Effect of PET Micro/Nanoplastics on Model Freshwater Zooplankton
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of PET Micro/Nanoplastics (PET-M/NPs)
2.3. Laser Diffraction (LD) and Zeta Potential Measurements
2.4. Transmission Electron Microscopy (TEM)
2.5. Atomic Force Microscopy (AFM)
2.6. Toxicity of PET Micro/Nanoplastics to Aquatic Ecosystem Organisms
2.6.1. Thamnotoxkit F
2.6.2. Daphtoxkit F
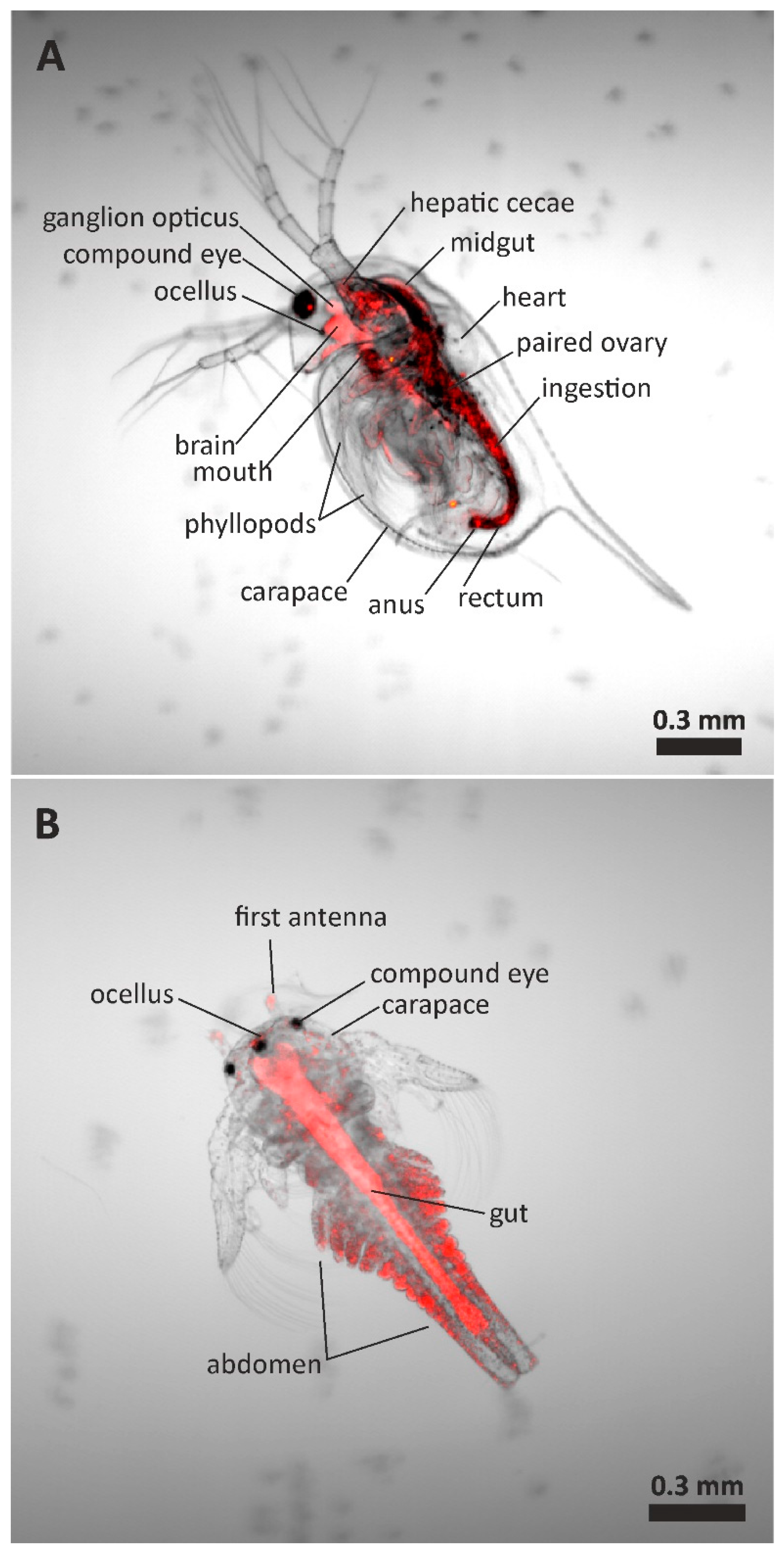
2.7. Confocal Microscopy Analysis
3. Results and Discussion
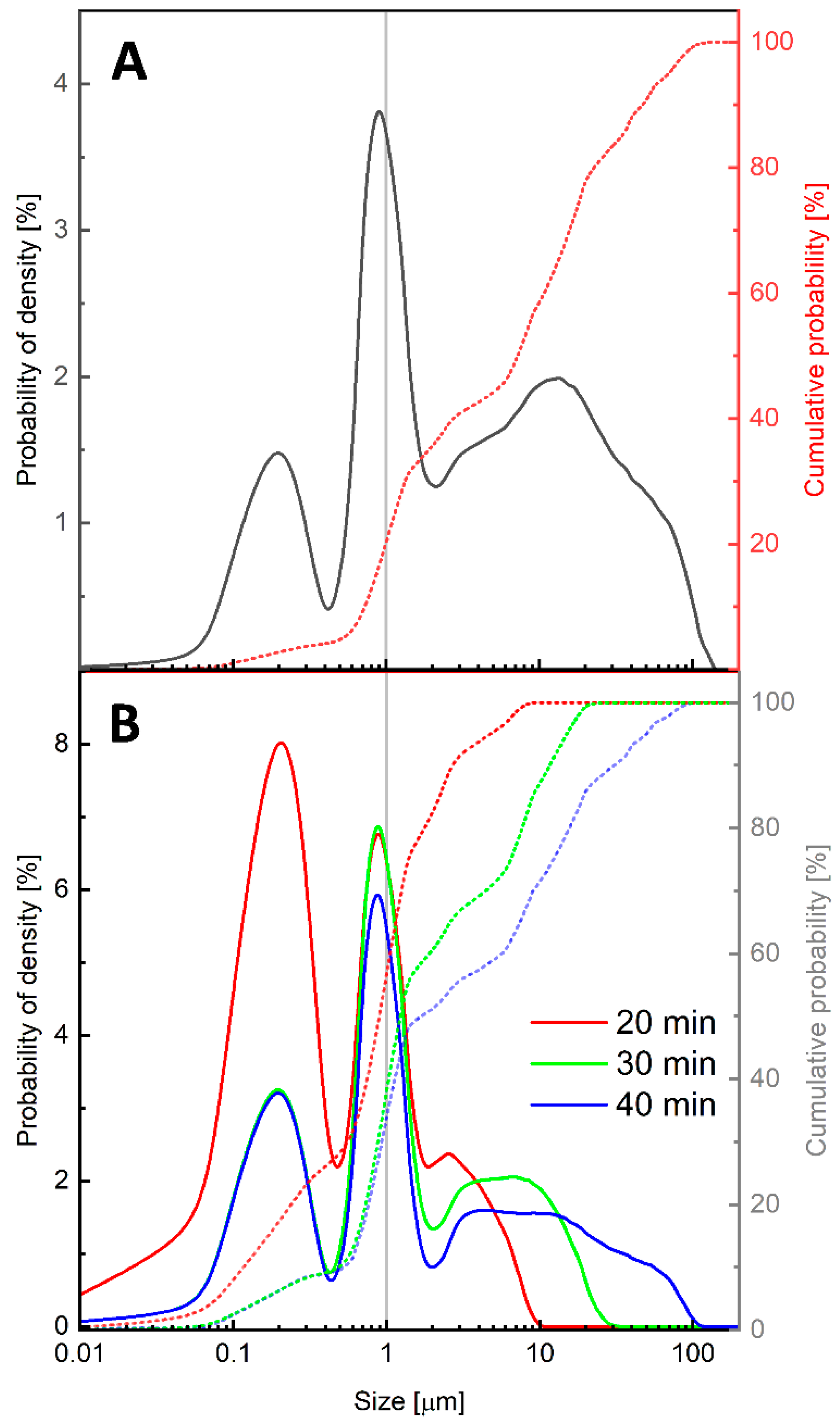
3.1. Preparation of PET Micro/Nanoplastics
3.2. Effect of PET NPs on Aquatic Organisms
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Chemanalyst, Discover Our Polyethylene Terephthalate (PET) Industry Tracking. 2024. Available online: https://www.chemanalyst.com/industry-report/polyethylene-terephthalate-market-2958 (accessed on 1 October 2024).
- Nisticò, R.; Magnacca, G.; Martorana, S. Surface Science in Hernioplasty: The Role of Plasma Treatments. Appl. Surf. Sci. 2017, 419, 860–868. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, M.; Zhou, M.; Gu, C.; Ye, Z.; Xiao, Y.; Zhou, Y.; Lang, M.; Tan, W.S. Fabrication and Evaluation of Modified Poly(Ethylene Terephthalate) Microfibrous Scaffolds for Hepatocyte Growth and Functionality Maintenance. Mater. Sci. Eng. C 2020, 109, 110523. [Google Scholar] [CrossRef] [PubMed]
- Zero Waste Europe. How Circular Is PET? Available online: https://zerowasteeurope.eu/library/how-circular-is-pet (accessed on 1 October 2024).
- Zhang, J.; Wang, L.; Kannan, K. Quantitative Analysis of Polyethylene Terephthalate and Polycarbonate Microplastics in Sediment Collected from South Korea, Japan and the USA. Chemosphere 2021, 279, 130551. [Google Scholar] [CrossRef]
- Zahari, N.Z.; Vincent, S.D.; Cleophas, F.N.; Budin, K.; Sabullah, M.K. Abundance, Distribution, and Characterization of Microplastics on Two Recreational Beaches in Kota Kinabalu, Sabah, Malaysia. Water 2023, 15, 2681. [Google Scholar] [CrossRef]
- Chae, Y.; Kim, D.; Kim, S.W.; An, Y.J. Trophic Transfer and Individual Impact of Nano-Sized Polystyrene in a Four-Species Freshwater Food Chain. Sci. Rep. 2018, 8, 284. [Google Scholar] [CrossRef]
- Magrì, D.; Sánchez-Moreno, P.; Caputo, G.; Gatto, F.; Veronesi, M.; Bardi, G.; Catelani, T.; Guarnieri, D.; Athanassiou, A.; Pompa, P.P. Laser Ablation as a Versatile Tool to Mimic Polyethylene Terephthalate Nanoplastic Pollutants: Characterization and Toxicology Assessment. ACS Nano 2018, 12, 7690–7700. [Google Scholar] [CrossRef]
- Caldwell, J.; Lehner, R.; Balog, S.; Rhême, C.; Gao, X.; Septiadi, D.; Weder, C.; Petri-Fink, A.; Rothen-Rutishauser, B. Fluorescent Plastic Nanoparticles to Track Their Interaction and Fate in Physiological Environments. Environ. Sci. Nano 2021, 8, 502–513. [Google Scholar] [CrossRef]
- Johnson, L.M.; Mecham, J.B.; Krovi, S.A.; Caffaro, M.M.M.; Aravamudhan, S.; Kovach, A.L.; Fennell, T.R.; Mortensen, N.P. Fabrication of Polyethylene Terephthalate (PET) Nanoparticles with Fluorescent Tracers for Studies in Mammalian Cells. Nanoscale Adv. 2021, 3, 339–346. [Google Scholar] [CrossRef]
- Rodríguez-Hernández, A.G.; Muñoz-Tabares, J.A.; Aguilar-Guzmán, J.C.; Vazquez-Duhalt, R. A Novel and Simple Method for Polyethylene Terephthalate (PET) Nanoparticle Production. Environ. Sci. Nano 2019, 6, 2031–2036. [Google Scholar] [CrossRef]
- Ji, Y.; Wang, C.; Wang, Y.; Fu, L.; Man, M.; Chen, L. Realistic Polyethylene Terephthalate Nanoplastics and the Size-and Surface Coating-Dependent Toxicological Impacts on Zebrafish Embryos. Environ. Sci. Nano 2020, 7, 2313–2324. [Google Scholar] [CrossRef]
- Bashirova, N.; Poppitz, D.; Klüver, N.; Scholz, S.; Matysik, J.; Alia, A. A Mechanistic Understanding of the Effects of Polyethylene Terephthalate Nanoplastics in the Zebrafish (Danio rerio) Embryo. Sci. Rep. 2023, 13, 1891. [Google Scholar] [CrossRef] [PubMed]
- Lionetto, F.; Esposito Corcione, C.; Messa, F.; Perrone, S.; Salomone, A.; Maffezzoli, A. The Sorption of Amoxicillin on Engineered Polyethylene Terephthalate Microplastics. J. Polym. Environ. 2023, 31, 1383–1397. [Google Scholar] [CrossRef]
- Pikuda, O.; Roubeau Dumont, E.; Chen, Q.; Macairan, J.R.; Robinson, S.A.; Berk, D.; Tufenkji, N. Toxicity of Microplastics and Nanoplastics to Daphnia magna: Current Status, Knowledge Gaps and Future Directions. TrAC-Trends Anal. Chem. 2023, 167, 117208. [Google Scholar] [CrossRef]
- Pochelon, A.; Stoll, S.; Slaveykova, V.I. Polystyrene Nanoplastic Behavior and Toxicity on Crustacean Daphnia magna: Media Composition, Size, and Surface Charge Effects. Environments 2021, 8, 101. [Google Scholar] [CrossRef]
- Lin, W.; Jiang, R.; Hu, S.; Xiao, X.; Wu, J.; Wei, S.; Xiong, Y.; Ouyang, G. Investigating the Toxicities of Different Functionalized Polystyrene Nanoplastics on Daphnia magna. Ecotoxicol. Environ. Saf. 2019, 180, 509–516. [Google Scholar] [CrossRef]
- De Felice, B.; Sugni, M.; Casati, L.; Parolini, M. Molecular, Biochemical and Behavioral Responses of Daphnia magna under Long-Term Exposure to Polystyrene Nanoplastics. Environ. Int. 2022, 164, 107264. [Google Scholar] [CrossRef]
- Kelpsiene, E.; Torstensson, O.; Ekvall, M.T.; Hansson, L.A.; Cedervall, T. Long-Term Exposure to Nanoplastics Reduces Life-Time in Daphnia magna. Sci. Rep. 2020, 10, 5979. [Google Scholar] [CrossRef]
- Rist, S.; Baun, A.; Hartmann, N.B. Ingestion of Micro- and Nanoplastics in Daphnia magna—Quantification of Body Burdens and Assessment of Feeding Rates and Reproduction. Environ. Pollut. 2017, 228, 398–407. [Google Scholar] [CrossRef]
- Saavedra, J.; Stoll, S.; Slaveykova, V.I. Influence of Nanoplastic Surface Charge on Eco-Corona Formation, Aggregation and Toxicity to Freshwater Zooplankton. Environ. Pollut. 2019, 252, 715–722. [Google Scholar] [CrossRef]
- Cooper, D.A.; Corcoran, P.L. Effects of Mechanical and Chemical Processes on the Degradation of Plastic Beach Debris on the Island of Kauai, Hawaii. Mar. Pollut. Bull. 2010, 60, 650–654. [Google Scholar] [CrossRef]
- Monira, S.; Roychand, R.; Hai, F.I.; Bhuiyan, M.; Pramanik, B.K. Microplastic Fragmentation into Nanoplastics by Water Shear Forces during Wastewater Treatment: Mechanical Insights and Theoretical Analysis. Environ. Pollut. 2025, 364, 125310. [Google Scholar] [CrossRef] [PubMed]
- Żandarek, J.; Żmudzki, P.; Obradović, D.; Lazović, S.; Bogojević, A.; Koszła, O.; Sołek, P.; Maciąg, M.; Płazińska, A.; Starek, M.; et al. Analysis of Pharmacokinetic Profile and Ecotoxicological Character of Cefepime and Its Photodegradation Products. Chemosphere 2024, 353, 141529. [Google Scholar] [CrossRef] [PubMed]
- Lionetto, F.; Lionetto, M.G.; Mele, C.; Corcione, C.E.; Bagheri, S.; Udayan, G.; Maffezzoli, A. Autofluorescence of Model Polyethylene Terephthalate Nanoplastics for Cell Interaction Studies. Nanomaterials 2022, 12, 1560. [Google Scholar] [CrossRef] [PubMed]
- Mark, J.E. Polymer Data Handbook; Oxford University Press: Oxford, UK, 2009; ISBN 0195181018. [Google Scholar]
- Hergenrother, W.L.; Nelson, C.J. Viscosity–Molecular Weight Relationship for Fractionated Poly (Ethylene Terephthalate). J. Polym. Sci. Polym. Chem. Ed. 1974, 12, 2905–2915. [Google Scholar] [CrossRef]
- Lionetto, F.; Corcione, C.E.; Rizzo, A.; Maffezzoli, A. Production and Characterization of Polyethylene Terephthalate Nanoparticles. Polymers 2021, 13, 3745. [Google Scholar] [CrossRef]
- Lewandowska, J.; Kȩpczyński, M.; Bednar, J.; Rza̧d, E.; Moravcikova, V.; Jachimska, B.; Nowakowska, M. Silicone-Stabilized Liposomes. Colloid Polym. Sci. 2010, 288, 37–45. [Google Scholar] [CrossRef]
- Microbiotests Thamnotoxkit F Test Procedure. Available online: https://www.microbiotests.com/wp-content/uploads/2019/04/Microbiotests-Thamnotoxkit-F-test-procedure-slide-show-thamnocephalus-toxicity-test.pdf (accessed on 1 November 2024).
- Microbiotests Microbiotests Daphtoxkit F Test Procedure. Available online: https://www.microbiotests.com/wp-content/uploads/2019/04/Microbiotests-Daphtoxkit-F-test-procedure-slide-show-daphnia-toxicity-test.pdf (accessed on 1 November 2024).
- Zhang, H.; Xu, X.; Tang, X.; Kong, F. Investigation of the Adsorption of Norfloxacin by Biodegradable and Non-Biodegradable Microplastics Aged by Chemical Oxidation. J. Polym. Environ. 2023, 31, 3894–3906. [Google Scholar] [CrossRef]
- Kepczyński, M.; Nawalany, K.; Jachimska, B.; Romek, M.; Nowakowska, M. Pegylated Tetraarylporphyrin Entrapped in Liposomal Membranes: A Possible Novel Drug-Carrier System for Photodynamic Therapy. Colloids Surf. B Biointerfaces 2006, 49, 22–30. [Google Scholar] [CrossRef]
- Dong, S.; Cai, W.; Xia, J.; Sheng, L.; Wang, W.; Liu, H. Aggregation Kinetics of Fragmental PET Nanoplastics in Aqueous Environment: Complex Roles of Electrolytes, PH and Humic Acid. Environ. Pollut. 2021, 268, 115828. [Google Scholar] [CrossRef]
- Piccardo, M.; Provenza, F.; Grazioli, E.; Cavallo, A.; Terlizzi, A.; Renzi, M. PET Microplastics Toxicity on Marine Key Species Is Influenced by PH, Particle Size and Food Variations. Sci. Total Environ. 2020, 715, 136947. [Google Scholar] [CrossRef]
- Provenza, F.; Piccardo, M.; Terlizzi, A.; Renzi, M. Exposure to Pet-Made Microplastics: Particle Size and PH Effects on Biomolecular Responses in Mussels. Mar. Pollut. Bull. 2020, 156, 111228. [Google Scholar] [CrossRef] [PubMed]
- Salam, M.; Zheng, H.; Liu, Y.; Zaib, A.; Rehman, S.A.U.; Riaz, N.; Eliw, M.; Hayat, F.; Li, H.; Wang, F. Effects of Micro(Nano)Plastics on Soil Nutrient Cycling: State of the Knowledge. J. Environ. Manag. 2023, 344, 118437. [Google Scholar] [CrossRef] [PubMed]
- Weber, A.; Schwiebs, A.; Solhaug, H.; Stenvik, J.; Nilsen, A.M.; Wagner, M.; Relja, B.; Radeke, H.H. Nanoplastics Affect the Inflammatory Cytokine Release by Primary Human Monocytes and Dendritic Cells. Environ. Int. 2022, 163, 107173. [Google Scholar] [CrossRef] [PubMed]
- Ebert, D. Ecology, Epidemiology, and Evolution of Parasitism in Daphnia; National Library of Medicine: Bethesda, MD, USA, 2005; pp. 1–25. [Google Scholar]
- Geller, W.; Müller, H. The Filtration Apparatus of Cladocera: Filter Mesh-Sizes and Their Implications on Food Selectivity. Oecologia 1981, 49, 316–321. [Google Scholar] [CrossRef]
- Fadare, O.O.; Wan, B.; Guo, L.H.; Xin, Y.; Qin, W.; Yang, Y. Humic Acid Alleviates the Toxicity of Polystyrene Nanoplastic Particles to: Daphnia magna. Environ. Sci. Nano 2019, 6, 1466–1477. [Google Scholar] [CrossRef]
- Masseroni, A.; Fossati, M.; Ponti, J.; Schirinzi, G.; Becchi, A.; Saliu, F.; Soler, V.; Collini, M.; Della Torre, C.; Villa, S. Sublethal Effects Induced by Different Plastic Nano-Sized Particles in Daphnia magna at Environmentally Relevant Concentrations. Environ. Pollut. 2024, 343, 123107. [Google Scholar] [CrossRef]
- Mattsson, K.; Johnson, E.V.; Malmendal, A.; Linse, S.; Hansson, L.A.; Cedervall, T. Brain Damage and Behavioural Disorders in Fish Induced by Plastic Nanoparticles Delivered through the Food Chain. Sci. Rep. 2017, 7, 11452. [Google Scholar] [CrossRef]


| Concentration a [mg/L] | T. platyurus | D. magna | ||
|---|---|---|---|---|
| # of Individuals b | 24 h Survivability [%] | # of Individuals b | 48 h Survivability [%] | |
| 1000 | 27 | 100% | 19 | 100% |
| 100 | 28 | 100% | 18 | 100% |
| 10 | 27 | 100% | 19 | 100% |
| 1 | 27 | 100% | 17 | 100% |
| 0.1 | 29 | 100% | 13 | 100% |
| Control | 18 | 100% | 18 | 94.4% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajtar, N.; Starek, M.; Vincenti, L.; Dąbrowska, M.; Romek, M.; Rinaldi, R.; Lionetto, F.; Kepczynski, M. Effect of PET Micro/Nanoplastics on Model Freshwater Zooplankton. Polymers 2025, 17, 1256. https://doi.org/10.3390/polym17091256
Rajtar N, Starek M, Vincenti L, Dąbrowska M, Romek M, Rinaldi R, Lionetto F, Kepczynski M. Effect of PET Micro/Nanoplastics on Model Freshwater Zooplankton. Polymers. 2025; 17(9):1256. https://doi.org/10.3390/polym17091256
Chicago/Turabian StyleRajtar, Natan, Małgorzata Starek, Lorenzo Vincenti, Monika Dąbrowska, Marek Romek, Rosaria Rinaldi, Francesca Lionetto, and Mariusz Kepczynski. 2025. "Effect of PET Micro/Nanoplastics on Model Freshwater Zooplankton" Polymers 17, no. 9: 1256. https://doi.org/10.3390/polym17091256
APA StyleRajtar, N., Starek, M., Vincenti, L., Dąbrowska, M., Romek, M., Rinaldi, R., Lionetto, F., & Kepczynski, M. (2025). Effect of PET Micro/Nanoplastics on Model Freshwater Zooplankton. Polymers, 17(9), 1256. https://doi.org/10.3390/polym17091256









