Recent Advances in Functional Biopolymer Films with Antimicrobial and Antioxidant Properties for Enhanced Food Packaging
Abstract
1. Introduction
1.1. Classification of Films: Biodegradable Films
1.2. Edible Films and Coatings
1.3. Active Packaging Films
1.4. Intelligent Packaging Films
1.5. Multilayer and Composite Films
2. Advances in Biopolymer Films for Antimicrobial and Antioxidant Food Packaging
2.1. Advanced Fabrication Methods for Enhancing Biopolymer-Based Food Packaging
2.1.1. Fabrication of Cellulose/Curcumin Composite Films
2.1.2. Physical and Functional Properties of the Films
2.1.3. Intelligent pH-Responsive Freshness Monitoring
2.1.4. Antimicrobial and Biodegradability Performance
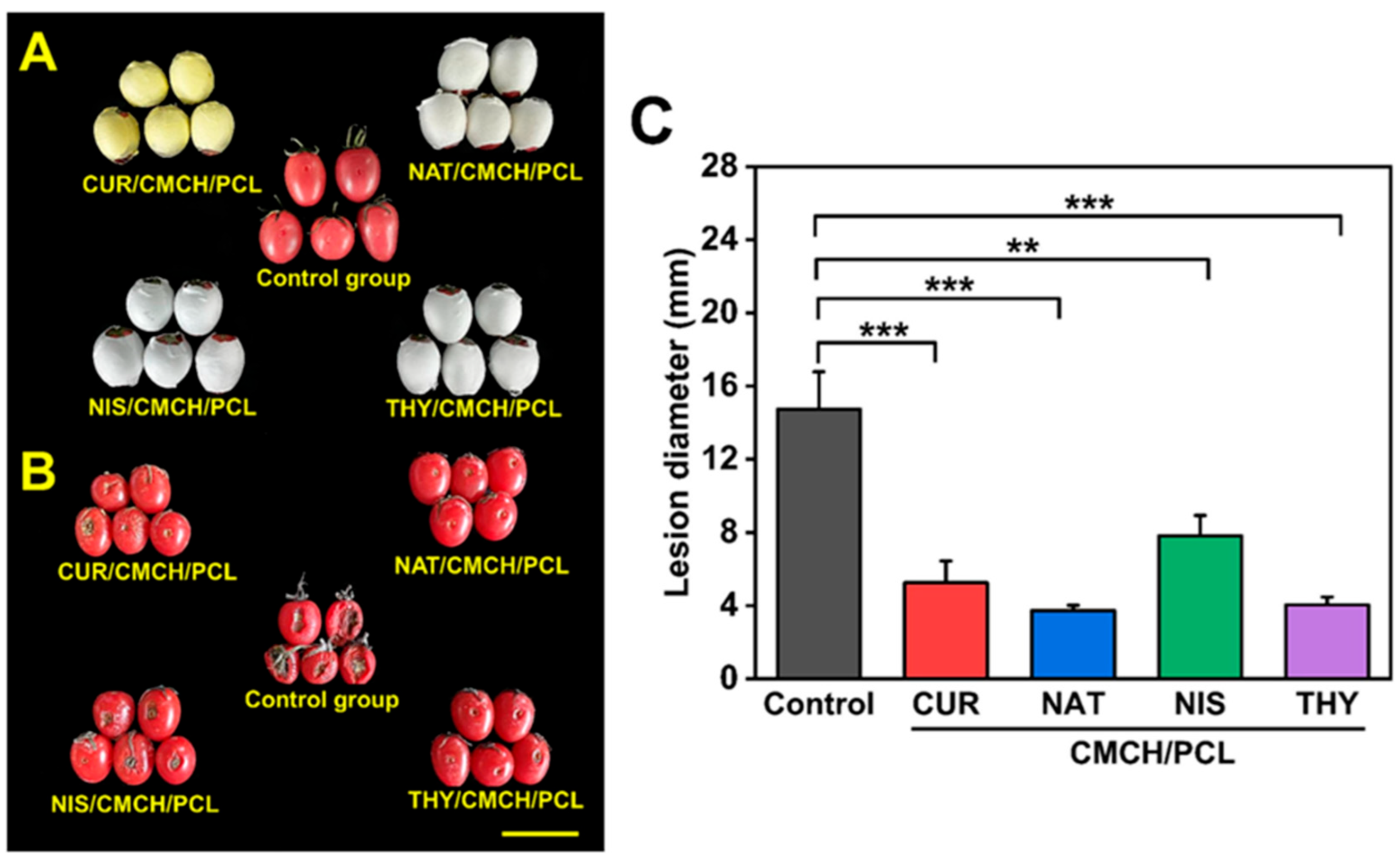
2.2. In Situ Fruit Packaging with Carboxymethyl Chitosan and Polycaprolactone
2.2.1. Fabrication and Characterization of In Situ Packaging
2.2.2. Effectiveness of In Situ Packaging on Fruit Preservation
2.2.3. Industrial Applications and Future Perspectives
2.3. Development of Eco-Friendly Polylactic Acid/Thermoplastic Starch Films Enhanced with Clove Essential Oil and Cochineal for Active and Intelligent Food Packaging
2.3.1. Fabrication and Structural Characterization of the PLA/TPS Films
2.3.2. Antibacterial Activity and Food Preservation Performance
2.3.3. Industrial Feasibility and Conclusion
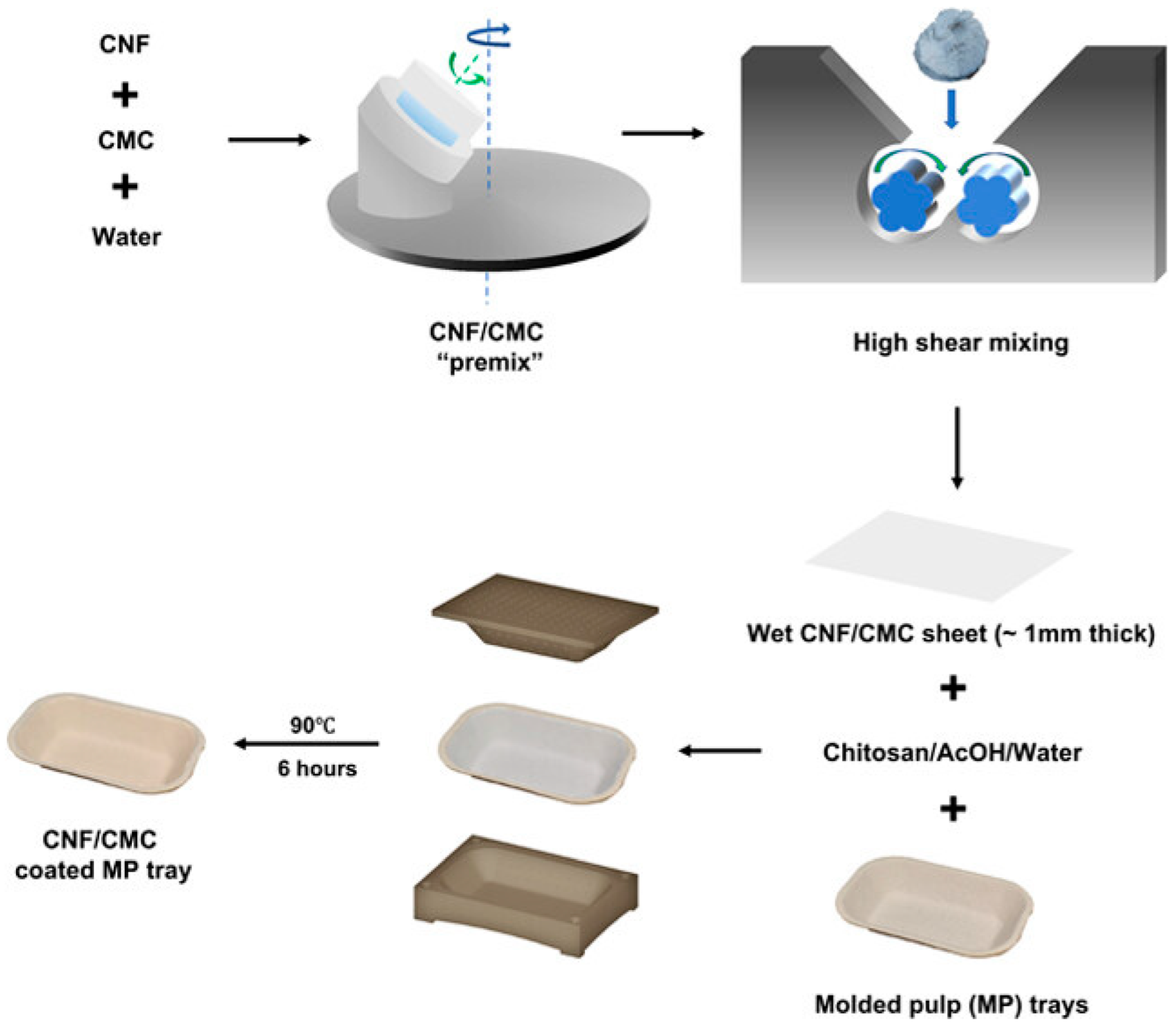
2.4. Development of PFAS-Free Cellulose Nanofibril-Based Food Packaging for Sustainable Applications
2.4.1. Structural Characterization and Material Performance
2.4.2. Barrier Properties and Food Preservation
2.4.3. Industrial Feasibility and Sustainability
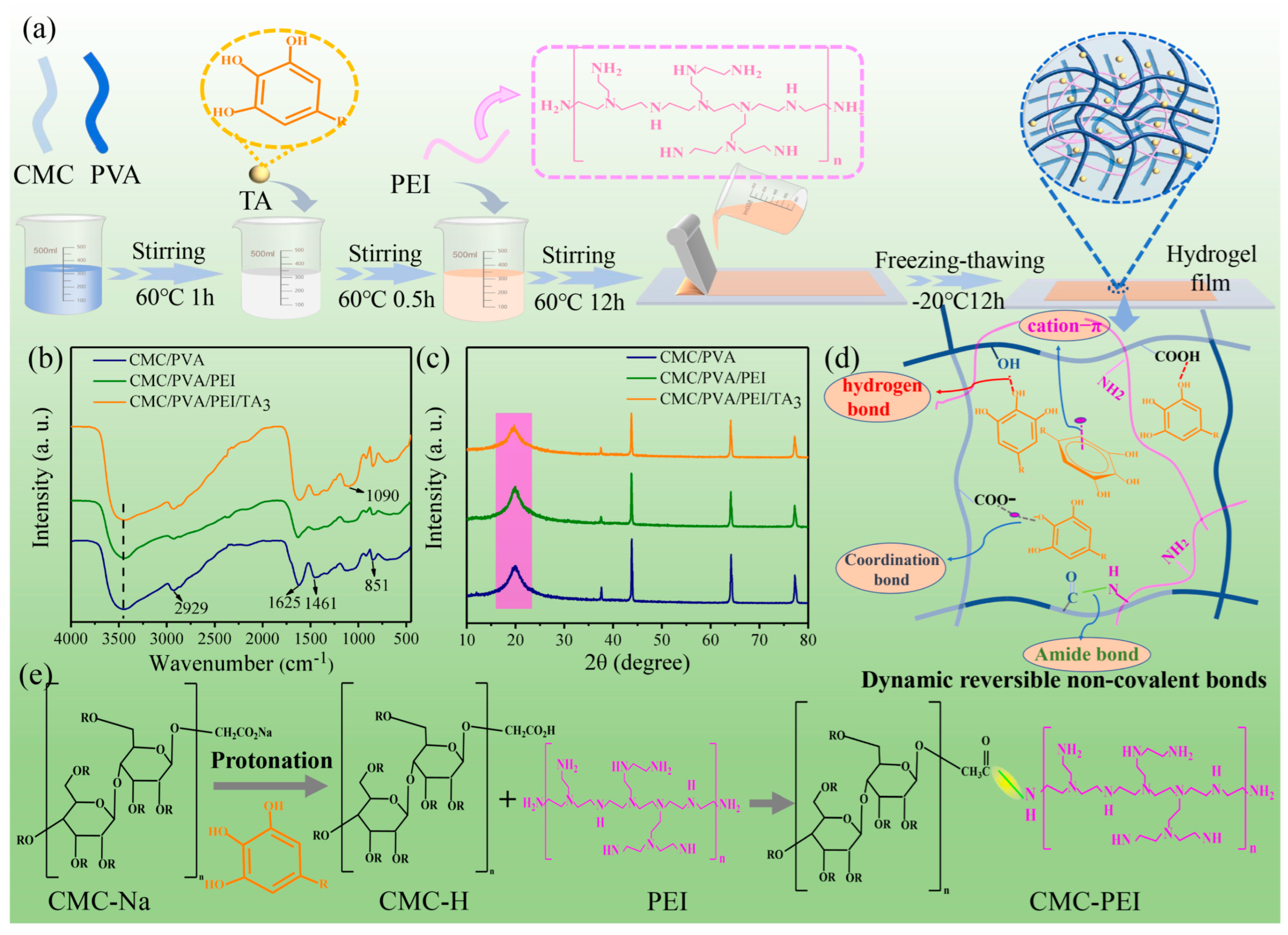
2.5. Development of High-Performance Carboxymethyl Cellulose-Based Hydrogel Film for Food Packaging and Preservation
2.5.1. Structural and Mechanical Properties
2.5.2. Barrier Performance and Antioxidant Properties
2.5.3. Self-Healing and Adhesion Performance
2.5.4. Food Preservation Efficacy and Industrial Viability
2.6. Development of Robust Cellulose/Carboxymethyl Chitosan Composite Films for Fresh Fruit Preservation
2.6.1. Structural and Mechanical Properties
2.6.2. Barrier and Antibacterial Performance
2.6.3. Food Preservation and Industrial Feasibility
2.6.4. Emerging Chitosan-Based Films for Food Packaging Applications
2.6.5. Fabrication and Functional Enhancement of Chitosan Films
2.6.6. Applications in Active and Intelligent Packaging
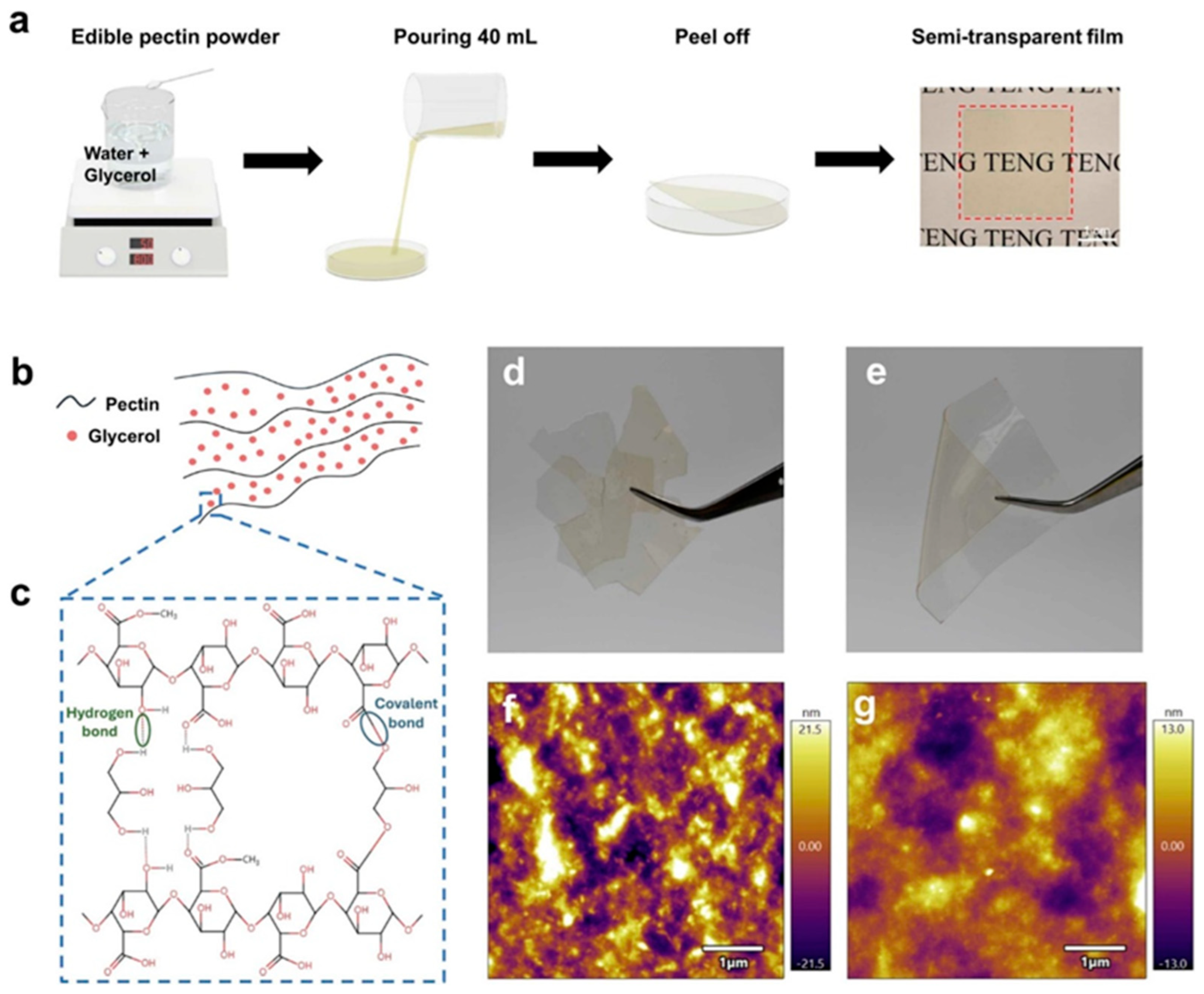
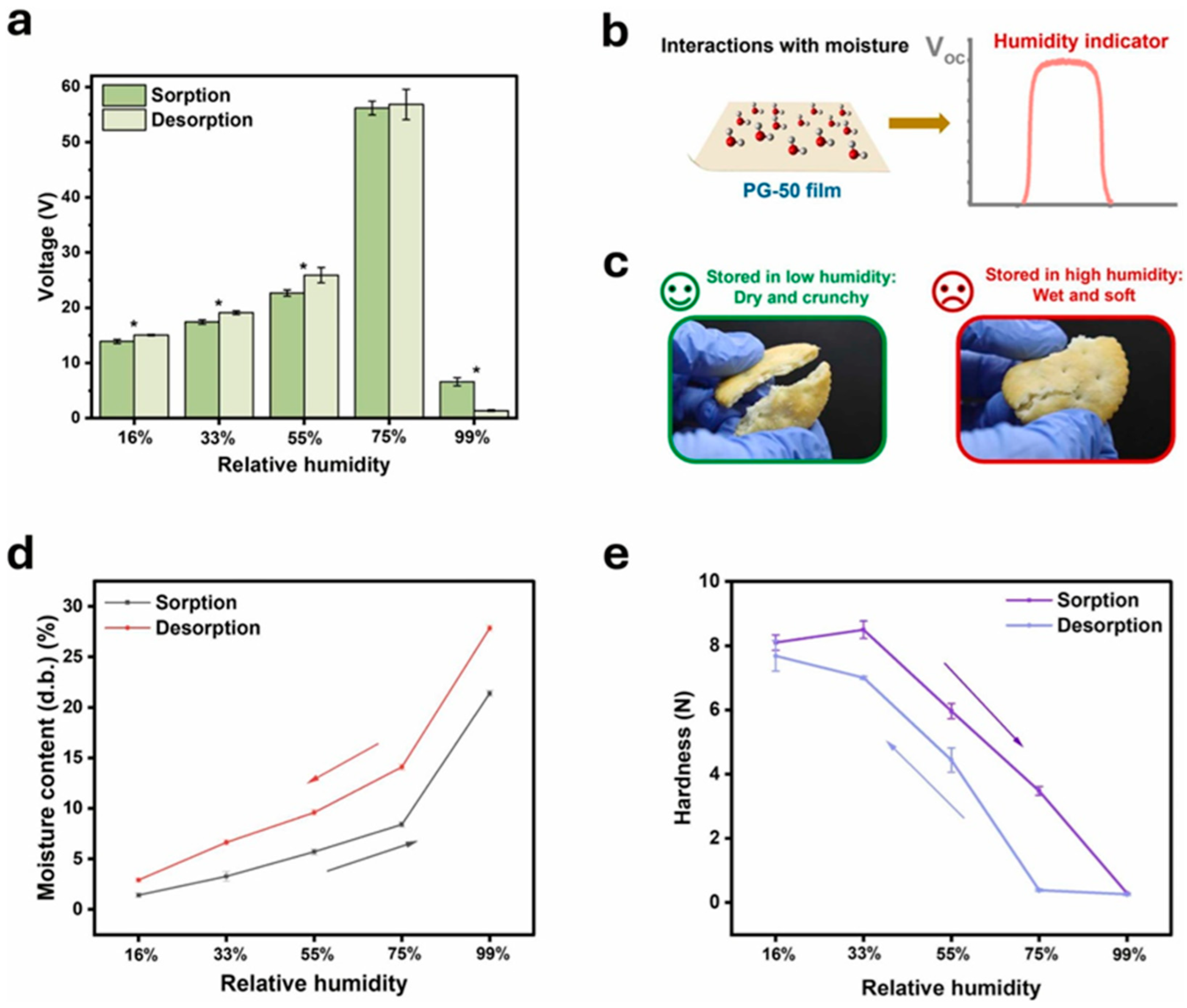
2.7. Triboelectric Nanogenerators for Intelligent Food Packaging and Energy Harvesting
2.7.1. Fabrication and Properties of Pectin-Based TENGs
2.7.2. Energy Harvesting and Humidity-Sensitive Food Monitoring
2.7.3. Triboelectric Food-Quality Sensors for Intelligent Packaging
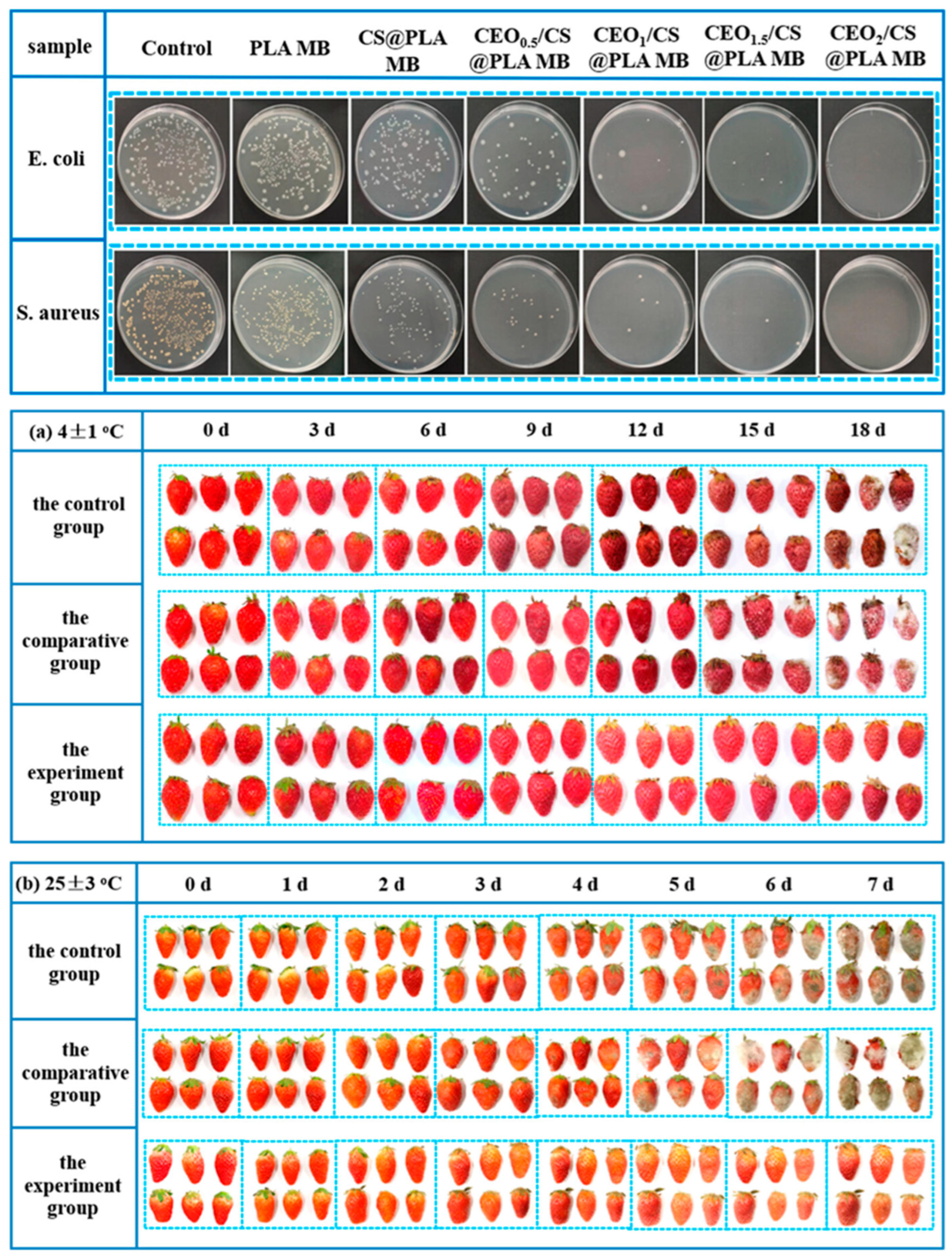
2.8. Development of Active Antibacterial CEO/CS@PLA Nonwovens for Food Preservation
2.8.1. Fabrication and Structural Properties
2.8.2. Antibacterial Performance and Food Preservation Efficiency
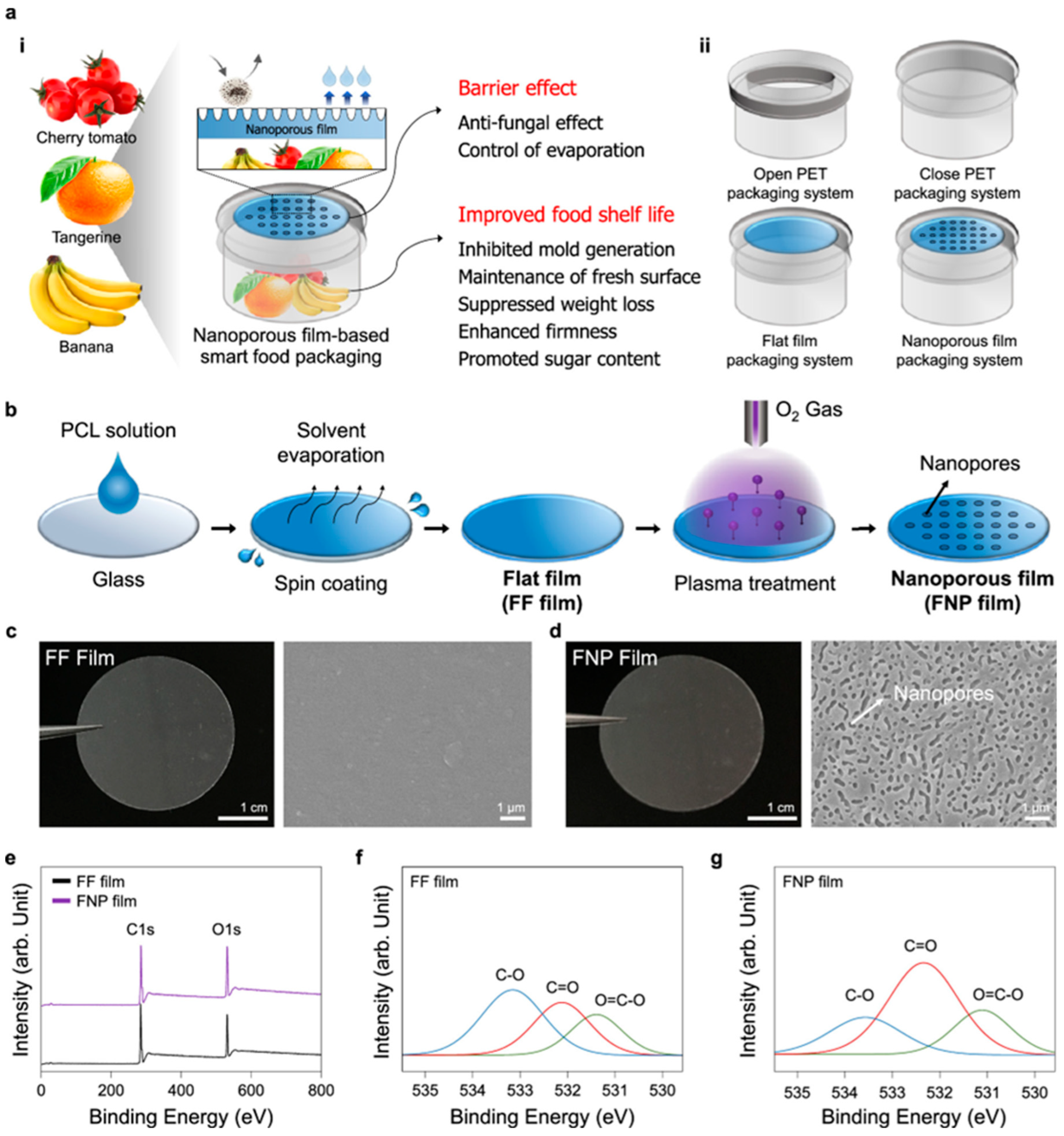
2.9. Biodegradable and Flexible Nanoporous Films for Active Food Packaging
2.9.1. Fabrication and Structural Characterization
2.9.2. Food Preservation and Antimicrobial Performance
2.10. Packaging Materials Based on Bacterial Nanocellulose
3. Conclusions and Perspective
Author Contributions
Funding
Conflicts of Interest
References
- Tian, B.; Liu, J.; Yang, W.; Wan, J.B. Biopolymer Food Packaging Films Incorporated with Essential Oils. J. Agric. Food Chem. 2023, 71, 1325–1347. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.; Li, W.; Wang, J.; Liu, Y. Functional Polysaccharide-Based Film Prepared from Chitosan and β-Acids: Structural, Physicochemical, and Bioactive Properties. Int. J. Biol. Macromol. 2021, 181, 966–977. [Google Scholar] [CrossRef]
- Amin, U.; Khan, M.U.; Majeed, Y.; Rebezov, M.; Khayrullin, M.; Bobkova, E.; Shariati, M.A.; Chung, I.M.; Thiruvengadam, M. Potentials of Polysaccharides, Lipids and Proteins in Biodegradable Food Packaging Applications. Int. J. Biol. Macromol. 2021, 183, 2184–2198. [Google Scholar] [CrossRef]
- Sharma, S.; Barkauskaite, S.; Jaiswal, A.K.; Jaiswal, S. Essential Oils as Additives in Active Food Packaging. Food Chem. 2021, 343, 128403. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Youngblood, J.P. Cellulose Nanofibril (CNF)-Coated PFAS-Free, Grease-Resistant All-Bio-Based Molded Pulp Containers for Food Packaging. ACS Appl. Polym. Mater. 2023, 5, 5696–5706. [Google Scholar] [CrossRef]
- Risch, S.J. Food Packaging History and Innovations. J. Agric. Food Chem. 2009, 57, 8089–8092. [Google Scholar] [CrossRef] [PubMed]
- Moon, R.J.; Martini, A.; Nairn, J.; Simonsen, J.; Youngblood, J. Cellulose Nanomaterials Review: Structure, Properties and Nanocomposites. Chem. Soc. Rev. 2011, 40, 3941–3994. [Google Scholar] [CrossRef]
- Rol, F.; Billot, M.; Bolloli, M.; Beneventi, D.; Bras, J. Production of 100% Cellulose Nanofibril Objects Using the Molded Cellulose Process: A Feasibility Study. Ind. Eng. Chem. Res. 2020, 59, 7670–7679. [Google Scholar] [CrossRef]
- Hurley, B.R.A.; Ouzts, A.; Fischer, J.; Gomes, T. Paper Presented at Iapri World Conference 2012 Effects of Private and Public Label Packaging on Consumer Purchase Patterns. Packag. Technol. Sci. 2013, 29, 399–412. [Google Scholar] [CrossRef]
- Chin, K.M.; Sung Ting, S.; Ong, H.L.; Omar, M. Surface Functionalized Nanocellulose as a Veritable Inclusionary Material in Contemporary Bioinspired Applications: A Review. J. Appl. Polym. Sci. 2018, 135, 46065. [Google Scholar] [CrossRef]
- Eyley, S.; Thielemans, W. Surface Modification of Cellulose Nanocrystals. Nanoscale 2014, 6, 7764–7779. [Google Scholar] [CrossRef]
- Oksman, K.; Aitomäki, Y.; Mathew, A.P.; Siqueira, G.; Zhou, Q.; Butylina, S.; Tanpichai, S.; Zhou, X.; Hooshmand, S. Review of the Recent Developments in Cellulose Nanocomposite Processing. Compos. Part A Appl. Sci. Manuf. 2016, 83, 2–18. [Google Scholar] [CrossRef]
- Clarkson, C.M.; El Awad Azrak, S.M.; Forti, E.S.; Schueneman, G.T.; Moon, R.J.; Youngblood, J.P. Recent Developments in Cellulose Nanomaterial Composites. Adv. Mater. 2021, 33, 14–17. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Seidi, F.; Ahmad, M.; Liu, Y.; Saeb, M.R.; Akbari, A.; Xiao, H. Recent Advances in Functional Cellulose-Based Films with Antimicrobial and Antioxidant Properties for Food Packaging. J. Agric. Food Chem. 2023, 71, 16469–16487. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, H.; Su, H.M.; Imani, S.M.; Alkhaldi, K.; Filipe, C.D.; Didar, T.F. Intelligent Food Packaging: A Review of Smart Sensing Technologies for Monitoring Food Quality. ACS Sens. 2019, 4, 808–821. [Google Scholar] [CrossRef] [PubMed]
- Ncube, L.K.; Ude, A.U.; Ogunmuyiwa, E.N.; Zulkifli, R.; Beas, I.N. Environmental Impact of Food Packaging Materials: A Review of Contemporary Development from Conventional Plastics to Polylactic Acid Based Materials. Materials 2020, 13, 4994. [Google Scholar] [CrossRef]
- Salimi, H.; Aryanasab, F.; Banazadeh, A.R.; Shabanian, M.; Seidi, F. Designing Syntheses of Cellulose and Starch Derivatives with Basic or Cationic N-Functions: Part I-Cellulose Derivatives. Polym. Adv. Technol. 2016, 27, 5–32. [Google Scholar] [CrossRef]
- Romão, S.; Bettencourt, A.; Ribeiro, I.A.C. Novel Features of Cellulose-Based Films as Sustainable Alternatives for Food Packaging. Polymers 2022, 14, 4968. [Google Scholar] [CrossRef]
- Casalini, S.; Giacinti Baschetti, M. The Use of Essential Oils in Chitosan or Cellulose-Based Materials for the Production of Active Food Packaging Solutions: A Review. J. Sci. Food Agric. 2023, 103, 1021–1041. [Google Scholar] [CrossRef]
- Huang, J.; Liu, Y.; Yang, L.; Zhou, F. Synthesis of Sulfonated Chitosan and Its Antibiofilm Formation Activity against E. Coli and S. Aureus. Int. J. Biol. Macromol. 2019, 129, 980–988. [Google Scholar] [CrossRef]
- Di, Y.; Long, G.; Zhang, H.; Li, Q. Preparation and Properties of Viscose Rayon/O-Carboxymethyl Chitosan Antibacterial Fibers. J. Eng. Fiber. Fabr. 2011, 6, 39–43. [Google Scholar] [CrossRef]
- Silvestre, C.; Duraccio, D.; Cimmino, S. Food packaging based on polymer nanomaterials. Prog. Polym. Sci. 2011, 36, 1766–1782. [Google Scholar] [CrossRef]
- Marsh, K.; Bugusu, B. Food Packaging—Roles, Materials, and Environmental Issues. J. Food Sci. 2007, 72, R39–R55. [Google Scholar] [CrossRef]
- Mahmud, J.; Sarmast, E.; Shankar, S.; Lacroix, M. Advantages of nanotechnology developments in active food packaging. Food Res. Int. 2022, 154, 111023. [Google Scholar] [CrossRef]
- Yan, M.R.; Hsieh, S.; Ricacho, N. Innovative Food Packaging, Food Quality and Safety, and Consumer Perspectives. Processes 2022, 10, 747. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Peponi, L.; Lopez, D.; Lopez, J.; Kenny, J.M. 12—An Overview of Nanoparticles Role in the Improvement of Barrier Properties of Bioplastics for Food Packaging Applications; Grumezescu, A.M., Ed.; Food Packaging; Academic Press: Cambridge, MA, USA, 2017; pp. 391–424. [Google Scholar] [CrossRef]
- Kuswandi, B.; Moradi, M. Improvement of Food Packaging Based on Functional Nanomaterial. In Nanotechnology: Applications in Energy, Drug and Food; Siddiquee, S., Melvin, G., Rahman, M., Eds.; Springer: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
- Wyrwa, J.; Barska, A. Innovations in the food packaging market: Active packaging. Eur. Food Res. Technol. 2017, 243, 1681–1692. [Google Scholar] [CrossRef]
- Basavegowda, N.; Baek, K.-H. Synergistic Antioxidant and Antibacterial Advantages of Essential Oils for Food Packaging Applications. Biomolecules 2021, 11, 1267. [Google Scholar] [CrossRef]
- Harish, R.; Nisha, K.D.; Prabakaran, S.; Sridevi, B.; Harish, S.; Navaneethan, M.; Ponnusamy, S.; Hayakawa, Y.; Vinniee, C.; Ganesh, M.R. Cytotoxicity Assessment of Chitosan Coated CdS Nanoparticles for Bio-Imaging Applications. Appl. Surf. Sci. 2020, 499, 143817. [Google Scholar] [CrossRef]
- Hussain, M.S.; Musharraf, S.G.; Bhanger, M.I.; Malik, M.I. Salicylaldehyde Derivative of Nano-Chitosan as an Efficient Adsorbent for Lead(II), Copper(II), and Cadmium(II) Ions. Int. J. Biol. Macromol. 2020, 147, 643–652. [Google Scholar] [CrossRef]
- Tran, T.N.; Doan, C.T.; Nguyen, V.B.; Nguyen, A.D.; Wang, S.L. Anti-Oxidant and Anti-Diabetes Potential of Water-Soluble Chitosan-Glucose Derivatives Produced by Maillard Reaction. Polymers 2019, 11, 1714. [Google Scholar] [CrossRef]
- Liao, Y.; Wang, C.; Huang, C.; Hussain Abdalkarim, S.Y.; Wang, L.; Chen, Z.; Yu, H.Y. Robust Cellulose/Carboxymethyl Chitosan Composite Films with High Transparency and Antibacterial Ability for Fresh Fruit Preservation. ACS Sustain. Chem. Eng. 2023, 11, 5908–5917. [Google Scholar] [CrossRef]
- Li, T.; Chen, C.; Brozena, A.H.; Zhu, J.Y.; Xu, L.; Driemeier, C.; Dai, J.; Rojas, O.J.; Isogai, A.; Wågberg, L.; et al. Developing Fibrillated Cellulose as a Sustainable Technological Material. Nature 2021, 590, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Rhim, J.W. Carboxymethyl Cellulose-Based Antioxidant and Antimicrobial Active Packaging Film Incorporated with Curcumin and Zinc Oxide. Int. J. Biol. Macromol. 2020, 148, 666–676. [Google Scholar] [CrossRef]
- Jiang, J.; Chen, X.; Zhang, G.L.; Hao, H.; Hou, H.M.; Bi, J. Preparation of Chitosan-Cellulose-Benzyl Isothiocyanate Nanocomposite Film for Food Packaging Applications. Carbohydr. Polym. 2022, 285, 119234. [Google Scholar] [CrossRef] [PubMed]
- Cano, A.; Contreras, C.; Chiralt, A.; González-Martínez, C. Using Tannins as Active Compounds to Develop Antioxidant and Antimicrobial Chitosan and Cellulose Based Films. Carbohydr. Polym. Technol. Appl. 2021, 2, 100156. [Google Scholar] [CrossRef]
- Yang, J.; Chen, Y.; Zhao, L.; Feng, Z.; Peng, K.; Wei, A.; Wang, Y.; Tong, Z.; Cheng, B. Preparation of a Chitosan/Carboxymethyl Chitosan/AgNPs Polyelectrolyte Composite Physical Hydrogel with Self-Healing Ability, Antibacterial Properties, and Good Biosafety Simultaneously, and Its Application as a Wound Dressing. Compos. Part B Eng. 2020, 197, 108139. [Google Scholar] [CrossRef]
- Ali, A.; Ahmed, S. A Review on Chitosan and Its Nanocomposites in Drug Delivery. Int. J. Biol. Macromol. 2018, 109, 273–286. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, H.Y.; Li, Y. Highly Efficient and Superfast Cellulose Dissolution by Green Chloride Salts and Its Dissolution Mechanism. ACS Sustain. Chem. Eng. 2020, 8, 18446–18454. [Google Scholar] [CrossRef]
- Abutalib, M.M.; Rajeh, A. Structural, Thermal, Optical and Conductivity Studies of Co/ZnO Nanoparticles Doped CMC Polymer for Solid State Battery Applications. Polym. Test. 2020, 91, 106803. [Google Scholar] [CrossRef]
- Qi, Y.; Lin, S.; Lan, J.; Zhan, Y.; Guo, J.; Shang, J. Fabrication of Super-High Transparent Cellulose Films with Multifunctional Performances via Postmodification Strategy. Carbohydr. Polym. 2021, 260, 117760. [Google Scholar] [CrossRef]
- Abdullah; Cai, J.; Hafeez, M.A.; Wang, Q.; Farooq, S.; Huang, Q.; Tian, W.; Xiao, J. Biopolymer-Based Functional Films for Packaging Applications: A Review. Front. Nutr. 2022, 9, 1000116. [Google Scholar] [CrossRef]
- Yildirim, S.; Röcker, B.; Pettersen, M.K.; Nilsen-Nygaard, J.; Ayhan, Z.; Rutkaite, R.; Radusin, T.; Suminska, P.; Marcos, B.; Coma, V. Active Packaging Applications for Food. Compr. Rev. Food Sci. Food Saf. 2018, 17, 165–199. [Google Scholar] [CrossRef] [PubMed]
- Medina-Jaramillo, C.; Ochoa-Yepes, O.; Bernal, C.; Famá, L. Active and Smart Biodegradable Packaging Based on Starch and Natural Extracts. Carbohydr. Polym. 2017, 176, 187–194. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.W. Preparation of Carrageenan-Based Functional Nanocomposite Films Incorporated with Melanin Nanoparticles. Colloids Surf. B Biointerfaces 2019, 176, 317–324. [Google Scholar] [CrossRef]
- Hernández-García, E.; Vargas, M.; González-Martínez, C.; Chiralt, A. Biodegradable Antimicrobial Films for Food Packaging: Effect of Antimicrobials on Degradation. Foods 2021, 10, 1256. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Woo, M.W. Transitioning of Petroleum-Based Plastic Food Packaging to Sustainable Bio-Based Alternatives. Sustain. Food Technol. 2024, 2, 548–566. [Google Scholar] [CrossRef]
- Alamri, M.S.; Qasem, A.A.A.; Mohamed, A.A.; Hussain, S.; Ibraheem, M.A.; Shamlan, G.; Alqah, H.A.; Qasha, A.S. Food Packaging’s Materials: A Food Safety Perspective. Saudi J. Biol. Sci. 2021, 28, 4490–4499. [Google Scholar] [CrossRef]
- Dodero, A.; Escher, A.; Bertucci, S.; Castellano, M.; Lova, P. Intelligent Packaging for Real-Time Monitoring of Food-Quality: Current and Future Developments. Appl. Sci. 2021, 11, 3532. [Google Scholar] [CrossRef]
- Vasile, C.; Baican, M. Progresses in Food Packaging, Food Quality, and Safetyzcontrolled-Release Antioxidant and/or Antimicrobial Packaging. Molecules 2021, 26, 1263. [Google Scholar] [CrossRef]
- Drago, E.; Campardelli, R.; Pettinato, M.; Perego, P. Innovations in Smart Packaging Concepts for Food: An Extensive Review. Foods 2020, 9, 1628. [Google Scholar] [CrossRef]
- Ali, S.S.; Abdelkarim, E.A.; Elsamahy, T.; Al-Tohamy, R.; Li, F.; Kornaros, M.; Zuorro, A.; Zhu, D.; Sun, J. Bioplastic Production in Terms of Life Cycle Assessment: A State-of-the-Art Review. Environ. Sci. Ecotechnol. 2023, 15, 100254. [Google Scholar] [CrossRef] [PubMed]
- Varghese, S.A.; Pulikkalparambil, H.; Promhuad, K.; Srisa, A.; Laorenza, Y.; Jarupan, L.; Nampitch, T.; Chonhenchob, V.; Harnkarnsujarit, N. Renovation of Agro-Waste for Sustainable Food Packaging: A Review. Polymers 2023, 15, 648. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Liu, X.; Guo, F.; Yu, Y.; Lu, J.; Li, Y.; Cheng, Q.; Peng, J.; Yu, G. Biodegradable Cellulose/Curcumin Films with Janus Structure for Food Packaging and Freshness Monitoring. Carbohydr. Polym. 2024, 324, 121516. [Google Scholar] [CrossRef]
- Kaur, S.; Modi, N.H.; Panda, D.; Roy, N. Probing the Binding Site of Curcumin in Escherichia Coli and Bacillus Subtilis FtsZ—A Structural Insight to Unveil Antibacterial Activity of Curcumin. Eur. J. Med. Chem. 2010, 45, 4209–4214. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Q.; Liu, S.; Ji, X.; Yang, G.; Chen, J. Lipase Induced Highly Hydrophobic Nanofibrillated Cellulose Film for Strain Sensor Application. Carbohydr. Polym. 2022, 284, 119193. [Google Scholar] [CrossRef]
- Zhu, M.; Ying, D.; Zhang, H.; Xu, X.; Chang, C. Self-Healable Hydrophobic Films Fabricated by Incorporating Natural Wax into Cellulose Matrix. Chem. Eng. J. 2022, 446, 136791. [Google Scholar] [CrossRef]
- Shen, C.; Yang, X.; Wang, D.; Li, J.; Zhu, C.; Wu, D.; Chen, K. Carboxymethyl Chitosan and Polycaprolactone-Based Rapid in-Situ Packaging for Fruit Preservation by Solution Blow Spinning. Carbohydr. Polym. 2024, 326, 121636. [Google Scholar] [CrossRef]
- Shen, C.; Yang, Z.; Rao, J.; Li, J.; Wu, D.; He, Y.; Chen, K. Development of a Thermally Conductive and Antimicrobial Nanofibrous Mat for the Cold Chain Packaging of Fruits and Vegetables. Mater. Des. 2022, 221, 110931. [Google Scholar] [CrossRef]
- Sela, A.; Shkuri, N.; Tish, N.; Vinokur, Y.; Rodov, V.; Poverenov, E. Carboxymethyl Chitosan-Quercetin Conjugate: A Sustainable One-Step Synthesis and Use for Food Preservation. Carbohydr. Polym. 2023, 316, 121084. [Google Scholar] [CrossRef]
- Mohammadi, M.; Fasihi, M. Eco-Friendly Polylactic Acid/Modified Thermoplastic Starch Films Enhanced with Clove Essential Oil and Cochineal for Dual-Functional Active and Intelligent Food Packaging. Carbohydr. Polym. 2025, 354, 123320. [Google Scholar] [CrossRef]
- Zhao, X.; Li, P.; Mo, F.; Zhang, Y.; Huang, Z.; Yu, J.; Zhou, L.; Bi, S.; Peng, S. Copolyester Toughened Poly(Lactic Acid) Biodegradable Material Prepared by in Situ Formation of Polyethylene Glycol and Citric Acid. RSC Adv. 2024, 14, 11027–11036. [Google Scholar] [CrossRef] [PubMed]
- Riahi, Z.; Khan, A.; Rhim, J.W.; Shin, G.H.; Kim, J.T. Gelatin/Poly(Vinyl Alcohol)-Based Dual Functional Composite Films Integrated with Metal-Organic Frameworks and Anthocyanin for Active and Intelligent Food Packaging. Int. J. Biol. Macromol. 2023, 249, 126040. [Google Scholar] [CrossRef] [PubMed]
- Ndwandwe, B.K.; Malinga, S.P.; Kayitesi, E.; Dlamini, B.C. Recent Developments in the Application of Natural Pigments as PH-Sensitive Food Freshness Indicators in Biopolymer-Based Smart Packaging: Challenges and Opportunities. Int. J. Food Sci. Technol. 2024, 59, 2148–2161. [Google Scholar] [CrossRef]
- Fenton, S.E.; Ducatman, A.; Boobis, A.; DeWitt, J.C.; Lau, C.; Ng, C.; Smith, J.S.; Roberts, S.M. Per- and Polyfluoroalkyl Substance Toxicity and Human Health Review: Current State of Knowledge and Strategies for Informing Future Research. Environ. Toxicol. Chem. 2021, 40, 606–630. [Google Scholar] [CrossRef]
- Zhang, Y.; Duan, C.; Bokka, S.K.; He, Z.; Ni, Y. Molded Fiber and Pulp Products as Green and Sustainable Alternatives to Plastics: A Mini Review. J. Bioresour. Bioprod. 2022, 7, 14–25. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhou, S.; Xia, X.; Tan, M.; Lv, Y.; Cheng, Y.; Tao, Y.; Lu, J.; Du, J.; Wang, H. High-Performance Carboxymethyl Cellulose-Based Hydrogel Film for Food Packaging and Preservation System. Int. J. Biol. Macromol. 2022, 223, 1126–1137. [Google Scholar] [CrossRef]
- Zhou, H.; Zhou, S.; Ji, X.; Zhao, Y.; Lv, Y.; Cheng, Y.; Tao, Y.; Lu, J.; Du, J.; Wang, H. High-Performance Cellulose Acetate-Based Gas Barrier Films via Tailoring Reduced Graphene Oxide Nanosheets. Int. J. Biol. Macromol. 2022, 209, 1450–1456. [Google Scholar] [CrossRef]
- He, B.; Wang, W.; Song, Y.; Ou, Y.; Zhu, J. Structural and Physical Properties of Carboxymethyl Cellulose/Gelatin Films Functionalized with Antioxidant of Bamboo Leaves. Int. J. Biol. Macromol. 2020, 164, 1649–1656. [Google Scholar] [CrossRef]
- Jin, Z.; Hu, G.; Zhao, K. Mannose-Anchored Quaternized Chitosan/Thiolated Carboxymethyl Chitosan Composite NPs as Mucoadhesive Carrier for Drug Delivery. Carbohydr. Polym. 2022, 283, 119174. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, M.; Woo, M.W.; Li, Y.; Han, W.; Dang, X. High-Mechanical Strength Carboxymethyl Chitosan-Based Hydrogel Film for Antibacterial Wound Dressing. Carbohydr. Polym. 2021, 256, 117590. [Google Scholar] [CrossRef]
- Wang, H.; Qian, J.; Ding, F. Emerging Chitosan-Based Films for Food Packaging Applications. J. Agric. Food Chem. 2018, 66, 395–413. [Google Scholar] [CrossRef] [PubMed]
- Cano Embuena, A.I.; Cháfer Nácher, M.; Chiralt Boix, A.; Molina Pons, M.P.; Borrás Llopis, M.; Beltran Martínez, M.C.; González Martínez, C. Quality of Goat′s Milk Cheese as Affected by Coating with Edible Chitosan-Essential Oil Films. Int. J. Dairy Technol. 2017, 70, 68–76. [Google Scholar] [CrossRef]
- Wang, H.; Guo, T.; Zhang, Y.; Zhang, Q.; Li, H. Rheological Properties, Antimicrobial Activity and Screen-Printing Performance of Chitosan-Pigment (FeO(OH)·xH2O) Composite Edible Ink. Prog. Org. Coat. 2017, 111, 75–82. [Google Scholar] [CrossRef]
- Jin, Z.; Fu, Y.; Zhao, H.; Ding, W.; Wang, Y.C. Carbohydrate Polymer-Based Triboelectric Devices for Energy Harvesting and Intelligent Packaging for Food-Quality Monitoring. Nano Energy 2025, 134, 110561. [Google Scholar] [CrossRef]
- Lin, S.; Xu, L.; Chi Wang, A.; Wang, Z.L. Quantifying Electron-Transfer in Liquid-Solid Contact Electrification and the Formation of Electric Double-Layer. Nat. Commun. 2020, 11, 399. [Google Scholar] [CrossRef]
- Deng, W.; Zhou, Y.; Zhao, X.; Zhang, S.; Zou, Y.; Xu, J.; Yeh, M.H.; Guo, H.; Chen, J. Ternary Electrification Layered Architecture for High-Performance Triboelectric Nanogenerators. ACS Nano 2020, 14, 9050–9058. [Google Scholar] [CrossRef]
- Sun, H.; Wang, B.; Xie, Y.; Li, F.; Xu, T.; Yu, B. Development of Active Antibacterial CEO/CS@PLA Nonwovens and the Application on Food Preservation. ACS Omega 2023, 8, 42907–42920. [Google Scholar] [CrossRef]
- Coyotl-Pérez, W.A.; Morales-Rabanales, Q.N.; Lozoya-Gloria, E.; Becerra-Martínez, E.; Ramírez-García, S.A.; Mosso-González, C.; Villa-Ruano, N. Fungistatic Films Containing Cinnamon Essential Oil: New Coatings to Preserve the Nutraceutical Content of Avocado Fruit against Fusariosis. Chem. Biodivers. 2022, 19, e202200441. [Google Scholar] [CrossRef]
- Huang, C.; Wang, X.; Yang, P.; Shi, S.; Duan, G.; Liu, X.; Li, Y. Size Regulation of Polydopamine Nanoparticles by Boronic Acid and Lewis Base. Macromol. Rapid Commun. 2023, 44, 2100916. [Google Scholar] [CrossRef]
- Kim, W.; Han, T.; Gwon, Y.; Park, S.; Kim, H.; Kim, J. Biodegradable and Flexible Nanoporous Films for Design and Fabrication of Active Food Packaging Systems. Nano Lett. 2022, 22, 3480–3487. [Google Scholar] [CrossRef]
- Adeyeye, S.A.O.; Ashaolu, T.J. Applications of Nano-Materials in Food Packaging: A Review. J. Food Process Eng. 2021, 44, e13708. [Google Scholar] [CrossRef]
- Samantaray, P.K.; Little, A.; Haddleton, D.M.; McNally, T.; Tan, B.; Sun, Z.; Huang, W.; Ji, Y.; Wan, C. Poly(Glycolic Acid) (PGA): A Versatile Building Block Expanding High Performance and Sustainable Bioplastic Applications. Green Chem. 2020, 22, 4055–4081. [Google Scholar] [CrossRef]
- Padrão, J.; Gonçalves, S.; Silva, J.P.; Sencadas, V.; Lanceros-Méndez, S.; Pinheiro, A.C.; Vicente, A.A.; Rodrigues, L.R.; Dourado, F. Bacterial Cellulose-Lactoferrin as an Antimicrobial Edible Packaging. Food Hydrocoll. 2016, 58, 126–140. [Google Scholar] [CrossRef]
- Salari, M.; Sowti Khiabani, M.; Rezaei Mokarram, R.; Ghanbarzadeh, B.; Samadi Kafil, H. Development and Evaluation of Chitosan Based Active Nanocomposite Films Containing Bacterial Cellulose Nanocrystals and Silver Nanoparticles. Food Hydrocoll. 2018, 84, 414–423. [Google Scholar] [CrossRef]
- Stroescu, M.; Isopencu, G.; Busuioc, C.; Stoica-Guzun, A. Antimicrobial Food Pads Containing Bacterial Cellulose and Polysaccharides; Springer: Cham, Switzerland, 2018; ISBN 9783319765730. [Google Scholar]













| Advantage | Description | Comparison with Other Methods |
|---|---|---|
| Barrier Function [22] | Blocks moisture, oxygen, light, and contaminants. | Thermal or pressure processing can kill microbes, but they do not protect post-treatment. |
| Shelf Life Extension [23] | Modified Atmosphere Packaging (MAP) slows microbial growth and oxidation. | Unlike natural preservatives, packaging can precisely control gas ratios over time. |
| Reduced Need for Additives [24] | Minimizes use of chemical preservatives, appealing to “clean label” trends. | Consumers prefer fewer artificial ingredients—packaging helps achieve that. |
| Temperature Insulation [25] | Some packaging can help maintain thermal conditions (e.g., thermal liners). | Cold chain systems need external power; packaging enhances passive protection. |
| Smart Monitoring Capabilities [25] | Embedded indicators can detect gas changes, microbial growth, or temp deviations. | Irradiation or preservatives do not provide real-time feedback. |
| Improved Logistics and Handling [26] | Better stacking, cushioning, and protection during transport. | Other methods do not address mechanical spoilage or crushing. |
| Sustainability and Waste Reduction [27] | Recyclable/compostable packaging and longer shelf life help reduce food waste. | Irradiation or HPP requires energy; biodegradable packaging offers a low-impact option. |
| Consumer Convenience [28] | Easy-open, resealable, portion-controlled, or microwavable packaging enhances usability. | Thermal or biocontrol methods do not offer functional consumer benefits. |
| Cost-Efficiency at Scale [29] | Once implemented, active packaging can be cheaper than continual application of preservatives. | High-pressure or irradiation methods can be expensive for small-scale producers. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Periyasamy, T.; Asrafali, S.P.; Lee, J. Recent Advances in Functional Biopolymer Films with Antimicrobial and Antioxidant Properties for Enhanced Food Packaging. Polymers 2025, 17, 1257. https://doi.org/10.3390/polym17091257
Periyasamy T, Asrafali SP, Lee J. Recent Advances in Functional Biopolymer Films with Antimicrobial and Antioxidant Properties for Enhanced Food Packaging. Polymers. 2025; 17(9):1257. https://doi.org/10.3390/polym17091257
Chicago/Turabian StylePeriyasamy, Thirukumaran, Shakila Parveen Asrafali, and Jaewoong Lee. 2025. "Recent Advances in Functional Biopolymer Films with Antimicrobial and Antioxidant Properties for Enhanced Food Packaging" Polymers 17, no. 9: 1257. https://doi.org/10.3390/polym17091257
APA StylePeriyasamy, T., Asrafali, S. P., & Lee, J. (2025). Recent Advances in Functional Biopolymer Films with Antimicrobial and Antioxidant Properties for Enhanced Food Packaging. Polymers, 17(9), 1257. https://doi.org/10.3390/polym17091257









