Organic Manure Amendment Fortifies Soil Health by Enriching Beneficial Metabolites and Microorganisms and Suppressing Plant Pathogens
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites and Sample Collection
2.2. Determination of Soil Physicochemical Properties and Enzyme Activities
2.3. Illumina Sequencing and Raw Data Processing
2.4. Metabolite Extraction and LC-MS Analyses
2.5. Validation Experiment for Beneficial Metabolites
2.6. Soil Health Index Calculation
2.7. Data Statistics and Analyses
3. Results
3.1. Effects of Organic Amendment on Soil Chemical Properties and Enzymatic Activity
3.2. Effects of Organic Amendment on Microbial Communities
3.3. Effects of Organic Amendment on Beneficial Microorganisms and Pathogens
3.4. Effects of Organic Amendment on Soil Metabolite Composition
3.5. The Soil Health Index and the Relationship Between Soil Properties, Enzymatic Activity, Soil Microbial Communities, and Metabolite Composition
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lehmann, J.; Bossio, D.A.; Kögel-Knabner, I.; Rillig, M.C. The concept and future prospects of soil health. Nat. Rev. Earth Environ. 2020, 1, 544–553. [Google Scholar] [CrossRef]
- Jansson, J.K.; Hofmockel, K.S. Soil microbiomes and climate change. Nat. Rev. Microbiol. 2020, 18, 35–46. [Google Scholar] [CrossRef]
- Philippot, L.; Chenu, C.; Kappler, A.; Rillig, M.C.; Fierer, N. The interplay between microbial communities and soil properties. Nat. Rev. Microbiol. 2024, 22, 226–239. [Google Scholar] [CrossRef]
- Bahram, M.; Hildebrand, F.; Forslund, S.K.; Anderson, J.L.; Soudzilovskaia, N.A.; Bodegom, P.M.; Bengtsson-Palme, J.; Anslan, S.; Coelho, L.P.; Harend, H.; et al. Structure and function of the global topsoil microbiome. Nature 2018, 560, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Sokol, N.W.; Slessarev, E.; Marschmann, G.L.; Nicolas, A.; Blazewicz, S.J.; Brodie, E.L.; Firestone, M.K.; Foley, M.M.; Hestrin, R.; Hungate, B.A.; et al. Life and death in the soil microbiome: How ecological processes influence biogeochemistry. Nat. Rev. Microbiol. 2022, 20, 415–430. [Google Scholar] [CrossRef]
- Hartmann, M.; Six, J. Soil structure and microbiome functions in agroecosystems. Nat. Rev. Earth Environ. 2023, 4, 4–18. [Google Scholar] [CrossRef]
- Averill, C.; Anthony, M.A.; Baldrian, P.; Finkbeiner, F.; van den Hoogen, J.; Kiers, T.; Kohout, P.; Hirt, E.; Smith, G.R.; Crowther, T.W. Defending Earth’s terrestrial microbiome. Nat. Microbiol. 2022, 7, 1717–1725. [Google Scholar] [CrossRef]
- Bünemann, E.K.; Bongiorno, G.; Bai, Z.; Creamer, R.E.; De Deyn, G.; de Goede, R.; Fleskens, L.; Geissen, V.; Kuyper, T.W.; Mäder, P.; et al. Soil quality—A critical review. Soil Biol. Biochem. 2018, 120, 105–125. [Google Scholar] [CrossRef]
- Rinot, O.; Levy, G.J.; Steinberger, Y.; Svoray, T.; Eshel, G. Soil health assessment: A critical review of current methodologies and a proposed new approach. Sci. Total Environ. 2019, 648, 1484–1491. [Google Scholar] [CrossRef] [PubMed]
- Chatzimpiros, P.; Harchaoui, S. Sevenfold variation in global feeding capacity depends on diets, land use and nitrogen management. Nat. Food 2023, 4, 372–383. [Google Scholar] [CrossRef] [PubMed]
- Xue, R.; Wang, C.; Zhao, L.; Sun, B.; Wang, B. Agricultural intensification weakens the soil health index and stability of microbial networks. Agric. Ecosyst. Environ. 2022, 339, 108118. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, J.; Manuel, D.-B.; Op de Beeck, M.; Shahbaz, M.; Chen, Y.; Deng, X.; Xu, Z.; Li, J.; Liu, Z. Rotation cropping and organic fertilizer jointly promote soil health and crop production. J. Environ. Manag. 2022, 315, 115190. [Google Scholar] [CrossRef]
- Wang, C.; Ma, Y.; He, W.; Kuzyakov, Y.; Bol, R.; Chen, H.; Fan, M. Soil quality and ecosystem multifunctionality after 13-year of organic and nitrogen fertilization. Sci. Total Environ. 2024, 931, 172789. [Google Scholar] [CrossRef]
- Zheng, X.; Wei, L.; Lv, W.; Zhang, H.; Zhang, Y.; Zhang, H.; Zhang, H.; Zhu, Z.; Ge, T.; Zhang, W. Long-term bioorganic and organic fertilization improved soil quality and multifunctionality under continuous cropping in watermelon. Agric. Ecosyst. Environ. 2024, 359, 108721. [Google Scholar] [CrossRef]
- Li, K.; Wang, C. Multiple soil quality assessment methods for evaluating effects of organic fertilization in wheat-maize rotation system. Eur. J. Agron. 2023, 150, 126929. [Google Scholar] [CrossRef]
- Walder, F.; Büchi, L.; Wagg, C.; Colombi, T.; Banerjee, S.; Hirte, J.; Mayer, J.; Six, J.; Keller, T.; Charles, R.; et al. Synergism between production and soil health through crop diversification, organic amendments and crop protection in wheat-based systems. J. Appl. Ecol. 2023, 60, 2091–2104. [Google Scholar] [CrossRef]
- Moorhead, D.; Cui, Y.; Sinsabaugh, R.; Schimel, J. Interpreting patterns of ecoenzymatic stoichiometry. Soil Biol. Biochem. 2023, 180, 108997. [Google Scholar] [CrossRef]
- Banerjee, S.; van der Heijden, M.G.A. Soil microbiomes and one health. Nat. Rev. Microbiol. 2023, 21, 6–20. [Google Scholar] [CrossRef]
- Mueller, U.G.; Sachs, J.L. Engineering Microbiomes to Improve Plant and Animal Health. Trends Microbiol. 2015, 23, 606–617. [Google Scholar] [CrossRef]
- Durán, P.; Thiergart, T.; Garrido-Oter, R.; Agler, M.; Kemen, E.; Schulze-Lefert, P.; Hacquard, S. Microbial Interkingdom Interactions in Roots Promote Arabidopsis Survival. Cell 2018, 175, 973–983.e14. [Google Scholar] [CrossRef]
- Thompson, L.R.; Sanders, J.G.; McDonald, D.; Amir, A.; Ladau, J.; Locey, K.J.; Prill, R.J.; Tripathi, A.; Gibbons, S.M.; Ackermann, G.; et al. A communal catalogue reveals Earth’s multiscale microbial diversity. Nature 2017, 551, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Ma, Q.; Marsden, K.A.; Chadwick, D.R.; Luo, Y.; Kuzyakov, Y.; Wu, L.; Jones, D.L. Microbial community succession in soil is mainly driven by carbon and nitrogen contents rather than phosphorus and sulphur contents. Soil Biol. Biochem. 2023, 180, 109019. [Google Scholar] [CrossRef]
- Li, P.; Tedersoo, L.; Crowther, T.W.; Dumbrell, A.J.; Dini-Andreote, F.; Bahram, M.; Kuang, L.; Li, T.; Wu, M.; Jiang, Y.; et al. Fossil-fuel-dependent scenarios could lead to a significant decline of global plant-beneficial bacteria abundance in soils by 2100. Nat. Food 2023, 4, 996–1006. [Google Scholar] [CrossRef]
- Põlme, S.; Abarenkov, K.; Henrik Nilsson, R.; Lindahl, B.D.; Clemmensen, K.E.; Kauserud, H.; Nguyen, N.; Kjøller, R.; Bates, S.T.; Baldrian, P.; et al. FungalTraits: A user-friendly traits database of fungi and fungus-like stramenopiles. Fungal Divers. 2020, 105, 1–16. [Google Scholar] [CrossRef]
- Bonanomi, G.; Lorito, M.; Vinale, F.; Woo, S.L. Organic Amendments, Beneficial Microbes, and Soil Microbiota: Toward a Unified Framework for Disease Suppression. Annu. Rev. Phytopathol. 2018, 56, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Wang, X.; Zhang, W.; Zhou, Z.; Ding, C.; Liao, Y.; Li, X. Persistent organic fertilization reinforces soil-borne disease suppressiveness of rhizosphere bacterial community. Plant Soil 2020, 452, 313–328. [Google Scholar] [CrossRef]
- Tao, C.; Li, R.; Xiong, W.; Shen, Z.; Liu, S.; Wang, B.; Ruan, Y.; Geisen, S.; Shen, Q.; Kowalchuk, G.A. Bio-organic fertilizers stimulate indigenous soil Pseudomonas populations to enhance plant disease suppression. Microbiome 2020, 8, 137. [Google Scholar] [CrossRef]
- Brown, R.W.; Reay, M.K.; Centler, F.; Chadwick, D.R.; Bull, I.D.; McDonald, J.E.; Evershed, R.P.; Jones, D.L. Soil metabolomics—Current challenges and future perspectives. Soil Biol. Biochem. 2024, 193, 109382. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, J.; Khashi u Rahman, M.; Gao, D.; Wei, Z.; Wu, F.; Dini-Andreote, F. Interspecific plant interaction via root exudates structures the disease suppressiveness of rhizosphere microbiomes. Mol. Plant 2023, 16, 849–864. [Google Scholar] [CrossRef] [PubMed]
- Wen, T.; Xie, P.; Liu, H.; Liu, T.; Zhao, M.; Yang, S.; Niu, G.; Hale, L.; Singh, B.K.; Kowalchuk, G.A.; et al. Tapping the rhizosphere metabolites for the prebiotic control of soil-borne bacterial wilt disease. Nat. Commun. 2023, 14, 4497. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, Ó.J.; Ospina, D.A.; Montoya, S. Compost supplementation with nutrients and microorganisms in composting process. Waste Manag. 2017, 69, 136–153. [Google Scholar] [CrossRef] [PubMed]
- Matamoros, V.; Casas, M.E.; Mansilla, S.; Tadić, Đ.; Cañameras, N.; Carazo, N.; Portugal, J.; Piña, B.; Díez, S.; Bayona, J.M. Occurrence of antibiotics in Lettuce (Lactuca sativa L.) and Radish (Raphanus sativus L.) following organic soil fertilisation under plot-scale conditions: Crop and human health implications. J. Hazard. Mater. 2022, 436, 129044. [Google Scholar] [CrossRef]
- Liu, J.; Shu, A.; Song, W.; Shi, W.; Li, M.; Zhang, W.; Li, Z.; Liu, G.; Yuan, F.; Zhang, S.; et al. Long-term organic fertilizer substitution increases rice yield by improving soil properties and regulating soil bacteria. Geoderma 2021, 404, 115287. [Google Scholar] [CrossRef]
- Cui, J.; Song, D.; Dai, X.; Xu, X.; He, P.; Wang, X.; Liang, G.; Zhou, W.; Zhu, P. Effects of long-term cropping regimes on SOC stability, soil microbial community and enzyme activities in the Mollisol region of Northeast China. Appl. Soil Ecol. 2021, 164, 103941. [Google Scholar] [CrossRef]
- Fan, F.; Yu, B.; Wang, B.; George, T.S.; Yin, H.; Xu, D.; Li, D.; Song, A. Microbial mechanisms of the contrast residue decomposition and priming effect in soils with different organic and chemical fertilization histories. Soil Biol. Biochem. 2019, 135, 213–221. [Google Scholar] [CrossRef]
- Bi, J.; Song, A.; Li, S.; Chen, M.; Wang, Y.; Wang, S.; Si, Z.; Wang, E.; Zhang, J.; Asante-Badu, B.; et al. Plant physiology, microbial community, and risks of multiple fungal diseases along a soil nitrogen gradient. Appl. Soil Ecol. 2022, 175, 104445. [Google Scholar] [CrossRef]
- Liu, Y.-X.; Chen, L.; Ma, T.; Li, X.; Zheng, M.; Zhou, X.; Chen, L.; Qian, X.; Xi, J.; Lu, H.; et al. EasyAmplicon: An easy-to-use, open-source, reproducible, and community-based pipeline for amplicon data analysis in microbiome research. iMeta 2023, 2, e83. [Google Scholar] [CrossRef] [PubMed]
- Want, E.J.; Masson, P.; Michopoulos, F.; Wilson, I.D.; Theodoridis, G.; Plumb, R.S.; Shockcor, J.; Loftus, N.; Holmes, E.; Nicholson, J.K. Global metabolic profiling of animal and human tissues via UPLC-MS. Nat. Protoc. 2013, 8, 17–32. [Google Scholar] [CrossRef] [PubMed]
- Ichihashi, Y.; Date, Y.; Shino, A.; Shimizu, T.; Shibata, A.; Kumaishi, K.; Funahashi, F.; Wakayama, K.; Yamazaki, K.; Umezawa, A.; et al. Multi-omics analysis on an agroecosystem reveals the significant role of organic nitrogen to increase agricultural crop yield. Proc. Natl. Acad. Sci. USA 2020, 117, 14552–14560. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Ye, G.; Kuzyakov, Y.; Liu, D.; Fan, J.; Ding, W. Long-term manure application increases soil organic matter and aggregation, and alters microbial community structure and keystone taxa. Soil Biol. Biochem. 2019, 134, 187–196. [Google Scholar] [CrossRef]
- Sheng, Y.; He, P.; Xu, X.; Liu, Y. A large-scale assessment on spatial variability of potato yield and soil chemical properties in northern China. Soil Tillage Res. 2023, 231, 105743. [Google Scholar] [CrossRef]
- Li, X.; Wang, D.; Ren, Y.; Wang, Z.; Zhou, Y. Soil quality assessment of croplands in the black soil zone of Jilin Province, China: Establishing a minimum data set model. Ecol. Indic. 2019, 107, 105251. [Google Scholar] [CrossRef]
- Li, X.; Li, H.; Yang, L.; Ren, Y. Assessment of Soil Quality of Croplands in the Corn Belt of Northeast China. Sustainability 2018, 10, 248. [Google Scholar] [CrossRef]
- Lai, J.; Zou, Y.; Zhang, J.; Peres-Neto, P.R. Generalizing hierarchical and variation partitioning in multiple regression and canonical analyses using the rdacca.hp R package. Methods Ecol. Evol. 2022, 13, 782–788. [Google Scholar] [CrossRef]
- Ren, J.; Liu, X.; Yang, W.; Yang, X.; Li, W.; Xia, Q.; Li, J.; Gao, Z.; Yang, Z. Rhizosphere soil properties, microbial community, and enzyme activities: Short-term responses to partial substitution of chemical fertilizer with organic manure. J. Environ. Manag. 2021, 299, 113650. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, P.; Yu, T.; Zang, H.; Zeng, Z.; Yang, Y. Manure replacement of chemical fertilizers can improve soil quality in the wheat-maize system. Appl. Soil Ecol. 2024, 200, 105453. [Google Scholar] [CrossRef]
- Hamedi, J.; Mohammadipanah, F. Biotechnological application and taxonomical distribution of plant growth promoting actinobacteria. J. Ind. Microbiol. Biotechnol. 2015, 42, 157–171. [Google Scholar] [CrossRef] [PubMed]
- Mehmood, M.A.; Fu, Y.; Zhao, H.; Cheng, J.; Xie, J.; Jiang, D. Enrichment of bacteria involved in the nitrogen cycle and plant growth promotion in soil by sclerotia of rice sheath blight fungus. Stress Biol. 2022, 2, 32. [Google Scholar] [CrossRef] [PubMed]
- Rani, V.; Bhatia, A.; Nain, L.; Tomar, G.S.; Kaushik, R. Methane utilizing plant growth-promoting microbial diversity analysis of flooded paddy ecosystem of India. World J. Microbiol. Biotechnol. 2021, 37, 56. [Google Scholar] [CrossRef]
- Chhetri, G.; Kim, I.; Kang, M.; Kim, J.; So, Y.; Seo, T. Devosia rhizoryzae sp. nov., and Devosia oryziradicis sp. nov., novel plant growth promoting members of the genus Devosia, isolated from the rhizosphere of rice plants. J. Microbiol. 2022, 60, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mukhia, S.; Kumar, A.; Kumari, P.; Kumar, R. Psychrotrophic plant beneficial bacteria from the glacial ecosystem of Sikkim Himalaya: Genomic evidence for the cold adaptation and plant growth promotion. Microbiol. Res. 2022, 260, 127049. [Google Scholar] [CrossRef] [PubMed]
- Sati, P.; Sharma, E.; Soni, R.; Dhyani, P.; Solanki, A.C.; Solanki, M.K.; Rai, S.; Malviya, M.K. Chapter 11—Bacterial endophytes as bioinoculant: Microbial functions and applications toward sustainable farming. In Microbial Endophytes and Plant Growth; Solanki, M.K., Yadav, M.K., Singh, B.P., Gupta, V.K., Eds.; Academic Press: New York, NY, USA, 2023; pp. 167–181. [Google Scholar]
- Palaniyandi, S.A.; Yang, S.H.; Zhang, L.; Suh, J.-W. Effects of actinobacteria on plant disease suppression and growth promotion. Appl. Microbiol. Biotechnol. 2013, 97, 9621–9636. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.N.; Sachan, S.G.; Verma, P.; Saxena, A.K. Prospecting cold deserts of north western Himalayas for microbial diversity and plant growth promoting attributes. J. Biosci. Bioeng. 2015, 119, 683–693. [Google Scholar] [CrossRef]
- Enebe, M.C.; Babalola, O.O. Functional diversity of bacterial communities in the rhizosphere of maize grown on a soil under organic and inorganic fertilization. Sci. Afr. 2022, 16, e01212. [Google Scholar] [CrossRef]
- Zhou, G.; Qiu, X.; Zhang, J.; Tao, C. Effects of seaweed fertilizer on enzyme activities, metabolic characteristics, and bacterial communities during maize straw composting. Bioresour. Technol. 2019, 286, 121375. [Google Scholar] [CrossRef]
- Tan, H.; Deng, Q.; Cao, L. Ruminant feces harbor diverse uncultured symbiotic actinobacteria. World J. Microbiol. Biotechnol. 2014, 30, 1093–1100. [Google Scholar] [CrossRef]
- de Gannes, V.; Eudoxie, G.; Hickey, W.J. Prokaryotic successions and diversity in composts as revealed by 454-pyrosequencing. Bioresour. Technol. 2013, 133, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, M.K.; Zhang, Z.; Wang, Q.; Shen, F.; Li, R.; Li, D.-S.; Ren, X.; Wang, M.; Chen, H.; Zhao, J. New insight with the effects of biochar amendment on bacterial diversity as indicators of biomarkers support the thermophilic phase during sewage sludge composting. Bioresour. Technol. 2017, 238, 589–601. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Wang, S.; Ni, W.; Rensing, C.; Xing, S. Enhanced Cd-Zn-Pb-contaminated soil phytoextraction by Sedum alfredii and the rhizosphere bacterial community structure and function by applying organic amendments. Plant Soil 2019, 444, 101–118. [Google Scholar] [CrossRef]
- Ma, L.-J.; van der Does, H.C.; Borkovich, K.A.; Coleman, J.J.; Daboussi, M.-J.; Di Pietro, A.; Dufresne, M.; Freitag, M.; Grabherr, M.; Henrissat, B.; et al. Comparative genomics reveals mobile pathogenicity chromosomes in Fusarium. Nature 2010, 464, 367–373. [Google Scholar] [CrossRef]
- Liu, Z.; Guo, Q.; Feng, Z.; Liu, Z.; Li, H.; Sun, Y.; Liu, C.; Lai, H. Long-term organic fertilization improves the productivity of kiwifruit (Actinidia chinensis Planch.) through increasing rhizosphere microbial diversity and network complexity. Appl. Soil Ecol. 2020, 147, 103426. [Google Scholar] [CrossRef]
- Tao, R.; Liang, Y.; Wakelin, S.A.; Chu, G. Supplementing chemical fertilizer with an organic component increases soil biological function and quality. Appl. Soil Ecol. 2015, 96, 42–51. [Google Scholar] [CrossRef]
- Liu, H.; Xiong, W.; Zhang, R.; Hang, X.; Wang, D.; Li, R.; Shen, Q. Continuous application of different organic additives can suppress tomato disease by inducing the healthy rhizospheric microbiota through alterations to the bulk soil microflora. Plant Soil 2018, 423, 229–240. [Google Scholar] [CrossRef]
- Couteaudier, Y.; Alabouvette, C. Quantitative comparison of Fusarium oxysporum competitiveness in relation to carbon utilization. FEMS Microbiol. Lett. 1990, 74, 261–267. [Google Scholar] [CrossRef]
- Xiong, W.; Li, R.; Ren, Y.; Liu, C.; Zhao, Q.; Wu, H.; Jousset, A.; Shen, Q. Distinct roles for soil fungal and bacterial communities associated with the suppression of vanilla Fusarium wilt disease. Soil Biol. Biochem. 2017, 107, 198–207. [Google Scholar] [CrossRef]
- Du, T.; Hu, Q.; He, H.; Mao, W.; Yang, Z.; Chen, H.; Sun, L.; Zhai, M. Long-term organic fertilizer and biofertilizer application strengthens the associations between soil quality index, network complexity, and walnut yield. Eur. J. Soil Biol. 2023, 116, 103492. [Google Scholar] [CrossRef]
- Liu, X.; Liu, H.; Zhang, Y.; Liu, C.; Liu, Y.; Li, Z.; Zhang, M. Organic amendments alter microbiota assembly to stimulate soil metabolism for improving soil quality in wheat-maize rotation system. J. Environ. Manag. 2023, 339, 117927. [Google Scholar] [CrossRef]
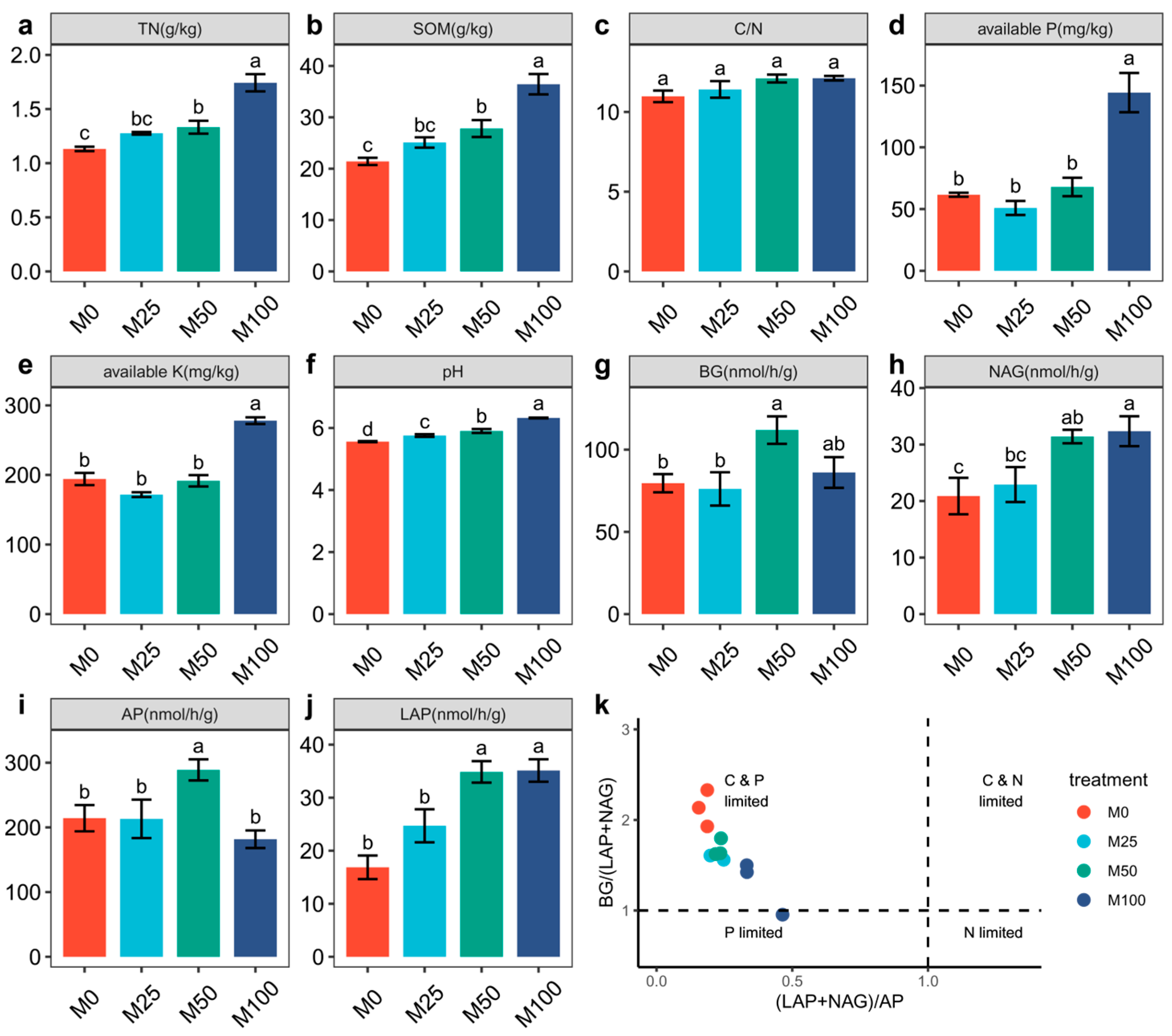
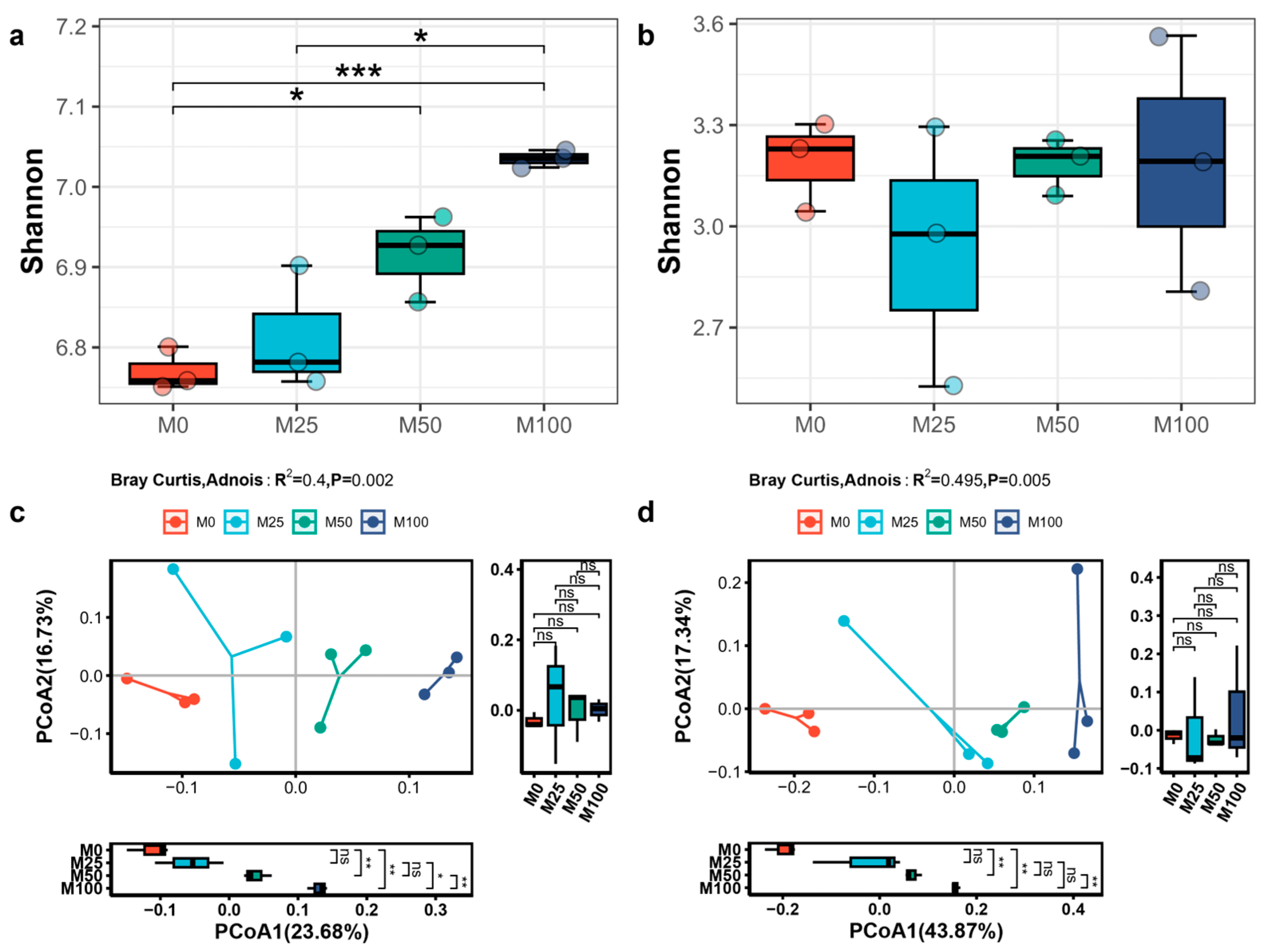
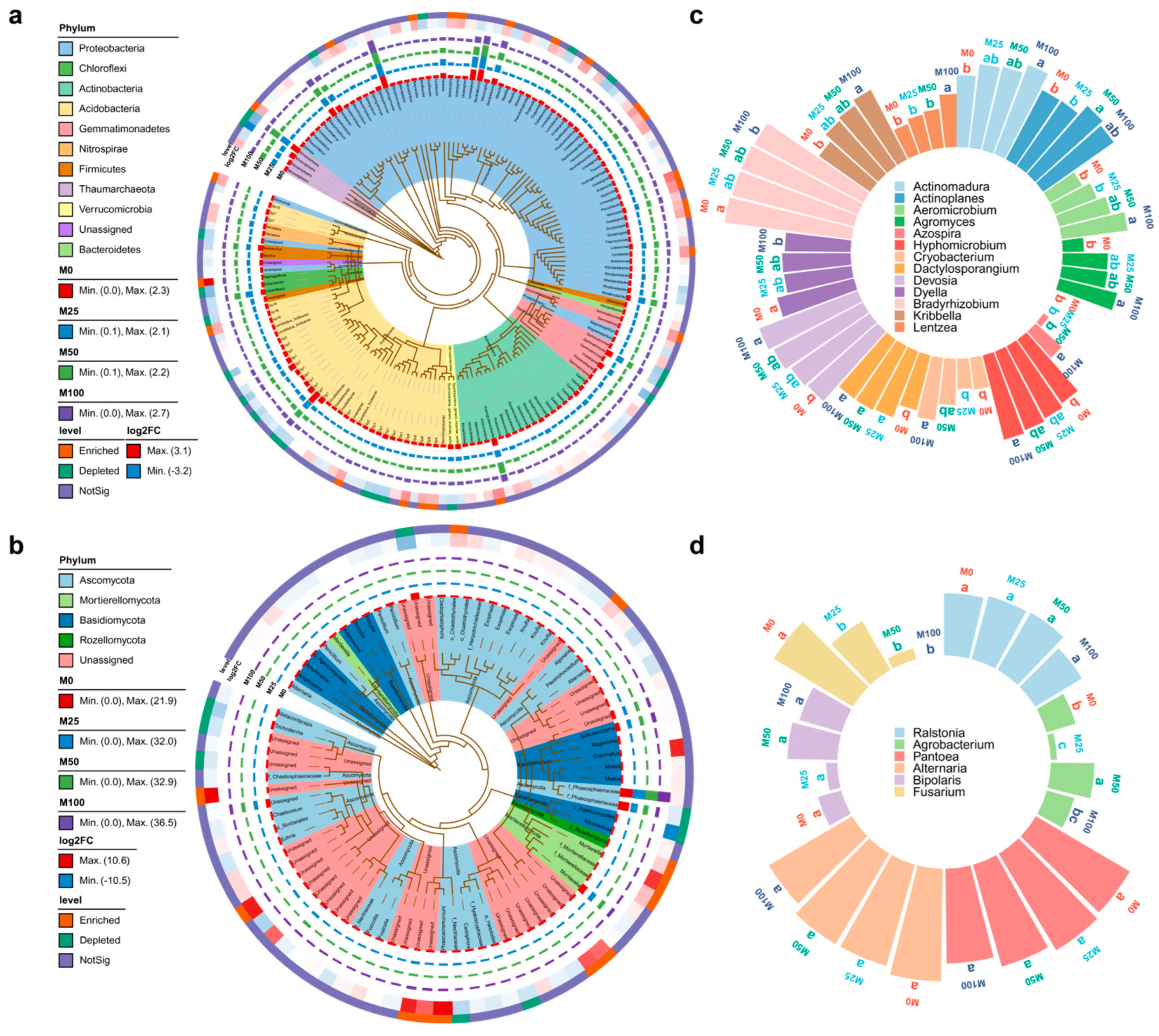
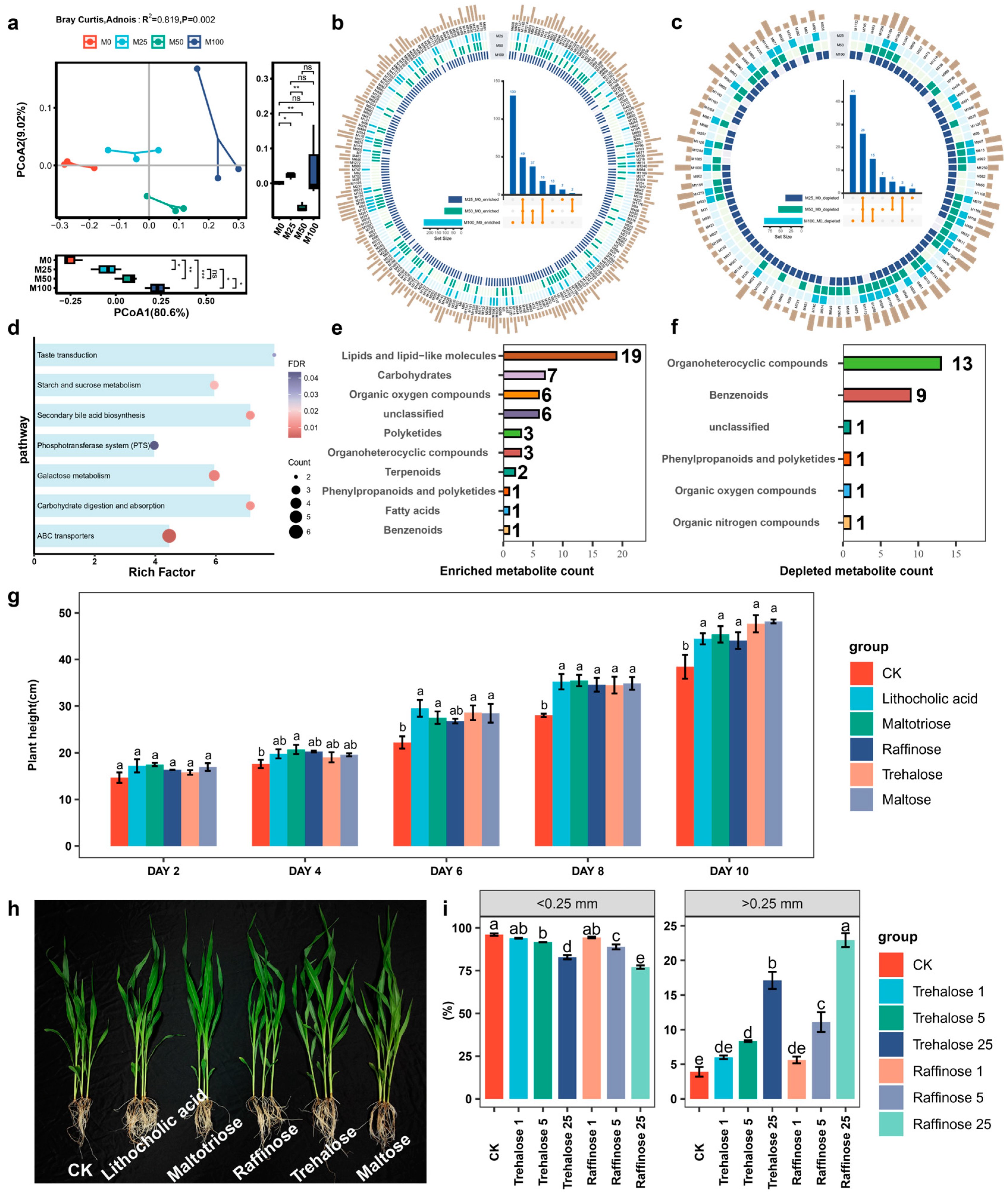

| Indicator | Weight | Function | x1 | x2 | x3 | x4 |
|---|---|---|---|---|---|---|
| total nitrogen | 0.201 | U (x) | 0.75 | 2.86 | ||
| soil organic matter | 0.201 | U (x) | 10 | 50 | ||
| available phosphorus | 0.201 | U (x) | 1.40 | 137.51 | ||
| available potassium | 0.201 | U (x) | 20 | 435 | ||
| pH | 0.196 | P (x) | 4.5 | 6.5 | 7.0 | 9.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, B.; Bi, J.; Qian, X.; Peng, C.; Sun, M.; Wang, E.; Liu, X.; Zeng, X.; Feng, H.; Song, A.; et al. Organic Manure Amendment Fortifies Soil Health by Enriching Beneficial Metabolites and Microorganisms and Suppressing Plant Pathogens. Agronomy 2025, 15, 429. https://doi.org/10.3390/agronomy15020429
Wei B, Bi J, Qian X, Peng C, Sun M, Wang E, Liu X, Zeng X, Feng H, Song A, et al. Organic Manure Amendment Fortifies Soil Health by Enriching Beneficial Metabolites and Microorganisms and Suppressing Plant Pathogens. Agronomy. 2025; 15(2):429. https://doi.org/10.3390/agronomy15020429
Chicago/Turabian StyleWei, Buqing, Jingjing Bi, Xueyan Qian, Chang Peng, Miaomiao Sun, Enzhao Wang, Xingyan Liu, Xian Zeng, Huaqi Feng, Alin Song, and et al. 2025. "Organic Manure Amendment Fortifies Soil Health by Enriching Beneficial Metabolites and Microorganisms and Suppressing Plant Pathogens" Agronomy 15, no. 2: 429. https://doi.org/10.3390/agronomy15020429
APA StyleWei, B., Bi, J., Qian, X., Peng, C., Sun, M., Wang, E., Liu, X., Zeng, X., Feng, H., Song, A., & Fan, F. (2025). Organic Manure Amendment Fortifies Soil Health by Enriching Beneficial Metabolites and Microorganisms and Suppressing Plant Pathogens. Agronomy, 15(2), 429. https://doi.org/10.3390/agronomy15020429







