Exogenous Melatonin Application Delays Senescence and Improves Postharvest Antioxidant Capacity in Blueberries
Abstract
1. Introduction
2. Materials and Methods
2.1. Fruit Selecting and Treatment
2.2. Determination of Physiological and Biochemical Indexes
2.3. Data Analysis
2.4. Transcriptome Data Analysis
2.4.1. Total RNA Extraction
2.4.2. Transcriptome Data Quality Assessment
2.4.3. Screening of DEGs and GO, KEGG Pathway Enrichment Analysis
2.5. Quantitative Real-Time PCR (RT-qPCR) Analysis
3. Results
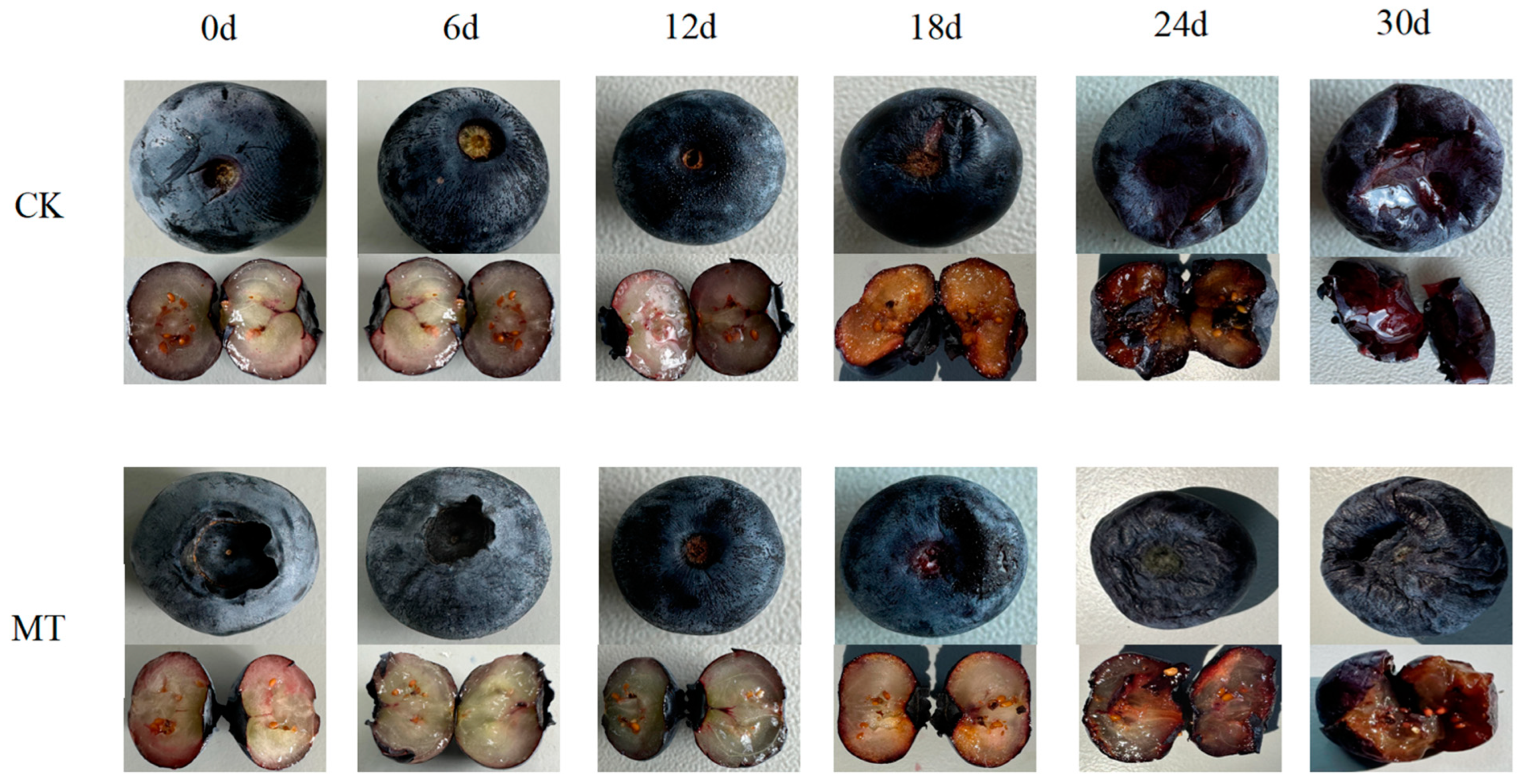
3.1. Effects of MT on the Appearance Changes of Blueberry
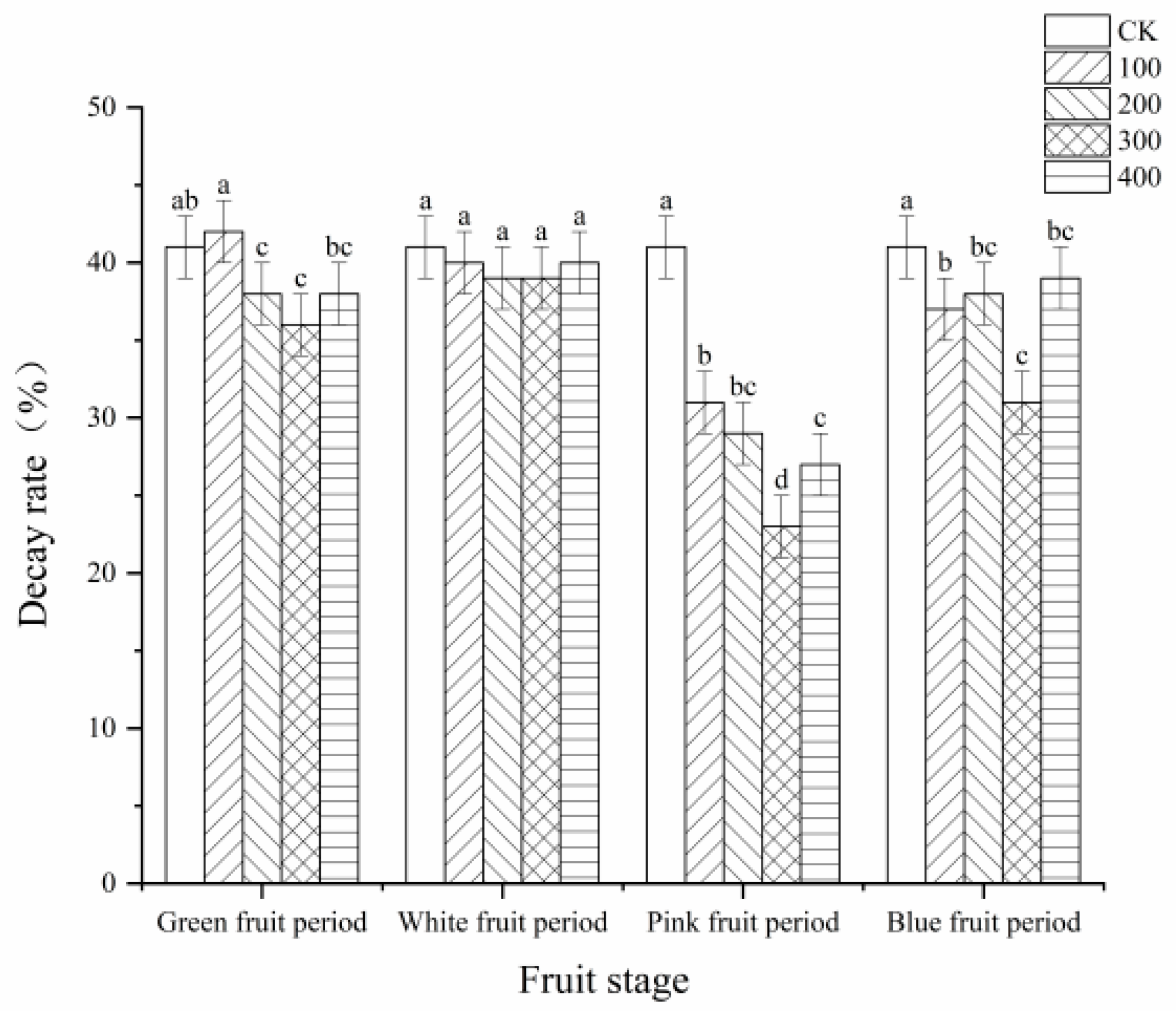
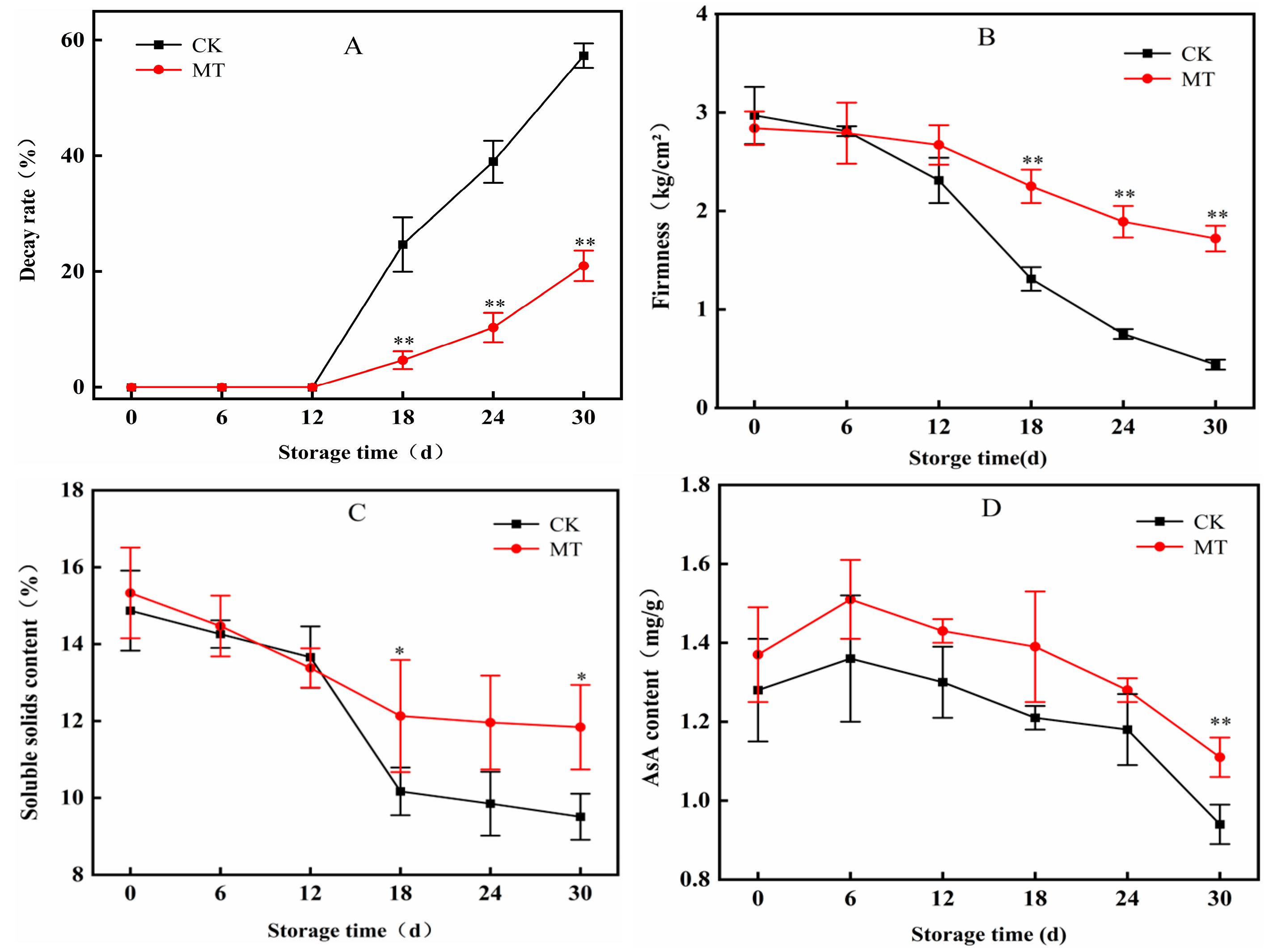
3.2. Effects of MT on the Decay Rate of Blueberry
3.3. Effects of MT on Firmness, Soluble Solids Content (TSS), and Ascorbic Acid (AsA) Levels in Blueberry
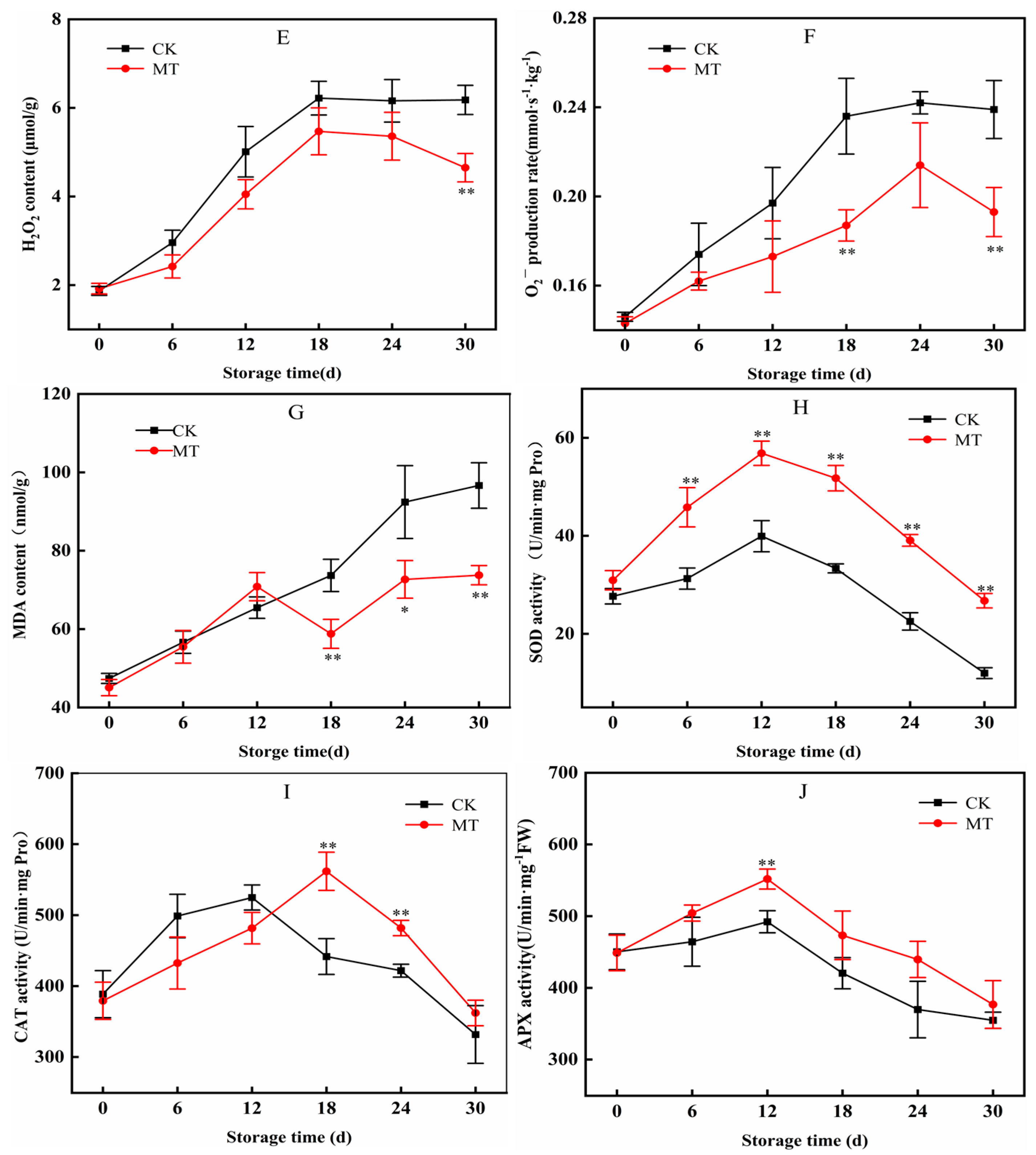
3.4. Effects of MT on the Levels of H2O2, O2−, and MDA in Blueberry
3.5. Effects of MT on SOD, CAT, and APX Activities in Blueberry
3.6. Transcriptome Results
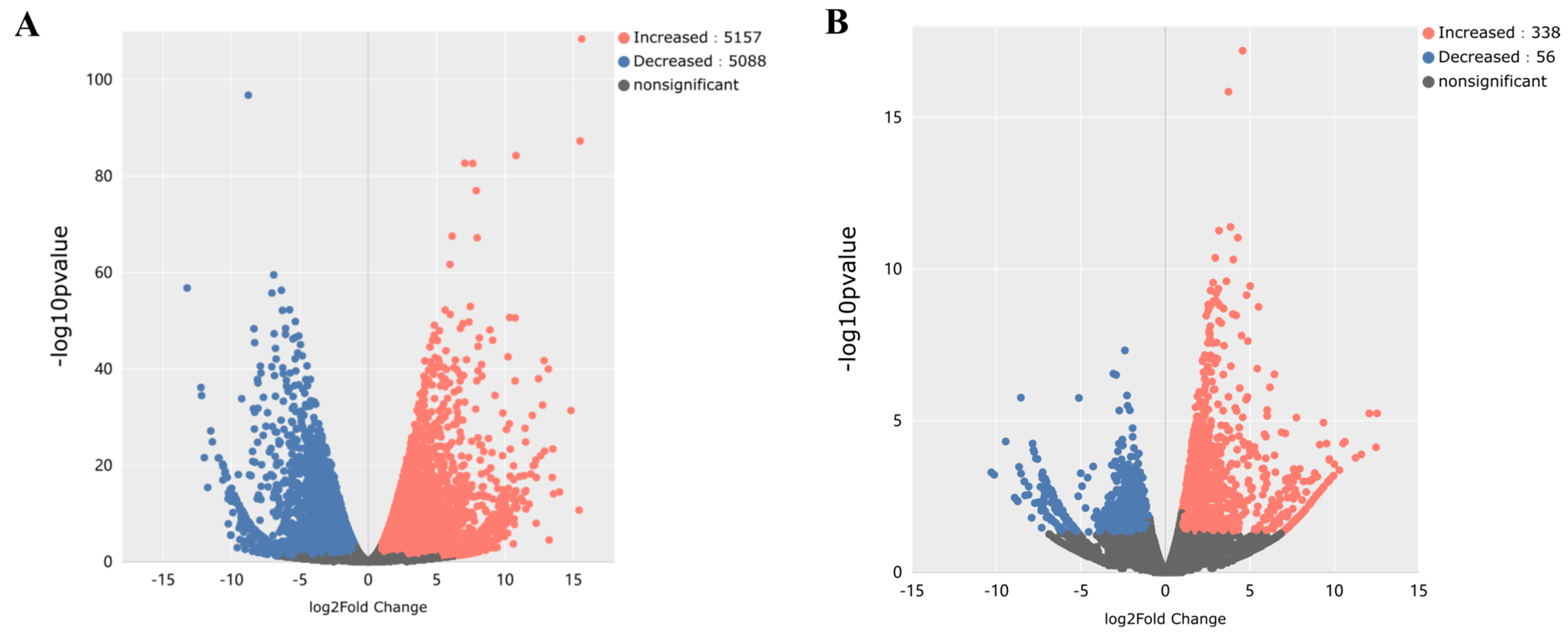
3.6.1. Screening Results of DEGs
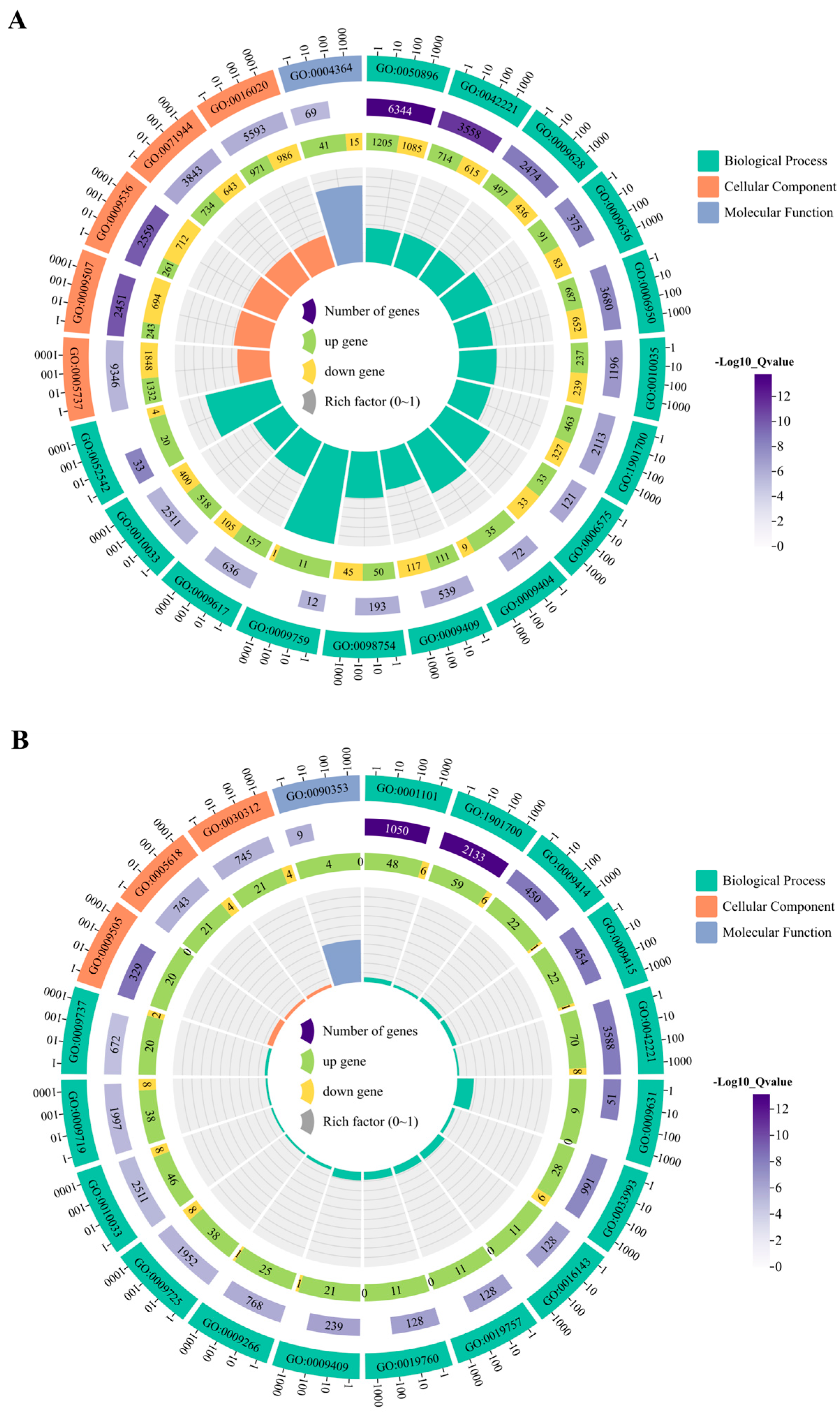
3.6.2. GO Pathway Enrichment Analysis
3.6.3. KEGG Pathway Enrichment Analysis
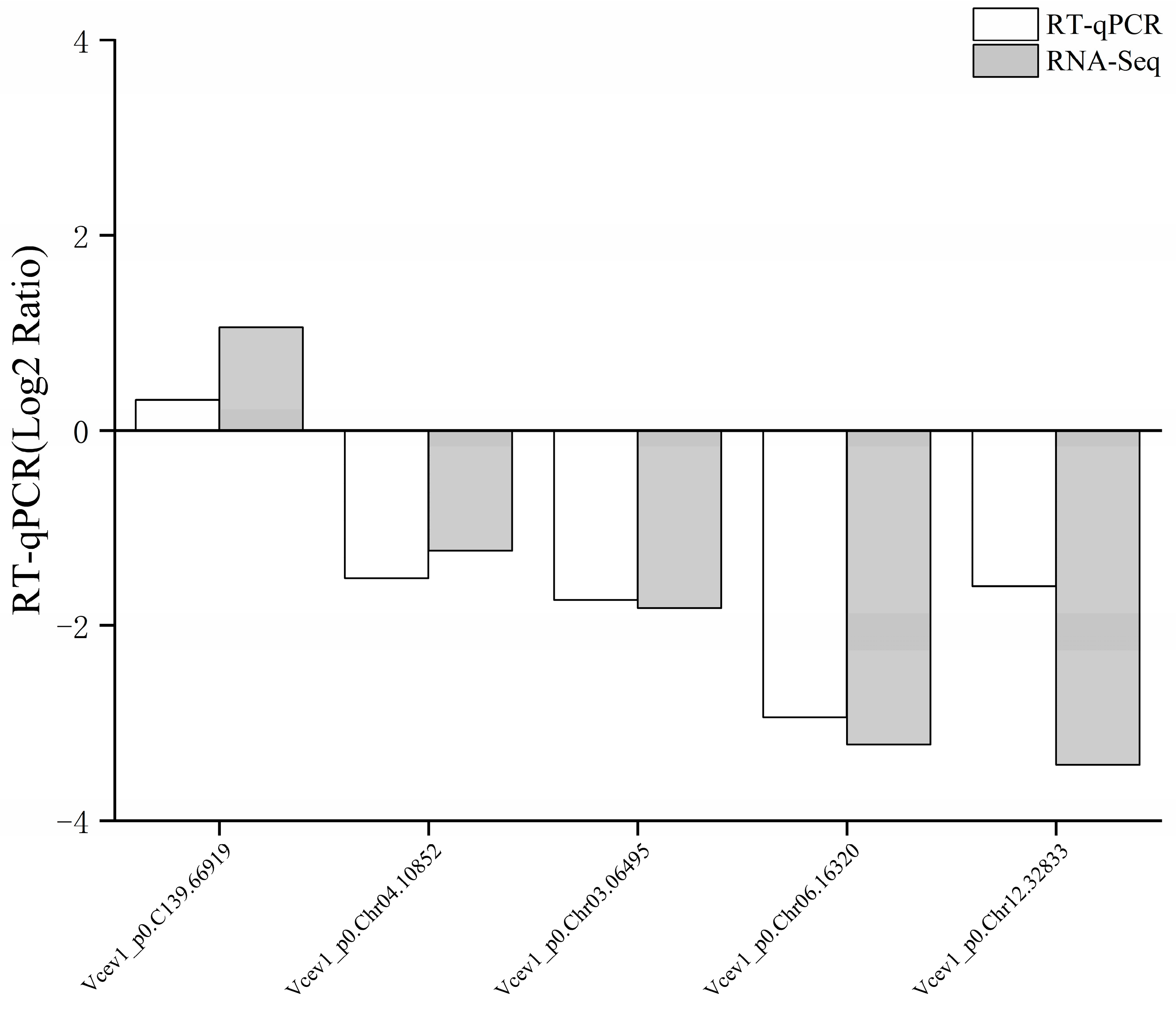
3.7. RT-qPCR Gene Expression Analysis
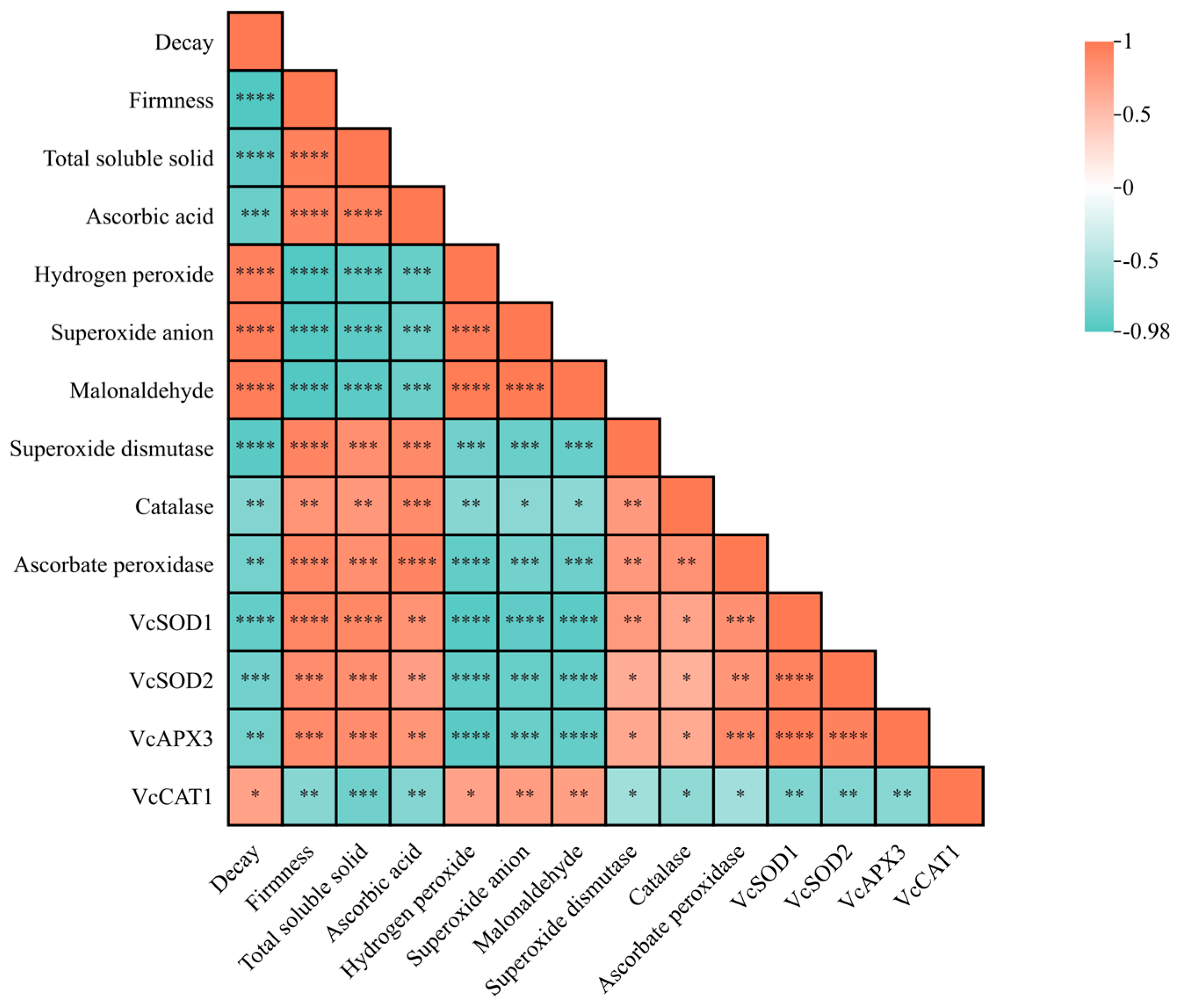
3.8. Correlation Analysis Between Physiological Parameters and Gene Expression in Blueberry Under MT Treatment
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| Sample | Raw Data Reads | Raw Data Bases | Raw Data q20% | Raw Data q30% | Clean Data Reads | Clean Data Bases | Clean Data q20% | Clean Data q30% | GC% |
|---|---|---|---|---|---|---|---|---|---|
| CK0 1 | 44797090 | 6.72G | 98.73% | 96.28% | 43679190 | 6.53G | 99.16% | 97.14% | 48.11% |
| CK0 2 | 48165052 | 7.22G | 98.81% | 96.50% | 47136994 | 7.05G | 99.19% | 97.26% | 47.87% |
| CK0 3 | 41617564 | 6.24G | 98.61% | 95.90% | 39841718 | 5.96G | 99.18% | 97.18% | 48.18% |
| CK30 1 | 48152262 | 7.22G | 97.63% | 93.22% | 47322728 | 7.06G | 98.07% | 94.00% | 47.80% |
| CK30 2 | 41163724 | 6.17G | 97.43 | 92.79% | 39950266 | 5.96G | 98.01% | 93.82% | 47.78% |
| CK30 3 | 38613096 | 5.79G | 97.43% | 92.8% | 36961558 | 5.51G | 98.10% | 94.08% | 47.84% |
| MT0 1 | 41304754 | 6.20G | 98.61% | 95.97% | 39893312 | 5.97G | 99.12% | 97.04% | 48.11% |
| MT0 2 | 42000580 | 6.30G | 98.62% | 96.00% | 40853662 | 6.11G | 99.10% | 96.95% | 49.00% |
| MT0 3 | 47947806 | 7.19G | 98.67% | 96.13% | 46536160 | 6.96G | 99.14% | 97.11% | 47.58% |
| MT30 1 | 46488732 | 6.97G | 97.48% | 92.92% | 45269410 | 6.74G | 98.05% | 93.96% | 48.06% |
| MT30 2 | 43662448 | 6.55G | 97.52% | 92.99% | 42685998 | 6.35G | 98.03% | 93.89% | 48.17% |
| MT30 3 | 45454054 | 6.82G | 97.52% | 93.02% | 42685998 | 6.67G | 98.00% | 93.83% | 47.94% |
| Gene | Primers | Sequence (5′ → 3′) |
|---|---|---|
| GAPDH | F | CGGCTACTTACGAGCAAATCAA |
| R | TTCAGTGTAGCCCAAAATTCCTTT | |
| Vcev1_p0.Chr03.06495 | F | CCGATGATACCACAGGCAAT |
| R | AGATCCCTCTTTCAGGACCACATTC | |
| Vcev1_p0.Chr12.32833 | F | ATTGCTAATGCTGATGGTAAGGTG |
| R | CCAGTCGTAAGGCTAAGTTCGTG | |
| Vcev1_p0.Chr06.16320 | F | TCTCAAACCCTAACAACAACCC |
| R | GTAACGACGCCTTCAACGGA | |
| Vcev1_p0.C139.66919 | F | GTGAAGTTTTACACCAGGGAGG |
| R | CTTGGGGTTAGGTTTAAGAGCA | |
| Vcev1_p0.Chr04.10852 | F | TCTCATCTGCGTGATGTTTTCTAC |
| R | AACCACTGCGTTCTGGTCTG |
References
- Duan, Y.; Tarafdar, A.; Chaurasia, D.; Singh, A.; Bhargava, P.C.; Yang, J.; Li, Z.; Ni, X.; Tian, Y.; Li, H.; et al. Blueberry fruit valorization and valuable constituents: A review. Int. J. Food Microbiol. 2022, 381, 109890. [Google Scholar] [CrossRef]
- Sater, H.; Ferrão, L.F.V.; Olmstead, J.; Munoz, P.R.; Bai, J.; Hopf, A.; Plotto, A. Exploring environmental and storage factors affecting sensory, physical and chemical attributes of six southern highbush blueberry cultivars. Sci. Hortic. 2021, 289, 110468. [Google Scholar] [CrossRef]
- Dong, M.; Tian, Y.; Dong, K.; Li, Y.; Sun, H. Isolation and Identification of Root-Associated Symbiotic Fungi of Lowbush Blueberry ’Meidong’. J. Jilin Agric. Univ. 2019, 41, 419–425. [Google Scholar] [CrossRef]
- Han, P.; Zhang, B.; Feng, X.; Huang, X.; Li, M.; Yang, F. Nutritional and Health Benefits of Blueberry and Its Development and Utilization. Food Ind. Technol. 2015, 6, 370–375+379. [Google Scholar] [CrossRef]
- Wang, C.; Gao, Y.; Tao, Y.; Wu, X.; Zhibo, C. Influence of γ-irradiation on the reactive-oxygen metabolism of blueberry fruit during cold storage. Innov. Food Sci. Emerg. Technol. 2017, 41, 397–403. [Google Scholar] [CrossRef]
- Mannozzi, C.; Tylewicz, U.; Chinnici, F.; Siroli, L.; Rocculi, P.; Dalla Rosa, M.; Romani, S. Effects of chitosan based coatings enriched with procyanidin by-product on quality of fresh blueberries during storage. Food Chem. 2018, 251, 18–24. [Google Scholar] [CrossRef]
- Kurniawan, O.; Wilson, K.; Mohamed, R.; Avis, T.J. Bacillus and Pseudomonas spp. provide antifungal activity against gray mold and Alternaria rot on blueberry fruit. Biol. Control 2018, 126, 136–141. [Google Scholar] [CrossRef]
- Chiabrando, V.; Giacalone, G. Shelf-life extension of highbush blueberry using 1-methylcyclopropene stored under air and controlled atmosphere. Food Chem. 2011, 126, 1812–1816. [Google Scholar] [CrossRef]
- Rivera, S.; Kerckhoffs, H.; Sofkova-Bobcheva, S.; Hutchins, D.; East, A. Influence of harvest maturity and storage technology on mechanical properties of blueberries. Postharvest Biol. Technol. 2022, 191, 111961. [Google Scholar] [CrossRef]
- Chea, S.; Yu, D.J.; Park, J.; Oh, H.D.; Chung, S.W.; Lee, H.J. Preharvest β-aminobutyric acid treatment alleviates postharvest deterioration of ‘Bluecrop’ highbush blueberry fruit during refrigerated storage. Sci. Hortic. 2019, 246, 95–103. [Google Scholar] [CrossRef]
- Debnath, B.; Islam, W.; Li, M.; Sun, Y.; Lu, X.; Mitra, S.; Hussain, M.; Liu, S.; Qiu, D. Melatonin Mediates Enhancement of Stress Tolerance in Plants. Int. J. Mol. Sci. 2019, 20, 1040. [Google Scholar] [CrossRef] [PubMed]
- Mauriz, J.L.; Collado, P.S.; Veneroso, C.; Reiter, R.J.; González-Gallego, J. A review of the molecular aspects of melatonin’s anti-inflammatory actions: Recent insights and new perspectives. J. Pineal Res. 2013, 54, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishna, A.; Giridhar, P.; Sankar, K.U.; Ravishankar, G.A. Melatonin and serotonin profiles in beans of Coffea species. J. Pineal Res. 2012, 52, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Sae-Teaw, M.; Johns, J.; Johns, N.P.; Subongkot, S. Serum melatonin levels and antioxidant capacities after consumption of pineapple, orange, or banana by healthy male volunteers. J. Pineal Res. 2013, 55, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Hernández-Ruiz, J. The Potential of Phytomelatonin as a Nutraceutical. Molecules 2018, 23, 238. [Google Scholar] [CrossRef]
- Rastegar, S.; Hassanzadeh Khankahdani, H.; Rahimzadeh, M. Effects of melatonin treatment on the biochemical changes and antioxidant enzyme activity of mango fruit during storage. Sci. Hortic. 2020, 259, 108835. [Google Scholar] [CrossRef]
- Shekari, A.; Hassani, R.N.; Aghdam, M.S.; Rezaee, M.; Jannatizadeh, A. The effects of melatonin treatment on cap browning and biochemical attributes of Agaricus bisporus during low temperature storage. Food Chem. 2021, 348, 129074. [Google Scholar] [CrossRef]
- Nawaz, M.A.; Huang, Y.; Bie, Z.; Ahmed, W.; Reiter, R.J.; Niu, M.; Hameed, S. Melatonin: Current Status and Future Perspectives in Plant Science. Front. Plant Sci. 2016, 6, 1230. [Google Scholar] [CrossRef]
- Guillén, F.; Medina-Santamarina, J.; García-Pastor, M.E.; Chen, N.J.; Uruu, G.; Paull, R.E. Postharvest melatonin treatment delays senescence and increases chilling tolerance in pineapple. LWT 2022, 169, 113989. [Google Scholar] [CrossRef]
- Fekry, W.M.E.; Rashad, Y.M.; Alaraidh, I.A.; Mehany, T. Exogenous Application of Melatonin and Methyl Jasmonate as a Pre-Harvest Treatment Enhances Growth of Barhi Date Palm Trees, Prolongs Storability, and Maintains Quality of Their Fruits under Storage Conditions. Plants 2022, 11, 96. [Google Scholar] [CrossRef]
- Waszczak, C.; Carmody, M.; Kangasjärvi, J. Reactive Oxygen Species in Plant Signaling. Annu. Rev. Plant Biol. 2018, 69, 209–236. [Google Scholar] [CrossRef] [PubMed]
- Rabiei, V.; Kakavand, F.; Zaare-Nahandi, F.; Razavi, F.; Aghdam, M.S. Nitric oxide and γ-aminobutyric acid treatments delay senescence of cornelian cherry fruits during postharvest cold storage by enhancing antioxidant system activity. Sci. Hortic. 2019, 243, 268–273. [Google Scholar] [CrossRef]
- Akram, N.A.; Shafiq, F.; Ashraf, M. Ascorbic Acid-A Potential Oxidant Scavenger and Its Role in Plant Development and Abiotic Stress Tolerance. Front. Plant Sci. 2017, 8, 613. [Google Scholar] [CrossRef] [PubMed]
- Charoenphun, N.; Pham, N.H.; Rattanawut, J.; Venkatachalam, K. Exogenous Application of Melatonin on the Preservation of Physicochemical and Enzymatic Qualities of Pepper Fruit from Chilling Injury. Horticulturae 2024, 10, 550. [Google Scholar] [CrossRef]
- Bal, E. Physicochemical changes in ‘Santa Rosa’ plum fruit treated with melatonin during cold storage. J. Food Meas. Charact. 2019, 13, 1713–1720. [Google Scholar] [CrossRef]
- Menaka, M.; Asrey, R.; Meena, N.K.; Vargheese, E.; Sethi, S.; Ahamad, S.; Goswami, A.K. Effect of melatonin on biochemical changes, antioxidant system and oxidative membrane damage of Indian guava (cv. Barafkhana) during cold storage. S. Afr. J. Bot. 2024, 169, 95–108. [Google Scholar] [CrossRef]
- Mohd Yusof, F.A.; Azman, E.M.; Mohd Adzahan, N.; Yusof, N.L. Effect of vacuum impregnation with melatonin, γ-aminobutyric acid, and oxalic acid on chilling injury and quality of carambola. Int. J. Food Sci. Technol. 2023, 58, 6432–6444. [Google Scholar] [CrossRef]
- Promyou, S.; Raruang, Y.; Chen, Z.-Y. Melatonin Treatment of Strawberry Fruit during Storage Extends Its Post-Harvest Quality and Reduces Infection Caused by Botrytis cinerea. Foods 2023, 12, 1445. [Google Scholar] [CrossRef] [PubMed]
- Bal, E.; Torçuk, A.İ.; Özer, C. Influence of Melatonin Treatments on Fruit Quality and Storage Life of Sweet Cherry cv. ‘Sweetheart’. Erwerbs-Obstbau 2022, 64, 127–133. [Google Scholar] [CrossRef]
- Kakaei, S.; Koushesh Saba, M.; Mansouri, S.; Darvishi, H. Melatonin postharvest spray influence on white mulberry browning, storage life, and biochemical changes. Postharvest Biol. Technol. 2024, 213, 112947. [Google Scholar] [CrossRef]
- Zhai, R.; Liu, J.; Liu, F.; Zhao, Y.; Liu, L.; Fang, C.; Wang, H.; Li, X.; Wang, Z.; Ma, F.; et al. Melatonin limited ethylene production, softening and reduced physiology disorder in pear (Pyrus communis L.) fruit during senescence. Postharvest Biol. Technol. 2018, 139, 38–46. [Google Scholar] [CrossRef]
- Xu, R.; Wang, L.; Li, K.; Cao, J.; Zhao, Z. Integrative transcriptomic and metabolomic alterations unravel the effect of melatonin on mitigating postharvest chilling injury upon plum (cv.Friar) fruit. Postharvest Biol. Technol. 2022, 186, 111819. [Google Scholar] [CrossRef]
- Belay, Z.A.; James Caleb, O. Role of integrated omics in unravelling fruit stress and defence responses during postharvest: A review. Food Chem. Mol. Sci. 2022, 5, 100118. [Google Scholar] [CrossRef]
- Li, J.; Lu, C.; Wu, L.; Li, J.; Li, Y.; Wang, Y. Effects of Exogenous Melatonin Treatment on Postharvest Quality and Storage Tolerance of Blueberry. J. Jilin Agric. Univ. 2025, 1–14. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, L.; Wang, Q.; Zuo, J. Low temperature conditioning combined with methyl jasmonate can reduce chilling injury in bell pepper. Sci. Hortic. 2019, 243, 434–439. [Google Scholar] [CrossRef]
- Yang, W.; Liu, Y.; Sang, Y.; Ma, Y.; Guo, M.; Bai, G.; Cheng, S.; Chen, G. Influences of ice-temperature storage on cell wall metabolism and reactive oxygen metabolism in Xinjiang (Diaogan) apricot. Postharvest Biol. Technol. 2021, 180, 111614. [Google Scholar] [CrossRef]
- Liu, C.; Zheng, H.; Sheng, K.; Liu, W.; Zheng, L. Effects of melatonin treatment on the postharvest quality of strawberry fruit. Postharvest Biol. Technol. 2018, 139, 47–55. [Google Scholar] [CrossRef]
- Zhao, H.; Lv, W.; Fan, Y.; Li, H. Gibberellic acid enhances postharvest toon sprout tolerance to chilling stress by increasing the antioxidant capacity during the short-term cold storage. Sci. Hortic. 2018, 237, 184–191. [Google Scholar] [CrossRef]
- Chen, H.; Gao, H.; Fang, X.; Ye, L.; Zhou, Y.; Yang, H. Effects of allyl isothiocyanate treatment on postharvest quality and the activities of antioxidant enzymes of mulberry fruit. Postharvest Biol. Technol. 2015, 108, 61–67. [Google Scholar] [CrossRef]
- Hu, Q.D.L. Biochemical Experiments; Chemical Industry Publishing House: Beijing, China, 2007. [Google Scholar]
- Cao, J.K.; Jiang, W.B.; Zhao, Y.M. Postharvest Physiological and Biochemical Experiments of Fruits and Vegetables; China Light Industry Press: Beijing, China, 2007. [Google Scholar]
- Zhang, M.; Zhang, Q.; Tian, C.; Liu, G.; Pan, Y.; Xu, X.; Shi, X.; Zhang, Z.; Meng, L. Physiological and Transcriptome Analyses of CaCl2 Treatment to Alleviate Chilling Injury in Pineapple. Plants 2022, 11, 2215. [Google Scholar] [CrossRef]
- Cortés-Montaña, D.; Bernalte-García, M.J.; Velardo-Micharet, B.; Serrano, M.; Serradilla, M.J. Impact of Pre-Storage Melatonin Application on the Standard, Sensory, and Bioactive Quality of Early Sweet Cherry. Foods 2023, 12, 1723. [Google Scholar] [CrossRef]
- Xie, J.; Qin, Z.; Pan, J.; Li, J.; Li, X.; Khoo, H.E.; Dong, X. Melatonin treatment improves postharvest quality and regulates reactive oxygen species metabolism in “Feizixiao” litchi based on principal component analysis. Front. Plant Sci. 2022, 13, 965345. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, P.; Jaleel, C.A.; Salem, M.A.; Nabi, G.; Sharma, S. Roles of enzymatic and nonenzymatic antioxidants in plants during abiotic stress. Crit. Rev. Biotechnol. 2010, 30, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Marak, K.A.; Mir, H.; Singh, P.; Siddiqui, M.W.; Ranjan, T.; Singh, D.R.; Siddiqui, M.H.; Irfan, M. Exogenous melatonin delays oxidative browning and improves postharvest quality of litchi fruits. Sci. Hortic. 2023, 322, 112408. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, M.; Zhang, W.; Gao, Y.; Ma, X.; Cheng, S.; Chen, G. Exogenous melatonin activates the antioxidant system and maintains postharvest organoleptic quality in Hami melon (Cucumis. melo var. inodorus Jacq.). Front. Plant Sci. 2023, 14, 1274939. [Google Scholar] [CrossRef] [PubMed]
- Sang, Y.; Liu, Y.; Tang, Y.; Yang, W.; Guo, M.; Chen, G. Transcriptome sequencing reveals mechanism of improved antioxidant capacity and maintained postharvest quality of winter jujube during cold storage after salicylic acid treatment. Postharvest Biol. Technol. 2022, 189, 111929. [Google Scholar] [CrossRef]
- Larrigaudière, C.; Vilaplana, R.; Soria, Y.; Recasens, I. Oxidative behaviour of Blanquilla pears treated with 1-methylcyclopropene during cold storage. J. Sci. Food Agric. 2004, 84, 1871–1877. [Google Scholar] [CrossRef]
- Gao, H.; Zhang, Z.K.; Chai, H.K.; Cheng, N.; Yang, Y.; Wang, D.N.; Yang, T.; Cao, W. Melatonin treatment delays postharvest senescence and regulates reactive oxygen species metabolism in peach fruit. Postharvest Biol. Technol. 2016, 118, 103–110. [Google Scholar] [CrossRef]
- Cuypers, A.; Karen, S.; Jos, R.; Kelly, O.; Els, K.; Tony, R.; Nele, H.; Nathalie, V.; Suzy, V.S.; Frank, V.B.; et al. The cellular redox state as a modulator in cadmium and copper responses in Arabidopsis thaliana seedlings. J. Plant Physiol. 2011, 168, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Miranda, S.; Vilches, P.; Suazo, M.; Pavez, L.; García, K.; Méndez, M.A.; González, M.; Meisel, L.A.; Defilippi, B.G.; del Pozo, T. Melatonin triggers metabolic and gene expression changes leading to improved quality traits of two sweet cherry cultivars during cold storage. Food Chem. 2020, 319, 126360. [Google Scholar] [CrossRef]
- Dong, Y.; Zhi, H.H.; Xu, J.; Zhang, L.H.; Liu, M.P.; Zong, W. Effect of methyl jasmonate on reactive oxygen species, antioxidant systems, and microstructure of Chinese winter jujube at two major ripening stages during shelf life. J. Hortic. Sci. Biotechnol. 2016, 91, 316–323. [Google Scholar] [CrossRef]
- Zhang, L.; Kou, X.; Huang, X.; Li, G.; Liu, J.; Ye, J. Peach-gum: A promising alternative for retarding the ripening and senescence in postharvest peach fruit. Postharvest Biol. Technol. 2020, 161, 111088. [Google Scholar] [CrossRef]
- Peng, Z.; Fu, D. Effects of 1-methylcyclopropene treatment on the quality of red ‘Fuji’ apples fruit during short-term storage. Food Qual. Saf. 2023, 7, fyac074. [Google Scholar] [CrossRef]
- Gleason, C.; Huang, S.; Thatcher, L.F.; Foley, R.C.; Anderson, C.R.; Carroll, A.J.; Millar, A.H.; Singh, K.B. Mitochondrial complex II has a key role in mitochondrial-derived reactive oxygen species influence on plant stress gene regulation and defense. Proc. Natl. Acad. Sci. USA 2011, 108, 10768–10773. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Huang, T.; Kou, X.; Zhang, Y.; Ba, L.; Wang, X.; Cao, S. MeJA enhances antioxidant activity and reduces membrane lipid degradation by maintaining energy charge levels in crystal grapes. Postharvest Biol. Technol. 2024, 216, 113078. [Google Scholar] [CrossRef]
- Leshem, Y.; Seri, L.; Levine, A. Induction of phosphatidylinositol 3-kinase-mediated endocytosis by salt stress leads to intracellular production of reactive oxygen species and salt tolerance. Plant J. 2007, 51, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Oikawa, K.; Goto-Yamada, S.; Hayashi, Y.; Takahashi, D.; Kimori, Y.; Shibata, M.; Yoshimoto, K.; Takemiya, A.; Kondo, M.; Hikino, K.; et al. Pexophagy suppresses ROS-induced damage in leaf cells under high-intensity light. Nat. Commun. 2022, 13, 7493. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Li, L.; Liu, Y.; Zhang, W.; Guo, M.; Chen, G. MeJA and MeSA alleviate black rot in winter jujube caused by Alternaria tenuissima by regulating membrane lipid and reactive oxygen metabolism. Postharvest Biol. Technol. 2025, 219, 113275. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Wang, Y.; Li, J.; Li, Y.; Lu, C.; Hou, Z.; Liu, H.; Wu, L. Exogenous Melatonin Application Delays Senescence and Improves Postharvest Antioxidant Capacity in Blueberries. Agronomy 2025, 15, 428. https://doi.org/10.3390/agronomy15020428
Li J, Wang Y, Li J, Li Y, Lu C, Hou Z, Liu H, Wu L. Exogenous Melatonin Application Delays Senescence and Improves Postharvest Antioxidant Capacity in Blueberries. Agronomy. 2025; 15(2):428. https://doi.org/10.3390/agronomy15020428
Chicago/Turabian StyleLi, Jie, Ying Wang, Jinying Li, Yanan Li, Chunze Lu, Zihuan Hou, Haiguang Liu, and Lin Wu. 2025. "Exogenous Melatonin Application Delays Senescence and Improves Postharvest Antioxidant Capacity in Blueberries" Agronomy 15, no. 2: 428. https://doi.org/10.3390/agronomy15020428
APA StyleLi, J., Wang, Y., Li, J., Li, Y., Lu, C., Hou, Z., Liu, H., & Wu, L. (2025). Exogenous Melatonin Application Delays Senescence and Improves Postharvest Antioxidant Capacity in Blueberries. Agronomy, 15(2), 428. https://doi.org/10.3390/agronomy15020428





