Identification of SbWRKY Transcription Factors in Scutellaria baicalensis Georgi under Drought Stress and Their Relationship with Baicalin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Screening of WRKYs in S. baicalensis and Prediction of Their Properties
2.3. Constructing a Phylogenetic Tree of SbWRKYs
2.4. Analysis of Conserved Structural Domains of SbWRKYs
2.5. Analyzing the Response of S. baicalensis Roots to Different Drought Stress Conditions Based on RNA-Seq Results
2.6. Extracting RNA and Performing Real-Time Fluorescence Quantitative PCR of S. baicalensis
2.7. Extraction and Content Determination of Baicalin
2.8. Data Processing
3. Results
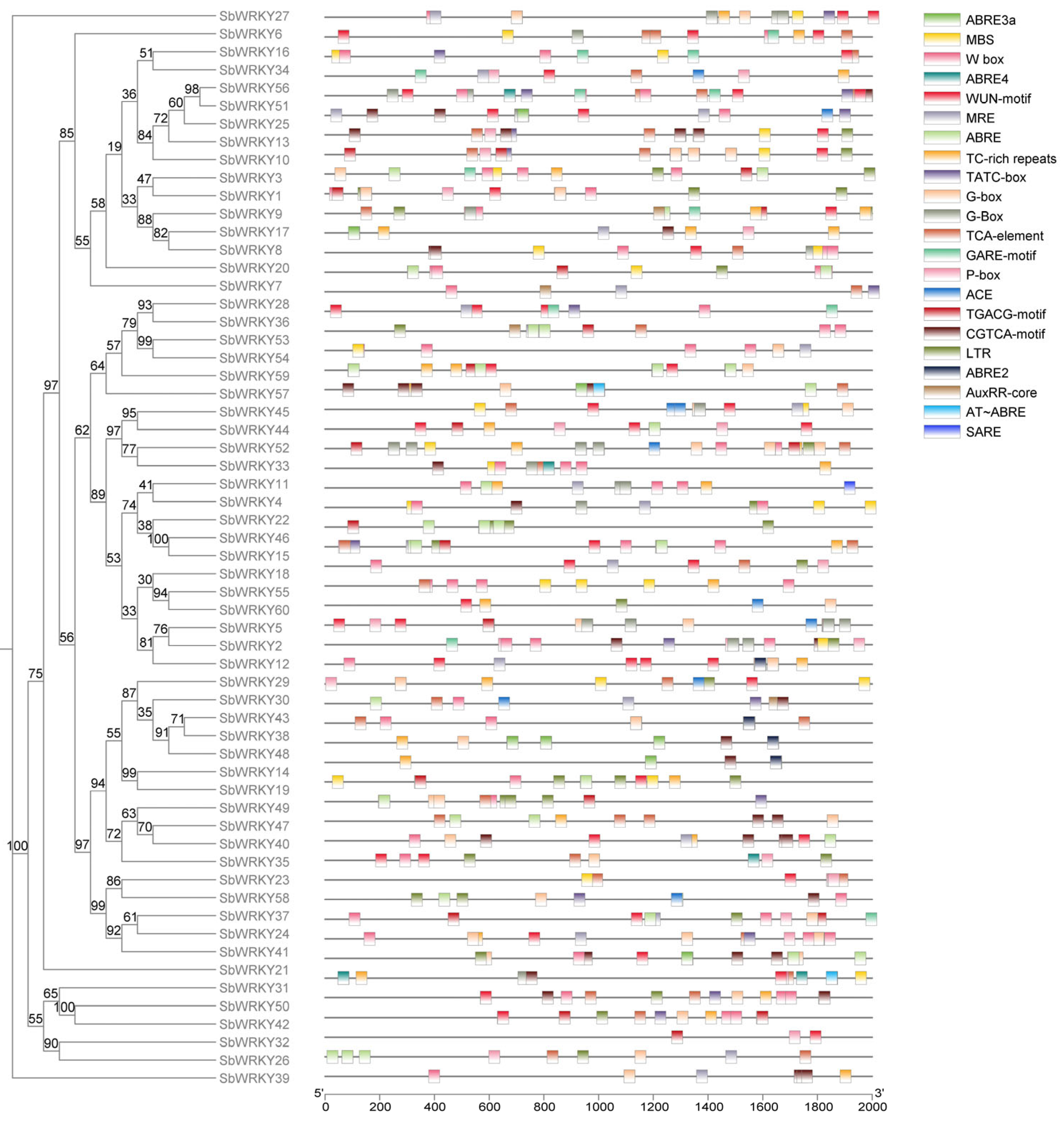
3.1. Phylogenetic Analysis of the SbWRKY Family
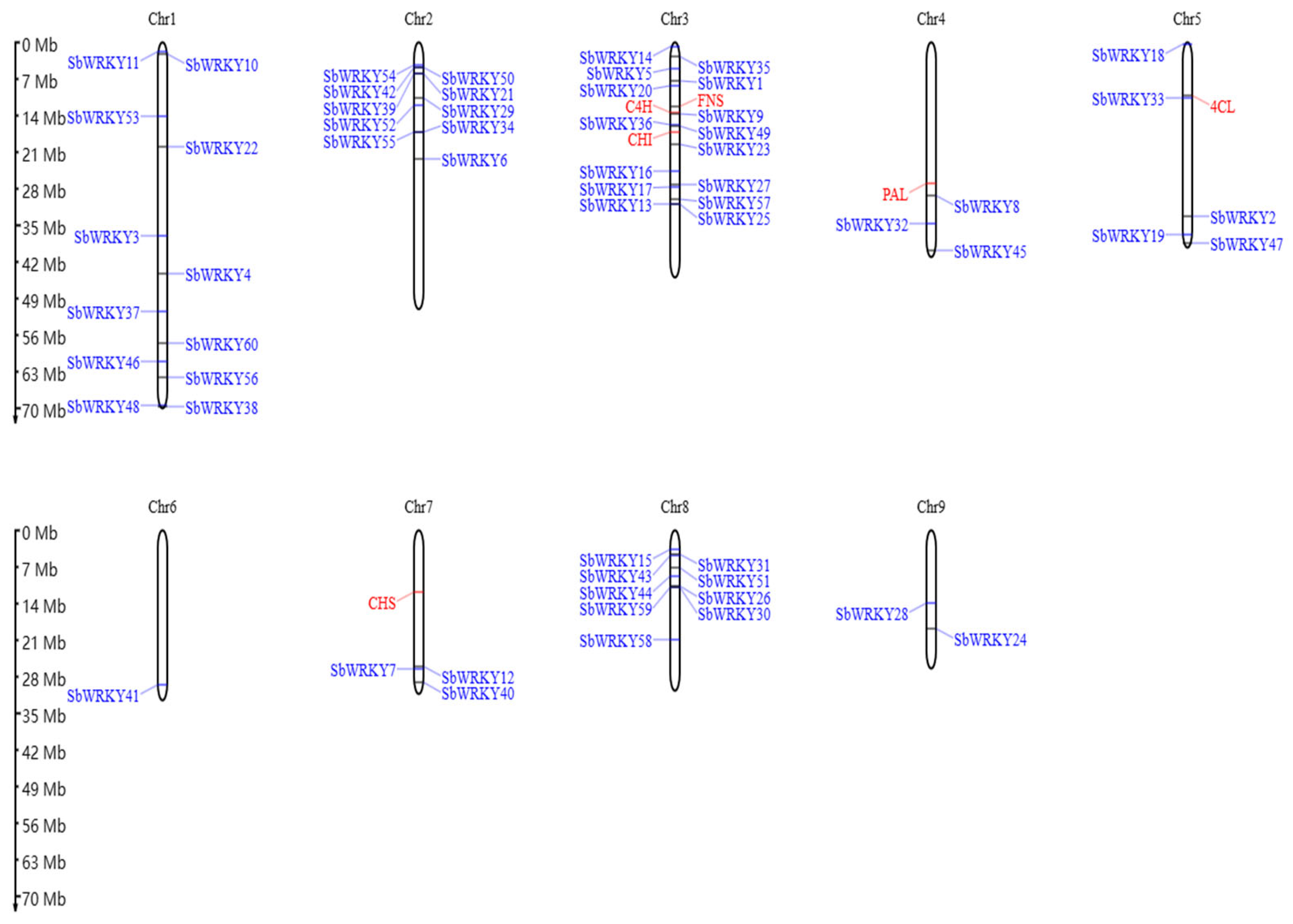
3.2. Chromosomal Localization in the Promoter of SbWRKY Gene and Key Enzyme Genes
3.3. Conserved Motifs and Gene Structure Analysis of SbWRKYs
3.4. Analysis of Cis-Regulatory Elements of the SbWRKY Promoter
3.5. Analysis of Cis-Acting Elements of Key Enzyme Genes of S. baicalensis
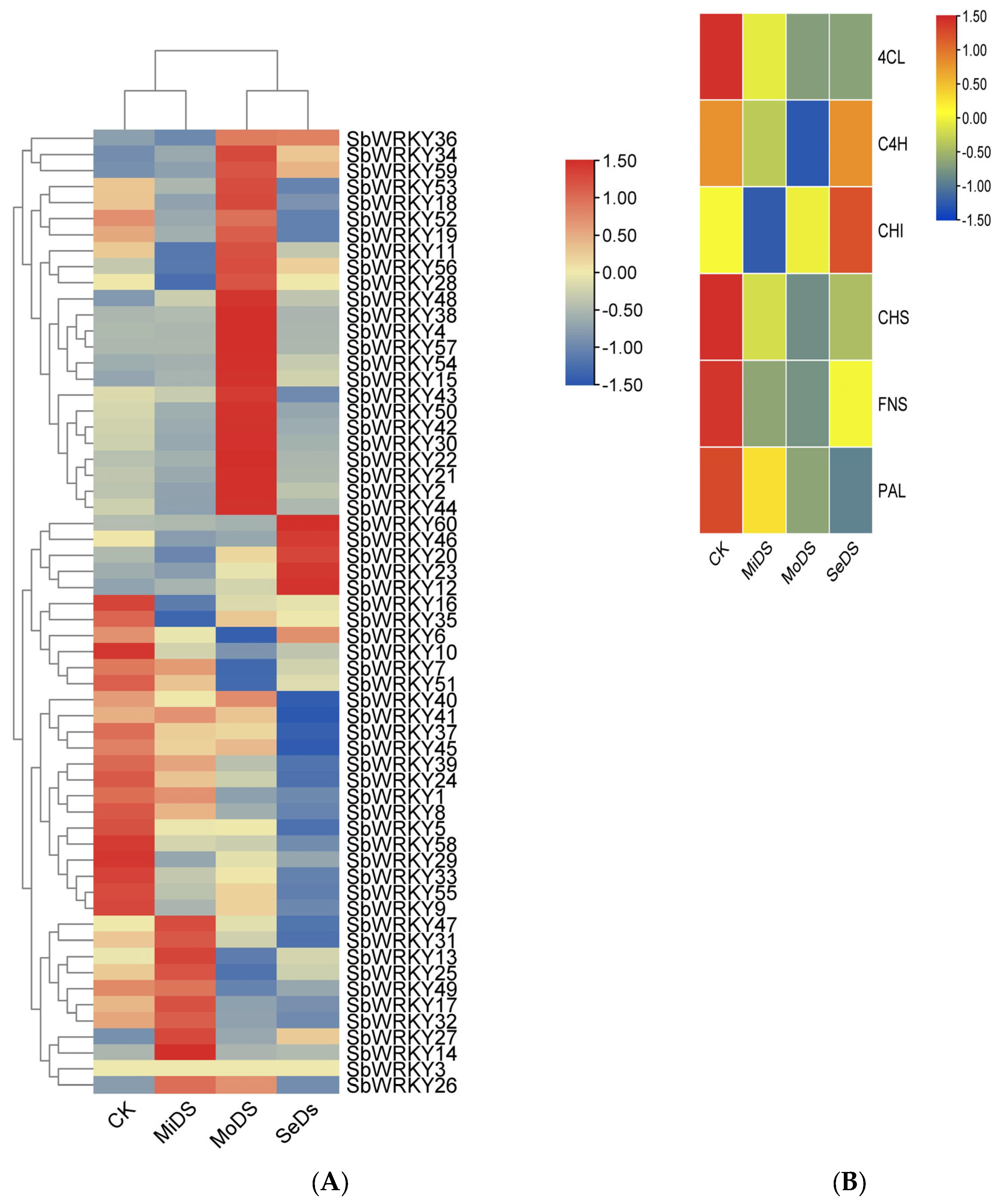
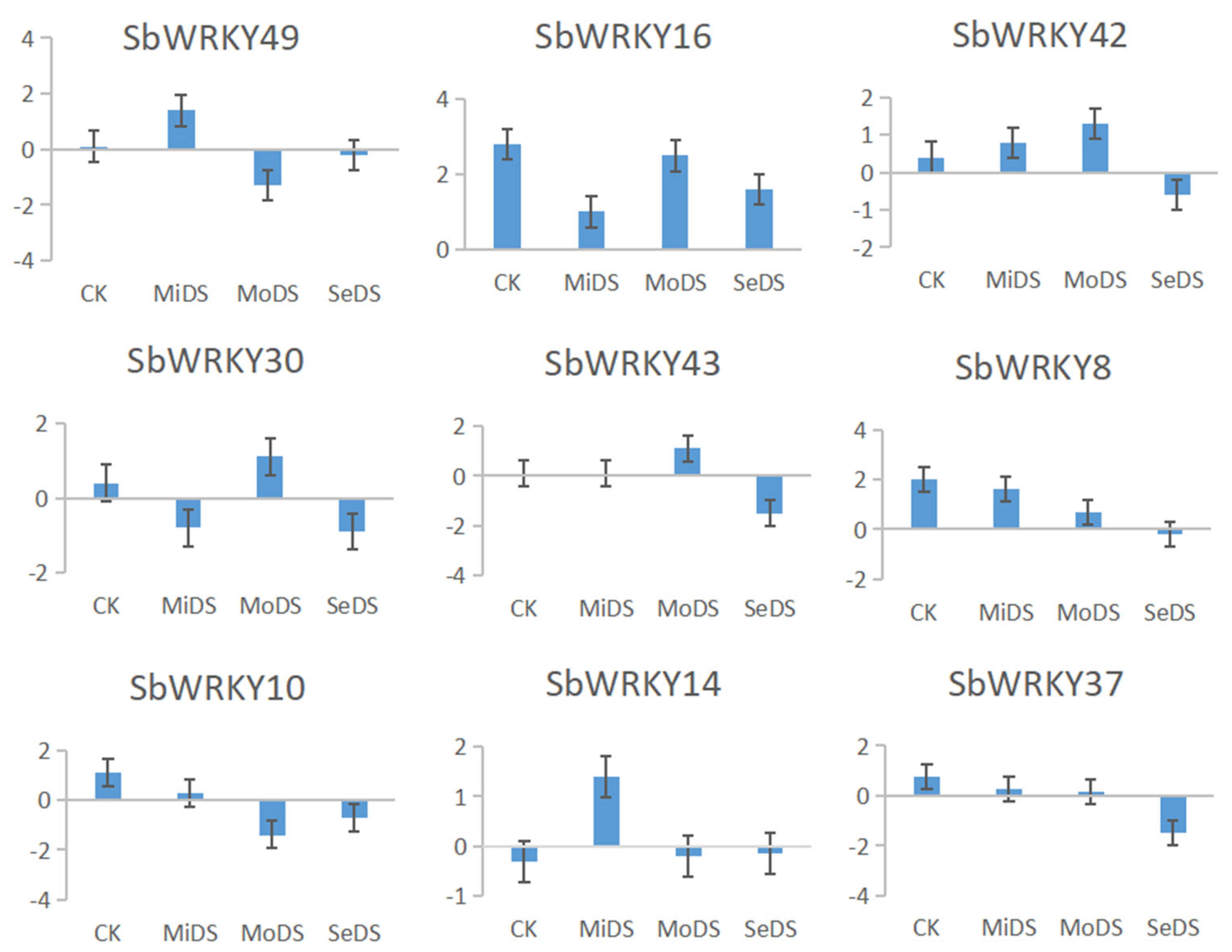
3.6. SbWRKYs and Key Enzyme Genes Expression Analysis under Drought Stress
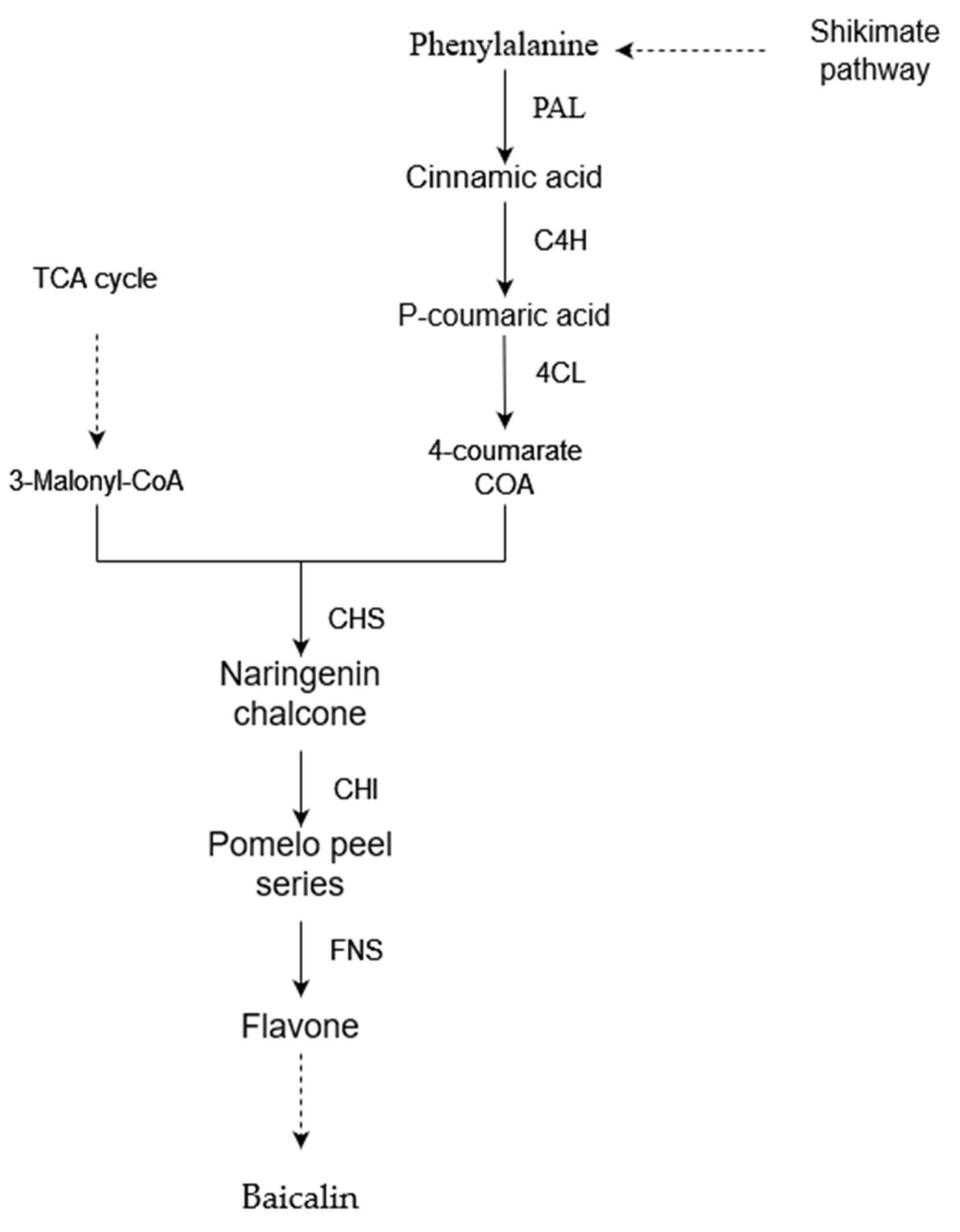
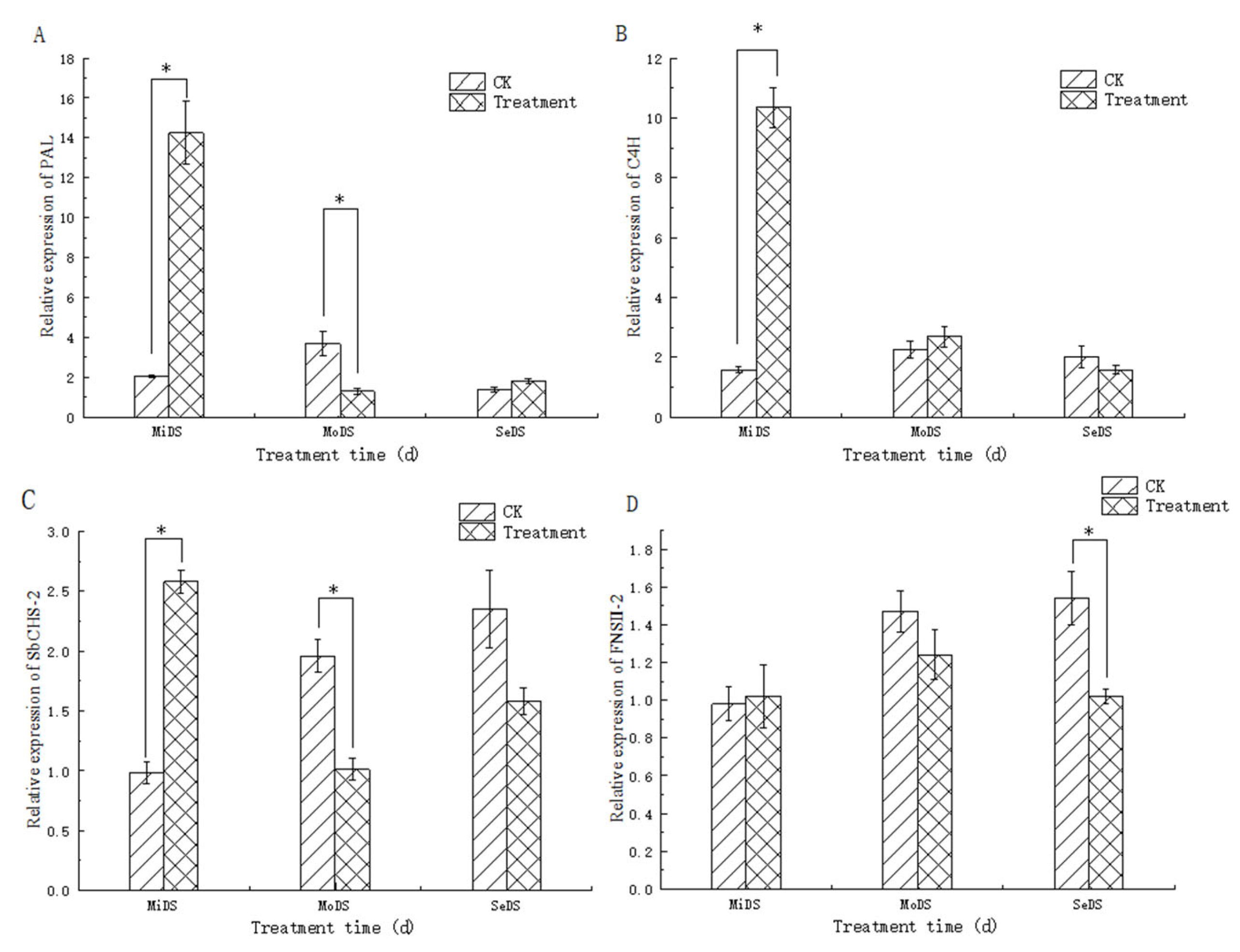
3.7. Effect of Drought Stress on the Expression of Key Enzyme Genes in S. baicalensis
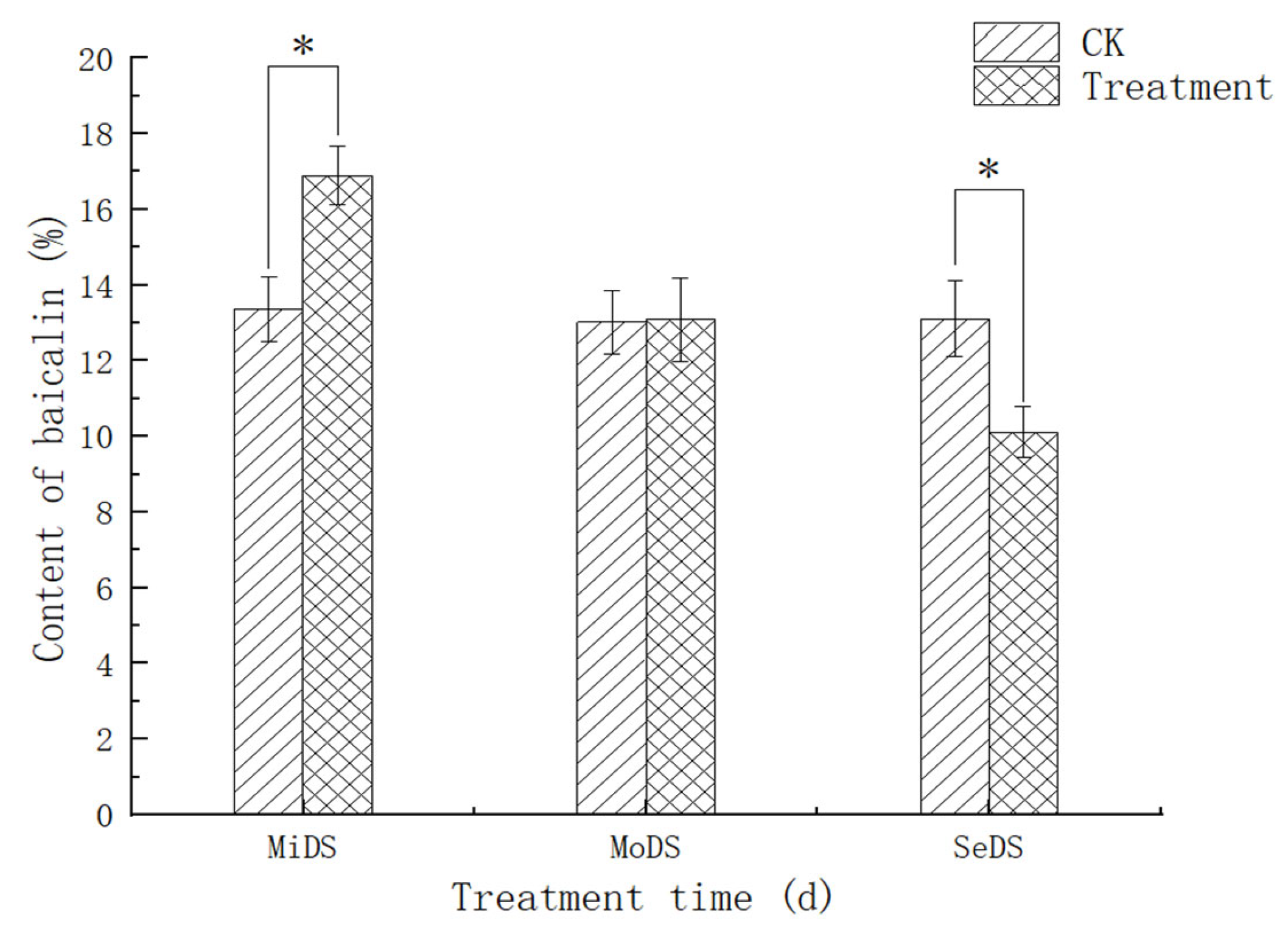
3.8. Effect of Drought Stress on the Content of Baicalin
3.9. Protein Interaction Network between SbWRKYs and Key Enzyme Genes
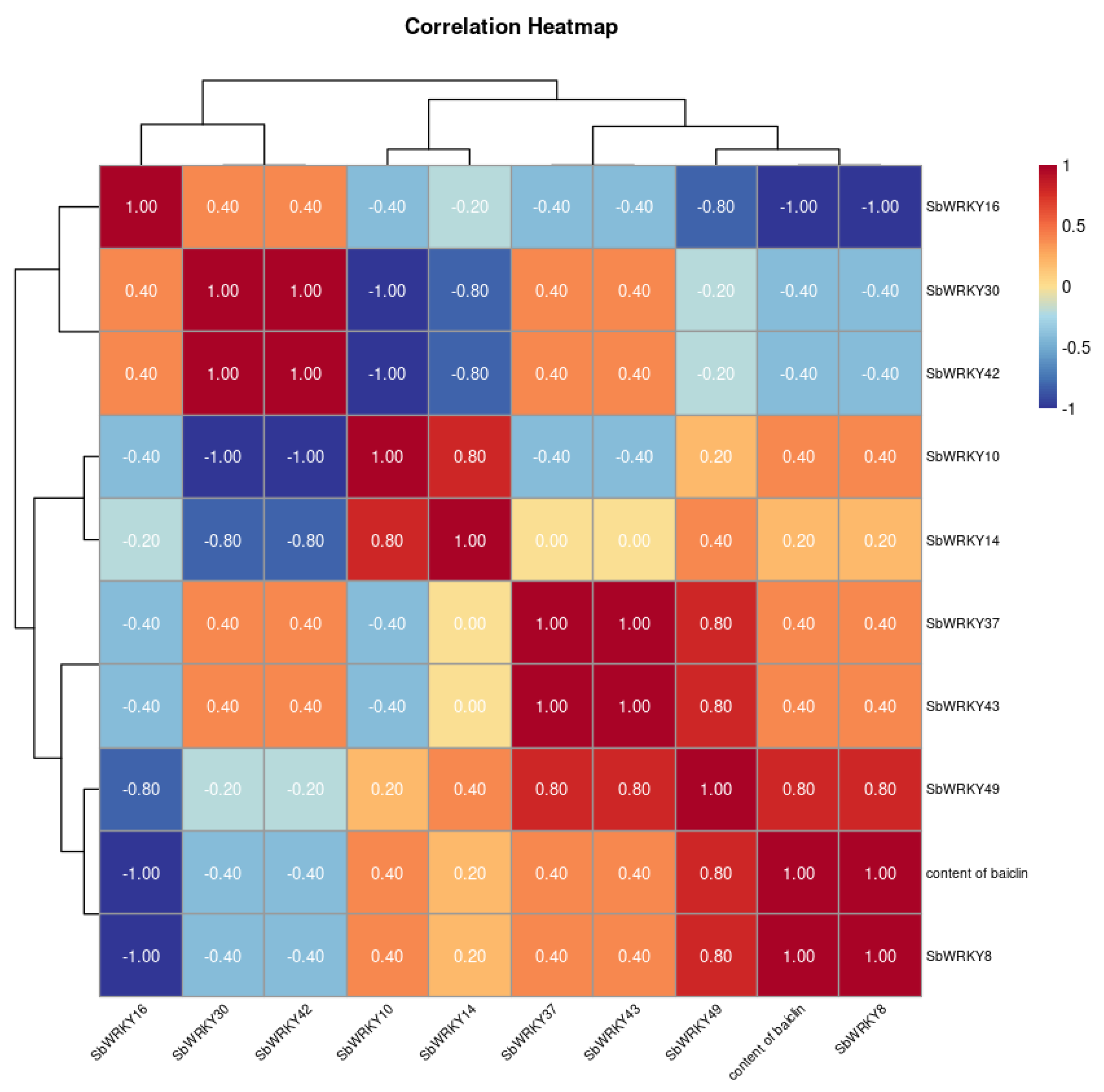
3.10. Correlation between Baicalin Content and SbWRKYs
3.11. Validation of RNA-seq Data via Quantitative qRT-PCR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ji, H.; Zhang, X.; Li, Y.; Li, H. Scutellaria baicalensis, a traditional Chinese medicine from Hebei Province. Mod. Rural. Sci. Technol. 2019, 1, 104–105. [Google Scholar]
- Qian, J.; Meng, W.; Zhao, J.; Wang, Y.; Jin, Y.; Zhan, Z. Herbal Textual Research on Scutellariae Radix in Famous Classical Formulas. Chin. J. Exp. Tradit. Med. Formulae 2023, 29, 84–93. [Google Scholar] [CrossRef]
- Wang, L. Study on Growth and Development Patterns and Medicinal Quality and Yield of Different Scutellaria baicalensis Georg. Master’s Thesis, Jinlin Agricultural University, Changchun, China, 2012. [Google Scholar]
- National Pharmacopoeia Committee. National Pharmacopoeia Committee Pharmacopoeia of the People’s Republic of China; China Medical Science and Technology Press: Beijing, China, 2020. [Google Scholar]
- Hao, W.; Guo, L.; Li, L.; Li, L.; Zhang, G.; Peng, L.; Gao, J.; Hu, B.; Yan, Y. Effects of different elevations and illumination on content of seven active components in Scutellaria baicalensis. Chin. Tradit. Herb. Drugs 2019, 50, 1472–1476. [Google Scholar]
- Liu, Y.; He, Z.; Xie, Y.; Su, L.; Zhang, R.; Wang, H.; Li, C.; Long, S. Drought Resistance Mechanisms of Phedimus aizoon L. Sci. Rep. 2021, 11, 13600. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z. Study on the Quality Formation of Lonicerae japonicae Flos under Salt Stress Based on Multi-Omics. Ph.D. Thesis, Nanjing University of Chinese Medicine, Nanjing, China, 2021. [Google Scholar]
- Zhang, Y.; Han, M.; Jiang, X.; Zhao, S.; Yang, L. Physiological ecology responses of Scutellaria baicalensis to drought and rewatering. China J. Chin. Mater. Medica 2013, 38, 3845–3850. [Google Scholar]
- Yao, H.; Wang, F.; Bi, Q.; Liu, H.; Liu, L.; Xiao, G.; Zhu, J.; Shen, H.; Li, H. Combined Analysis of Pharmaceutical Active Ingredients and Transcriptomes of Glycyrrhiza Uralensis under PEG6000-Induced Drought Stress Revealed Glycyrrhizic Acid and Flavonoids Accumulation via JA-Mediated Signaling. Front. Plant. Sci. 2022, 13, 920172. [Google Scholar] [CrossRef]
- Cheng, L.; Han, M.; Yang, L.; Li, Y.; Sun, Z.; Zhang, T. Changes in the Physiological Characteristics and Baicalin Biosynthesis Metabolism of Scutellaria baicalensis Georgi under Drought Stress. Ind. Crops Prod. 2018, 122, 473–482. [Google Scholar] [CrossRef]
- Fang, Y.; Cui, M.; Liu, J.; Pei, T.; Wei, Y.; Zhao, Q. Study advance in biosynthesis of flavone from Scutellaria. China J. Chin. Mater. Medica 2020, 45, 4819–4826. [Google Scholar] [CrossRef]
- Cheng, L. Physiological and Ecological Changes of Scutellaria baicalensis Georgi under Drought Stress and Mlecular Ecological Mechanism of Baicalin Biosynthesis. Ph.D. Thesis, Jinlin Agricultural University, Changchun, China, 2018. [Google Scholar]
- Shulse, C.N.; Cole, B.J.; Ciobanu, D.; Lin, J.; Yoshinaga, Y.; Gouran, M.; Turco, G.M.; Zhu, Y.; O’Malley, R.C.; Brady, S.M.; et al. High-Throughput Single-Cell Transcriptome Profiling of Plant Cell Types. Cell Rep. 2019, 27, 2241–2247.e4. [Google Scholar] [CrossRef] [PubMed]
- Ryu, K.H.; Zhu, Y.; Schiefelbein, J. Plant Cell Identity in the Era of Single-Cell Transcriptomics. Annu. Rev. Genet. 2021, 55, 479–496. [Google Scholar] [CrossRef]
- Raines, T.; Blakley, I.C.; Tsai, Y.-C.; Worthen, J.M.; Franco-Zorrilla, J.M.; Solano, R.; Schaller, G.E.; Loraine, A.E.; Kieber, J.J. Characterization of the Cytokinin-Responsive Transcriptome in Rice. BMC Plant Biol. 2016, 16, 260. [Google Scholar] [CrossRef]
- Lian, T.; Wang, X.; Li, S.; Jiang, H.; Zhang, C.; Wang, H.; Jiang, L. Comparative Transcriptome Analysis Reveals Mechanisms of Folate Accumulation in Maize Grains. Int. J. Mol. Sci. 2022, 23, 1708. [Google Scholar] [CrossRef]
- Nakayama, R.; Safi, M.T.; Ahmadzai, W.; Sato, K.; Kawaura, K. Comparative Transcriptome Analysis of Synthetic and Common Wheat in Response to Salt Stress. Sci. Rep. 2022, 12, 11534. [Google Scholar] [CrossRef]
- Xia, K.; Sun, H.-X.; Li, J.; Li, J.; Zhao, Y.; Chen, L.; Qin, C.; Chen, R.; Chen, Z.; Liu, G.; et al. The Single-Cell Stereo-Seq Reveals Region-Specific Cell Subtypes and Transcriptome Profiling in Arabidopsis Leaves. Dev. Cell 2022, 57, 1299–1310.e4. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishna, A.; Ravishankar, G.A. Influence of Abiotic Stress Signals on Secondary Metabolites in Plants. Plant Signal. Behav. 2011, 6, 1720–1731. [Google Scholar] [CrossRef]
- Rushton, P.J.; Somssich, I.E.; Ringler, P.; Shen, Q.J. WRKY Transcription Factors. Trends Plant Sci. 2010, 15, 247–258. [Google Scholar] [CrossRef]
- Lee, F.C.; Yeap, W.C.; Appleton, D.R.; Ho, C.-L.; Kulaveerasingam, H. Identification of Drought Responsive Elaeis Guineensis WRKY Transcription Factors with Sensitivity to Other Abiotic Stresses and Hormone Treatments. BMC Genom. 2022, 23, 164. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Ma, S.; Ye, N.; Jiang, M.; Cao, J.; Zhang, J. WRKY Transcription Factors in Plant Responses to Stresses. J. Integr. Plant Biol. 2017, 59, 86–101. [Google Scholar] [CrossRef] [PubMed]
- Eulgem, T.; Rushton, P.J.; Robatzek, S.; Somssich, I.E. The WRKY Superfamily of Plant Transcription Factors. Trends Plant Sci. 2000, 5, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Pandey, B.; Grover, A.; Sharma, P. Molecular Dynamics Simulations Revealed Structural Differences among WRKY Domain-DNA Interaction in Barley (Hordeum vulgare). BMC Genom. 2018, 19, 132. [Google Scholar] [CrossRef]
- Song, H.; Sun, W.; Yang, G.; Sun, J. WRKY Transcription Factors in Legumes. BMC Plant. Biol. 2018, 18, 243. [Google Scholar] [CrossRef]
- Su, M.; Zuo, W.; Wang, Y.; Liu, W.; Zhang, Z.; Wang, N.; Chen, X. The WKRY Transcription Factor MdWRKY75 Regulates Anthocyanins Accumulation in Apples (Malus domestica). Funct. Plant Biol. 2022, 49, 799–809. [Google Scholar] [CrossRef]
- Chang, X.; Yang, Z.; Zhang, X.; Zhang, F.; Huang, X.; Han, X. Transcriptome-Wide Identification of WRKY Transcription Factors and Their Expression Profiles under Different Stress in Cynanchum Thesioides. PeerJ 2022, 10, e14436. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Liu, Y.; Wu, C.; Chen, S.; Wang, Z.; Yang, Z.; Qin, S.; Huang, L. Water Deficit Affected Flavonoid Accumulation by Regulating Hormone Metabolism in Scutellaria baicalensis Georgi Roots. PLoS ONE 2012, 7, e42946. [Google Scholar] [CrossRef] [PubMed]
- Abdullah-Zawawi, M.-R.; Ahmad-Nizammuddin, N.-F.; Govender, N.; Harun, S.; Mohd-Assaad, N.; Mohamed-Hussein, Z.-A. Comparative Genome-Wide Analysis of WRKY, MADS-Box and MYB Transcription Factor Families in Arabidopsis and Rice. Sci. Rep. 2021, 11, 19678. [Google Scholar] [CrossRef]
- Li, W.; Pang, S.; Lu, Z.; Jin, B. Function and Mechanism of WRKY Transcription Factors in Abiotic Stress Responses of Plants. Plants 2020, 9, 1515. [Google Scholar] [CrossRef]
- Mukhi, N.; Brown, H.; Gorenkin, D.; Ding, P.; Bentham, A.R.; Stevenson, C.E.M.; Jones, J.D.G.; Banfield, M.J. Perception of Structurally Distinct Effectors by the Integrated WRKY Domain of a Plant Immune Receptor. Proc. Natl. Acad Sci. USA 2021, 118, e2113996118. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Jia, J.; Jiang, X.; Wang, Y.; Zhao, J.; Chen, Y.; Huang, S.; Wu, S.; Dong, Y. The evolution, characterization and transcriptional responses to multiple stresses of the WRKY genes in Chenopodium quinoa. Acta Prataculturae Sin. 2022, 31, 168–182. [Google Scholar]
- Zhang, C.; Wang, W.; Wang, D.; Hu, S.; Zhang, Q.; Wang, Z.; Cui, L. Genome-Wide Identification and Characterization of the WRKY Gene Family in Scutellaria baicalensis Georgi under Diverse Abiotic Stress. Int. J. Mol. Sci. 2022, 23, 4225. [Google Scholar] [CrossRef]
- Cao, X.; Hu, Y.; Song, J.; Feng, H.; Wang, J.; Chen, L.; Wang, L.; Diao, X.; Wan, Y.; Liu, S.; et al. Transcriptome Sequencing and Metabolome Analysis Reveals the Molecular Mechanism of Drought Stress in Millet. Int. J. Mol. Sci. 2022, 23, 10792. [Google Scholar] [CrossRef]
- Park, S.Y.; Jeong, D.-H. Comprehensive Analysis of Rice Seedling Transcriptome during Dehydration and Rehydration. Int. J. Mol. Sci. 2023, 24, 8439. [Google Scholar] [CrossRef]
- Wu, W.; Zhu, S.; Xu, L.; Zhu, L.; Wang, D.; Liu, Y.; Liu, S.; Hao, Z.; Lu, Y.; Yang, L.; et al. Genome-Wide Identification of the Liriodendron Chinense WRKY Gene Family and Its Diverse Roles in Response to Multiple Abiotic Stress. BMC Plant. Biol. 2022, 22, 25. [Google Scholar] [CrossRef]
- Han, Z.; Guo, H.; Chang, B.; Hu, S.; Han, M.; Yang, L. Extracting Technology of Baicalin by Microwave from Scutellaria baicalensis Georgi. Lishizhen Med. Mater. Medica 2011, 22, 2840–2841. [Google Scholar]
- Zhang, T.; Li, Z.; Wu, G. Role of WRKY Transcription Factor in Plant Response to Stresses. Biotechnol. Bull. 2021, 37, 203–215. [Google Scholar] [CrossRef]
- Cheng, X.; Zhao, Y.; Jiang, Q.; Yang, J.; Zhao, W.; Taylor, I.A.; Peng, Y.-L.; Wang, D.; Liu, J. Structural Basis of Dimerization and Dual W-Box DNA Recognition by Rice WRKY Domain. Nucleic. Acids Res. 2019, 47, 4308–4318. [Google Scholar] [CrossRef]
- Di, P.; Wang, P.; Yan, M.; Han, P.; Huang, X.; Yin, L.; Yan, Y.; Xu, Y.; Wang, Y. Genome-Wide Characterization and Analysis of WRKY Transcription Factors in Panax Ginseng. BMC Genom. 2021, 22, 834. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Yuan, Y.; Chen, M.; Shuai, L.; Xiao, Q.; Lin, S. Effects of PEG stress on flavonoids accumulation and related gene expression in suspension of Scutellaria baicalensis. Zhongguo Zhong Yao Za Zhi 2011, 36, 2157–2161. [Google Scholar] [PubMed]
- Hsin, K.-T.; Hsieh, M.-C.; Lee, Y.-H.; Lin, K.-C.; Cheng, Y.-S. Insight into the Phylogeny and Binding Ability of WRKY Transcription Factors. Int. J. Mol. Sci. 2022, 23, 2895. [Google Scholar] [CrossRef]
- Gu, L.; Dou, L.; Guo, Y.; Wang, H.; Li, L.; Wang, C.; Ma, L.; Wei, H.; Yu, S. The WRKY Transcription Factor GhWRKY27 Coordinates the Senescence Regulatory Pathway in Upland Cotton (Gossypium hirsutum L.). BMC Plant. Biol. 2019, 19, 116. [Google Scholar] [CrossRef] [PubMed]
- Jimmy, J.L.; Babu, S. Variations in the Structure and Evolution of Rice WRKY Genes in Indica and Japonica Genotypes and Their Co-Expression Network in Mediating Disease Resistance. Evol. Bioinform. 2019, 15, 1176934319857720. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, N.; Zhao, S. Functional Characterization of a WRKY Family Gene Involved in Somatic Embryogenesis in Panax Ginseng. Protoplasma 2020, 257, 449–458. [Google Scholar] [CrossRef]
- Hu, Q.; Ao, C.; Wang, X.; Wu, Y.; Du, X. GhWRKY1-like, a WRKY Transcription Factor, Mediates Drought Tolerance in Arabidopsis via Modulating ABA Biosynthesis. BMC Plant. Biol. 2021, 21, 458. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-T.; Ru, J.-N.; Liu, Y.-W.; Li, M.; Zhao, D.; Yang, J.-F.; Fu, J.-D.; Xu, Z.-S. Maize WRKY Transcription Factor ZmWRKY106 Confers Drought and Heat Tolerance in Transgenic Plants. Int. J. Mol. Sci. 2018, 19, 3046. [Google Scholar] [CrossRef]
- Gulzar, F.; Fu, J.; Zhu, C.; Yan, J.; Li, X.; Meraj, T.A.; Shen, Q.; Hassan, B.; Wang, Q. Maize WRKY Transcription Factor ZmWRKY79 Positively Regulates Drought Tolerance through Elevating ABA Biosynthesis. Int. J. Mol. Sci. 2021, 22, 10080. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Liu, F.; Chao, D.; Xin, B.; Liu, K.; Cao, S.; Chen, X.; Peng, L.; Zhang, B.; Fu, S.; et al. The WRKY Transcription Factor OsWRKY54 Is Involved in Salt Tolerance in Rice. Int. J. Mol. Sci. 2022, 23, 11999. [Google Scholar] [CrossRef]
- Ayoub Khan, M.; Dongru, K.; Yifei, W.; Ying, W.; Penghui, A.; Zicheng, W. Characterization of WRKY Gene Family in Whole-Genome and Exploration of Flowering Improvement Genes in Chrysanthemum Lavandulifolium. Front. Plant Sci. 2022, 13, 861193. [Google Scholar] [CrossRef]
- Su, L.; Wang, P.; Yang, Y.; Ren, F.; Wang, Y.; Chen, W. Genome-wide Identification and Analysis of WRKY Transcription Factors in Graoe. Heilongjiang Agric. Sci. 2019, 1, 13–22. [Google Scholar]
- Agurla, S.; Gahir, S.; Munemasa, S.; Murata, Y.; Raghavendra, A.S. Mechanism of Stomatal Closure in Plants Exposed to Drought and Cold Stress. Adv. Exp. Med. Biol. 2018, 1081, 215–232. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, J.; Zhou, X.; Liu, S.; Zhuang, Y. Identification of WRKY Gene Family and Characterization of Cold Stress-Responsive WRKY Genes in Eggplant. PeerJ 2020, 8, e8777. [Google Scholar] [CrossRef]
- Du, Z.; You, S.; Zhao, X.; Xiong, L.; Li, J. Genome-Wide Identification of WRKY Genes and Their Responses to Chilling Stress in Kandelia Obovata. Front. Genet. 2022, 13, 875316. [Google Scholar] [CrossRef]
- Chanwala, J.; Satpati, S.; Dixit, A.; Parida, A.; Giri, M.K.; Dey, N. Genome-Wide Identification and Expression Analysis of WRKY Transcription Factors in Pearl Millet (Pennisetum glaucum) under Dehydration and Salinity Stress. BMC Genom. 2020, 21, 231. [Google Scholar] [CrossRef] [PubMed]
- Park, N.I.; Xu, H.; Li, X.; Kim, Y.S.; Lee, M.Y.; Park, S.U. Overexpression of Phenylalanine Ammonia-Lyase Improves Flavones Production in Transgenic Hairy Root Cultures of Scutellaria baicalensis. Process Biochem. 2012, 47, 2575–2580. [Google Scholar] [CrossRef]
- Xu, H.; Park, N.I.; Li, X.; Kim, Y.K.; Lee, S.Y.; Park, S.U. Molecular Cloning and Characterization of Phenylalanine Ammonia-Lyase, Cinnamate 4-Hydroxylase and Genes Involved in Flavone Biosynthesis in Scutellaria baicalensis. Bioresour. Technol. 2010, 101, 9715–9722. [Google Scholar] [CrossRef] [PubMed]


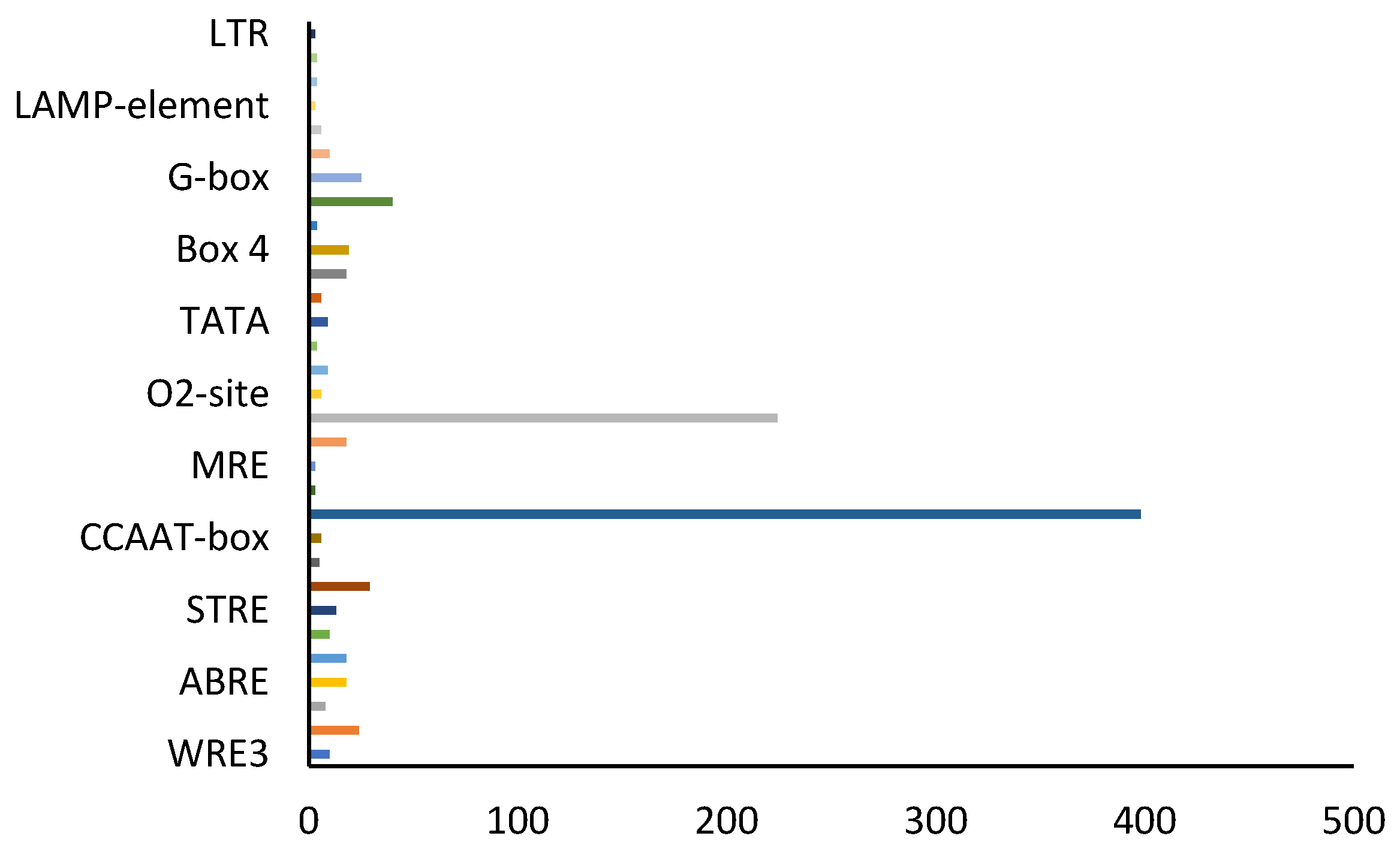

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, L.; Yu, J.; Zhang, L.; Yao, Y.; Sun, Z.; Han, M.; Zhang, Y.; Yang, L. Identification of SbWRKY Transcription Factors in Scutellaria baicalensis Georgi under Drought Stress and Their Relationship with Baicalin. Agronomy 2023, 13, 2564. https://doi.org/10.3390/agronomy13102564
Cheng L, Yu J, Zhang L, Yao Y, Sun Z, Han M, Zhang Y, Yang L. Identification of SbWRKY Transcription Factors in Scutellaria baicalensis Georgi under Drought Stress and Their Relationship with Baicalin. Agronomy. 2023; 13(10):2564. https://doi.org/10.3390/agronomy13102564
Chicago/Turabian StyleCheng, Lin, Jingjing Yu, Lichao Zhang, Yanying Yao, Zhuo Sun, Mei Han, Yonggang Zhang, and Limin Yang. 2023. "Identification of SbWRKY Transcription Factors in Scutellaria baicalensis Georgi under Drought Stress and Their Relationship with Baicalin" Agronomy 13, no. 10: 2564. https://doi.org/10.3390/agronomy13102564
APA StyleCheng, L., Yu, J., Zhang, L., Yao, Y., Sun, Z., Han, M., Zhang, Y., & Yang, L. (2023). Identification of SbWRKY Transcription Factors in Scutellaria baicalensis Georgi under Drought Stress and Their Relationship with Baicalin. Agronomy, 13(10), 2564. https://doi.org/10.3390/agronomy13102564



