Fine and Coarse Carbonaceous Aerosol in Houston, TX, during DISCOVER-AQ
Abstract
1. Introduction
2. Methods and Materials
2.1. Sampling
2.2. Sample and Measurement Analysis
2.2.1. Bulk Carbon Analysis
2.2.2. BC Corrections
2.2.3. Radiocarbon Analysis of TC
2.3. 14C Source Apportionment of TC
3. Results and Discussion
3.1. Bulk Carbon Measurements
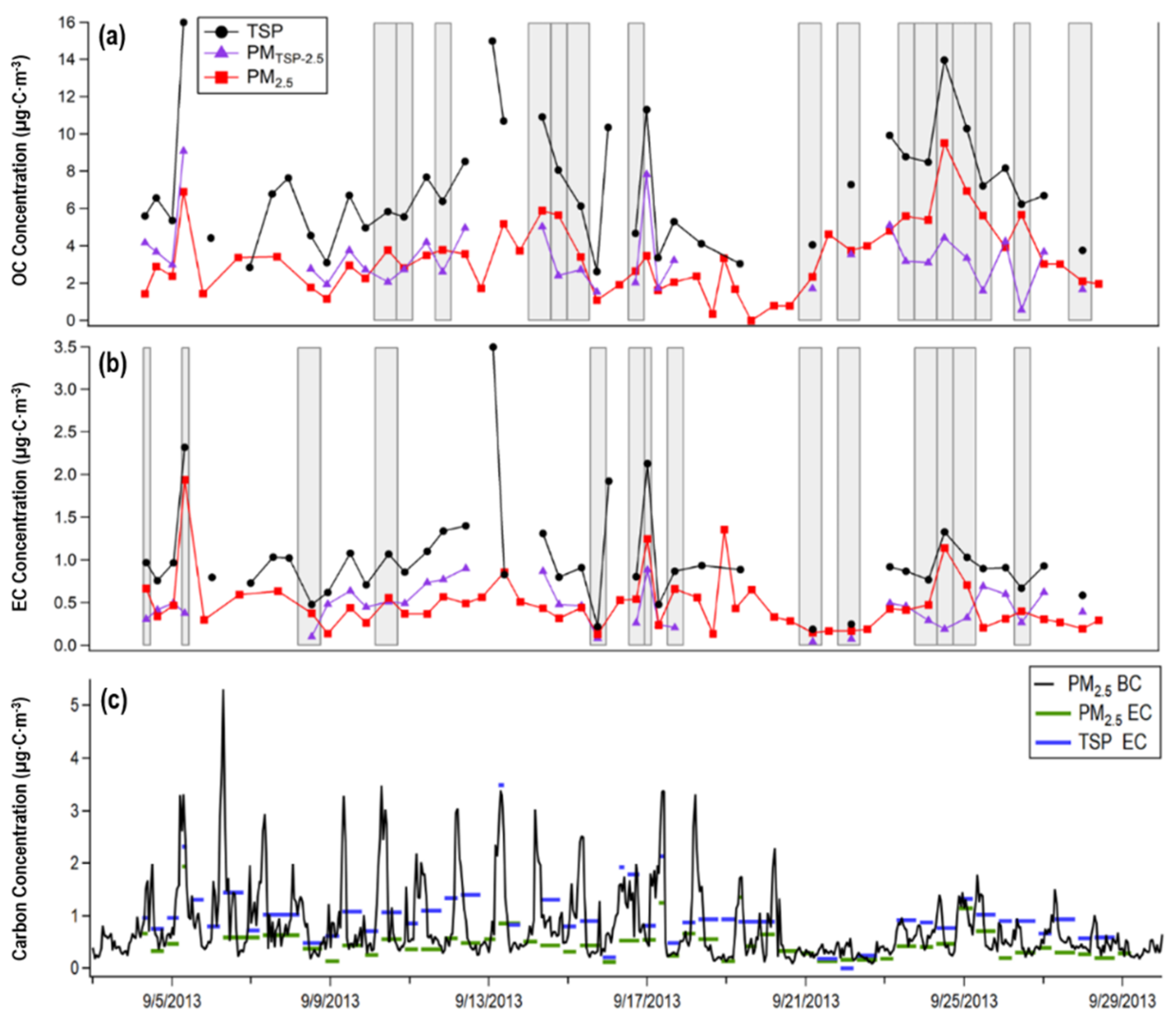
3.1.1. Carbonaceous Aerosols Trends of PM2.5 at MT
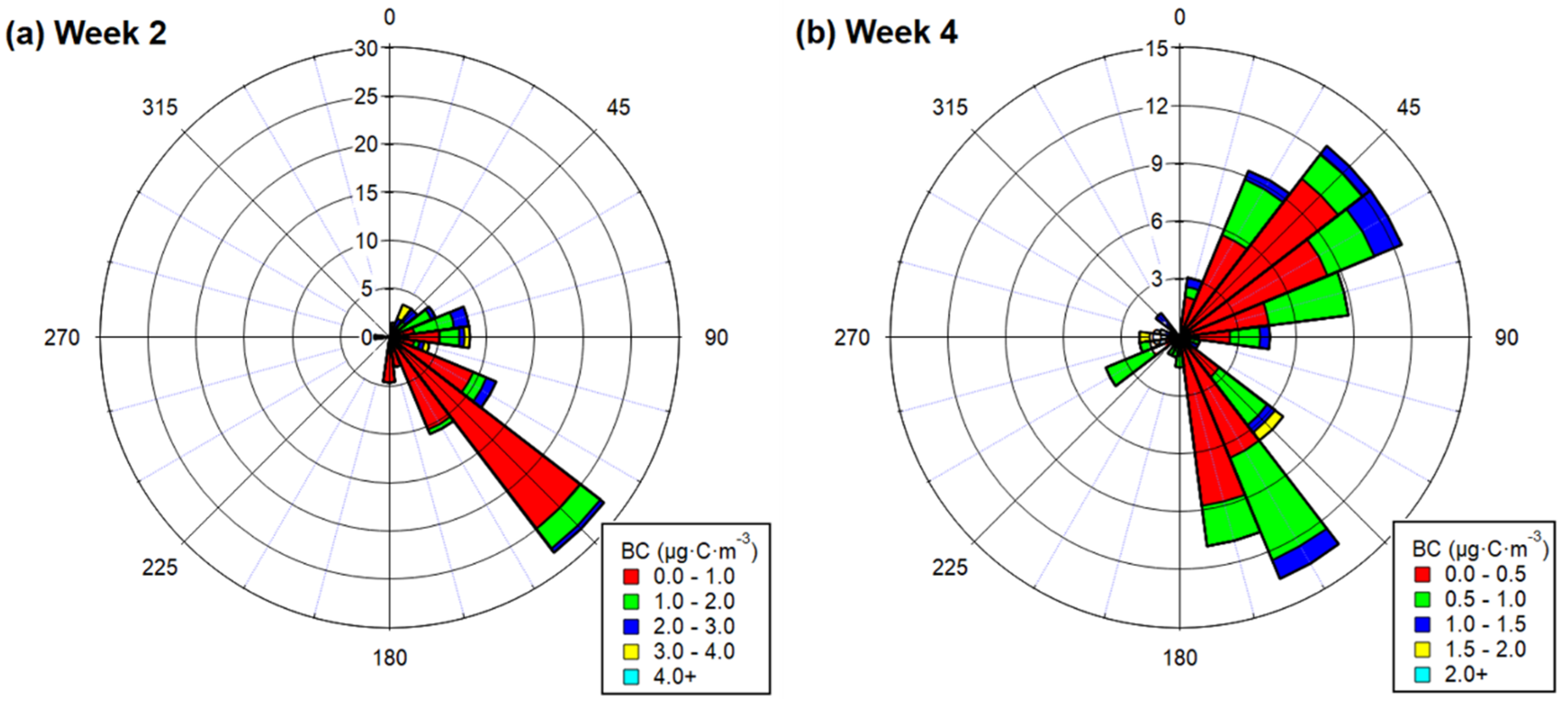
3.1.2. BC and EC Comparison
3.1.3. Carbonaceous Aerosol Trends of TSP
3.1.4. Comparison of Carbonaceous Aerosols Between PM2.5 and TSP
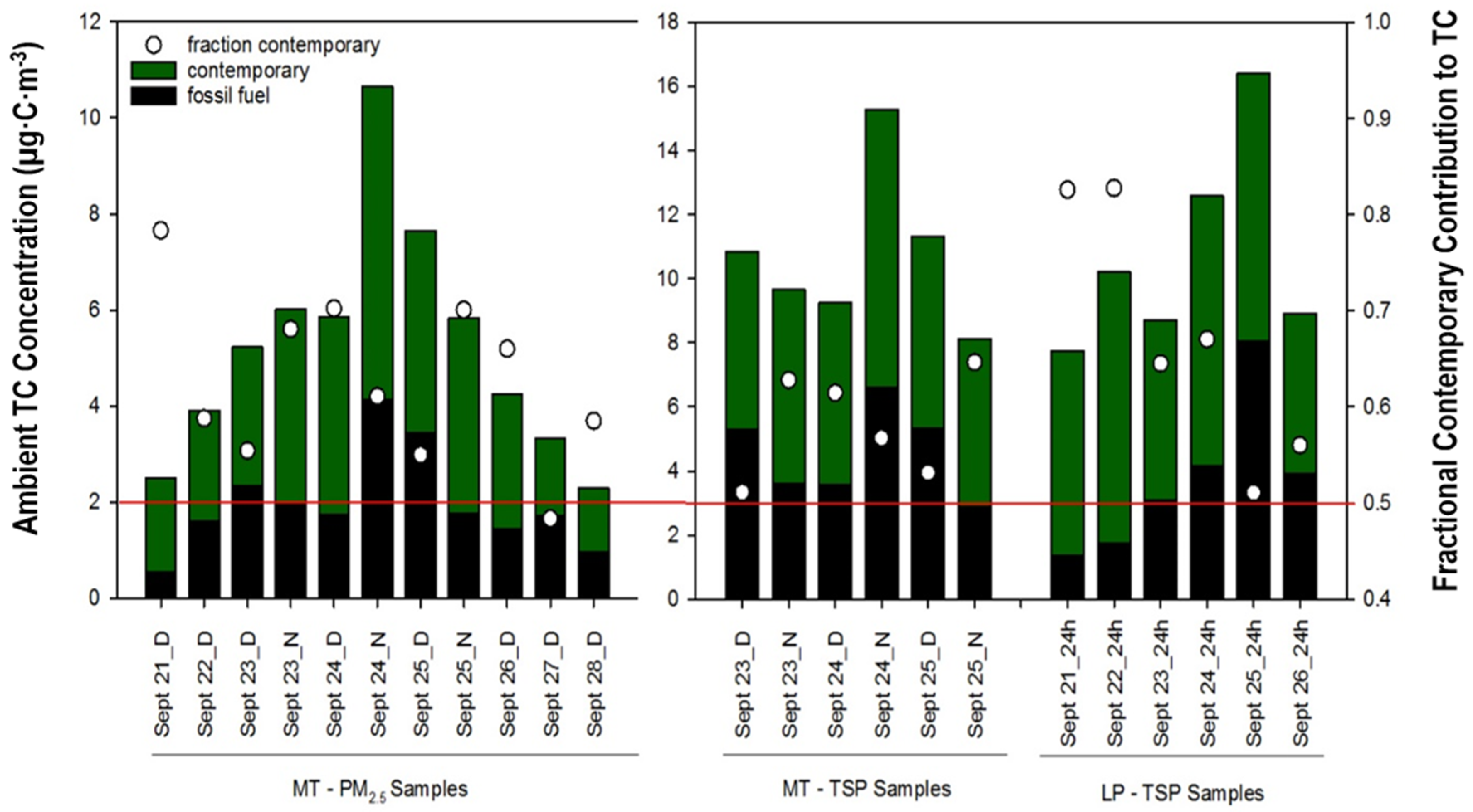
3.2. 14C-based Apportionment of PM2.5 and TSP
Fossil and Contemporary Carbon for TSP versus PM2.5
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chung, C.E.; Ramanathan, V.; Decremer, D. Observationally constrained estimates of carbonaceous aerosol radiative forcing. Proc. Natl. Acad. Sci. USA 2012, 109, 11624–11629. [Google Scholar] [CrossRef]
- Spracklen, D.; Carslaw, K.; Pöschl, U.; Rap, A.; Forster, P. Global cloud condensation nuclei influenced by carbonaceous combustion aerosol. Atmos. Chem. Phys. 2011, 11, 9067–9087. [Google Scholar] [CrossRef]
- Novakov, T.; Penner, J. Large contribution of organic aerosols to cloud-condensation-nuclei concentrations. Nature 1993, 365, 823–826. [Google Scholar] [CrossRef]
- Kanakidou, M.; Seinfeld, J.; Pandis, S.; Barnes, I.; Dentener, F.; Facchini, M.; Dingenen, R.V.; Ervens, B.; Nenes, A.; Nielsen, C. Organic aerosol and global climate modelling: A review. Atmos. Chem. Phys. 2005, 5, 1053–1123. [Google Scholar] [CrossRef]
- Tsigaridis, K.; Daskalakis, N.; Kanakidou, M.; Adams, P.; Artaxo, P.; Bahadur, R.; Balkanski, Y.; Bauer, S.; Bellouin, N.; Benedetti, A. The AeroCom evaluation and intercomparison of organic aerosol in global models. Atmos. Chem. Phys. 2014, 14, 10845–10895. [Google Scholar] [CrossRef]
- Dockery, D.W.; Pope, C.A. Acute respiratory effects of particulate air pollution. Ann. Rev. Public Health 1994, 15, 107–132. [Google Scholar] [CrossRef]
- Brook, R.D.; Rajagopalan, S.; Pope, C.A.; Brook, J.R.; Bhatnagar, A.; Diez-Roux, A.V.; Holguin, F.; Hong, Y.; Luepker, R.V.; Mittleman, M.A. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation 2010, 121, 2331–2378. [Google Scholar] [CrossRef]
- Laden, F.; Neas, L.M.; Dockery, D.W.; Schwartz, J. Association of fine particulate matter from different sources with daily mortality in six US cities. Environ. Health Perspect. 2000, 108, 941. [Google Scholar] [CrossRef]
- Delfino, R.J.; Murphy-Moulton, A.M.; Becklake, M.R. Emergency room visits for respiratory illnesses among the elderly in Montreal: Association with low level ozone exposure. Environ. Res. 1998, 76, 67–77. [Google Scholar] [CrossRef]
- Chen, R.; Zhao, Z.; Kan, H. Heavy smog and hospital visits in Beijing, China. Am. J. Respir. Crit. Care Med. 2013, 188, 1170–1171. [Google Scholar] [CrossRef]
- United States Census Burea: QuickFacts Houston City, Texas. Available online: https://www.census.gov/quickfacts/fact/table/houstoncitytexas/PST045219 (accessed on 28 January 2020).
- Sullivan, D.W.; Price, J.H.; Lambeth, B.; Sheedy, K.A.; Savanich, K.; Tropp, R.J. Field study and source attribution for PM2.5 and PM10 with resulting reduction in concentrations in the neighborhood north of the Houston Ship Channel based on voluntary efforts. J. Air Waste Manag. Assoc. 2013, 63, 1070–1082. [Google Scholar] [CrossRef] [PubMed]
- Wallace, H.W.; Sanchez, N.P.; Flynn, J.H.; Erickson, M.H.; Lefer, B.L.; Griffin, R.J. Source apportionment of particulate matter and trace gases near a major refinery near the Houston Ship Channel. Atmos. Environ. 2018, 173, 16–29. [Google Scholar] [CrossRef]
- Zhang, X.; Craft, E.; Zhang, K. Characterizing spatial variability of air pollution from vehicle traffic around the Houston Ship Channel area. Atmos. Environ. 2017, 161, 167–175. [Google Scholar] [CrossRef]
- Schulze, B.C.; Wallace, H.W.; Bui, A.T.; Flynn, J.H.; Erickson, M.H.; Alvarez, S.; Dai, Q.; Usenko, S.; Sheesley, R.J.; Griffin, R.J. The impacts of regional shipping emissions on the chemical characteristics of coastal submicron aerosols near Houston, TX. Atmos. Chem. Phys. 2018, 18, 14217–14241. [Google Scholar] [CrossRef]
- Park, C.; Schade, G.W.; Boedeker, I. Characteristics of the flux of isoprene and its oxidation products in an urban area. J. Geophys. Res. Atmos. 2011, 116, D21303. [Google Scholar] [CrossRef]
- Bean, J.K.; Faxon, C.B.; Leong, Y.J.; Wallace, H.W.; Cevik, B.K.; Ortiz, S.; Canagaratna, M.R.; Usenko, S.; Sheesley, R.J.; Griffin, R.J. Composition and Sources of Particulate Matter Measured near Houston, TX: Anthropogenic-Biogenic Interactions. Atmosphere 2016, 7, 73. [Google Scholar] [CrossRef]
- Leong, Y.; Sanchez, N.; Wallace, H.; Cevik, B.K.; Hernandez, C.; Han, Y.; Flynn, J.; Massoli, P.; Floerchinger, C.; Fortner, E. Overview of surface measurements and spatial characterization of submicrometer particulate matter during the DISCOVER-AQ 2013 campaign in Houston, TX. J. Air Waste Manag. Assoc. 2017, 67, 854–872. [Google Scholar] [CrossRef]
- DISCOVER-AQ. Available online: https://discover-aq.larc.nasa.gov/ (accessed on 1 August 2015).
- Yoon, S.; Ortiz, S.; Clark, A.E.; Barrett, T.E.; Usenko, S.; Duvall, R.M.; Ruiz, L.H.; Bean, J.K.; Faxon, C.B.; Flynn, J.; et al. Apportioned primary and secondary organic aerosol during pollution events of DISCOVER-AQ Houston. Atmos. Environ. under review.
- Mazzuca, G.M.; Ren, X.; Loughner, C.P.; Estes, M.; Crawford, J.H.; Pickering, K.E.; Weinheimer, A.J.; Dickerson, R.R. Ozone production and its sensitivity to NOx and VOCs: Results from the DISCOVER-AQ field experiment, Houston 2013. Atmos. Chem. Phys. 2016, 16, 14463–14474. [Google Scholar] [CrossRef]
- Baier, B.C.; Brune, W.H.; Lefer, B.L.; Miller, D.O.; Martins, D.K. Direct ozone production rate measurements and their use in assessing ozone source and receptor regions for Houston in 2013. Atmos. Environ. 2015, 114, 83–91. [Google Scholar] [CrossRef]
- Hansen, A.; Rosen, H.; Novakov, T. Aethalometer—An Instrument for the Real-Time Measurement of Optical Absorption by Aerosol Particles; Lawrence Berkeley Lab.: Berkeley, CA, USA, 1983. [Google Scholar]
- Chow, J.C.; Watson, J.G.; Crow, D.; Lowenthal, D.H.; Merrifield, T. Comparison of IMPROVE and NIOSH carbon measurements. Aerosol Sci. Technol. 2001, 34, 23–34. [Google Scholar] [CrossRef]
- Clark, A.E.; Yoon, S.; Sheesley, R.J.; Usenko, S. Pressurized liquid extraction technique for the analysis of pesticides, PCBs, PBDEs, OPEs, PAHs, alkanes, hopanes, and steranes in atmospheric particulate matter. Chemosphere 2015, 137, 33–44. [Google Scholar] [CrossRef]
- Birch, M.; Cary, R. Elemental carbon-based method for monitoring occupational exposures to particulate diesel exhaust. Aerosol Sci. Technol. 1996, 25, 221–241. [Google Scholar] [CrossRef]
- Schauer, J.J. Evaluation of elemental carbon as a marker for diesel particulate matter. J. Expo. Sci. Environ. Epidemiol. 2003, 13, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Barrett, T.E.; Sheesley, R.J. Urban impacts on regional carbonaceous aerosols: Case study in central Texas. J. Air Waste Manag. Assoc. 2014, 64, 917–926. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cachier, H.; Bremond, M.-P.; Buat-Menard, P. Determination of atmospheric soot carbon with a simple thermal method. Tellus B 1989, 41, 379–390. [Google Scholar] [CrossRef]
- Tian, S.; Pan, Y.; Liu, Z.; Wen, T.; Wang, Y. Reshaping the size distribution of aerosol elemental carbon by removal of coarse mode carbonates. Atmos. Environ. 2019, 214, 116852. [Google Scholar] [CrossRef]
- Edgerton, E.S.; Casuccio, G.S.; Saylor, R.D.; Lersch, T.L.; Hartsell, B.E.; Jansen, J.J.; Hansen, D.A. Measurements of OC and EC in coarse particulate matter in the southeastern United States. J. Air Waste Manag. Assoc. 2009, 59, 78–90. [Google Scholar] [CrossRef]
- Snyder, D.C.; Schauer, J.J. An inter-comparison of two black carbon aerosol instruments and a semi-continuous elemental carbon instrument in the urban environment. Aerosol Sci. Technol. 2007, 41, 463–474. [Google Scholar] [CrossRef]
- Schmid, O.; Artaxo, P.; Arnott, W.; Chand, D.; Gatti, L.V.; Frank, G.; Hoffer, A.; Schnaiter, M.; Andreae, M. Spectral light absorption by ambient aerosols influenced by biomass burning in the Amazon Basin. I: Comparison and field calibration of absorption measurement techniques. Atmos. Chem. Phys. 2006, 6, 3443–3462. [Google Scholar] [CrossRef]
- Stuiver, M.; Polach, H.A. Discussion; reporting of C-14 data. Radiocarbon 1977, 19, 355–363. [Google Scholar] [CrossRef]
- Zotter, P.; El-Haddad, I.; Zhang, Y.; Hayes, P.L.; Zhang, X.; Lin, Y.H.; Wacker, L.; Schnelle-Kreis, J.; Abbaszade, G.; Zimmermann, R. Diurnal cycle of fossil and nonfossil carbon using radiocarbon analyses during CalNex. J. Geophys. Res. Atmos. 2014, 119, 6818–6835. [Google Scholar] [CrossRef]
- Gustafsson, Ö.; Kruså, M.; Zencak, Z.; Sheesley, R.J.; Granat, L.; Engström, E.; Praveen, P.; Rao, P.; Leck, C.; Rodhe, H. Brown clouds over South Asia: Biomass or fossil fuel combustion? Science 2009, 323, 495–498. [Google Scholar] [CrossRef] [PubMed]
- Chow, J.C.; Watson, J.G.; Fujita, E.M.; Lu, Z.; Lawson, D.R.; Ashbaugh, L.L. Temporal and spatial variations of PM2.5 and PM10 aerosol in the Southern California air quality study. Atmos. Environ. 1994, 28, 2061–2080. [Google Scholar] [CrossRef]
- Benetello, F.; Squizzato, S.; Hofer, A.; Masiol, M.; Khan, M.B.; Piazzalunga, A.; Fermo, P.; Formenton, G.M.; Rampazzo, G.; Pavoni, B. Estimation of local and external contributions of biomass burning to PM 2.5 in an industrial zone included in a large urban settlement. Environ. Sci. Pollut. Res. 2017, 24, 2100–2115. [Google Scholar] [CrossRef]
- Turpin, B.J.; Huntzicker, J.J. Identification of secondary organic aerosol episodes and quantitation of primary and secondary organic aerosol concentrations during SCAQS. Atmos. Environ. 1995, 29, 3527–3544. [Google Scholar] [CrossRef]
- Ram, K.; Sarin, M. Day–night variability of EC, OC, WSOC and inorganic ions in urban environment of Indo-Gangetic Plain: Implications to secondary aerosol formation. Atmos. Environ. 2011, 45, 460–468. [Google Scholar] [CrossRef]
- Zhang, Y.; Zotter, P.; Perron, N.; Prévôt, A.; Wacker, L.; Szidat, S. Fossil and non-fossil sources of different carbonaceous fractions in fine and coarse particles by radiocarbon measurement. Radiocarbon 2013, 55, 1510–1520. [Google Scholar] [CrossRef]
- Hong, L.; Liu, G.; Zhou, L.; Li, J.; Xu, H.; Wu, D. Emission of organic carbon, elemental carbon and water-soluble ions from crop straw burning under flaming and smoldering conditions. Particuology 2017, 31, 181–190. [Google Scholar] [CrossRef]
- Viana, M.; Maenhaut, W.; Brink, H.T.; Chi, X.; Weijers, E.; Querol, X.; Alastuey, A.; Mikuška, P.; Večeřa, Z. Comparative analysis of organic and elemental carbon concentrations in carbonaceous aerosols in three European cities. Atmos. Environ. 2007, 41, 5972–5983. [Google Scholar] [CrossRef]
- Zeng, T.; Wang, Y. Nationwide summer peaks of OC/EC ratios in the contiguous United States. Atmos. Environ. 2011, 45, 578–586. [Google Scholar] [CrossRef]
- Blanchard, C.L.; Hidy, G.M.; Tanenbaum, S.; Edgerton, E.; Hartsell, B.; Jansen, J. Carbon in southeastern US aerosol particles: Empirical estimates of secondary organic aerosol formation. Atmos. Environ. 2008, 42, 6710–6720. [Google Scholar] [CrossRef]
- Kim, B.M.; Teffera, S.; Zeldin, M.D. Characterization of PM25 and PM10 in the South Coast air basin of Southern California: Part 1—Spatial variations. J. Air Waste Manag. Assoc. 2000, 50, 2034–2044. [Google Scholar] [CrossRef] [PubMed]
- Rattigan, O.V.; Felton, H.D.; Bae, M.-S.; Schwab, J.J.; Demerjian, K.L. Multi-year hourly PM2.5 carbon measurements in New York: Diurnal, day of week and seasonal patterns. Atmos. Environ. 2010, 44, 2043–2053. [Google Scholar] [CrossRef]
- Czader, B.H.; Choi, Y.; Li, X.; Alvarez, S.; Lefer, B. Impact of updated traffic emissions on HONO mixing ratios simulated for urban site in Houston, Texas. Atmos. Chem. Phys. 2015, 15, 1253–1263. [Google Scholar] [CrossRef]
- Levy, M.E.; Zhang, R.; Khalizov, A.F.; Zheng, J.; Collins, D.R.; Glen, C.R.; Wang, Y.; Yu, X.Y.; Luke, W.; Jayne, J.T. Measurements of submicron aerosols in Houston, Texas during the 2009 SHARP field campaign. J. Geophys. Res. Atmos. 2013, 118, 10518–10534. [Google Scholar] [CrossRef]
- Shakya, K.M.; Louchouarn, P.; Griffin, R.J. Lignin-derived phenols in Houston aerosols: Implications for natural background sources. Environ. Sci. Technol. 2011, 45, 8268–8275. [Google Scholar] [CrossRef]
- Kavouras, I.G.; Stephanou, E.G. Particle size distribution of organic primary and secondary aerosol constituents in urban, background marine, and forest atmosphere. J. Geophys. Res. Atmos. 2002, 107, AAC 7-1–AAC 7-12. [Google Scholar] [CrossRef]
- Shiraiwa, M.; Yee, L.D.; Schilling, K.A.; Loza, C.L.; Craven, J.S.; Zuend, A.; Ziemann, P.J.; Seinfeld, J.H. Size distribution dynamics reveal particle-phase chemistry in organic aerosol formation. Proc. Natl. Acad. Sci. USA 2013, 110, 11746–11750. [Google Scholar] [CrossRef]
- Offenberg, J.H.; Baker, J.E. Aerosol size distributions of elemental and organic carbon in urban and over-water atmospheres. Atmos. Environ. 2000, 34, 1509–1517. [Google Scholar] [CrossRef]
- Shahid, I.; Kistler, M.; Mukhtar, A.; Ghauri, B.M.; Ramirez-Santa Cruz, C.; Bauer, H.; Puxbaum, H. Chemical characterization and mass closure of PM10 and PM2.5 at an urban site in Karachi–Pakistan. Atmos. Environ. 2016, 128, 114–123. [Google Scholar] [CrossRef]
- Lee, T.; Yu, X.-Y.; Ayres, B.; Kreidenweis, S.M.; Malm, W.C.; Collett, J.L., Jr. Observations of fine and coarse particle nitrate at several rural locations in the United States. Atmos. Environ. 2008, 42, 2720–2732. [Google Scholar] [CrossRef]
- Caicedo, V.; Rappenglueck, B.; Cuchiara, G.; Flynn, J.; Ferrare, R.; Scarino, A.; Berkoff, T.; Senff, C.; Langford, A.; Lefer, B. Bay Breeze and Sea Breeze Circulation Impacts on the Planetary Boundary Layer and Air Quality From an Observed and Modeled DISCOVER-AQ Texas Case Study. J. Geophys. Res. Atmos. 2019, 124, 7359–7378. [Google Scholar] [CrossRef]
- Dunker, A.M.; Koo, B.; Yarwood, G. Source apportionment of organic aerosol and ozone and the effects of emission reductions. Atmos. Environ. 2019, 198, 89–101. [Google Scholar] [CrossRef]

| Site | Size Fraction | Sampler Type | Samples | Sample Duration | Analysis |
|---|---|---|---|---|---|
| MT | PM2.5 | Tisch | 4–28 September | morning, afternoon, day | OC EC |
| 21–28 September | day | 14C | |||
| MV URG | 6–28 September | 24-h | OC EC | ||
| 23–25 September | 24-h | 14C | |||
| LP | TSP | HV Tisch | 4–28 September | morning, afternoon, day, night, 24-h | OC EC |
| 23–25 September | day and night | 14C | |||
| HV Tisch | 21–26 September | 24-h | OC EC, 14C |
| PM Type | Sample Type | Sample No. | Average OC (±SD) (µg·C·m−3) | Max. OC Sample | Max. OC Conc (µg·C·m−3) |
|---|---|---|---|---|---|
| PM2.5 | morning | 4 | 3.79 ± 2.27 | 5 September | 6.90 ± 0.71 |
| afternoon | 3 | 2.06 ± 0.72 | 4 September | 2.89 ± 0.27 | |
| day | 19 | 3.55 ± 1.56 | 25 September | 6.94 ± 0.44 | |
| night 1 | 22 | 3.21 ± 2.25 | 24 September | 9.51 ± 0.89 | |
| TSP | morning | 5 | 11.64 ± 4.13 | 5 September | 15.98 ± 1.14 |
| afternoon | 3 | 6.87 ± 3.67 | 13 September | 10.69 ± 0.78 | |
| day | 16 | 7.24 ± 2.12 | 14 September | 10.92 ± 0.60 | |
| night | 16 | 6.07 ± 2.78 | 24 September | 13.95 ± 0.83 |
| PM Type | Sample Type | Sample No. | Average EC (±SD) (µg·C·m−3) | Max. EC Sample | Max. EC Conc (µg·C·m−3) |
|---|---|---|---|---|---|
| PM2.5 | morning | 4 | 1.30 ± 0.52 | September 5 | 1.94 ± 0.40 |
| afternoon | 3 | 0.34 ± 0.10 | September 19 | 0.43 ± 0.14 | |
| day | 19 | 0.43 ± 0.18 | September 13 | 0.86 ± 0.12 | |
| night 1 | 22 | 0.40 ± 0.24 | September 24 | 1.14 ± 0.21 | |
| TSP | morning | 5 | 2.17 ± 0.90 | September 13 | 3.49 ± 0.46 |
| afternoon | 3 | 0.69 ± 0.18 | September 13 | 0.83 ± 0.28 | |
| day | 16 | 0.87 ± 0.34 | September 12 | 1.40 ± 0.16 | |
| night | 16 | 0.84 ± 0.26 | September 11 | 1.34 ± 0.20 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoon, S.; Usenko, S.; Sheesley, R.J. Fine and Coarse Carbonaceous Aerosol in Houston, TX, during DISCOVER-AQ. Atmosphere 2020, 11, 482. https://doi.org/10.3390/atmos11050482
Yoon S, Usenko S, Sheesley RJ. Fine and Coarse Carbonaceous Aerosol in Houston, TX, during DISCOVER-AQ. Atmosphere. 2020; 11(5):482. https://doi.org/10.3390/atmos11050482
Chicago/Turabian StyleYoon, Subin, Sascha Usenko, and Rebecca J. Sheesley. 2020. "Fine and Coarse Carbonaceous Aerosol in Houston, TX, during DISCOVER-AQ" Atmosphere 11, no. 5: 482. https://doi.org/10.3390/atmos11050482
APA StyleYoon, S., Usenko, S., & Sheesley, R. J. (2020). Fine and Coarse Carbonaceous Aerosol in Houston, TX, during DISCOVER-AQ. Atmosphere, 11(5), 482. https://doi.org/10.3390/atmos11050482







