Nanobacterial Cellulose from Kombucha Fermentation as a Potential Protective Carrier of Lactobacillus plantarum under Simulated Gastrointestinal Tract Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Kombucha Bacterial Cellulose Production and Purification
2.2. Analysis of Kombucha Bacterial Cellulose Properties
2.2.1. Morphological Structure
2.2.2. Cellulose Type and Crystallite Size
2.2.3. Determination of Surface Area, Pore Volume, and Pore Size
2.3. Immobilization of Lactobacillus plantarum TISTR 541 Cells on Kombucha Bacterial Cellulose
2.3.1. Lactobacillus plantarum Strain
2.3.2. Preparation of L. plantarum for Immobilization
2.3.3. Immobilization
2.3.4. Enumeration of Immobilized Cells on KBC
2.3.5. Visualization of Immobilized L. plantarum Cells on KBC under SEM
2.4. Investigation of the Tolerance of Immobilized L. plantarum Cells to Acid and Bile Salts
3. Results
3.1. Bacterial Cellulose Production from Kombucha
3.2. Morphological Structure and Physical Properties of Kombucha Bacterial Cellulose
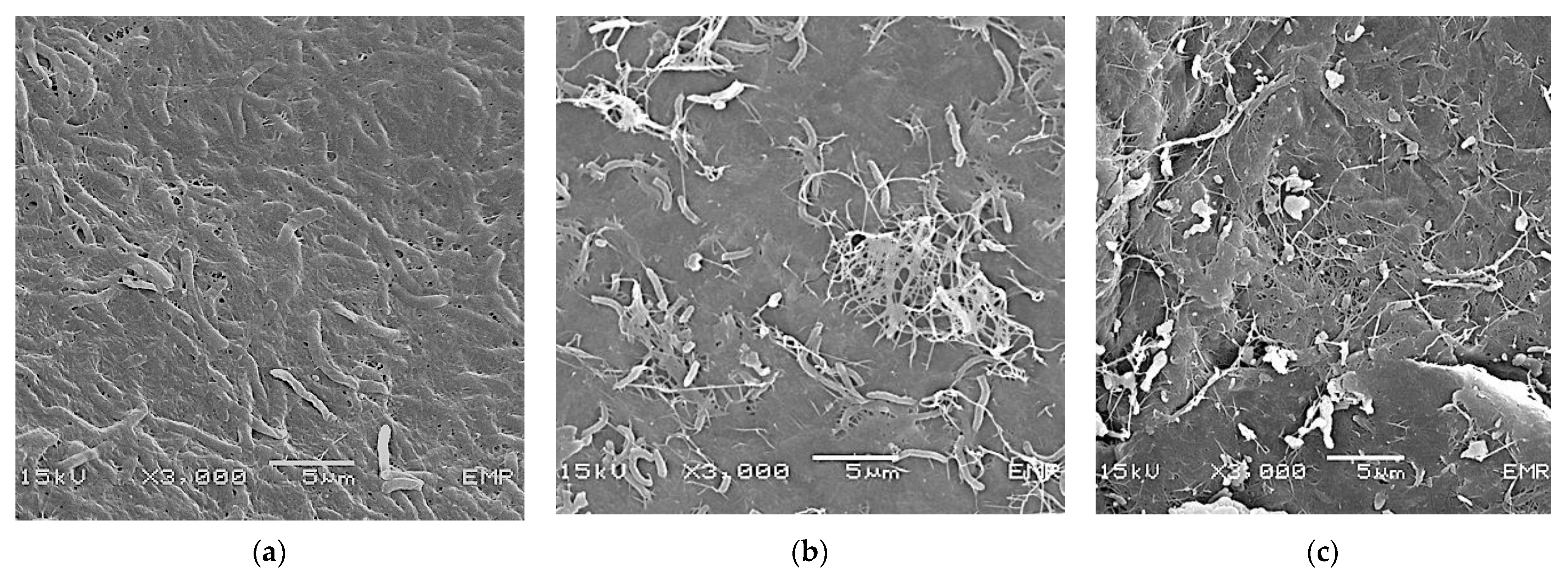
3.2.1. Morphological Structure of Kombucha Bacterial Cellulose
3.2.2. Cellulose Type and Crystallite Size of KBC
3.2.3. Surface Area, Pore Volume, and Pore Size of Kombucha Bacterial Cellulose
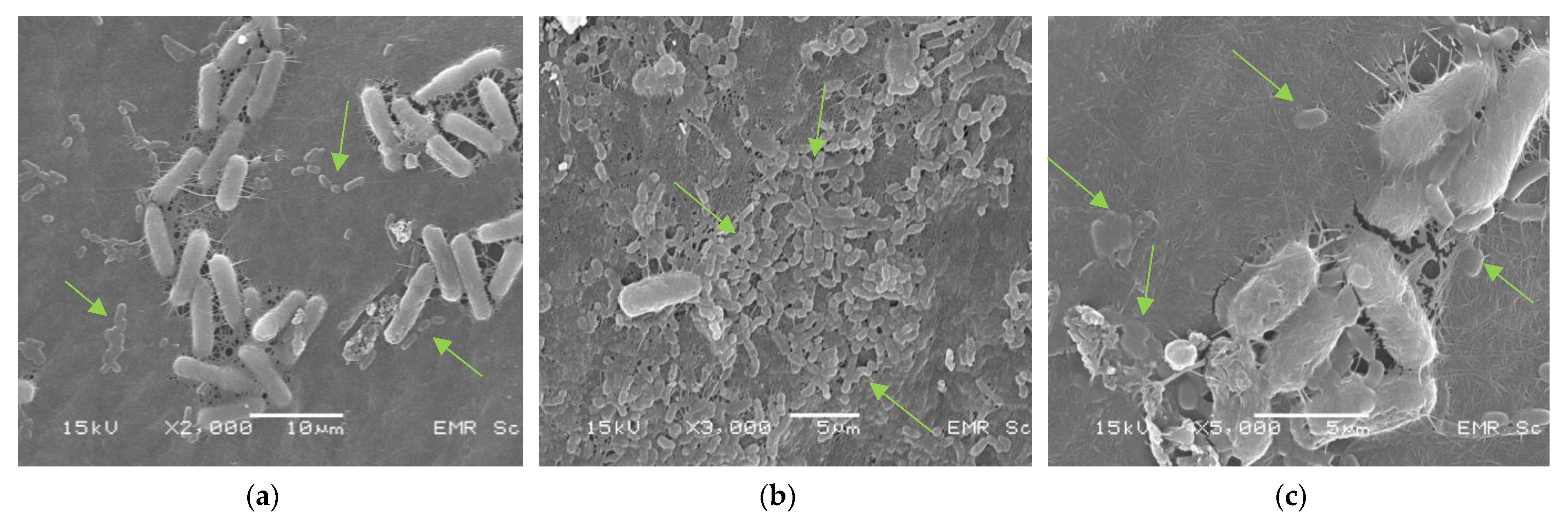
3.3. Immobilization of L. plantarum TISTR 541 Cells on Kombucha Bacterial Cellulose
3.4. Tolerance of Immobilized Lactobacillus plantarum TISTR 541 Cells to Acid and Bile Salts
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mariano, M.; El Kissi, N.; Dufresne, A. Cellulose nanocrystals and related nanocomposites: Review of some properties and challenges. J. Polym. Sci. Part B Polym. Phys. 2014, 52, 791–806. [Google Scholar] [CrossRef]
- Shankaran, D.R. Cellulose nanocrystals for health care applications. In Book Applications of Nanomaterials; Bhagyaraj, S.M., Oluwafemi, O.M., Kalarikkal, N., Thomas, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 415–459. ISBN 978-0-08-101971-9. [Google Scholar]
- Nasir, M.; Hashim, R.; Sulaiman, O.; Asim, M. Nanocellulose: Preparation methods and applications. In Book Cellulose-Reinforced Nanofibre Composites; Jawaid, M., Boufi, S., Khalil, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 261–276. ISBN 978-0-08-100957-4. [Google Scholar]
- Torres, F.G.; Arroyo, J.J.; Troncoso, O.P. Bacterial cellulose nanocomposites: An all-nano type of material. Mater. Sci. Eng. C 2019, 98, 1277–1293. [Google Scholar] [CrossRef]
- Future, M.R. Nanocellulose Market Size to Surpass USD 1100 Million by 2030 at 19.9% CAGR—Report by Market Research Future (MRFR). Available online: https://www.globenewswire.com/news-release/2023/02/08/2603861/0/en/Nanocellulose-Market-Size-to-Surpass-USD-1100-Million-by-2030-at-19-9-CAGR-Report-by-Market-Research-Future-MRFR.html (accessed on 10 February 2023).
- Park, J.K.; Jung, J.Y.; Khan, T. Bacterial cellulose. In Handbook of Hydrocolloids; Phillips, G.O., Williams, P.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2009; pp. 724–739. ISBN 978-1-84569-414-2. [Google Scholar]
- Karim, Z.; Afrin, S. Bacterial cellulose. In Book Cellulose-Reinforced Nanofibre Composites; Jawaid, M., Boufi, S., Khalil, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 327–340. ISBN 978-0-08-100957-4. [Google Scholar]
- Lee, K.Y.; Buldum, G.; Mantalaris, A.; Bismarck, A. More than meets the eye in bacterial cellulose: Biosynthesis, Bioprocessing, and Applications in Advanced Fiber Composites. Macromol. Biosci. 2014, 14, 10–32. [Google Scholar] [CrossRef] [PubMed]
- Jayani, T.; Sanjeev, B.; Marimuthu, S.; Uthandi, S. Bacterial cellulose nano fiber (BCNF) as carrier support for the immobilization of probiotic, Lactobacillus acidophilus 016. Carbohyr. Polym. 2020, 250, 116965. [Google Scholar] [CrossRef] [PubMed]
- Lappa, I.K.; Kachrimanidou, V.; Alexandri, M.; Papadaki, A.; Kopsahelis, N. Novel probiotic/bacterial cellulose biocatalyst for the development of functional dairy beverage. Foods 2022, 11, 2586. [Google Scholar] [CrossRef] [PubMed]
- Jagannath, A.; Raju, P.S.; Bawa, A.S. Comparative evaluation of bacterial cellulose (nata) as a cryoprotectant and carrier support during the freeze drying process of probiotic lactic acid bacteria. LWT—Food Sci. Technol. 2010, 43, 1197–1203. [Google Scholar] [CrossRef]
- Fijałkowski, K.; Peitler, D.; Rakoczy, R.; Żywicka, A. Survival of probiotic lactic acid bacteria immobilized in different forms of bacterial cellulose in simulated gastric juices and bile salt solution. LWT—Food Sci. Technol. 2016, 68, 322–328. [Google Scholar] [CrossRef]
- Amarasekara, A.S.; Wang, D.; Grady, T.L. A comparison of kombucha SCOBY bacterial cellulose purification methods. SN Appl. Sci. 2020, 2, 240. [Google Scholar] [CrossRef]
- Kitwetcharoen, H.; Phung, L.T.; Klanrit, P.; Thanonkeo, S.; Tippayawat, P.; Yamada, M.; Thanonkeo, P. Kombucha healthy drink—Recent advances in production, chemical composition and health benefits. Fermentation 2023, 9, 48. [Google Scholar] [CrossRef]
- Laavanya, D.; Shirkole, S.; Balasubramanian, P. Current challenges, applications and future perspectives of SCOBY cellulose of kombucha fermentation. J. Clean. Prod. 2021, 295, 126454. [Google Scholar] [CrossRef]
- Orlando, I.; Basnett, P.; Nigmatullin, R.; Wang, W.; Knowles, J.C.; Roy, I. Chemical modification of bacterial cellulose for the development of an antibacterial wound dressing. Front. Bioeng. Biotechnol. 2020, 8, 557885. [Google Scholar] [CrossRef] [PubMed]
- Avcioglu, N.H.; Birben, M.; Seyis Bilkay, I. Optimization and physicochemical characterization of enhanced microbial cellulose production with a new kombucha consortium. Process Biochem. 2021, 108, 60–68. [Google Scholar] [CrossRef]
- Sheykhnazari, S.; Tabarsa, T.; Ashori, A.; Shakeri, A.; Golalipour, M. Bacterial synthesized cellulose nanofibers; effects of growth times and culture mediums on the structural characteristics. Carbohydr. Polym. 2011, 86, 1187–1191. [Google Scholar] [CrossRef]
- Herigstad, B.; Hamilton, M.; Heersink, J. How to optimize the drop plate method for enumerating bacteria. J. Microbiol. Methods 2001, 44, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.; Wu, X.; Zhu, J.; Sun, B.; Zhang, Y.Y.; Miller, C. Bacterial cellulose membrane—A new support carrier for yeast immobilization for ethanol fermentation. Process Biochem. 2011, 46, 2054–2058. [Google Scholar] [CrossRef]
- Yim, S.M.; Song, J.E.; Kim, H.R. Production and characterization of bacterial cellulose fabrics by nitrogen sources of tea and carbon sources of sugar. Process Biochem. 2017, 59, 26–36. [Google Scholar] [CrossRef]
- Żywicka, A.; Peitler, D.; Rakoczy, R.; Junka, A.F.; Fijałkowski, K. Wet and dry forms of bacterial cellulose synthetized by different strains of Gluconacetobacter xylinus as carriers for yeast immobilization. Appl. Biochem. Biotechnol. 2016, 180, 805–816. [Google Scholar] [CrossRef]
- Jayabalan, R.; Marimuthu, S.; Swaminathan, K. Changes in content of organic acids and tea polyphenols during kombucha tea fermentation. Food Chemis. 2007, 102, 392–398. [Google Scholar] [CrossRef]
- Chakravorty, S.; Bhattacharya, S.; Chatzinotas, A.; Chakraborty, W.; Bhattacharya, D.; Gachhui, R. Kombucha tea fermentation: Microbial and biochemical dynamics. Int. J. Food Microbiol. 2016, 220, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Dima, S.O.; Panaitescu, D.M.; Orban, C.; Ghiurea, M.; Doncea, S.M.; Fierascu, R.; Nistor, C.; Alexandrescu, E.; Nicolae, C.A.; Trică, B. Bacterial nanocellulose from side-streams of kombucha beverages production: Preparation and physical-chemical properties. Polymers 2017, 9, 374. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Liu, B.Y. Changes in major components of tea fungus metabolites during prolonged fermentation. J. Appl. Microbiol. 2000, 89, 834–839. [Google Scholar] [CrossRef]
- Ayed, L.; Ben Abid, S.; Hamdi, M. Development of a beverage from red grape juice fermented with the kombucha consortium. Ann. Microbiol. 2017, 67, 111–121. [Google Scholar] [CrossRef]
- Antolak, H.; Piechota, D.; Kucharska, A. Kombucha tea—A double power of bioactive compounds from tea and symbiotic culture of bacteria and yeasts (SCOBY). Antioxidants 2021, 10, 1541. [Google Scholar] [CrossRef] [PubMed]
- Diez-Ozaeta, I.; Astiazaran, O.J. Recent advances in kombucha tea: Microbial consortium, chemical parameters, health implications and biocellulose production. Int. J. Food Microbiol. 2022, 377, 109783. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, T.Y.A.; Di Monte, M.V.; Peltzer, M.A.; Salvay, A.G. Bacterial cellulose films production by kombucha symbiotic community cultured on different herbal infusions. Food Chemis. 2022, 372, 131346. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.F.S.; Almeida, F.C.G.; Vinhas, G.M.; Sarubbo, L.A. Production of bacterial cellulose by Gluconacetobacter hansenii using corn steep liquor as nutrient sources. Front. Microbiol. 2017, 8, 20–27. [Google Scholar] [CrossRef]
- Leonarski, E.; Cesca, K.; Zanella, E.; Stambuk, B.U.; Oliveira, D.; Poletto, P. Production of kombucha-like beverage and bacterial cellulose by acerola by product as raw material. LWT 2021, 135, 110075. [Google Scholar] [CrossRef]
- Machado, R.T.A.; Gutierrez, J.; Tercjak, A.; Trovatti, E.; Uahib, F.G.M.; Moreno, G.d.P.; Nascimento, A.P.; Berreta, A.A.; Ribeiro, S.J.L.; Barud, H.S. Komagataeibacter rhaeticus as an alternative bacteria for cellulose production. Carbohydr. Polym. 2016, 152, 841–849. [Google Scholar] [CrossRef]
- Machado, R.T.A.; Meneguin, A.B.; Sábio, R.M.; Franco, D.F.; Antonio, S.G.; Gutierrez, J.; Tercjak, A.; Berretta, A.A.; Ribeiro, S.J.L.; Lazarini, S.C. Komagataeibacter rhaeticus grown in sugarcane molasses-supplemented culture medium as a strategy for enhancing bacterial cellulose production. Ind. Crops Prod. 2018, 122, 637–646. [Google Scholar] [CrossRef]
- Lin, S.P.; Huang, Y.H.; Hsu, K.D.; Lai, Y.J.; Chen, Y.K.; Cheng, K.C. Isolation and identification of cellulose-producing strain Komagataeibacter intermedius from fermented fruit Juice. Carbohydr. Polym. 2016, 151, 827–833. [Google Scholar] [CrossRef]
- Lotfiman, S.; Awang, B.D.R.; Ti, T.B.; Kamarudin, S.; Nikbin, S. Influence of date syrup as a carbon source on bacterial cellulose production by Acetobacter xylinum 0416. Adv. Polym. Technol. 2018, 37, 1085–1091. [Google Scholar] [CrossRef]
- Zhou, L.L.; Sun, D.P.; Hu, L.Y.; Li, Y.W.; Yang, J.Z. Effect of addition of sodium alginate on bacterial cellulose production by Acetobacter xylinum. J. Ind. Microbiol. Biotechnol. 2007, 34, 483–489. [Google Scholar] [CrossRef]
- Revin, V.; Liyaskina, E.; Nazarkina, M.; Bogatyreva, A.; Shchankin, M. Cost-effective production of bacterial cellulose using acidic food industry by-products. Braz. J. Microbiol. 2018, 49, 151–159. [Google Scholar] [CrossRef]
- Soemphol, W.; Hongsachart, P.; Tanamool, V. Production and characterization of bacterial cellulose produced from agricultural by-product by Gluconacetobacter Strains. Mater. Today Proc. 2018, 5, 11159–11168. [Google Scholar] [CrossRef]
- Oliver, O.H.; Geng, S.; Espinach, F.X.; Oksman, K.; Vilaseca, F. Bacterial cellulose network from kombucha fermentation impregnated with emulsion-polymerized poly (methyl methacrylate) to form nanocomposite. Polymers 2021, 13, 664. [Google Scholar] [CrossRef]
- Huang, Y.; Zhu, C.; Yang, J.; Nie, Y.; Chen, C.; Sun, D. Recent advances in bacterial cellulose. Cellulose 2014, 21, 1–30. [Google Scholar] [CrossRef]
- Barkhordari, S.; Yadollahi, M.; Namazi, H. PH Sensitive nanocomposite hydrogel beads based on carboxymethyl cellulose/layered double hydroxide as drug delivery systems. J. Polym. Res. 2014, 21, 454. [Google Scholar] [CrossRef]
- Thomas, D.; Latha, M.S.; Thomas, K.K. Synthesis and in vitro evaluation of alginate-cellulose nanocrystal hybrid nanoparticles for the controlled oral delivery of rifampicin. J. Drug Deliv. Sci. Technol. 2018, 46, 392–399. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Gidley, M.J.; Dykes, G.A. Potential of a nisin-containing bacterial cellulose film to inhibit Listeria Monocytogenes on processed meats. Food Microbiol. 2008, 25, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Carmona, M.; Gálvez-Gómez, M.P.; González-Perez, L.; Pinedo-Rangel, V.; Pineda-Vasquez, T.; Hotza, D. Production of bacterial cellulose hydrogel and its evaluation as a proton exchange membrane. J. Polym. Environ. 2023. [Google Scholar] [CrossRef]
- Shi, Z.; Zhang, Y.; Phillips, G.O.; Yang, G. Utilization of bacterial cellulose in food. Food Hydrocoll. 2014, 35, 539–545. [Google Scholar] [CrossRef]
- Azeredo, H.M.C.; Barud, H.; Farinas, C.S.; Vasconcellos, V.M.; Claro, A.M. Bacterial cellulose as a raw material for food and food packaging applications. Front. Sustain. Food Syst. 2019, 3, 7. [Google Scholar] [CrossRef]
- Savedboworn, W.; Teawsomboonkit, K.; Surichay, S.; Riansa-ngawong, W.; Rittisak, S.; Charoen, R.; Phattayakorn, K. Impact of protectants on the storage stability of freeze-dried probiotic Lactobacillus Plantarum. Food Sci. Biotechnol. 2019, 28, 795–805. [Google Scholar] [CrossRef] [PubMed]
- Guan, Z.W.; Yu, E.Z.; Feng, Q. Soluble dietary fiber, one of the most important nutrients for the gut microbiota. Molecules 2021, 26, 6802. [Google Scholar] [CrossRef] [PubMed]
- Płoska, J.; Garbowska, M.; Pluta, A.; Stasiak-Różańska, L. Bacterial cellulose—Innovative biopolymer and possibilities of its applications in dairy industry. Int. Dairy J. 2023, 140, 105586. [Google Scholar] [CrossRef]


| Time of Fermentation (Days) | Kombucha Bacterial Cellulose | Kombucha Broth | ||||||
|---|---|---|---|---|---|---|---|---|
| WW (g) | Moisture Content (%) | DW (g) | Yield of DW (%) | pH | Acetic Acid Conc. (g/L) | Number of Microorganisms (log CFU/mL) | ||
| Yeasts | AAB * | |||||||
| 0 | - | - | - | - | 3.6 ± 0.1 | 1.44 ± 0.08 | 5.1 ± 0.4 | 3.6 ± 0.1 |
| 7 | 1162 ± 28 | 89.7 | 120 ± 10 | 0.9 | 3.2 ± 0.0 | 3.42 ± 0.08 | 7.0 ± 0.5 | 7.9 ± 0.1 |
| 14 | 2258 ± 19 | 76.5 | 530 ± 21 | 3.9 | 3.0 ± 0.0 | 6.24 ± 0.33 | 7.1 ± 0.1 | 7.5 ± 0.4 |
| 30 | 3931 ± 43 | 77.6 | 880 ± 27 | 6.5 | 2.3 ± 0.0 | 19.50 ± 0.58 | 6.4 ± 0.2 | 4.3 ± 0.2 |
| Time (Day) | Total Pore Volume (cc/g) | Average Pore Size (nm) | Surface Area (m2/g) |
|---|---|---|---|
| 7 | 0.0451 | 4.865 | 18.52 |
| 14 | 0.0345 | 5.674 | 12.16 |
| 30 | 0.0616 | 6.189 | 19.91 |
| Form of L. plantarum Culture | Initial No. of L. plantarum Cells before Freeze-Drying (log CFU/mL) | No. of L. plantarum Cells after Freeze-Drying (log CFU/g) | Survival of L. plantarum Cells after Being Exposed to Simulated GI Tract Conditions | |||
|---|---|---|---|---|---|---|
| MRS (pH 2.0) | MRS with 0.3% (w/v) Bile Salt | |||||
| Number (log CFU/g) | Log Reduction * | Number (log CFU/g) | Log Reduction * | |||
| Non-immobilized culture | 15.55 ± 0.12 | 5.20 ± 0.02 | ND ** | - | ND ** | - |
| Immobilized culture on KBC | 16.20 ± 0.03 | 7.98 ± 0.19 | 5.16 ± 0.62 | 2.82 | 2.94 ± 0.21 | 5.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Charoenrak, S.; Charumanee, S.; Sirisa-ard, P.; Bovonsombut, S.; Kumdhitiahutsawakul, L.; Kiatkarun, S.; Pathom-Aree, W.; Chitov, T.; Bovonsombut, S. Nanobacterial Cellulose from Kombucha Fermentation as a Potential Protective Carrier of Lactobacillus plantarum under Simulated Gastrointestinal Tract Conditions. Polymers 2023, 15, 1356. https://doi.org/10.3390/polym15061356
Charoenrak S, Charumanee S, Sirisa-ard P, Bovonsombut S, Kumdhitiahutsawakul L, Kiatkarun S, Pathom-Aree W, Chitov T, Bovonsombut S. Nanobacterial Cellulose from Kombucha Fermentation as a Potential Protective Carrier of Lactobacillus plantarum under Simulated Gastrointestinal Tract Conditions. Polymers. 2023; 15(6):1356. https://doi.org/10.3390/polym15061356
Chicago/Turabian StyleCharoenrak, Sonthirat, Suporn Charumanee, Panee Sirisa-ard, Sittisin Bovonsombut, Ladapa Kumdhitiahutsawakul, Suwalee Kiatkarun, Wasu Pathom-Aree, Thararat Chitov, and Sakunnee Bovonsombut. 2023. "Nanobacterial Cellulose from Kombucha Fermentation as a Potential Protective Carrier of Lactobacillus plantarum under Simulated Gastrointestinal Tract Conditions" Polymers 15, no. 6: 1356. https://doi.org/10.3390/polym15061356
APA StyleCharoenrak, S., Charumanee, S., Sirisa-ard, P., Bovonsombut, S., Kumdhitiahutsawakul, L., Kiatkarun, S., Pathom-Aree, W., Chitov, T., & Bovonsombut, S. (2023). Nanobacterial Cellulose from Kombucha Fermentation as a Potential Protective Carrier of Lactobacillus plantarum under Simulated Gastrointestinal Tract Conditions. Polymers, 15(6), 1356. https://doi.org/10.3390/polym15061356










