Abstract
Hypophthalmichthys molitrix is one of the four most important fish in China and has high breeding potential. However, simple sequence repeat (SSR) markers developed on H. molitrix genome level for genetic diversity analysis are limited. In this study, the distribution characteristics of SSRs in the assembled H. molitrix genome were analyzed, and new markers were developed to preliminarily evaluate the genetic diversity of the four breeding populations. A total of 368,572 SSRs were identified from the H. molitrix genome. The total length of SSRs was 6,492,076 bp, accounting for 0.77% of the total length of the genome sequence. The total frequency and total density were 437.73 loci/Mb and 7713.16 bp/Mb, respectively. Among the 2–6 different nucleotide repeat types, SSRs were dominated by di-nucleotide repeats (204,873, 55.59%), and AC/GT was the most abundant motif. The number of SSRs on each chromosome was positively correlated with the length. The 13 pairs of markers developed were used to analyze the genetic diversity of four cultivated populations in Hubei Province. The results showed that the genetic diversity of the four populations was low, and the ranges of alleles (Na), effective alleles (Ne), observed heterozygosity (Ho), and Shannon’s index information (I) were 3.538–4.462, 2.045–2.461, 0.392–0.450, and 0.879–0.954, respectively. Genetic variation occurs mainly among individuals within populations (95.35%). UPGMA tree and Bayesian analysis showed that four populations could be divided into two different branches. Therefore, the genome-wide SSRs were effectively in genetic diversity analysis on H. molitrix.
1. Introduction
Silver carp (H. molitrix), as one of the four dominant fish in China, are mainly fed on phytoplankton and live in the upper and middle layers of water. They are widely distributed in ponds, lakes, rivers, and other major freshwater ecosystems in China and Asia [1]. Due to its fast growth, low breeding cost, high economic benefit, and purification role of water quality, it has become an important freshwater economic fish [2]. Before the implementation of the ten-year fishing ban in the Yangtze River, more attention was paid to the analysis of genetic diversity and genetic structure of natural populations, and less attention was paid to the genetic background of breeding populations. Moreover, breeding populations might affect the genetic diversity and adaptability of natural populations through the hatchery release of H. molitrix [3]. Therefore, it is necessary to further develop effective genetic markers to evaluate the genetic diversity of H. molitrix breeding populations.
Microsatellites, also known as simple sequence repeats (SSRs), are DNA sequences composed of 1–6 bases as repeat units, which widely exist in the coding and non-coding regions of eukaryotic and prokaryotic genomes [4,5]. SSRs have been widely used in genetic relationship identification [6], population genetic structure analysis [7], genetic breeding [8], molecular-marker-assisted selection [9], gender marker detection [10], genetic linkage map construction [11], and other studies because of their high polymorphism, codominant inheritance, and easy detection. Traditional SSR markers are mainly developed by enrichment methods, genomic–SSR hybrid screening, and primer sharing of related species, but these methods are complicated in operation, long experimental cycle, and are easily affected by some human factors [12]. In recent years, with the rapid development of sequencing technology, a lot of the genomic information of non-model organisms has been reported, which provides basic data for the mass development of SSR markers [13].
So far, limited previous studies focused on SSRs markers development of H. molitrix. One hundred and fifty-nine SSR sites were obtained by transcriptome sequences of H. molitrix [14]. Guo et al. [15] developed 134 polymorphic SSR markers and used 40 pairs for population genetic diversity analysis. However, the screening and development of SSR markers at the H. molitrix genome level have not been studied. In the present study, we screened SSR repeats at the whole H. molitrix genome level (unpublished, in our lab), analyzed the number, frequency, and type of SSRs, developed 13 SSR markers, and analyzed the genetic diversity of four H. molitrix cultivated populations. The study provides SSR characteristics of the H. molitrix genome and useful markers for analysis in genetic diversity and germplasm resources protection and utilization.
2. Materials and Methods
2.1. Sample Collections and DNA Extraction
One hundred and twenty samples of four cultured H. molitrix populations were collected from Shishou (SS), Wuhan (WH), Xiaochang (XC), and Yaowan (YW) (Table 1), respectively, in Hubei Province in 2021. The tail fins were sampled and stored in anhydrous ethanol at −20 °C. Genomic DNA of samples were extracted using a high-salt method [16]. After extraction, the quality of the DNA was detected by 1% agarose gel electrophoresis and a UV gel imaging system. DNA concentrations were measured by NanoPhotometer® spectrophotometer (IMPLEN, München, Germany) and diluted with sterile double-distilled water to 50 ng/μL (Table 1).

Table 1.
Sample information of the cultured H. molitrix populations in this study.
2.2. Identification of Genome-Wide SSRs
MISA 2.1 software (Leibniz institute, IPK, Germany. http://pgrc.ipk-gatersleben.de/misa/ (accessed on 16 April 2021)) was used to search SSRs in the H. molitrix genome. SSR screening criteria were as follows: di-nucleotide repeats more than 6 times, tri-nucleotide repeats more than 5 times, tetra-nucleotide repeats more than 4 times, and penta- and hexa-nucleotide repeats more than 3 times. Compound SSRs were defined as the interval between two repeat motifs less than 100 bp. Due to the principle of complementary base pairing, the same kind of repetitive SSRs were merged as a repetitive representation. Di-nucleotide AC (AC/TG/CA/GT), tri-, tetra-, penta-, and hexa-nucleotides follow the same principles.
2.3. Primer Design for Genome-Wide SSRs
SSR primers were designed using the Primer 3.0 software according to the flanking sequence of SSRs. The design principles of primers were as follows: the primer sequence from the core sequence was 50~80 bases, the PCR amplification product was from 100 to 400 bp, and the annealing temperature was from 50 °C to 60 °C. GC content ranged from 40% to 60%.
2.4. Verification of SSRs Using PCR Amplification
Ninety-six pairs of SSR primers with three bases and above were designed and synthesized by Tianyi Huiyuan Biotech Company, Wuhan, China (Table S1). The PCR reaction system contained 5.0 μL 2 × Taq PCR Master Mix, 1 μL template DNA (20 ng/μL), 0.5 μL of each primer (10 μL/mol), and DNase-/RNase-free deionized water 3.0 μL. Two-stage amplification programs were used. In the first stage, the pre-denaturation at 95 °C for 5 min caused the annealing temperature to gradually decrease from 62 °C to 52 °C, with a total of 10 cycles. The second stage included 25 amplification cycles, and the annealing temperature was 52 °C. In these two stages, the denaturation and extension steps remained unchanged for 30 s at 95 °C and 72 °C, respectively. After the second stage, the final extension was carried out at 72 °C for 20 min. Ninety-six pairs of primers were selected for PCR amplification and detected by 1% agarose gel electrophoresis. Finally, 13 SSR markers were obtained (Table 2). The PCR products were subjected to SSR analysis on an ABI 3730xl instrument, and then the genotype data were read using GeneMarker (Applied Biosystems).

Table 2.
Information of SSR markers analyzed in this study from H. molitrix.
2.5. Genetic Analysis
POPGENE 1.32 [17] was used to calculate the number of alleles (Na), the number of effective alleles (Ne), expected heterozygosity (He), observed heterozygosity (Ho), Shannon’s index information (I), and Nei’s genetic distance. The polymorphism information content (PIC) was calculated by Cervus 3.0 software (Kruuk, Australian National University, Australian) [18]. The genetic differentiation index (Fst) of each population was calculated using Arlequin version 3.5 [19] and the molecular variance analysis (AMOVA) was performed.
The phylogenetic tree was constructed based on Nei’s genetic distance and an unweighted pair-group method with arithmetic mean using MEGA 5.0 [20]. Structure v2.3.4 [21] was used to evaluate the genetic relationship between populations. Based on the Bayesian model, the clustering value (K value) was found based on the hybrid model. The length of the burn-in period at the beginning of Markov Chain Monie Carfo (MCMC) was set to 50,000 times, and the range of K value was set to 1–8. Each K value was repeated 20 times. The analysis results were submitted to Structure Harvester (http://taylor0.biology.ucla.edu/struct harvest/ (accessed on 13 April 2022)) to determine the best K value, and then CLUMPP1.1.2 software (Rosenberg, Oxford University Press, USA) [22] was used for repeated clustering analysis. Finally, DISTRUCT1.1 [23] was used for visualization.
3. Results
3.1. Identification of SSRs in the H. molitrix Genome
A total of 368,572 SSR repeats were screened in the 842.01 Mb genome of H. molitrix. The total length of the identified SSRs was 6,492,076 bp, accounting for 0.77% of the total length of the whole genome. The average length of SSRs was 84.66 bp, the frequency was 437.73 loci/Mb, and the density was 7713.16 bp/Mb (Table 3). Di-nucleotide repeats (204873) accounted for 55.59% of the total number of SSRs, followed by tetr- (70,012,19%), pent- (44,921,12.19%), tri- (38,048,10.32%), and hexa-nucleotide repeats (10,718,2.90%). The highest frequency and density were Din- (243.31 loci/Mb, 5832.79 bp/Mb), followed by tetra- (83.15 loci/Mb, 846.65 bp/Mb), penta- (53.35 loci/Mb, 392.48 bp/Mb), and hexa-nucleotides (12.73 loci/Mb, 84.38 bp/Mb) (Table 3 and Table S2).

Table 3.
Information of SSR repeats in H. molitrix.
Among different repeat types, AC (89,924) had the largest number of di-nucleotide repeats, accounting for 43.89%, followed by AT and AG, which were 40.6% and 15.4%, respectively. CG had the lowest proportion (0.1%). The highest frequency type of tri-nucleotide is AAT (24,409), accounting for 64.15%, followed by AAC (12.34%), AAG (5.36%), and the remaining repetitive sequences are relatively few. Among the tetra-, penta-, and hexa-nucleotide repeats, the highest number was AAAT (24%), AAAAT (28.2%), and AAAAAT (31.6%), respectively (Figure 1).
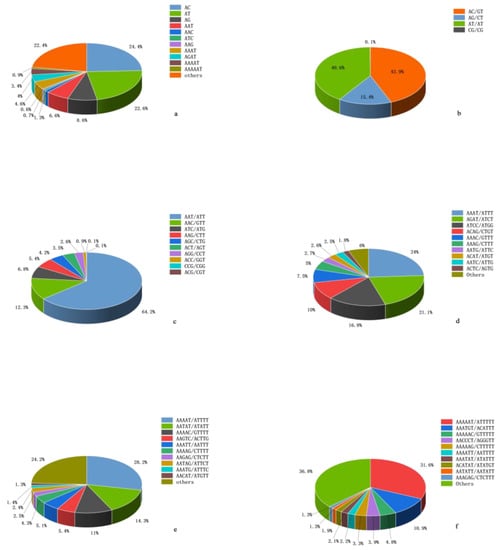
Figure 1.
Motif proportions of different repeat types in H. molitrix genome. (a) The most abundant SSRs motifs in the H. molitrix genome; (b) mono-nucleotide repeat types, (c) tri-nucleotide repeat types, (d) tera-nucleotide repeat types, (e) penta-nucleotide repeat types, and (f) hexa-nucleotide repeat types.
Among the di-nucleotide repeat types, AC (106.80 loci/Mb) had the highest distribution frequency, followed by AT (98.72 loci/Mb), AG (37.53 loci/Mb), and CG (0.27 loci/Mb). AT (3045.83 bp/Mb) had the highest density, followed by AC (2111.18 bp/Mb), AG (672.34 bp/Mb), and CG (3.44 bp/Mb). Among the tri-nucleotide repeat types, AAT (28.99 loci/Mb, 365.86 bp/Mb) had the highest distribution frequency and density, followed by AAC (5.58 loci/Mb, 63.67 bp/Mb), ATC (3.05 loci/Mb, 37.90 bp/Mb), and AAG (2.42 loci/Mb, 29.29 bp/Mb). Among the tetra-nucleotide repeat types, the distribution frequency of AAAT (19.97 loci/Mb) was the highest, followed by AGAT (17.52 loci/Mb), while the density of AAAT (178.24 bp/Mb) was lower than that of AGAT (208.83 bp/Mb). AAAAT (15.04 loci/Mb, 111.11 bp/Mb) and AAAAAT (4.02 loci/Mb, 24.32 bp/Mb) had the highest frequency and density in penta- and hexa-nucleotide repeat types (Table 4 and Table S2).

Table 4.
The most abundant motif categories in the H. molitrix genome SSRs.
3.2. The Distributions of Copy Numbers in Different SSR Repeat Types in H. molitrix Genome
In the H. molitrix genome, the copy number of di-nucleotide repeats ranged from 6 to 29 times, which accounted for 95.74% of the total di-nucleotide SSRs, and 6 repeat types were the most abundant (47,731) and accounted for 23.3%. The copy number of tri-nucleotide repeats was mainly concentrated in 5–10 times, accounting for 97.72% of the total number of tri-nucleotide SSRs, of which 5 repeats were the most (18,401), accounting for 48.36% of the total. The copy number of tetra-nucleotide repeats was largely concentrated in 4–8 times, accounting for 95.64% of the total number of tetra-nucleotide SSRs, of which 4 repeats were the most (35,997), accounting for 51.42% of the total number. The copy number of penta-nucleotide repeats was mostly concentrated in 3–5 times, accounting for 96.37% of the total number of penta-nucleotide SSRs, of which 3 repeats (33,442) accounted for 74.45%. The 3–5 times copy number of hexa-nucleotide repeats dominated, which accounted for 98.9% of the total hexa-nucleotide SSRs. Among them, the number of three repeats were the most (9683), accounting for 90.34% of the total number (Figure 2, Table S3).
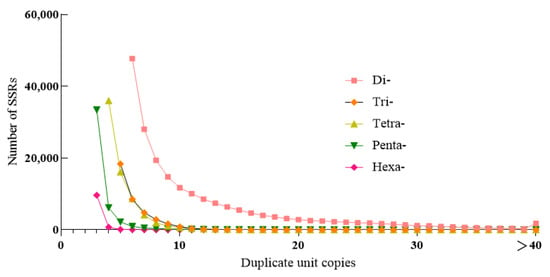
Figure 2.
Different copy number distribution of H. molitrix SSRs.
3.3. Distribution of SSRs on Chromosomes
The total length of 24 assembled chromosomes in H. molitrix accounted for 95.87% of the total assembled sequences. A total of 260,712 SSRs were screened, of which the largest number (15,637) was located in chromosome 1, accounting for 6%, followed by chromosome 2 (15,610) and chromosome 4 (14,548), accounting for 5.98% and 5.58%, respectively. The number of SSRs on chromosome 24 was the least (7650), accounting for 2.93% (Figure 3, Table S4). Linear regression analysis was performed using SPSS, and the results showed that the total number of SSRs was positively correlated with chromosome length (R = 0.969, p < 0.01).
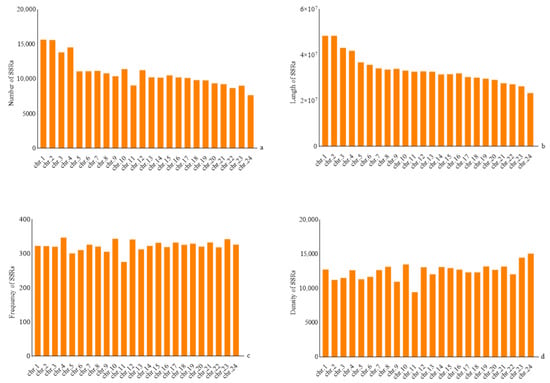
Figure 3.
Analysis of SSR frequencies on H. molitrix chromosomes: (a) number of SSRs, (b) length of SSRs, (c) frequency of SSRs, and (d) density of SSRs.
The frequencies of SSRs on 24 different chromosomes of H. molitrix were also different. The average frequency of SSRs was 323.22 loci/Mb. The highest frequency of SSRs was 347.31 loci/Mb on chromosome 4, followed by 343.67 loci/Mb on chromosome 10 and 342.63 loci/Mb on chromosome 23, and the lowest frequency was 276.45 loci/Mb on chromosome 11 (Figure 3, Table S4).
3.4. Screening of Polymorphic SSR Sites
A total of 56 alleles were detected in 13 SSR markers, observed number of alleles (Na) ranged from 2 to 7, effective numbers of alleles (Ne) ranged from 1.052 to 4.765, observed heterozygosity (Ho) ranged from 0.017 to 0.683, expected heterozygosity (He) ranged from 0.049 to 0.800, Shannon’s index information (I) ranged from 0.133 to 1.747, and polymorphism information content (PIC) ranged from 0.048 to 0.768 (Table 5).

Table 5.
Characteristics of 13 polymorphic SSR sites in H. molitrix.
3.5. Population Genetic Diversity Analysis
Na in four H. molitrix populations ranged from 3.538 (YW) to 4.462 (XC), with an average of 4.116; Ne ranged from 2.045 (YW) to 2.461 (WH), with an average of 2.307; Ho ranged from 0.392 to 0.450, with an average of 0.4138; He ranged from 0.402 (XC) to 0.504 (WH) with an average of 0.457, and the mean value of He was greater than that of Ho, indicating that the proportion of homozygotes was greater than that of heterozygotes. Shannon’s index information (I) ranged from 0.879 to 0.954, with an average of 0.911. The Fixation Index (Fst) in the group was between 0.084 (SS) and 0.178 (YW) (Table 6).

Table 6.
Statistical values of genetic diversity of 13 SSR sites in four H. molitrix populations.
3.6. Genetic Differentiation in Four Populations
Analysis molecular of variance (AMOVA) was used to detect genetic differentiation among populations. The results showed that 95.35% of genetic variation was within populations, while only 4.65% was among populations. The Fst value among populations was 0.04650 (p < 0.001) (Table 7). Genetic differentiation index between YW and WH populations was the highest, while it was the lowest between XC and SS (Table 8). Based on Nei’s genetic distance, a population phylogenetic tree was constructed by the UPGMA method. The cluster results showed that SS, XC, and WH populations were clustered into one clade, and the YW population was clustered in another (Figure 4). A structure harvester was used to determine the best k value of 2, and it is inferred that the four populations can contain all individuals with the greatest possibility (Table S5). Different colors in the clustering diagram represented different groups. The results showed that the YW population was significantly different from the SS, WH, and XC populations, and it was divided into two different groups (Figure 5).

Table 7.
Molecular variance (AMOVA) results of four H. molitrix populations.

Table 8.
Genetic differentiation index (Fst) (above diagonal) and Nei’s genetic distance (below diagonal).
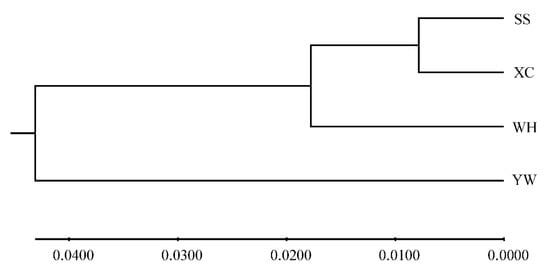
Figure 4.
UPGMA tree based on the genetic distance among four H. molitrix populations.
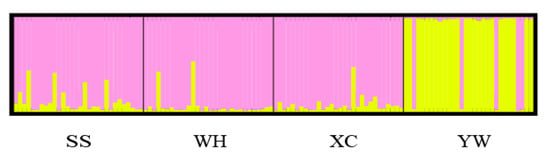
Figure 5.
Structure analysis of H. molitrix populations using genotype data from 13 SSR sites. (K = 2).
4. Discussion
In the H. molitrix genome, 368,572 SSRs were screened and accounted for 0.77% of the total genome length. The genome-wide SSRs content was similar to that of Takifugu rubripes (0.77%) and Takifugu flavidus (0.73%) [24]. It was higher than some lepidopteran insects (0.13–0.61%) [25] and birds (0.13–0.49%) [26], but lower than mammals such as Homo sapiens (3%) [27], Bos mutus (5.8%), Bubalus bubalis (5.69%) [28], and some macaque species (4.96–5.18%) [29]. These differences were caused due to studying species, genome size, and setting parameters for SSR screening.
The frequency of SSRs in H. molitrix was 437.73 loci/Mb, lower than that in Cyprinus carpio (621.95 loci/Mb), Oncorhynchus kisutch (461.35 loci/Mb), and Cynoglossus semilaevis (3445.94 loci/Mb) [30], and higher than that in some birds (80.9–256.9 loci/Mb) [26] and Lateolabrax maculatus (425.06 loci/Mb) [31]. The density (7713.16 bp/Mb) was higher than that of Ctenopharyngodon idella (1425.35 bp/Mb) [32] and lower than that of Monopterus albus (10,259 bp/Mb) [33].
Studies have shown that the longer the species evolution, the more SSR repeats of low repeat units there are. [34]. The dominant repeat type of SSRs in the H. molitrix genome is a di-nucleotide, which is consistent with that of most aquatic organisms, such as Pelteobagrus fulvidraco [35], Hemibagrus wyckioides [36], and L. maculatus [31]. This may be related to the evolutionary time of species.
Among the di-nucleotide repeat types, AC/GT had the largest number, which was consistent with most vertebrates [37]. The distribution of SSRs in different species exhibits certain differences, but the G/C bases in the genome are generally low [26]. AAT/ATT, AAAT/ATTT, AAAAT/ATTTT, and AAAAAT/ATTTTT are the dominant tri-, tera-, penta-, and hexa-nucleotide repeat types, respectively. A/T bases accounted for the majority of SSRs in the whole genome, while G/C content was less. This is similar to the results of H. wyckioides [36], C. carpio [38], and Misgurnus anguillicaudatus [39]. It was speculated that the CpG di-nucleotide sequence of cytosine (C) usually methylated, and then went through deamination to generate thymine (T) [40]. In addition, sequences containing A/T were prone to base sliding during replication, and G/C content was negatively correlated with the probability of replication sliding [41].
The number of SSR repeat copies in the H. molitrix genome was mainly concentrated between 3 and 29. The repeat number gradually decreased with the increase in the number of repeat unit copies, which was consistent with the distribution of SSRs in most genomes. The number of SSR repeats decreased with the increase in repeat length, because the longer the repeat length, the higher the possibility of mutation [42]. A large number of studies have shown that the number of SSRs on different biological chromosomes is correlated with their length. Linear analysis showed that the chromosome length of H. molitrix was positively correlated with the number of SSRs (R = 0.969, p < 0.01). The longer the chromosome, the higher the microsatellite content [43]. The frequency and density of SSRs was not correlated with chromosome length, which was relevant to the long-term evolution of species in 14 fish species [30].
Genetic diversity is an important genetic index to evaluate population adaptability, which can be estimated by the observed number of alleles, effective numbers of alleles, observed heterozygosity, and expected heterozygosity [44,45]. Many studies have shown that unintentional parental selection and inbreeding in the process of reproduction can lead to a decrease in the genetic diversity of populations. Our present results showed that the average values of Na, Ne, Ho, He, and I were 3.538–4.462, 2.045–2.461, 0.392–0.450, 0.402–0.5, and 0.879–0.954, respectively, which indicated that the genetic diversity among the four populations was low. This is consistent with [46] in Guangxi-cultivated H. molitrix populations. Therefore, the genetic diversity of the four cultivated H. molitrix populations in this experiment is low, which is very unfavorable to the protection of germplasm resources, and more scientific breeding measures should be carried out in the process of reproduction to improve the genetic diversity.
Fst is usually used to evaluate genetic differentiation among populations [47]. When 0 < FST < 0.05, there was no differentiation among populations, when 0.05 < FST < 0.15, there was moderate differentiation between groups, and when 0.25 < FST < 1, there was a high differentiation between groups [48]. Among these populations, the Fst values of the YW population and the other three populations (SS, WH, and XC) were 0.05402, 0.08709, and 0.06319, respectively. The YW population exhibited moderate differentiation from the other three populations. This is consistent with the results of the UPGMA tree and cluster diagram.
5. Conclusions
In conclusion, this study analyzed the number, frequency, distribution, and type of SSRs in the whole genome of H. molitrix, screened and developed SSR markers at the level of the whole genome for the first time, and then analyzed the genetic diversity of four breeding populations. It was found that the genetic diversity of these four populations was low. Therefore, developing new SSR markers from the H. molitrix genome will provide a basis for genetic diversity analysis, the formulation of more scientific breeding measures, the protection and development of germplasm resources, and the realization and development of a sustainable aquaculture.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/genes13071267/s1, Table S1: 96 pairs of primer information, Table S2: Summary of SSR motifs and repeats, Table S3: The distributions of copy numbers in different SSR repeat types in the H. molitrix genome, Table S4: Microsatellite distribution characteristics on 24 chromosomes, Figure S1: The curve of change of Delta K with the changing K value.
Author Contributions
Conceptualization, H.S., X.L. (Xiangzhong Luo) and H.L.; methodology, H.S. and T.Z.; software, Y.W., X.L. (Xiaohui Li) and Y.C.; validation, G.Z., Y.C. and H.L.; formal analysis, Y.W. and H.L.; investigation, Y.W. and X.L. (Xiaohui Li); resources, X.L. (Xiangzhong Luo); data curation, Y.W. and H.L.; writing—original draft preparation, Y.W.; writing—review and editing, H.L.; visualization, Y.C.; supervision, H.L.; project administration, G.Z. and H.L.; funding acquisition, H.L. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the China Agriculture Research System of MOF and MARA (CARS-45), Central Public-interest Scientific Institution Basal Research Fund, CAFS (2020TD33), Engineering Research Centre of the Ministry of Education for Wetland Ecology and Agriculture Use of Wetland, Ministry of Education (Yangtze University) (KFT202006), and National Freshwater Aquatic Germplasm Resource Center (FGRC18537).
Institutional Review Board Statement
The animal study was approved by the animal care regulations of the Yangtze River Fisheries Research Institute, Chinese Academy of Fishery Sciences (approval number 2020098).
Informed Consent Statement
Not applicable.
Data Availability Statement
The experimental data involved in this article can be obtained by the corresponding author.
Acknowledgments
Thanks to Liguo Huang and Junqiao Chen for collecting these samples.
Conflicts of Interest
The authors report no conflict of interest.
References
- Sha, H.; Luo, X.Z.; Wang, D.; Zou, G.W.; Liang, H.W. New insights to protection and utilization of silver carp (Hypophthalmichthys molitrix) in Yangtze River based on microsatellite analysis. Fish. Res. 2021, 241, 105997. [Google Scholar] [CrossRef]
- Liang, H.W.; Li, Z.; Luo, X.Z.; Pan, G.B.; Zou, G.W. Morphological differences and discriminant analysis between Changfeng and Yangtze river silver carp. Acta Hydrobiol. Sin. 2015, 39, 6. [Google Scholar]
- Fang, D.A.; Luo, Y.T.; Xu, D.P.; Yang, X.W.; Wang, X.H. Relationship between genetic risk and stock enhancement of the silver carp (Hypophthalmichthys molitrix) in the Yangtze River. Fish. Res. 2021, 235, 105829. [Google Scholar] [CrossRef]
- Toth, G. Microsatellites in Different Eukaryotic Genomes: Survey and Analysis. Genome Res. 2000, 10, 967. [Google Scholar] [CrossRef]
- Li, Z.; Chen, F.; Huang, C.; Zheng, W.; Zhou, R. Genome-wide mapping and characterization of microsatellites in the swamp eel genome. Sci. Rep. 2017, 7, 3157. [Google Scholar] [CrossRef]
- Zane; Nelson; Jones; Avise. Microsatellite assessment of multiple paternity in natural populations of a live-bearing fish, Gambusia holbrooki. J. Evol. Biol. 2010, 12, 61–69. [Google Scholar] [CrossRef]
- Nikolic, N.; Fve, K.; Chevalet, C.; Hyheim, B.; Riquet, J. A set of 37 microsatellite DNA markers for genetic diversity and structure analysis of Atlantic salmon Salmo salar populations. J. Fish Biol. 2009, 74, 458–466. [Google Scholar] [CrossRef]
- Chen, S.L.; Ji, X.S.; Shao, C.W.; Li, W.L.; Yang, J.F.; Liang, Z.; Liao, X.L.; Xu, G.B.; Xu, Y.; Song, W.T. Induction of Mitogynogenetic Diploids and Identification of WW Super-female Using Sex-Specific SSR Markers in Half-Smooth Tongue Sole (Cynoglossus semilaevis). Mar. Biotechnol. 2012, 14, 120–128. [Google Scholar] [CrossRef]
- Mastrochirico-Filho, V.A.; Pazo, F.D.; Hata, M.E.; Villanova, G.V.; Hashimoto, D.T. Assessing Genetic Diversity for a Pre-Breeding Program in Piaractus mesopotamicus by SNPs and SSRs. Genes 2019, 10, 668. [Google Scholar] [CrossRef]
- Shen, X.; Yang, G.; Liu, Y.; Liao, M.; Wang, X.; Zhu, M.; Song, W.; Zou, G.; Wei, Q.; Wang, D. Construction of genetic linkage maps of guppy (Poecilia reticulata) based on AFLP and microsatellite DNA markers. Aquaculture 2007, 271, 178–187. [Google Scholar] [CrossRef]
- Alcivar-Warren, A.; Meehan-Meola, D.; Won, S.; Xu, Z.; Zuniga, G. ShrimpMap: A low-density, microsatellite-based linkage map of the pacific whiteleg shrimp, Litopenaeus vannamei: Identification of sex-linked markers in linkage group 4. J. Shellfish Res. 2017, 26, 1259–1277. [Google Scholar] [CrossRef]
- Yu, F.; Wang, B.H.; Feng, S.P.; Wang, J.Y.; Wu, L. Development, characterization, and cross-species/genera transferability of SSR markers for rubber tree (Hevea brasiliensis). Plant Cell Rep. 2011, 30, 335–344. [Google Scholar] [CrossRef]
- Zalapa, J.E.; Cuevas, H.; Zhu, H.; Steffan, S.; Senalik, D.; Zeldin, E.; Mccown, B.; Harbut, R.; Simon, P. Using next-generation sequencing approaches to isolate simple sequence repeat (SSR) loci in the plant sciences. Am. J. Bot. 2012, 99, 193–208. [Google Scholar] [CrossRef]
- Feng, X.; Yu, X.; Fu, B.; He, S.; Tong, J.; Feng, X.; Yu, X.; Fu, B.; He, S.; Tong, J. Development of 159 transcript-associated microsatellite markers in silver carp (Hypophthalmichthys molitrix). Conserv. Genet. Resour. 2014, 6, 111–113. [Google Scholar] [CrossRef][Green Version]
- Guo, W.; Yu, X.; Tong, J.; Guo, W.; Yu, X.; Tong, J. Development of 134 novel polynucleotide-repeat microsatellite markers in silver carp (Hypophthalmichthys molitrix). Conserv. Genet. Resour. 2013, 5, 525–528. [Google Scholar] [CrossRef]
- Aljanabi, S.M.; Martinez, I. Universal and rapid salt-extraction of high quality gnomic DNA for PCR-based techniques. Nucleic Acids Res. 1997, 25, 4692–4693. [Google Scholar] [CrossRef]
- Yeh, C.; Boule, T. POPGENE-1.32: A Free Program for the Analysis of Genetic Variation among and within Populations Using Co-Dominant and Dominant Markers; Department of Renewable Resources at the University of Alberta: Edmonton, AB, Canada, 2000. [Google Scholar]
- Marshall, T.C.; Slate, J.; Kruuk, L.; Pemberton, J.M. Statistical confidence for likelihood-based paternity inference in natural populations. Mol. Ecol. 1998, 7, 639–655. [Google Scholar] [CrossRef]
- Excoffier, L.; Lischer, H.E.L. Arlequin suite ver 3.5: A new series of programs to perform population genetics analyses under Linux and Windows. Mol. Ecol. Resour. 2010, 10, 564–567. [Google Scholar] [CrossRef]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular Evolutionary Genetics Analysis Using Maximum Likelihood, Evolutionary Distance, and Maximum Parsimony Methods. Mol. Biol. Evol. 2011, 28, 2731. [Google Scholar] [CrossRef]
- Smouse, P.E.; Peakall, R. Spatial autocorrelation analysis of individual multiallele and multilocus genetic structure. Heredity 1999, 82, 561–573. [Google Scholar] [CrossRef]
- Rosenberg, J. CLUMPP: A cluster matching and permutation program for dealing with label switching and multimodality in analysis of population structure. Bioinformatics 2007, 23, 1801–1806. [Google Scholar]
- Rosenberg, N.A. distruct: A program for the graphical display of population structure. Mol. Ecol. Notes 2004, 4, 137–138. [Google Scholar] [CrossRef]
- Xu, J.J.; Zheng, X.; Zhang, X.Y.; Wang, T.; Yin, S.W. Analysis of Distribution Characteristics of Microsatellites in Four Genomes of Puffer Fish. Genom. Appl. Biol. 2021, 40, 1441–1451. [Google Scholar]
- Gan, L.P.; Tian, H.T.; Heng, L.H. Distribution Regularities of SSR in the Whole Genomes of the Six Lepi-doptera Insects. Genom. Appl. Biol. 2021, 40, 1022–1030. [Google Scholar]
- Yan, Y.F.; Yue, B.S.; Huang, J.; Jian, Z.Y.; Li, W.J. Genome-wide distribution and organization of microsatellites in six species of birds. Biochem. Syst. Ecol. 2016, 67, 95–102. [Google Scholar]
- Subramanian, S.; Mishra, R.K.; Singh, L. Genome-wide analysis of microsatellite repeats in humans: Their abundance and density in specific genomic regions. Genome Biol. 2003, 4, R13. [Google Scholar] [CrossRef]
- Qi, W.; Jiang, X.; Du, L.; Xiao, G. Distribution regularities and comparative analysis of microsatellite in the whole genomes of yak and water buffalo. Genom. Appl. Biol. 2015, 34, 1406–1412. [Google Scholar]
- Liu, S.; Hou, W.; Sun, T.; Xu, Y.; Li, P.; Yue, B.; Fan, Z.; Li, J. Genome-wide mining and comparative analysis of microsatellites in three macaque species. Mol. Genet. Genom. 2017, 292, 537–550. [Google Scholar] [CrossRef]
- Lei, Y.; Zhou, Y.; Price, M.; Song, Z. Genome-wide characterization of microsatellite DNA in fishes: Survey and analysis of their abundance and frequency in genome-specific regions. BMC Genom. 2021, 22, 421. [Google Scholar] [CrossRef]
- Fan, S.A.; Huang, H.; Liu, Y.; Wang, P.A.; Zhao, C.A.; Yan, L.A.; Qiao, X.C.; Qiu, L. Genome-wide identification of microsatellite and development of polymorphic SSR markers for spotted sea bass (Lateolabrax maculatus). Aquacult. Rep. 2021, 20, 100677. [Google Scholar]
- Huang, W.J.; Guo, X.Z.; Zhang, Z.H.; Dong, Q.; Xiong, X.M.; Gao, Z.X. Analysis of microsatellite in the entire grass carp (Ctenopharyngodon idella) M genome and the application in parentage identification. J. Fish. China 2022, 46, 161–172. [Google Scholar]
- Tian, H.F.; Hu, Q.M.; Li, Z. Genome-wide identification of simple sequence repeats and development of polymorphic SSR markers in swamp eel (Monopterus albus). Sci. Prog. 2021, 104, 368504211035597. [Google Scholar] [CrossRef]
- Dreisigacker, S.; Zhang, P.; Warburton, M.L.; Ginkel, M.V.; Hoisington, D.; Bohn, M.; Melchinger, A.E. SSR and Pedigree Analyses of Genetic Diversity among CIMMYT Wheat Lines Targeted to Different Megaenvironments. Crop Sci. 2004, 44, 381–388. [Google Scholar] [CrossRef]
- Xu, J.J.; Zheng, X.; Li, J.; Yi, S.W.; Wang, T. Distribution Characteristics of Whole Genome Microsatellite of Pelteobagrus fulvidraco. Genom. Appl. Biol. 2020, 39, 5488–5498. [Google Scholar]
- Zhou, Y.L.; Wu, J.J.; Wang, Z.W.; Li, G.H.; Gui, J.F. Microsatellite polymorphism and genetic differentiation of different populations screened from genome survey sequencing in red-tail catfish (Hemibagrus wyckioides). Aquacult. Rep. 2021, 19, 100614. [Google Scholar] [CrossRef]
- Chistiakov, D.A.; Hellemans, B.; Volckaert, F. Microsatellites and their genomic distribution, evolution, function and applications: A review with special reference to fish genetics. Aquaculture 2006, 255, 1–29. [Google Scholar] [CrossRef]
- Ji, P.; Zhang, Y.; Chao, L.; Zhao, Z.; Sun, X. High Throughput Mining and Characterization of Microsatellites from Common Carp Genome. Int. J. Mol. Sci. 2012, 13, 9798–9807. [Google Scholar] [CrossRef]
- Huang, G.; Cao, J.; Chen, C.; Wang, M.; Liu, Z.; Gao, F.; Yi, M.; Chen, G.; Lu, M. Genome survey of Misgurnus anguillicaudatus to identify genomic information, simple sequence repeat (SSR) markers, and mitochondrial genome. Mol. Biol. Rep. 2022, 49, 2185–2196. [Google Scholar] [CrossRef]
- Gartler, S. Analysis of CpG Suppression in Methylated and Nonmethylated Species. Proc. Natl. Acad. Sci. USA 1992, 89, 957–961. [Google Scholar]
- Christian, S.; Diethard, T. Slippage synthesis of simple sequence DNA. Nucleic Acids Res. 1992, 2, 211–215. [Google Scholar]
- Wierdl, M.; Dominska, M. Microsatellite Instability in Yeast: Dependence on the Length of the Microsatellite. Genetics 1997, 146, 769. [Google Scholar] [CrossRef]
- Hancock, J.M. Simple sequences and the expanding genome. BioEssays 1996, 18, 421–425. [Google Scholar] [CrossRef] [PubMed]
- Shete, S.; Tiwari, H.; Elston, R.C. On Estimating the Heterozygosity and Polymorphism Information Content Value. Theor. Popul. Biol. 2000, 57, 265–271. [Google Scholar] [CrossRef]
- Beardmore, J.A.; Mair, G.C.; Lewis, R.I. Biodiversity in aquatic systems in relation to aquaculture. Aquac. Res. 1997, 28, 829–839. [Google Scholar] [CrossRef]
- Ye, X.; Wei, L.; Liang, K.; Zhang, S.; Teng, Z.Z. Genetic diversity analysis in changfeng silver carp and guangxi local silver carp. Genom. Appl. Biol. 2019, 38, 100–108. [Google Scholar]
- Wright, S. Evolution and Genetics of Populations; University of Chicago Press: Chicago, IL, USA, 1978; pp. 439–459. [Google Scholar]
- Balloux, F.; Lugon-Moulin, N. The estimation of population differentiation with microsatellite markers. Mol. Ecol. 2002, 11, 55–65. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).