Abstract
The pisco industry in Peru generates large amounts of grape pomace, which is a natural source of bioactive compounds with potential nutraceutical applications. Hot pressurized liquid extraction (HPLE) with water-ethanol solvent mixtures (20–60%) at high temperatures (100–160 °C) was applied to recover polyphenols from the skin and seeds of a Peruvian pisco-industry grape-pomace waste. At the same HPLE conditions (60% ethanol, 160 °C), the seed fraction extracts contained ~6 times more total polyphenol and presented ~5 times more antioxidant activity than the extract from the skin fraction. The lowest ethanol concentration (20%) and the highest temperature (160 °C) achieved the highest recovery of flavanols with 163.61 µg/g dw from seeds and 10.37 µg/g dw from skins. The recovery of phenolic acids was maximized at the highest ethanol concentration and temperature with 45.34 µg/g dw from seeds and 6.93 µg/g dw from skins. Flavonols were only recovered from the skin, maximized (17.53 µg/g dw) at 20% of ethanol and the highest temperature. The recovery of specific polyphenols is maximized at specific extraction conditions. These conditions are the same for seed and skin extractions. This alternative method can be used in other agroindustrial wastes in order to recover bioactive compounds with potential applications in the pharmaceutical and food industry.
1. Introduction
Peru produces ~9.5 million L of pisco from Vitis vinifera L. cv. Negra Criolla grape [1], whose production generates significant quantities of grape pomace (skin and seeds), which is an agroindustrial waste without any commercial value causing an environmental management problem [2]. However, both skin and seed fractions of this residue present high concentrations of different specific families of phenolic acids, flavanols, flavonols, and stilbenes [3].
Although the molecular weight of polyphenols can vary between 200 Da and 3500 kDa, the presence of hydroxyl groups in their chemical structure confers bioactive properties related to the prevention of degenerative diseases [4,5,6]. These bioactive compounds are used to produce functional ingredients for the food, nutraceutical, and pharmaceutical industries [7]. For example, phenolic acids like gallic adic has been used to promote the apoptosis in prostate cancer cells [8]. Flavanols like catechin present a notably antioxidant effect and a strong inhibition activities on bacteria, viruses, and fungi [9]. Flavonols like quercetin have been used for treating inflammatory disorders and cardiovascular diseases [10]. Thus, the demand and the international price of polyphenol extracts (which depend on their purity) have been grown significantly in recent years [11]. For example, the price of quercetin (≥95%), resveratrol (≥99%), and epigallocatechin (≥98%) are $1200, $1600, and $33,000 per gram, respectively [12]. Hence, developing sustainable technologies for the optimal extraction of these compounds is still attractive and challenging.
Conventional solid-liquid extraction at an atmospheric pressure is a widely used technology to recover polyphenols from different plant matrices [13]. Typically, this process uses toxic solvents such as acetone, methanol, and hexane, limiting their food products’ applicability. This conventional extraction also suffers from long processing times (>4 h), consumption of high amounts of solvents, and low yields [14,15]. Alternative methods such as Hot Pressurized Liquid Extraction (HPLE) and Ultrasound Assisted Extraction (UAE) combined with food-grade solvents allow us to not only reduce process times but also to increase yields significantly compared to conventional extraction [16,17]. Although both HPLE and UAE methods are recognized as sustainable technologies, it has been shown that HPLE production costs are ~50% lower than UAE for polyphenols’ extraction [18,19].
HPLE is a method that works at high pressures (~10 atm) and temperatures, typically between 60 and 250 °C, can use food-grade solvents, and its yields overcome conventional extraction [13,20]. Mariotti-Celis et al. [21], using a water-ethanol mixture (15%) at 90 °C in HPLE, recovered about two times more total polyphenols from grape pomace than atmospheric extraction with water-acetone (60%) at 30 °C. HPLE extraction conditions can be tuned to favor the recovery of specific polyphenol families such as phenolic acids, flavanols, flavonols, and stilbenes [22]. However, the total polyphenol content of these extracts is relatively low (50–70 mg GAE/g dw) compared to extracts obtained from fresh grapes (>150 mg GAE/g dw) [21,22,23]. The use of resins as absorbent material in a subsequent stage can increase the concentration of these compounds and reduce the presence of undesirable compounds (glucose and fructose) [21,24].
Previous HPLE studies have focused on the recovery of polyphenols from grape pomace of the wine industry. However, none of them have dealt with optimizing the process conditions to recover polyphenols from the Peruvian pisco industry waste [21,22,25,26]. Few of these studies considered the phenolic composition of both grape pomace fractions (skin and seed) separately [23,27]. Therefore, in this research, we evaluated the effect of water-ethanol mixtures (20–60%) at high temperatures (100–160 °C) on the recovery of the total polyphenol content, antioxidant capacity, and specific families of polyphenols of extracts obtained separately from the skins and seeds of Negra Criolla pomace, which is an agro-industrial residue from the Peruvian pisco industry.
2. Materials and Methods
2.1. Grape Pomace
Negra Criolla grape pomace (~5 kg) was collected after the processing of pisco, from The Antonio Biondi e Hijos Winery, Moquegua Region, Peru. The samples were frozen immediately (−20 °C). Before the extraction process, the samples were reduced to a particle size of ~2 mm using a grinder (TSM6A013B Bosch, Gerlingen, Germany).
2.2. Chemical Reagents
Folin-Ciocalteu reagents, sodium hydroxide, DPPH (2.2-Diphenyl-1-picrylhydrazyl), AAPH (2,2′-azobis (2-methyl-propanimidamide) dihydrochloride), and Trolox were acquired from Sigma Aldrich Chemical Co. (St. Louis, MO, USA). Solvents such as ethanol (≥99%) acetone (≥98%) were acquired from J. T. Baker Chemical Co. (Temixco, Mexico). Specific polyphenols, such as gallic acid (98%), ellagic acid (≥97%), protocatechuic acid (≥97%), caffeic acid (≥95%), chlorogenic acid (≥98%), coumaric acid (≥95%), catechin (≥97%), epicatechin (≥98%), epigallocatechin (≥97%), gallocatechin (≥97%), kaempferol (≥97%), and quercetin (≥95%), were purchased from Sigma Aldrich Chemical Co. (St. Louis, MO, USA).
2.3. Pressurized Liquid Extraction Process
A dried sample of ~5 g was placed in an extraction cell (100 mL) and submitted to HPLE in an Accelerated Solvent Extraction system (ASE 150, Dionex, Thermofisher, San Jose, CA, USA). The pressurized extraction (~10 atm) of polyphenols was performed using different mixtures of water-ethanol (20–60%) and high temperatures (100–160 °C). For all obtained extracts, the static extraction time was 5 min with 250 s of nitrogen purge. For control, a conventional extraction process was performed using acetone (60%) at 30 °C for 1 h. After the extraction, the samples were collected and stored in amber vials at −20 °C for the later chemical analysis.
2.4. Determination of Total Polyphenols Fraction (TPF)
The extracts obtained were analyzed to determine the total polyphenols content according to the method proposed by Singleton et al. [28]. To summarize, 4.25 mL of extract, 0.25 mL of Folin-Ciocalteu reagent (1:1 v/v), and 0.5 mL of sodium carbonate (10% w/v) were mixed. The absorbance was measured at 765 nm (UV Visible Spectrometer Genesis 150, Thermo Fisher, San Jose, CA, USA) after a reaction time of 1 h at 20 °C in the dark. The results were expressed as mg of Gallic acid equivalent (GAE) per gram of dried grape pomace.
2.5. Determination of Antioxidant Capacity by DPPH
The antioxidant capacity by DPPH of the extracts was determined with the proposed method by Brand-Williams et al. [29]. In summary, 0.1 mL of diluted extract and 3.9 mL of DPPH solution (0.1 mM) were mixed, and then the mixture was protected from light for 30 min at room temperature. The reduction of the DPPH radical was determined at 517 nm (Visible Genesis 150 UV Spectrometer, Thermo Fisher, San Jose, CA, USA). Simultaneously, 3.9 mL of methanol and 0.1 mL of extract were used as a blank and 3.9 mL of DPPH solution and 0.1 mL of methanol were used as part of the control. Trolox standars were diluted in methanol and used as a positive control. Finally, The IC50 of the sample was measured as the polyphenols concentration necessary to inhibit 50% of the DPPH radical activity.
2.6. Determination of Antioxidant Capacity by ORAC
The ORAC (Oxygen Radical Absorbance Capacity) of the obtained extracts was performed using a fluorescence microplate reader (S1LFTA, Biotek Instruments Inc, Winooski, VT, USA), according to the methodology proposed by Chirinos et al. [30]. The AAPH was used as a generator of peroxyl radicals, Trolox was used as a standard, and fluorescein was used as a fluorescence emitter. Previously, 48 nM fluorescein solution and 153 nM AAPH solution were diluted in a PBS buffer solution (pH 7.4). A sample (blank) of 25 µL of standard Trolox solution or diluted sample was mixed and incubated for 10 min at 37 °C before the automatic injection into the microplate reader. Fluorescence readings were taken at 485 nm (λ: excitation) and 520 nm (emission) every minute for 50 min. The final ORAC values were calculated using the net area under the decay curve and expressed as µmol Trolox equivalents (ET) per gram of skin and seed of grape pomace.
2.7. Polyphenols Profile
The quantification of specific polyphenols was carried out utilizing the methodology proposed by Chirinos et al. [31]. The obtained extracts were previously filtered using a Millipore filter type GV (0.22 µm). Then 20 µL of the samples were injected into the HPLC system (Waters 2695, Waters, Milford, MA, USA) equipped with a photodiode array detector (PAD) (Waters, Milford, MA, USA). An X-terra RP18 (5 µm, 250 mm × 4.6 mm) column (Waters, Milford, MA, USA) was used for phenolic separation at 30 °C. The mobile phase was made from a solvent (A) water:formic acid (95:5, v/v, pH 2) and a solvent (B) acetonitrile. The following gradient was applied: 0–15% B the first 40 min, 15–45% B the next 45 min, and 45–100% B the next 10 min. The flow rate was set to 0.5 mL/min. Finally, polyphenols were identified and quantified by comparing the retention time and area under the curve of different polyphenol standards (gallic acid, ellagic acid, protocatechuic acid, caffeic acid, chlorogenic acid, coumaric acid, catechin, epicatechin, epigallocatechin, gallocatechin, kaempferol, and quercetin). The analyses were performed in triplicate, and the results were expressed as µg of specific polyphenol per gram of dried sample.
2.8. Statistical Analysis
A 2k factorial design with three central points was considered to assess the effect of temperature and ethanol concentration on response variables. Results were presented as mean and coefficient variation. The analysis of variance (ANOVA) and Tukey test were applied to the response variables (p < 0.05) using the Statgraphics Plus statistical program for Windows 4.0 (Statpoint Technologies, Inc., Warrenton, VA, USA).
3. Results and Discussion
3.1. Chemical Characterization of Extracts
The total polyphenols content (TPC) of the extracts obtained from the skins (0.46–1.98 mg GAE/g dw) and seeds (5.66–12.54 mg GAE/g dw) fractions presented significant differences (p < 0.05) (Table 1). In general, an increase in the ethanol content during HPLE allowed a greater recovery of total polyphenols. For example, when ethanol increased from 20% to 60% at 160 °C, the recovery of polyphenols increased by 37% and 18% for skins and seeds extracts, respectively (Table 1).

Table 1.
Chemical characterization of extracts obtained by the HPLE process.
The positive effect of ethanol on total polyphenols recovery was also reported by Mariotti-Celis et al. [21], who found low ethanol concentrations (15%) at 90 °C improved the recovery of polyphenols by 40% compared to pure water under the same conditions. Wijngaard and Brunton [32] found that ethanol in the range of 15% to 50% at 175 °C improved the polyphenols’ recovery by ~12%. Ethanol as a co-solvent in HPLE reduces the solvents’ polarity, improving the solubility of phenolic compounds through dipole-dipole molecular interactions and London dispersion forces [22].
On the other hand, the use of 40% ethanol at 130 °C only recovered ~40% of the total polyphenol content reached when using 60% ethanol at 160 °C (Table 1). In this regard, in previous works, we found that an increase in the temperature and concentration of ethanol favors a greater recovery of polyphenols due to a reduction in the solvent polarity and a greater ability of ethanol to establish intermolecular interactions with polyphenols [21,22].
The use of 60% ethanol at 100 °C allowed us to recover a similar total poly-phenol content compared to the use of 40% ethanol at 130 °C (Table 1). Therefore, the addition of ethanol as a co-solvent during the HPLE process allows us to reduce the temperature from 130 °C to 100 °C without decreasing the recovery yield of total polyphenols.
In turn, when temperature increased from 100 °C to 160 °C using ethanol (60%), the recovery of polyphenols improved ~2.8 and ~1.7 times for skin and seed, respectively (Table 1). High temperatures (>100 °C) increase the kinetic energy of the solvent molecules, favoring the matrix rupture and improving the extraction of phenolic compounds [21,25].
The best process conditions to recover total polyphenols from both skins and seeds fractions were 60% ethanol and 160 °C. Under these conditions, skin and seed yields were ~2.5 and ~1.5 higher, respectively, than conventional extraction with acetone (CE) (Figure 1a). Acetone at atmospheric conditions has a lower polarity (ε: 21.30) than ethanol (ε: 25.02) [33], but ethanol creates more hydrogen bonds with other molecules (α: 0.83) than acetone (α: 0.08) [34]. These specific ethanol-polyphenols interactions can explain the obtained high yields.
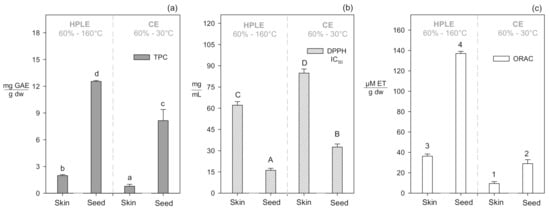
Figure 1.
Chemical characterization of the extracts obtained by the HPLE process and conventional extraction with acetone (CE) for TPC (a), DPPH (b), and ORAC (c). The significant differences (p < 0.05) between the process conditions and grape pomace samples (seed or skin) were established with different lower case letters, capital letters, and numbers for TPC, DPPH, and ORAC, respectively.
It is worth mentioning that others solvents, such as methanol, are more effective than ethanol in HPLE of polyphenols [35]. However, methanol is an objectionable solvent for obtaining both food ingredients and nutraceuticals because of its toxicity.
DPPH and ORAC methods evaluate the capacity of the polyphenols to inhibit a specific radical. The DPPH method evaluates polyphenols’ capacity to neutralize DPPH, which is a free radical that differs from other biologically generated reactive species. The ORAC method measures polyphenols’ capacity to neutralize peroxyl radicals, similar to the free radicals generated within an organism [36,37]. We applied both methods as complementary approximation tests to correlate the antioxidant capacity of polyphenols present in our extracts. HPLE extracts obtained from the seeds and skins of Negra Criolla grape pomace using 60% of ethanol at 160 °C presented the highest inhibiton capacity on DPPH radical with IC50 values of 62.12 mg/mL and 16.16 mg/mL, respectively (Table 1). These skins and seeds extracts were 1.4 and 2 times more effective to inhibit the DPPH radical, respectively, than those obtained using conventional extraction with acetone (60%, 30 °C) (Figure 1b). HPLE extracts obtained under the same conditions (60%, 160 °C) presented the highest ORAC values as well, with 26.33 µMTE/g dw and 187.65 µMTE/g dw for skins and seeds extracts, respectively (Table 1). These HPLE extracts’ capacities to inhibit the peroxyl radical (ORAC) were about four and ten times higher for skins and seeds, respectively, than the corresponding acetone extracts (Figure 1c).
Applying high temperatures (>120 °C) during HPLE favors the recovery of polyphenols but also the formation of some undesirable antioxidant compounds, increasing the free radicals’ inhibition capacity of the extracts [38]. Although the obtained absolute DPPH and ORAC values were different, the observed trend for the antioxidant capacity was the same with both methods.
3.2. Polyphenol Profile
The contents of some target phenolic acids, flavanols, and flavonols present in the extracts were quantified (Table 2). Seed extracts contained much more of these polyphenols than skins extracts.

Table 2.
Chemical characterization of extracts obtained from Negra Criolla seed and skins using ethanol at high temperatures.
Gallic acid was the most abundant phenolic acid in our extracts. Increasing ethanol concentration (from 20% to 60%) raises total phenolic acid recovery from skins and seeds (Table 2). Seed extracts obtained at 160 °C with 60% EtOH contained the highest amount of gallic acid (21.6 µg/gdw). This concentration is 10 times higher than that of skins extracts obtained under the same conditions. It has been reported previously that the seeds of grape pomace are rich in gallic acid and are typically galloylated with polymers of high molecular weight (tannins) [4,5]. High temperatures in HPLE promote the hydrolysis of these polymers, increasing the gallic acid release [7,18,19].
At the same temperature, the recovery of the flavanols catechin and epicatechin from seeds is favored at the lowest ethanol concentration evaluated (Table 2). The highest amounts of catechin (96.3 µg/gdw) and epicatechin (41.7 µg/gdw) were recovered from the seed fraction at 20% EtOH and 160 °C. These recoveries were ~90 and ~6 times higher, respectively, than those obtained from the skin fraction under the same conditions (Table 2). In a previous study [22], we observed a similiar behaviour for the HPLE of flavanols from Carmenere grape pomace, in which their recovery was favoured for ethanol contents up to 32.2%. When higher ethanol amounts were added to the HPLE solvent, the flavanols recovery diminished abruptly, suggesting that the threshold polarity of flavonols could be reached in a range of 20–32.5% of ethanol addition. Observed differences can be atributed not only to the variety of grape but also to the extracted fraction of grape pomace [23]. The seeds and skins of grape pomace also contain polymers of high molecular weight. Those in seeds (procyanidins) are mainly formed from catechin and epicatechin monomers [39], while those in skins (prodelphinidins) are mainly formed from epigallocatechin and gallocatechin monomers [40]. In HPLE, high temperatures favor the release of these monomers into the extracts [7].
Only the skin extracts contained detectable amounts of the target flavonols. The skin extracts obtained at 20% EtOH and 160 °C contained the highest quantities of quercetin (12.4 µg/gdw) and kaempferol (2.9 µg/gdw) (Table 2). Huaman-Castilla et al. [22] argued that the presence of ketone groups in the chemical structure of flavonols favors the polar interactions with water molecules. Consequently, these compounds’ solubility is higher at low ethanol concentrations.
In Figure 2, we summarize the observations above, associating ethanol content in the solvent with the extracted matrix (skins, seeds) and the specific polyphenol families considered in this study (phenolic acids, flavanols, flavonols).
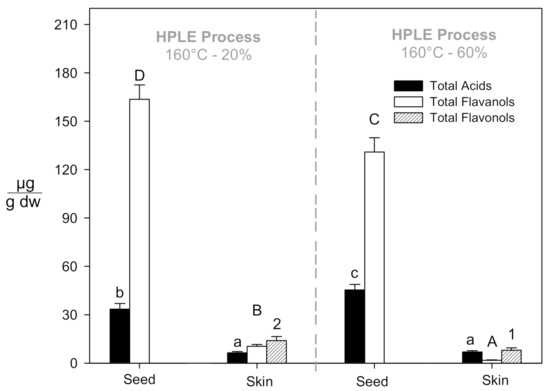
Figure 2.
Specific polyphenol families recovered in the HPLE extracts at the highest temperature and maximum and minimum ethanol concentrations. The significant differences (p < 0.05) between the different process conditions and grape pomace samples (seed or skin) were established with different lower-case letters, capital letters, and numbers for total acids, total flavanols, and total flavonols, respectively.
The best HPLE conditions to obtain phenolic acids were 60% EtOH and 160 °C, yielding 44.6 µg/gdw and 6.3 µg/gdw of phenolic acid from the seeds and skins, respectively. Flavanols recovery was maximized at 20% EtOH and 160 °C, yielding 10.37 µg/gdw and 163.61 µg/gdw of total flavanols from the skin and seeds fractions, respectively. Flavonols were only detected in skin extracts, reaching the highest recovery (17.35 µg/g) with 20% EtOH and 160 °C.
4. Conclusions
Compared to the conventional extraction (1 atm, 60% acetone, 30 °C), high concentrations of ethanol (60%) and high temperatures (160 °C) in HPLE recovered ~2.5 and ~1.5 more polyphenols from skins and seeds, respectively, of Negra Criolla grape pomace. These HPLE extracts also showed higher antioxidant activity than conventional extracts, 45% and 58% higher DPPH, and about four and ten higher ORAC for skin and seeds, respectively.
The recovery of phenolic acids from Negra Criolla grape pomace was maximized in HPLE at 60% EtOH and 160 °C, for both the skins (6.93 µg/g dw) and seeds fractions (45.34 µg/g dw). These seed extracts contained the highest amount of gallic acid (21.6 µg/gdw) and 10 times more than skin extracts. Flavanols recovery was favored at the lowest ethanol content, and the highest temperature tested. The seed extracts obtained under these conditions contained 96.3 µg/gdw of catechin and 41.72 µg/gdw of epicatechin. Flavonols, such as quercetin and kaempferol, were only detected in skin extracts, and the lowest ethanol concentrations (20%) and the highest temperature (160 °C) favored their recovery (17.53 µg/gdw).
Compared to conventional extraction with acetone, HPLE operated with water-ethanol mixtures and at high temperatures, recovered more polyphenols, and yielded extracts with higher antioxidant capacity from a local agro-industrial waste, such as Negra Criolla grape pomace. The selective polyphenols recovery can be tuned by merely changing the ethanol content of the HPLE solvent. The seeds of Negra Criolla grape pomace are an excellent source of phenolic acids and flavonols, while only the skin of this native Peruvian agro-industrial waste contains flavonols.
Author Contributions
Designed the experiments and analyzed the data, E.E.A.-A., N.C.L.-C., and N.L.H.-C. Performed the experiments, E.E.A.-A., O.M.L.-V., and N.C.L.-C. Wrote and revised the paper, J.R.P.-C., M.M.-C., E.E.A.-A., N.L.H.-C., and M.S.M.-C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received funding from Universidad Nacional de Moquegua-Perú through Resolution C.O. N° 562-2017-UNAM.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- CONAPISCO (Comisión Nacional del Pisco) Estadisticas. Available online: https://conapisco.org.pe/estadisticas.html (accessed on 10 January 2021).
- Maicas, S. Sustainability of Wine Production. Sustainability 2020, 12, 559. [Google Scholar] [CrossRef]
- Xia, E.-Q.; Deng, G.-F.; Guo, Y.-J.; Li, H.-B. Biological Activities of polyphenols from grapes. Int. J. Mol. Sci. 2014, 11, 622–646. [Google Scholar] [CrossRef] [PubMed]
- Huaman-Castilla, N.L.; Mariotti-Celis, M.S.; Perez-Correa, J.R. Polyphenols of Carménère grapes. Mini. Rev. Org. Chem. 2017, 14, 176–186. [Google Scholar] [CrossRef] [PubMed]
- Obreque-Slier, E.; Peña-Neira, Á.; López-Solís, R. Precipitation of low molecular weight phenolic compounds of grape seeds cv. Carménère (Vitis vinifera L.) by whole saliva. Eur. Food Res. Technol. 2011, 232, 113–121. [Google Scholar] [CrossRef]
- Peixoto, C.M.; Dias, M.I.; Alves, M.J.; Calhelha, R.C.; Barros, L.; Pinho, S.P.; Ferreira, I.C.F.R. Grape pomace as a source of phenolic compounds and diverse bioactive properties. Food Chem. 2018, 253, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Huamán-Castilla, N.L.; Campos, D.; García-Ríos, D.; Parada, J.; Martínez-Cifuentes, M.; Mariotti-Celis, M.S.; Pérez-Correa, J.R. Chemical properties of vitis vinifera Carménère pomace extracts obtained by hot pressurized liquid extraction, and their inhibitory effect on type 2 diabetes mellitus related enzymes. Antioxidants 2021, 10, 472. [Google Scholar] [CrossRef] [PubMed]
- Reddivari, L.; Vanamala, J.; Safe, S.H.; Miller, J.C. The bioactive compounds α-chaconine and gallic acid in potato extracts decrease survival and induce apoptosis in LNCaP and PC3 prostate cancer cells. Nutr. Cancer 2010, 62, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.N.D.; Nascimento, T.D.; Gondim, B.; Velo, M.; Rêgo, R.D.A.; Neto, R.D.; Machado, J.; Silva, M.D.; Araújo, H.; Fonseca, M.; et al. Catechins as model bioactive compounds for biomedical applications. Curr. Pharm. Des. 2020, 26, 4032–4047. [Google Scholar] [CrossRef]
- Batiha, G.E.S.; Beshbishy, A.M.; Ikram, M.; Mulla, Z.S.; El-Hack, M.E.A.; Taha, A.E.; Algammal, A.M.; Elewa, Y.H.A. The pharmacological activity, biochemical properties, and pharmacokinetics of the major natural polyphenolic flavonoid: Quercetin. Foods 2020, 9, 374. [Google Scholar] [CrossRef]
- Grand View Research Polyphenols Market Size, Share & Trends Analysis Report By Product (Grape Seed, Green Tea, Cocoa), By Application (Beverages, Food, Feed, Dietary Supplements, Cosmetics), And Segment Forecasts, 2019–2025. Available online: https://www.grandviewresearch.com/industry-analysis/polyphenols-market-analysis (accessed on 15 January 2021).
- SIGMA ALDRICH Catalog Products. Available online: http://www.sigmaaldrich.com/ (accessed on 6 March 2021).
- Ameer, K.; Shahbaz, H.M.; Kwon, J.H. Green extraction methods for polyphenols from plant matrices and their byproducts: A Review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 295–315. [Google Scholar] [CrossRef]
- Ajila, C.M.; Brar, K.; Verma, M. Extraction and analysis of polyphenols: Recent trends. Crit. Rev. Biotechnol. 2011, 31, 227–249. [Google Scholar] [CrossRef]
- Osorio-tobo, J.F. Recent advances and comparisons of conventional and alternative extraction techniques of phenolic compounds. J. Food Sci. Technol. 2020, 57, 4299–4315. [Google Scholar] [CrossRef]
- Putnik, P.; Barba, F.J.; Španić, I.; Zorić, Z.; Dragović-Uzelac, V.; Kovačević, D.B. Green extraction approach for the recovery of polyphenols from Croatian olive leaves (Olea europea). Food Bioprod. Process. 2017, 106, 19–28. [Google Scholar] [CrossRef]
- Bubalo, M.C.; Vidović, S.; Redovniković, I.R.; Jokić, S. Green solvents for green technologies. J. Chem. Technol. Biotechnol. 2015, 90, 1631–1639. [Google Scholar] [CrossRef]
- Santos, D.T.; Veggi, P.C.; Meireles, M.A.A. Optimization and economic evaluation of pressurized liquid extraction of phenolic compounds from jabuticaba skins. J. Food Eng. 2012, 108, 444–452. [Google Scholar] [CrossRef]
- Santos, D.T.; Veggi, P.C.; Meireles, M.A.A. Extraction of antioxidant compounds from Jabuticaba (Myrciaria cauliflora) skins: Yield, composition and economical evaluation. J. Food Eng. 2010, 101, 23–31. [Google Scholar] [CrossRef]
- Mustafa, A.; Turner, C. Pressurized liquid extraction as a green approach in food and herbal plants extraction: A review. Anal. Chim. Acta 2011, 703, 8–18. [Google Scholar] [CrossRef]
- Mariotti-Celis, M.S.; Martínez-Cifuentes, M.; Huamán-Castilla, N.; Pedreschi, F.; Iglesias-Rebolledo, N.; Pérez-Correa, J.R. Impact of an integrated process of hot pressurised liquid extraction–macroporous resin purification over the polyphenols, hydroxymethylfurfural and reducing sugars content of Vitis vinifera ‘Carménère’ pomace extracts. Int. J. Food Sci. Technol. 2018, 53, 1072–1078. [Google Scholar] [CrossRef]
- Huaman-Castilla, N.L.; Mart, M.; Camilo, C.; Pedreschi, F.; Mariotti-Celis, M.; Pérez-Correa, J.R. The impact of temperature and ethanol concentration on the global recovery of specific polyphenols in an integrated HPLE/RP process on Carménère pomace extracts. Molecules 2019, 24, 3145. [Google Scholar] [CrossRef]
- Duba, K.S.; Casazza, A.A.; Mohamed, H.B.; Perego, P.; Fiori, L. Extraction of polyphenols from grape skins and defatted grape seeds using subcritical water: Experiments and modeling. Food Bioprod. Process. 2015, 94, 29–38. [Google Scholar] [CrossRef]
- Niculescu, V.C.; Miricioiu, M.; Geana, E.I.; Ionete, R.E.; Paun, N.; Parvulescu, V. Silica mesoporous materials—An efficient sorbent for wine polyphenols separation. Rev. Chim. 2019, 70, 1513–1517. [Google Scholar] [CrossRef]
- Vergara-Salinas, J.R.; Bulnes, P.; Zúñiga, M.C.; Pérez-Jiménez, J.; Torres, J.L.; Mateos-Martín, M.L.; Agosin, E.; Pérez-Correa, J.R. Effect of pressurized hot water extraction on antioxidants from grape pomace before and after enological fermentation. J. Agric. Food Chem. 2013, 61, 6929–6936. [Google Scholar] [CrossRef] [PubMed]
- Otero-Pareja, M.J.; Casas, L.; Fernández-Ponce, M.T.; Mantell, C.; Martinez de la Ossa, E.J. Green extraction of antioxidants from different varieties of red grape pomace. Molecules 2015, 20, 9686–9702. [Google Scholar] [CrossRef]
- Ju, Z.Y.; Howard, L.R. Effects of solvent and temperature on pressurized liquid extraction of anthocyanins and total phenolics from dried red grape skin. J. Agric. Food Chem. 2003, 51, 5207–5213. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, J.A., Jr. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Chirinos, R.; Campos, D.; Warnier, M.; Pedreschi, R.; Rees, J.F.; Larondelle, Y. Antioxidant properties of mashua (Tropaeolum tuberosum) phenolic extracts against oxidative damage using biological in vitro assays. Food Chem. 2008, 111, 98–105. [Google Scholar] [CrossRef]
- Chirinos, R.; Pedreschi, R.; Rogez, H.; Larondelle, Y.; Campos, D. Phenolic compound contents and antioxidant activity in plants with nutritional and/or medicinal properties from the Peruvian Andean region. Ind. Crops Prod. 2013, 47, 145–152. [Google Scholar] [CrossRef]
- Wijngaard, H.; Brunton, N. The optimization of extraction of antioxidants from apple pomace by pressurized liquids. J. Agric. Food Chem. 2009, 57, 10625–10631. [Google Scholar] [CrossRef]
- Mohsen-Nia, M.; Amiri, H.; Jazi, B. Dielectric constants of water, methanol, ethanol, butanol and acetone: Measurement and computational study. J. Solut. Chem. 2010, 39, 701–708. [Google Scholar] [CrossRef]
- Jessop, P.G.; Jessop, D.A.; Fu, D.; Phan, L. Solvatochromic parameters for solvents of interest in green chemistry. Green Chem. 2012, 14, 1245–1259. [Google Scholar] [CrossRef]
- Piñeiro, Z.; Palma, M.; Barroso, C.G. Determination of catechins by means of extraction with pressurized liquids. J. Chromatogr. A 2004, 1026, 19–23. [Google Scholar] [CrossRef]
- Roy, M.K.; Koide, M.; Rao, T.P.; Okubo, T.; Ogasawara, Y.; Juneja, L.R. ORAC and DPPH assay comparison to assess antioxidant capacity of tea infusions: Relationship between total polyphenol and individual catechin content. Int. J. Food Sci. Nutr. 2010, 61, 109–124. [Google Scholar] [CrossRef]
- Karadag, A.; Ozcelik, B.; Saner, S. Review of methods to determine antioxidant capacities review of methods to determine antioxidant capacities. Food Anal. Methods 2009, 2, 41–60. [Google Scholar] [CrossRef]
- Plaza, M.; Abrahamsson, V.; Turner, C. Extraction and neoformation of antioxidant compounds by pressurized hot water extraction from apple byproducts. J. Agric. Food Chem. 2013, 61, 5500–5510. [Google Scholar] [CrossRef]
- Fuleki, T.; Ricardo da Silva, J.M. Catechin and procyanidin composition of seeds from grape cultivars grown in Ontario. J. Agric. Food Chem. 1997, 45, 1156–1160. [Google Scholar] [CrossRef]
- Ćurko, N.; Ganić, K.K.; Gracin, L.; Dapić, M.; Jourdes, M.; Teissedre, P.L. Characterization of seed and skin polyphenolic extracts of two red grape cultivars grown in Croatia and their sensory perception in a wine model medium. Food Chem. 2014, 145, 15–22. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).