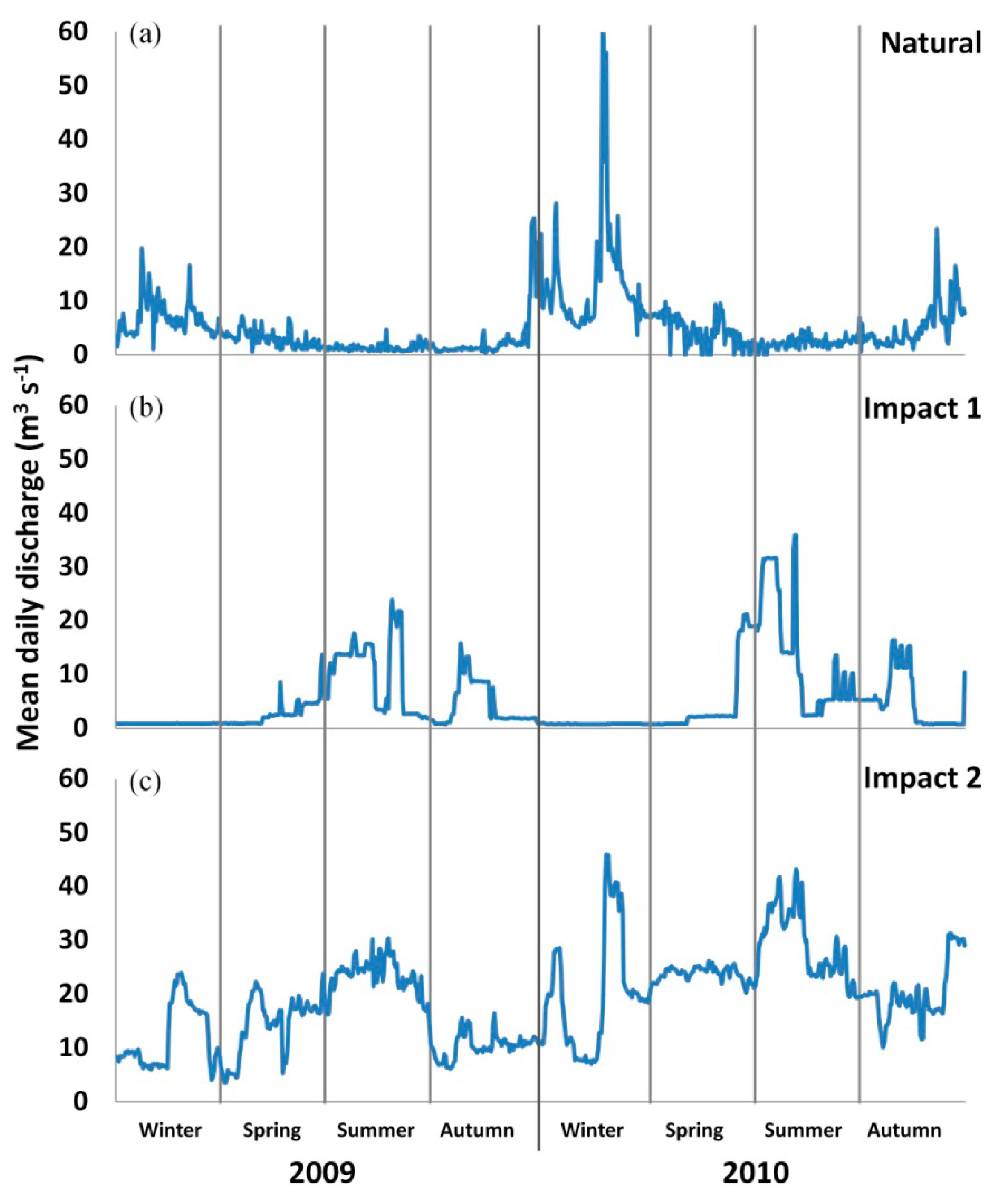
3.2. Environmental Factors
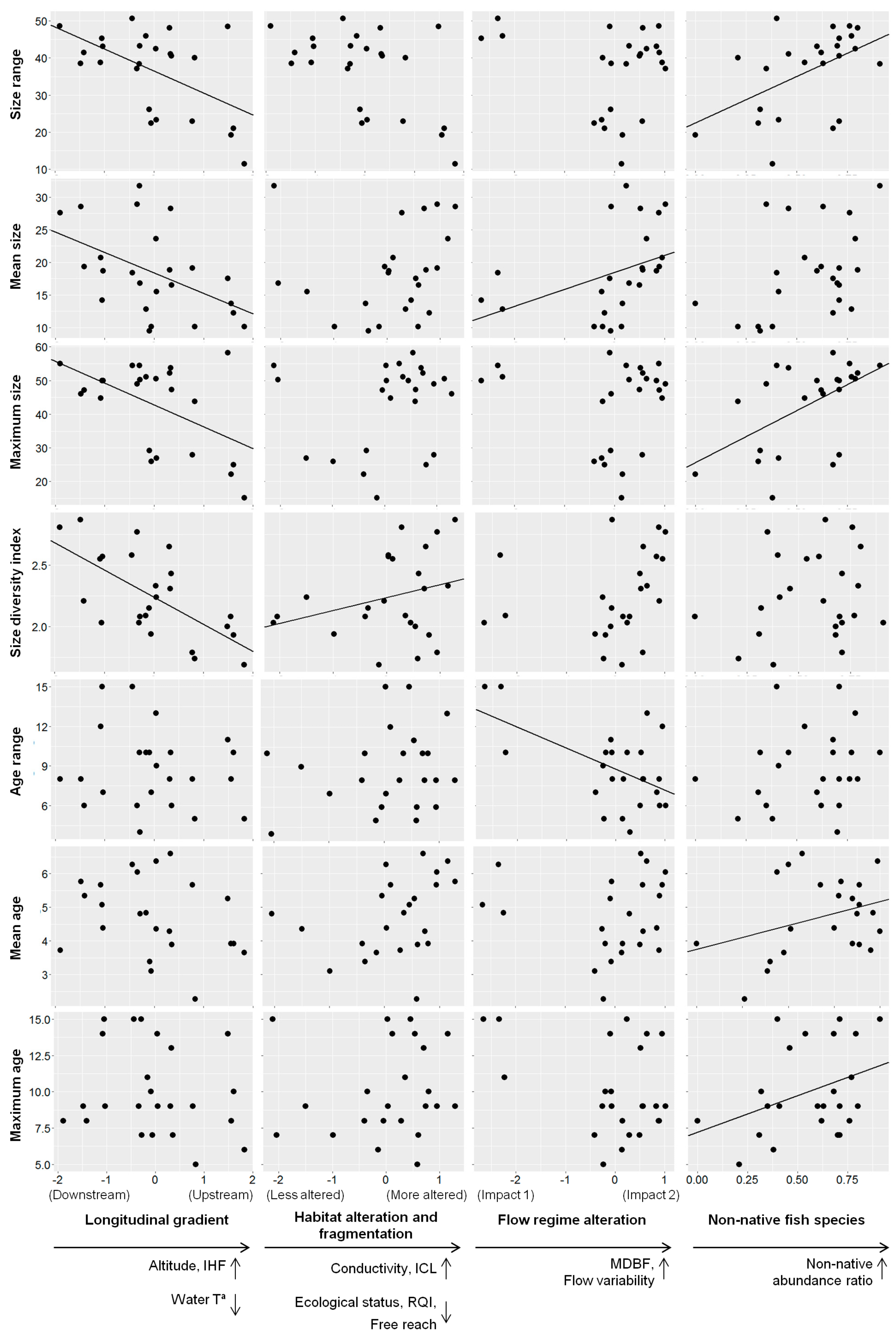
3.3. Effects of Environmental Factors on Population Traits
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Srinivasan, V.; Lambin, E.F.; Gorelick, S.M.; Thompson, B.H.; Rozelle, S. The Nature and Causes of the Global Water Crisis: Syndromes from a Meta-Analysis of Coupled Human-Water Studies. Water Resour. Res. 2012, 48, W10516. [Google Scholar] [CrossRef]
- Sabater, S.; Bregoli, F.; Acuña, V.; Barceló, D.; Elosegi, A.; Ginebreda, A.; Marcé, R.; Muñoz, I.; Sabater-Liesa, L.; Ferreira, V. Effects of Human-Driven Water Stress on River Ecosystems: A Meta-Analysis. Sci. Rep. 2018, 8, 11462. [Google Scholar] [CrossRef]
- Veldkamp, T.I.E.; Wada, Y.; Aerts, J.C.J.H.; Döll, P.; Gosling, S.N.; Liu, J.; Masaki, Y.; Oki, T.; Ostberg, S.; Pokhrel, Y.; et al. Water Scarcity Hotspots Travel Downstream Due to Human Interventions in the 20th and 21st Century. Nat. Commun. 2017, 8, 15697. [Google Scholar] [CrossRef] [PubMed]
- Vörösmarty, C.J.; Green, P.; Salisbury, J.; Lammers, R.B. Global Water Resources: Vulnerability from Climate Change and Population Growth. Science. 2000, 289, 284–288. [Google Scholar] [CrossRef]
- Dudgeon, D.; Arthington, A.H.; Gessner, M.O.; Kawabata, Z.-I.; Knowler, D.J.; Lévêque, C.; Naiman, R.J.; Prieur-Richard, A.-H.; Soto, D.; Stiassny, M.L.J.; et al. Freshwater Biodiversity: Importance, Threats, Status and Conservation Challenges. Biol. Rev. 2006, 81, 163–182. [Google Scholar] [CrossRef] [PubMed]
- Hooke, J.M. Human Impacts on Fluvial Systems in the Mediterranean Region. Geomorphology 2006, 79, 311–335. [Google Scholar] [CrossRef]
- Brink, K.; Gough, P.; Royte, J.; Schollema, P.P.; Wanningen, H. From Sea to Source: Protection and Restoration of Fish Migration in Rivers Worldwide; World Fish Migration Foundation: Groningen, The Netherlands, 2016. [Google Scholar]
- Maceda-Veiga, A. Towards the Conservation of Freshwater Fish: Iberian Rivers as an Example of Threats and Management Practices. Rev. Fish. Biol. Fish. 2013, 23, 1–22. [Google Scholar] [CrossRef]
- Vidal-Abarca, M.R.; Suárez, M.L.; Ramírez-Díaz, L. Ecology of Spanish Semiarid Streams. Limnetica 1992, 8, 151–160. [Google Scholar]
- Gasith, A.; Resh, V.H. Streams in Mediterranean Climate Regions: Abiotic Influences and Biotic Responses to Predictable Seasonal Events. Annu. Rev. Ecol. Syst. 1999, 30, 51–81. [Google Scholar] [CrossRef]
- Hershkovitz, Y.; Gasith, A. Resistance, Resilience, and Community Dynamics in Mediterranean-Climate Streams. Hydrobiologia 2013, 719, 59–75. [Google Scholar] [CrossRef]
- Encina, L.; Rodríguez, A.; Granado-Lorencio, C. The Iberian Ichthyfauna: Ecological Contributions. Limnetica 2006, 25, 349–368. [Google Scholar] [CrossRef]
- Alexandre, C.M.; Ferreira, M.T.; Almeida, P.R. Fish Assemblages in Non-Regulated and Regulated Rivers from Permanent and Temporary Iberian Systems. River Res. Appl. 2012, 29, 1042–1058. [Google Scholar] [CrossRef]
- Ormerod, S.J. Current Issues with Fish and Fisheries: Editor’s Overview and Introduction. J. Appl. Ecol. 2003, 40, 204–213. [Google Scholar] [CrossRef]
- Kottelat, M.; Freyhof, J. Handbook of European Freshwater Fishes; Publications Kottelat: Cornol, Switzerland, 2007; ISBN 978-2-8399-0298-4. [Google Scholar]
- Clavero, M.; Hermoso, V.; Levin, N.; Kark, S. Geographical Linkages between Threats and Imperilment in Freshwater Fish in the Mediterranean Basin. Divers. Distrib. 2010, 16, 744–754. [Google Scholar] [CrossRef]
- Bonada, N.; Resh, V.H. Mediterranean-Climate Streams and Rivers: Geographically Separated but Ecologically Comparable Freshwater Systems. Hydrobiologia 2013, 719, 1–29. [Google Scholar] [CrossRef]
- Bunn, S.E.; Arthington, A.H. Basic Principles and Ecological Consequences of Altered Flow Regimes for Aquatic Biodiversity. Environ. Manage. 2002, 30, 492–507. [Google Scholar] [CrossRef] [PubMed]
- Poff, N.L.; Zimmerman, J.K.H. Ecological Responses to Altered Flow Regimes: A Literature Review to Inform the Science and Management of Environmental Flows. Freshw. Biol. 2010, 55, 194–205. [Google Scholar] [CrossRef]
- Lytle, D.A.; Poff, N.L. Adaptation to Natural Flow Regimes. Trends Ecol. Evol. 2004, 19, 94–100. [Google Scholar] [CrossRef]
- Hermoso, V.; Clavero, M. Threatening Processes and Conservation Management of Endemic Freshwater Fish in the Mediterranean Basin: A Review. Mar. Freshw. Res. 2011, 62, 244–254. [Google Scholar] [CrossRef]
- Fornaroli, R.; Muñoz-Mas, R.; Martínez-Capel, F. Fish Community Responses to Antecedent Hydrological Conditions Based on Long-Term Data in Mediterranean River Basins (Iberian Peninsula). Sci. Total Environ. 2020, 728, 138052. [Google Scholar] [CrossRef] [PubMed]
- Belletti, B.; Leaniz, C.G.D.; Jones, J.; Bizzi, S.; Börger, L.; Segura, G.; Castelletti, A.; van de Bund, W.; Aarestrup, K.; Barry, J.; et al. More than One Million Barriers Fragment Europe’s Rivers. Nature 2020, 7838, 436–441. [Google Scholar] [CrossRef]
- Branco, P.; Amaral, S.D.; Ferreira, M.T.; Santos, J.M. Do Small Barriers Affect the Movement of Freshwater Fish by Increasing Residency? Sci. Total Environ. 2017, 581–582, 486–494. [Google Scholar] [CrossRef]
- Alexandre, C.M.; Ferreira, M.T.; Almeida, P.R. Life History of a Cyprinid Species in Non-Regulated and Regulated Rivers from Permanent and Temporary Mediterranean Basins. Ecohydrology 2014, 8, 1137–1153. [Google Scholar] [CrossRef]
- Merciai, R.; Bailey, L.L.; Bestgen, K.R.; Fausch, K.D.; Zamora, L.; Sabater, S.; García-Berthou, E. Water Diversion Reduces Abundance and Survival of Two Mediterranean Cyprinids. Ecol. Freshw. Fish. 2017, 27, 481–491. [Google Scholar] [CrossRef]
- Bernardo, J.M.; Ilhéu, M.; Matono, P.; Costa, A.M. Interannual Variation of Fish Assemblage Structure in a Mediterranean River: Implications of Streamflow on the Dominance of Native or Exotic Species. River Res. Appl. 2003, 19, 521–532. [Google Scholar] [CrossRef]
- Ilhéu, M.; Matono, P.; Bernardo, J.M. Invasibility of Mediterranean-Climate Rivers by Non-Native Fish: The Importance of Environmental Drivers and Human Pressures. PLoS ONE 2014, 9, e109694. [Google Scholar] [CrossRef]
- Schinegger, R.; Palt, M.; Segurado, P.; Schmutz, S. Untangling the Effects of Multiple Human Stressors and Their Impacts on Fish Assemblages in European Running Waters. Sci. Total Environ. 2016, 573, 1079–1088. [Google Scholar] [CrossRef]
- Segurado, P.; Almeida, C.; Neves, R.; Ferreira, M.T.; Branco, P. Understanding Multiple Stressors in a Mediterranean Basin: Combined Effects of Land Use, Water Scarcity and Nutrient Enrichment. Sci. Total Environ. 2018, 624, 1221–1233. [Google Scholar] [CrossRef] [PubMed]
- Colin, N.; Villéger, S.; Wilkes, M.; de Sostoa, A.; Maceda-Veiga, A. Functional Diversity Measures Revealed Impacts of Non-Native Species and Habitat Degradation on Species-Poor Freshwater Fish Assemblages. Sci. Total Environ. 2018, 625, 861–871. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Pérez, A.; Oliva-Paterna, F.J.; Colin, N.; Torralva, M.; Górski, K. Functional Response of Fish Assemblage to Multiple Stressors in a Highly Regulated Mediterranean River System. Sci. Total Environ. 2020, 730, 138989. [Google Scholar] [CrossRef] [PubMed]
- Bruno, D.; Belmar, O.; Sánchez-Fernández, D.; Guareschi, S.; Millán, A.; Velasco, J. Responses of Mediterranean Aquatic and Riparian Communities to Human Pressures at Different Spatial Scales. Ecol. Indic. 2014, 45, 456–464. [Google Scholar] [CrossRef]
- Belmar, O.; Bruno, D.; Martínez-Capel, F.; Barquín, J.; Velasco, J. Effects of Flow Regime Alteration on Fluvial Habitats and Riparian Quality in a Semiarid Mediterranean Basin. Ecol. Indic. 2013, 30, 52–64. [Google Scholar] [CrossRef]
- Amat-Trigo, F. Biological Response of Invasive Fish in a Highly Regulated Mediterranean River Basin. Ph.D. Thesis, Univerisity of Murcia, Murcia, Spain, 2018. [Google Scholar]
- Amat-Trigo, F.; Torralva, M.; Sánchez-Pérez, A.; Ruiz-Navarro, A.; Oliva-Paterna, F.J. Effects of Flow Regulation along Longitudinal Gradient on Size-Related Metrics of Fish Populations from a Mediterranean Basin. Fishes Mediterr. Environ. 2016, 2016.010, 4. [Google Scholar] [CrossRef]
- Grindlay, A.L.; Zamorano, M.; Rodríguez Muñoz, I.; Molero, E.; Urrea, M.A. Implementation of the European Water Framework Directive: Integration of Hydrological and Regional Planning at the Segura River Basin, Southeast Spain. Land use policy 2011, 28, 242–256. [Google Scholar] [CrossRef]
- Oliva-Paterna, F.J.; Verdiell-Cubedo, D.; Ruiz-Navarro, A.; Torralva, M. La Ictiofauna Continental de La Cuenca Del Río Segura (S.E. Península Ibérica): Décadas Después de Mas (1986). An. Biol. 2014, 36, 37–45. [Google Scholar] [CrossRef]
- Encina, L.; Granado-Lorencio, C. Seasonal Changes in Condition, Nutrition, Gonad Maturation and Energy Content in Barbel, Barbus Sclateri, Inhabiting a Fluctuating River. Environ. Biol. Fishes 1997, 50, 75–84. [Google Scholar] [CrossRef]
- Herrera, M.; Hernando, J.A.; Fernández-Delgado, C.; Bellido, M. Age, Growth and Reproduction of the Barbel, Barbus Sclateri (Günther, 1868), in a First-Order Stream in Southern Spain. J. Fish. Biol. 1988, 33, 371–381. [Google Scholar] [CrossRef]
- Torralva, M. Biología de Babus Sclateri Günther, 1868 (Pisces, Cyprinidae) En Dos Cursos de Agua Con Distinto Grado de Regulación En La Cuenca Del Río Segura (S.E. de España). Ph.D. Thesis, Univerisity of Murcia, Murcia, Spain, 1996. [Google Scholar]
- Martínez-Morales, I.; José Oliva-Paterna, F.J.; Verdiell-Cubedo, D.; Torralva, M. Inventario y Estado de Conservación de La Fauna Piscícola En La Cuenca Alta Del Río Segura (SE Península Ibérica). An. Biol. 2010, 32, 47–58. [Google Scholar]
- Oliva-Paterna, F.J.; Miñano, P.A.; Torralva, M. Habitat Quality Affects the Condition of Barbus Sclateri in Mediterranean Semi-Arid Streams. Environ. Biol. Fishes 2003, 67, 13–22. [Google Scholar] [CrossRef]
- De Miguel, R.J.; Oliva-Paterna, F.J.; Gálvez-Bravo, L.; Fernández-Delgado, C. Habitat Quality Affects the Condition of Luciobarbus Sclateri in the Guadiamar River (SW Iberian Peninsula): Effects of Disturbances by the Toxic Spill of the Aznalcóllar Mine. Hydrobiologia 2013, 700, 85–97. [Google Scholar] [CrossRef][Green Version]
- Torralva, M.; Angeles Puig, M.; Fernández-Delgado, C. Effect of River Regulation on the Life-History Patterns of Barbus Sclateri in the Segura River Basin (South-East Spain). J. Fish. Biol. 1997, 51, 300–311. [Google Scholar] [CrossRef]
- Torralva, M.; Oliva-Paterna, F.J.; Andreu-Soler, A.; Verdiell-Cubedo, D.; Miñano, P.A.; Egea-Serrano, A. Atlas de Distribución de Los Peces Epicontinentales de La Región de Murcia; Dirección General del Medio Natural (CARM): Murcia, Spain, 2005; ISBN MU-2362-2005. [Google Scholar]
- Oliva-Paterna, F.J.; Zamora-Marín, J.M.; Franco Galera, J.M.; Zamora-López, A.; Sánchez-Pérez, A.; Amat-Trigo, F.; Guillén Beltrán, A.; Guerrero Gómez, A.; Torralva, M. Peces Dulceacuícolas de La Cuenca Del Río Segura; Asociación de Naturalistas del Sureste (ANSE): Murcia, Spain, 2019; ISBN 978-84-09-07845-5. [Google Scholar]
- Bergerot, B.; Hugueny, B.; Belliard, J. Relating Life-History Traits, Environmental Constraints and Local Extinctions in River Fish. Freshw. Biol. 2015, 60, 1279–1291. [Google Scholar] [CrossRef]
- Cooke, S.J.; Paukert, C.; Hogan, Z. Endangered River Fish: Factors Hindering Conservation and Restoration. Endanger. Species Res. 2012, 17, 179–191. [Google Scholar] [CrossRef]
- CHS (Confederación Hidrográfica del Segura). Plan. Hidrológico de La Cuenca Del Segura 2009-2015. Anejo 7: Inventario de Presiones; Ministerio de Medio Ambiente, 2013. Available online: https://www.chsegura.es/export/sites/chs/descargas/planificacionydma/planificacion/docsdescarga/Anejo_07_Inventario_de_presiones.pdf (accessed on 12 September 2018).
- CHS (Confederación Hidrográfica del Segura). Plan. Hidrológico de La Demarcación Del Segura 2015/21. 2015. Available online: https://www.chsegura.es/export/sites/chs/descargas/planificacionydma/planificacion15-21/docsdescarga/docplan1521/01_MEMORIA/Memoria_PHDS2015_21.pdf (accessed on 12 September 2018).
- Pellicer-Martínez, F.; Martínez-Paz, J.M. Probabilistic Evaluation of the Water Footprint of a River Basin: Accounting Method and Case Study in the Segura River Basin, Spain. Sci. Total Environ. 2018, 627, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Belmar, O.; Velasco, J.; Martínez-Capel, F. Hydrological Classification of Natural Flow Regimes to Support Environmental Flow Assessments in Intensively Regulated Mediterranean Rivers, Segura River Basin (Spain). Environ. Manage. 2011, 47, 992–1004. [Google Scholar] [CrossRef] [PubMed]
- Piqué, G.; Batalla, R.J.; Sabater, S. Hydrological Characterization of Dammed Rivers in the NW Mediterranean Region. Hydrol. Process. 2016, 30, 1691–1707. [Google Scholar] [CrossRef]
- Pardo, I.; Alvarez, M.; Casas, J.; Moreno, J.L.; Vivas, S.; Bonada, N.; Alba-Tercedor, J.; Jaimez-Cuellar, P.; Moya, G.; Prat, N.; et al. El Hábitat de Los Ríos Mediterráneos. Diseño de Un Índice de Diversidad de Hábitat. Limnetica 2002, 21, 115–133. [Google Scholar]
- González del Tánago, M.; García de Jalón, D. Riparian Quality Index (RQI): A Methodology for Characterising and Assessing the Environmental Conditions of Riparian Zones. Limnetica 2011, 30, 235–254. [Google Scholar]
- CHS (Confederación Hidrográfica del Segura). Estudio General Sobre La Demarcación Hidrográfica Del Segura. 2007. Available online: https://www.chsegura.es/export/sites/chs/descargas/planificacionydma/planificacion/docsdescarga/Proyecto_de_participacion_publica_v4.pdf (accessed on 12 September 2018).
- González Fernández, G.; Rodríguez Muñoz, I.; Seisdedos Fidalgo, P.; Pérez Cardenal, D.; Miguélez Carbajo, D.; Gallego García, R. Diseño de Índices Para El Análisis de La Conectividad Longitudinal En La Cuenca Del Duero. In Proceedings of the Actas del I Congreso Ibérico de Restauración Fluvial (RESTAURARÍOS), León, Spain, 2011; pp. 378–385. [Google Scholar]
- Marsh, N.A.; Stewardson, M.J.; Kennard, M.J. River Analysis Package; Monash University: Melbourne, 2003. [Google Scholar]
- CEN (Comité Européen de Normaliation). Water Quality: Sampling of Fish with Electricity. European Standard EN—14011:2003; European Committee for Standarization: Brussels, Belgium, 2003. [Google Scholar]
- Oliva-Paterna, F.J.; Vila-Gispert, A.; Torralva, M. Condition of Barbus Sclateri from Semi-Arid Aquatic Systems: Effects of Habitat Quality Disturbances. J. Fish. Biol. 2003, 63, 699–709. [Google Scholar] [CrossRef][Green Version]
- Miñano, P.A.; Oliva-Paterna, F.J.; Fernández-Delgado, C.; Torralva, M. Edad y Crecimiento de Barbus Graellsii Steindachner, 1866 y Chondrostoma Miegii, Steindachner, 1866 (Pisces, Cyprinidae) En El Río Cinca (Cuenca Hidrográfica Del Ebro, NE España). Misc. Zool. 2000, 23, 9–19. [Google Scholar]
- Musk, R.S.; Britton, J.R.; Axford, S.N. The Effect of Subjective Fish Scale Ageing on Growth and Recruitment Analyses: A Case Study from the UK. Acta Ichthyol. Piscat. 2006, 36, 81–84. [Google Scholar] [CrossRef][Green Version]
- Masó, G.; Latorre, D.; Tarkan, A.S.; Vila-Gispert, A.; Almeida, D. Inter-Population Plasticity in Growth and Reproduction of Invasive Bleak, Alburnus Alburnus (Cyprinidae, Actinopterygii), in Northeastern Iberian Peninsula. Folia Zool. 2016, 65, 10–14. [Google Scholar] [CrossRef]
- Amat-Trigo, F.; Torralva, M.; Ruiz-Navarro, A.; Oliva-Paterna, F.J. Colonization and Plasticity in Population Traits of the Invasive Alburnus Alburnus along a Longitudinal River Gradient in a Mediterranean River Basin. Aquat. Invasions 2019, 14, 310–331. [Google Scholar] [CrossRef]
- Hickley, P.; Dexter, K.F. A Comparative Index for Quantifying Growth in Length of Fish. Fish. Manag. 1979, 10, 147–151. [Google Scholar] [CrossRef]
- Walford, L.A. A New Graphic Method of Describing the Growth of Animals. Biol. Bull. 1946, 90, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Berthou, E.; Moreno-Amich, R. Multivariate Analysis of Covariance in Morphometric Studies of Reproductive Cycle. Can. J. Fish. Aquat. Sci. 1993, 50, 1394–1399. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach, 2nd ed.; Springer: New York, NY, USA, 2002; ISBN 0-387-95364-7. [Google Scholar]
- Barton, K. MuMIn: Multi-Model Inference. R Package. 2018. Available online: http://Mumin.r-Forge.r-Project.Org/ (accessed on 30 June 2020).
- Quinn, G.P.; Keough, M.J. Experimental Design and Data Analysis for Biologists; Cambridge University Press: Cambridge, UK, 2002; ISBN 978-0-511-07812-5. [Google Scholar]
- Pringle, C.M. Hydrologic Connectivity and the Management of Biological Reserves: A Global Perspective. Ecol. Appl. 2001, 11, 981–998. [Google Scholar] [CrossRef]
- Magalhães, M.F.; Batalha, D.C.; Collares-Pereira, M.J. Gradients in Stream Fish Assemblages across a Mediterranean Landscape: Contributions of Environmental Factors and Spatial Structure. Freshw. Biol. 2002, 47, 1015–1031. [Google Scholar] [CrossRef]
- Radinger, J.; Alcaraz-Hernández, J.D.; García-Berthou, E. Environmental and Spatial Correlates of Hydrologic Alteration in a Large Mediterranean River Catchment. Sci. Total Environ. 2018, 639, 1138–1147. [Google Scholar] [CrossRef]
- Hermoso, V.; Clavero, M.; Blanco-Garrido, F.; Prenda, J. Assessing Freshwater Fish Sensitivity to Different Sources of Perturbation in a Mediterranean Basin. Ecol. Freshw. Fish. 2009, 18, 269–281. [Google Scholar] [CrossRef]
- Mas-Martí, E.; García-Berthou, E.; Sabater, S.; Tomanova, S.; Muñoz, I. Comparing Fish Assemblages and Trophic Ecology of Permanent and Intermittent Reaches in a Mediterranean Stream. Hydrobiologia 2010, 657, 167–180. [Google Scholar] [CrossRef]
- Hughes, S.J.; Santos, J.M.; Ferreira, M.T.; Mendes, A. Evaluating the Response of Biological Assemblages as Potential Indicators for Restoration Measures in an Intermittent Mediterranean River. Environ. Manage. 2010, 46, 285–301. [Google Scholar] [CrossRef] [PubMed]
- Wootton, R.J. Ecology of Teleost Fishes; Chapman and Hall: London, UK, 1998. [Google Scholar]
- Alexandre, C.M.; Sales, S.; Ferreira, M.T.; Almeida, P.R. Food Resources and Cyprinid Diet in Permanent and Temporary Mediterranean Rivers with Natural and Regulated Flow. Ecol. Freshw. Fish. 2014, 24, 629–645. [Google Scholar] [CrossRef]
- Poff, N.L. Landscape Filters and Species Traits: Towards Mechanistic Understanding and Prediction in Stream Ecology. J. North. Am. Benthol. Soc. 1997, 16, 391–409. [Google Scholar] [CrossRef]
- Poff, N.L.; Richter, B.D.; Arthington, A.H.; Bunn, S.E.; Naiman, R.J.; Kendy, E.; Acreman, M.; Apse, C.; Bledsoe, B.P.; Freeman, M.C.; et al. The Ecological Limits of Hydrologic Alteration (ELOHA): A New Framework for Developing Regional Environmental Flow Standards. Freshw. Biol. 2010, 55, 147–170. [Google Scholar] [CrossRef]
- Ribeiro, F.; Elvira, B.; Collares-Pereira, M.J.; Moyle, P.B. Life-History Traits of Non-Native Fishes in Iberian Watersheds across Several Invasion Stages: A First Approach. Biol. Invasions 2008, 10, 89–102. [Google Scholar] [CrossRef]
- Clavero, M.; Hermoso, V.; Aparicio, E.; Godinho, F.N. Biodiversity in Heavily Modified Waterbodies: Native and Introduced Fish in Iberian Reservoirs. Freshw. Biol. 2013, 58, 1190–1201. [Google Scholar] [CrossRef]
- Tedesco, P.A.; Sagnes, P.; Laroche, J. Variability in the Growth Rate of Chub Leuciscus Cephalus along a Longitudinal River Gradient. J. Fish. Biol. 2009, 74, 312–319. [Google Scholar] [CrossRef]
- Hermoso, V.; Clavero, M.; Blanco-Garrido, F.; Prenda, J. Invasive Species and Habitat Degradation in Iberian Streams: An Analysis of Their Role in Freshwater Fish Diversity Loss. Ecol. Appl. 2011, 21, 175–188. [Google Scholar] [CrossRef] [PubMed]
- Lytle, D.A. Disturbance Regimes and Life-History Evolution. Am. Nat. 2001, 157, 525–536. [Google Scholar] [CrossRef]
- Mims, M.C.; Olden, J.D. Life History Theory Predicts Fish Assemblage Response to Hydrologic Regimes. Ecology 2012, 93, 35–45. [Google Scholar] [CrossRef]
- Vila-Gispert, A.; Moreno-Amich, R. Mass-Length Relationship of Mediterranean Barbel as an Indicator of Environmental Status in South-West European Stream Ecosystems. J. Fish. Biol. 2001, 59, 824–832. [Google Scholar] [CrossRef]
- Langerhans, R.B. Predictability of Phenotypic Differentiation across Flow Regimes in Fishes. Integr. Comp. Biol. 2008, 48, 750–768. [Google Scholar] [CrossRef]
- Alexandre, C.M.; Quintella, B.R.; Ferreira, A.F.; Romão, F.A.; Almeida, P.R. Swimming Performance and Ecomorphology of the Iberian Barbel Luciobarbus Bocagei (Steindachner, 1864) on permanent and temporary rivers. Ecol. Freshw. Fish 2014, 23, 244–258. [Google Scholar] [CrossRef]
- Boavida, I.; Santos, J.M.; Ferreira, M.T.; Pinheiro, A. Barbel Habitat Alterations Due to Hydropeaking. J. Hydro-Environ. Res. 2015, 237–247. [Google Scholar] [CrossRef]
- Oliveira, J.M.; Ferreira, A.P.; Ferreira, M.T. Intrabasin Variations in Age and Growth of Barbus Bocagei Populations. J. Appl. Ichthyol. 2002, 18, 134–139. [Google Scholar] [CrossRef]
- Merciai, R.; Molons-Sierra, C.; Sabater, S.; García-Berthou, E. Water Abstraction Affects Abundance, Sizestructure and Growth of Two Threatened Cyprinid Fishes. PLoS ONE 2017, 12, e0175932. [Google Scholar] [CrossRef] [PubMed]
- Marr, S.M.; Olden, J.D.; Leprieur, F.; Arismendi, I.; Ćaleta, M.; Morgan, D.L.; Nocita, A.; Šanda, R.; Serhan Tarkan, A.; García-Berthou, E. A Global Assessment of Freshwater Fish Introductions in Mediterranean-Climate Regions. Hydrobiologia 2013, 719, 317–329. [Google Scholar] [CrossRef]
- Leunda, P.M. Impacts of Non-Native Fishes on Iberian Freshwater Ichthyofauna: Current Knowledge and Gaps. Aquat. Invasions 2010, 5, 239–262. [Google Scholar] [CrossRef]
- Martínez-Fernández, V.; Solana-Gutiérrez, J.; García de Jalón, D.; Alonso, C. Sign, Strength and Shape of Stream Fish-Based Metric Responses to Geo-Climatic and Human Pressure Gradients. Ecol. Indic. 2019, 104, 86–95. [Google Scholar] [CrossRef]
- Clavero, M.; García-Berthou, E. Homogenization Dynamics and Introduction Routes of Invasive Freshwater Fish in the Iberian Peninsula. Ecol. Appl. 2006, 16, 2313–2324. [Google Scholar] [CrossRef]
- Cucherousset, J.; Olden, J.D. Ecological Impacts of Non-Native Freshwater Fishes. Fisheries 2011, 36, 215–230. [Google Scholar] [CrossRef]
- García-Berthou, E.; Almeida, D.; Benejam, L.; Magellan, K.; Bae, M.J.; Casals, F.; Merciai, R. Impacto Ecológico de Los Peces Continentales Introducidos En La Península Ibérica. Ecosistemas 2015, 24, 36–42. [Google Scholar] [CrossRef]
- Rincón, P.A.; Velasco, J.C.; González-Sánchez, N.; Pollo, C. Fish Assemblages in Small Streams in Western Spain: The Influence of an Introducer Predator. Arch. Fur Hydrobiol. 1990, 118, 81–91. [Google Scholar]
- Domínguez, J.; Pena, J.C. Alimentación de Lucio Exos Lucius En Un Área de Reciente Colonización (Cuenca Del Esla, Noroeste de España). Variaciones En Función de La Talla. Ecología 2001, 15, 293–308. [Google Scholar]
- Pérez-Bote, J.L.; Roso, R. Diet of the Introduced Pikeperch Sander Lucioperca (L.) (Osteichthyes, Percidae) in a Recent Colonised Reservoir in South-Western Iberian Peninsula. Ital. J. Zool. 2012, 79, 617–626. [Google Scholar] [CrossRef]
- Bravo, R.; Soriguer, M.C.; Villar, N.; Hernando, J.A. The Dynamics of Fish Populations in the Palancar Stream, a Small Tributary of the River Guadalquivir, Spain. Acta Oecologica 2001, 22, 9–20. [Google Scholar] [CrossRef]
- Dettori, E.E.; Balestrieri, A.; Zapata-Pérez, V.M.; Bruno, D.; Rubio-Saura, N.; Robledano-Aymerich, F. Distribution and Diet of Recovering Eurasian Otter (Lutra Lutra) along the Natural-to-Urban Habitat Gradient (River Segura, SE Spain). Urban Ecosyst. 2021. [Google Scholar] [CrossRef]
- Ruiz-Navarro, A.; Gillingham, P.K.; Robert Britton, J. Shifts in the Climate Space of Temperate Cyprinid Fishes Due to Climate Change Are Coupled with Altered Body Sizes and Growth Rates. Glob. Chang. Biol. 2016, 22, 3221–3232. [Google Scholar] [CrossRef] [PubMed]
- IPCC. Climate Change and Land: An IPCC Special Report on Climate Change, Desertification, Land Degradation, Sustainable Land Management, Food Security, and Greenhouse Gas Fluxes in Terrestrial Ecosystems; Shukla, P.R., Skea, J., Calvo Buendia, E., Masson-Delmotte, V., Pörtner, H.-O., Roberts, D.C., Zhai, P., Slade, R., Connors, S., van Diemen, R., et al., Eds.; Intergovernmental Panel on Climate Change: Geneva, Switzerland, 2019. [Google Scholar]
| Sampling Site | Altitude | Ecological Status | IHF | RQI | Conductivity | Water Temperature | Free Reach | ICL | MDBF | Flow Variability |
|---|---|---|---|---|---|---|---|---|---|---|
| MU01 | 560 | 2 | 72 | 55 | 602 | 14.7 | 4.13 | 255 | 1.59 | −2.44 |
| MU02 | 540 | 2 | 81 | 85 | 619 | 14.7 | 2.43 | 255 | 1.59 | −2.44 |
| MU03 | 520 | 2 | 81 | 85 | 619 | 14.7 | 5.39 | 255 | 1.59 | −2.44 |
| MU04 | 480 | 3 | 69 | 43 | 639 | 14.7 | 3.25 | 205 | 8.58 | −1.71 |
| MU05 | 460 | 3 | 75 | 53 | 674 | 14.7 | 2.02 | 205 | 8.58 | −1.71 |
| MU06 | 430 | 3 | 70 | 64 | 688 | 14.7 | 3.67 | 205 | 8.58 | −1.71 |
| MU07 | 396 | 3 | 72 | 46 | 724 | 14.7 | 3.79 | 205 | 8.58 | −1.71 |
| MU08 | 354 | 3 | 70 | 34 | 1303 | 14.7 | 2.29 | 205 | 8.58 | −1.71 |
| MU09 | 350 | 4 | 67 | 39 | 1109 | 15.4 | 3.54 | 138 | 14.04 | −1.62 |
| SE01 | 860 | 3 | 78 | 94 | 364 | 14. 3 | 25.88 | 84 | 2.91 | −3.14 |
| SE02 | 685 | 1 | 61 | 98 | 387 | 14.3 | 32.33 | 84 | 2.91 | −3.14 |
| SE03 | 491 | 1 | 63 | 87 | 390 | 14.3 | 12.58 | 84 | 2.91 | −3.14 |
| SE04 | 470 | 1 | 67 | 78 | 340 | 13.8 | 30.02 | 0 | 4.14 | −2.58 |
| SE05 | 452 | 1 | 68 | 80 | 401 | 13.8 | 30.02 | 0 | 4.14 | −2.58 |
| SE06 | 432 | 1 | 78 | 98 | 401 | 13.8 | 30.02 | 0 | 4.14 | −2.58 |
| SE07 | 363 | 2 | 67 | 77 | 447 | 14.1 | 7.73 | 126 | 1.77 | −13.10 |
| SE08 | 325 | 2 | 73 | 66 | 492 | 14.1 | 5.96 | 126 | 1.77 | −13.10 |
| SE09 | 306 | 2 | 58 | 69 | 579 | 14.1 | 1.7 | 126 | 1.77 | −13.10 |
| SE10 | 290 | 2 | 62 | 32 | 756 | 15.9 | 7.18 | 126 | 14.04 | −1.62 |
| SE11 | 260 | 3 | 57 | 44 | 803 | 15.9 | 10.64 | 90 | 14.04 | −1.62 |
| SE12 | 200 | 2 | 66 | 70 | 861 | 16.4 | 4.98 | 106 | 14.22 | −1.55 |
| SE13 | 148 | 2 | 58 | 45 | 1139 | 16.4 | 6.88 | 365 | 15.14 | −1.47 |
| SE14 | 112 | 4 | 64 | 36 | 1157 | 16.8 | 4.36 | 365 | 2.46 | −3.31 |
| TAI | 640 | 2 | 61 | 43 | 566 | 14.7 | 15.67 | 58 | - | - |
| TUS | 809 | 1 | 84 | 65 | 432 | 14.5 | 12.81 | 76 | 1.59 | −2.44 |
| Env. Variables | PC1 | PC2 | PC3 |
|---|---|---|---|
| Ecological status | 0.817 | −0.150 | 0.202 |
| Fluvial Habitat Index (IHF) | 0.081 | 0.882 | 0.036 |
| Riparian Quality Index (RQI) | −0.648 | 0.427 | −0.286 |
| Altitude | −0.339 | 0.832 | −0.071 |
| Free reach | −0.909 | 0.052 | 0.087 |
| Longitudinal connectivity (ICL) | 0.869 | 0.021 | −0.068 |
| Conductivity | 0.750 | −0.443 | 0.393 |
| Water temperature | 0.520 | −0.534 | 0.466 |
| Mean daily base flow (MDBF) | 0.170 | −0.473 | 0.700 |
| Flow variability | −0.038 | 0.164 | 0.950 |
| Proportion of variance | 0.366 | 0.241 | 0.191 |
| SIZE-RELATED PARAMETERS | |||||
| Size range | Longitudinal gradient + Non-native species | ||||
| Estimate | Std. Error | t value | Pr(>|t|) | ||
| (Intercept) | 26.264 | 5.239 | 5.013 | 5.81 × 10−5 | *** |
| Longitudinal gradient | −4.660 | 1.921 | −2.426 | 0.024 | * |
| Non-native species | 18.278 | 8.792 | 2.079 | 0.050 | • |
| Mean size | Longitudinal gradient + Flow regime alteration | ||||
| Estimate | Std. Error | t value | Pr(>|t|) | ||
| (Intercept) | 18.420 | 1.114 | 16.537 | 1.62 × 10−13 | *** |
| Longitudinal gradient | −3.097 | 1.119 | −2.768 | 0.0115 | * |
| Flow regime alteration | 2.578 | 1.117 | 2.307 | 0.0313 | * |
| Maximum size | Longitudinal gradient + Non-native species | ||||
| Estimate | Std. Error | t value | Pr(>|t|) | ||
| (Intercept) | 29.456 | 5.913 | 4.981 | 6.27 × 10−5 | *** |
| Longitudinal gradient | −4.812 | 2.168 | −2.220 | 0.038 | * |
| Non-native species | 23.839 | 9.924 | 2.402 | 0.026 | * |
| Size diversity index | Longitudinal gradient + Habitat alteration and fragmentation | ||||
| Estimate | Std. Error | t value | Pr(>|t|) | ||
| (Intercept) | 2.231 | 0.051 | 43.977 | <2 × 10−16 | *** |
| Longitudinal gradient | −0.023 | 0.051 | −4.434 | 0.0002 | *** |
| Habitat alteration and fragmentation | 0.117 | 0.056 | 2.098 | 0.048 | * |
| ABUNDANCE (CPUE) | |||||
| CPUE | Null | ||||
| Estimate | Std. Error | t value | Pr(>|t|) | ||
| (Intercept) | 61.525 | 7.786 | 7.902 | 5.3 × 10−8 | *** |
| AGE-RELATED PARAMETERS | |||||
| Age range | Flow regime alteration | ||||
| Estimate | Std. Error | t value | Pr(>|t|) | ||
| (Intercept) | 8.765 | 0.519 | 16.900 | 4.36 × 10−14 | *** |
| Flow regime alteration | −1.586 | 0.520 | −3.048 | 0.006 | ** |
| Mean age | Non-native species | ||||
| Estimate | Std. Error | t value | Pr(>|t|) | ||
| (Intercept) | 3.237 | 0.749 | 4.324 | 2.73 × 10−4 | *** |
| Non-native species | 2.985 | 1.245 | 2.397 | 0.025 | * |
| Maximum age | Non-native species | ||||
| Estimate | Std. Error | t value | Pr(>|t|) | ||
| (Intercept) | 7.206 | 1.658 | 4.345 | 2.59 × 10−4 | *** |
| Non-native species | 5.060 | 2.759 | 1.834 | 0.080 | • |
| SOMATIC CONDITION | |||||
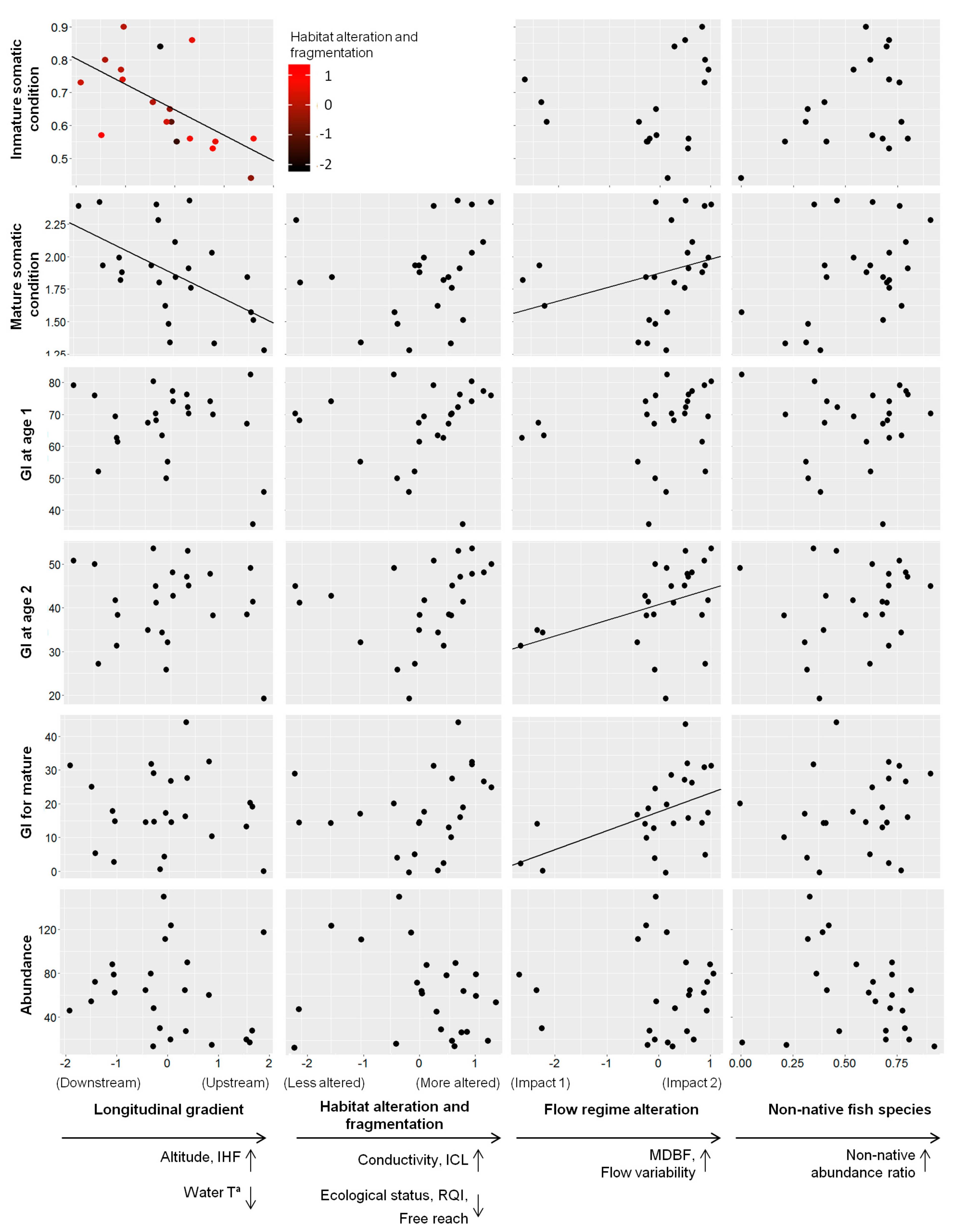
| Immature somatic condition | Longitudinal gradient * Habitat alteration and fragmentation | ||||
| Estimate | Std. Error | t value | Pr(>|t|) | ||
| (Intercept) | 0.642 | 0.023 | 28.162 | 9.97 × 10−14 | *** |
| Longitudinal gradient | −0.119 | 0.028 | −4.291 | 0.0007 | *** |
| Habitat alteration and fragmentation | −0.015 | 0.028 | −0.552 | 0.589 | |
| L. gradient: H. alter. and fragment. | 0.110 | 0.043 | 2.578 | 0.022 | * |
| Mature somatic condition | Longitudinal gradient + Flow regime alteration | ||||
| Estimate | Std. Error | t value | Pr(>|t|) | ||
| (Intercept) | 1.869 | 0.058 | 32.211 | <2 × 10−16 | *** |
| Longitudinal gradient | −0.188 | 0.058 | −3.228 | 0.004 | ** |
| Flow regime alteration | 0.107 | 0.058 | 1.844 | 0.080 | • |
| GROWTH | |||||
| Growth index (GI) at age 1 | Null | ||||
| Estimate | Std. Error | t value | Pr(>|t|) | ||
| (Intercept) | 66.676 | 2.387 | 27.930 | <2 × 10−16 | *** |
| Growth index (GI) at age 2 | Flow regime alteration | ||||
| Estimate | Std. Error | t value | Pr(>|t|) | ||
| (Intercept) | 40.724 | 1.723 | 23.639 | <2 × 10−16 | *** |
| Flow regime alteration | 3.607 | 1.728 | 2.087 | 0.049 | * |
| Growth index (GI) for mature | Flow regime alteration | ||||
| Estimate | Std. Error | t value | Pr(>|t|) | ||
| (Intercept) | 18.165 | 2.042 | 8.894 | 9.73 × 10−9 | *** |
| Flow regime alteration | 5.614 | 2.049 | 2.740 | 0.012 | * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Pérez, A.; Oliva-Paterna, F.J.; Amat-Trigo, F.; Torralva, M. Variability in Population Traits of a Sentinel Iberian Fish in a Highly Modified Mediterranean-Type River. Water 2021, 13, 747. https://doi.org/10.3390/w13060747
Sánchez-Pérez A, Oliva-Paterna FJ, Amat-Trigo F, Torralva M. Variability in Population Traits of a Sentinel Iberian Fish in a Highly Modified Mediterranean-Type River. Water. 2021; 13(6):747. https://doi.org/10.3390/w13060747
Chicago/Turabian StyleSánchez-Pérez, Ana, Francisco J. Oliva-Paterna, Fátima Amat-Trigo, and Mar Torralva. 2021. "Variability in Population Traits of a Sentinel Iberian Fish in a Highly Modified Mediterranean-Type River" Water 13, no. 6: 747. https://doi.org/10.3390/w13060747
APA StyleSánchez-Pérez, A., Oliva-Paterna, F. J., Amat-Trigo, F., & Torralva, M. (2021). Variability in Population Traits of a Sentinel Iberian Fish in a Highly Modified Mediterranean-Type River. Water, 13(6), 747. https://doi.org/10.3390/w13060747






