Chemical Characterization of Brown and Red Seaweed from Southern Peru, a Sustainable Source of Bioactive and Nutraceutical Compounds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Seaweed Collection
2.2. Chemicals and Analytic Reagents
2.3. Proximal Analysis
2.4. Determination of Amino Acids
2.5. Determination of Fatty Acids
2.6. Determination of Tocopherols
2.7. Determination of Minerals
2.8. Conventional Extraction and Determination of Total Polyphenols (TPC)
2.9. Determination of Antioxidant Capacity by ORAC
2.10. Statistical Analysis
3. Results and Discussion
3.1. Proximal Characterization of Seaweeds
3.2. Composition of Amino Acids
3.3. Composition of Saturated and Unsaturated Fatty Acids
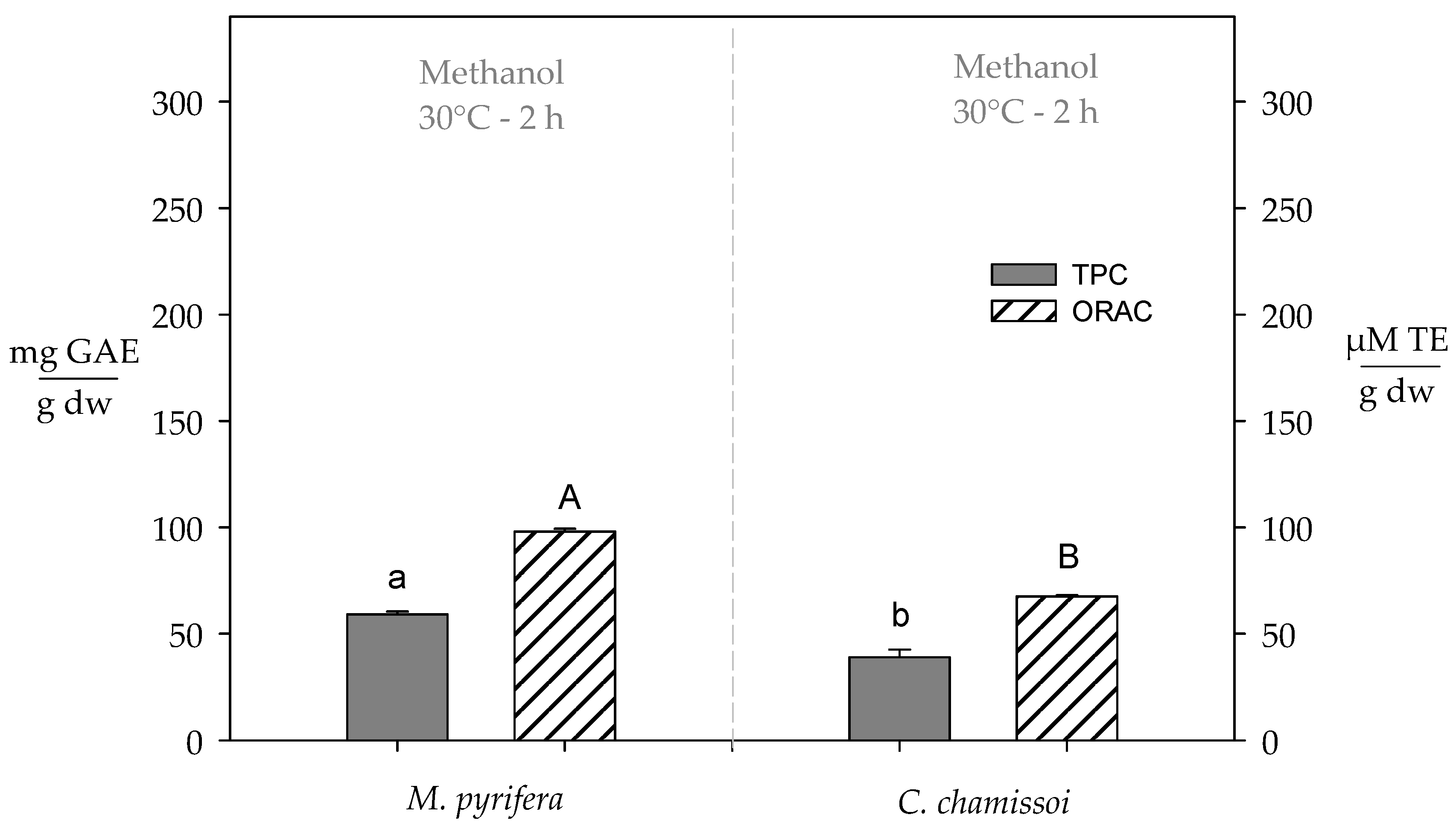
3.4. Total Polyphenol Content and Antioxidant Capacity of Extracts Obtained from Brown (M. pyrifera) and Red (C. chamissoi) Peruvian Seaweed
3.5. Composition of Minerals
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cotas, J.; Leandro, A.; Monteiro, P.; Pacheco, D.; Figueirinha, A.; Goncąlves, A.M.M.; Da Silva, G.J.; Pereira, L. Seaweed phenolics: From extraction to applications. Mar. Drugs 2020, 18, 384. [Google Scholar] [CrossRef] [PubMed]
- Tejada, A.G. Evaluacion de la Variacion Temporal de Biomasa de Algas Marinas Lessonia Nigrescens “Aracanto Negro” en el litoral sur del Peru y Propuesta de Manejo. Master’s Thesis, Ciencias Universidad Nacional San Agustin de Arequipa, Arequipa, Peru, 2019. [Google Scholar]
- El-Said, G.F.; El-Sikaily, A. Chemical composition of some seaweed from Mediterranean Sea coast, Egypt. Environ. Monit. Assess. 2013, 185, 6089–6099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- FAO (Food and Agriculture Organization). Alcance de la Industria de las Algas Marinas. Available online: http://www.fao.org/3/y5600s/y5600s07.htm (accessed on 20 January 2021).
- Salehi, B.; Sharifi-rad, J.; Seca, A.M.L.; Pinto, D.C.G.A. Current Trends on Seaweeds: Looking at Chemical. Molecules 2019, 24, 4182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olsson, J.; Toth, G.B.; Albers, E. Biochemical composition of red, green and brown seaweeds on the Swedish west coast. J. Appl. Phycol. 2020, 32, 3305–3317. [Google Scholar] [CrossRef]
- Machado, M.; Machado, S.; Pimentel, F.B.; Freitas, V.; Alves, R.C.; Oliveira, M.B.P.P. Amino acid profile and protein quality assessment of macroalgae produced in an integrated multi-trophic aquaculture system. Foods 2020, 9, 1382. [Google Scholar] [CrossRef]
- Pedroso, J.A.B.; Zampieri, T.T.; Donato, J. Reviewing the effects of l-leucine supplementation in the regulation of food intake, energy balance, and glucose homeostasis. Nutrients 2015, 7, 3914–3937. [Google Scholar] [CrossRef] [Green Version]
- Singhal, N.K.; Freeman, E.; Arning, E.; Wasek, B.; Clements, R.; Sheppard, C.; Blake, P.; Bottiglieri, T.; McDonough, J. Dysregulation of methionine metabolism in multiple sclerosis. Neurochem. Int. 2018, 112, 1–4. [Google Scholar] [CrossRef]
- Chen, S.F.; Pan, M.X.; Tang, J.C.; Cheng, J.; Zhao, D.; Zhang, Y.; Liao, H.B.; Liu, R.; Zhuang, Y.; Zhang, Z.F.; et al. Arginine is neuroprotective through suppressing HIF-1α/LDHA-mediated inflammatory response after cerebral ischemia/reperfusion injury. Mol. Brain 2020, 13, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.-Y.; Kim, E.-H. Therapeutic Effects of Amino Acids in Liver Diseases: Current Studies and Future Perspectives. J. Cancer Prev. 2019, 24, 72–78. [Google Scholar] [CrossRef]
- Lafarga, T.; Acién-Fernández, F.G.; Garcia-Vaquero, M. Bioactive peptides and carbohydrates from seaweed for food applications: Natural occurrence, isolation, purification, and identification. Algal Res. 2020, 48, 1–10. [Google Scholar] [CrossRef]
- Stiger-Pouvreau, V.; Bourgougnon, N.; Deslandes, E. Chapter 8—Carbohydrates from Seaweeds. In Seaweed in Health and Disease Prevention; Fleurence, J., Levine, I., Eds.; Academic Press: San Diego, CA, USA, 2016; pp. 223–274. [Google Scholar]
- Gosch, B.J.; Magnusson, M.; Paul, N.A.; de Nys, R. Total lipid and fatty acid composition of seaweeds for the selection of species for oil-based biofuel and bioproducts. GCB Bioenergy 2012, 4, 919–930. [Google Scholar] [CrossRef] [Green Version]
- Susanto, E.; Fahmi, A.S.; Abe, M.; Hosokawa, M.; Miyashita, K. Lipids, Fatty Acids, and Fucoxanthin Content from Temperate and Tropical Brown Seaweeds. Aquat. Procedia 2016, 7, 66–75. [Google Scholar] [CrossRef]
- Kendel, M.; Wielgosz-Collin, G.; Bertrand, S.; Roussakis, C.; Bourgougnon, N.B.; Bedoux, G. Lipid composition, fatty acids and sterols in the seaweeds ulva armoricana, and solieria chordalis from brittany (France): An analysis from nutritional, chemotaxonomic, and antiproliferative activity perspectives. Mar. Drugs 2015, 13, 5606–5628. [Google Scholar] [CrossRef]
- Hwang, E.S.; Thi, N. Do Effects of extraction and processing methods on antioxidant compound contents and radical scavenging activities of laver (Porphyra tenera). Prev. Nutr. Food Sci. 2014, 19, 40–48. [Google Scholar] [CrossRef]
- Pacheco, L.V.; Parada, J.; Pérez-Correa, J.R.; Mariotti-Celis, M.S.; Erpel, F.; Zambrano, A.; Palacios, M. Bioactive Polyphenols from Southern Chile Seaweed as Inhibitors of Enzymes for Starch Digestion. Mar. Drugs 2020, 18, 353. [Google Scholar] [CrossRef]
- Circuncisão, A.R.; Catarino, M.D.; Cardoso, S.M.; Silva, A.M.S. Minerals from macroalgae origin: Health benefits and risks for consumers. Mar. Drugs 2018, 16, 400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rupérez, P. Mineral content of edible marine seaweeds. Food Chem. 2002, 79, 23–26. [Google Scholar] [CrossRef]
- Roleda, M.Y.; Marfaing, H.; Desnica, N.; Jónsdóttir, R.; Skjermo, J.; Rebours, C.; Nitschke, U. Variations in polyphenol and heavy metal contents of wild-harvested and cultivated seaweed bulk biomass: Health risk assessment and implication for food applications. Food Control 2019, 95, 121–134. [Google Scholar] [CrossRef]
- Horwitz, W.; Latimer, G. Official Methods of Analysis of AOAC International, 18th ed.; AOAC International: Gaithersburg, MA, USA, 2005. [Google Scholar]
- González-Castro, M.J.; López-Hernández, J.; Simal-Lozano, J.; Oruña-Concha, M.J. Determination of Amino Acids in Green Beans by Derivatization with Phenylisothiocianate and High-Performance Liquid Chromatography with Ultraviolet Detection. J. Chromatogr. Sci. 1997, 35, 181–185. [Google Scholar] [CrossRef] [Green Version]
- Frank, D.; Sandra, P.; Philip, W. Improving the Analysis of Fatty Acid Methyl Esters Using Retention Time Locked Methods and Retention Time Databases; Agilent Technologies, Inc.: Wilmington, DE, USA, 2002; pp. 1–10. [Google Scholar]
- Lampi, A.M. Tocopherols and Tocotrienols in Vegetable Oils and Fats by HPLC, 3rd ed.; AOCS American Oil Chemists Society: Urbana, IL, USA, 1993. [Google Scholar]
- Jorhem, L. Determination of metals in foods by atomic absorption spectrometry after dry ashing. J AOAC Int. 2000, 83, 1204. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Segovia, I.; Lerma-García, M.J.; Fuentes, A.; Barat, J.M. Characterization of Spanish powdered seaweeds: Composition, antioxidant capacity and technological properties. Food Res. Int. 2018, 111, 212–219. [Google Scholar] [CrossRef]
- Chun, S.S.; Vattem, D.A.; Lin, Y.T.; Shetty, K. Phenolic antioxidants from clonal oregano (Origanum vulgare) with antimicrobial activity against Helicobacter pylori. Process Biochem. 2005, 40, 809–816. [Google Scholar] [CrossRef]
- Davalos, A.; Gómez, C.; Begoña, B. Extending Applicability of the Oxygen Radical Absorbance Capacity (ORAC–Fluorescein) Assay. J. Agric. Food Chem. 2004, 52, 48–54. [Google Scholar] [CrossRef]
- Vásquez, V.; Martínez, R.; Bernal, C. Enzyme-assisted extraction of proteins from the seaweeds Macrocystis pyrifera and C hondracanthus chamissoi: Characterization of the extracts and their bioactive potential. J. Appl. Phycol. 2019, 31, 1999–2010. [Google Scholar] [CrossRef]
- Gamero-vega, G.; Palacios-palacios, M.; Quitral, V. Nutritional Composition and Bioactive Compounds of Red Seaweed: A Mini-Review. J. Food Nutr. Res. 2020, 8, 431–440. [Google Scholar] [CrossRef]
- Leyton, A.; Pezoa-conte, R.; Barriga, A.; Buschmann, A.H.; Mäki-arvela, P.; Mikkola, J.; Lienqueo, M.E. Identi fi cation and ef fi cient extraction method of phlorotannins from the brown seaweed Macrocystis pyrifera using an orthogonal experimental design. ALGAL 2016, 16, 201–208. [Google Scholar] [CrossRef]
- Misurcova, L. Chemical Composition of Seaweeds. In Handbook of Marine Macroalgae: Biotechnology and Applied Phycology; Kim, S.K., Ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2018; pp. 171–192. [Google Scholar]
- Hotimchenko, S. V Fatty Acid Composition of Algae from Habitats with Varying Amounts of Illumination. Biol. Chem. 2002, 28, 218–220. [Google Scholar]
- Harrysson, H. Production of protein extracts from Swedish red, green, and brown seaweeds, Porphyra umbilicalis Kützing, Ulva lactuca Linnaeus, and Saccharina latissima (Linnaeus) J.V. Lamouroux using three different methods. J. Appl. Phycol. 2018, 30, 3565–3580. [Google Scholar] [CrossRef] [Green Version]
- Astorga-españa, M.S.; Rodríguez-galdón, B.; Rodríguez-rodríguez, E.M.; Díaz-romero, C. Journal of Food Composition and Analysis Amino acid content in seaweeds from the Magellan Straits (Chile). J. Food Compos. Anal. 2016, 53, 77–84. [Google Scholar] [CrossRef]
- Burghardt, R.C.; Johnson, G.A. Proline and hydroxyproline metabolism: Implications for animal and human nutrition. HHS Public Access 2013, 40, 1053–1063. [Google Scholar]
- Razak, M.A.; Begum, P.S.; Viswanath, B.; Rajagopal, S. Multifarious Beneficial Effect of Nonessential Amino Acid, Glycine: A Review. Oxidative Med. Cell. Longev. 2017, 2017, 1–10. [Google Scholar] [CrossRef]
- Shannon, E.; Abu-Ghannam, N. Seaweeds as nutraceuticals for health and nutrition. Phycologia 2019, 58, 563–577. [Google Scholar] [CrossRef] [Green Version]
- Ortiz, J.; Romero, N.; Robert, P.; Araya, J.; Lopez-Hernández, J.; Bozzo, C.; Navarrete, E.; Osorio, A.; Rios, A. Dietary fiber, amino acid, fatty acid and tocopherol contents of the edible seaweeds Ulva lactuca and Durvillaea antarctica. Food Chem. 2006, 99, 98–104. [Google Scholar] [CrossRef]
- Sánchez-Machado, D.I.; López-Cervantes, J.; López-Hernández, J.; Paseiro-Losada, P. Fatty acids, total lipid, protein and ash contents of processed edible seaweeds. Food Chem. 2004, 85, 439–444. [Google Scholar] [CrossRef]
- Herrero, M.; Mendiola, J.; Plaza, M.; Ibañez, E. Screening for Bioactive Compounds from Algae. In Advanced Biofuels and Bioproducts; Lee, J.W., Ed.; Springer: New York, NY, USA, 2012; pp. 833–872. [Google Scholar]
- Biancarosa, I.; Belghit, I.; Bruckner, C.G.; Liland, N.S.; Waagbø, R.; Amlund, H.; Heesch, S.; Lock, E.J. Chemical characterization of 21 species of marine macroalgae common in Norwegian waters: Benefits of and limitations to their potential use in food and feed. J. Sci. Food Agric. 2018, 98, 2035–2042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Description | C. chamissoi | M. pyrifera | ||
|---|---|---|---|---|
| Mean | CV | Mean | CV | |
| Moisture (%) | 11.69 a | 0.04 | 6.37 b | 0.05 |
| Protein (%) | 12.16 a | 0.05 | 5.86 b | 0.04 |
| Fat (%) | 0.16 b | 0.09 | 0.74 a | 0.08 |
| Ash (%) | 13.34 b | 0.03 | 43.74 a | 0.02 |
| Carbohidrates (%) | 62.65 a | 0.00 | 43.29 b | 0.02 |
| Description | M. pyrifera | C. chamissoi | ||
|---|---|---|---|---|
| Mean (mg 100 g−1, dw) | CV | Mean (mg 100 g−1, dw) | CV | |
| Essential amino acids | ||||
| Histidine | 4.91 a | 0.10 | 12.14 b | 0.06 |
| Threonine | 9.91 a | 0.15 | 15.67 a | 0.09 |
| Valine | 5.10 a | 0.22 | 19.45 b | 0.10 |
| Methionine | 8.96 a | 0.12 | 11.76 a | 0.13 |
| Phenylalanine | 6.02 a | 0.14 | 18.18 a | 0.12 |
| Isoleucine | 3.58 a | 0.13 | 13.23 b | 0.10 |
| Leucine | 11.83 a | 0.13 | 38.93 b | 0.09 |
| Lysine | 4.66 a | 0.05 | 19.43 b | 0.12 |
| Non-essential amino acids | ||||
| Aspartic Acid | 7.41 a | 0.14 | 21.29 b | 0.12 |
| Glutamic Acid | 15.26 a | 0.11 | 42.72 b | 0.10 |
| Serine | 15.52 a | 0.14 | 57.66 b | 0.08 |
| Glycine | 20.60 a | 0.11 | 59.04 b | 0.05 |
| Arginine | 11.04 a | 0.13 | 50.36 b | 0.07 |
| Alanine | 13.88 a | 0.12 | 28.56 a | 0.09 |
| Tyrosine | 4.38 a | 0.14 | 10.72 a | 0.09 |
| Cystine | ND | ND | ||
| Proline | 22.70 a | 0.14 | 59.81 b | 0.04 |
| Description | M. pyrifera | C. chamissoi | ||
|---|---|---|---|---|
| Fatty Acids (%) | Mean | CV | Mean | CV |
| Arachidonic acid (C20:4, ω 6) | 15.77 | 0.03 | ND | |
| Stearic acid (C18:0) | 10.31 a | 0.02 | 17.22 b | 0.02 |
| Omega 9 (C18:1, ω 9) | 16.40 a | 0.02 | 15.52 a | 0.04 |
| Palmitoleic acid (C16:1, ω 7) | ND | 2.76 | 0.01 | |
| Palmitic acid (C16:0) | 24.69 a | 0.03 | 27.91 b | 0.02 |
| Myristic acid (C14:0) | 10.77 | 0.06 | ND | 0.03 |
| Total saturated | 49.14 a | 0.02 | 45.13 b | 0.02 |
| Total monounsaturated | 16.40 a | 0.04 | 18.29 b | 0.03 |
| Total polyunsaturated | 15.77 | 0.03 | ND | |
| Tocopherols (µg 100 g−1, dw) | ||||
| α-Tocopherol | 2.14 a | 0.14 | 1.21 b | 0.05 |
| δ-Tocopherol | 0.23 a | 0.11 | 0.12 b | 0.02 |
| Description | M. pyrifera | C. chamissoi | ||
|---|---|---|---|---|
| Minerals | Mean (mg kg−1, dw) | CV | Mean (mg kg−1, dw) | CV |
| Copper | 13.19 a | 0.03 | 8.41 a | 0.04 |
| Cadmium | 6.62 a | 0.02 | 1.57 b | 0.02 |
| Nickel | 6.30 a | 0.00 | 3.72 b | 0.01 |
| Calcium | 657.17 a | 0.02 | 413.77 b | 0.02 |
| Magnesium | 912.80 a | 0.03 | 739.48 b | 0.02 |
| Phosphorus | 98.82 a | 0.04 | 119.53 a | 0.04 |
| Iodine | 2.04 a | 0.00 | 0.37 b | 0.01 |
| Arsenic | 0.21 a | 0.01 | 0.32 a | 0.03 |
| Manganese | 143.33 a | 0.02 | 4.81 b | 0.02 |
| Iron | 46.36 a | 0.02 | 8.61 b | 0.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vilcanqui, Y.; Mamani-Apaza, L.O.; Flores, M.; Ortiz-Viedma, J.; Romero, N.; Mariotti-Celis, M.S.; Huamán-Castilla, N.L. Chemical Characterization of Brown and Red Seaweed from Southern Peru, a Sustainable Source of Bioactive and Nutraceutical Compounds. Agronomy 2021, 11, 1669. https://doi.org/10.3390/agronomy11081669
Vilcanqui Y, Mamani-Apaza LO, Flores M, Ortiz-Viedma J, Romero N, Mariotti-Celis MS, Huamán-Castilla NL. Chemical Characterization of Brown and Red Seaweed from Southern Peru, a Sustainable Source of Bioactive and Nutraceutical Compounds. Agronomy. 2021; 11(8):1669. https://doi.org/10.3390/agronomy11081669
Chicago/Turabian StyleVilcanqui, Yesica, Luis Omar Mamani-Apaza, Marcos Flores, Jaime Ortiz-Viedma, Nalda Romero, María Salomé Mariotti-Celis, and Nils Leander Huamán-Castilla. 2021. "Chemical Characterization of Brown and Red Seaweed from Southern Peru, a Sustainable Source of Bioactive and Nutraceutical Compounds" Agronomy 11, no. 8: 1669. https://doi.org/10.3390/agronomy11081669
APA StyleVilcanqui, Y., Mamani-Apaza, L. O., Flores, M., Ortiz-Viedma, J., Romero, N., Mariotti-Celis, M. S., & Huamán-Castilla, N. L. (2021). Chemical Characterization of Brown and Red Seaweed from Southern Peru, a Sustainable Source of Bioactive and Nutraceutical Compounds. Agronomy, 11(8), 1669. https://doi.org/10.3390/agronomy11081669








