Pyrolyzed Biomass Filler for PLA-Based Food Packaging
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Preparation of the Biomass
2.1.2. PLA Sample Preparation
2.2. Biomass and PLA Composite Characterization
2.2.1. Determination of Water Content
2.2.2. Determination of Extractables in Ethanol
2.2.3. Determination of Lignin Content
2.2.4. Determination of Carbohydrate Content
2.2.5. Determination of Ash Content
2.2.6. Fourier Transform Infrared Spectroscopy (FT-IR)
2.2.7. Thermal Properties
2.2.8. Mechanical Properties
2.2.9. Surface Analysis Equipment
2.2.10. Rheological Properties
3. Results and Discussion
3.1. Characterization of Biomass Waste
3.1.1. Analysis by Classes of Chemical Compounds of Biomass Waste Samples
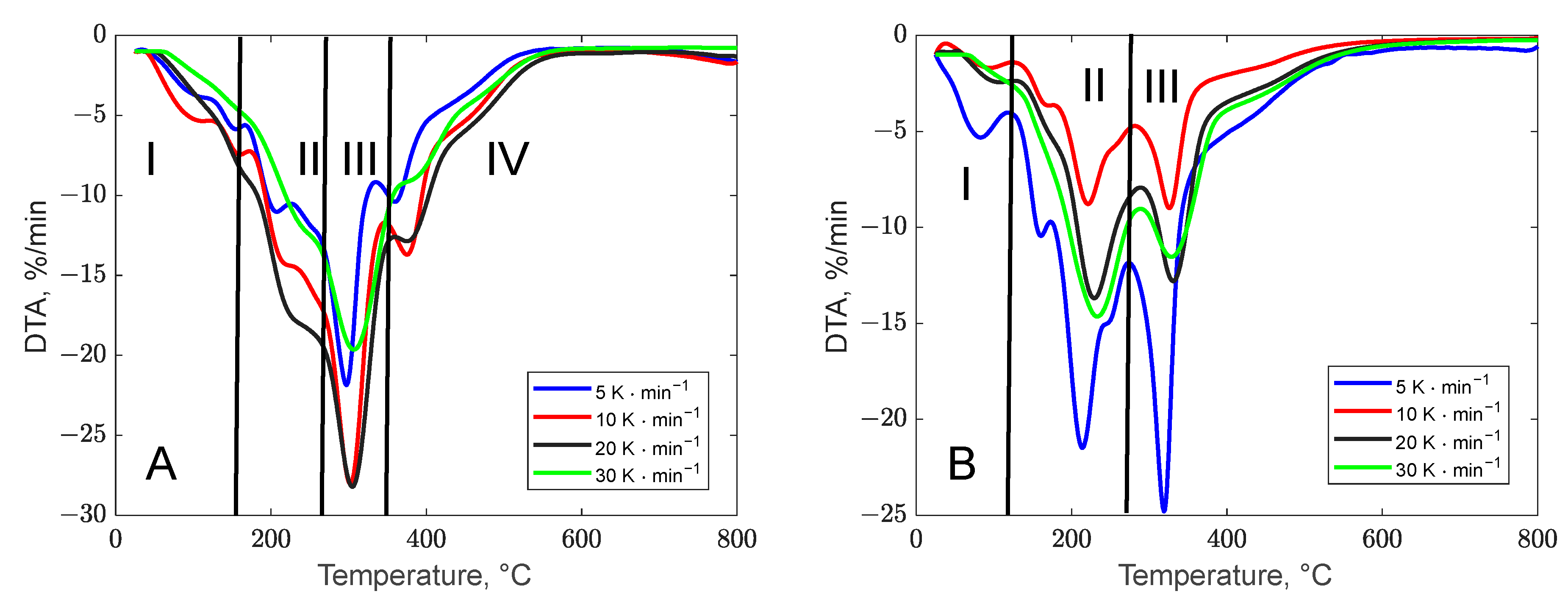
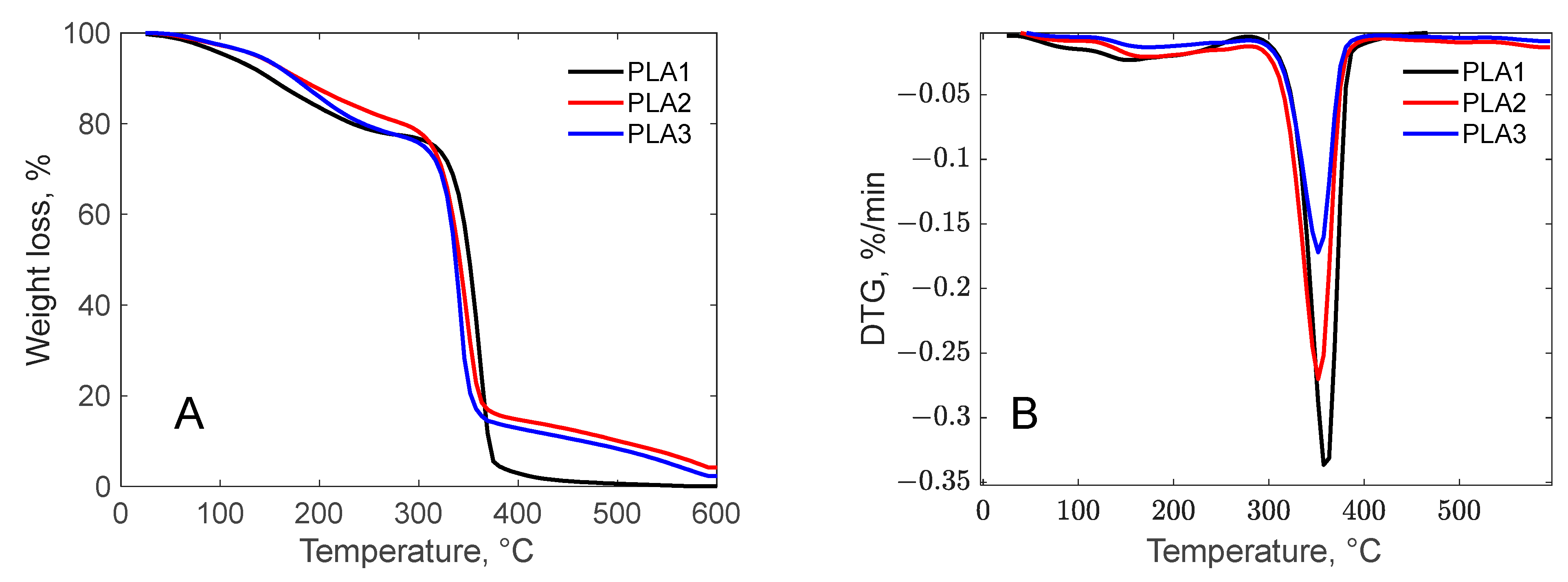
3.1.2. Thermogravimetric Profile of the Biomass Waste Samples
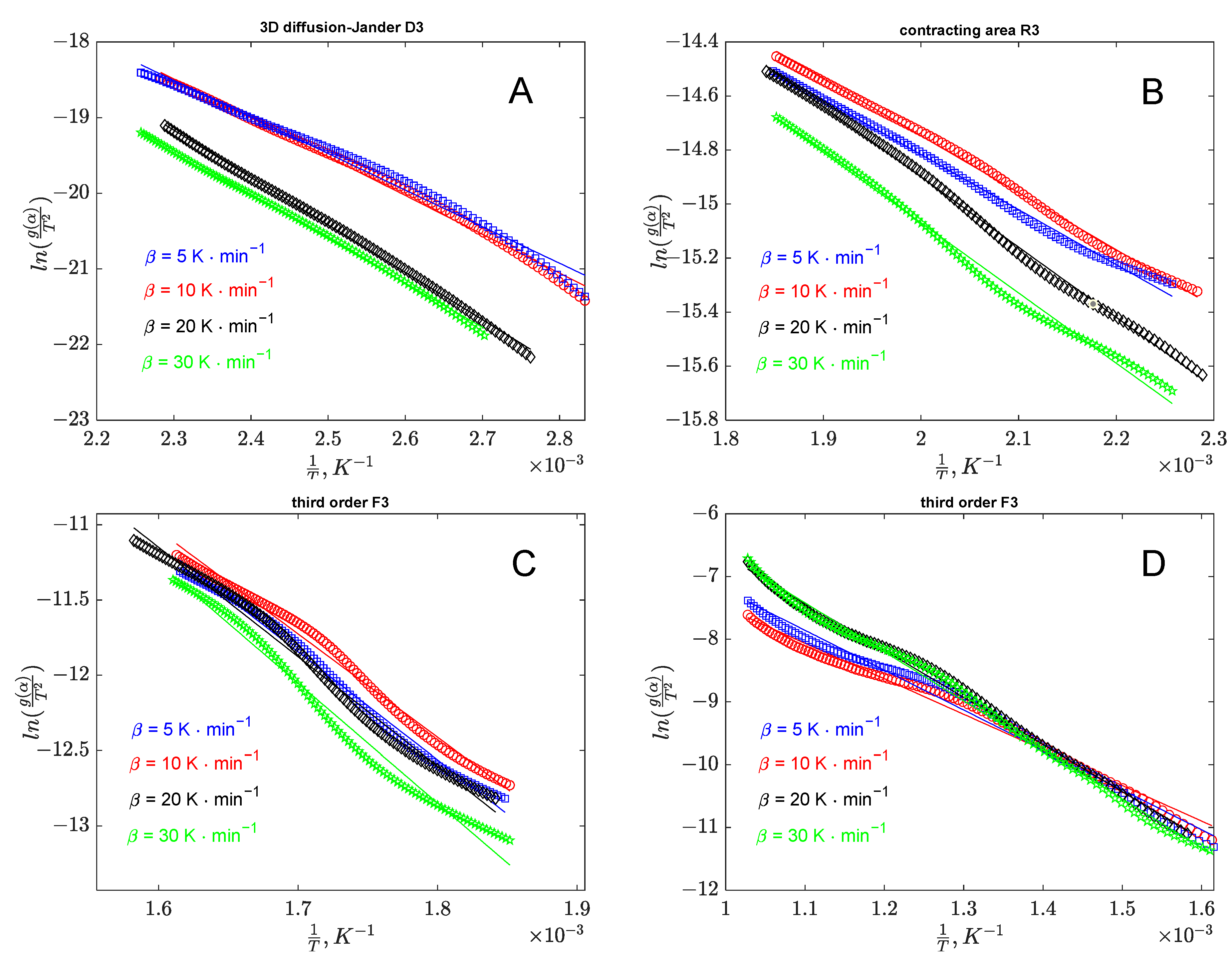
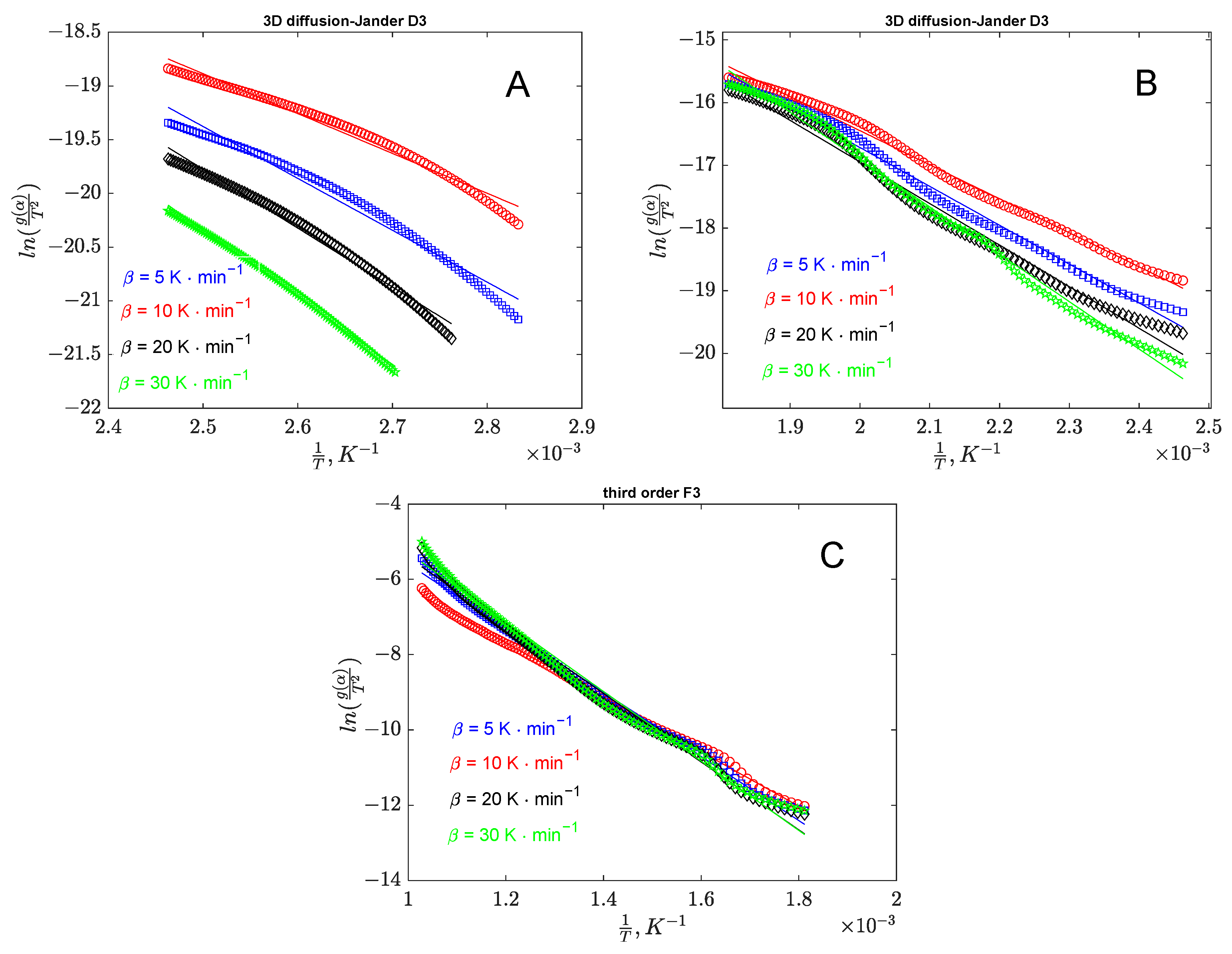
3.1.3. Thermal Decomposition Kinetic Modeling
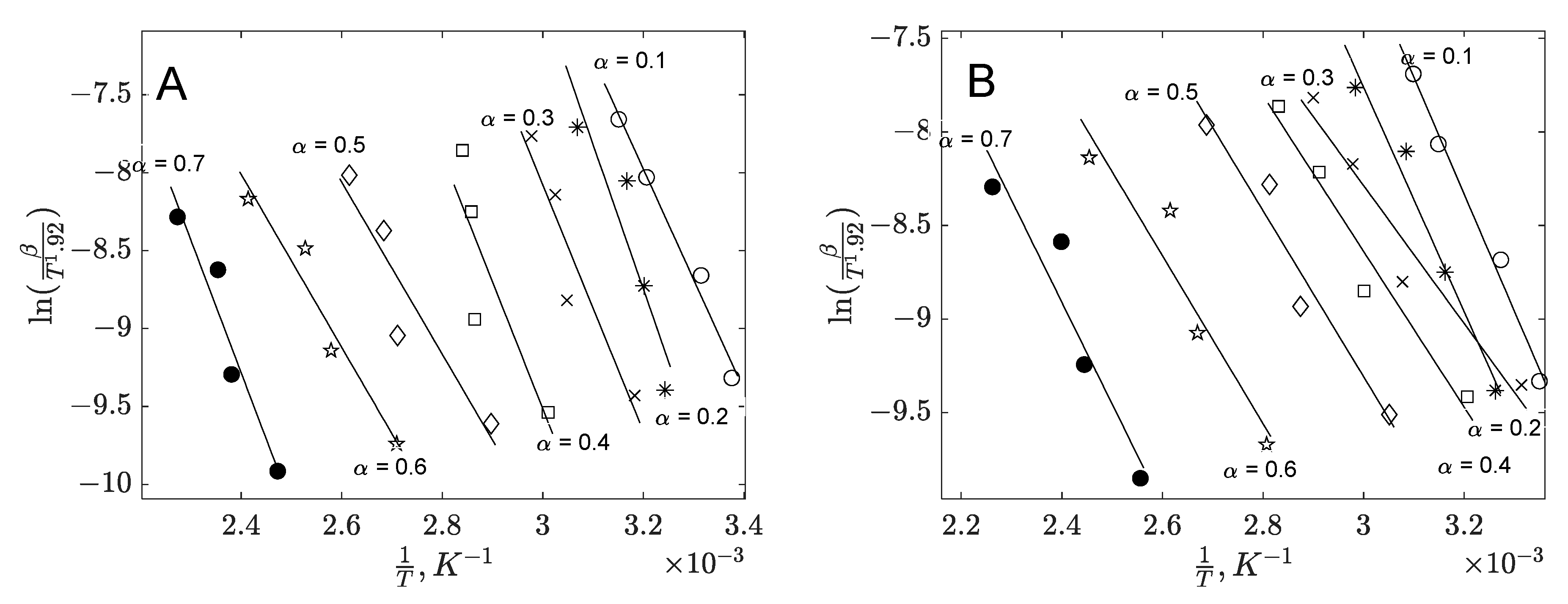
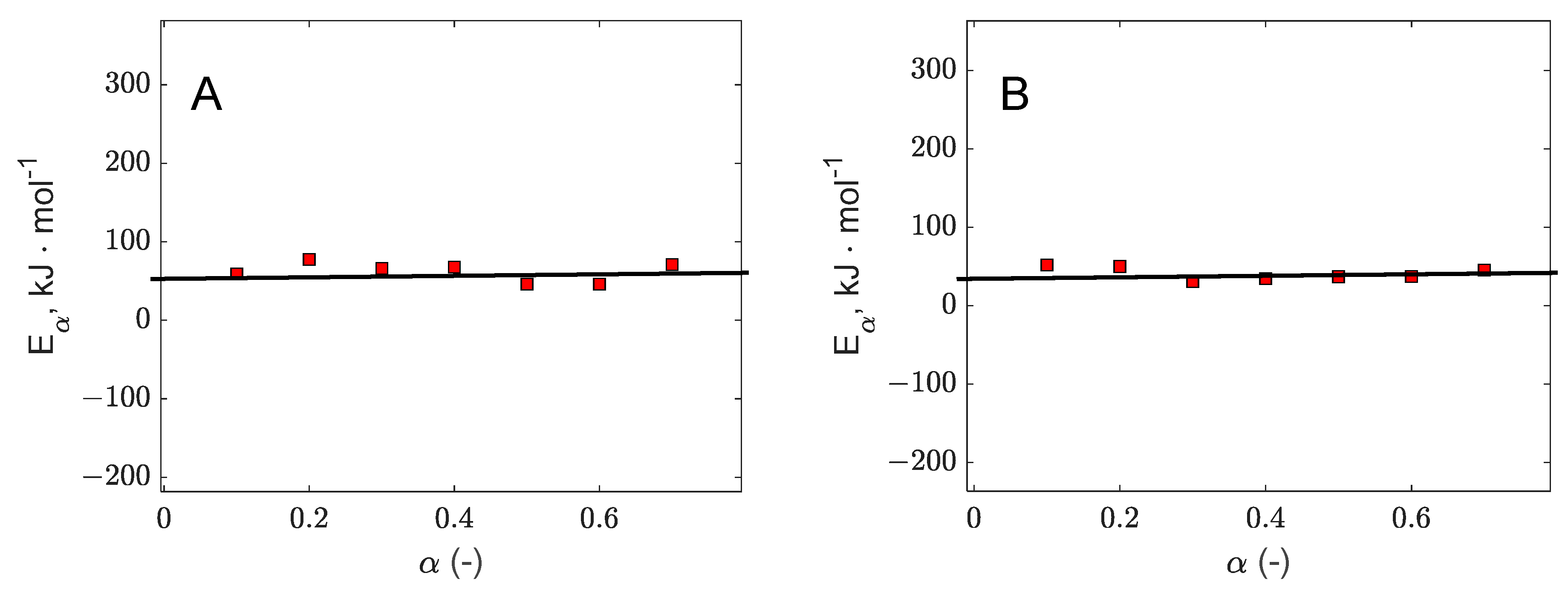
3.1.4. Model-Free Methods
3.1.5. Model-Fit Methods
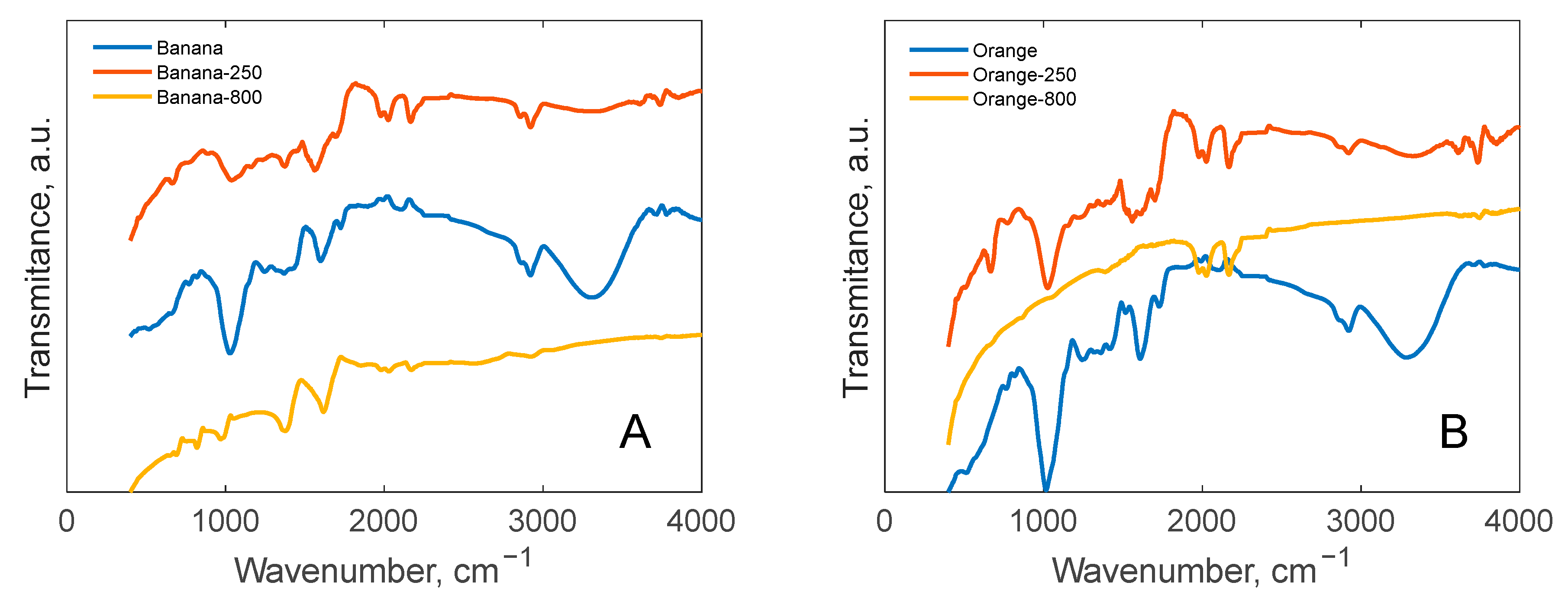
3.2. FT-IR Analysis
3.3. Influence of Biochar on Thermal and Mechanical Properties of PLA Samples
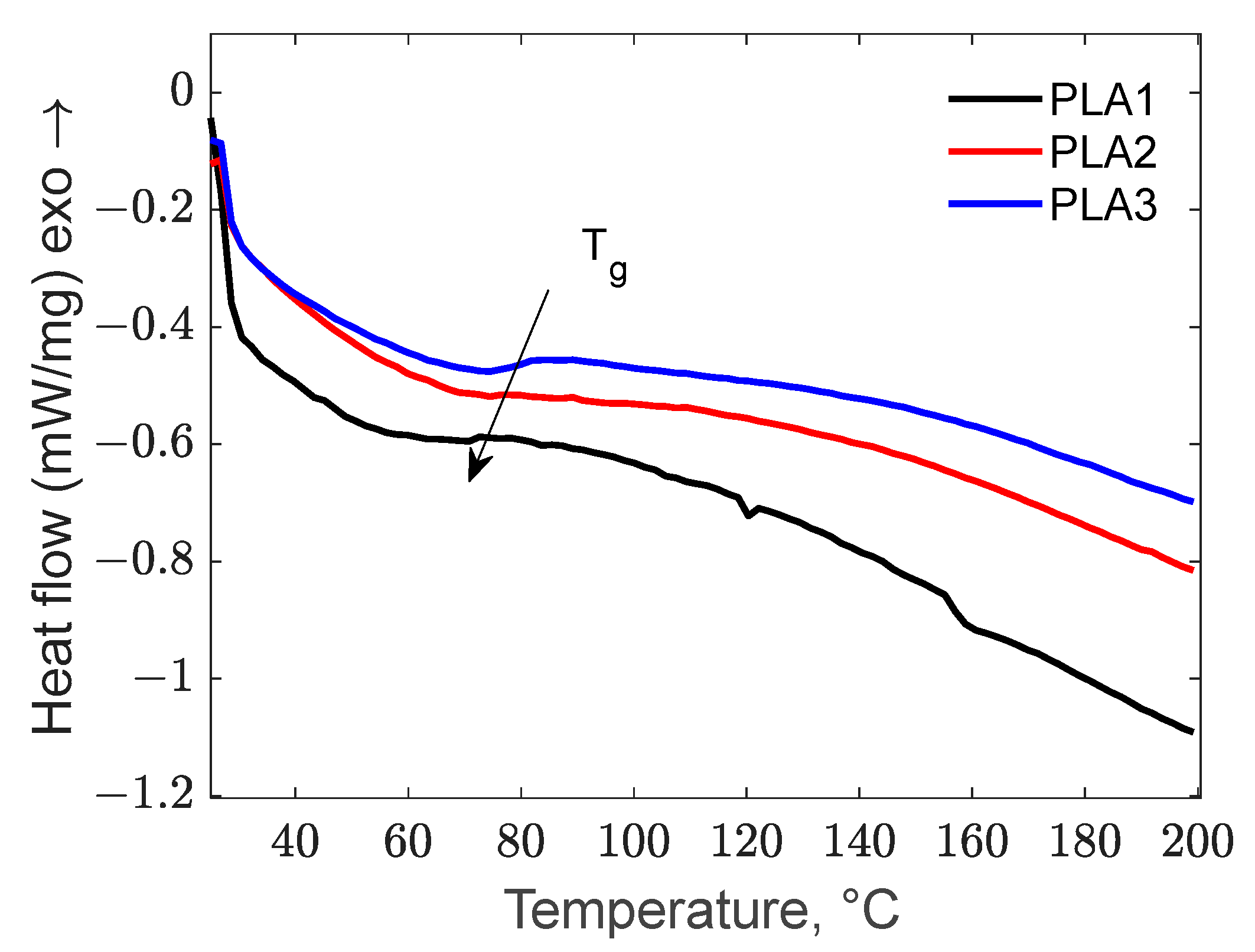
3.3.1. The Influence of Biochar Content on Thermal Properties
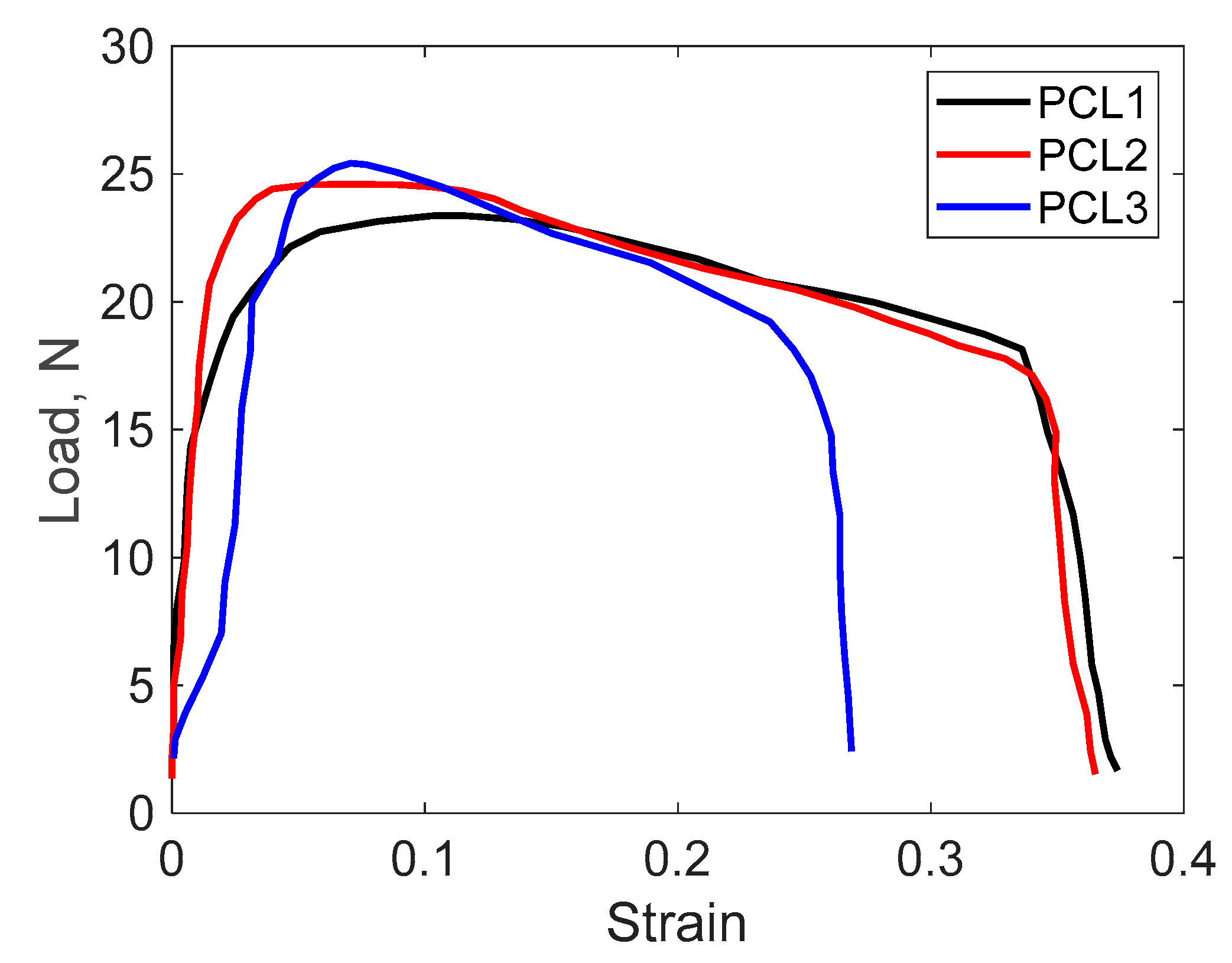
3.3.2. Tensile Test Results
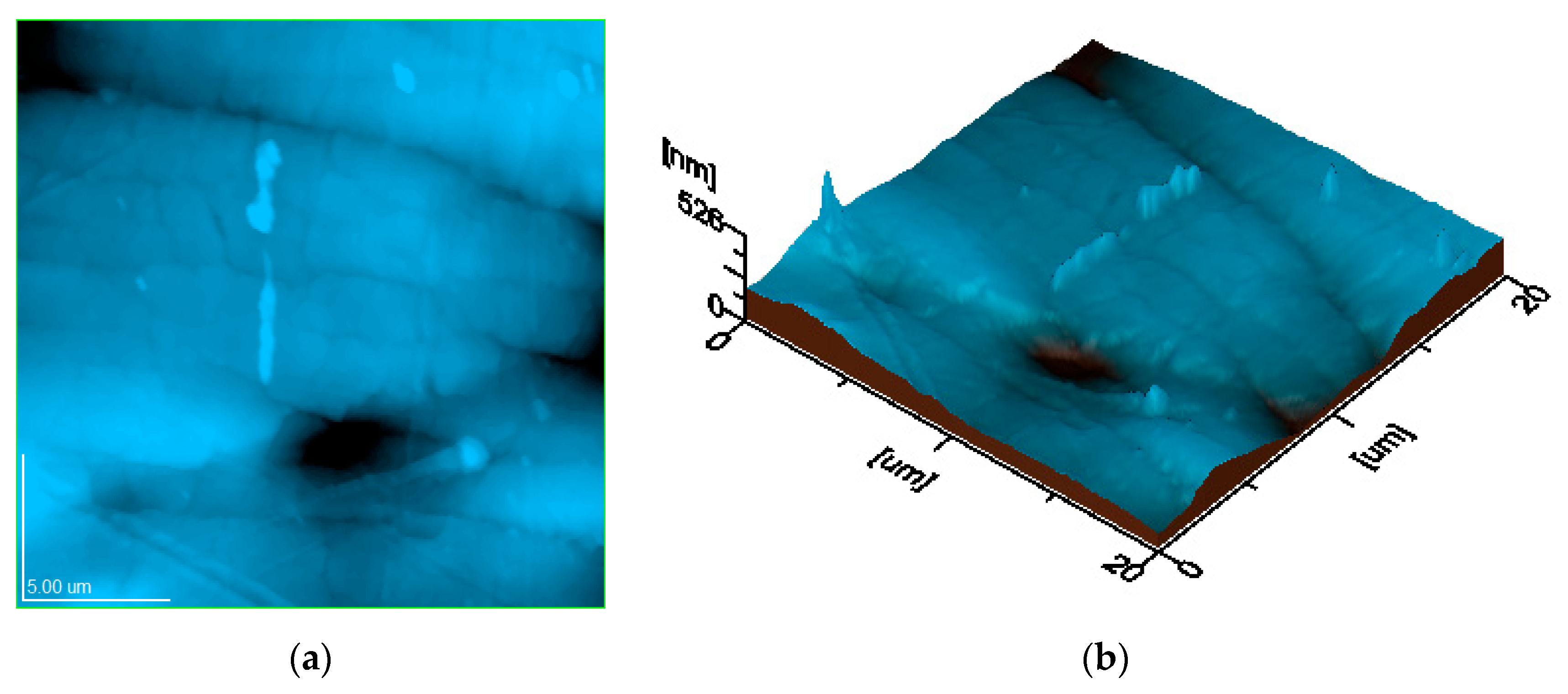
3.3.3. Surface Analysis Results
3.3.4. The Influence of Biochar Content on Rheological Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Lepak-Kuc, S.; Kiciński, M.; Michalski, P.P.; Pavlov, K.; Giorcelli, M.; Bartoli, M.; Jakubowska, M. Innovative Biochar-Based Composite Fibres from Recycled Material. Materials 2021, 14, 5304. [Google Scholar] [CrossRef] [PubMed]
- Zouari, M.; Mikuljan, M.; Schwarzkopf, M. Impact of biochar on the fungal decay resistance of hemp-polylactic acid composites against wood basidiomycetes. Front. Mater. 2023, 10, 1129303. [Google Scholar] [CrossRef]
- Oprescu, E.-E.; Enascuta, E.C.; Vasilievici, G.; Banu, N.D.; Banu, I. Preparation of magnetic biochar for nitrate removal from aqueous solutions. React. Kinet. Mech. Catal. 2022, 135, 2629–2642. [Google Scholar] [CrossRef]
- Gheorghe, C.G.; Pantea, O.; Matei, V.; Bombos, D.; Borcea, A.F. Testing the Biodegradation of Contaminated Water with Petroleum Products Through Conventional Treatment in Comparison with Treatment Through Biological Sludge Enriched with Activated Charcoal. Rev. Chim. 2011, 62, 655–658. [Google Scholar]
- Bondarev, A.; Gheorghe, C.-G. Adsorptive removal of crystal violet dye from aqueous solutions using natural resource systems. Desalin. Water Treat. 2022, 264, 215–223. [Google Scholar] [CrossRef]
- Popovici, D.R.; Gheorghe, C.G.; Dușescu-Vasile, C.M. Assessment of the Active Sludge Microorganisms Population During Wastewater Treatment in a Micro-Pilot Plant. Bioengineering 2024, 11, 1306. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulou, K.; Klonos, P.A.; Kyritsis, A.; Tarani, E.; Chrissafis, K.; Mašek, O.; Tsachouridis, K.; Anastasiou, A.D.; Bikiaris, D.N. Synthesis and Characterization of PLA/Biochar Bio-Composites Containing Different Biochar Types and Content. Polymers 2025, 17, 263. [Google Scholar] [CrossRef]
- Feliz Florian, G.; Ragoubi, M.; Leblanc, N.; Taouk, B.; Abdelouahed, L. Biochar Production and Its Potential Application for Biocomposite Materials: A Comprehensive Review. J. Compos. Sci. 2024, 8, 220. [Google Scholar] [CrossRef]
- Alhelal, A.; Mohammed, Z.; Jeelani, S.; Rangari, V.K. 3D printing of spent coffee ground derived biochar reinforced epoxy composites. J. Compos. Mater. 2021, 55, 3651–3660. [Google Scholar] [CrossRef]
- Călin, C.; Sîrbu, E.-E.; Tănase, M.; Győrgy, R.; Popovici, D.R.; Banu, I. A Thermogravimetric Analysis of Biomass Conversion to Biochar: Experimental and Kinetic Modeling. Appl. Sci. 2024, 14, 9856. [Google Scholar] [CrossRef]
- Xia, Y.; Qian, S.; Zhang, X.; Zhang, Z.; Zhu, C. Biochar as an efficient reinforcing agent for poly(lactic acid)/poly(ε-caprolactone) biodegradable composites with high robustness and thermo-resistance. Ind. Crops Prod. 2024, 219, 119049. [Google Scholar] [CrossRef]
- Kopitzky, R. Poly(Lactic Acid)–Poly(Butylene Succinate)–Sugar Beet Pulp Composites; Part I: Mechanics of Composites with Fine and Coarse Sugar Beet Pulp Particles. Polymers 2021, 13, 2531. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Fishman, M.L.; Hicks, K.B.; Liu, C.-K. Biodegradable Composites from Sugar Beet Pulp and Poly(lactic acid). J. Agric. Food Chem. 2005, 53, 9017–9022. [Google Scholar] [CrossRef]
- Jamshidian, M.; Tehrany, E.A.; Imran, M.; Jacquot, M.; Desobry, S. Poly-Lactic Acid: Production, Applications, Nanocomposites, and Release Studies. Compr. Rev. Food Sci. Food Saf. 2010, 9, 552–571. [Google Scholar] [CrossRef] [PubMed]
- Rasal, R.M.; Hirt, D.E. Toughness decrease of PLA-PHBHHx blend films upon surface-confined photopolymerization. J. Biomed. Mater. Res. Part A 2009, 88A, 1079–1086. [Google Scholar] [CrossRef]
- Banu, I.; Stanciu, N.D.; Puaux, J.-P.; Bozga, G. A study of L-lactide ring-opening polymerization in molten state. U.P.B. Sci. Bull. Ser. B 2009, 71, 35–46. [Google Scholar]
- Ho, M.-P.; Lau, K.-T.; Wang, H.; Hui, D. Improvement on the properties of polylactic acid (PLA) using bamboo charcoal particles. Compos. Part B Eng. 2015, 81, 14–25. [Google Scholar] [CrossRef]
- George, J.; Jung, D.; Bhattacharyya, D. Improvement of Electrical and Mechanical Properties of PLA/PBAT Composites Using Coconut Shell Biochar for Antistatic Applications. Appl. Sci. 2023, 13, 902. [Google Scholar] [CrossRef]
- Vidakis, N.; Petousis, M.; Sagris, D.; David, C.; Mountakis, N.; Spiridaki, M.; Maravelakis, E.; Charitidis, C.; Stratakis, E. Enhancing biocomposite critical quality indicators (CQIs): The impact of biochar content in additive manufacturing. Biochar 2025, 7, 22. [Google Scholar] [CrossRef]
- Arrigo, R.; Bartoli, M.; Malucelli, G. Poly(lactic Acid)–Biochar Biocomposites: Effect of Processing and Filler Content on Rheological, Thermal, and Mechanical Properties. Polymers 2020, 12, 892. [Google Scholar] [CrossRef]
- Zouari, M.; Devallance, D.B.; Marrot, L. Effect of Biochar Addition on Mechanical Properties, Thermal Stability, and Water Resistance of Hemp-Polylactic Acid (PLA) Composites. Materials 2022, 15, 2271. [Google Scholar] [CrossRef] [PubMed]
- Aup-Ngoen, K.; Noipitak, M. Effect of carbon-rich biochar on mechanical properties of PLA-biochar composites. Sustain. Chem. Pharm. 2020, 15, 100204. [Google Scholar] [CrossRef]
- Day, R.; Adhikari, S.; Peng, Y. Properties of polylactic acid and biochar-based composites for environment-friendly plant containers. Clean. Eng. Technol. 2024, 23, 100850. [Google Scholar] [CrossRef]
- Anerao, P.; Kulkarni, A.; Munde, Y.; Shinde, A.; Das, O. Biochar reinforced PLA composite for fused deposition modelling (FDM): A parametric study on mechanical performance. Compos. Part C Open Access 2023, 12, 100406. [Google Scholar] [CrossRef]
- Gazzotti, S.; Rampazzo, R.; Hakkarainen, M.; Bussini, D.; Ortenzi, M.A.; Farina, H.; Lesma, G.; Silvani, A. Cellulose nanofibrils as reinforcing agents for PLA-based nanocomposites: An in situ approach. Compos. Sci. Technol. 2019, 171, 94–102. [Google Scholar] [CrossRef]
- Huang, C.-C.; Chang, C.-W.; Jahan, K.; Wu, T.-M.; Shih, Y.-F. Effects of the Grapevine Biochar on the Properties of PLA Composites. Materials 2023, 16, 816. [Google Scholar] [CrossRef]
- Fabijański, M.; Gabriołek, A.P.; Jaszczuk, J.; Nowakowski, K.Z.; Plichta, A.; Richert, J.; Sielska, K.M. Material Composition, Method of Its Production and Packaging Made of It. Patent no. 244616. 2021. Available online: https://ewyszukiwarka.pue.uprp.gov.pl/search/pwp-details/P.437251?lng=en (accessed on 7 April 2025).
- Koutoulis, A.S.; Giannakas, A.E.; Lazaridis, D.G.; Kitsios, A.-P.; Karabagias, V.K.; Giannakas, A.E.; Ladavos, A.; Karabagias, I.K. Preparation and Characterization of PLA-Based Films Fabricated with Different Citrus Species Peel Powder. Coatings 2024, 14, 1311. [Google Scholar] [CrossRef]
- Raimondo, M.; Caracciolo, F.; Cembalo, L.; Chinnici, G.; Pecorino, B.; D’Amico, M. Making Virtue Out of Necessity: Managing the Citrus Waste Supply Chain for Bioeconomy Applications. Sustainability 2018, 10, 4821. [Google Scholar] [CrossRef]
- Alzate Acevedo, S.; Díaz Carrillo, Á.J.; Flórez-López, E.; Grande-Tovar, C.D. Recovery of Banana Waste-Loss from Production and Processing: A Contribution to a Circular Economy. Molecules 2021, 26, 5282. [Google Scholar] [CrossRef]
- Kim, J.; Lee, J.; Lee, S.; Kwon, O.; Lee, J.; Kim, J. Enhanced biodegradability and thermal insulation capability of polylactic acid reinforced with calcium-crosslinked orange peel biochar. Ind. Crops Prod. 2025, 226, 120666. [Google Scholar] [CrossRef]
- Sambudi, N.S.; Lin, W.Y.; Harun, N.Y.; Mutiari, D. Modification of Poly(lactic acid) with Orange Peel Powder as Biodegradable Composite. Polymers 2022, 14, 4126. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, S.; Kumar, D.; Mahakur, V.K.; Bhowmik, S. Utilization of banana peel as bio-filler to develop bio-composite materials and characterize their physical, mechanical, thermal, and tribological characteristics. Polym. Bull. 2024, 81, 16175–16196. [Google Scholar] [CrossRef]
- Komal, U.K.; Lila, M.K.; Singh, I. PLA/banana fiber based sustainable biocomposites: A manufacturing perspective. Compos. Part B Eng. 2020, 180, 107535. [Google Scholar] [CrossRef]
- Nguyen, T.A.; Nguyen, T.H. Study on Mechanical Properties of Banana Fiber-Reinforced Materials Poly (Lactic Acid) Composites. Int. J. Chem. Eng. 2022, 2022, 8485038. [Google Scholar] [CrossRef]
- ASTM E1690_08; Standard Test Method for Determination of Ethanol Extractives in Biomass. ASTM International: Conshohocken, PA, USA, 2021. [CrossRef]
- Carrier, M.; Loppinet-Serani, A.; Denux, D.; Lasnier, J.-M.; Ham-Pichavant, F.; Cansell, F.; Aymonier, C. Thermogravimetric analysis as a new method to determine the lignocellulosic composition of biomass. Biomass Bioenergy 2011, 35, 298–307. [Google Scholar] [CrossRef]
- E-1721; Standard Test Method for Determination of Acid-Insoluble Residue in Biomass. ASTM International: Conshohocken, PA, USA, 2024.
- ASTM E1755-01(2020); Standard Test Method for Determination of Acid-Insoluble Residue in Biomass. ASTM International: Conshohocken, PA, USA, 2024.
- Reza, M.S.; Ahmed, A.; Caesarendra, W.; Abu Bakar, M.S.; Shams, S.; Saidur, R.; Aslfattahi, N.; Azad, A.K. Acacia Holosericea: An Invasive Species for Bio-char, Bio-oil, and Biogas Production. Bioengineering 2019, 6, 33. [Google Scholar] [CrossRef]
- EN ISO 527-3; Plastics—Determination of Tensile Properties Part 3: Test Conditions for Films and Sheets. International Organization for Standardization: Geneva, Switzerland, 2018. Available online: https://www.iso.org/standard/70307.html (accessed on 7 April 2025).
- Moldovan, A.; Sarosi, I.; Cuc, S.; Prodan, D.; Taut, A.C.; Petean, I.; Bombos, D.; Doukeh, R.; Nemes, O.; Man, S.C. Development and characterization of PLA food packaging composite. J. Therm. Anal. Calorim. 2024. [Google Scholar] [CrossRef]
- Ahmed, A.; Bakar, M.S.A.; Razzaq, A.; Hidayat, S.; Jamil, F.; Amin, M.N.; Sukri, R.S.; Shah, N.S.; Park, Y.-K. Characterization and Thermal Behavior Study of Biomass from Invasive Acacia mangium Species in Brunei Preceding Thermochemical Conversion. Sustainability 2021, 13, 5249. [Google Scholar] [CrossRef]
- Müsellim, E.; Tahir, M.H.; Ahmad, M.S.; Ceylan, S. Thermokinetic and TG/DSC-FTIR study of pea waste biomass pyrolysis. Appl. Therm. Eng. 2018, 137, 54–61. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, J.; Evrendilek, F.; Zhang, X.; Buyukada, M. TG-FTIR and Py-GC/MS analyses of pyrolysis behaviors and products of cattle manure in CO2 and N2 atmospheres: Kinetic, thermodynamic, and machine-learning models. Energy Convers. Manag. 2019, 195, 346–359. [Google Scholar] [CrossRef]
- Da Silva, M.D.; Da Boit Martinello, K.; Knani, S.; Lütke, S.F.; Machado, L.M.M.; Manera, C.; Perondi, D.; Godinho, M.; Collazzo, G.C.; Silva, L.F.O.; et al. Pyrolysis of citrus wastes for the simultaneous production of adsorbents for Cu(II), H2, and d-limonene. Waste Manag. 2022, 152, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Ayala, J.R.; Montero, G.; Coronado, M.A.; García, C.; Curiel-Alvarez, M.A.; León, J.A.; Sagaste, C.A.; Montes, D.G. Characterization of Orange Peel Waste and Valorization to Obtain Reducing Sugars. Molecules 2021, 26, 1348. [Google Scholar] [CrossRef]
- Saffe, A.; Fernandez, A.; Mazza, G.; Rodriguez, R. Prediction of regional agro-industrial wastes characteristics by thermogravimetric analysis to obtain bioenergy using thermal process. Energy Explor. Exploit. 2018, 37, 544–557. [Google Scholar] [CrossRef]
- Emiola-Sadiq, T.; Zhang, L.; Dalai, A.K. Thermal and Kinetic Studies on Biomass Degradation via Thermogravimetric Analysis: A Combination of Model-Fitting and Model-Free Approach. ACS Omega 2021, 6, 22233–22247. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, G.R.; Mahmoud, R.K.; Shaban, M.; Fahim, I.S.; Abd El-Salam, H.M.; Mahmoud, H.M. Towards a circular economy: Valorization of banana peels by developing bio-composites thermal insulators. Sci. Rep. 2023, 13, 12756. [Google Scholar] [CrossRef] [PubMed]
- Pereira, P.H.F.; Maia, L.S.; da Silva, A.I.C.; Silva, B.A.R.; Pinhati, F.R.; de Oliveira, S.A.; Rosa, D.S.; Mulinari, D.R. Prospective Life Cycle Assessment Prospective (LCA) of Activated Carbon Production, Derived from Banana Peel Waste for Methylene Blue Removal. Adsorption 2024, 30, 1081–1101. [Google Scholar] [CrossRef]
- Singh, S.; Wu, C.; Williams, P.T. Pyrolysis of waste materials using TGA-MS and TGA-FTIR as complementary characterisation techniques. J. Anal. Appl. Pyrolysis 2012, 94, 99–107. [Google Scholar] [CrossRef]
- Zlatanović, S.; Ostojić, S.; Micić, D.; Rankov, S.; Dodevska, M.; Vukosavljević, P.; Gorjanović, S. Thermal behaviour and degradation kinetics of apple pomace flours. Thermochim. Acta 2019, 673, 17–25. [Google Scholar] [CrossRef]
- Çepelioğullar, Ö.; Haykırı-Açma, H.; Yaman, S. Kinetic modelling of RDF pyrolysis: Model-fitting and model-free approaches. Waste Manag. 2016, 48, 275–284. [Google Scholar] [CrossRef]
- Li, B.; Ng, J.-H.; Woon, K.S.; Chong, W.W.F.; Ng, K.L.A.; Lee, C.T.; Chiong, M.C.; Nge, K.S.; Mong, G.R. Comparative analysis of kinetic model-fitting methods and selection priority for horse manure pyrolysis. Sustain. Chem. Pharm. 2024, 39, 101590. [Google Scholar] [CrossRef]
- Friedman, H.L. Kinetics of thermal degradation of char-forming plastics from thermogravimetry. Application to a phenolic plastic. J. Polym. Sci. Part C Polym. Symp. 1964, 6, 183–195. [Google Scholar] [CrossRef]
- Ozawa, T. A New Method of Analyzing Thermogravimetric Data. Bull. Chem. Soc. Jpn. 1965, 38, 1881–1886. [Google Scholar] [CrossRef]
- Flynn, J.H.; Wall, L.A. General Treatment of the Thermogravimetry of Polymers. J. Res. Natl. Bur. Stand. A Phys. Chem. 1966, 70A, 487–523. [Google Scholar] [CrossRef]
- Kissinger, H.E. Variation of Peak Temperature With Heating Rate in Differential Thermal Analysis. J. Res. Natl. Bur. Stand. 1956, 57, 217–221. [Google Scholar] [CrossRef]
- Starink, M.J. Activation energy determination for linear heating experiments: Deviations due to neglecting the low temperature end of the temperature integral. J. Mater. Sci. 2007, 42, 483–489. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Burnham, A.K.; Criado, J.M.; Pérez-Maqueda, L.A.; Popescu, C.; Sbirrazzuoli, N. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim. Acta 2011, 520, 1–19. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Dollimore, D. Linear and Nonlinear Procedures in Isoconversional Computations of the Activation Energy of Nonisothermal Reactions in Solids. J. Chem. Inf. Comput. Sci. 1996, 36, 42–45. [Google Scholar] [CrossRef]
- Balogun, A.O.; Lasode, O.A.; McDonald, A.G. Devolatilisation kinetics and pyrolytic analyses of Tectona grandis (teak). Bioresour. Technol. 2014, 156, 57–62. [Google Scholar] [CrossRef]
- Liu, Z.; Ku, X.; Wang, Z. Mechanism insights into hardwood lignin pyrolysis via ReaxFF molecular dynamics simulations. Biomass Bioenergy 2025, 199, 107938. [Google Scholar] [CrossRef]
- Debiagi, P.; Piazza, V.; Papagni, M.; Beretta, A.; Frassoldati, A.; Faravelli, T. Cellulose pyrolysis kinetic model: Detailed description of volatile species. Proc. Combust. Inst. 2024, 40, 105651. [Google Scholar] [CrossRef]
- Luangkiattikhun, P.; Tangsathitkulchai, C.; Tangsathitkulchai, M. Non-isothermal thermogravimetric analysis of oil-palm solid wastes. Bioresour. Technol. 2008, 99, 986–997. [Google Scholar] [CrossRef] [PubMed]
- Ko, K.-H.; Rawal, A.; Sahajwalla, V. Analysis of thermal degradation kinetics and carbon structure changes of co-pyrolysis between macadamia nut shell and PET using thermogravimetric analysis and 13C solid state nuclear magnetic resonance. Energy Convers. Manag. 2014, 86, 154–164. [Google Scholar] [CrossRef]
- Pistor, V.; Ornaghi, F.G.; Ornaghi, H.L., Jr.; Zattera, A.J. Degradation kinetic of epoxy nanocomposites containing different percentage of epoxycyclohexyl—POSS. Polym. Compos. 2012, 33, 1224–1232. [Google Scholar] [CrossRef]
- Grioui, N.; Halouani, K.; Zoulalian, A.; Halouani, F. Thermogravimetric analysis and kinetics modeling of isothermal carbonization of olive wood in inert atmosphere. Thermochim. Acta 2006, 440, 23–30. [Google Scholar] [CrossRef]
- Tripathi, M.; Sahu, J.N.; Ganesan, P. Effect of process parameters on production of biochar from biomass waste through pyrolysis: A review. Renew. Sust. Energ. Rev. 2016, 55, 467–481. [Google Scholar] [CrossRef]
- Vassileva, P.; Tumbalev, V.; Kichukova, D.; Voykova, D.; Kovacheva, D.; Spassova, I. Study on the Dye Removal from Aqueous Solutions by Graphene-Based Adsorbents. Materials 2023, 16, 5754. [Google Scholar] [CrossRef] [PubMed]
- Patra, B.R.; Nanda, S.; Dalai, A.K.; Meda, V. Slow pyrolysis of agro-food wastes and physicochemical characterization of biofuel products. Chemosphere 2021, 285, 131431. [Google Scholar] [CrossRef] [PubMed]
- Qian, S.; Kong, Y.; Cheng, H.; Tu, S.; Zhai, C. Interfacial interaction improvement of polylactic acid/bamboo-char biocomposites for high toughness, good strength, and excellent thermal stability. Surf. Interfaces 2023, 42, 103315. [Google Scholar] [CrossRef]
- Lekrine, A.; Belaadi, A.; Dembri, I.; Jawaid, M.; Ismail, A.S.; Abdullah, M.M.S.; Chai, B.X.; Al-Khawlani, A.; Ghernaout, D. Thermomechanical and structural analysis of green hybrid composites based on polylactic acid/biochar/treated W. filifera palm fibers. J. Mater. Res. Technol. 2024, 30, 9656–9667. [Google Scholar] [CrossRef]
- Avram, S.E.; Barbu Tudoran, L.; Cuc, S.; Borodi, G.; Birle, B.V.; Petean, I. Microstructural Investigations Regarding Sustainable Recycling of Ceramic Slurry Collected from Industrial Waste Waters. Sustainability 2024, 16, 1123. [Google Scholar] [CrossRef]
- Avram, S.E.; Birle, B.V.; Tudoran, L.B.; Borodi, G.; Petean, I. Investigation of Used Water Sediments from Ceramic Tile Fabrication. Water 2024, 16, 1027. [Google Scholar] [CrossRef]
- Avram, S.E.; Tudoran, L.B.; Borodi, G.; Filip, M.R.; Ciotlaus, I.; Petean, I. Physicochemical Aspects Regarding the Sustainable Conversion of Carwash Slurry as Coverage Admixture for Landfills. Sustainability 2025, 17, 2906. [Google Scholar] [CrossRef]
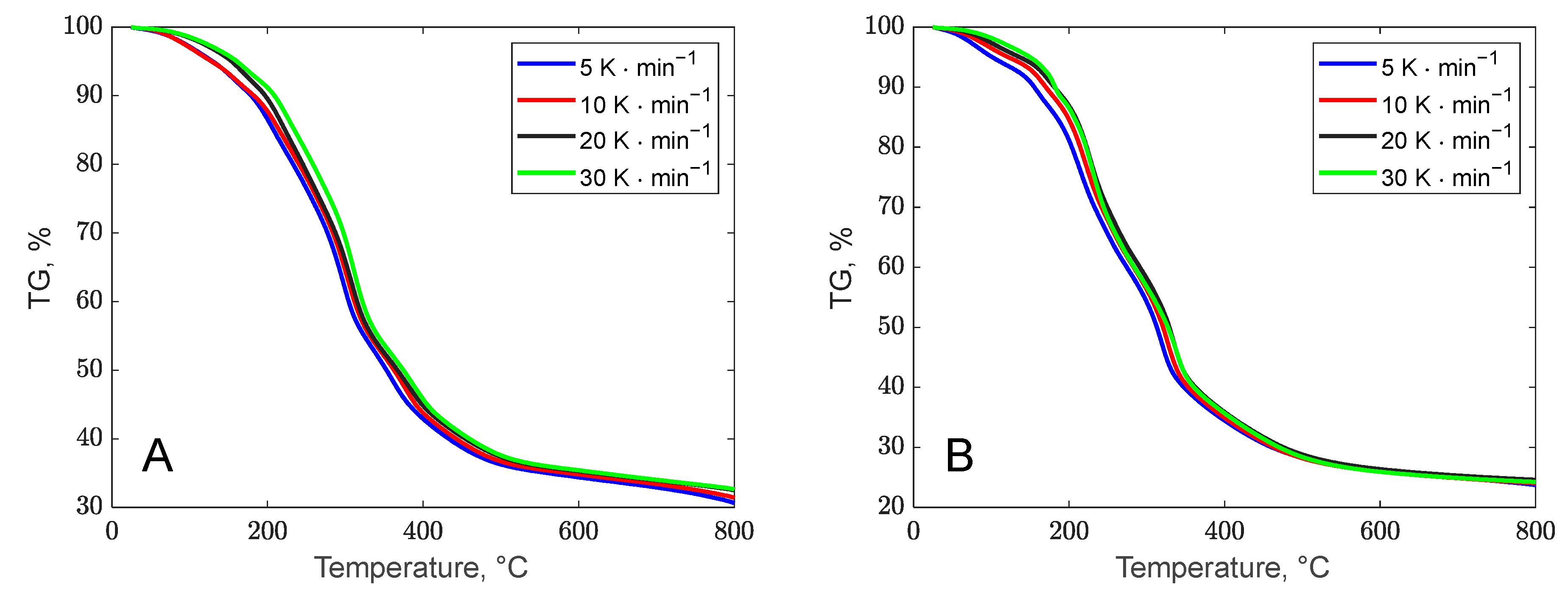




| Biomass Waste | Banana Peels | Orange Peels |
|---|---|---|
| Moisture, wt.% | 2.50 | 3.26 |
| Ethanol extractables, wt.% | 14.35 | 12.57 |
| Lignin, wt.% | 11.68 | 1.21 |
| Carbohydrates, wt.% | 66.71 | 80.62 |
| Ash, wt.% | 4.72 | 2.29 |
| Peel Type | Heating Rate, °C·min−1 | Temperature of the Peaks, °C | , %·min−1 | Material Left, % |
|---|---|---|---|---|
| Banana | 5 | 152 206.4 298.3 359.7 | 5.82 11.1 21.8 10.4 | 32.8 |
| 10 | 155.1 216.4 301.3 375.2 | 7.41 14.2 28.5 13.7 | 33.4 | |
| 20 | 156.5 226.1 305.6 375.9 | 8.06 17.6 28.2 12.9 | 33.9 | |
| 30 | 157.3 237.9 306.8 382.3 | 4.58 12.0 19.6 9.03 | 34.1 | |
| Orange | 5 | 80.3 215.4 319 | 5.31 21.43 24.72 | 25.1 |
| 10 | 93.5 221.5 325.8 | 1.84 8.77 8.97 | 24.8 | |
| 20 | 105.2 232.1 332.3 | 2.43 13.7 12.7 | 25.3 | |
| 30 | 100.72 232.3 330.5 | 2.41 14.7 11.53 | 24.8 |
| Mathematical Model | |||
|---|---|---|---|
| Power/Exponential | |||
| Power law | P2 | ||
| P3 | |||
| P4 | |||
| Random nucleation and nuclei growth | |||
| Avrami–Erofeev | A2 | ||
| A3 | |||
| A4 | |||
| Contraction models (geometrical) | |||
| Contracting area | R2 | ||
| Contracting volume | R3 | ||
| Diffusion models | |||
| 1D diffusion | D1 | ||
| 2D diffusion | D2 | ||
| 3D diffusion (Jander) | D3 | ||
| 3D diffusion (Zhuravlev–Lesokhin–Tempelman) | D4 | ||
| Reaction models | |||
| Zero order | F0 | 1 | |
| First order | F1 | ||
| Second order | F2 | ||
| Third order | F3 | ||
| Banana Peel | Orange Peel | |||||
|---|---|---|---|---|---|---|
| Slope | Intercept | R2 | Slope | Intercept | R2 | |
| 0.1 | −7091.3 | 14.715 | 0.983 | −6256.9 | 11.688 | 0.988 |
| 0.2 | −9279.4 | 20.946 | 0.852 | −6001.9 | 10.244 | 0.974 |
| 0.3 | −7912.1 | 15.657 | 0.892 | −3686.9 | 2.7736 | 0.947 |
| 0.4 | −8138.6 | 14.901 | 0.744 | −4163.7 | 3.8521 | 0.958 |
| 0.5 | −5518.8 | 6.2879 | 0.877 | −4439.9 | 4.0106 | 0.947 |
| 0.6 | −5551.6 | 5.3139 | 0.945 | −4488 | 3.0068 | 0.909 |
| 0.7 | −8515.4 | 11.155 | 0.931 | −5446.9 | 4.1624 | 0.907 |
| Banana Peel Kinetic Parameters | |||
|---|---|---|---|
| Stage I | Stage II | Stage III | Stage IV |
R2 = 0.994 | R2 = 0.996 | R2 = 0.989 | R2 = 0.991 |
| −3D diffusion (Jander) | –Contracting volume | –Third order | –Third order |
| Orange Peel Kinetic Parameters | |||
| Stage I | Stage II | Stage III | |
R2 = 0.984 | R2 = 0.992 | R2 = 0.993 | |
| −3D diffusion (Jander) | −3D diffusion (Jander) | –Third order | |
| Sample | Tensile Strength (MPa) | Maximum Load (N) | Elongation at Break (mm) | Young’s Modulus (MPa) | Stress at Yield (MPa) |
|---|---|---|---|---|---|
| PCL1 | 2.621 ± 0.11 | 23.904 ± 1.21 | 14.179 ± 1.53 | 194.334 ± 8.26 | 1.341 ± 0.16 |
| PCL2 | 2.957 ± 0.13 | 24.607 ± 2.43 | 10.666 ± 1.46 | 317.839 ± 10.1 | 0.591 ± 0.05 |
| PCL3 | 3.432 ± 0.15 | 26.147 ± 2.65 | 7.240 ± 0.95 | 388.314 ± 12.4 | 2.088 ± 0.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joe, A.-C.; Tănase, M.; Călin, C.; Sîrbu, E.-E.; Banu, I.; Bomboș, D.; Cuc, S. Pyrolyzed Biomass Filler for PLA-Based Food Packaging. Polymers 2025, 17, 1327. https://doi.org/10.3390/polym17101327
Joe A-C, Tănase M, Călin C, Sîrbu E-E, Banu I, Bomboș D, Cuc S. Pyrolyzed Biomass Filler for PLA-Based Food Packaging. Polymers. 2025; 17(10):1327. https://doi.org/10.3390/polym17101327
Chicago/Turabian StyleJoe, Andreea-Cătălina, Maria Tănase, Catalina Călin, Elena-Emilia Sîrbu, Ionuț Banu, Dorin Bomboș, and Stanca Cuc. 2025. "Pyrolyzed Biomass Filler for PLA-Based Food Packaging" Polymers 17, no. 10: 1327. https://doi.org/10.3390/polym17101327
APA StyleJoe, A.-C., Tănase, M., Călin, C., Sîrbu, E.-E., Banu, I., Bomboș, D., & Cuc, S. (2025). Pyrolyzed Biomass Filler for PLA-Based Food Packaging. Polymers, 17(10), 1327. https://doi.org/10.3390/polym17101327








