Abstract
The salinity of water and soil is a constraint that has an extreme effect on germination and the establishment of crops. Therefore, it is pivotal to boost crop salt tolerance in global semi-arid regions. By mixing Si in an ME medium, a new complex of nanoparticles (Si-CTS-HPC-ME NPs) was synthesized, and we investigated the role of Si-CTS-HPC-ME NPs on Cyamopsis tetragonoloba germination and tolerance against salinity stress. Thus, this study examined the influence of Si-CTS-HPC-ME NPs at different concentrations (N1: 0, N2: 40 and N3: 80 mg L−1) on some germination and seedling growth parameters and the ion homeostasis of Cyamopsis tetragonoloba L. (cluster bean) seedlings under three salinity levels (S1: 0, S2: 6 and S3: 12 dS m−1). With increasing salinity, the energy of germination (GE), index of germination (GI), index of vitality (VI), seedling vigor index (SVI), fresh weight (SFW) and dry (SDW) weight of seedlings, plumule length (PL), and radicle length (RL) parameters gradually decreased, while the mean germination time (MGT) and coefficient of velocity of germination (CVG) increased in salt-stressed cluster bean seedlings in comparison to the control. However, the usage of Si-CTS-HPC-ME NPs was effective in enhancing cluster bean tolerance to salinity by enhancing total phenols and flavonoids and improving K+, Si, and Ca2+ uptake, thus reducing lipid peroxidation, decreasing sodium ion uptake and potassium leakage, and promoting germination parameters compared with non-NP-treated seedlings. Meanwhile, 40 mg L−1 Si-CTS-HPC-ME NPs exhibited an effective response in saline conditions compared with the other NP treatments. Consequently, the application of Si-CTS-HPC-ME NPs in salt-stressed cluster bean seedlings can serve as an effective technique to enhance salinity tolerance in saline conditions under arid and semi-arid climatic conditions.
1. Introduction
Water and soil salinity can be a serious challenge to the germination and establishment of crops [1]. Salinity stress decreases the hydration of cells and delays the onset of germination under arid and semi-arid climatic conditions, thus posing a significant threat to sustainable agriculture and food security [2]. One of the problems of salt stress is the increase in sodium ion uptake, which can lead to potassium leakage from cells. This accumulation can disrupt the K+/Na+ balance and reduce morpho-physiological and biochemical responses in crops [3]. Generally, an increase in sodium uptake boosts the ion toxicity in crops, leading to induced ionic, osmotic and oxidative stresses. Oxidative stress can lead to free radical production, such as hydroxyl radical (OH•), hydrogen peroxide (H2O2) and superoxide anion radical (O2−•). These free radicals in crops have the ability to alter redox potential and increase lipid peroxidation [4,5]. On the other hand, crops can reduce the harmful effects of salinity by improving secondary metabolites (phenol and flavonoid contents) and enzymatic antioxidants [4,5]. The establishment of effective approaches is, therefore, essential to ensure continuous crop production [6].
An approach to diminishing the negative impacts of salinity and elevating the growth of plants involves the usage of plant growth-stimulating substances, which are associated with mechanisms of salt tolerance due to their regulation of ionic homeostasis and redox metabolism [7,8]. Silicon (Si) and melatonin (ME) are plant growth-stimulating substances. These substances aid in enhancing the germination of seeds, growth of seedlings, and relative water content [9,10]. In addition, they can enhance the ROS scavenging capacity and regulate both the non-enzymatic and enzymatic antioxidant systems in crops [10,11]. The application of Si or ME can improve ion homeostasis and enhance the compartmentalization of ions into vacuoles by improving cellular functions and decreasing the sodium uptake in the cytoplasm [9,12].
A new strategy to alleviate salinity stress is nanoparticle (NP) application, which facilitates the uptake of plant growth-stimulating substances by crops and improved stress tolerance [13]. This approach is an effective strategy that enhances the efficacy of agrochemicals and decreases agrochemical waste in the environment [14,15]. Applying plant growth-stimulating substances such as silicon and melatonin has been found to improve stress tolerance in crops [9,10,16,17,18]. The application of synthesized NPs for the better absorption and transfer of silicon and melatonin could be beneficial to seedling growth in salt conditions. Numerous studies have indicated that the application of NPs increases seed germination (Phaseolus vulgaris), plant growth (bitter melon) and antioxidant enzymatic activities (spearmint) [14,15,19].
Cyamopsis tetragonoloba L. (cluster bean), belonging to the Leguminosae, is an annual plant with industrial properties [20]. This plant is originally native to Africa and the South Asian subcontinent [21]. It is also a drought- and salt-tolerant summer annual legume [20]. It is used in petrol, paper, textiles, pharmaceutics, folk medicine as a digestive stimulant, as an anti-ulcer treatment, a cooling agent, an anti-hyperglycemic substance, an anti-secretion agent, and a cathartic factor, and it is also used in the food industry. This plant is popular all over the world due to its low input and lower care costs [20]. It has gained global recognition for its carbohydrate content (gum) and has been applied in the food industry. There is not, however, much information concerning NPs’ impact on the germination of seeds, growth of seedlings and ion homeostasis of cluster beans, particularly in salinity conditions. Hence, the purpose of this study was to assess the protective role of silicon–melatonin nanoparticles (Si-CTS-HPC-ME NPs) in diminishing the adverse effects of salinity on the germination of seeds, the growth of seedling parameters and the maintenance of ion homeostasis in Cyamopsis tetragonoloba L.
2. Materials and Methods
2.1. Silicon–Melatonin Nanoparticle (Si-CTS-HPC-ME NP) Synthesis
To prepare Si-CTS-HPC-ME NPs, 0.1 g of Chitosan (CTS; Sigma Aldrich, Burlington, MA, USA) powder was added to acetic acid (1%) and stirred for 5 min. Separately, 0.1 g ME (Sigma Aldrich, Burlington, MA, USA) was dissolved in ethanol and then added to distilled water, with stirring for 5 min. Subsequently, 10 mL of the ME solution was added to the CTS solution and stirred for 3 h. In parallel, 2 g Na2SiO3 (Si; Sigma Aldrich, Burlington, MA, USA) and 0.2 g hydroxypropyl cellulose (HPC; Sigma Aldrich, Burlington, MA, USA) were each dissolved in distilled water and gradually added to the ME-CTS solution. This Si-CTS-HPC-ME solution was then treated with tripolyphosphate (TPP; Sigma Aldrich, Burlington, MA, USA) as an ionic cross-linker. To this end, 5 mL TPP solution was added to the Si-CTS-HPC-ME solution and stirred for 12 h. The appearance of flocculated particles was observed upon TPP addition. The resulting mixture was rinsed with distilled water to remove any unreacted material, yielding purified NPs. Finally, Si-CTS-HPC-ME NPs were freeze-dried.
2.2. Silicon–Melatonin Nanoparticle (Si-CTS-HPC-MEl NP) Characterization
Si-CTS-HPC-ME NPs were further characterized using a variety of analytical techniques, including X-ray diffraction (XRD), scanning electron microscopy (SEM), energy-dispersive spectroscopy (EDS), and Fourier transform infrared spectroscopy (FTIR). The structural properties of the samples were determined by analyzing XRD patterns obtained using a Siemens D8 Advance spectrometer at room temperature, within a range of 2θ = 10° to 70°, with an XPert-PRO diffractometer (XRD; Panalytical, Almelo, The Netherlands). A scanning electron microscope (SEM; Hitachi Model E-1010, Ibaraki, Japan) was employed to examine the surface morphology and elemental composition of Si-CTS-HPC-ME NPs. Additionally, energy-dispersive X-ray spectrometer (EDS; Oxford Instruments, High Wycombe, UK), integrating with the SEM device, was used to analyze the elements of Si-CTS-HPC-ME NPs. Fourier transform infrared (FTIR) analysis was conducted to identify the functional groups present in the raw materials and NPs, within a spectral range of 500–3500 cm−1, using a Bruker TENSOR II spectrometer (Bruker, Ettlingen, Germany).
2.3. Experimental Design
The present investigation was conducted at the Physiology Laboratory of the School of Agriculture, Shiraz University, Iran, in June 2024. Cluster bean seeds (cv. BR-2017) were obtained from the Department of Horticultural Sciences and Engineering, Ardakan University, Iran. This study was designed as a factorial experiment based on a completely randomized design (CRD) with three replicates. Various treatments, including different concentrations of NaCl (S1: 0, S2: 6 and S3: 12 dS m−1) and Si-CTS-HPC-ME NPs (N1: 0, N2: 40 and N3: 80 mg L−1), were prepared in distilled water.
The seeds were sterilized with 3% sodium hypochlorite solution for three minutes and then washed three times with distilled water. Subsequently, the seeds were soaked in distilled water for 1 h (seed imbibition) [22,23]. Following imbibition, the seeds were germinated in petri plates with one layer of filter paper, and 10 mL of the respective treatments was applied for 7 days under growth conditions (25 °C, 600 μmol m−2 s−1 PAR, and 75% relative humidity). Each treatment was repeated three times, with 25 seeds per replication, totaling 675 seeds for the experiment. The solutions of distilled water, NaCl and Si-CTS-HPC-ME NPs were replaced daily. To avoid the position effect, all petri plates were shifted daily in the growth chamber. Germination parameters were recorded over the 7-day germination period, and seedlings were collected for biochemical analyses on the 7th day after germination.
2.4. Determination of Seed Germination Parameters
Germination percentage (GP), energy of germination (GE), index of germination (GI), index of vitality (VI), index of seedling vigor (SVI), mean germination time (MGT) and coefficient of velocity of germination (CVG) were determined according to the procedure described by Ghosh et al. [22], Alsaeedi et al. [24], Cheng et al. [25], Ren et al. [26], Irik and Bikmaz [27], Zhang et al. [28] and Kader et al. [29]. Plumule and radicle length (PL and RL) and fresh weight of the seedling (SFW) were measured after 7 days of germination, while the dry weight of seedling (SDW) was determined after drying at 80 °C for 48 h.
where Gt represents the number of seeds germinated on day t, Dt is the day t, and S is the average radicle length.
where Ni is the number of newly germinated seeds at time Ti
where N is number of seeds germinated each day and T is the number of days from seeding corresponding to N.
2.5. Malondialdehyde (MDA) Determination
MDA, an indicator of membrane lipid peroxidation, was quantified using the method developed by Heath and Packer [30]. For this determination, 1 mL of supernatant was mixed with 20% trichloroacetic acid (TCA) and 0.5% thiobarbituric acid (TBA), and the mixture was boiled at 95 °C for 30 min. After boiling, the mixture was cooled on ice and centrifuged at 10,000 rpm for 10 min. The absorbance of the resulting mixture was measured at 450, 532 as well as 600 nm. MDA concentration was calculated by using the following formula.
2.6. Total Phenolic and Flavonoid Contents
To extract phenolic compounds from the dry seedlings of cluster bean, 0.5 g of dry seedlings was combined with 50 mL of cold methanol (80%) at 4 °C and then centrifuged at 10,000× g for 20 min. The resulting supernatants (50 µL) were mixed with 1 mL of Folin-Ciocalteu phenol reagent, followed by addition of 1 mL of 10% sodium carbonate solution. This mixture was used to determine the total phenols, according to Wong-Paz et al. [31], and the absorbance was measured at 760 nm using a spectrophotometer (7315 UV/visible Spectrophotometer, Jenway, UK). The concentration of total phenols was calculated using a calibration curve of gallic acid (y = 0.1632x, R2 = 0.996) and expressed as μg gallic acid equivalents (GAE) per g DW. For the extraction of total flavonoids, 0.5 g of dry seedlings was extracted with 50 mL of cold methanol (80%) at 4 °C and then filtered. The filtrate (0.5 mL) was mixed with 150 µL of 10% aluminum chloride and 2 mL of 1 M sodium hydroxide. The mixture was used to determine total flavonoid content, according to the method of Czerniewicz et al. [32]. The absorbance was measured at 510 nm using the spectrophotometer (7315 UV/visible Spectrophotometer, Jenway, UK), and the concentration of flavonoids was calculated using a quercetin standard dilution series and a calibration curve (y = 117.58x, R2 = 0.9798). Results were expressed as mg quercetin equivalents (QUE) g−1 DW.
2.7. Ion Analysis
Sodium, potassium, silicate and calcium concentrations in the dried seedling samples were determined using an atomic absorption spectrometer [33]. Powdered samples (1 g) were washed in a muffle furnace at 250 °C for 1 h, followed by 550 °C for 3 h. The resulting ash was dissolved in 5 mL of 2N HCl, and the mixture was filtered into a 50 mL volumetric flask. The final volume of the filtrate was adjusted to 50 mL by boiling with distilled water [33].
2.8. Statistical Analysis
The results were analyzed using one-way analysis of variance (ANOVA) with SAS v. 9.1 software (SAS Institute 2003, Inc., Cary, NC, USA). Mean comparisons were conducted using the LSD test (p < 0.05). Pearson’s correlation coefficient, heat maps and principal component analysis (PCA) were generated using the Origin Pro software, ver. 2022.
3. Results
3.1. Characterization of Si-CTS-HPC-ME NPs
The XRD patterns of melatonin, sodium metasilicate, and Si-CTS-HPC-ME NPs are presented in Figure 1a. The XRD pattern of melatonin displays sharp peaks at diffraction angles of 16.5°, 24.4°, 25.2°, and 26.2°, indicating its crystalline structure. These reflections signify the structural order within the melatonin crystal lattice. The intensity and sharpness of these peaks suggest the high purity of the sample and the presence of well-ordered crystallites, making the material suitable for applications requiring crystallinity. The XRD pattern of sodium metasilicate reveals its crystalline structure, with sharp peaks observed at 2θ = 29°, 34.8°, 37.2°, and 52.1°. These peaks closely correspond to the crystallographic planes of the anhydrous phase (Na2SiO3) and indicate high crystallinity and purity. The absence of additional peaks implies a lack of impurities or secondary phases in the sample. Significant structural changes are evident in the composite. In the 2θ = 20–38° range, the sharp peaks corresponding to the crystalline phases of sodium metasilicate and melatonin have transitioned into a broad and low-intensity peak, indicating reduced crystallinity and enhanced amorphous characteristics. Additionally, a broad but high-intensity peak is observed in the 2θ range of 10–20°, aligning with the characteristic peak of sodium metasilicate (Figure 1a).
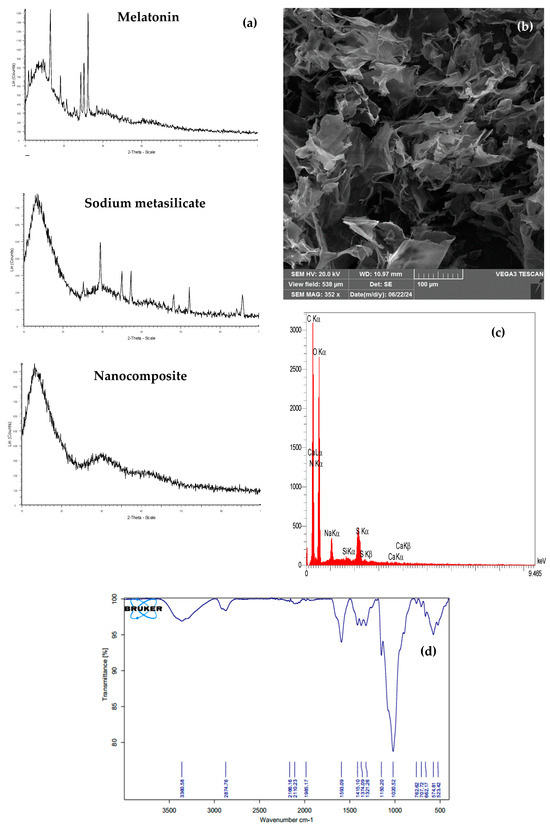
Figure 1.
Characterization of Si-CTS-HPC-ME NPs. (a) XRD pattern of melatonin, sodium metasilicate and Si-CTS-HPC-ME NPs, (b) SEM images, (c) EDS and (d) FTIR peaks.
The analysis of SEM images of the chitosan/melatonin/sodium metasilicate composite revealed structural similarities to previously reported three-dimensional porous features of chitosan/melatonin composites. Specifically, voids and pores are formed, which are largely attributed to synthesis methods such as lyophilization. However, in this composite, thin and irregular shell-like plates are observed instead of an interconnected network of pores. It appears that the presence of sodium metasilicate plays a critical role in altering the morphology by influencing the crystallization behavior and arrangement of components, thereby replacing the typical dense, polyhedral particles of sodium metasilicate with shell-like structures (Figure 1b).
The EDS analysis of the Si-CTS-HPC-ME NP nanocomposite revealed a composition predominantly comprising carbon (C), oxygen (O), and sulfur (S), along with significant amounts of nitrogen (N), sodium (Na), silicon (Si), and trace calcium (Ca). The high intensities of carbon and oxygen result from the organic matrix formed by chitosan, hydroxypropyl cellulose (HPC), and melatonin, which are rich in these elements. The presence of nitrogen confirms the contribution of chitosan and melatonin, both of which contain nitrogen-bearing functional groups. The lower levels of sodium and silicon arise from the limited incorporation of sodium metasilicate (Na2SiO3) during synthesis and the partial removal of these compounds during the washing steps. The prominent sulfur signal indicates successful cross-linking facilitated by tripolyphosphate (TPP), highlighting its effective interaction with the chitosan backbone. Finally, the trace amount of calcium originates from minor impurities in the raw materials and the synthesis process (Figure 1c).
Analysis of the FTIR spectrum of the Si-CTS-HPC-ME NPs composite revealed the presence of key functional groups associated with its components and their interactions. Peaks observed in the range of 500–700 cm−1 correspond to Si–O and Si–O–Si vibrations of sodium metasilicate, while a strong peak at 1020 cm−1 confirms the stretching vibrations of C–O and Si–O bonds. The peak at 1593 cm−1 is attributed to the amine groups of chitosan and the aromatic rings of melatonin. In the range of 1900–2200 cm−1, the peaks are assigned to sodium metasilicate and melatonin. These peaks likely indicate specific silicate-related vibrations of sodium metasilicate and weak molecular interactions associated with melatonin. The broad peak at 3360 cm−1 arises from the combined stretching vibrations of −OH and −NH2 groups in chitosan and melatonin. The hydrogen bonding interactions between these components result in a broadening and slight shift in the peaks, indicating strong chemical interactions within the composite (Figure 1d).
3.2. Effect of Si-CTS-HPC-ME NPs on the Parameters of Germination
The results indicated that the interaction between Si-CTS-HPC-ME NPs and salinity stress was significant for germination parameters, excluding germination percentage (GP), of cluster bean (Table 1; Figure 2 and Figure 3a–g). As shown in Figure 3b–g, the germination parameters (GE, GI, VI, SVI, MGT and CVG) were negatively affected by S2 and S3 salinity levels compared to the S1 level. Si-CTS-HPC-ME NPs significantly (p < 0.05) promoted the germination-related parameters, relative to salinity stress. Specifically, compared to the S1 treatment under the N1 condition, GI decreased by 13.5 and 19.9% under S2 and S3 treatments, respectively. Additionally, GE was significantly reduced by 5.3% at the S3 salinity level. MGT exhibited an increasing trend, rising by 69.6 and 19.6%, under S2 and S3 salinity levels, respectively, compared to the S1 treatment. A considerable reduction in SVI (12.2%), VI (39.3%) and CVG (43.6%) was observed under the S3 salinity level compared to the S1 level (Figure 3e–g). The results (Figure 3a–g) indicated that Si-CTS-HPC-ME NPs significantly (p < 0.05) mitigated the negative effect of S2 and S3 salinity levels and enhanced the germination parameters compared to the S1 treatment. Moreover, N2 treatments exhibited higher recovery at the S2 salinity level than at the S3 salinity level. Under S2 and S3 salinity levels, N2 treatments increased GI by 10.3 and 15.6% and GE by 1.3 and 5.6%, respectively, compared with the N1 treatment.

Table 1.
Results of the analysis of variance (ANOVA) of Si-CTS-HPC-ME NPs, salinity stress and their interaction effects on the germination parameters, total phenol and flavonoid, MDA and Na+/K+/Si/Ca2+ concentrations of cluster bean.
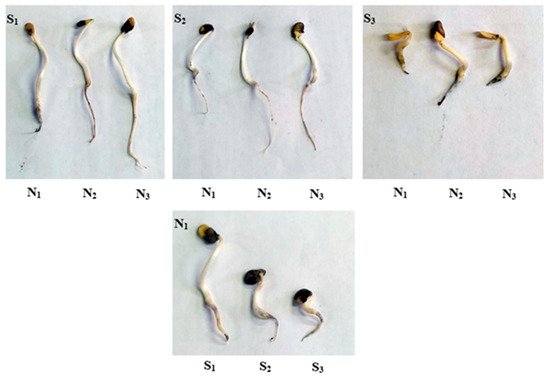
Figure 2.
The effect of Si-CTS-HPC-ME NPs application (N1: 0, N2: 40 and N3: 80 mg L−1) and salinity stress (S1: 0, S2: 6 and S3: 12 dS m−1) on the phenotypic changes of cluster bean seedlings.
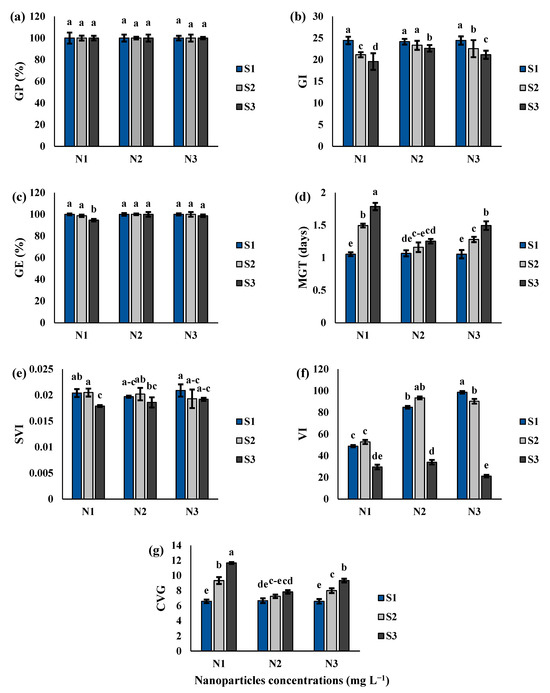
Figure 3.
The effect of Si-CTS-HPC-ME NPs application (N1: 0, N2: 40 and N3: 80 mg L−1) and salinity stress (S1: 0, S2: 6 and S3: 12 dS m−1) on the percentage of germination (GP) (a), index of germination (GI) (b), energy of germination (GE) (c), mean germination time (MGT) (d), index of seedling vigor (SVI) (e), index of vitality (VI) (f) and coefficient of velocity of germination (CVG) (g) of cluster bean seedlings. Different letters in the columns show significant differences (p < 0.05) according to the LSD test.
3.3. Effect of Si-CTS-HPC-ME NPs on the SFW, SDW, PL and RL Parameters
SFW, SDW, PL and RL parameters were measured in the cluster bean seedlings under various salinity levels and Si-CTS-HPC-ME NP applications (Figure 4a–d). The interaction of Si-CTS-HPC-ME NPs and salinity stress significantly affected the SFW, SDW, PL and RL parameters of cluster bean seedlings (Table 1; Figure 4a–d). As the salinity treatment progressed, the SFW, SDW, PL, and RL parameters decreased. In the N1 treatment, the values of SFW, SDW, PL and RL parameters decreased by 6.7 and 26.5%, 20.7 and 81.5%, 16 and 72% and 20 and 40%, respectively, under S2 and S3 salinity levels compared to S1. However, N2 application led to a significant increase in the values of SFW by 1.1- and 1.2-fold; SDW by 1.5- and 3.2-fold; and PL by 1.5- and 2.00-fold under S2 and S3 salinity levels, respectively, compared to the N1 treatment (Figure 4a–d).
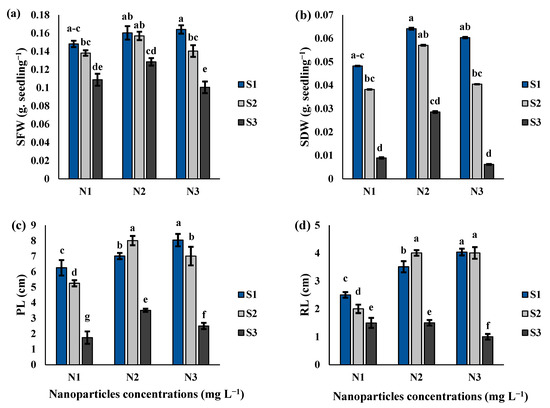
Figure 4.
The effect of Si-CTS-HPC-ME NPs application (N1: 0, N2: 40 and N3: 80 mg L−1) and salinity stress (S1: 0, S2: 6 and S3: 12 dS m−1) on the fresh (SFW) (a) and dry (SDW) (b) weight, length of plumule (PL) (c) and length of radicle (RL) (d) of cluster bean seedlings. Different letters in the columns show significant differences (p < 0.05) according to the LSD test.
3.4. Effect of Si-CTS-HPC-ME NPs on Total Phenol and Flavonoid
The interaction effect of Si-CTS-HPC-ME NPs and salinity stress significantly affected the values of phenols and flavonoids in cluster bean seedlings (Table 1; Figure 5a,b). Salinity levels increased total phenol and flavonoid content, with Si-CTS-HPC-ME NP-treated seedlings showing higher phenol and flavonoid levels than those in the N1 treatment (Figure 5a,b). Under S2 and S3 salinity levels, the application of N2 and N3 treatments increased the total phenol content by 1.9- and 3.5-fold, respectively, as compared to the N1 treatment. Additionally, the total flavonoid content increased by 1.1- and 1.4-fold and 1.0- and 1.4-fold, respectively, under N2 and N3 treatments, compared to the N1 treatment (Figure 5a,b).
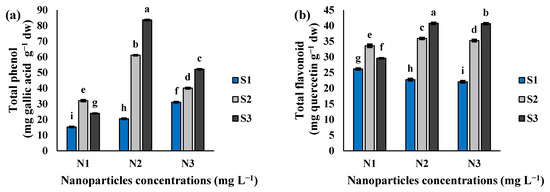
Figure 5.
The effect of Si-CTS-HPC-ME NPs application (N1: 0, N2: 40 and N3: 80 mg L−1) and salinity stress (S1: 0, S2: 6 and S3: 12 dS m−1) on the total phenol (a) and total flavonoid (b) of cluster bean seedlings. Different letters in the columns show significant differences (p < 0.05) according to the LSD test.
3.5. Effect of Si-CTS-HPC-ME NPs on Malondialdehyde Content (MDA)
MDA content in cluster bean seedlings was significantly affected by the interaction between Si-CTS-HPC-ME NPs and salinity stress (Table 1; Figure 6). The highest MDA level (3.6 nmol g−1 fw) was observed in the combination of the S3 salinity level and the N1 treatment (Figure 6). Under S2 and S3 salinity levels, the application of N2 and N3 treatments decreased the MDA content by 33.9 and 49.9% and 28.7 and 50.6%, respectively, compared to the N1 treatment (Figure 6).
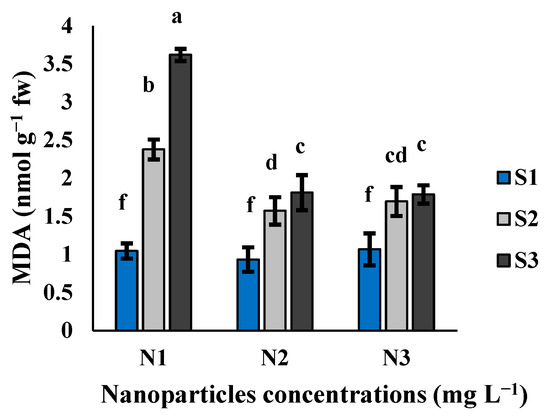
Figure 6.
The effect of Si-CTS-HPC-ME NP application (N1: 0, N2: 40 and N3: 80 mg L−1) and salinity stress (S1: 0, S2: 6 and S3: 12 dS m−1) on the MDA content of cluster bean seedlings. Different letters in the columns show significant differences (p < 0.05) according to the LSD test.
3.6. Effect of Si-CTS-HPC-ME NPs on Na+/K+/Si/Ca2+ Concentrations
The interaction of Si-CTS-HPC-ME NPs and salinity stress exhibited a significant influence on the concentrations of Na+, K+, Si, and Ca2+ in cluster bean seedlings (Table 1; Figure 7a–d). In the N1 treatment, S2 and S3 salinity levels decreased the values of K+ by 21.2% and 46.7%, Si by 19.9% and 49.7%, and Ca2+ by 23.2 and 51.0%, respectively, compared to the S1 salinity level (Figure 7a,c,d). With increasing salinity to S2 and S3 levels, the concentration of Na+ in the N1 treatment increased by 4.5- and 6.9-fold, compared to the S1 salinity level (Figure 7b). The application of 40 mg L−1 Si-CTS-HPC-ME NPs was found to enhance K+, Si and Ca2+ more efficiently compared to 80 mg L−1 Si-CTS-HPC-ME NPs. Specifically, 40 mg/L Si-CTS-HPC-ME NP application significantly improved the concentrations of K+ by 21.5 and 55.7%, Si by 12.4 and 37.6%, and Ca2+ by 19.4 and 39.9%, under S2 and S3 salinity levels, respectively, compared to the N1 treatment (Figure 7a,c,d). Under S2 and S3 salinity levels, 80 mg/L Si-CTS-HPC-ME NPs enhanced the concentrations of K+ by 9.7 and 28.5%, Si by 3.6 and 16.5%, and Ca2+ by 10.8 and 32.1%, compared to the N1 treatment (Figure 7a,c,d). Interestingly, different results were observed in the concentration of Na+, with significant decreases of 17.3 and 28.5% under S2 and S3 salinity levels, compared to the N1 treatment after being treated with 40 mg L−1 Si-CTS-HPC-ME NPs (Figure 7b).
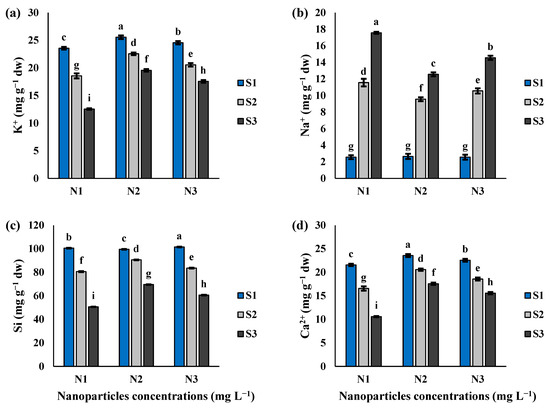
Figure 7.
The effect of Si-CTS-HPC-ME NP application (N1: 0, N2: 40 and N3: 80 mg L−1) and salinity stress (S1: 0, S2: 6 and S3: 12 dS m−1) on K+ (a), Na+ (b), Si (c) and Ca2+ (d) of cluster bean seedlings. Different letters in the columns show significant differences (p < 0.05) according to the LSD test.
3.7. Pearson’s Correlation Coefficient Combined with Heat Maps and Principal Component Analysis (PCA)
Pearson’s correlation coefficient, combined with heat maps, demonstrated a positive correlation among SFW, SDW, PL, RL, K+, Si and Ca2+. Additionally, a strong positive correlation was observed among Na+, MDA, Ph and FLV. Finally, the findings indicated a negative correlation between the two groups mentioned above (Figure 8a).
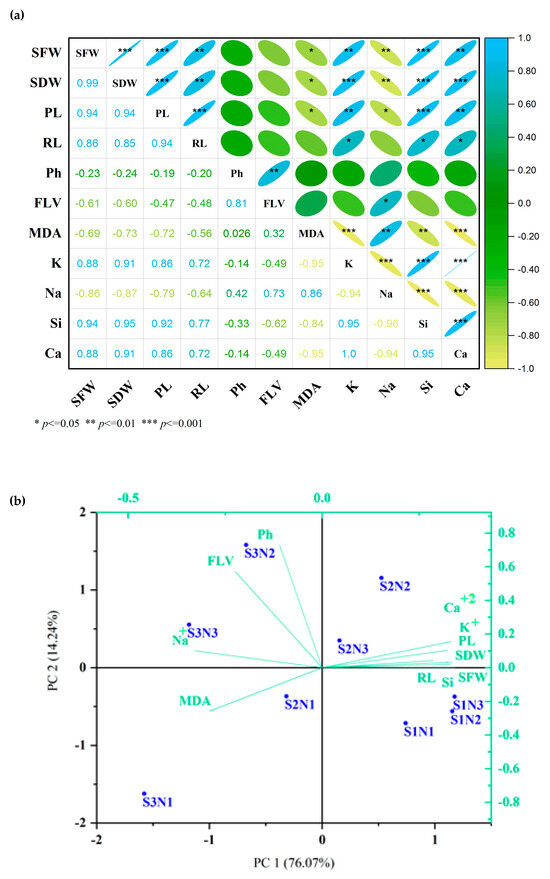
Figure 8.
Pearson’s correlation coefficient combined with heat maps (a) and the principal component analysis (PCA) (b) for the measured parameters of the related Si-CTS-HPC-ME NP-treated cluster seedlings exposed to salinity stress. Plumule length (PL), radicle length (RL), fresh (SFW) and dry (SDW) weight of seedling, malondialdehyde (MDA), total phenol (Ph) and flavonoid (FLV), sodium (Na+), potassium (K+), silicate (Si) and calcium (Ca2+) concentrations of cluster bean seedlings.
The PCA indicated that both components accounted for 90.31% of the total variation, with 76.07% and 14.24% of the complete variability. The application of 40 mg L−1 Si-CTS-HPC-ME NPs in this experiment demonstrated a positive effect on the parameters of germination and seedling growth of cluster beans. Ionic stress indicators, such as MDA, Na+, FLV and Ph, clustered together and were proximate to the S3 treatment. Conversely, Ca2+, K+, Si, PL, RL, SDW and SFW clustered together and were proximate to the low salinity level (Figure 8b). The PCA plot also demonstrated a positive correlation among the parameters related to salt tolerance improvement and Ca2+, K+, Si, PL, RL, SDW, as well as the SFW (Figure 8b). Conversely, a negative correlation was observed between MDA and Na+ with Ca2+, K+, Si, PL, RL, SDW and SFW. Notably, S2 and S3 salinity levels were associated with ionic stress. However, the use of 40 mg L−1 Si-CTS-HPC-ME NPs under salt conditions demonstrated a strong association with FLV and Ph, suggesting the efficacy of 40 mg L−1 Si-CTS-HPC-ME NPs in reducing salinity stress (Figure 8b).
4. Discussion
The primary objective of this study was to synthesize Si-CTS-HPC-ME nanoparticles with high purity and to properly characterize these NPs. The XRD result reflects the high proportion of sodium metasilicate (approximately 20-times that of melatonin) and the influence of the chemical synthesis process, which preserves partial crystallinity. These observations highlight strong interactions between the composite components and structural rearrangements induced by the chemical synthesis, leading to a reduction in crystalline order at the microscopic scale. In the present composition, the interaction of sodium metasilicate with the organic matrix of chitosan and melatonin inhibited particle growth and aggregation, resulting in a uniform and distinct structure. This structure consists of shell-like plates with large and irregular pores, which are clearly visible in the SEM images. These plates, characterized by limited dimensions and irregular distribution, are associated with the specific composition of the composite and its synthesis method. Furthermore, the absence of agglomeration indicates a uniform distribution of the constituent phases within the composite, contributing to its structural stability. The morphological features, including large pores and shell-like plates, highlight the potential applicability of this composite in biological and industrial fields. The EDS results confirm the successful fabrication of the nanocomposite and the expected elemental distribution based on the synthesis protocol. Overall, these results suggest the formation of new hydrogen bonds and chemical interactions that contribute to the stabilization and reinforcement of the composite structure.
Since abiotic stresses such as exposure to salinity have a notable effect on seed germination and embryonic development, it is increasingly necessary to understand the potential of NP usage [15,22,34]. Their antioxidant properties, modulation of membrane transporters’ efficiency, and biosynthesis of secondary metabolites position NPs as a key component in increasing stress tolerance against abiotic stresses. Therefore, the composition of nanoparticles can significantly influence their efficiency in improving stress tolerance [15,22,34].
Salinity stress significantly reduces seed germination and seedling growth parameters by decreasing the water uptake and nutrient absorption efficiency and altering the ion balance within the seed [5,14]. The reduction in the germination index, index of vitality, index of seedling vigor, seedling and radicle length, fresh and dry weight of seedlings are common phenomena under salinity stress in crops [23,24,25,26,27]. In this context, the negative impacts of salt stress on the germination of seed and seedling growth parameters are likely due to its initial effect on water and nutrient uptake, which subsequently changes biochemical, physiological, and structural properties in the germination stage, leading to decreased seed germination [23,27]. Cluster beans are known to exhibit high tolerance to salinity stress in arid and semi-arid areas of the world. However, if the salinity stress exceeds the tolerance limits (8 dS m−1), different negative and toxic impacts begin to appear in different parts of the cluster bean. In the present study, 6 dS m−1 salinity level (S2) exposure significantly decreased the seed germination and seedling growth parameters of cluster beans, while the 12 dS m−1 salinity level (S3) exerted a greater decreasing impact in comparison to other salinity levels. Consistent with the present results, previous studies have indicated that salinity stress can induce a notable inhibitory impact on seed germination and seedling growth parameters of white clover [23], wheat [22] and barley [35].
Earlier studies suggested that NPs (e.g., chitosan–melatonin nanoparticles and zinc-loaded mesoporous silica nanoparticles) may be crucial in diminishing the negative impacts of salt stress in crops [15,22]. In the present study, the use of Si-CTS-HPC-ME NPs in the germination stage of cluster beans at a concentration of 40 mg L−1 significantly diminished the damage caused by S2 and S3 salinity levels’ exposure while improving the measured seed germination and seedling growth parameters relative to N1 treatment. In agreement with the present results, Farooq et al. [36] also reported an increase in the physiological attributes of Brassica napus under arsenic stress following the application of melatonin–selenium nanoparticles. This is supported by the study of Gohari et al. [15], which indicates that chitosan–melatonin nanoparticles may help to alleviate salinity stress in spearmint plants. Both of these reports and the current results suggest that nanoparticles may act as an innovative protective agent for crops, with greater effectiveness in mitigating stress in combinations of nanomaterials, as compared to the application alone [15,36].
Salinity levels trigger changes in secondary metabolites to reduce lipid peroxidation in crops. The MDA content, a good indicator of lipid peroxidation and oxidative damage, decreases under salinity stress. Secondary metabolites become pivotal under salinity stress, involving the synthesis of phytochemicals such as polyphenols and flavonoids to counterbalance the oxidative stress imposed by the salinity levels [37]. In the present study, the secondary metabolites in cluster bean seedlings were increased along with salinity levels as a result of the improved levels of phenols and flavonoids. Published reports have also shown that salinity stress causes oxidative stress by influencing membrane integrity and electrolyte leakage [22]. Similar conclusions have also been reported by Khan et al. [34] for pearl millet, Moradbeygi et al. [38] for Moldavian balm (Dracocephalum moldavica), and Abdoli et al. [39] for Ajowan (Trachyspermum ammi L.) under salinity stress. Elevated levels of total phenolic and flavonoids compounds have been described as being advantageous for scavenging the excessive ROS in crops, thereby decreasing the effect of salinity stress and MDA contents in crops [34,37]. In our experiment, total phenolic and flavonoids compounds were significantly changed and improved in cluster bean seedlings treated with N2 and N3, as compared to the N1 treatment under salinity stress condition. Another research study also confirmed that the application of nanoparticles promoted the synthesis of phytochemicals such as polyphenols and flavonoids, while it reduced the MDA content under salinity stress, which was consistent with the present results [34,37,38,39].
Ion homeostasis is crucial for maintaining the integrity of the plasma membrane and an equilibrium of ions in plant cells [40,41]. The impact of salinity stress on Na+, K+, Si, and Ca2+ concentrations is essential for stress tolerance [41,42]. In this study, salinity stress diminished the K+, Si and Ca2+ uptake, while it increased Na+ uptake in cluster bean seedlings. However, the improvement in K+, Si and Ca2+ uptake and the decrease in Na+ uptake following the application of Si-CTS-HPC-ME NPs may be intended to ensure that nutrients are kept at specific concentrations to reduce or prevent salinity toxicity. Farouk and AlAmri [42] also observed the improved ionic regulation of canola (Brassica napus) with 10 mg L−1 Zn NP treatment, which was consistent with the results obtained for cluster beans in the present study. In addition, the 20 mM Ag NP treatment in pearl millet (Pennisetum glaucum) significantly regulated the ion balance in comparison to the control crops [34]. Applying copper nanoparticles in tomato (Lycopersicon esculentum) also improved the K+/Na+ ratio by modulating ion uptake and vacuolar compartmentalization [36]. In another study, Abdoli et al. [39] reported the enhancement of K+ uptake, K+/Na+ ratio and Fe content in ajowan (Trachyspermum ammi L.) treated with 3 mM Fe2O3 NPs under 4, 8 and 12 dS m−1 salinity levels, which was consistent with the present findings.
5. Conclusions
This study highlighted the crucial role of ion homeostasis in improving the salt tolerance of cluster bean seedlings treated with Si-CTS-HPC-ME NPs. Si-CTS-HPC-ME NP-treated cluster bean seedlings exhibited better biochemical adaptation, as evidenced by increased total phenol and flavonoid levels and reduced MDA, which alleviated the harmful impacts of salinity stress and improved seed germination and seedling growth parameters. This study also provided noteworthy evidence showing the positive impacts of Si-CTS-HPC-ME NPs on reducing salinity stress. This stress alone severely decreased germination parameters, increased lipid peroxidation, and raised sodium ion uptake and potassium leakage in cluster bean seedlings. It also altered Si and Ca2+ uptake in the seedlings. However, the application of Si-CTS-HPC-ME NPs improved germination and seedling growth parameters and maintained ion homeostasis. Notably, 40 mg L−1 Si-CTS-HPC-ME NPs appeared to be a more efficacious approach for improving the tolerance of cluster bean seedlings under salinity stress. Treating seedlings with 40 mg L−1 Si-CTS-HPC-ME NPs after sowing under salinity stress may, therefore, prove to be advantageous as a straightforward, user-friendly, and cost-effective method, contributing to heightened agricultural yields and the production of safe uncontaminated food.
Author Contributions
Conceptualization, M.A. and S.A.K.; methodology, M.A., S.A.K., S.S. (Samad Sabbaghi), S.S. (Shima Sayahi) and A.A.; software, M.A.; validation, M.A., S.A.K. and S.S. (Samad Sabbaghi); formal analysis, M.A. and S.S. (Shima Sayahi); investigation, S.A.K. and S.S. (Samad Sabbaghi); resources, S.A.K. and S.S. (Samad Sabbaghi); data curation, M.A.; writing—original draft preparation, M.A., S.A.K. and A.A.; writing—review and editing, M.A., S.A.K. and B.A.L.; supervision, S.A.K.; project administration, S.A.K. and S.S. (Samad Sabbaghi); funding acquisition, B.A.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Iran National Science Foundation (INSF), grant number 4016025.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding authors upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Parihar, P.; Singh, S.; Singh, R.; Singh, V.P.; Prasad, S.M. Effect of salinity stress on plants and its tolerance strategies: A review. Environ. Sci. Pollut. Res. 2015, 22, 4056–4075. [Google Scholar] [CrossRef]
- Isayenkov, S.V.; Maathuis, F.J. Plant salinity stress: Many unanswered questions remain. Front. Plant Sci. 2019, 10, 80. [Google Scholar] [CrossRef] [PubMed]
- Balasubramaniam, T.; Shen, G.; Esmaeili, N.; Zhang, H. Plants’ response mechanisms to salinity stress. Plants 2023, 12, 2253. [Google Scholar] [CrossRef] [PubMed]
- Aliyari Rad, S.; Dehghanian, Z.; Asgari Lajayer, B.; Nobaharan, K.; Astatkie, T. Mitochondrial respiration and energy production under some abiotic stresses. J. Plant Growth Regul. 2022, 41, 3285–3299. [Google Scholar] [CrossRef]
- Zia-ur-Rehman, M.; Anayatullah, S.; Irfan, E.; Hussain, S.M.; Rizwan, M.; Sohail, M.I.; Jafir, M.; Ahmad, T.; Usman, M.; Alharby, H.F. Nanoparticles assisted regulation of oxidative stress and antioxidant enzyme system in plants under salt stress: A review. Chemosphere 2023, 314, 137649. [Google Scholar] [CrossRef]
- Botella, M.A.; Rosado, A.; Bressan, R.A.; Hasegawa, P.M. Plant adaptive responses to salinity stress. In Plant Abiotic Stress, 2nd ed.; Jenks, M.A., Hasegawa, P.M., Eds.; Wiley: Hoboken, NJ, USA, 2005; pp. 37–70. [Google Scholar] [CrossRef]
- Rodríguez-Seijo, A.; Soares, C.; Ribeiro, S.; Amil, B.F.; Patinha, C.; Cachada, A.; Fidalgo, F.; Pereira, R. Nano-Fe2O3 as a tool to restore plant growth in contaminated soils–Assessment of potentially toxic elements (bio) availability and redox homeostasis in Hordeum vulgare L. J. Hazard. Mater. 2022, 425, 127999. [Google Scholar] [CrossRef] [PubMed]
- Jalil, S.; Alghanem, S.M.; Al-Huqail, A.A.; Nazir, M.M.; Zulfiqar, F.; Ahmed, T.; Fidalgo, F.; Jin, X. Zinc oxide nanoparticles mitigated the arsenic induced oxidative stress through modulation of physio-biochemical aspects and nutritional ions homeostasis in rice (Oryza sativa L.). Chemosphere 2023, 338, 139566. [Google Scholar] [CrossRef]
- Alinia, M.; Kazemeini, S.A.; Dadkhodaie, A.; Sepehri, M.; Mahjenabadi, V.A.J.; Amjad, S.F.; Poczai, P.; El-Ghareeb, D.; Bassouny, M.A.; Abdelhafez, A.A. Co-application of ACC deaminase-producing rhizobial bacteria and melatonin improves salt tolerance in common bean (Phaseolus vulgaris L.) through ion homeostasis. Sci. Rep. 2022, 12, 22105. [Google Scholar] [CrossRef]
- Alinia, M.; Kazemeini, S.A.; Meftahizadeh, H.; Mastinu, A. Alleviating salinity stress in Cyamopsis tetragonoloba L. seedlings through foliar application of silicon or melatonin in arid and semi-desert environments. S. Afr. J. Bot. 2024, 174, 347–359. [Google Scholar] [CrossRef]
- Zhang, Y.; Fan, Y.; Rui, C.; Zhang, H.; Xu, N.; Dai, M.; Chen, X.; Lu, X.; Wang, D.; Wang, J.; et al. Melatonin improves cotton salt tolerance by regulating ROS scavenging system and Ca2+ signal transduction. Front. Plant Sci. 2021, 12, 693690. [Google Scholar] [CrossRef]
- Younes, N.A.; El-Sherbiny, M.; Alkharpotly, A.A.; Sayed, O.A.; Dawood, A.F.; Hossain, M.A.; Abdelrhim, A.S.; Dawood, M.F. Rice-husks synthesized-silica nanoparticles modulate silicon content, ionic homeostasis, and antioxidants defense under limited irrigation regime in eggplants. Plant Stress 2024, 11, 100330. [Google Scholar] [CrossRef]
- Etesami, H.; Fatemi, H.; Rizwan, M. Interactions of nanoparticles and salinity stress at physiological, biochemical and molecular levels in plants: A review. Ecotoxicol. Environ. Saf. 2021, 225, 112769. [Google Scholar] [CrossRef] [PubMed]
- Sheikhalipour, M.; Esmaielpour, B.; Behnamian, M.; Gohari, G.; Giglou, M.T.; Vachova, P.; Rastogi, A.; Marian, B.; Skalicky, M. Chitosan–selenium nanoparticle (Cs–Se NP) foliar spray alleviates salt stress in bitter melon. Nanomaterials 2021, 11, 684. [Google Scholar] [CrossRef] [PubMed]
- Gohari, G.; Farhadi, H.; Panahirad, S.; Zareei, E.; Labib, P.; Jafari, H.; Mahdavinia, G.; Hassanpouraghdam, M.B.; Ioannou, A.; Kulak, M.; et al. Mitigation of salinity impact in spearmint plants through the application of engineered chitosan-melatonin nanoparticles. Int. J. Biol. Macromol. 2023, 224, 893–907. [Google Scholar] [CrossRef]
- Alinia, M.; Kazemeini, S.A.; Sepehri, M.; Dadkhodaie, A. Simultaneous application of rhizobium strain and melatonin improves the photosynthetic capacity and induces antioxidant defense system in common bean (Phaseolus vulgaris L.) under salinity stress. J. Plant Growth Regul. 2022, 41, 1367–1381. [Google Scholar] [CrossRef]
- Guo, S.; Wang, X.; Li, X.; Ma, Y.; Yang, J.; Fu, B.; Li, S. Melatonin and calcium synergistically improve salt tolerance in alfalfa (Medicago sativa. L). Ind. Crops Prod. 2025, 224, 120322. [Google Scholar] [CrossRef]
- Fahad, S.; Muhammad, I.; Zhang, S.; Alwahibi, M.S.; Elshikh, M.S.; Wang, J. Impact of melatonin application on wheat agronomic traits under abiotic stress: A meta-analysis. J. Agron. Crop Sci. 2025, 211, e70016. [Google Scholar] [CrossRef]
- Santo Pereira, A.E.; Silva, P.M.; Oliveira, J.L.; Oliveira, H.C.; Fraceto, L.F. Chitosan nanoparticles as carrier systems for the plant growth hormone gibberellic acid. Colloids Surf. B Biointerfaces 2017, 150, 141–152. [Google Scholar] [CrossRef]
- Bhatt, R.K.; Jukanti, A.K.; Roy, M.M. Cluster bean [Cyamopsis tetragonoloba (L.) Taub.], an important industrial arid legume: A review. Legume Res. 2017, 40, 207–214. [Google Scholar] [CrossRef]
- Teja, R.R.; Saidaiah, P.; Kumar, A.K.; Geetha, A.; Bhasker, K. Stability analysis of yield and yield attributing traits of promising genotypes of cluster bean [Cyamopsis tetragonoloba (L.) Taub.]. Legume Res. 2022, 45, 536–544. [Google Scholar] [CrossRef]
- Ghosh, D.; Das, T.; Paul, P.; Dua, T.K.; Roy, S. Zinc-loaded mesoporous silica nanoparticles mitigate salinity stress in wheat seedlings through silica-zinc uptake, osmotic balance, and ROS detoxification. Plant Physiol. Biochem. 2024, 211, 108693. [Google Scholar] [CrossRef] [PubMed]
- Cheng, B.; Hassan, M.J.; Peng, D.; Huang, T.; Peng, Y.; Li, Z. Spermidine or spermine pretreatment regulates organic metabolites and ions homeostasis in favor of white clover seed germination against salt toxicity. Plant Physiol. Biochem. 2024, 207, 108379. [Google Scholar] [CrossRef] [PubMed]
- Alsaeedi, A.H.; El-Ramady, H.; Alshaal, T.; El-Garawani, M.; Elhawat, N.; Almohsen, M. Engineered silica nanoparticles alleviate the detrimental effects of Na+ stress on germination and growth of common bean (Phaseolus vulgaris). Environ. Sci. Pollut. Res. Int. 2017, 24, 21917–21928. [Google Scholar] [CrossRef] [PubMed]
- Cheng, B.; Hassan, M.J.; Feng, G.; Zhao, J.; Liu, W.; Peng, Y.; Li, Z. Metabolites reprogramming and Na+/K+ transportation associated with putrescine-regulated white clover seed germination and seedling tolerance to salt toxicity. Front. Plant Sci. 2022, 13, 856007. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Wang, W.; He, J.; Zhang, L.; Wei, Y.; Yang, M. Nitric oxide alleviates salt stress in seed germination and early seedling growth of pakchoi (Brassica chinensis L.) by enhancing physiological and biochemical parameters. Ecotoxicol. Environ. Saf. 2020, 187, 109785. [Google Scholar] [CrossRef]
- Irik, H.A.; Bikmaz, G. Effect of different salinity on seed germination, growth parameters and biochemical contents of pumpkin (Cucurbita pepo L.) seeds cultivars. Sci. Rep. 2024, 14, 6929. [Google Scholar] [CrossRef]
- Zhang, S.; Hu, J.; Zhang, Y.; Xie, X.J.; Knapp, A. Seed priming with brassinolide improves lucerne (Medicago sativa L.) seed germination and seedling growth in relation to physiological changes under salinity stress. Aust. J. Agric. Res. 2007, 58, 811–815. [Google Scholar] [CrossRef]
- Kader, M.A. A comparison of seed germination calculation formulae and the associated interpretation of resulting data. J. Proc. R. Soc. New South Wales 2005, 138, 65–75. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Wong-Paz, J.E.; Muñiz-Márquez, D.B.; Aguilar-Zárate, P.; Rodríguez-Herrera, R.; Aguilar, C.N. Microplate quantification of total phenolic content from plant extracts obtained by conventional and ultrasound methods. Phytochem. Anal. 2014, 25, 439–444. [Google Scholar] [CrossRef]
- Czerniewicz, P.; Sytykiewicz, H.; Durak, R.; Borowiak-Sobkowiak, B.; Chrzanowski, G. Role of phenolic compounds during antioxidative responses of winter triticale to aphid and beetle attack. Plant Physiol. Biochem. 2017, 118, 529–540. [Google Scholar] [CrossRef] [PubMed]
- Horneck, D.A.; Hanson, D. Handbook of reference methods for plant analysis. In Determination of Potassium and Sodium by Flame Emission Spectrophotometry, 2nd ed.; Kalra, P.Y., Ed.; CRC Press: New York, NY, USA, 1998; pp. 153–157. [Google Scholar]
- Khan, I.; Raza, M.A.; Awan, S.A.; Shah, G.A.; Rizwan, M.; Ali, B.; Tariq, R.; Hassan, M.J.; Alyemeni, M.N.; Brestic, M.; et al. Amelioration of salt induced toxicity in pearl millet by seed priming with silver nanoparticles (AgNPs): The oxidative damage, antioxidant enzymes and ions uptake are major determinants of salt tolerant capacity. Plant Physiol. Biochem. 2020, 156, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Ben Youssef, R.; Jelali, N.; Acosta Motos, J.R.; Abdelly, C.; Albacete, A. Salicylic acid seed priming: A key frontier in conferring salt stress tolerance in barley seed germination and seedling growth. Agronomy 2025, 15, 154. [Google Scholar] [CrossRef]
- Farooq, M.A.; Islam, F.; Ayyaz, A.; Chen, W.; Noor, Y.; Hu, W.; Hannan, F.; Zhou, W. Mitigation effects of exogenous melatonin-selenium nanoparticles on arsenic-induced stress in Brassica napus. Environ. Pollut. 2022, 292, 118473. [Google Scholar] [CrossRef] [PubMed]
- Kiani, R.; Arzani, A.; Mirmohammady Maibody, S.A.M. Polyphenols, flavonoids, and antioxidant activity involved in salt tolerance in wheat, Aegilops cylindrica and their amphidiploids. Front. Plant Sci. 2021, 12, 646221. [Google Scholar] [CrossRef]
- Moradbeygi, H.; Jamei, R.; Heidari, R.; Darvishzadeh, R. Fe2O3 nanoparticles induced biochemical responses and expression of genes involved in rosmarinic acid biosynthesis pathway in Moldavian balm under salinity stress. Physiol. Plant. 2020, 169, 555–570. [Google Scholar] [CrossRef]
- Abdoli, S.; Ghassemi-Golezani, K.; Alizadeh-Salteh, S. Responses of ajowan (Trachyspermum ammi L.) to exogenous salicylic acid and iron oxide nanoparticles under salt stress. Environ. Sci. Pollut. Res. 2020, 27, 36939–36953. [Google Scholar] [CrossRef]
- Pérez-Labrada, F.; López-Vargas, E.R.; Ortega-Ortiz, H.; Cadenas-Pliego, G.; Benavides-Mendoza, A.; Juárez-Maldonado, A. Responses of tomato plants under saline stress to foliar application of copper nanoparticles. Plants 2019, 8, 151. [Google Scholar] [CrossRef]
- Junedi, M.A.; Mukhopadhyay, R.; Manjari, K.S. Alleviating salinity stress in crop plants using new engineered nanoparticles (ENPs). Plant Stress 2023, 9, 100184. [Google Scholar] [CrossRef]
- Farouk, S.; Al-Amri, S.M. Exogenous zinc forms counteract NaCl-induced damage by regulating the antioxidant system, osmotic adjustment substances, and ions in canola (Brassica napus L. cv. Pactol) plants. J. Soil Sci. Plant Nutr. 2019, 19, 887–899. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).