Abstract
The excessive use of conventional fertilizers has led to low nutrient-use efficiency and significant environmental challenges. To address these limitations, this study aimed to evaluate the effects of Fe2O3 and CuO nanoparticles (NPs) as potential nanofertilizers, on the soil chemical composition, nutrient fractionation, enzyme activity, and Lepidium sativum L. growth. The results of the study showed that Fe2O3-NPs improved nitrogen bioavailability and enhanced plant biomass, particularly at low to moderate doses. CuO-NPs, in contrast, reduced nitrogen and phosphorus mobility and showed phytotoxic effects at high concentrations. Enzyme activity was suppressed at high NP levels, likely due to oxidative stress. Nutrient fractionation revealed the increased immobilization of phosphorus and the moderate mobilization of potassium and copper, depending on NP type. Based on the results, Fe2O3-NPs show potential as a nanofertilizer for enhancing soil fertility and plant growth in sandy loam soils, whereas CuO-NPs require caution due to toxicity risks. Future research should focus on long-term environmental impact, optimal NP concentrations, and their interaction with soil microbial communities.
1. Introduction
Agriculture has been essential for human survival for centuries. With population growth and technological development, plant cultivation methods have undergone significant changes. For years, plant growth has been supported by fertilizers that not only improve the quality of crops but also the soil. Conventional fertilizers support plant nutrition; however, owing to their low absorption, they may also have many negative effects on the environment, such as groundwater pollution, the eutrophication of surface waters, and the degradation of soil quality. All these disturbances are the result of inappropriate fertilizer release in relation to plant uptake [1]. Currently, efforts are being made to achieve ideal agriculture, which is based on sustainable cultivation that promotes management through the reasonable use of natural resources, as well as rational fertilization, that should balance the nutritional needs of plants and, at the same time, not create high reserves of nutrients in the soil [1]. According to this concept, plants should be fertilized using multipurpose preparations characterized by the controlled and slow release of nutrients. The most commonly used fertilizers contain nitrogen (N), phosphorus (P), and potassium (K), which are essential for plant growth. N is an element that enters the soil through atmospheric precipitation, human activity, and the decomposition of organic matter. It is most easily absorbed by plants in the form of N-NO3− and N-NH4+ [2]. The use of N in fertilizers significantly improves plant yields. However, it is estimated that plants use only half of the nitrogen supplied through fertilization [2]. The bioavailability of P in soil depends on environmental conditions, mainly pH and organic matter content. P is used in soluble forms in fertilizers. However, even under optimal environmental conditions, it is taken up by plants in amounts of approximately 30% of the total P subjected to the soil [3]. K is the most abundant inorganic cation and a key element that significantly contributes to the physiological processes that support plant growth and development. Its bioavailability in soil is dependent on the soil organic matter content and clay mineral type [4]. Its application directly to the soil improves plant yield; however, the efficiency of K fertilizer is in the range of 30–50% [5].
Currently, an increase in the use of nanoparticles in agronomy has been observed worldwide. The main advantages of nanoformulation are its increased efficiency in plant nutrition compared to conventional chemicals used in fertilization. Moreover, the limited negative impact of nanoparticles on the environment, achieved by decreasing the concentration of slowly released nutritional compounds, was observed. The properties of nanoagrochemicals are conducive to their use as nanofertilizers and/or nanopesticides that selectively deliver various macromolecules [6]. However, the impact of these compounds on the food chain through bioaccumulation by plants should be carefully evaluated before their application.
Nanoparticles that enter the soil system may undergo several processes that determine their bioavailability and toxicity. In agrotechnology, nanoparticles enter the soil through seed treatment or as nanofertilizers during plant cultivation. Numerous studies have demonstrated that iron oxide nanoparticles (Fe2O3-NPs) exert significant positive effects on the physiological and agronomic parameters of crop species. The application of Fe2O3-NPs has been shown to enhance seed germination rates, likely due to improved nutrient availability and stimulation of metabolic activity during early developmental stages. Furthermore, these nanoparticles contributed to increased root and shoot biomass, indicating their role in promoting vegetative growth and nutrient assimilation in plants. Enhancements in chlorophyll content and photosynthetic efficiency have also been reported, reflecting improved Fe uptake and the subsequent optimization of the photosynthetic machinery. Collectively, these physiological improvements translate into increased yield quality and quantity across several major crops, including wheat (Triticum aestivum), maize (Zea mays), soybean (Glycine max), and rice (Oryza sativa). These findings underscore the potential of Fe2O3 nanoparticles as effective nanofertilizers for sustainable crop production [7,8,9].
Copper oxide nanoparticles (CuO-NPs) have emerged as promising nanofertilizers because of their potential to enhance plant growth, physiological function, and stress resilience. Their application has been shown to improve seed germination, biomass accumulation, photosynthetic efficiency and antioxidant enzyme activity in various crop species. For instance, seed priming with CuO-NPs at a concentration of 4 mg/L significantly increased the shoot and root lengths, net photosynthetic rate, internal CO2 concentration, and proline content in Brassica juncea. Additionally, the activities of antioxidant enzymes, such as superoxide dismutase, catalase, and peroxidase, were enhanced, indicating improved oxidative stress management [10]. The foliar application of CuO-NPs also had a positive effect. In Brassica juncea, spraying with 8 mg/L CuO-NPs increased the leaf area, chlorophyll content, photosynthetic rate, and stomatal conductance. These enhancements were accompanied by elevated activities of nitrate reductase and carbonic anhydrase, enzymes crucial for nitrogen assimilation and CO2 fixation, respectively [10]. However, the effects of CuO NPs were concentration dependent. While low concentrations promote growth, high doses can be toxic. In barley (Hordeum vulgare), low concentrations (10–100 mg/L) of CuO-NPs enhanced germination and seedling growth, whereas concentrations above 500 mg/L inhibited these parameters [11]. These findings underscore the potential of both Fe2O3-NPs and CuO-NPs as nanofertilizers to enhance crop productivity and stress tolerance. Nevertheless, the careful optimization of the application rates and methods is essential to maximize the benefits and minimize potential adverse effects.
Upon the introduction of nanoparticles into the agroenvironment, they undergo various physicochemical transformations that promote their accumulation in soil matrices. These modifications were influenced by the aggregate state of the nanoparticles. Furthermore, soil properties such as pH, dissolved organic matter content, moisture level, and soil redox potential modulate the behavior of metal-based nanoparticles, which may potentially result in the release of free metal ions [6]. However, still limited information exists on how specific metal oxide nanoparticles influence nutrient dynamics, enzyme activity, and plant uptake in soil systems. A critical gap remains in understanding how Fe2O3-NPs and CuO -NPs affect the bioavailability and fractionation of key macronutrients and their impact on plant growth and soil health under agronomic conditions. This study evaluates the dual effects of Fe2O3-NPs and CuO-NPs on soil chemistry and Lepidium sativum L. growth. The innovative aspects of this work lie in its comprehensive assessment of N, P, K, Fe and Cu fractionation, soil enzyme activity (dehydrogenase, urease and acid phosphatase), and plant nutrient uptake in response to nanoparticle exposure. This dual-nanoparticle approach shows the possibility and safety of Fe2O3-NPs and CuO-NPs applications in sustainable agriculture.
This study aimed to determine the concentration and bioavailability of Fe2O3-NPs and CuO-NPs after their application to the soil. The evaluation of how the tested nanoparticles affected the extractable and bioavailable amounts of key macronutrients, such as N, P, and K, in soil, along with other macro- and microelements, was performed. The concentrations of elements accumulated by Lepidium sativum L. during cultivation in soil supplemented with Fe2O3-NPs or/and CuO-NPs at different concentrations were determined.
2. Materials and Methods
2.1. Nanoparticles
The study was conducted using commercially available Fe2O3-NPs and CuO-NPs (Sigma-Aldrich, St. Louis, MO, USA) with the following characteristics: Fe2O3-NPs with an average particle size below 50 nm (BET) and CuO-NPs with a particle size below 50 nm (TEM).
To disperse Fe2O3-NPs and CuO-NPs and prevent the formation of aggregates, the nanoparticles were dissolved in deionized water to obtain solutions with specified concentrations and subjected to ultrasonication at a frequency of 22 kHz and an ultrasonic intensity of 7 W/cm2 for 5 min, with agitation intervals of 10 s followed by 30 s of rest.
2.2. Soil
The soil used in the study was collected from the surface area (0–30 cm) in Lodz, Poland, in April 2023. Soil was air-dried and sieved through a 2 mm mesh and then subjected to physicochemical analysis, including texture analysis, pH, organic carbon (Corg), total nitrogen (Ntotal), nitrate (N-NO3−), ammonium (N-NH4+), and available phosphorus (Pavail). Soil was also analyzed for the content of the chosen macro- and microelements. The analysis was preceded by soil mineralization.
Soil texture analysis was performed in accordance with ISO 11277:2009 [12]. Further analysis included pH determination according to ISO 10390:2021, Corg in relation to the procedure described in ISO 14235:1998, Ntotal, N-NO3−, and N-NH4+ according to ISO 14256-1:2003, and Pavail in accordance with ISO 11263:1994 [13,14,15,16]. The pseudototal concentration of chosen macro- and microelements was determined by flame atomic absorption spectroscopy method (F-AAS) in soil samples prepared by acid digestion in aqua regia according to ISO 11466:1995/EN 13657 [17].
2.3. Soil Experiments
One kilogram of air-dried soil was placed in a plastic pot and watered with deionized water to obtain a humidity of 35%. After 7 days, the soil humidity was checked and replenished in case of losses to maintain the required level of humidity. Next, Fe2O3-NPs and CuO-NPs were added separately as solutions to obtain concentrations of 10, 100, and 300 mg kg−1 soil dry weight. The investigations were also performed in soil samples where Fe2O3-NPs and CuO-NPs were added simultaneously at a concentration of 10 mg kg−1 of Fe2O3-NPs + 10 mg kg−1 of CuO-NPs, 10 mg kg−1 of Fe2O3-NPs + 100 mg kg−1 of CuO-NPs, 100 mg kg−1 of Fe2O3-NPs + 10 mg kg−1 of CuO-NPs, and 100 mg kg−1 of Fe2O3-NPs + 100 mg kg−1 of CuO-NPs. The soil sample without nanoparticles served as control. The experimental design is shown in Figure 1. The soil samples supplemented with nanoparticles were mixed to obtain homogenized samples and were left for stabilization for 14 days, maintaining the soil humidity at 35%, temperature 20 ± 2 °C, with limited light exposure. After the stabilization period, soil samples were subjected to fractionation analysis. Each soil sample (variant of the experiment) was prepared in triplicate.
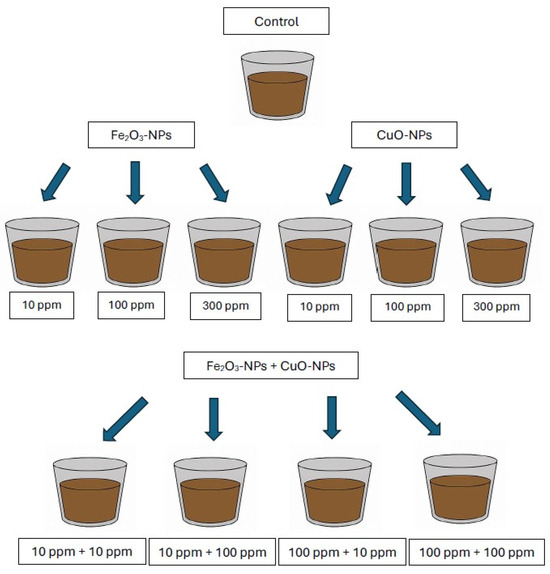
Figure 1.
Experimental design.
2.4. Fractionation Analysis
Sequential chemical analysis was performed in accordance with the procedure described by Tessier et al. (1979), which separates metals into exchangeable (F1), carbon bond (F2), reducible (F3), oxidizable (F4) and residual (F5) fractions [18]. In the study, the same extraction reagents and sequence were applied, but instead of measuring only trace metals, the extracted solutions were also analyzed for Ntotal, Pavail, K, Fe and Cu concentrations with a procedure described in Section 2.2.
For Ntotal, each extract was analyzed according to ISO 14256-1:2003. Although ISO 14256-1:2003 is conventionally applied to bulk soil samples for mineral nitrogen determination, in this study KCl extraction and colorimetric/reduction-based detection protocol to quantify NO3−, NO2−, and NH4+ within each Tessier extraction fraction were adopted. N in the mineral forms N-NO3−, N-NO2−, and N-NH4+ was extracted from soil using KCl solution. N-NO3− was reduced to N-NO2−, and N-NO2− was then coupled with N-(1-naphthyl)ethylenediamine dihydrochloride (NED) reagent to form a colored complex, which was measured colorimetrically at 540 nm. N-NH4+ was determined colorimetrically (660 nm) in a separate portion of the extract with the use of the indophenol blue method. Ntotal means the sum of N-NO3−, N-NO2−, and N-NH4+.
For Pavail measurements, soil extracts obtained in accordance with the Tessier procedure [18] underwent persulfate-sulphuric acid digestion (5% K2S2O8 + H2SO4 conc.; autoclaved at 120 °C for 60 min) to hydrolyze organic phosphates, polyphosphates, and colloidal P into orthophosphate (PO43−). Digested solutions were then cooled, diluted with deionized water, and neutralized to pH 5–7 using 10 M NaOH to ensure optimal conditions for colorimetric analysis. Pavail in all fractions was quantified colorimetrically at 880 nm as orthophosphate-P via the ascorbic acid-molybdenum blue method (ISO 11263:1994).
Concentrations of K, Cu and Fe in sequential extracts were determined directly by F-AAS following the Tessier fractionation procedure. For F1–F4 fractions, extracts were subjected to F-AAS analysis after centrifugation and filtration, while F5 aqua regia digestion was performed in accordance with ISO 11466: 1995/EN 13657 prior to F-AAS analysis.
Based on the analyses conducted, the mobility factor (MF) for individual elements and their chemical forms was determined using the following Formula (1) [19]:
where
MF = [(F1 + F2)/(F1 + F2 +F3 +F4 +F5)] ∗ 100%
MF: Mobility factor expressed as a percentage.
F1, …, F5—content of the element in the fraction.
2.5. Soil Enzymatic Activity
Soil enzymatic activity was estimated by determining the activity of dehydrogenase, urease, and acid phosphatase in soil extracts. Dehydrogenase activity (DHA) was determined according to the method described by Casida et al. (1964) [20] by colorimetric measurement of triphenyl formazan (TPF) obtained from the reduction in triphenyl tetrazolium chloride (TTC). Urease activity (UA) was measured based on the colorimetric method of ammonium determination that occurred after enzymatic hydrolysis of urea [21]. Acid phosphatase activity (ACP) was analyzed using p-nitrophenyl phosphate (pNPP) as a substrate. The enzyme hydrolyzes pNPP to p-nitrophenol (pNP), which was measured spectrophotometrically [22].
2.6. Greenhouse Studies
Soil prepared as described in Section 2.3 was used for greenhouse studies. Ten grams of Lepidum sativum L. seeds was sown in control soil samples, as well as in soil samples supplemented with nanoparticles: in soil with Fe2O3-NPs at different concentrations, CuO-NPs at different concentrations, and where Fe2O3-NPs + CuO-NPs were added simultaneously. The experiments were conducted under controlled conditions with a day/night system of 22/19 °C, a photoperiod of 14 h, and soil humidity of 35%. After 14 days, the plants were harvested, washed to remove soil particles, weighed, and subjected to further analysis. The experiments were performed in triplicate.
2.7. Plant Analysis
After plant cultivation, the fresh biomass of the aboveground parts of L. sativum L. was determined. Next, 1 g of air-dried plant aboveground parts was subjected to acid mineralization according to Chang et al. (2006) [23]. After mineralization, the concentrations of K, Fe, and Cu were determined using F-AAS. The total nitrogen content in plant shoots was determined according to the Kjeldahl method described in detail in the AOAC (2006) Standard Method 9784 [24]. The phosphorus content in plant shoots was determined using a colorimetric method based on the molybdenum blue assay [25].
2.8. Statistics
Statistical analysis was performed using ANOVA Excel Data Analysis, where data were expressed as means of triplicate datasets ± standard deviations (SD). To compare the means between groups, analysis of variance was performed. Statistical significance was set at p < 0.05. A multiple range test based on Fischer’s last significant procedure was performed to identify the means that were significantly different from others.
3. Results
3.1. Soil Analysis
Physicochemical soil analysis included soil texture, pH, and concentration of macro- and microelements in soil. The results of this stage of the experiment for untreated soil sample (control) are presented in Table 1. Based on the content of sand, silt, and clay, the soil was classified as sandy loam [26]. The soil pH (H2O) indicated that the soil used in this study was classified as slightly acidic. The results presented in Table 1 show that soil organic carbon (SOC) was at a moderate level, which suggests that the requirements for plant cultivation should be covered. In the present study, SOM was equal to 2.9–3.1%. SOM factors show various stages of organic matter decomposition, including fresh plant and animal residues, decomposing organic material, and stable humus. The concentration of N total in soil indicated low to moderate nitrogen content. On the other hand, the amount of P available in the soil was classified as high, which confirmed that the P level was sufficient for optimal plant growth. Further analysis was performed to determine other elements that support plant growth during cultivation. Based on the results presented in Table 1, the Fe concentrations are typical for mineral soils. The concentration of other soil elements indicated the balanced nutritional status of the soil. Most of the tested elements were at the optimal level, which promoted the proper supply of soil nutrients needed for the proper functioning of plants.

Table 1.
Physicochemical properties of soil (control sample).
3.2. Element Distribution in Soil
Figure 2a–c presents the concentration of three main nutritional elements (N, P, K) in different soil fractions for untreated soil (control) and soil supplemented by Fe2O3-NPs and CuO-NPs in different concentrations. Figure 2d,e shows the results of Fe and Cu fractionation in different variants of experiment.
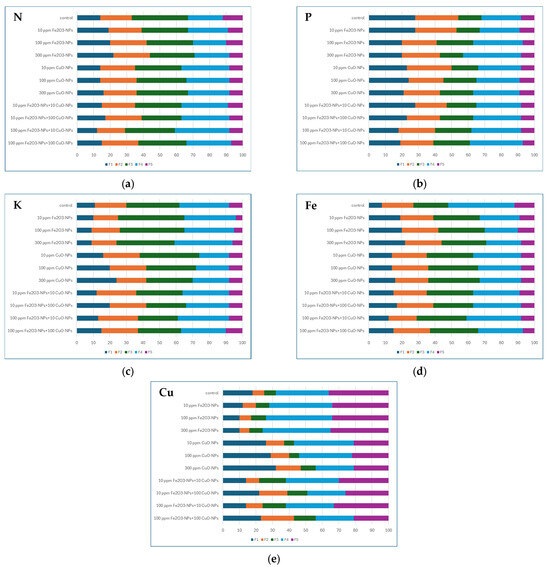
Figure 2.
Percentage concentration of N total, P available, K, Fe and Cu in soil fractions F1–F5 (F1-water soluble, F2-acid soluble, F3-organic matter bound, F4-strongly complexed, F5-residual): (a) for N; (b) for P; (c) for K; (d) for Fe; (e) for Cu.
As can be seen in Figure 2a, for the control treatment, over 33.0% of N total constituted the bioavailable form as water (F1) and acid-soluble (F2) fractions. Increasing amounts of N total in F1 showed not only the amount of bioavailable N but also allowed estimating the risk of its leaching for soils of low sorption capacity like sandy loam. The results of the study showed that F4 and F5 fractions constituted 33.0% of total N. Application of Fe2O3-NPs and CuO-NPs affected the fractionation of all tested elements, regardless of the NP concentrations. Introducing Fe2O3-NPs increased N fractions recognized as available for plants when compared to the control. The higher the concentration of Fe2O3-NPs applied to the soil, the more bioavailable fractions of N occurred. The results of the study showed an increase in F4 when compared to the control, regardless of the concentration of Fe2O3-NPs. The slight increase in N in F2 was also observed; however, it was dependent on the concentration of Fe2O3-NPs used in the study. Analysis of CuO-NPs on N in soil showed that F1 of N increased only when CuO-NPs was added to the soil in a concentration of 300 ppm. Other CuO-NPs treatments did not bring significant changes in F1 for N. A significant increase in N concentration in F4 was observed regardless of CuO-NP concentration. Fractionation analysis of N concentrtion in soil after CuO-NPs application showed that F2, F3 and F5 fractions were kept at the same level when compared to control, regardless of the CuO-NP concentration. Combined use of Fe2O3-NPs and CuO-NPs in different concentrations also affects the fractionation of N in soil. As can be seen in Figure 2, the general increase in N in F1, F2 and F4 occurred when compared to the control. On the other hand, the organic matter fraction (F3) and residual fraction (F5) of N decreased as follows: 11.8–29.4% and 25.0–41.7%, in relation to the control.
Other analyzed element was P (Figure 2b). Fractionation analysis of P shows that soil is characterized by a high potential of P supply to plants. Based on the results presented in Figure 2 for the control treatment, F1 > F2 > F4 > F3 > F5. P bound to organic matter and P strongly complexed constituted as follows over 24% and over 14% of total P in soil. This suggests the increasing soil microbial activity and potential of long-term P delivery to plants during the nutrition process. The lowest amount of P was the residual fraction (F5). Application of Fe2O3-NPs decreased the mobile and semi-mobile fractions (F1, F2, F4) by about 1.3–9.0%, regardless of NP concentration when compared to the control. The highest decrease in potentially bioavailable forms of P was observed for the 300 ppm Fe2O3-NPs treatment. Introducing CuO-NPs also decreased the level of P in mobile and semi-mobile fractions. The decrease was on the level of 2.6–9.0% in relation to the control (Figure 2b). Simultaneous application of Fe2O3-NPs and CuO-NPs also influenced the P distribution in soil fractions. In general, the concentration of P in F3 and F4 fractions increased, while P in F1 and F5 was kept at the same level, regardless of NP concentration.
K fractionation analysis showed that K concentration in soil fractions (control) was as follows: F3 > F4 > F2 > F1 > F5. This sequence indicates that the dominant pool of K is the organic matter-bound fraction, which is not easily available for plants. However, the mobile and semi-mobile fractions constitute 60.0% of total K in soil. Application of Fe2O3-NPs kept the sequence of K fractionation in soil at the same scheme as for control treatment (Figure 2c). The difference occurred in the percentage of mobile fractions in the soil, which were dependent on the concentration of applied Fe2O3-NPs. The lower dosage of Fe2O3-NPs decreased mobile and semi-mobile K fractionations about 6.7% when compared to the control. The highest concentration of Fe2O3-NPs kept the fractions of K considered as bioavailable at the same level as for the control. The application of Fe2O3-NPs had an insignificant influence on K fractionation in soil. Introducing CuO-NPs to soil changed the K fractionation in soil. The mobile and semi-mobile fractions (F1, F2, F4) of K increased for medium and the highest CuO-NP concentration as follows—3.3% and 6.7%—respectively. Moreover, the difference in K distribution among the fractions was observed. For 100 ppm CuO-NPs the dominant fractions were F3 > F2 > F4 > F1 > F5, while for 300 ppm CuO-NPs the treatments were F3 > F1 > F4 > F2 > F5. Simultaneous application of Fe2O3-NPs and CuO-NPs showed that the increase in K concentration in mobile and semi-mobile soil fractions, regardless variant of experiment in relation to control. The most significant increase in K concentration in mentioned fractions was observed for 10 ppm Fe2O3-NPs + 100 ppm CuO-NPs treatment when compared to control.
3.3. Influence of Fe2O3-NPs and CuO-NPs on Fe and Cu Fractionation
Further analysis included the fractionation of Fe and Cu in control soil samples. The results of this stage of experiments are presented in Figure 2d,e. Fe fractionation analysis of control sample showed that water (F1) and acid-soluble (F2) fractions constituted over 27.0% of total Fe concentration in soil. The highest concentration of Fe was determined in the organic matter bound fraction (F3), which constituted over 34.0% of total Fe present in the soil. F3 fraction is a reserve of Fe which can be transformed into easily bioavailable forms.
The incorporation of NPs into the soil altered the distribution of Fe among the soil fractions relative to the control, regardless of NPs type or concentration (Figure 2d). In general, across all experimental treatments, the concentrations of Fe in fractions F1 and F3 increased, whereas decreases were observed in F4 and F5. The concentration of Fe in fraction F2 remained largely unchanged. A comparative analysis of all treatment variants indicated that only the treatment with 10 ppm Fe2O3-NPs combined with 100 ppm CuO-NPs slightly increased the levels of Fe in the mobile and semi-mobile soil fractions. The incorporation of CuO-NPs into the soil did not significantly affect the distribution of Fe among the soil fractions.
Cu fractionation in control soil was as follows: F5 > F4 > F1 > F2 = F3 (Figure 2e). Comparative analysis of results showed that the residual form of Cu in soil is dominant. The water-soluble fraction of Cu was at a moderate level, which can be beneficial from the point of view of their nutrition (Figure 2e). The application of Fe2O3-NPs alone did not significantly alter the distribution of Cu among the soil fractions compared to the control. However, notable changes were observed in treatments involving CuO-NPs, independent of NP concentration. For both low and medium CuO-NP concentrations, the Cu fractionation followed the order: F4 > F1 > F5 > F2 > F3. In contrast, the application of 300 ppm CuO-NPs resulted in the distribution: F1 > F4 > F2 > F5 > F3. Across all CuO-NP concentrations, the combined mobile and semi-mobile fractions (F1 and F2) accounted for approximately 70.0–73.0% of total soil Cu. Simultaneous application of Fe2O3-NPs with 100 ppm CuO-NPs affected Cu distribution among soil fractions only at higher CuO-NP concentrations, relative to the control.
To summarize this part of the investigation, the mobility factor (MF) of nutrient fractionation was calculated, with results presented in Table 2. Comparative analysis revealed that P exhibited the highest MF, while Fe and Cu showed decreasing mobility in the control treatment. Application of Fe2O3-NPs increased Fe availability but simultaneously reduced the mobility of K and Cu, likely due to antagonistic interactions and nutrient competition. In contrast, CuO-NPs enhanced Cu mobility and indirectly promoted K mobility; however, this effect was concentration-dependent. The simultaneous incorporation of Fe2O3-NPs and CuO-NPs either harmonized or suppressed nutrient mobility, depending on the dosage.

Table 2.
Mobility factor (MF) [%] for each variant of experiment.
3.4. Effect of NPs on Soil Enzyme Activity
Activity of dehydrogenase (DHA), urease (UA) and acid phosphatase (ACP) in soil under different treatments was determined. The results are given in Table 3.

Table 3.
Activity of soil enzymes for different variants of experiment (mean (n = 3) ± SD). Different letters indicate significant difference between the series: DHA (letters a–d); UA (letters m, n and p); ACP (letters w, x and z) (p < 0.05).
According to results presented in Table 3, DHA was dependent on the NPs type and their concentration. Introducing Fe2O3-NPs to the soil did not bring any significant influence on DHA at lower concentrations when compared to control. This implies that low concentrations of Fe2O3-NPs are microbiologically tolerable. However, the highest concentration of Fe2O3-NPs caused inhibition of DHA over 12.6% in relation to the control, which can be related to the potentially toxic effect of Fe2O3-NPs in a concentration of 300 ppm. Similar observations were noticed after CuO-NPs soil incorporation. NPs at low concentrations did not influence DHA, while their moderate concentrations stimulated enzyme production. However, DHA decreased over 30.3% after 300 ppm of CuO-NPs treatment in relation to the control. Mixed treatments of soil with NPs showed the decrease of DHA of 6.2–32.9% depending on the concentration when compared to control.
The other analyzed enzyme was urease. Application of both Fe2O3-NPs and CuO-NPs influenced the UA when compared to control. Regardless of NP type, low and moderate doses of NPs stimulated this enzyme activity. Incorporation of 300 ppm Fe2O3-NPs and 300 ppm CuO-NPs decreased UA as follows: 18.1% and 16.5%, respectively, when compared to the control. Simultaneously applying Fe2O3-NPs and CuO-NPs at a dose of 100 ppm each lowered UA about 22.8% in relation to the control.
ACP in soil under NPs treatment showed that low (10 ppm) and moderate (100 ppm) concentrations of Fe2O3-NPs and CuO-NPs did not significantly influence ACP activity. The highest NPs dose used in the study contributed to an ACP activity decrease in the range of 10.4–10.8% in relation to the control.
3.5. L. sativum L. Growth Under NPs Treatment
One of the goals of the presented study was to examine the effect of NPs application on L. sativum L. growth and concentration of key elements in its aboveground tissues According to Table 4, fresh biomass of plant shoots decreased significantly for 100 ppm and 300 ppm of CuO-NPs treatments as follows: 5.2% and 13.2%, respectively, in relation to control. Fe2O3-NPs stimulated plant growth, especially in low and moderate concentrations. After 10 and 100 ppm Fe2O3-NPs soil incorporation, plant biomass increased 4.8% and 4.3%, respectively. The simultaneous application of Fe2O3-NPs and CuO-NPs significantly affected biomass production only for the variant with a concentration of 10 ppm Fe2O3-NPs and 100 ppm CuO-NPs, which can be correlated with increased toxicity of CuO-NPs. Further analysis of key macronutrients in L. sativum L. shoots showed the increase in N concentration for variants of the experiment when Fe2O3-NPs were incorporated into the soil, regardless of NP concentration. Comparative analysis of NPK accumulation reflects the trends obtained for the fresh biomass of plants grown in the presence of NPs. As shown in Table 4, N concentration in plant shoots increased over 30% for Fe2O3-NPs treatments, regardless of the used concentration when compared to the control. The significant increase in N accumulation was also observed for simultaneous application of NPs for treatments where 100 ppm of Fe2O3-NPs was added to soil. However, the increase was at the level of 15.5–18.0% when compared to the control. The increase in P concentration in plant shoots was observed in all variants of experiments; however, only CuO-NPs application at higher concentrations significantly increased P amounts in plant cells. The results also indicate the changes in K accumulation in plant shoots cultivated in the soil with CuO-NPs when compared to the control. Comparative analysis of Fe and Cu concentration in L. sativum L. shoots showed higher accumulation of both Fe and Cu in L. sativum L. shoots exposed to Fe2O3-NPs and CuO-NPs, respectively.

Table 4.
L. sativum L. shoot analysis under different variants of cultivation (mean (n = 3) ± SD). Different letters indicate significant difference (p < 0.05) for: fresh biomass (letters a–d); N (letters i and j); P (letters m and n); Fe (letter w, x and z), Cu (letters r, s and t).
4. Discussion
4.1. Effect of NPs on Soil Fractionation and Enzymatic Activity
The soil used in the study was classified as sandy loam. This type of soil has good water retention, which affects the simplicity of water absorption by plant roots and, at the same time, the uptake of nutrients dissolved in water. However, sandy loam can be less effective at retaining nutrients, suggesting that fertilization may be needed [27]. Sandy loam, in which the dominant fraction is sand, shows good aeration due to the pore size distribution and hydraulic conductivity of the soil. This property provides the appropriate amount of oxygen for plant roots and, therefore, affects the amount of energy required by the plant to absorb water and nutrients [28]. The soil type and its pH can influence the bioavailability of soil elements by increasing the availability of phosphorus (P), iron (Fe), and manganese (Mn) and decreasing the availability of other elements such as molybdenum (Mo) and calcium (Ca) [29]. Soil pH determined in KCl confirmed that the soil was slightly acidic. The difference between the pH of H2O and the pH of KCl indicates that the soil had a dominant negative charge, which is typical for organic and clay soils and can be potentially beneficial for nutrient cation retention. The physicochemical analysis of soil showed that soil organic matter (SOM) was at a good level; however, it should be noted that extensive, long-term agricultural practices may be supported by adding natural substances that increase the SOM level, such as manure or compost [27]. Soil characteristics showed the low amount of total N. In accordance with Stevenson and Cole (1999) [30], soils with a total nitrogen below 0.1% are often considered nitrogen deficient. N total represents the nitrogen reserves in the soil in both organic and inorganic forms. A more precise analysis provided in this study showed that the nitrate (N-NO3−) form was dominant. Nitrate is a mobile form of nitrogen in soil and can be easily absorbed by plants; however, nitrogen in this form can also be leached from the soil, especially from soils with an increased amount of sand in their texture. Further soil analysis showed high levels of available P, which suggests that additional P fertilization is not required for this soil due to the possibility of negative environmental impacts such as water eutrophication [31]. The other key element for plant growth and development is K. According to Johnston (2001) [32], the level of K in soil can be classified as high and is adequate for soil used in agronomy practices [32].
Concentration of soil elements showed that Fe amount in soil was typical for mineral soils [33]. Fe in the soil forms various compounds, including silicates, sulfates, iron oxides, and hydroxides. Its availability to plants is determined by soil properties such as pH, redox potential, and organic matter content [34]. The Cu concentration in the tested soil was relatively low but within the range for natural soils. According to Kumar et al. (2019) [35], a Cu concentration below 10 mg kg−1 in soil can limit its bioavailability for plants. Moreover, a low amount of SOM can cause a deficiency of Cu accumulation in plant tissues, resulting in photosynthesis disturbance [35]. Other macroelements, the concentrations of which in soil were analyzed in the study, were sodium (Na), magnesium (Mg), and calcium (Ca). Based on the presented results, Na concentration was typical for unpolluted soils. Although Na is not required for most plant species, its increased levels in soil can make it difficult for K, Ca, and Mg uptake, and as a result, can cause disturbances in plant ion regulation. The Mg concentration in the tested soil was high. According to Gransee and Fuhrs (2013), the bioavailability of Mg depends on the soil pH and its physicochemical properties [36]. Mg is taken up from soil by plant roots with the use of both active and passive mechanisms. When the concentration of Mg in soil is high, the dominant process is passive diffusion based on the concentration gradient [37]. It should also be noticed that increasing concentrations of K+ and NH4+ in soil can constitute competition with Mg2+ for absorption sites in plant roots, which leads to the so-called ionic antagonism and limits the uptake of Mg [37]. Ca concentration in tested soil was high, which suggests suitable buffering soil properties and its ability for stabilizing soil texture [38]. Increasing amounts of Ca should not influence plant growth and development; however, there is a need for monitoring the plant uptake of Mg, Fe, zinc (Zn) and Mn (manganese). Molybdenum (Mo) is the element that catalyzes the reactions of nitrate transformation in plants and the binding of atmospheric nitrogen by rhizobia [39]. Although plant requirements on Mo are low, it is taken up from soil; therefore, its concentration in soil is required for proper plant development. The concentration of this element in soil used in the study is typical for mineral soil. Average concentrations of Mn in soils range from 200 to 2000 mg kg−1 soil. The analysis provided in this study showed that soil had an average amount of Mn; however, uptake of this element by plants is dependent on soil pH and redox potential. In acidic soils Mn can be released, which increases its toxicity, especially in anaerobic conditions [40]. The analysis of this part of the study indicated the balanced nutritional status of the soil. Most of the tested elements were at the optimal level, which promotes the proper supply of soil nutrients needed for the proper functioning of plants.
Soil concentration of elements that meet the nutritional requirements of plants is an important issue in the context of soil fertility management and effective fertilization. Sequential chemical fractionation is often used for the determination of bioavailable forms of elements in soil, as well as for estimating the resources of nutrients in active (labile) vs. reserve (stable) fractions. This parameter can also be used for assessment of fertilization efficiency. F1 showed not only the amount of bioavailable elements as water soluble but also allowed estimating the risk of their leaching for soils of low sorption capacity like sandy loam. F2 is called acid soluble and represents the labile fraction of the element that is bound with organic matter. That concentration of elements present in the F2 fraction can be easily solubilized. F3 constitutes the organic matter bound fraction. Elements that are found in F3 are beneficial for the long-term maintenance of element resources in the soil, providing their stable source. The fractions strongly complexed (F4) and residual (F5) show the amount of element absorbed on the surface of clay minerals as well as highly bound by minerals and organic matter. Both fractions are considered as low bioavailable for plants. According to the presented results, the concentration of all tested elements varies depending on the variant of the experiment.
N bioavailability is a critical factor limiting ecosystem productivity. It is an essential element for the synthesis of amino acids, peptides, and enzymes, as well as chlorophyll in plants. The limitation of bioavailable N results in decreasing photosynthesis and disturbance of plant growth and development [41]. In the present study, Fe was added to the soil in the form of Fe2O3-NPs. Fe oxides are known to play a crucial role in the formation and the stability of microaggregates in soils, which affects N bioavailability [41]. Fe2O3-NPs influence on N soil fractionation can be related to processes that occur in soil, like mineralization. Based on the results of Kamran et al. (2020), Fe2O3-NPs decreased N mineralization by 24–35%, significantly reducing available nitrogen pools [42]. Decreasing N mineralization is mainly due to reducing the activity of soil enzymes like urease and nitrate reductase. Application of CuO-NPs also influenced N fractionation by decreasing its mobile and semi-mobile fractions. The results of the present study stay in agreement with the results presented by Jośko et al. (2019), who showed that CuO-NPs exposure reduced available N in drier soils [43]. Furthermore, Liu et al. (2021) suggest the interaction of CuO-NPs with soil organic matter in lower CuO-NPs dosages, which may result in N immobilization in organic soil fractions or inhibition of N conversion to bioavailable forms [44]. The combined effect of Fe2O3-NPs and CuO-NPs on N fractionation is dependent on NP concentration, soil texture, and microbial response. Wei et al. (2021) demonstrated that exposure to CuO-NPs and γ-Fe2O3-NPs negatively affected microbial communities, especially those responsible for the nitrification process in soil [45]. This, in consequence, led to a reduction in the NO3− form of N and, at the same time, a reduction in microbial biomass N. The study of Maity et al. (2021) showed that combined use of Fe2O3-NPs and CuO-NPs inhibited activity of enzymes responsible for the denitrification process in soil, like nitrate reductase and nitrite reductase [46]. As the effect of the immobilization of bioavailable N fractions was observed, especially in soils with low organic matter. The results of the present study showed that simultaneous application of Fe2O3-NPs and CuO-NPs increased the available fractions of N in soil. The explanation of this finding exists in redox modulation and enhanced mineralization processes as well as nutrient desorption dynamics. Fe2O3-NPs can participate in redox buffering, particularly in anaerobic soil conditions. When both Fe3+ and Cu2+ from their oxide form of NPs are present, their combined redox activity may modulate oxygen availability and favor the mineralization of organic N, increasing NH4+ and NO3− levels [45]. Enhanced mineralization can also be a result of promoting nitrifying and ammonifying microbes that are resistant to Cu and Fe stress. On the other hand, the high surface area of Fe2O3-NPs and CuO-NPs and their metal-oxide active sites can desorb previously bound organic N from soil particles and act as a slow-release source of N in soil that enhances the bioavailable fractions of N [47].
Further investigations concerned the influence of NPs on soil enzyme activity. Soil enzymatic activity provides valuable insight into biological health, fertility, and soil biochemical functioning. It also serves as an indicator of microbial activity and nutrient cycling, particularly under environmentally altered conditions caused by nanoparticle amendments. Urease is a key soil enzyme responsible for nitrogen transformation. Its main role consists of taking part in N cycling by hydrolysis of urea fertilizers commonly applied in agroecosystems. Urease is secreted extracellularly by soil microorganisms like Urobacterium and Urobacillus, and its activity is highly sensitive to soil management because it is influenced by soil factors such as cropping history, organic matter content, soil depth, management practices, heavy metals and environmental factors like temperature and pH [48]. According to the results of the present study, NPs influenced UA in relation to control. Both Fe and Cu are essential trace elements for microbes. At low concentrations they can enhance metalloenzyme activity, including oxidases and reductases, and support microbial biomass growth. These, in consequence, positively affect the N metabolism efficiency and stimulate UA [49]. The toxic effect of Fe2O3-NPs and CuO-NPs used separately and simultaneously was observed only for the highest concentrations used in the study. According to Kumar et al. (2019), a high amount may damage cell membranes and cause protein oxidation due to oxidative stress [50]. Both NPs used in the study are known as compounds that can generate reactive oxygen species (ROS), like hydrogen peroxide, hydroxyl radicals or superoxide radicals. Moreover, cations released from Fe2O3-NPs and CuO-NPs can directly interact with active sites of urease and displace Ni2+ or bind non-specifically to the active site [51].
The other key macroelement that is essential for plant growth and development is P. P presence is required for numerous physiological and biochemical plant processes, including energy transfer (ATP), photosynthesis, signal transduction, nucleic acid synthesis, and membrane integrity [52]. P is taken up from soil by plants due to the diffusion to the root as well as root adaptations like increasing the root surface area or rhizosphere acidification by root exudation of organic acids. The possibility of P uptake from soil also exists in phosphatase enzyme secretion that is used for solubilization of P bound to organic matter [53]. Therefore, the uptake of P from the soil by plants depends on its bioavailability. Soil fractionation analysis indicated that the concentration of P in mobile and semi-mobile soil fractions decreased after Fe2O3-NPs application. These findings stay in agreement with research of Koopmans et al. (2020), who showed that application of Fe2O3-NPs is a method of P immobilization in soil [54]. Fe2O3-NPs have a high specific surface area and surface hydroxyl groups, which are active sites for P binding, which leads to the formation of very stable bonds of Fe-O-P and, as a consequence, reduces P solubility and mobility. Furthermore, Fe2+ or Fe3+ released from Fe2O3-NPs can react with soluble phosphate to form low-solubility iron phosphate minerals like FePO4·2H2O that are insoluble in neutral and acid solutions, thus reducing P bioavailability [55]. Similarly, introducing CuO-NPs also led to a decrease in the amount of bioavailable P, regardless of NP concentration. These results stay in agreement with results presented by Suazo-Hernandez et al. (2023), who showed that after CuO-NPs soil incorporation, the complex Cu-O-P can be formed that decreases water-extractable (mobile) and bicarbonate-extractable (semi-mobile) phosphorus [56]. Moreover, Cu2+ ions that are released from CuO-NPs may participate with phosphate as Cu-phosphate minerals with reduced P mobility [57]. Combined use of NPs increased P concentration in F3 and F4. This observation can be explained by synergistic immobilization of P when simultaneous Fe2O3-NPs and CuO-NPs were introduced to soil. When NPs are applied together, the reactive surface area of soil particles increases, which contributes to stronger ligand exchange reactions and the formation of insoluble complexes [54]. Moreover, the ions that are formed from NPs compounds formulate low-soluble minerals that are not available for plants. Simultaneous incorporation of Fe2O3-NPs and CuO-NPs into the soil can also affect soil pH. CuO-NPs tend to acidify the rhizosphere slightly, while Fe2O3-NPs may buffer or neutralize soil pH depending on dose. Even slight pH changes enhance P sorption to Fe/Al oxides and promote precipitation reactions. As a result, increased retention of P in non-labile forms, especially in acidic and neutral soils, occurs [56].
Acid phosphatase is an enzyme whose role includes supporting the conversion of organic P compounds into inorganic phosphates, which are directly available to plants and soil organisms. It plays a central role in the P cycle, particularly in acidic soils, where its activity is most effective [58]. Comparative analysis of obtained results indicated that only the highest NPs treatment decreased ACP when compared to control. Decreasing ACP activity can be correlated with the generation of ROS, which provides damage to microbial cells that are responsible for ACP production. Under oxidative stress proteins can be oxidized, which leads to denaturation and loss of function. The results obtained in the study stay in agreement with the findings of Kumar et al. (2019), who stated ROS from nanoparticles disrupt microbial membranes, cellular respiration, and enzyme secretion [50]. These authors observed significant suppression of enzyme activity, especially for ACP due to oxidative stress and metal–protein interactions.
Another enzyme that constitutes the indicator of microbial activity is dehydrogenase. This is an enzyme that oxidizes soil organic matter by transferring protons and electrons from substrates to acceptors, and it is considered to exist as an integral part of intact cells but does not accumulate extra-cellularly in the soil [59]. Determining its activity is an indicator of the intensity of the respiratory metabolism of soil microorganisms, primarily bacteria and actinomycetes. High DHA indicates a large number of soil microorganisms inhabiting this environment [50]. The results of the present study showed that application of Fe2O3-NPs in a concentration of 300 ppm partially inhibited DHA. This result stays in agreement with results presented by Kumar et al. (2019), who showed that higher Fe2O3-NP concentrations act as alternate electron acceptors to hydrogen, due to which the DHA might have fallen down [50]. Similar observations were noticed for CuO-NPs soil incorporation at the highest concentration, which suggests that CuO-NPs can cause oxidative stress to microbes, impairing damage to membrane integrity and respiration of soil microorganisms as well as affecting enzyme biosynthesis [60]. Mixed treatments of soil with NPs also showed the decrease in DHA, which can be related to combined oxidative stress and ion antagonism, especially for high concentrations of Fe2O3-NPs and CuO-NPs [48].
To ensure the appropriate quantity and quality of the crop K is required. Although it does not form plant tissues, its role includes activation of enzymes involved in energy metabolism, starch synthesis and protein production, as well as participation in photosynthesis and ATP production by enhancing CO2 fixation and regulation of ATP synthesis and transport. K is also known as a stabilizer of membranes and plant enzyme activity, which is very important for improving plant tolerance to abiotic stress like drought, frost or salinity [61]. The present study showed that the dominant pool of K in the control soil sample is the organic matter-bound fractions, not easily available for plants. Incorporation of NPs affected K fractionation, regardless of NP type and concentration, when compared to control. Low doses of Fe2O3-NPs decreased K in mobile and semi-mobile fractions, while the highest concentration of Fe2O3-NPs had an insignificant influence on K fractionation in relation to control. This finding stays in agreement with results presented by Bidast et al. (2022), who showed that K was not strongly adsorbed or immobilized by Fe2O3-NPs [62]. K remained soluble and did not precipitate with Fe or form stable mineral phases with Fe from Fe2O3-NPs. On the other hand, the differences in K soil fractionation occurred after CuO-NPs treatment. Increased K in F2 and F1 fractions in soil can be explained by the competition of Cu2+ with K+ for exchange sites, replacing K+ from exchange complexes (CEC sites), and pushing it into solution (F1) [57].
Further investigation was conducted to analyze the fractionation of Fe and Cu following the application of NPs to the soil. The results showed that the use of Fe2O3-NPs increased Fe concentrations in F1 and F3 fractions, while its decrease in F4 and F5 was observed when compared to the control. This increase is likely related to the partial dissolution of Fe2O3-NPs under slightly acidic soil conditions. Under such conditions, Fe3+ can be released and subsequently adsorbed onto exchangeable sites or bind to easily reducible Fe/Mn oxides, resulting in increased Fe concentrations in F1 and F2 [63]. The amount of Fe bound to organic matter (F3) was less affected, likely because Fe2O3-NPs were not strongly associated with organic matter or redox-active sulfur species. This observation is consistent with the findings of Liu et al. (2019), who reported minimal changes in oxidizable metal content following NPs treatment [44]. Moreover, Wyszkowski and Kordala (2025) observed significant reductions in Fe concentrations within the residual fraction (F5) when Fe-bearing amendments were introduced into acidic soils. CuO-NPs did not significantly influence Fe fractionation in soil [64]. Analysis of Cu fractionation in the control treatment revealed that almost half the concentration of Cu was present in the strongly complexed and residual fractions, which are characterized by low bioavailability to plants. Application of CuO-NPs increased Cu concentration in mobile and semi-mobile fractions, which may be attributed to the partial dissolution of CuO-NPs in acidic environments and/or surface interactions. Consistent with Guo et al. (2022), CuO-NPs significantly increased the exchangeable (F1) and reducible (F2) Cu fractions [65]. Although CuO-NPs are only sparingly soluble, their increased dissolution may result from enhanced adsorption on cation exchange sites and weak binding to Fe/Mn oxides. All the findings on the influence of NPs on soil element fractionation are well illustrated as MF. MF reflects how readily a nutrient is available or mobile in the soil–plant system, typically indicating its potential uptake by plants. A higher MF percentage signifies greater mobility or availability.
4.2. NPs Impact on Plant Growth and Nutrient Accumulation
Influencing both soil element fractionation and its enzymatic activity by NPs was reflected in L. sativum L. growth and development. These experiments were provided to assess the best experimental conditions with NPs fertilization potential. In accordance with results, only CuO-NPs negatively influenced plant biomass when compared to the control. The study of Pelegrino et al. (2020) showed the inhibition of Lactuca sativa L. growth after exposition to CuO-NPs [66]. Similar findings were observed in the study of Santhos et al. (2021), who showed Sesbania virgata biomass reduction after CuO-NPs treatments in concentrations of 300 and 400 ppm [67]. This research indicates that higher amounts of CuO-NPs can cause oxidative stress with a reduction in plant growth as a result of changes in plant cells. On the other hand, Fe2O3-NPs stimulated plant growth, especially in low and moderate concentrations. A similar tendency was observed by Ghafoor et al. (2024), who demonstrated that Fe2O3-NPs at 50–100 ppm enhanced biomass, chlorophyll content, and macro/micronutrient uptake (e.g., N, K, Fe) in Sorghum bicolor grown under cadmium (Cd) and zinc (Zn) contamination [68]. Comparative analysis of NPK accumulation reflects the trends obtained for the fresh biomass of plants grown in the presence of NPs. The significant increase in N accumulation was observed for the Fe2O3-NPs variant of experiments, regardless of NP concentration. These findings were correlated with an increase in MF of N in soil for the mentioned treatments. An increased amount of N in plant tissues suggests that soluble forms of N, like nitrate or ammonium, were easily absorbed by plant roots. The study also showed that after Fe2O3-NPs treatments, accumulation of P did not change significantly in relation to the control. The study of Feng et al. (2022) for another type of Fe-NPs showed the increase in P amount in Triticum aestivum in both stems and leaves for NP concentrations of 200 and 500 mg/L [7]. This can be explained by the formation of Fe-phosphate species with a mechanism similar to that presented by Dimkpa et al. (2013) for Zn and Cu-NPs and allows for the explanation of the increase in P uptake by L. sativum L. shoots exposed to CuO-NPs [69]. Application of CuO-NPs not only increased the accumulation of P by plant aboveground parts but also led to an increase in K concentration. These results stay in agreement with the findings of Lung et al. (2021), who showed that the application of CuO-NPs caused the accumulation of K in Triticum aestivum plants, which was related to increased MF [70]. Although the increase in K amount in plant shoots after Fe2O3-NPs was statistically insignificant, the slight increase in values was observed. As shown by Feng et al. (2022), the positive effects of treatment with Fe3O4-NPs on K accumulation could be due to iron-dependent activation of NADPH oxidases since the activity of these enzymes is essential for controlling intracellular K+ homeostasis via ROS-gated ion channels [7]. The present study showed higher accumulation of both Fe and Cu in L. sativum L. shoots exposed to Fe2O3-NPs and CuO-NPs, respectively. This finding stays in agreement with the investigation of Hu et al. (2017) on Citrus maxima using foliar-applied γFe2O3-NPs, where an increase in Fe was observed as dose dependent [71]. Similarly, the study of Xiong et al. (2017) on lettuce and cabbage showed the increase in Cu accumulation after CuO-NPs treatments [72]. However, it was also shown that increasing amounts of Cu can be phytotoxic with a reduction in plant biomass, as in the present study.
Considering the above, the most favorable L. sativum L. growth and development occurred after introducing Fe2O3-NPs into the soil at lower and medium concentrations. However, determining the fertilizing potential of NPs on the quality and quantity of the obtained crop is not possible at this stage of the research due to limited data.
Despite the potential of Fe2O3-NPs and CuO-NPs as nano-fertilizers, several practical limitations must be addressed before field-scale deployment. Key constraints include high production costs, unresolved regulatory frameworks for environmental risk assessment, and dose-dependent phytotoxicity—particularly for CuO-NPs, which consistently reduce plant biomass above 100 ppm. Environmental persistence raises concerns: CuO-NPs pose ecotoxicity risks to soil microbiota and aquatic systems via runoff, while Fe2O3-NPs may chronically immobilize P (as observed in the fractionation studies), limiting bioavailability for subsequent crops. Efficiency is also highly dependent on soil type—sandy textures increasing leaching risks and acidic soils enhancing metal ion dissolution.
Future research should prioritize controlling ion release kinetics after NPs soil incorporation and improve nutrient bioavailability. It is important to provide long-term field studies tracking soil microbiome resilience, enzyme activity recovery, and NPs accumulation for establishment environmental safety thresholds. The solution for long nutrient release with decreasing risk of nutrient leaching may be the use of biochar, chitosan or other substances, that help to precise application NPs with optimalization technological efficiency.
5. Conclusions
This study comprehensively evaluated the impact of Fe2O3-NPs and CuO-NPs on the physicochemical properties of sandy loam soil, nutrient fractionation, enzymatic activity, and Lepidium sativum L. growth. The soil, characterized as slightly acidic sandy loam, presented favorable conditions for plant development, although there were limitations in N availability and potential nutrient leaching due to its texture. The application of Fe2O3-NPs and CuO-NPs significantly influenced the mobility and bioavailability of macro- and micronutrients, particularly N and P. Notably, Fe2O3-NPs enhanced N availability and promoted plant biomass accumulation at lower and moderate concentrations, suggesting their potential as a nanofertilizer. Conversely, CuO-NPs showed phytotoxic effects at higher doses, primarily due to oxidative stress and enzyme inhibition.
The comparative analysis of treatment variants in this study demonstrated that Fe2O3-NPs applied individually—particularly at low and moderate concentrations (10 and 100 ppm)—resulted in the most favorable outcomes in terms of nutrient bioavailability, soil enzyme activity, and L. sativum L. growth. These treatments increased the concentration of N in bioavailable soil fractions (F1, F2, F4), enhanced enzymatic activities at tolerable levels, and significantly improved plant biomass and N accumulation in aboveground tissues. In contrast, CuO-NPs, when applied alone at higher concentrations (300 ppm), were associated with reduced N and P mobility, suppressed enzymatic activity (especially DHA and UA), and caused phytotoxic effects reflected in decreased plant biomass. The combined application of Fe2O3-NPs and CuO-NPs yielded mixed results. While certain combinations (e.g., 10 ppm Fe2O3-NPs + 100 ppm CuO-NPs) slightly improved nutrient mobility factors and nutrient accumulation in plant tissues, they also posed higher risks of enzyme inhibition and Cu toxicity. These synergistic treatments did not consistently outperform the single Fe2O3-NPs applications and, in some cases, induced antagonistic interactions that limited nutrient bioavailability and enzyme activity. Overall, the results support the conclusion that individual application of Fe2O3-NPs at 10–100 ppm is more effective and agronomically favorable than either CuO-NPs alone or mixed nanoparticle treatments, highlighting the importance of selective nanoparticle use and concentration control in soil–plant systems. Changes in nutrient fractionation, especially the shift toward less mobile P forms and increased exchangeable N fractions, were accompanied by measurable shifts in enzymatic activity—namely, the inhibition of DHA and ACP at high NP concentrations. It should be emphasized that long-term exposure to metal oxide NPs, such as Fe2O3-NPs and CuO-NPs, poses sustained risks to soil microbial functions. While Fe2O3-NPs exhibit relatively lower toxicity due to their low solubility and role as micronutrients, chronic accumulation can alter microbial diversity. Conversely, CuO-NPs demonstrate higher toxicity via continuous ion release (Cu2+), disrupting cell membranes and inducing oxidative stress. Furthermore, CuO-NPs can accumulate in soil, causing legacy effects that inhibit microbial recovery for long periods after exposure.
Despite some benefits of NPs application, the study underscores the importance of concentration control and soil condition monitoring to avoid unintended ecological consequences. Among all tested treatments, the individual application of Fe2O3-NPs at 100 ppm emerged as the most effective variant for improving soil fertility and enhancing plant performance. Based on the presented results, Fe2O3-NPs at 100 ppm can be recommended as a promising nanofertilizer for future applications in sustainable crop production, particularly in sandy loam soils with a low N concentration. However, further long-term studies are essential to determine safe application thresholds, environmental implications, and agronomic effectiveness across diverse soil types and crops.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The author declares no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| NPs | Nanoparticles |
| BET | Brunauer–Emmett–Teller method |
| TEM | Transmission Electron Microscopy |
| F-AAS | Flame-Atomic Absorption Spectroscopy |
| F1 | Water soluble soil fraction |
| F2 | Acid soluble soil fraction |
| F3 | Organic matter bound soil fraction |
| F4 | Strongly complexed soil fraction |
| F5 | Residual soil fraction |
| DHA | Dehydrogenase activity |
| UA | Urease activity |
| ACP | Acid phosphatase activity |
References
- Zulfiqar, F.; Navarro, M.; Ashraf, M.; Akram, N.A.; Munne-Bosch, S. Nanofertilizer use for sustainable agriculture: Advantages and limitations. Plant Sci. 2019, 289, 110270. [Google Scholar] [CrossRef]
- Iavicoli, I.; Leso, V.; Beezhold, D.H.; Shvedova, A.A. Nanotechnology in agriculture: Opportunities, toxicological implications, and occupational risk. Toxicol. Appl. Pharmacol. 2017, 329, 96–111. [Google Scholar] [CrossRef]
- Oberdorster, G.; Stone, V.; Donaldson, K. Toxicology of nanoparticles: A historical perspective. Nanotoxicology 2007, 1, 2–25. [Google Scholar] [CrossRef]
- Bader, B.R.; Taban, S.K.; Fahmi, A.H.; Abood, M.A.; Hamdi, G.J. Potassium availability in soil amended with organic matter and phosphorous fertiliser under water stress during maize (Zea mays L.) growth. J. Saudi Soc. Agric. Sci. 2021, 20, 390–394. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Almutairi, K.F.; Alotaibi, M.; Shami, A.; Alhammad, B.A.; Battaglia, M.L. Nano-Fertilization as an Emerging Fertilization Technique: Why Can Modern Agriculture Benefit from Its Use? Plants 2021, 10, 2. [Google Scholar] [CrossRef] [PubMed]
- Rajput, V.; Minkina, T.; Mazarji, M.; Shende, S.; Sushkova, S.; Mandzhieva, S.; Burachevskaya, M.; Chaplygin, V.; Singh, A.; Jatav, H. Accumulation of nanoparticles in the soil-plant systems and their effects on human health. Ann. Agric. Sci. 2020, 65, 137–143. [Google Scholar] [CrossRef]
- Feng, Y.; Kreslavski, V.D.; Shmarev, A.N.; Ivanov, A.A.; Zharmukhamedov, S.K.; Kosobryukhov, A.; Yu, M.; Allakhverdiev, S.I.; Shabala, S. Effects of Iron Oxide Nanoparticles (Fe3O4) on Growth, Photosynthesis, Antioxidant Activity and Distribution of Mineral Elements in Wheat (Triticum aestivum) Plants. Plants 2022, 11, 1894. [Google Scholar] [CrossRef]
- Yang, X.; Alidoust, D.; Wang, C. Effects of iron oxide nanoparticles on the mineral composition and growth of soybean (Glycine max L.) plants. Acta Physiol. Plant 2020, 42, 128. [Google Scholar] [CrossRef]
- Ullah, J.; Gul, A.; Khan, I.; Shehzad, J.; Kausar, R.; Ahmed, M.S.; Batool, S.; Hasan, M.; Ghorbanpour, M.; Mustafa, G. Green synthesized iron oxide nanoparticles as a potential regulator of callus growth, plant physiology, antioxidative and microbial contamination in Oryza sativa L. BMC Plant Biol. 2024, 24, 939. [Google Scholar] [CrossRef]
- Faraz, A.; Faizan, M.; Rajput, V.D.; Minkina, T.; Hayat, S.; Faisal, M.; Alatar, A.A.; Abdel-Salam, E.M. CuO Nanoparticle-Mediated Seed Priming Improves Physio-Biochemical and Enzymatic Activities of Brassica juncea. Plants 2023, 12, 803. [Google Scholar] [CrossRef]
- Kadri, O.; Karmous, I.; Kharbech, O.; Arfaoui, H.; Chaoui, A. Cu and CuO Nanoparticles Affected the Germination and the Growth of Barley (Hordeum vulgare L.) Seedling. Bull. Environ. Contam. Toxicol. 2022, 108, 585–593. [Google Scholar] [CrossRef]
- ISO 11277:2009; Soil Quality—Determination of Particle Size Distribution in Mineral Soil Material—Method by Sieving and Sedimentation. ISO: Geneva, Switzerland, 2009.
- ISO 10390:2021; Soil, Sludge and Treated Biowaste—Determination of pH. ISO: Geneva, Switzerland, 2021.
- ISO 14235:1998; Soil Quality—Determination of Organic Carbon by Sulfochromic Oxidation. ISO: Geneva, Switzerland, 1998.
- ISO 14256-1:2003; Soil Quality—Determination of Nitrate, Nitrite and Ammonium in Field-Moist Soils by Extraction with Potassium Chloride Solution—Part 1: Manual Method. ISO: Geneva, Switzerland, 2003.
- ISO 11263:1994; Soil Quality—Determination of Phosphorus—Spectrometric Determination of Phosphorus Soluble in Sodium Hydrogen Carbonate Solution. ISO: Geneva, Switzerland, 1994.
- ISO 11466:1995/EN 13657; Soil Quality—Extraction of Trace Elements Soluble in Aqua Regia. ISO: Geneva, Switzerland, 1995.
- Tessier, P.; Campbell, P.G.C.; Bisson, M. Sequential extraction procedure for the speciation of particulate trace metals. Anal. Chem. 1979, 51, 844–851. [Google Scholar] [CrossRef]
- Nannoni, F.; Protano, G.; Riccobono, F. Fractionation and geochemical mobility of heavy elements in soils of a mining area in northern Kosovo. Geoderma 2011, 161, 63–73. [Google Scholar] [CrossRef]
- Casida, L.E.; Klein, D.A.; Santoro, T. Soil dehydrogenase activity. Soil Sci. 1964, 98, 371–376. [Google Scholar] [CrossRef]
- Kandeler, E.; Gerber, H. Short-term assay of soil urease activity using colorimetric determination of ammonium. Biol. Fert. Soils 1988, 6, 68–72. [Google Scholar] [CrossRef]
- Tabatabai, M.A.; Bremner, J.M. Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol. Biochem. 1969, 1, 301–307. [Google Scholar] [CrossRef]
- Chang, Y.-H.; Xue, G.-Q.; Song, H.; Di, D.L. Determination of the amount of 13 metal elements in the rape pollen from different regions by flame atomic absorption spectrophotometry. Guang Pu Xue Yu Guang Pu Fen Xi Guang Pu 2006, 27, 1235–1238. [Google Scholar]
- AOAC International. Official Methods of Analysis of AOAC International, 18th ed.; Rev. 2; AOAC International: Gaithersburg, MD, USA, 2006; Official Method 978.04–Nitrogen (Total) in Plants: Kjeldahl Method. [Google Scholar]
- Wieczorek, D.; Żyszka-Haberecht, B.; Kafka, A.; Lipok, J. Determination of phosphorus compounds in plant tissues: From colorimetry to advanced instrumental analytical chemistry. Plant Methods 2022, 18, 22. [Google Scholar] [CrossRef]
- Soil Survey Staff. Soil Taxonomy: A Basic System of Soil Classification for Making and Interpreting Soil Surveys; USDA, Natural Resources Conservation Service: Washington, DC, USA, 1999.
- Brady, N.C.; Weil, R.R. The Nature and Properties of Soils, 15th ed.; Pearson: Upper Saddle River, NJ, USA, 2016. [Google Scholar]
- Rawls, W.J.; Brakensiek, D.L.; Saxtonn, K.E. Estimation of soil water properties. Trans. ASAE 1982, 25, 1316–1320. [Google Scholar] [CrossRef]
- Thomas, G.W. Soil pH and soil acidity. In Methods of Soil Analysis Part 3: Chemical Methods; Soil Science Society of America; Sparks, D.L., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 1996; pp. 475–490. [Google Scholar]
- Stevenson, F.J.; Cole, M.A. Cycles of Soil: Carbon, Nitrogen, Phosphorus, Sulfur, Micronutrients; John Wiley & Sons: Hoboken, NJ, USA, 1999. [Google Scholar]
- Sharpley, A.N.; Chapra, S.C.; Wedepohl, R.; Sims, J.T.; Daniel, T.C.; Reddy, K.R. Managing Agricultural Phosphorus for Protection of Surface Waters: Issues and Options. J. Environ. Qual. 1994, 23, 437–451. [Google Scholar] [CrossRef]
- Johnston, A.E. Principles of crop nutrient management. In Balanced Fertilization; IFA: Paris, France, 2001. [Google Scholar]
- Mielki, G.F.; Novais, R.F.; Ker, J.C.; Vergütz, L.; de Castro, G.F. Iron Availability in Tropical Soils and Iron Uptake by Plants. Rev. Bras. Ciênc Solo 2016, 40, e0150174. [Google Scholar] [CrossRef]
- Kabata-Pendias, A.; Pendias, H. Biogeochemistry of Trace Elements; Polish Scientific Publishing Company: Warsaw, Poland, 1999. [Google Scholar]
- Kumar, V.; Pandita, S.; Singh Sidhu, G.P.; Sharma, A.; Khanna, K.; Kaur, P.; Bali, A.S.; Setia, R. Copper bioavailability, uptake, toxicity and tolerance in plants: A comprehensive review. Chemosphere 2021, 262, 127810. [Google Scholar] [CrossRef] [PubMed]
- Gransee, A.; Führs, H. Magnesium mobility in soils as a challenge for soil and plant analysis, magnesium fertilization and root uptake under adverse growth conditions. Plant Soil 2013, 368, 5–21. [Google Scholar] [CrossRef]
- Ahmed, N.; Zhang, B.; Bozdar, B.; Chachar, S.; Rai, M.; Li, J.; Li, Y.; Hayat, F.; Chachar, Z.; Tu, P. The power of magnesium: Unlocking the potential for increased yield, quality, and stress tolerance of horticultural crops. Front. Plant Sci. 2023, 14, 285512. [Google Scholar] [CrossRef] [PubMed]
- White, P.J. Calcium in Plants. Ann. Bot. 2003, 92, 487–511. [Google Scholar] [CrossRef]
- Mendel, R.R.; Schwarz, G. Molybdoenzymes and Molybdenum Cofactor in Plants. Crit. Rev. Plant Sci. 1999, 18, 33–69. [Google Scholar] [CrossRef]
- Rashed, M.; Hoque, T.; Jahangir, M.; Hashem, M. Manganese as a Micronutrient in Agriculture: Crop Requirement and Management. J. Environ. Sci. Nat. Resour. 2021, 12, 225–242. [Google Scholar] [CrossRef]
- Slimani, I.; Zhu-Barker, X.; Lazicki, P.; Horwath, W. Reviews and syntheses: Iron–a driver of nitrogen bioavailability in soils? Biogeosciences 2023, 20, 3873–3894. [Google Scholar] [CrossRef]
- Kamran, M.; Ali, H.; Saeed, M.F.; Bakhat, H.F.; Hassan, Z.; Tahir, M.; Abbas, G.; Naeem, M.A.; Rashid, M.I.; Shah, G.M. Unraveling the toxic effects of iron oxide nanoparticles on nitrogen cycling through manure-soil-plant continuum. Ecotoxicol. Environ. Saf. 2020, 205, 111099. [Google Scholar] [CrossRef]
- Jośko, I. Copper and zinc fractionation in soils treated with CuO and ZnO nanoparticles: The effect of soil type and moisture content. Sci. Total Environ. 2019, 653, 822–832. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Pan, B.; Zhang, X.; Zhang, H.; Steinberg, C.E.W.; Qiu, H.; Vijver, M.G.; Peijnenburg, W.J.G.M. Application of low dosage of copper oxide and zinc oxide nanoparticles boosts bacterial and fungal communities in soil. Sci. Total Environ. 2021, 757, 143807. [Google Scholar] [CrossRef]
- Wei, X.; Cao, P.; Wang, G.; Liu, Y.; Song, J.; Han, J. CuO, ZnO, and γ-Fe2O3 nanoparticles modified the underground biomass and rhizosphere microbial community of Salvia miltiorrhiza (Bge.) after 165-day exposure. Ecotoxicol. Environ. Saf. 2021, 217, 112232. [Google Scholar] [CrossRef] [PubMed]
- Maity, D.; Gupta, U.; Saha, S. Biosynthesized metal oxide nanoparticles for sustainable agriculture: Next-generation nanotechnology for crop production, protection and management. Nanoscale 2022, 14, 13950–13989. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.-M.; Jin, S.-Y.; Wang, Y.-J.; Wang, P.; Weng, N.-Y.; Wang, Y. Assessing the Impact of Iron-based Nanoparticles on pH, Dissolved Organic Carbon, and Nutrient Availability in Soils. Soil Sediment Contam. 2012, 21, 101–114. [Google Scholar] [CrossRef]
- Maurya, S.; Abraham, J.S.; Somasundaram, S.; Toteja, R.; Gupta, R.; Makhija, S. Indicators for assessment of soil quality: A mini-review. Environ. Monit. Assess. 2020, 192, 604. [Google Scholar] [CrossRef]
- He, S.; Feng, Y.; Ren, H.; Zhang, Y.; Gu, N.; Lin, X. The impact of iron oxide magnetic nanoparticles on the soil bacterial community. J. Soils Sediments 2011, 11, 1408–1417. [Google Scholar] [CrossRef]
- Kumar, A.; Rakshit, R.; Bhowmik, A.; Mandal, N.; Das, A.; Adhikary, S. Nanoparticle-Induced Changes in Resistance and Resilience of Sensitive Microbial Indicators towards Heat Stress in Soil. Sustainability 2019, 11, 862. [Google Scholar] [CrossRef]
- Burns, R.G. Enzyme activity in soil: Location and a possible role in microbial ecology. Soil Biol. Biochem. 1982, 14, 423–427. [Google Scholar] [CrossRef]
- Balemi, T.; Negisho, K. Management of soil phosphorus and plant adaptation mechanisms to phosphorus stress for sustainable crop production: A review. J. Soil Sci. Plant Nutr. 2012, 12, 547–562. [Google Scholar] [CrossRef]
- Richardson, A.E.; Hocking, P.J.; Simpson, R.J.; George, T.S. Plant mechanisms to optimise access to soil phosphorus. Crop Pasture Sci. 2009, 60, 124. [Google Scholar] [CrossRef]
- Koopmans, G.F.; Hiemstra, T.; Vaseur, C.; Chardon, W.J.; Voegelin, A.; Groenenberg, J.E. Use of iron oxide nanoparticles for immobilizing phosphorus in-situ: Increase in soil reactive surface area and effect on soluble phosphorus. Sci. Total Environ. 2020, 711, 135220. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Ali, B.; Adrees, M.; Arshad, M.; Hussain, A.; Zia ur Rehman, M.; Waris, A.A. Zinc and iron oxide nanoparticles improved the plant growth and reduced the oxidative stress and cadmium concentration in wheat. Chemosphere 2019, 214, 269–277. [Google Scholar] [CrossRef]
- Suazo-Hernández, J.; Sans-Serramitjana, E.; de la Luz Mora, M.; Fuentes, B.; de los Ángeles Sepúlveda, M.; Silva-Yumi, J.; Celletti, S.; Celi, L.; Rivas, S.; Ruiz, A. Assessment of the Effects of ZnO and CuO Engineered Nanoparticles on Physicochemical Properties of Volcanic Ash Soil and Phosphorus Availability. Environments 2024, 11, 208. [Google Scholar] [CrossRef]
- Jośko, I.; Dobrzyńska, J.; Dobrowolski, R.; Kusiak, M.; Terpiłowski, K. The effect of pH and ageing on the fate of CuO and ZnO nanoparticles in soils. Sci. Total Environ. 2020, 721, 137771. [Google Scholar] [CrossRef] [PubMed]
- Nannipieri, P.; Giagnoni, L.; Landi, L.; Renella, G. Phosphatase activity and P transformation in soil. In Soil Biology: Enzymes in The Environment; Shukla, G., Varma, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; Volume 22, pp. 215–243. [Google Scholar]
- Utobo, E.B.; Tewati, L. Soil enzymes as bioindicators of soil ecosystem status. Appl. Ecol. Environ. Res. 2014, 13, 147169. [Google Scholar]
- Adetunji, C.O.; Olaniyan, O.T.; Anani, O.A.; Olisaka Frances, N.; Inobeme, A.; Bodunrinde, R.E.; Adetunji, J.B.; Singh, K.R.; Palnam, W.D.; Singh, R.P. Current Scenario of Nanomaterials in the Environmental, Agricultural, and Biomedical Fields. In Nanomaterials in Bionanotechnology; CRC Press: Boca Raton, FL, USA, 2021; pp. 129–158. [Google Scholar]
- Azizah, N.; Nihayati, E.; Khotimah, H.; Rohmah, S.; Widaryanto, E.; Sugito, Y.; Kurniawan, S. Impact of potassium fertilization on yield, nutrient use and response efficiency, and antioxidant content of red ginger (Zingiber officinale var. rubrum Theilade). Chil. J. Agric. Res. 2022, 82, 380–389. [Google Scholar] [CrossRef]
- Bidast, S.; Golchin, A.; Baybordi, A.; Naidu, R. Effects of Fe oxide-based nanoparticles on yield and nutrient content of corn in Cobalt-contaminated soils. Environ. Technol. Inn. 2022, 26, 102314. [Google Scholar] [CrossRef]
- Yin, H.; Zhou, C.; Wang, J.; Yin, M.; Wu, Z.; Song, N.; Song, X.; Shangguan, Y.; Sun, Z.; Zong, Q.; et al. Fe-CGS Effectively Inhibits the Dynamic Migration and Transformation of Cadmium and Arsenic in Soil. Toxics 2024, 12, 273. [Google Scholar] [CrossRef]
- Wyszkowski, M.; Kordala, N. The Role of Organic Materials in Shaping the Content of Trace Elements in Iron-Contaminated Soil. Materials 2025, 18, 1522. [Google Scholar] [CrossRef]
- Guo, M.; Tong, H.; Cai, D.; Zhang, W.; Yuan, P.; Shen, Y.; Peng, C. Effect of wetting-drying cycles on the Cu bioavailability in the paddy soil amended with CuO nanoparticles. J. Hazard. Mater. 2022, 436, 129119. [Google Scholar] [CrossRef]
- Pelegrino, M.T.; Kohatsu, M.Y.; Seabra, A.B.; Monteiro, L.R.; Gomes, D.G.; Oliveira, H.C.; Rolim, W.R.; de Jesus, T.A.; Batista, B.L.; Lange, C.N. Effects of copper oxide nanoparticles on growth of lettuce (Lactuca sativa L.) seedlings and possible implications of nitric oxide in their antioxidative defense. Environ. Monit. Assess. 2020, 192, 232. [Google Scholar] [CrossRef] [PubMed]
- Santos, E.S.D.; Graciano, D.E.; Falco, W.F.; Caires, A.R.L.; de Arruda, E.J. Effects of copper oxide nanoparticles on germination of Sesbania virgata (FABACEAE) plants. An. Acad. Bras. Ciênc 2021, 93, e20190739. [Google Scholar] [CrossRef] [PubMed]
- Ghafoor, A.; Latif, M.; Ali, S.; Munir, M.; Sattar, M.N.; Alshehri, M.A. Exploring Metal Based Nanoparticles for Boosting Plant Tolerance to Heavy Metals and Trace Element Contamination. Phyton 2024, 93, 2683–2705. [Google Scholar] [CrossRef]
- Dimkpa, C.O.; Latta, D.E.; McLean, J.E.; Britt, D.W.; Boyanov, M.I.; Anderson, A.J. Fate of CuO and ZnO Nano- and Microparticles in the Plant Environment. Environ. Sci. Technol. 2013, 47, 4734–4742. [Google Scholar] [CrossRef] [PubMed]
- Lung, I.; Opriş, O.; Soran, M.-L.; Culicov, O.; Ciorîță, A.; Stegarescu, A.; Zinicovscaia, I.; Yushin, N.; Vergel, K.; Kacso, I.; et al. The Impact Assessment of CuO Nanoparticles on the Composition and Ultrastructure of Triticum aestivum L. Int. J. Environ. Res. Public Health 2021, 18, 6739. [Google Scholar] [CrossRef]
- Hu, J.; Guo, H.; Li, J.; Wang, Y.; Xiao, L.; Xing, B. Interaction of γ-Fe2O3 nanoparticles with Citrus maxima leaves and the corresponding physiological effects via foliar application. J. Nanobiotechnol. 2017, 15, 51. [Google Scholar] [CrossRef]
- Xiong, T.; Dumat, C.; Dappe, V.; Vezin, H.; Schreck, E.; Shahid, M.; Pierart, A.; Sobanska, S. Copper Oxide Nanoparticle Foliar Uptake, Phytotoxicity, and Consequences for Sustainable Urban Agriculture. Environ. Sci. Technol. 2017, 51, 5242–5251. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).