The Effect of Complexed, Nanosized, and Conventional Zinc Sources Applied at Varying Rates to an Acidic Mediterranean Soil on Two Successive Lettuce Crops
Abstract
1. Introduction
2. Materials and Methods
2.1. Original Soil Characterisation
2.2. Zinc Fertiliser Application in Soil and First Lettuce Crop
2.3. Second Lettuce Crop and Effect of Ageing Zn
2.4. Plant Analyses
2.5. Soil Analysis
2.6. Statistical Analysis
3. Results
3.1. Effect of Treatments on the Lettuce Plant
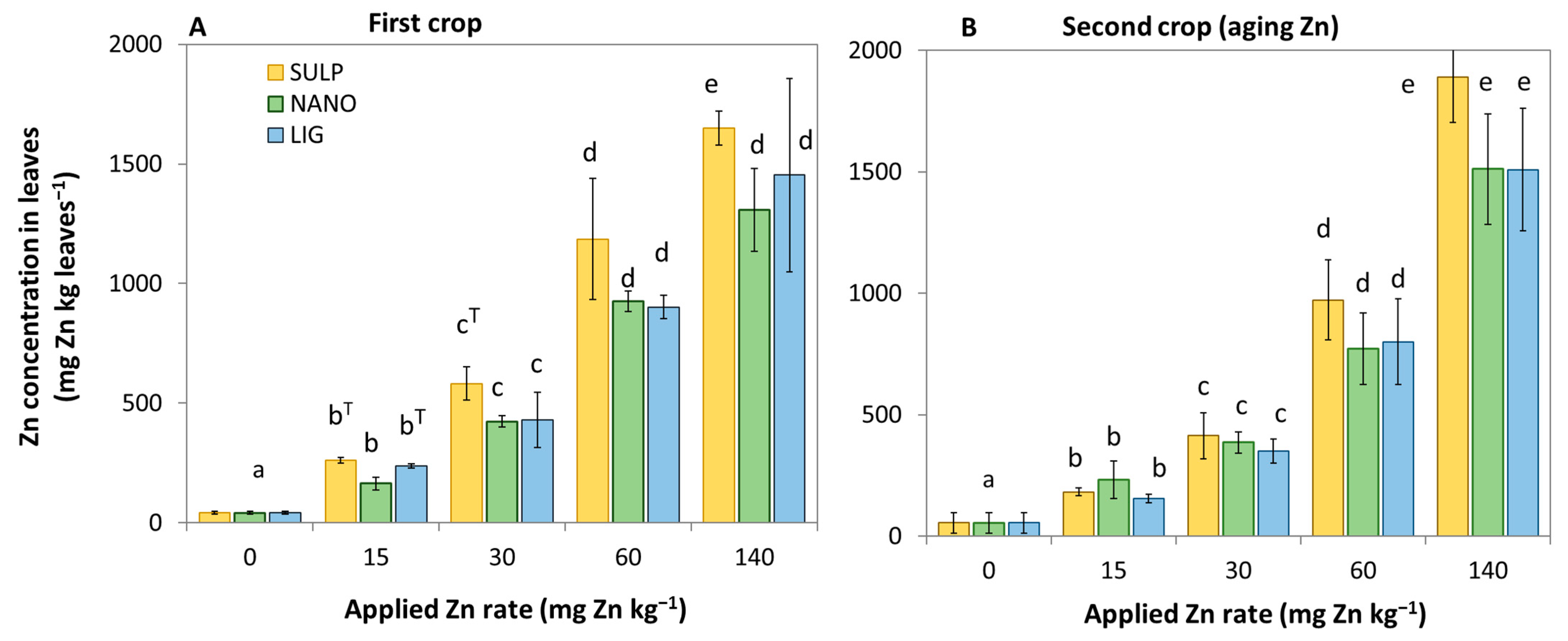
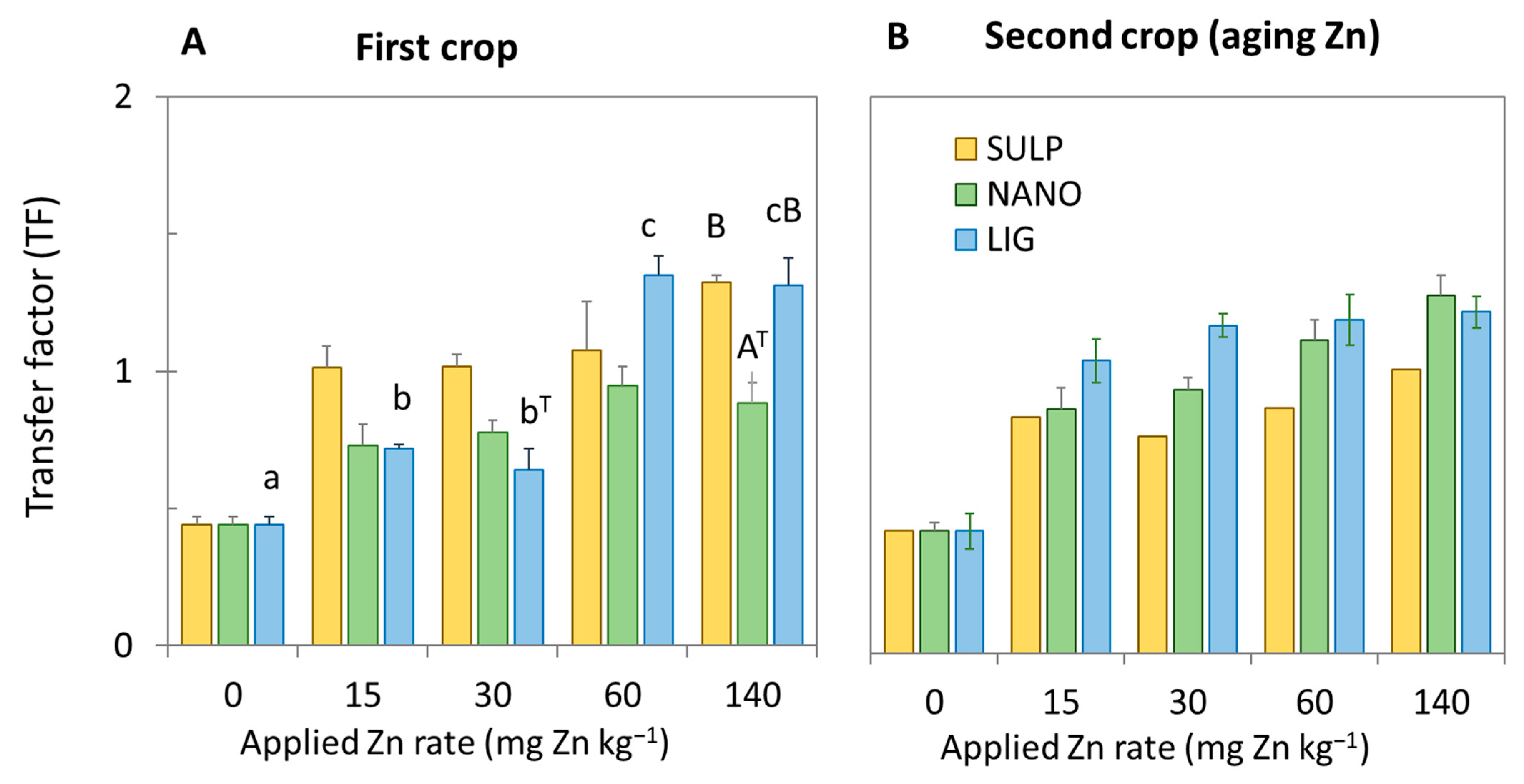
3.2. Dietary and Health Risks in Humans
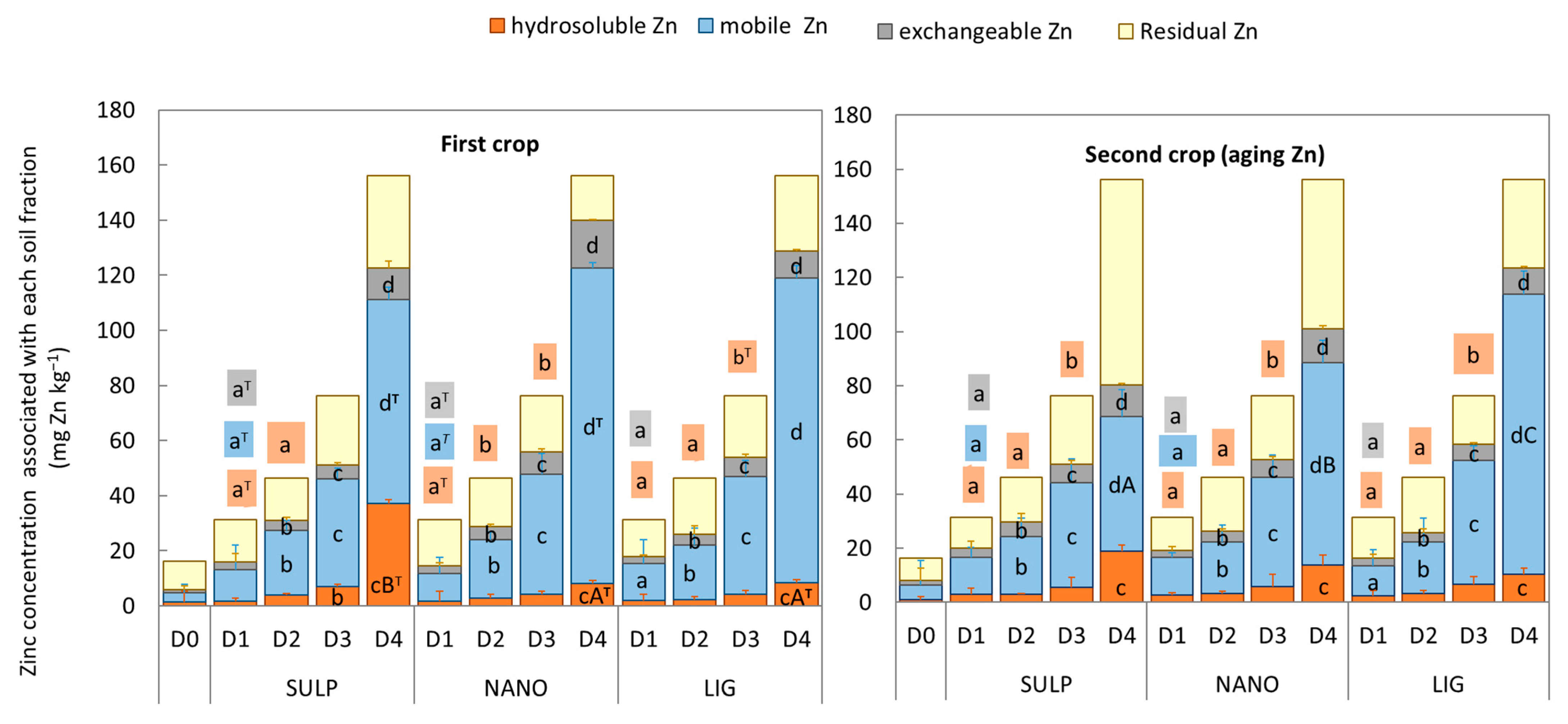
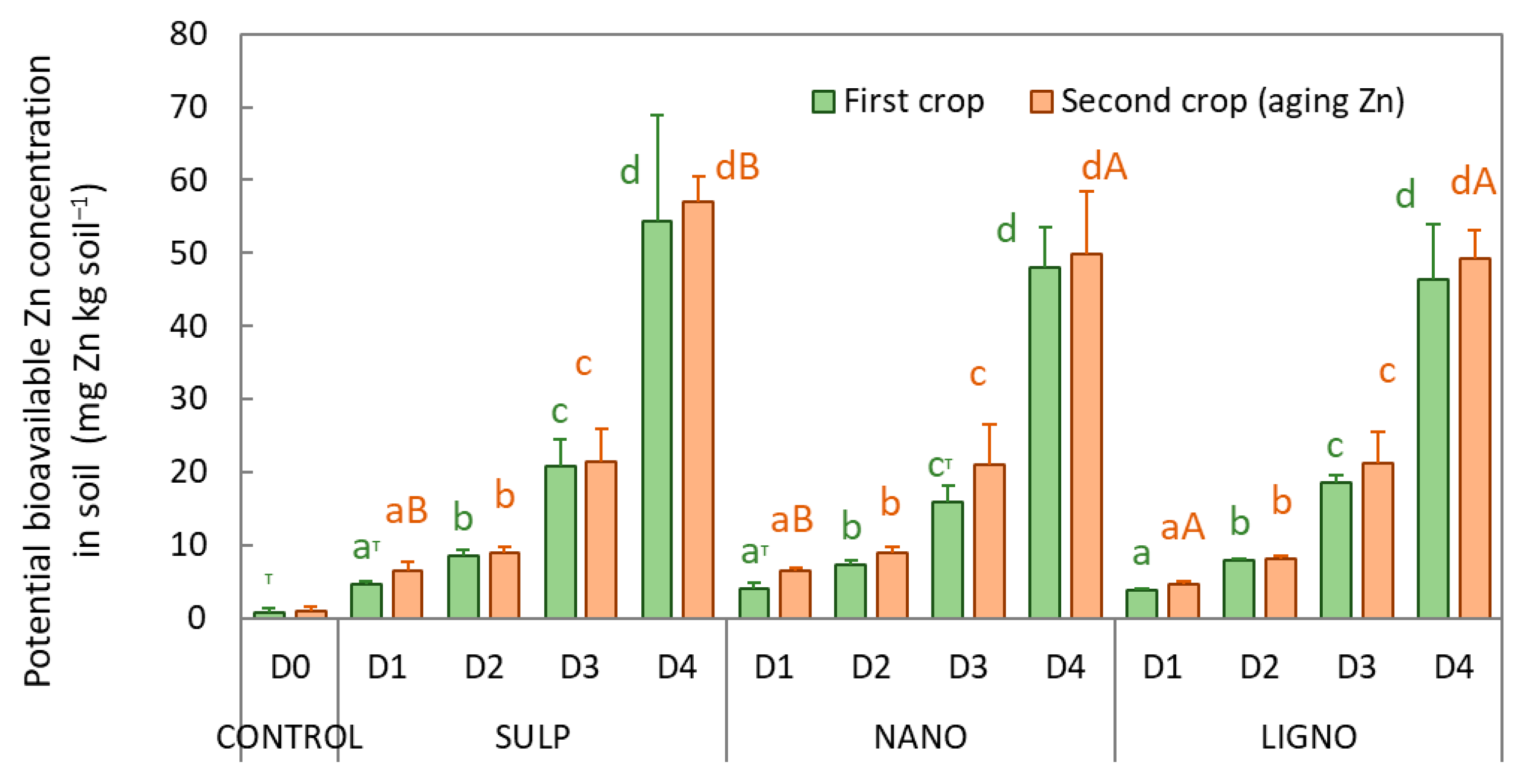
3.3. Zinc Status in Soil
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SULP | Zn sulphate heptahydrate |
| LIG | Zn complex: Zn lignosulphonate |
| NANO | commercial ZnO nanoparticle |
| TF | root–shoot translocation factor |
| BF | bioconcentration factor or transfer factor from soil to plant |
| DDI | human daily dietary intake |
| RDA | recommended dietary allowance |
| FM | fresh matter |
References
- Cakmak, I.; Kutman, U.B. Agronomic Biofortification of Cereals with Zinc: A Review. Eur. J. Soil Sci. 2018, 69, 172–180. [Google Scholar] [CrossRef]
- Brown, K.H.; Rivera, J.A.; Bhutta, Z.; Gibson, R.S.; King, J.C.; Lönnerdal, B.; Ruel, M.T.; Sandtröm, B.; Wasantwisut, E.; Hotz, C.; et al. International Zinc Nutrition Consultative Group (IZiNCG) Technical Document #1. Assessment of the Risk of Zinc Deficiency in Populations and Options for Its Control. Food Nutr. Bull. 2004, 25, S99–S203. [Google Scholar] [PubMed]
- Hess, S.Y.; King, J.C. Effects of Maternal Zinc Supplementation on Pregnancy and Lactation Outcomes. Food Nutr. Bull. 2009, 30, 60–78. [Google Scholar] [CrossRef]
- Allouche-Fitoussi, D.; Breitbart, H. The Role of Zinc in Male Fertility. Int. J. Mol. Sci. 2020, 21, 7796. [Google Scholar] [CrossRef]
- Global Nutrition Report Nourishing the SDGs. 2017. Available online: https://media.globalnutritionreport.org/documents/Global_Nutrition_Report_2017.pdf (accessed on 19 February 2025).
- Noulas, C.; Tziouvalekas, M.; Karyotis, T. Zinc in Soils, Water and Food Crops. J. Trace Elem. Med. Biol. 2018, 49, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Ryu, M.S.; Aydemir, A.T.B. Zinc. In Present Knowledge in Nutrition; Marriott, B.P., Birt, D.F., Stallings, V.A., Yates, A.A., Eds.; Academic Press: London, UK, 2020. [Google Scholar]
- EFSA. Panel on Dietetic Products Nutrition and Alergies Scientific Opinion on Dietary Reference Values for Zinc. EFSA J. 2014, 12, 3844. [Google Scholar] [CrossRef]
- Wessells, K.R.; Brown, K.H. Estimating the Global Prevalence of Zinc Deficiency: Results Based on Zinc Availability in National Food Supplies and the Prevalence of Stunting. PLoS ONE 2012, 7, e50568. [Google Scholar] [CrossRef]
- Pluhator, M.M.; Thomson, A.B.R.; Fedorak, R.N. Clinical Aspects of Trace Elements: Zinc in Human Nutrition—Assessment of Zinc Status. Can. J. Gastroenterol. 1996, 10, 37–42. [Google Scholar] [CrossRef][Green Version]
- Smolders, E.; Buekers, J.; Oliver, I.; McLaughlin, M.J. Soil Properties Affecting Toxicity of Zinc to Soil Microbial Properties in Laboratory-Spiked and Field-Contaminated Soils. Environ. Toxicol. Chem. 2004, 23, 2633–2640. [Google Scholar] [CrossRef]
- Cogger, C.; Brown, S. Soil Formation and Nutrient Cycling BT. In Sowing Seeds in the City: Ecosystem and Municipal Services; Brown, S., McIvor, K., Hodges Snyder, E., Eds.; Springer: Dordrecht, The Netherlands, 2016; pp. 25–52. ISBN 978-94-017-7453-6. [Google Scholar]
- Marschner, H. Mineral Nutrition of Higher Plants, 3rd ed.; Academic Press: Cambridge, MA, USA, 2012. [Google Scholar]
- Bhardwaj, A.K.; Chejara, S.; Malik, K.; Kumar, R.; Kumar, A.; Yadav, R.K. Agronomic Biofortification of Food Crops: An Emerging Opportunity for Global Food and Nutritional Security. Front. Plant Sci. 2022, 13, 1055278. [Google Scholar] [CrossRef]
- Wenxiao, L.; Geng, H.; Zhou, B.; Chen, H.; Yuan, R.; Ma, C.; Liu, R.; Xing, B.; Wang, F. The Behavior, Transport, and Positive Regulation Mechanism of ZnO Nanoparticles in a Plant-Soil-Microbe Environment. Environ. Pollut. 2022, 315, 120368. [Google Scholar] [CrossRef]
- Thounaojam, T.C.; Meetei, T.T.; Devi, Y.B.; Panda, S.K.; Upadhyaya, H. Zinc Oxide Nanoparticles (ZnO-NPs): A Promising Nanoparticle in Renovating Plant Science. Acta Physiol. Plant. 2021, 43, 136. [Google Scholar] [CrossRef]
- Tiede, K.; Boxall, A.B.A.; Tear, S.P.; Lewis, J.; David, H.; Hassellöv, M. Detection and Characterization of Engineered Nanoparticles in Food and the Environment. Food Addit. Contam.-Part A Chem. Anal. Control Expo. Risk Assess. 2008, 25, 795–821. [Google Scholar] [CrossRef]
- Alloway, B.J. Zinc in Soils and Crop Nutrition, 2nd ed.; International Zinc Association: Brussels, Belgium; Paris, France, 2008. [Google Scholar]
- FAOSTAT. The Food and Agriculture Organization (FAO). Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 19 February 2025).
- U.S. EPA. Ecological Effects Test Guidelines: Seed Germination/Root Elongation Toxicity Test. U. S. Environmental Protection Agency: Washington, DC, USA, 1996; pp. 1–8. Available online: https://www.epa.gov/test-guidelines-pesticides-and-toxic-substances/series-850-ecological-effects-test-guidelines (accessed on 19 February 2025).
- Martins, M.R.; Zanatta, M.C.K.; Pires, M.S.G. Sustainable Agricultural Use of Sewage Sludge: Impacts of High Zn Concentration on Folsomia candida, Enchytraeus crypticus, Lactuca sativa, and Phaseolus vulgaris. Environ. Monit. Assess. 2023, 195, 359. [Google Scholar] [CrossRef]
- FAO. Base Referencial Mundial del Recurso Suelo. Sistema Internacional de Clasificación de Suelos; Organización de las Naciones Unidas para la Alimentación y la Agricultura, Ed.; Organización de las Naciones Unidas para la Alimentación y la Agricultura: Rome, Italy, 2015; ISBN 9789253083695. [Google Scholar]
- Ramos, C.; Pomares, F. Abonado de Los Cultivos Hortícolas. Guía Práctica la Fertilización Racional de los Cultivos en España. Parte II. 2010, pp. 181–192. Available online: https://www.mapa.gob.es/es/agricultura/publicaciones/01_FERTILIZACI%C3%93N(BAJA)_tcm30-57890.pdf (accessed on 19 February 2025).
- Khan, A.; Khan, S.; Alam, M.; Amjad, M. Toxic Metal Interactions Affect the Bioaccumulation and Dietary Intake of Macro- and Micro-Nutrients. Chemosphere 2016, 146, 121–128. [Google Scholar] [CrossRef]
- Khan, S.; Rehman, S.; Zeb Khan, A.; Amjad Khan, M.; Tahir Shah, M. Soil and Vegetables Enrichment with Heavy Metals from Geological Sources in Gilgit, Northern Pakistan. Ecotoxicol. Environ. Saf. 2010, 73, 1820–1827. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency. Reference Dose (RfD): Description and Use in Health Risk Assessments; United States Environmental Protection Agency: Washington, DC, USA, 1993.
- Beesley, L.; Moreno-Jimenez, E.; Clemente, R.; Lepp, N.; Dickinson, N. Mobility of Arsenic, Cadmium and Zinc in a Multi-Element Contaminated Soil Profile Assessed by in-Situ Soil Pore Water Sampling, Column Leaching and Sequential Extraction. Environ. Pollut. 2010, 158, 155–160. [Google Scholar] [CrossRef]
- Kraus, U.; Wiegand, J. Long-Term Effects of the Aznalcóllar Mine Spill—Heavy Metal Content and Mobility in Soils and Sediments of the Guadiamar River Valley (SW Spain). Sci. Total Environ. 2006, 367, 855–871. [Google Scholar] [CrossRef]
- Feng, M.H.; Shan, X.Q.; Zhang, S.Z.; Wen, B. Comparison of a Rhizosphere-Based Method with Other One-Step Extraction Methods for Assessing the Bioavailability of Soil Metals to Wheat. Chemosphere 2005, 59, 939–949. [Google Scholar] [CrossRef]
- Van Ewijk, P.; Hoekstra, J. Calculation of the EC50 and Its Confidence Interval When Subtoxic Stimulus Is Present. Ecotoxicol. Env. Saf. 1993, 25, 25–32. [Google Scholar] [CrossRef]
- Haanstra, L.; Doelman, P.; Oude Voshaar, J.H. The Use of Sigmoidal Dose Response Curves in Soil Ecotoxicological Research. Plant Soil 1985, 84, 293–297. [Google Scholar]
- Singh, A.; Sharma, R.K.; Agrawal, M.; Marshall, F.M. Health Risk Assessment of Heavy Metals via Dietary Intake of Foodstuffs from the Wastewater Irrigated Site of a Dry Tropical Area of India. Food Chem. Toxicol. 2010, 48, 611–619. [Google Scholar] [CrossRef]
- Schoofs, H.; Schmit, J.; Rink, L. Zinc Toxicity: Understanding the Limits. Molecules 2024, 29, 3130. [Google Scholar] [CrossRef]
- IZA. Zinc Fact Sheet Zinc Fertilizer Overview; The International Zinc Association: Durham, NC, USA, 2015; Available online: https://crops.zinc.org/wp-content/uploads/sites/11/2016/12/Zinc-Fertilizer-Overview-General-Pop-June-2015.pdf (accessed on 19 February 2025).
- Kochian, L.V. Zinc Absorption from Hydroponic Solutions by Plant Roots. In Zinc in Soils and Plants: Proceedings of the International Symposium on “Zinc in Soils and Plants” held at the University of Western Australia, 27–28 September 1993; Robson, A.D., Ed.; Springer: Dordrecht, The Netherlands, 1993; pp. 45–57. ISBN 978-94-011-0878-2. [Google Scholar]
- Wang, H.; Inukai, Y.; Yamauchi, A. Root Development and Nutrient Uptake. CRC. Crit. Rev. Plant Sci. 2006, 25, 279–301. [Google Scholar] [CrossRef]
- Sadeghzadeh, B. A Review of Zinc Nutrition and Plant Breeding. J. Soil Sci. Plant Nutr. 2013, 13, 905–927. [Google Scholar] [CrossRef]
- Norvell, W.A. Reactions of Metal Chelates in Soils. In Micronutrients in Agriculture. SSSA Book; Mortvedt, J., Ed.; Soil Science Society of America, Inc.: Madison, WI, USA, 1991; pp. 187–227. [Google Scholar]
- Da Cruz, T.N.M.; Savassa, S.M.; Montanha, G.S.; Ishida, J.K.; de Almeida, E.; Tsai, S.M.; Lavres Junior, J.; Pereira de Carvalho, H.W. A New Glance on Root-to-Shoot in Vivo Zinc Transport and Time-Dependent Physiological Effects of ZnSO4 and ZnO Nanoparticles on Plants. Sci. Rep. 2019, 9, 10416. [Google Scholar] [CrossRef]
- De Francisco, M.; Fernandes-Silva, P.; Durães, L.; Romeiro, A.; Alvarez-Torrellas, S.; Almendros, P. Influence of the Application of Different Zinc Oxide Nanoparticles on a Lettuce Crop Grown in an Acidic Mediterranean Soil. Horticulturae 2024, 10, 681. [Google Scholar] [CrossRef]
- Fageria, N. Maximizing Crop Yields; CRC Press: Boca Raton, FL, USA, 1992. [Google Scholar]
- Kicińska, A.; Wikar, J. The Effect of Fertilizing Soils Degraded by the Metallurgical Industry on the Content of Elements in Lactuca sativa L. Sci. Rep. 2021, 11, 4072. [Google Scholar] [CrossRef]
- Obrador, A.; González, D.; Almendros, P.; García-Gomez, C.; Fernandez, M.D. Assessment of Phytotoxicity and Behavior of 1-Year-Aged Zn in Soil from ZnO Nanoparticles, Bulk ZnO, and Zn Sulfate in Different Soil- Plant Cropping Systems: From Biofortification to Toxicity. J. Soil Sci. Plant Nutr. 2022, 22, 150–164. [Google Scholar] [CrossRef]
- Balafrej, H.; Bogusz, D.; El Abidine Triqui, Z.; Guedira, A.; Bendaou, N.; Smouni, A.; Fahr, M. Zinc Hyperaccumulation in Plants: A Review. Plants 2020, 9, 562. [Google Scholar] [CrossRef]


| Daily Dietary Intake Values (mg d−1) | Health Risk Index (HRI) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| First Crop | Second Crop 1 | First Crop | Second Crop 1 | ||||||
| MIN 2 | MAX 2 | MIN 2 | MAX 2 | MIN 2 | MAX 2 | MIN 2 | MAX 2 | ||
| Control | D0 | 0.010 | 0.020 | 0.013 | 0.026 | 0.033 | 0.068 | 0.043 | 0.088 |
| SULP | D1 | 0.062 | 0.126 | 0.043 | 0.088 | 0.205 | 0.419 | 0.143 | 0.293 |
| SULP | D2 | 0.137 | 0.281 | 0.098 | 0.200 | 0.458 | 0.935 | 0.326 | 0.665 |
| SULP | D3 | 0.280 | 0.572 | 0.230 | 0.469 | 0.933 | 1.908 | 0.765 | 1.565 |
| SULP | D4 | 0.390 | 0.796 | 0.446 | 0.912 | 1.298 | 2.654 | 1.488 | 3.041 |
| NANO | D1 | 0.039 | 0.079 | 0.055 | 0.112 | 0.129 | 0.264 | 0.183 | 0.374 |
| NANO | D2 | 0.100 | 0.205 | 0.091 | 0.186 | 0.334 | 0.682 | 0.304 | 0.621 |
| NANO | D3 | 0.218 | 0.446 | 0.182 | 0.372 | 0.728 | 1.488 | 0.606 | 1.240 |
| NANO | D4 | 0.309 | 0.631 | 0.357 | 0.729 | 1.029 | 2.103 | 1.189 | 2.431 |
| LIG | D1 | 0.056 | 0.115 | 0.037 | 0.075 | 0.187 | 0.383 | 0.122 | 0.249 |
| LIG | D2 | 0.102 | 0.208 | 0.083 | 0.169 | 0.338 | 0.692 | 0.276 | 0.564 |
| LIG | D3 | 0.213 | 0.435 | 0.189 | 0.386 | 0.709 | 1.450 | 0.630 | 1.288 |
| LIG | D4 | 0.343 | 0.701 | 0.356 | 0.728 | 1.143 | 2.337 | 1.188 | 2.428 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Francisco, M.; Ortiz, R.; Obrador, A.; Gonzalez, D.; Gascó, G.; Almendros, P. The Effect of Complexed, Nanosized, and Conventional Zinc Sources Applied at Varying Rates to an Acidic Mediterranean Soil on Two Successive Lettuce Crops. Agronomy 2025, 15, 896. https://doi.org/10.3390/agronomy15040896
de Francisco M, Ortiz R, Obrador A, Gonzalez D, Gascó G, Almendros P. The Effect of Complexed, Nanosized, and Conventional Zinc Sources Applied at Varying Rates to an Acidic Mediterranean Soil on Two Successive Lettuce Crops. Agronomy. 2025; 15(4):896. https://doi.org/10.3390/agronomy15040896
Chicago/Turabian Stylede Francisco, Marina, Raquel Ortiz, Ana Obrador, Demetrio Gonzalez, Gabriel Gascó, and Patricia Almendros. 2025. "The Effect of Complexed, Nanosized, and Conventional Zinc Sources Applied at Varying Rates to an Acidic Mediterranean Soil on Two Successive Lettuce Crops" Agronomy 15, no. 4: 896. https://doi.org/10.3390/agronomy15040896
APA Stylede Francisco, M., Ortiz, R., Obrador, A., Gonzalez, D., Gascó, G., & Almendros, P. (2025). The Effect of Complexed, Nanosized, and Conventional Zinc Sources Applied at Varying Rates to an Acidic Mediterranean Soil on Two Successive Lettuce Crops. Agronomy, 15(4), 896. https://doi.org/10.3390/agronomy15040896







