Drought Stress Affects Spectral Separation of Maize Infested by Western Corn Rootworm
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Germplasm
2.1.1. AAPF Experimental Design
2.1.2. GH Experimental Design
2.2. Spectral Data Collection and Calculation of Indices
2.3. Maize Physiological Reference Measurements
2.3.1. Chlorophyll Content and Gas Exchange
2.3.2. Water Status and Leaf Thickness
2.4. Chemometric Modeling
2.5. Root Injury Assessment
2.6. Statistical Analyses
3. Results
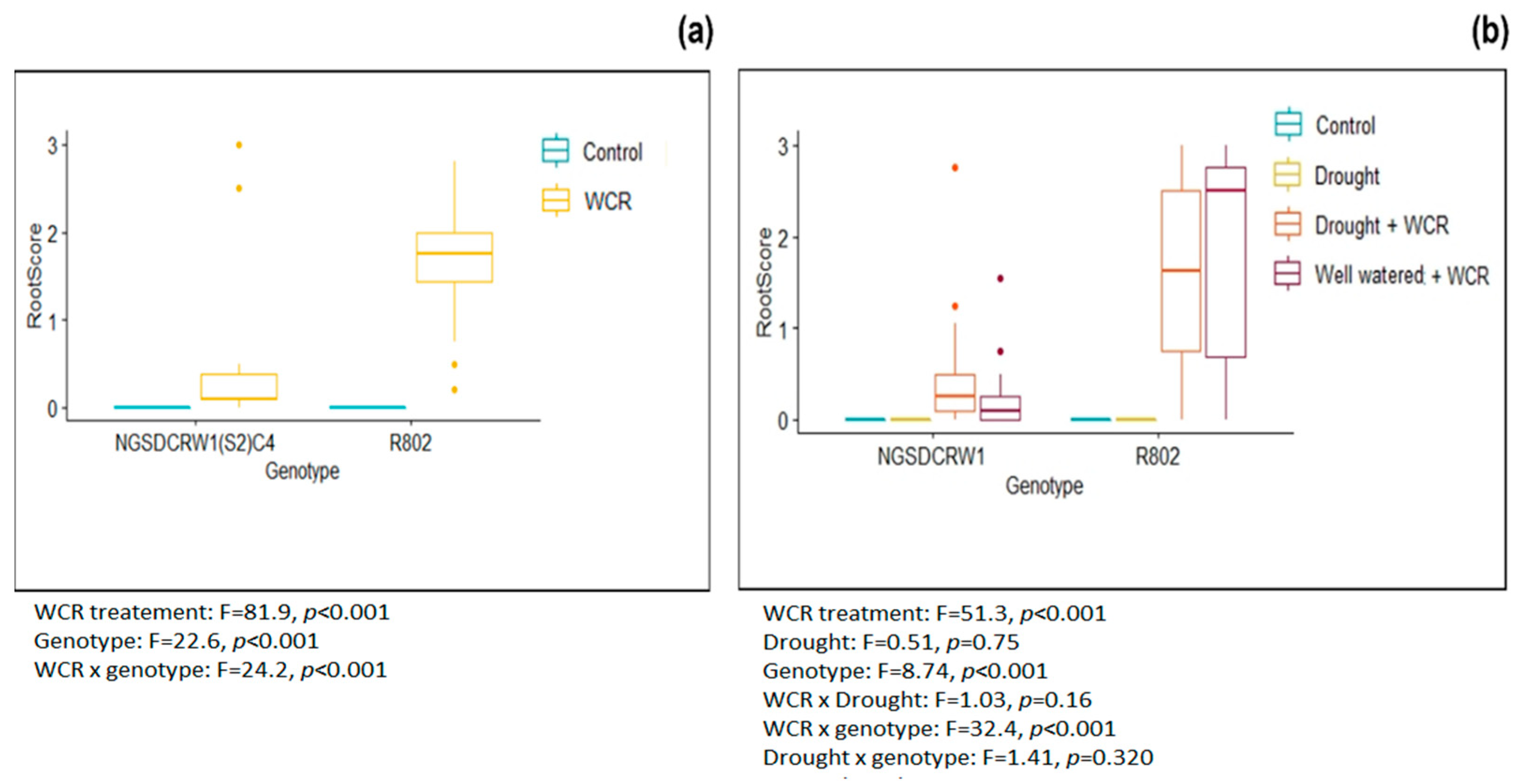
3.1. Root Injury Assessment
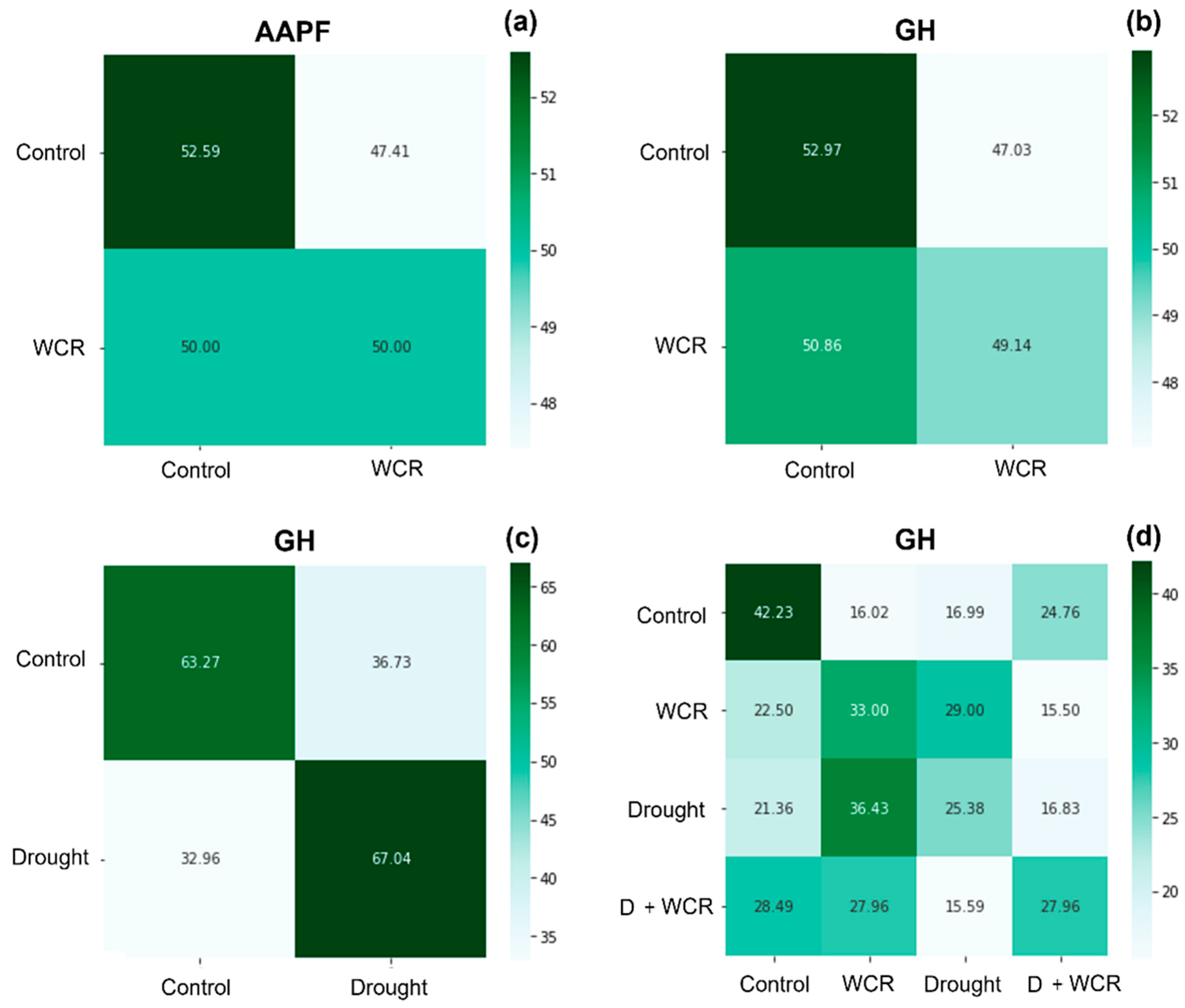
3.2. Spectral Separation and Classification among Different Treatment Combinations
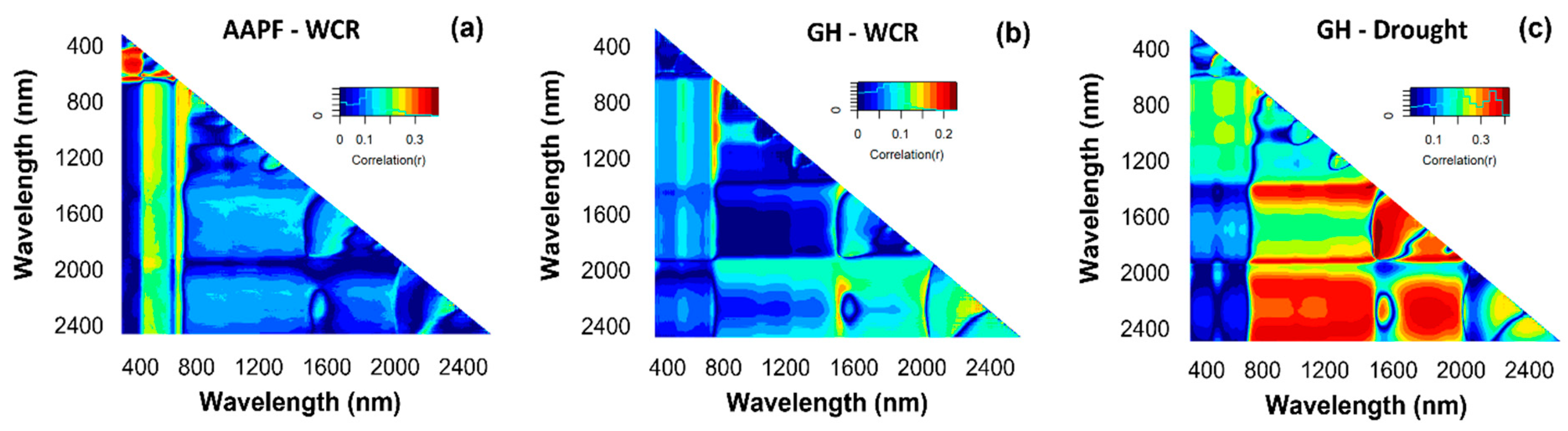
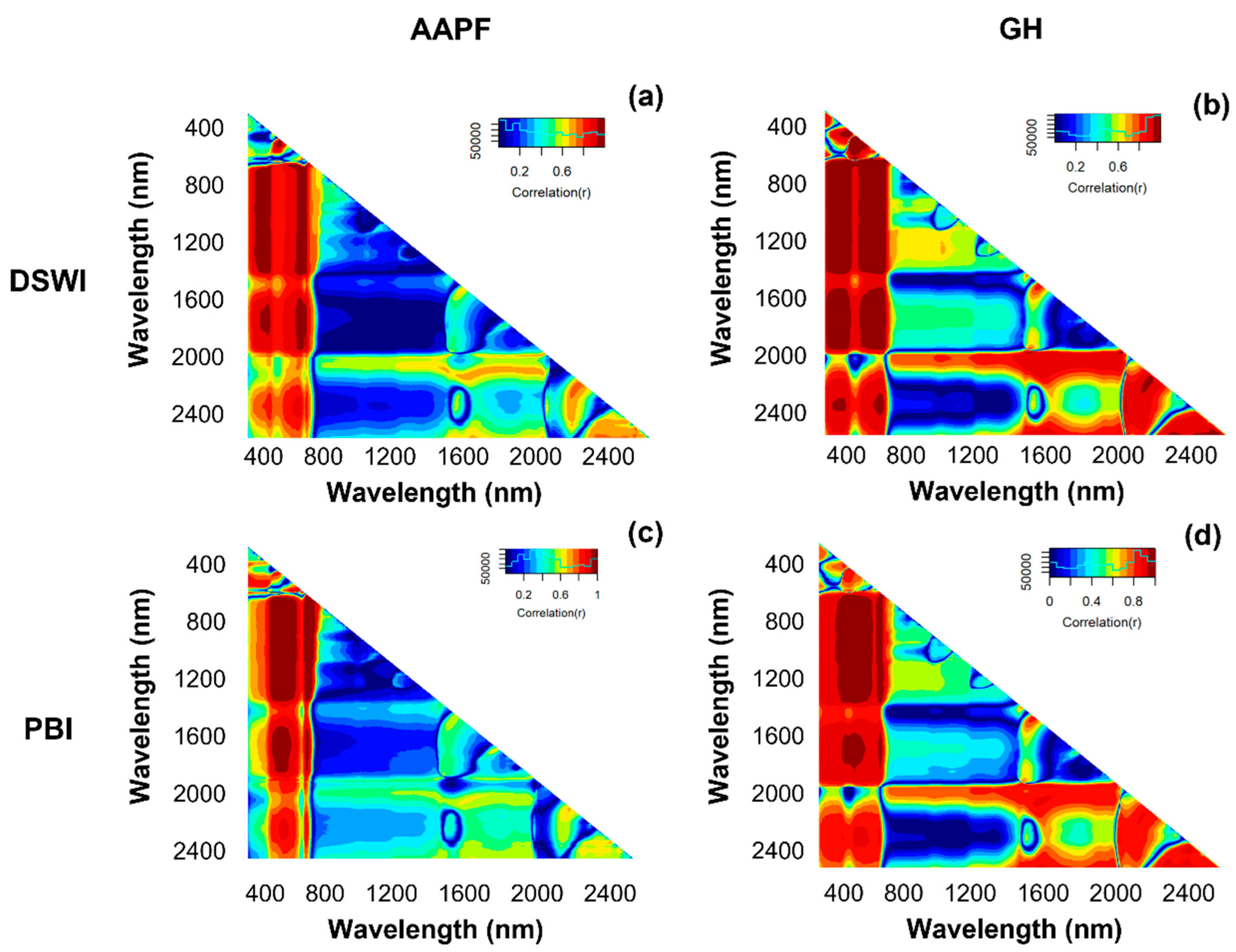
3.3. Relationships between the Normalized Differential Spectral Index (NDSI), Treatment Combinations, and Vegetation Indices (VI)
3.4. Functional Traits
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Example Image of Root Damage and Analysis of Root Damage

Appendix B. Post Hoc Analysis of Significant PERMANOVA Terms, Vegetation Indices, Chemometric Modeling Performance Metrics, and Functional Trait Values Calculated from the Spectral Data
| Experiment | WCR | posthoc comparison | Genotype | posthoc comparison | ||||
| AAPF | WCR+ | a | NGS | a | ||||
| WCR− | b | R802 | b | |||||
| Experiment | Drought | posthoc comparison | Genotype | posthoc comparison | WCR × drought | posthoc comparison | Drought × genotype | posthoc comparison |
| GH | Drought+ | a | NGS | a | WCR−, Drought− | a | NGS,0 | a |
| WCR+, Drought− | bc | NGS,1 | b | |||||
| Drought− | b | R802 | b | WCR−, Drought+ | c | R802,0 | a | |
| WCR+, Drought+ | c | R802,1 | a |
| Genotype | Treatments | PRI | NDWI | DSWI | PBI | ARI | HI | WBI | MCARI | |
|---|---|---|---|---|---|---|---|---|---|---|
| AAPF | NGS | Control | 0.004 ± 0.002 | 0.046 ± 0.001 | 4.823 ± 0.103 | 2.996 ± 0.083 | 0.334 ± 0.016 | 0.032 ± 0.005 | 0.956 ± 0.001 | 0.154 ± 0.009 |
| WCR | 0.001 ± 0.001 | 0.044 ± 0.001 | 4.685 ± 0.092 | 2.720 ± 0.074 | 0.317 ± 0.014 | 0.0321 ± 0.005 | 0.957 ± 0.001 | 0.187 ± 0.008 | ||
| R802 | Control | 0.007 ± 0.001 | 0.048 ± 0.001 | 4.695 ± 0.090 | 2.933 ± 0.073 | 0.329 ± 0.016 | 0.046 ± 0.005 | 0.953 ± 0.001 | 0.161 ± 0.008 | |
| WCR | 0.009 ± 0.002 | 0.049 ± 0.001 | 4.776 ± 0.106 | 2.924 ± 0.083 | 0.324 ± 0.014 | 0.041 ± 0.006 | 0.954 ± 0.001 | 0.168 ± 0.009 | ||
| GH | NGS | Control | 0.019 ± 0.001 | 0.046 ± 0.002 | 4.852 ± 0.238 | 3.760 ± 0.106 | 0.384 ± 0.025 | 0.064 ± 0.007 | 0.954 ± 0.002 | 0.070 ± 0.003 |
| WCR | 0.016 ± 0.001 | 0.048 ± 0.002 | 4.224 ± 0.238 | 3.362 ± 0.106 | 0.353 ± 0.025 | 0.042 ± 0.007 | 0.953 ± 0.002 | 0.078 ± 0.003 | ||
| Drought | 0.009 ± 0.001 | 0.043 ± 0.001 | 3.473 ± 0.168 | 2.903 ± 0.075 | 0.251 ± 0.018 | 0.015 ± 0.005 | 0.957 ± 0.002 | 0.080 ± 0.002 | ||
| WCR + Drought | 0.008 ± 0.001 | 0.045 ± 0.001 | 3.378 ± 0.168 | 2.877 ± 0.075 | 0.233 ± 0.018 | 0.013 ± 0.005 | 0.955 ± 0.002 | 0.077 ± 0.002 | ||
| R802 | Control | 0.018 ± 0.001 | 0.050 ± 0.002 | 4.716 ± 0.238 | 3.445 ± 0.106 | 0.319 ± 0.025 | 0.068 ± 0.007 | 0.949 ± 0.001 | 0.080 ± 0.003 | |
| WCR | 0.017 ± 0.001 | 0.041 ± 0.001 | 4.191 ± 0.238 | 3.199 ± 0.106 | 0.282 ± 0.025 | 0.057 ± 0.007 | 0.951 ± 0.001 | 0.081 ± 0.003 | ||
| Drought | 0.015 ± 0.009 | 0.044 ± 0.001 | 4.491 ± 0.168 | 3.425 ± 0.075 | 0.325 ± 0.018 | 0.059 ± 0.005 | 0.957 ± 0.001 | 0.076 ± 0.002 | ||
| WCR + Drought | 0.016 ± 0.001 | 0.044 ± 0.001 | 4.685 ± 0.170 | 3.378 ± 0.075 | 0.337 ± 0.018 | 0.013 ± 0.005 | 0.955 ± 0.001 | 0.083 ± 0.002 |
| Calibration | CV | EV | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Trait | LV | R2 | RMSE | NRMSE (%) | Bias | R2 | RMSE | NRMSE (%) | Bias | R2 | RMSE | NRMSE (%) | Bias |
| A | 17 | 0.94 ± 0.00 | 3.71 ± 0.13 | 8.5 | 1.05 × 10−14 ± 0.00 | 0.84 ± 0.04 | 6.21 ± 0.68 | 14.3 | 0.311 ± 1.21 | 0.77 | 8.07 | 18.1 | 0.66 |
| E | 15 | 0.90 ± 0.00 | 0.47 ± 0.01 | 9.7 | −1.5 × 10−16 ± 0.00 | 0.79 ± 0.05 | 0.68 ± 0.07 | 14.1 | 0.003 ± 0.13 | 0.65 | 1.03 | 18.1 | 0.04 |
| gs | 17 | 0.90 ± 0.00 | 0.05 ± 0.00 | 7.6 | 1.39 × 10−17 ± 0.00 | 0.75 ± 0.05 | 0.08 ± 0.00 | 12.8 | 0.001 ± 0.01 | 0.65 | 0.1 | 20.4 | −0.01 |
| RWC | 15 | 0.91 ± 0.01 | 0.04 ± 0.00 | 5.3 | 3.89 × 10−18 ± 0.00 | 0.79 ± 0.08 | 0.06 ± 0.01 | 7.6 | 0.001 ± 0.01 | 0.86 | 0.05 | 9.8 | −0.01 |
| Ci | 18 | 0.94 ± 0.05 | 18.36 ± 0.69 | 6.5 | −6.2 × 10−15 ± 0.00 | 0.84 ± 0.05 | 30.02 ± 3.23 | 10.6 | 0.379 ± 5.01 | 0.73 | 47.36 | 16.1 | −1.93 |
| Tleaf | 19 | 0.96 ± 0.00 | 0.47 ± 0.01 | 5.1 | 1.3 × 10−16 ± 0.00 | 0.88 ± 0.03 | 0.86 ± 0.10 | 9.3 | −0.026 ± 0.16 | 0.71 | 1.31 | 14.6 | 0.39 |
| SLA | 13 | 0.78 ± 0.02 | 22.3 ± 0.83 | 4.4 | −6.18 × 10−16 ± 0.00 | 0.60 ± 0.09 | 31.8 ± 3.58 | 6.2 | 0.082 ± 6.89 | 0.67 | 28.56 | 5.7 | 1.98 |
| WUE | 17 | 0.85 ± 0.01 | 14.50 ± 0.45 | 10.6 | 1.1 × 10−14 ± 0.00 | 0.65 ± 0.09 | 22.77 ± 2.08 | 17.6 | −0.534 ± 4.57 | 0.50 | 30.99 | 34.3 | 3.57 |
| LWP | 9 | 0.78 ± 0.01 | 2.48 ± 0.07 | 3.5 | −3.8 × 10−16 ± 0.00 | 0.71 ± 0.06 | 2.91 ± 0.30 | 4.1 | −0.038 ± 0.61 | 0.50 | 4.07 | 20.4 | 0.42 |
| LOP | 17 | 0.84 ± 0.02 | 49.71 ± 1.50 | 5.4 | 5.51 × 10−14 ± 0.00 | 0.64 ± 0.11 | 78.72 ± 6.36 | 8.6 | −1.880 ± 12.51 | 0.47 | 85.52 | 16.0 | 7.32 |
| SPAD | 11 | 0.73 ± 0.01 | 2.41 ± 0.06 | 8.4 | −1.7 × 10−18 ± 0.00 | 0.61 ± 0.09 | 2.93 ± 0.28 | 10.3 | −0.060 ± 0.56 | 0.33 | 4.62 | 20.2 | 0.48 |
| Genotype | Treatments | A | Transp | Ci | RWC | Tleaf | Cond | SLA | |
|---|---|---|---|---|---|---|---|---|---|
| AAPF | NGS | Control | 10.4 ± 4.9 | 0.63 ± 0.7 | 273.3 ± 33.2 | 0.79 ± 0.06 | 27.1 ± 0.2 | −0.03 ± 0.06 | 344.7 ± 28.5 |
| WCR | 7.7 ± 4.9 | 0.23 ± 0.7 | 302.9 ± 33.2 | 0.71 ± 0.06 | 28.4 ± 0.2 | −0.09 ± 0.06 | 309.9 ± 28.5 | ||
| R802 | Control | 10.0 ± 4.9 | 0.76 ± 0.6 | 264.0 ± 31.8 | 0.84 ± 0.05 | 30.0 ± 0.2 | −0.06 ± 0.05 | 413.0 ± 28.95 | |
| WCR | 7.5 ± 4.9 | 0.63 ± 0.7 | 314.1 ± 33.2 | 0.76 ± 0.06 | 30.5 ± 0.2 | −0.14 ± 0.06 | 405.7 ± 28.9 | ||
| GH | NGS | Control | 37.1 ± 2.2 | 3.98 ± 0.2 | 156.5 ± 15.2 | 0.95 ± 0.02 | 28.5 ± 0.5 | 0.37 ± 0.02 | 364.8 ± 17.4 |
| WCR | 36.9 ± 2.1 | 3.80 ± 0.2 | 147.0 ± 14.8 | 0.95 ± 0.02 | 528.5 ± 0.5 | 0.35 ± 0.02 | 424.1 ± 11.9 | ||
| Drought | 5.1 ± 1.5 | 0.63 ± 0.1 | 305.9 ± 10.5 | 0.68 ± 0.01 | 28.5 ± 0.4 | 0.05 ± 0.01 | 390.7 ± 16.8 | ||
| WCR + Drought | 6.96 ± 1.5 | 0.79 ± 0.1 | 272.4 ± 10.5 | 0.70 ± 0.01 | 28.7 ± 0.4 | 0.06 ± 0.01 | 397.9 ± 11.9 | ||
| R802 | Control | 37.5 ± 2.1 | 4.01 ± 0.2 | 165.4 ± 14.8 | 0.94 ± 0.02 | 28.0 ± 0.5 | 0.40 ± 0.02 | 378.3 ± 16.8 | |
| WCR | 33.3 ± 2.1 | 3.71 ± 0.2 | 164.9 ± 14.8 | 0.95 ± 0.02 | 28.6 ± 0.5 | 0.33 ± 0.02 | 422.1 ± 11.9 | ||
| Drought | 17.4 ± 1.5 | 1.82 ± 0.1 | 181.1 ± 10.6 | 0.88 ± 0.01 | 28.1 ± 0.4 | 0.16 ± 0.01 | 411.0 ± 16.7 | ||
| WCR+Drought | 18.1 ± 1.5 | 1.93 ± 0.1 | 181.1 ± 10.6 | 0.90 ± 0.01 | 28.5 ± 0.4 | 0.16 ± 0.01 | 433.6 ± 12.2 |
References
- Conklin, A.R.; Stilwell, T. (Eds.) Grain Crops. In World Food: Production and Use; Wiley: Hoboken, NJ, USA, 2007; pp. 77–127. [Google Scholar]
- Shiferaw, B.; Prasanna, B.M.; Hellin, J.; Bänziger, M. Crops that feed the world 6. Past successes and future challenges to the role played by maize in global food security. Food Secur. 2011, 3, 307–327. [Google Scholar] [CrossRef]
- Erenstein, O.; Jaleta, M.; Sonder, K.; Mottaleb, K.; Prasanna, B.M. Global maize production, consumption and trade: Trends and R&D implications. Food Secur. 2022, 14, 1295–1319. [Google Scholar]
- FAO. Statistical Yearbook of the Food and Agricultural Organization for Feeding the World. In FAO Statistical Yearbook 2013. 2013. Available online: www.fao.org/giews/english/fo/index.htm (accessed on 15 February 2019).
- Spencer, J.L.; Hibbard, B.E.; Moeser, J.; Onstad, D.W. Behavior and ecology of the western corn rootworm (Diabrotica virgifera virgifera LeConte). Agric. For. Entomol. 2009, 11, 9–27. [Google Scholar]
- Rice, M.E. Transgenic rootworm corn: Assessing potential agronomic, economic, and environmental benefits. Plant Health Prog. 2004, 5, 12. [Google Scholar] [CrossRef]
- Wechsler, S.; Smith, D. Has resistance taken root in US corn fields? Demand for insect control. Am. J. Agric. Econ. 2018, 100, 1136–1150. [Google Scholar] [CrossRef]
- Tinsley, N.A.; Estes, R.E.; Gray, M.E. Validation of a nested error component model to estimate damage caused by corn rootworm larvae. J. Appl. Entomol. 2013, 137, 161–169. [Google Scholar] [CrossRef]
- Jakka, S.R.K.; Shrestha, R.B.; Gassmann, A.J. Broad-spectrum resistance to Bacillus thuringiensis toxins by western corn rootworm (Diabrotica virgifera virgifera). Sci. Rep. 2016, 6, 27860. [Google Scholar] [CrossRef] [PubMed]
- Cullen, E.M.; Gray, M.E.; Gassmann, A.J.; Hibbard, B.E. Resistance to Bt corn by western corn rootworm (Coleoptera: Chrysomelidae) in the U.S. Corn Belt. J. Integr. Pest Manag. 2013, 4, D1–D6. [Google Scholar] [CrossRef]
- Gassmann, A.J.; Shrestha, R.B.; Jakka, S.R.K.; Dunbar, M.W.; Clifton, E.H.; Paolino, A.R.; Ingber, D.A.; French, B.W.; Masloski, K.E.; Dounda, J.W.; et al. Evidence of resistance to Cry34/35Ab1 corn by western corn rootworm (Coleoptera: Chrysomelidae): Root injury in the field and larval survival in plant-based bioassays. J. Econ. Entomol. 2016, 109, 1872–1880. [Google Scholar] [CrossRef]
- Gassmann, A.J.; Petzold-Maxwell, J.L.; Keweshan, R.S.; Dunbar, M.W. Western corn rootworm and Bt maize. GM Crops Food 2012, 3, 235–244. [Google Scholar] [CrossRef]
- Moar, W.; Khajuria, C.; Pleau, M.; Ilagan, O.; Chen, M.; Jiang, C.; Prics, P.; McNulty, B.; Clark, T.; Head, G. Cry3Bb1-Resistant western corn rootworm, Diabrotica virgifera virgifera (LeConte) does not exhibit cross-resistance to DvSnf7 dsRNA. PLoS ONE 2017, 12, e0169175. [Google Scholar] [CrossRef]
- Sankaran, S.; Mishra, A.; Ehsani, R.; Davis, C. A review of advanced techniques for detecting plant diseases. Comput. Electron. Agric. 2010, 72, 1–13. [Google Scholar] [CrossRef]
- Nowatzki, T.M.; Tollefson, J.J.; Calvin, D.D. Development and validation of models for predicting the seasonal emergence of corn rootworm (Coleoptera: Chrysomelidae) beetles in Iowa. Environ. Entomol. 2002, 31, 864–873. [Google Scholar] [CrossRef]
- Aslam, M.; Maqbool, M.A.; Cengiz, R. Drought stress in Maize (Zea Mays L.); Springer International Publishing: Cham, Switzerland, 2015. [Google Scholar]
- Mahmoud, A.M.B. Effects of Western Corn Rootworm Larval Feeding, Drought, and Their Interaction on Maize Performance and Rootworm Development. Ph.D. Thesis, University of Missouri, Columbia, MO, USA, 2015. [Google Scholar]
- Mahmoud, M.A.B.; Sharp, R.E.; Oliver, M.J.; Finke, D.L.; Bohn, M.; Ellersieck, M.R.; Hibbard, B.E. Response of maize hybrids with and without rootworm- and drought-tolerance to rootworm infestation under well-watered and drought conditions. J. Econ. Entomol. 2018, 111, 193–208. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, M.A.B.; Sharp, R.E.; Oliver, M.J.; Finke, D.L.; Ellersieck, M.R.; Hibbard, B.E. The effect of western corn rootworm (Coleoptera: Chrysomelidae) and water deficit on maize performance under controlled conditions. J. Econ. Entomol. 2016, 10, 684–698. [Google Scholar] [CrossRef]
- Mahlein, A.-K.; Steiner, U.; Hillnhütter, C.; Dehne, H.-W.; Oerke, E.-C. Hyperspectral imaging for small-scale analysis of symptoms caused by different sugar beet diseases. Plant Methods 2012, 8, 3. [Google Scholar] [CrossRef] [PubMed]
- Mutka, A.M.; Bart, R.S. Image-based phenotyping of plant disease symptoms. Front. Plant Sci. 2015, 5, 734. [Google Scholar] [CrossRef]
- Cotrozzi, L.; Couture, J.J.; Cavender-Bares, J.; Kingdon, C.C.; Fallon, B.; Pilz, G.; Pellegrini, E.; Nalia, C.; Townsend, P.A. Using foliar spectral properties to assess the effects of drought on plant water potential. Tree Physiol. 2017, 184, 1582–1591. [Google Scholar] [CrossRef]
- Susič, N.; Žibrat, U.; Širca, S.; Strajnar, P.; Razinger, J.; Knapič, M.; Vončina, M.; Urek, G.; Gerič Stare, B. Discrimination between abiotic and biotic drought stress in tomatoes using hyperspectral imaging. Sens. Actuators B Chem. 2018, 273, 842–852. [Google Scholar] [CrossRef]
- Zarco-Tejada, P.J.; Camino, C.; Beck, P.S.A.; Calderon, R.; Hornero, A.; Hernández-Clemente, R.; Kattenborn, T.; Montes-Borrego, B.; Susca, L.; Morelli, M.; et al. Previsual symptoms of Xylella fastidiosa infection revealed in spectral plant-trait alterations. Nat. Plants 2018, 4, 432–439. [Google Scholar] [CrossRef]
- Couture, J.J.; Singh, A.; Charkowski, A.O.; Groves, R.L.; Gray, S.M.; Bethke, P.C.; Townsend, P.A. Integrating spectroscopy with potato disease management. Plant Dis. 2018, 102, 2233–2240. [Google Scholar] [CrossRef] [PubMed]
- do Prado Ribeiro, L.; Klock, A.L.S.; Filho, J.A.W.; Tramontin, M.A.; Trapp, M.A.; Mithöfer, A.; Nansen, C. Hyperspectral imaging to characterize plant-plant communication in response to insect herbivory. Plant Methods 2018, 14, 54. [Google Scholar] [CrossRef] [PubMed]
- Nagasubramanian, K.; Jones, S.; Sarkar, S.; Singh, A.K.; Singh, A.; Ganapathysubramanian, B. Hyperspectral band selection using genetic algorithm and support vector machines for early identification of charcoal rot disease in soybean stems. Plant Methods 2018, 14, 86. [Google Scholar] [CrossRef] [PubMed]
- Campos-Medina, V.A.; Cotrozzi, L.; Stuart, J.J.; Couture, J.J. Spectral characterization of wheat functional trait responses to Hessian fly: Mechanisms for trait-based resistance. PLoS ONE 2019, 14, e0219431. [Google Scholar] [CrossRef]
- Yao, Z.; Lei, Y.; He, D. Early visual detection of wheat stripe rust using visible/near-infrared hyperspectral imaging. Sensors 2019, 19, 952. [Google Scholar] [CrossRef]
- Cotrozzi, L.; Couture, J.J. Hyperspectral assessment of plant responses to multi-stress environments: Prospects for managing protected agrosystems. Plants People Planet 2020, 2, 244–258. [Google Scholar] [CrossRef]
- Fallon, B.; Yang, A.; Lapadat, C.; Armour, I.; Juzwik, J.; Montgomery, R.A.; Cavender-Bares, J. Spectral differentiation of oak wilt from foliar fungal disease and drought is correlated with physiological changes. Tree Physiol. 2020, 40, 377–390. [Google Scholar] [CrossRef]
- Gold, K.M.; Townsend, P.A.; Chlus, A.; Herrmann, I.; Couture, J.J.; Larson, E.R.; Gevens, A.J. Hyperspectral measurements enable pre-symptomatic detection and differentiation of contrasting physiological effects of late blight and early blight in potato. Remote Sens. 2020, 12, 286. [Google Scholar] [CrossRef]
- Gold, K.M.; Townsend, P.A.; Herrmann, I.; Gevens, A.J. Investigating potato late blight physiological differences across potato cultivars with spectroscopy and machine learning. Plant Sci. 2020, 295, 110316. [Google Scholar] [CrossRef]
- Pérez-Roncal, C.; López-Maestresalas, A.; Lopez-Molina, C.; Jarén, C.; Urrestarazu, J.; Santesteban, L.G.; Arazuri, S. Hyperspectral imaging to assess the presence of powdery mildew (Erysiphe necator) in cv. Carignan noir grapevine bunches. Agronomy 2020, 10, 88. [Google Scholar] [CrossRef]
- Calamita, F.; Imran, H.A.; Vescovo, L.; Mekhalfi, M.L.; La Porta, N. Early identification of root rot disease by using hyperspectral reflectance: The case of pathosystem grapevine/armillaria. Remote Sens. 2021, 13, 2436. [Google Scholar] [CrossRef]
- Reynolds, G.J.; Windels, C.E.; MacRae, I.V.; Laguette, S. Remote sensing for assessing rhizoctonia crown and root rot severity in sugar beet. Plant Dis. 2012, 96, 497–505. [Google Scholar] [CrossRef]
- Pozdnyakova, L.; Oudemans, P.V.; Hughes, M.G.; Giménez, D. Estimation of spatial and spectral properties of phytophthora root rot and its effects on cranberry yield. Comput. Electron. Agric. 2003, 37, 57–70. [Google Scholar] [CrossRef]
- Omer, M.; Locke, J.C.; Frantz, J.M. Using leaf temperature as a nondestructive procedure to detect root rot stress in geranium. HortTechnology 2015, 17, 532–536. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, M.; Zhu, J.; Geng, S. Spectral prediction of Phytophthora infestans infection on tomatoes using artificial neural network (ANN). Int. J. Remote Sens. 2008, 29, 1693–1706. [Google Scholar] [CrossRef]
- Weksler, S.; Rozenstein, O.; Haish, N.; Moshelion, M.; Wallach, R.; Ben-dor, E. Pepper plants leaf spectral reflectance changes as a result of root rot damage. Remote Sens. 2021, 13, 980. [Google Scholar] [CrossRef]
- Penuelas, J.; Filella, I.; Gamon, J.A. Assessment of photosynthetic radiation-use efficiency with spectral reflectance. New Phyt. 1995, 131, 291–296. [Google Scholar] [CrossRef]
- Gao, B.-C. NDWI—A normalized difference water index for remote sensing of vegetation liquid water from space. Remote Sens. Env. 1996, 58, 257–266. [Google Scholar] [CrossRef]
- Apan, A.; Held, A.; Phinn. S.; Markley, J. Detecting sugarcane “orange rust” disease using EO1 Hyperion hyperspectral imagery. Inter. J. Remote Sens. 2004, 25, 489–498. [Google Scholar] [CrossRef]
- Rama Rao, N.; Garg, P.; Ghosh, S.; Dadhwal, V. Estimation of leaf total chlorophyll and nitrogen concentrations using hyperspectral satellite imagery. J. Agi. Sci. 2008, 146, 65–75. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Merzlyak, M.N.; Chivkunova, O.B. Optical properties and nondestructive estimation of anthocyanin content in plant leaves. Photochem Photobiol. 2001, 74, 38–45. [Google Scholar] [CrossRef]
- Mahlein, A.-K.; Rumpf, T.; Welke, P.; Dehne, H.-W.; Plümer, L.; Steiner, U.; Oerke, E.-C. Development of spectral indices for detecting and identifying plant diseases. 2013, 128, 21–30. [Google Scholar] [CrossRef]
- Penuelas, J.; Filella, I.; Biel, C.; Serrano, L.; Savé, R. The reflectance at 950-970 nm región as an indicator of plant water status. Inter. J. Remote Sens. 1993, 14, 1887–1905. [Google Scholar] [CrossRef]
- Daughtry, C.S.T.; Walthall, C.L.; Kim, M.S.; de Colstoun, E.B.; McMurtery, J.E., III. Estimating corn leaf chlorophyll concentration from leaf and canopy reflectance. Remote Sens. Environ. 2000, 74, 229–239. [Google Scholar] [CrossRef]
- Stanton, K.M.; Mickelbart, M.V. Maintenance of water uptake and reduced water loss contribute to water stress tolerance of Spiraea alba Du Roi and Spiraea tomentosa L. Hort. Res. 2014, 1, 14033. [Google Scholar] [CrossRef][Green Version]
- Wold, S.; Ruhe, A.; Wold, H.; Dunn, W. The collinearity problem in linear regression: The partial least squares (PLS) approach to generalized inverses. SIAM J. Sci. Stat. Comput. 1984, 5, 735–743. [Google Scholar] [CrossRef]
- Wold, S.; Sjöström, M.; Eriksson, L. PLS-regression: A basic tool of chemometrics. Chemom. Intell. Lab. Syst. 2001, 58, 109–130. [Google Scholar] [CrossRef]
- Grossman, Y.L.; Ustin, S.L.; Jacquemoud, S.; Sanderson, E.W.; Schmuck, G.; Verdebout, J. Critique of stepwise multiple linear regression for the extraction of leaf biochemistry information from leaf reflectance data. Remote Sens. Environ. 1996, 56, 182–193. [Google Scholar] [CrossRef]
- Chen, S.; Hong, X.; Harris, C.J.; Sharkey, P.M. Sparse modeling using orthogonal forward regression with PRESS statistic and regularization. IEEE Trans. Syst. Man Cybern. Part B 2004, 34, 898–911. [Google Scholar] [CrossRef] [PubMed]
- Oleson, J.D.; Park, Y.L.; Nowatzki, T.M.; Tollefson, J.J. Node-injury scale to evaluate root injury by corn rootworms (Coleoptera: Chrysomelidae). J. Econ. Entomol. 2005, 98, 1–8. [Google Scholar] [CrossRef]
- Anderson, M.J. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 2001, 26, 32–46. [Google Scholar]
- Rumpf, T.; Mahlein, A.K.; Steiner, U.; Oerke, E.C.; Dehne, H.W.; Plümer, L. Early detection and classification of plant diseases with Support Vector Machines based on hyperspectral reflectance. Comput. Electron. Agric. 2010, 74, 91–99. [Google Scholar] [CrossRef]
- Filion, M.; Dutilleul, P.; Potvin, C. Optimum experimental design for free-air carbon dioxide enrichment (FACE) studies. Glob. Chang. Biol. 2000, 6, 843–854. [Google Scholar] [CrossRef]
- Galiene, A.; D’Ascenzo, N.; Stagnari, F.; Pagnani, G.; Xie, Q.; Pisante, M. Past and future of plant stress detection: An overview from remote sensing to positron emission tomography. Front. Plant Sci. 2021, 11, 609155. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, B.; Kumar, R. Sensing methodologies in agriculture for monitoring biotic stress in plants due to pathogens and pests. Inventions 2021, 6, 29. [Google Scholar] [CrossRef]
- Lowe, A.; Harrison, N.; French, A.P. Hyperspectral image analysis techniques for the detection and classification of the early onset of plant disease and stress. Plant Methods 2017, 13, 80. [Google Scholar] [CrossRef]
- Abdulridha, J.; Ehsani, R.; Abd-Elrahman, A.; Ampatzidis, Y. A remote sensing technique for detecting laurel wilt disease in avocado in presence of other biotic and abiotic stresses. Comput. Electron. Agric. 2019, 156, 549–557. [Google Scholar] [CrossRef]
- Bravo, C.; Moshou, D.; West, J.; Mccartney, A.; Ramon, H. Early disease detection in wheat fields using spectral reflectance. Biosyst. Eng. 2003, 84, 137–145. [Google Scholar] [CrossRef]
- Moshou, D.; Bravo, C.; West, J.; Wahlen, T.; McCartney, A.; Ramon, H. Automatic detection of ‘yellow rust’ in wheat using reflectance measurements and neural networks. Comput. Electron. Agric. 2004, 44, 173–188. [Google Scholar] [CrossRef]
- Wang, D.; Kurle, J.E.; Estevez De Jensen, C.; Percich, J.A. Radiometric assessment of tillage and seed treatment effect on soybean root rot caused by Fusarium spp. in central Minnesota. Plant Soil 2004, 258, 319–331. [Google Scholar] [CrossRef]
- Chávez-Arias, C.C.; Ligarreto-Moreno, G.A.; Ramírez-Godoy, A.; Restrepo-Díaz, H. Maize responses challenged by drought, elevated daytime temperature and arthropod herbivory stresses: A physiological, biochemical and molecular view. Front. Plant Sci. 2021, 12, 702841. [Google Scholar] [CrossRef] [PubMed]
- Riedell, W.E.; Reese, R.N. Maize morphology and shoot CO2 assimilation after root damage by western corn rootworm larvae. Crop Sci. 1999, 39, 1332–1340. [Google Scholar] [CrossRef]

| Index | Name | Formula | Estimated Parameter | Reference |
|---|---|---|---|---|
| PRI | Photochemical Reflectance Index | (R531 − R570)/(R531 + R570) | Photosynthesis | [41] |
| NDWI | Normalized Difference Water Index | (R860 − R1240)/(R860 + R1240) | Leaf water content | [42] |
| DSWI | Disease Water Index | R800/R1660 | Detect specific disease and pests | [43] |
| PBI | Plant Biochemical Index | R810/R560 | Plant biochemicals | [44] |
| ARI | Anthocyanin Reflectance Index | (1/R550) − (1/R700) | Pigments | [45] |
| HI | Health Index | (R534 − R698)/(R534 + R698) − ½ × (R704) | Vegetation health | [46] |
| WBI | Water Band Index | R970/R902 | Plant water relation | [47] |
| MCARI | Modified Chlorophyll Absorption Reflectance Index | [(R700 − R670) − 0.2 × (R700 − R550)] × (R700/R670) | Chlorophyl Green Leaf Area Index | [48] |
| Experiment | Treatment | df | PRI | NDWI | DSWI | PBI | ARI | HI | WBI | MCARI |
|---|---|---|---|---|---|---|---|---|---|---|
| AAPF | WCR | 1,89 | 0.460 | 0.339 | 0.799 | 0.055 | 0.475 | 0.290 | 0.315 | 0.024 |
| Genotype | 1,89 | <0.001 | <0.001 | 0.300 | 0.002 | 0.333 | 0.056 | <0.001 | 0.491 | |
| WCR × genotype | 1,89 | 0.323 | 0.447 | 0.450 | 0.590 | 0.291 | 0.720 | 0.868 | 0.148 | |
| GH | WCR | 1,237 | 0.210 | 0.637 | 0.068 | 0.007 | 0.226 | 0.146 | 0.921 | 0.247 |
| Drought | 1,237 | <0.001 | 0.006 | <0.001 | <0.001 | 0.006 | <0.001 | 0.003 | 0.445 | |
| Genotype | 1,237 | <0.001 | 0.450 | <0.001 | 0.045 | 0.541 | <0.001 | 0.093 | 0.076 | |
| WCR × drought | 1,237 | 0.545 | 0.928 | 0.047 | 0.077 | 0.431 | 0.073 | 0.582 | 0.717 | |
| WCR × genotype | 1,237 | 0.438 | 0.390 | 0.567 | 0.714 | 0.777 | 0.380 | 0.206 | 0.490 | |
| Drought × genotype | 1,237 | <0.001 | 0.343 | 0.004 | <0.001 | <0.001 | <0.001 | 0.374 | 0.230 | |
| WCR × drought × genotype | 1,237 | 0.841 | 0.462 | 0.756 | 0.682 | 0.578 | 0.842 | 0.556 | 0.230 |
| Experiment | Treatment | df | A | Transp | gs | Ci | RWC | Tleaf | SLA |
| AAPF | WCR | 1,89 | 0.178 | 0.115 | 0.115 | 0.229 | 0.195 | 0.710 | 0.457 |
| Genotype | 1,89 | 0.380 | 0.852 | 0.938 | 0.977 | 0.410 | <0.001 | 0.004 | |
| Genotype × WCR | 1,89 | 0.424 | 0.716 | 0.536 | 0.756 | 0.932 | 0.027 | 0.628 | |
| GH | WCR | 1,237 | 0.851 | 0.809 | 0.187 | 0.187 | 0.306 | 0.409 | 0.291 |
| Drought | 1,237 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.956 | 0.001 | |
| Genotype | 1,237 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.486 | 0.107 | |
| WCR × Drought | 1,237 | 0.152 | 0.139 | 0.056 | 0.056 | 0.570 | 0.956 | 0.081 | |
| WCR × Genotype | 1,237 | 0.277 | 0.638 | 0.354 | 0.354 | 0.813 | 0.563 | 0.284 | |
| Drought × Genotype | 1,237 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.978 | 0.997 | |
| WCR × Drought × Genotype | 1,237 | 0.699 | 0.970 | 0.675 | 0.675 | 0.680 | 0.810 | 0.460 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peron-Danaher, R.; Cotrozzi, L.; Masjedi, A.; Enders, L.S.; Krupke, C.H.; Mickelbart, M.V.; Couture, J.J. Drought Stress Affects Spectral Separation of Maize Infested by Western Corn Rootworm. Agronomy 2023, 13, 2562. https://doi.org/10.3390/agronomy13102562
Peron-Danaher R, Cotrozzi L, Masjedi A, Enders LS, Krupke CH, Mickelbart MV, Couture JJ. Drought Stress Affects Spectral Separation of Maize Infested by Western Corn Rootworm. Agronomy. 2023; 13(10):2562. https://doi.org/10.3390/agronomy13102562
Chicago/Turabian StylePeron-Danaher, Raquel, Lorenzo Cotrozzi, Ali Masjedi, Laramy S. Enders, Christian H. Krupke, Michael V. Mickelbart, and John J. Couture. 2023. "Drought Stress Affects Spectral Separation of Maize Infested by Western Corn Rootworm" Agronomy 13, no. 10: 2562. https://doi.org/10.3390/agronomy13102562
APA StylePeron-Danaher, R., Cotrozzi, L., Masjedi, A., Enders, L. S., Krupke, C. H., Mickelbart, M. V., & Couture, J. J. (2023). Drought Stress Affects Spectral Separation of Maize Infested by Western Corn Rootworm. Agronomy, 13(10), 2562. https://doi.org/10.3390/agronomy13102562







