Effect of Differentiated Nitrogen Fertilization on the Enzymatic Activity of the Soil for Sweet Potato (Ipomoea batatas L. [Lam.]) Cultivation
Abstract
1. Introduction
- the first alternative hypothesis assumes that increasing nitrogen fertilization against the background of constant phosphorus-potassium fertilization will optimize the biological balance in the soil by changing the activity of selected soil enzymes (dehydrogenase, phosphatase, urease and protease), against the null hypothesis that nitrogen fertilization does not cause significant changes in their activity;
- the second alternative hypothesis assumes that increasing nitrogen fertilization against the background of constant phosphorus-potassium fertilization will change the nitrogen forms in the soil, against the null hypothesis that nitrogen fertilization will not change the balance of nitrogen forms in the soil;
- the third alternative hypothesis assumes that increasing nitrogen fertilization against the background of constant phosphorus-potassium fertilization will improve the C:N ratio, against the null hypothesis that fertilization with this component will not change the C:N ratio.
2. Materials and Methods
2.1. Field Research
2.2. Soil Sampling
2.3. Methodology for the Assessment of Soil Samples
2.4. Testing the Enzymatic Activity and Physico-chemical Characteristics of Soil
- the activity of dehydrogenases was expressed in cm3 H2, required to reduce triphenyltetrazole chloride (TTC) to TFP (triphenyl formazan) [28];
- phosphatases, in moles of p-nitrophenol (PNP), obtained from 4-nitrophenyl phosphate sodium [29];
- urease, in mg N-NH4+ obtained from hydrolysed urea [31];
- The activity of the tested enzymes was analyzed in soil with natural humidity, and the results were converted into absolutely dry soil mass.
- Simultaneously, the samples were tested for the content of total organic carbon, pHKCl and mineral forms of nitrogen (NH4+-N and NO3− N) [23].
- dehydrogenases (ADh), by the Thalmann method [28], using a 1% solution of TTC as a substrate and 96-h incubation at 37 °C, expressing their activity in cm3 H2 kg−1 d−1 (for 1 kg of soil in 24 h).
- phosphatase (AF), by the method of Tabatabai and Bremner [29], using 0.8% sodium p-nitrophenyl phosphate as a substrate and a 1hour incubation at 37 °C; enzyme activity was expressed in mmol PNP kg−1 h−1 (p-nitrophenol per 1 kg of soil in 1 h);
- urease (AU), by the method of Zantu and Bremner [30], using a 2.5% urea solution as a substrate and an 18-h incubation at 37 °C, expressing the enzyme activity in mg N-NH4 + kg−1 h−1 N-NH4 + (for 1 kg of soil in 1 h);
- proteases (AP), by the method of Ladd and Butler [31], using a 1% solution of sodium caseinate as a substrate and using a 1-h incubation at 50 °C, expressing the enzyme activity in mg of tyrosine kg−1 h−1 (per 1 kg of soil in 1 h).
2.5. Statistical Analysis
2.6. Weather Conditions
3. Results
3.1. Soil Characteristics Prior to the Experiment
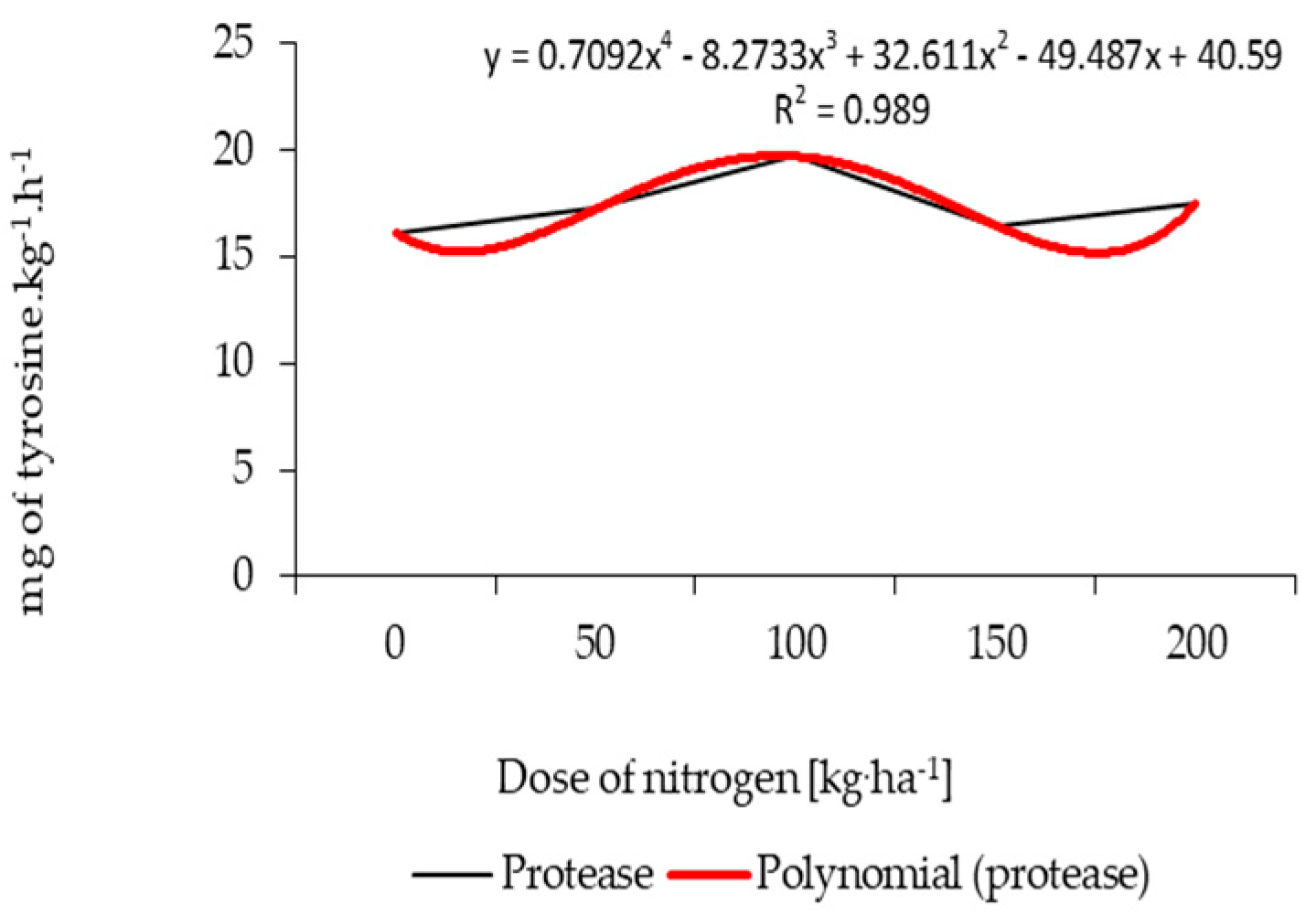
3.2. Variability of Soil Enzymatic Activity
3.3. Variability of Physico-Chemical Characteristics of Soil
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| Adh | dehydrogenase |
| AF | phosphatases |
| AP | protease |
| AU | urease |
| DEA | denitrifying enzyme activity |
| DP | denitrification potential |
| RESP | anaerobic respiration C-carbon |
| C:N | Carbon to Nitrogen ratio |
| Fm | maximum chlorophyll fluorescence |
| N | nitrogen |
| N-NH4 | Ammonium-Nitrogen |
| N-NO3 | Nitrate-NO3 |
| NPK | multicomponent mineral fertilizers containing nitrogen (N), phosphorus (P) and potassium (K) in a form available to plants |
| NUE | nutrient use efficiency |
| PLFA | phospholipid fatty acid |
| PS II (Y) | actual photochemical efficiency of photosystem PSII |
| qP | photochemical chlorophyll fluorescence quenching |
References
- Hawkesford, M.; Kopriva, S.; De Kok, L.J. Efficiency in the use of nutrients in plants—Concepts and approaches. Jumper 2014. [Google Scholar] [CrossRef]
- Brentrup, F.; Palliere, C. Efficiency of Nitrogen Consumption as an Agri-Environmental Indicator. 2017. Available online: http://www.oecd.org/tad/sustainable-agriculture/44810433.pdf (accessed on 23 June 2020).
- Duncan, E.G.; O’Sullivan, C.A.; Roper, M.M.; Biggs, J.S.; Peoples, M.B. Wpływ jednoczesnego stosowania azotu z fosforem, potasem i siarką na pozorną wydajność stosowania nawozów azotowych, plon ziarna i zawartość białka w pszenicy. Field Crops Res. 2018, 226, 56–65. [Google Scholar] [CrossRef]
- Rietra, R.P.J.J.; Heinen, M.; Dimkpa, C.O.; Bindraban, P.S. Effect of nutrient and antagonistic synergism on yields and fertilizer use efficiency. Commun. Soil. Sci. Plant Anal. 2017, 48, 1895–1920. [Google Scholar] [CrossRef]
- Sawicka, B.; Michałek, W.; Pszczółkowski, P.; Danilčenko, H. Variation in productivity of sweet potato (Ipomoea batatas L. [Lam.]) under different conditions of nitrogen fertilization. Zemdirb. Agric. 2018, 105, 149–158. [Google Scholar] [CrossRef]
- Sawicka, B. Efficiency of nutrient utilization for food security, sustainable development and resilience. In Encyclopedia of UN Sustainable Development Goals; Leal Filho, W., Azul, A., Brandli, L., Özuyar, P., Wall, T., Zero, H., Eds.; Springer: Cham, Switzerland, 2020; pp. 1–18. [Google Scholar] [CrossRef]
- Solano, J.; Corrêa, C.; Gouveia, A.; Evangelista, R.; Cardoso, A.; Ming, L.C. The quality of sweet potatoes with different doses and separate application of nitrogen fertilizer. Acta Hortic. 2018, 1194, 79–84. [Google Scholar] [CrossRef]
- Council Directive of 12 December 1991 Concerning the Protection of Waters against Pollution Caused by Nitrates from Agricultural Sources (91/676/EEC). 1991. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX:31991L0676 (accessed on 14 December 2020).
- Regulation of the Minister of the Environment of 23 December 2002 on Detailed Requirements to be Met by Action Programs Aimed at Limiting the Outflow of Nitrogen from Agricultural Sources, OJ 2003 No. 4 item 44; 2002. Available online: https://isap.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=WDU20030040044 (accessed on 14 December 2020). (In Polish)
- Regulation of the Minister of the Environment of 12 February 2020 on Detailed Requirements to be Met by Action Programs Aimed at Limiting the Outflow of Nitrogen from Agricultural Sources, OJ 2020 No. 4 item 243; 2020. Available online: https://isap.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=WDU20200000243 (accessed on 14 December 2020). (In Polish)
- Barabasz, W.; Albińska, D.; Jaśkowska, M.; Lipiec, J. Biological effects of mineral nitrogen fertilization on soil microorganisms. Pol. J. Environ. Stud. 2002, 11, 193–198. [Google Scholar]
- Bielińska, E.J.; Mocek, A.; Paul-Lis, M. Impact of tillage system cultivation on enzymatic activity of typologically diverse soils. J. Res. Appl. Agric. Eng. 2008, 53, 10–13. [Google Scholar]
- Wang, L.; Zhao, Y.; Al-Kaisi, M.; Yang, J.; Chen, Y.; Sui, P. Impact of seven different crop rotations on selected indicators of soil condition and wheat productivity. Agronomy 2020, 10, 235. [Google Scholar] [CrossRef]
- Gostkowska, K.; Furczak, J.; Domżał, H.; Bielińska, E.J. Suitability of some biochemical and microbiological tests for the degradation degree of podzolic soil on the background of it differentiated usage. Pol. J. Soil Sci. 1998, 30, 69–78. [Google Scholar]
- Sekaran, U.; McCoy, C.; Kumar, S.; Subramanian, S. Soil microbial community and enzymatic activity in the response to nitrogen management and landscape sites in switch grass (Panicum virgatum L.). Bioenergy 2019, 1–16. [Google Scholar] [CrossRef]
- Bielińska, E.J.; Stankowski, S.; Maciorowski, R.; Meller, E.; Tomaszewicz, T.; Honzik, R.; Ustiak, S. Biological activity and certain physico-chemicalproperties of reclamation layers and brown coalblanket claystones of “strymickavysypka” object (Chomutov, Czech Republic). In Proceedings of the ISTRO-Conference Brno 2005, Troubsko, June 29–July 1 2005; Section II—Poster Presentation; International Soil Tillage Research Organization, Research Institute for Fodder Crops, Ltd.: Troubsko, Czech Republic, 2005; pp. 143–146. ISBN 80-86908-01-1. [Google Scholar]
- Błońska, E.; Lasota, J.; Zwydak, M. The relationship between soil properties, enzyme activity and land use. For. Res. Pap. 2017, 78, 39–44. [Google Scholar] [CrossRef]
- Krochmal-Marczak, B.; Cebulak, T.; Kapusta, I.; Oszmiański, J.; Kaszuba, J.; Żurek, N. The Content of Phenolic Acids and Flavonols in the Leaves of Nine Varieties of Sweet Potatoes (Ipomoea batatas L.) Depending on Their Development, Grown in Central Europe. Molecules 2020, 25, 3473. [Google Scholar] [CrossRef] [PubMed]
- Krochmal-Marczak, B.; Sawicka, B.; Tobiasz-Salach, R. Impact of cultivations technology on the yield of sweet potato (Ipomoea batatas L.). Emir. J. Food Agric. 2018, 30, 978–983. [Google Scholar]
- WRB. World Reference Base for Soil Resources 2014 International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; World Soil Resources Reports 106; Food and Agriculture Organization of The United Nations: Rome, Italy, 2014. [Google Scholar]
- Bleinholder, H.; Weber, E.; Feller, C.; Hess, M.; Wicke, H.; Meier, U.; Boom, T.; Lancashire, P.D.; Buhr, L.; Hack, H.; et al. Growth Stages of Mono- and Dicotyledonous Plants; BBCH Monograph; Uwe, M., Ed.; Blackwell Wissenschafts-Verlag: Pairs, France, 2001; pp. 1–160. [Google Scholar]
- Ngailo, S.; Shimelis, H.; Sibiya, J.; Mtunda, K. Growing sweet potatoes for resistance to sweet potato viral disease and increasing yields: Advances and challenges. Afr. J. Agric. Res. 2013, 8, 3202–3215. [Google Scholar] [CrossRef]
- Mocek, A. Soil Science; State Scientific Publisher: Warszawa, Poland, 2015. [Google Scholar]
- PN-R-04020, 1994+AZ1. In Chemical and Agricultural Analysis of Soil; Polish Committee for Standardization: Warszawa, Poland, 2004.
- PN-R-04023. In Chemical and Agricultural Analysis of Soil. Determination of Available Phosphorus Content in Mineral Soils; Polish Committee for Standardization: Warsaw, Poland, 1996.
- PN–R–0403. In Chemical and Agricultural Analysis of Soil, Sampling; Polish Committee for Standardization: Warsaw, Poland, 1997.
- Fertilization Recommendations, Part I. Limits for the Evaluation of the Content in Macro-Soils Micronutrients; Institute of Soil Science and Plant Cultivation: Puławy, Poland, 1990; pp. 1–26.
- Thalmann, A. ZurMethodik der Bestimmung der Dehydrogenase Aktivität in Boden MittelsTriphenyltetrazoliumchlorid (TTC). Landwirtschaft. Forsh. 1968, 21, 249–284. [Google Scholar]
- Tabatabai, M.A.; Bremner, J.M. The use of p-nitrophenyl phosphate to determine soil phosphatase activity. Soil Boil. Biochem. 1969, 1, 301–307. [Google Scholar] [CrossRef]
- Zantua, M.I. Urease Activity in Soils; Retrospective Theses and Dissertations an authorized administrator of Iowa State University Digital Repository 6234; Iowa State University: Ames, IA, USA, 1976. [Google Scholar]
- Ladd, J.N.; Butler, J.H.A. Short-Term Assays of Soil Proteolytic Enzyme Activities Using Proteins and Dipeptide Derivatives as Substrates. Soil Biol. Biochem. 1972, 4, 19–30. [Google Scholar] [CrossRef]
- Von Mersi, W.; Schinner, F. An improved and accurate method for determining the dehydrogenase activity of soil with iodonitrotetrazolium chloride. Biol. Fertil. Soils 1991, 11, 216–220. [Google Scholar] [CrossRef]
- Wolińska, A. Dehydrogenase activity of soil enzymes and oxygen availability in the reoxidation process of selected mineral Polish soils. Dissertations and Monographs. Acta Agroph. 2010, 180, 1234–4125. [Google Scholar]
- SAS. Enterprise 4.2 Program SAS/STAT®9.2. Users Guide; SAS: Raleigh, NC, USA, 2008. [Google Scholar]
- Trętowski, J.; Wójcik, A.R. Methodology of Agricultural Experiments; Agricultural and Pedagogical University in Siedlce: Siedlce, Poland, 1991; pp. 331–334. [Google Scholar]
- Warzyński, H.; Sosnowska, A.; Harasimiuk, A. Effect of variable content of organic matter and carbonates on results of determination of granulometric composition by means of Casagrande’s areometric method in modification by Prószyński. Soil Sci. Ann. 2018, 69, 39–48. [Google Scholar] [CrossRef]
- Natywa, M.; Sawicka, A.; Wolna-Murawka, A. Microbial and enzymatic activity in the soil under maize crop in relation to differentiated nitrogen fertilisation. Water Environ. Rural Areas 2010, 10, 111–120. [Google Scholar]
- Bielińska, E.J.; Mocek-Płóciniak, A. Impact of the tillage system on the soil enzymatic activity. Arch. Environ. Protect. 2012, 38, 75–82. [Google Scholar] [CrossRef]
- Koper, J.; Piotrowska, A.; Siwik, A. The index of soil fertility in a long-term experiment with crop-rotation and monoculture. Probl.NotebooksProgr. Agric. Sci. 1999, 465, 461–470. [Google Scholar]
- Majchrzak, L.; Sawinska, Z.; Natywa, M.; Skrzypczak, G.; Głowicka-Wołoszyn, R. Impact of Different Tillage Systems on Soil Dehydrogenase Activity and Spring Wheat Infection. J. Agric. Sci. Technol. 2016, 18, 1871–1881. [Google Scholar]
- Vetanovetz, R.; Peterson, J. Effect of carbon source and nitrogen on urease activity in a sphagnum peat medium. Commun. Soil Sci. Plant Anal. 1992, 23, 379–388. [Google Scholar] [CrossRef]
- Burns, R.G.; DeForest, J.L.; Marxsen, J.; Sinsabaugh, R.L.; Stromberger, M.E.; Wallenstein, M.D.; Weintraub, M.N.; Zoppini, A.M. Soil enzymes in a changing environment: Current knowledge and future directions. Soil Biol. Biochem. 2013, 58, 216–234. [Google Scholar] [CrossRef]
- Trevors, J.T. Effect of substrate concentration, inorganic nitrogen, O2 concentration, temperature and pH on dehydrogenises activity in soil. Plant Soil 1984, 77, 285–293. [Google Scholar] [CrossRef]
- Brzezińska, M.; Włodarczyk, T. Enzymes of intracellular redox transformations (oxidoreductases). Acta Agroph. 2005, 3, 11–26. [Google Scholar]
- Yang, L.; Li, T.; Lemcoff, J.H.; Cohen, S. Fertilization regulates soil enzymatic activity and fertility dynamics in a cucumber field. Sci. Hortic. 2008, 116, 21–26. [Google Scholar] [CrossRef]
- Kucharski, J.; Ciećko, Z.; Niewolak, T.; Niklewska-Larska, T. Activity of microorganisms in soils classified as various agricultural utility complexes fertilized with mineral nitrogen. Acta Acad. Agric. Tech. Olst. Agric. 1996, 62, 25–35. [Google Scholar]
- Smyk, B.; Różycki, E.; Barabasz, W. Impact apply mineral nitrogen fertilizers (N and NPK) on occurrence of nitroisoamines and mycotoxins in mountain soils grassy ecosystems. Probl. Notebooks Progr. Agric. Sci. 1989, 380, 151. [Google Scholar]
- Natywa, M.; Selwet, M.; Maciejewski, T. Effect of some agrotechnical factors on the number and activity soil microorganisms. Frag. Agron. 2014, 31, 56–63. [Google Scholar]
- Tarafdar, J.C.; Classen, N. Organic phosphorus compounds as a phosphorus source for higher plants through the activity of phosphates produced by plant roots and microorganisms. Biol. Fert. Soils 1988, 5, 308–312. [Google Scholar] [CrossRef]
- Šimek, M.; Hopkins, D.W. Regulation of potential denitrification by soil pH in long-term fertilized arable soils. Biol. Fert. Soils 1999, 30, 41–47. [Google Scholar] [CrossRef]
- Nazarkiewicz, M.; Kaniuczak, J. Effect of liming and mineral fertilization on the enzymatic activity of grey-brown podzolic soils formed from loess. Pol. J. Soil Sci. 2008, 12, 2001–2007. [Google Scholar]
- Koper, J.; Lemanowicz, J.; Igras, J. The effect of fertilization on the phosphatase activity and the content of some phosphorus forms. Ann. Univ. Mariae Curie Skłodowska 2004, 49, 679–686. [Google Scholar]
- Mijangos, I.; Perez, R.; Albizu, I.; Garbisu, C. Effects of fertilization and tillage on soil biological parameters. Enzyme Microb. Technol. 2006, 40, 100–106. [Google Scholar] [CrossRef]
- Mona, E.E.; Ibrahim, S.A.; Manal, M. Combined effect of NPK levels and foliar nutritional compounds on growth and yield parameters of potato plants (Solanum tuberosum L.). Afr. J. Microbiol. Res. 2012, 6, 5100–5109. [Google Scholar]
- Acosta-Martinez, V.; Cano, A.; Johnson, J. Simultaneous determination of the activity of many soil enzymes for biogeochemical indicators of soil health. Appl. Soil Ecol. 2018, 126, 121–128. [Google Scholar] [CrossRef]
- Ishaq, U.M.; Umara, B.; Armanto, H.M.; Adzemi, M.A. BRIS Soil Suitability Assessment 2 on Sweet Potato in Merang—Terengganu Region of Malaysia. J. Biol. Agric. Healthcar. 2014, 4, 11–19. [Google Scholar]
- Futa, B.; Bielińska, E.J.; Mocek-Płóciniak, A. The use of enzymatic tests to assess the quality of arable soils along main thoroughfares inulin. J. Res. Appl. Agric. Eng. 2016, 61, 94–97. [Google Scholar]
- Siwik-Ziomek, A.; Szczepanek, M. Soil Extracellular Enzyme Activities and Uptake of N by Oilseed Rape Depending on Fertilization and Seaweed Biostimulant Application. Agronomy 2019, 9, 480. [Google Scholar] [CrossRef]
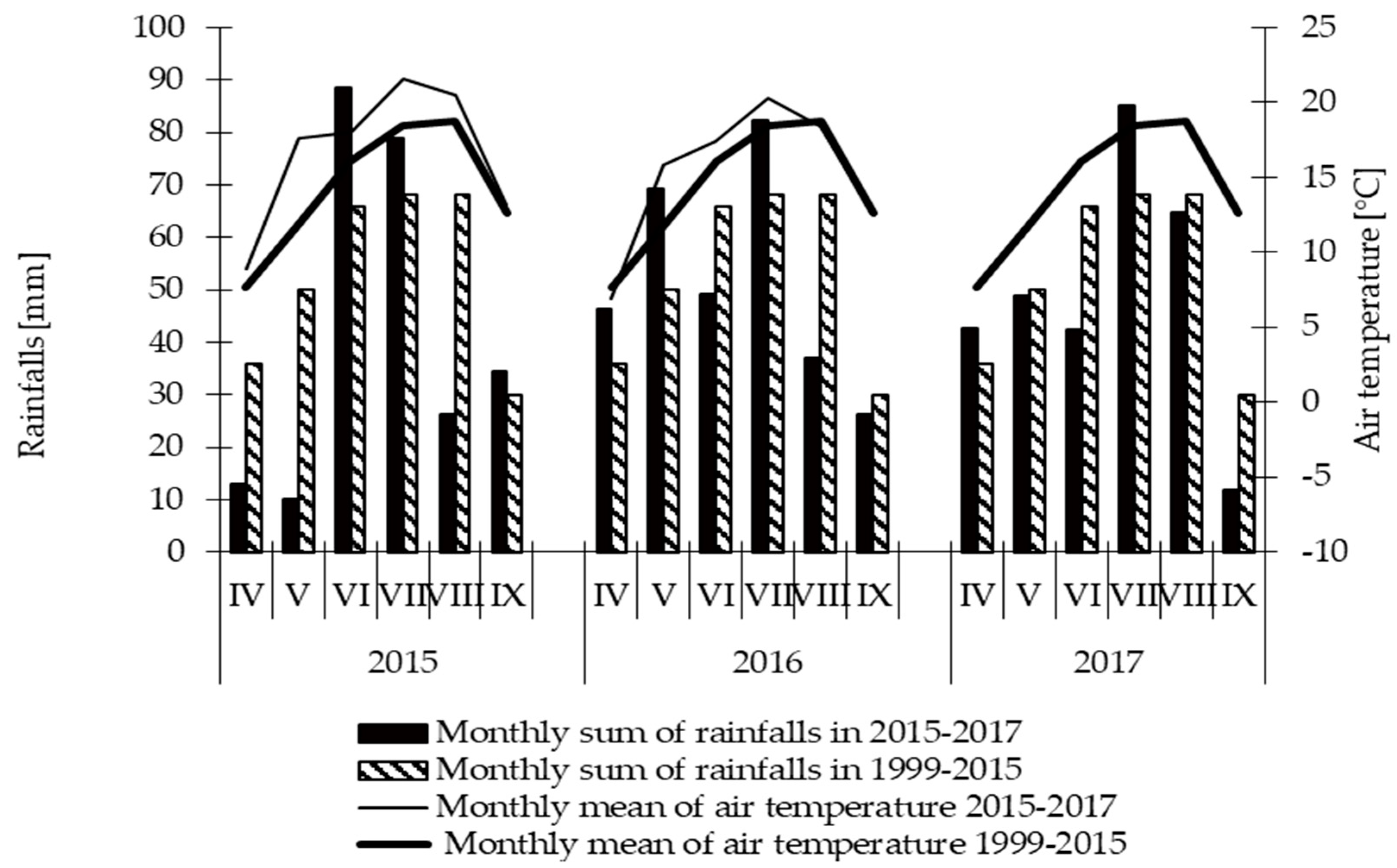
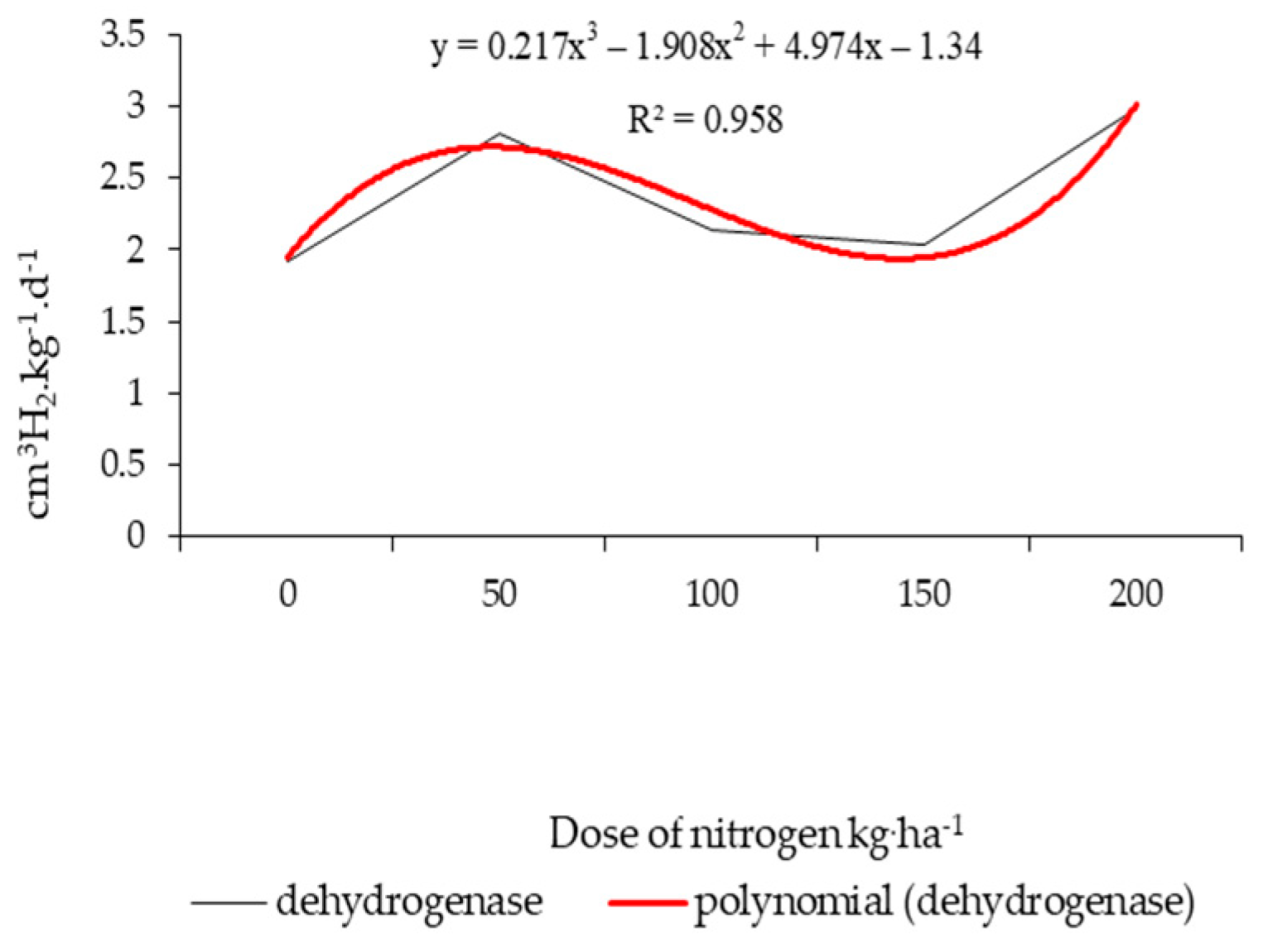
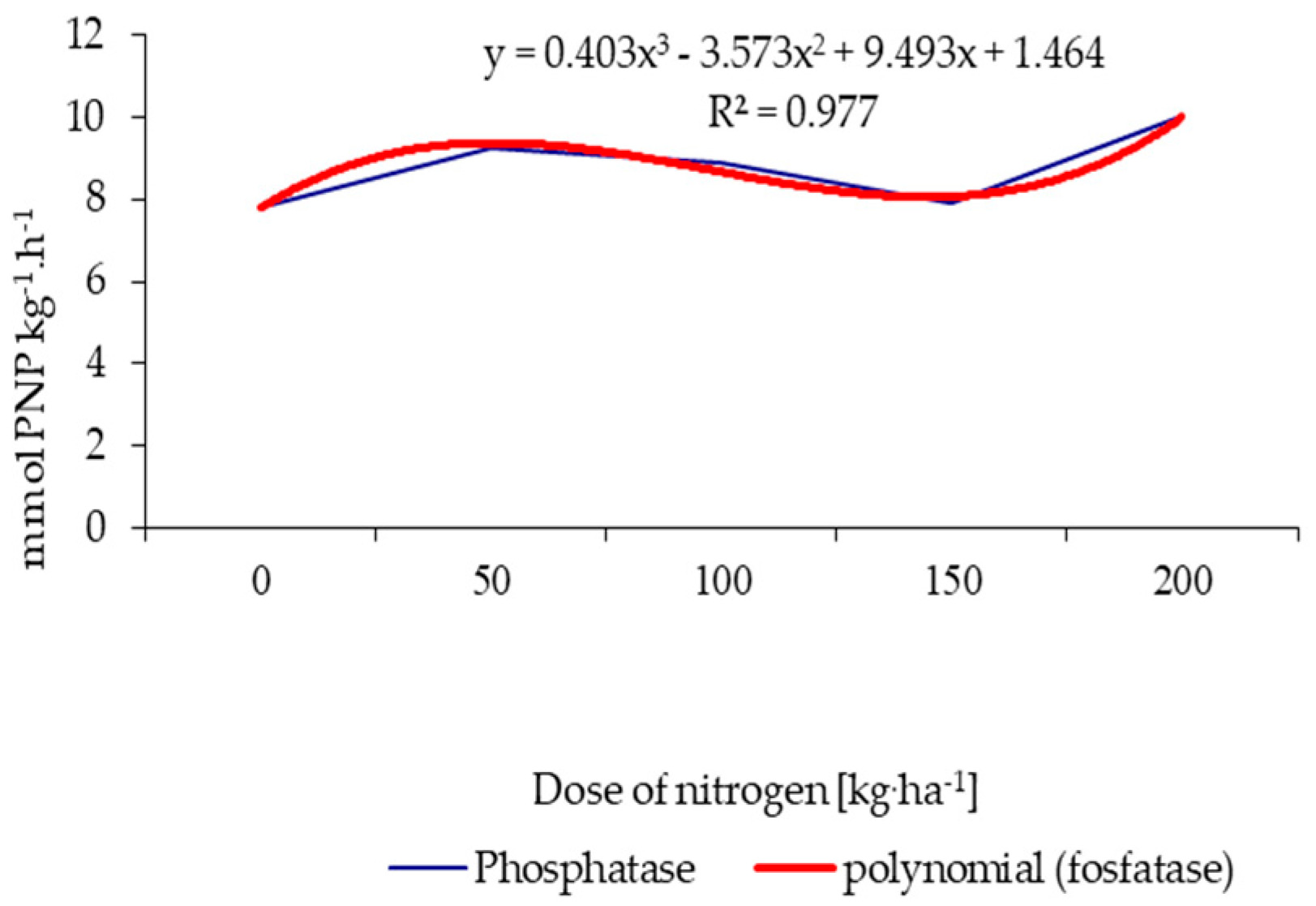
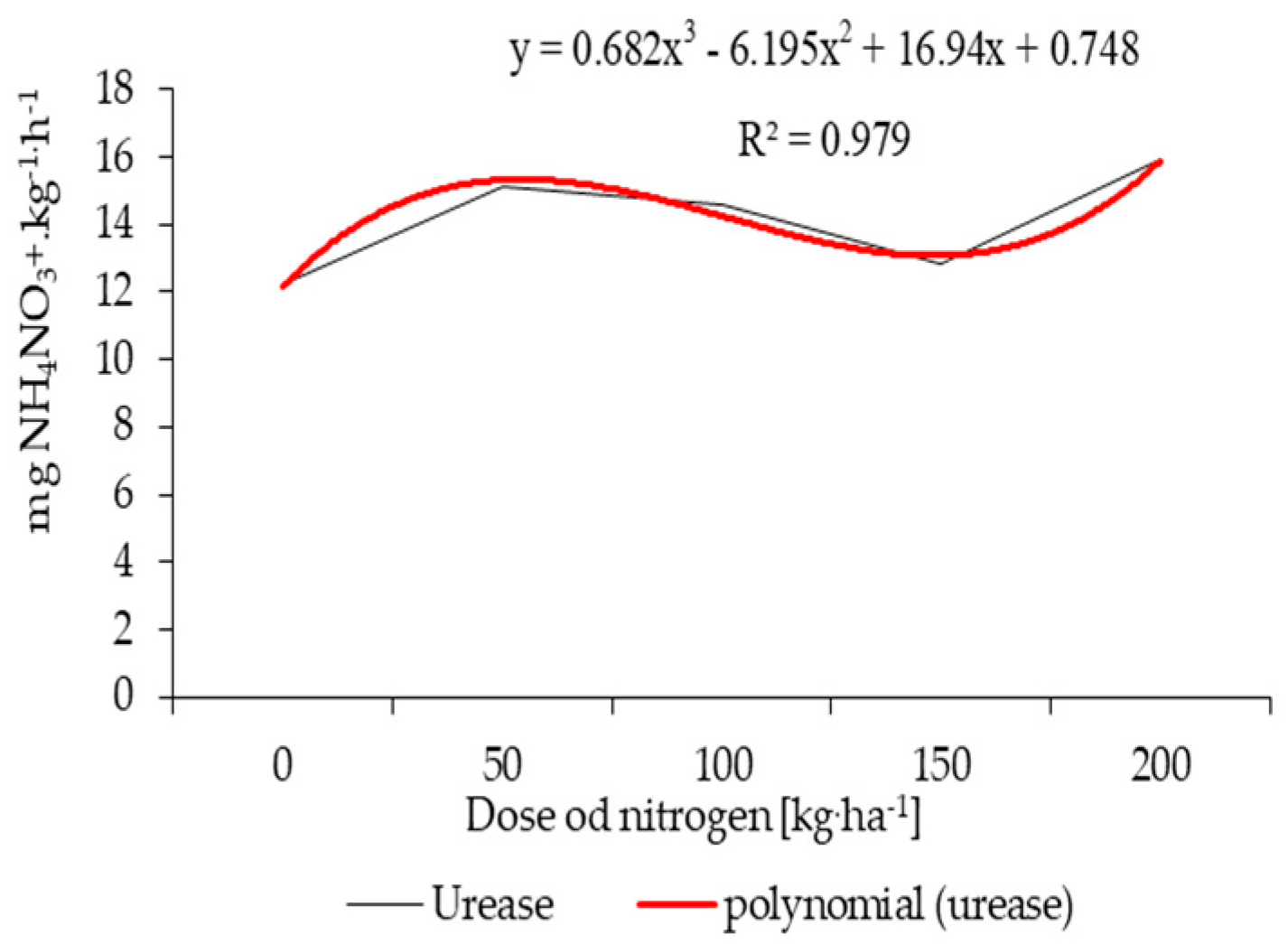
| Properties | Unit | 2015 | 2016 | 2017 |
|---|---|---|---|---|
| pH | H2O | 8.90 | 8.70 | 8.67 |
| pH | KCl | 8.30 | 8.10 | 8.25 |
| Corg. | % | 27.18 | 26.87 | 27.09 |
| N total | % | 2.60 | 2.55 | 3.03 |
| C:N | 10.45 | 10.54 | 10.58 | |
| Available phosphorus | mg kg−1 | 834.0 | 803.1 | 812.5 |
| Available potasium | mg kg−1 | 1232.0 | 1104.6 | 1167.1 |
| Available magnesium | mg kg−1 | 226.0 | 201.7 | 213.7 |
| P total | g kg−1 | 9.00 | 8.16 | 8.39 |
| K total | g kg−1 | 14.10 | 12.9 | 13.12 |
| Mg total | g kg−1 | 3.80 | 3.12 | 3.54 |
| Ca total | g kg−1 | 0.25 | 0.44 | 0.54 |
| Year | Composition of the Granulometric Fractions (%) | Soil Classification | ||
|---|---|---|---|---|
| Sand 2.0–0.05 | Silt 0.05–0.002 | Clay <0.002 | ||
| mm | ||||
| 2015 | 67.14 | 30.29 | 2.56 | medium soil |
| 2016 | 67.04 | 30.24 | 2.72 | medium soil |
| 2017 | 67.47 | 30.45 | 2.08 | medium soil |
| Average | 67.22 | 30.33 | 2.45 | medium soil |
| Year | Content of Available Macronutrients (mg 100 g−1 soil) | Humus Content (%) | pH (KCl) | Micronutrients Content (mg kg−1 soil) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| P2O5 | K2O | Mg | Cu | Mn | Zn | Fe | B | |||
| 2015 | 20.2 | 13.0 | 7.7 | 0.93 | 5.90 | 7.60 | 320 | 39.98 | 3770 | 7.31 |
| 2016 | 18.8 | 11.1 | 7.1 | 1.08 | 5.79 | 4.85 | 335 | 56.87 | 3899 | 5.37 |
| 2017 | 24.1 | 11.6 | 6.4 | 1.05 | 6.65 | 8.90 | 171 | 41.04 | 3596 | 6.06 |
| Average | 21.0 | 11.9 | 7.1 | 1.02 | 6.11 | 7.12 | 275 | 45.96 | 3755 | 6.25 |
| Experimental Factors | Biological Activity of Soil | ||||
|---|---|---|---|---|---|
| Dehydrogenase (cm3 H2 kg−1 d−1) | Phosphatase (mmol PNP kg−1 h−1) | Urease (mg NH4NO3 + kg−1 h−1) | Protease (mg tyrosine kg−1 h−1) | ||
| Nitrogen fertilization (kg ha−1) | 0 | 1.92 | 7.82 | 12.23 | 16.15 |
| 50 | 2.81 | 9.25 | 15.10 | 17.22 | |
| 100 | 2.14 | 8.87 | 14.56 | 17.77 | |
| 150 | 2.04 | 7.94 | 12.86 | 16.47 | |
| 200 | 2.99 | 10.04 | 15.94 | 17.49 | |
| HSD0.05 | 0.20 | 0.73 | 1.82 | 1.42 | |
| Years | 2015 | 2.41 | 8.92 | 14.17 | 16.63 |
| 2016 | 2.37 | 8.56 | 14.15 | 17.52 | |
| 2017 | 2.36 | 8.87 | 14.09 | 16.91 | |
| HSD0.05 | ns * | 0.35 | ns * | 0.85 | |
| Average | 2.38 | 8.78 | 14.14 | 17.02 | |
| Experimental Factors | C (g kg−1) | Total Nitrogen (g kg−1) | C:N Ratio | N-NO3− (mg kg−1) | N-NH4+ (mg kg−1) | pHKCl | |
|---|---|---|---|---|---|---|---|
| Nitrogen fertilization (kg ha−1) | 0 | 11.50 | 0.95 | 12.11 | 12.76 | 19.28 | 6.80 |
| 50 | 11.22 | 0.98 | 11.45 | 6.27 | 32.38 | 7.08 | |
| 100 | 13.56 | 1.18 | 11.46 | 5.83 | 30.21 | 6.98 | |
| 150 | 11.38 | 1.04 | 10.95 | 15.15 | 35.52 | 6.70 | |
| 200 | 12.92 | 1.22 | 10.59 | 14.18 | 36.43 | 6.33 | |
| HSD0.05 | 0.61 | 0.05 | 0.56 | 0.54 | 1.53 | 0.34 | |
| Years | 2015 | 12.01 | 1.07 | 11.22 | 10.67 | 30.65 | 7.06 |
| 2016 | 12.22 | 1.06 | 11.53 | 10.90 | 30.76 | 6.68 | |
| 2017 | 12.13 | 1.08 | 11.23 | 10.94 | 30.88 | 6.60 | |
| HSD0.05 | ns * | ns | ns | ns | ns | 0.20 | |
| Average | 12.12 | 1.07 | 11.31 | 11.31 | 30.76 | ||
| Specification | Adh | AF | AU | AP | N-NO3 | N-NH4 | pH | C | N | C:N |
|---|---|---|---|---|---|---|---|---|---|---|
| Adh | 1.00 | |||||||||
| AF | 0.85 ** | 1.00 | ||||||||
| AU | 0.90 ** | 0.91 ** | 1.00 | |||||||
| AP | 0.41 * | 0.49 * | 0.59 ** | 1.00 | ||||||
| N-NO3 | −0.12 | −0.22 | −0.35 * | −0.39 * | 1.00 | |||||
| N-NH4 | 0.59 ** | 0.55 ** | 0.64 ** | 0.38 * | 0.10 | 1.00 | ||||
| pH | −0.07 | 0.10 | 0.00 | −0.08 | −0.13 | −0.03 | 1.00 | |||
| C | 0.39 * | 0.46 ** | 0.50 ** | 0.53 ** | −0.25 | 0.21 | 0.08 | 1.00 | ||
| N | 0.38 * | 0.60 ** | 0.66 ** | 0.49 * | 0.00 | 0.56 ** | 0.06 | 0.80 ** | 1.00 | |
| C:N | −0.56 ** | −0.51 ** | −0.56 ** | −0.38 * | −0.37 * | −0.88 ** | −0.01 | −0.23 | −0.64 ** | 1.00 |
| Specification | Adh | AF | AU | AP | N-NO3 | N-NH4 | pH | C | N | C:N |
|---|---|---|---|---|---|---|---|---|---|---|
| Average | 2.38 | 8.78 | 14.14 | 17.02 | 10.84 | 30.76 | 7.77 | 12.12 | 1.07 | 11.28 |
| Median | 2.13 | 8.85 | 14.55 | 16.78 | 12.79 | 32.38 | 7.10 | 11.51 | 1.04 | 11.30 |
| Statistical deviations | 0.45 | 0.94 | 1.44 | 0.95 | 4.13 | 6.45 | 1.38 | 0.98 | 0.11 | 0.56 |
| Kurtosis | −1.83 | −0.25 | −1.63 | 3.55 | −2.00 | 0.06 | −1.01 | −1.61 | −1.79 | −0.80 |
| Skewness | 0.43 | −0.36 | −0.18 | 1.73 | −0.34 | −1.13 | 0.81 | 0.57 | 0.29 | 0.16 |
| Minimum | 1.88 | 6.87 | 12.15 | 16.02 | 5.72 | 17.93 | 6.20 | 11.14 | 0.94 | 10.40 |
| Maximum | 3.04 | 10.18 | 16.02 | 19.69 | 15.39 | 37.59 | 10.39 | 13.68 | 1.24 | 12.30 |
| Coefficient of variation (%) | 18.91 | 10.67 | 10.16 | 5.61 | 38.09 | 20.96 | 17.72 | 8.09 | 10.53 | 4.96 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sawicka, B.; Krochmal-Marczak, B.; Pszczółkowski, P.; Bielińska, E.J.; Wójcikowska-Kapusta, A.; Barbaś, P.; Skiba, D. Effect of Differentiated Nitrogen Fertilization on the Enzymatic Activity of the Soil for Sweet Potato (Ipomoea batatas L. [Lam.]) Cultivation. Agronomy 2020, 10, 1970. https://doi.org/10.3390/agronomy10121970
Sawicka B, Krochmal-Marczak B, Pszczółkowski P, Bielińska EJ, Wójcikowska-Kapusta A, Barbaś P, Skiba D. Effect of Differentiated Nitrogen Fertilization on the Enzymatic Activity of the Soil for Sweet Potato (Ipomoea batatas L. [Lam.]) Cultivation. Agronomy. 2020; 10(12):1970. https://doi.org/10.3390/agronomy10121970
Chicago/Turabian StyleSawicka, Barbara, Barbara Krochmal-Marczak, Piotr Pszczółkowski, Elżbieta Jolanta Bielińska, Anna Wójcikowska-Kapusta, Piotr Barbaś, and Dominika Skiba. 2020. "Effect of Differentiated Nitrogen Fertilization on the Enzymatic Activity of the Soil for Sweet Potato (Ipomoea batatas L. [Lam.]) Cultivation" Agronomy 10, no. 12: 1970. https://doi.org/10.3390/agronomy10121970
APA StyleSawicka, B., Krochmal-Marczak, B., Pszczółkowski, P., Bielińska, E. J., Wójcikowska-Kapusta, A., Barbaś, P., & Skiba, D. (2020). Effect of Differentiated Nitrogen Fertilization on the Enzymatic Activity of the Soil for Sweet Potato (Ipomoea batatas L. [Lam.]) Cultivation. Agronomy, 10(12), 1970. https://doi.org/10.3390/agronomy10121970









