A Comparison of the Effect of Cellulose Nanocrystals (CNCs) and Polyethylene Glycol (PEG) as Additives in Ultrafiltration Membranes (PES-UF): Characterization and Performance
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Membrane Fabrication
2.3. Membrane Characterization
2.4. Analysis
3. Results and Discussion
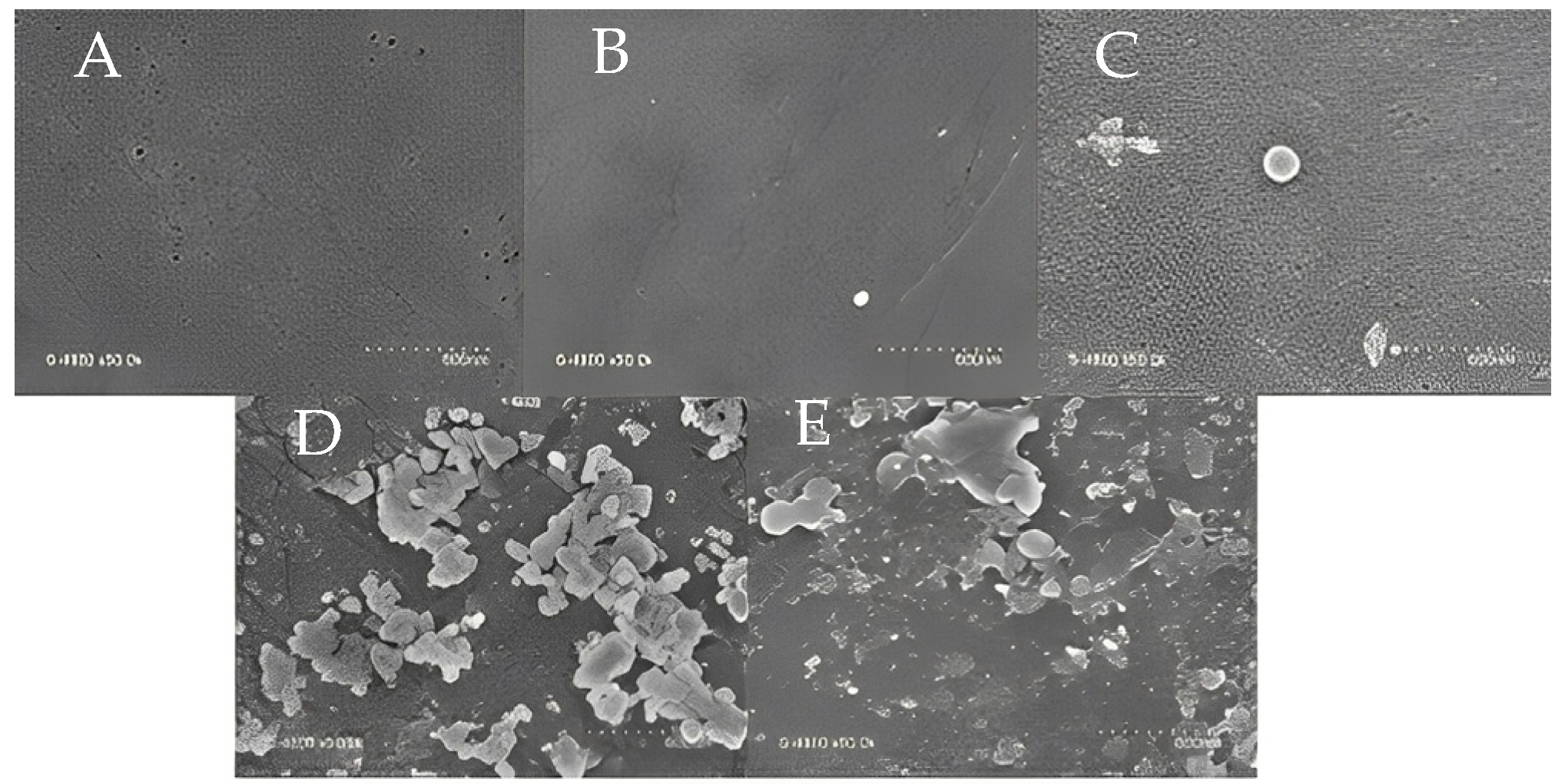
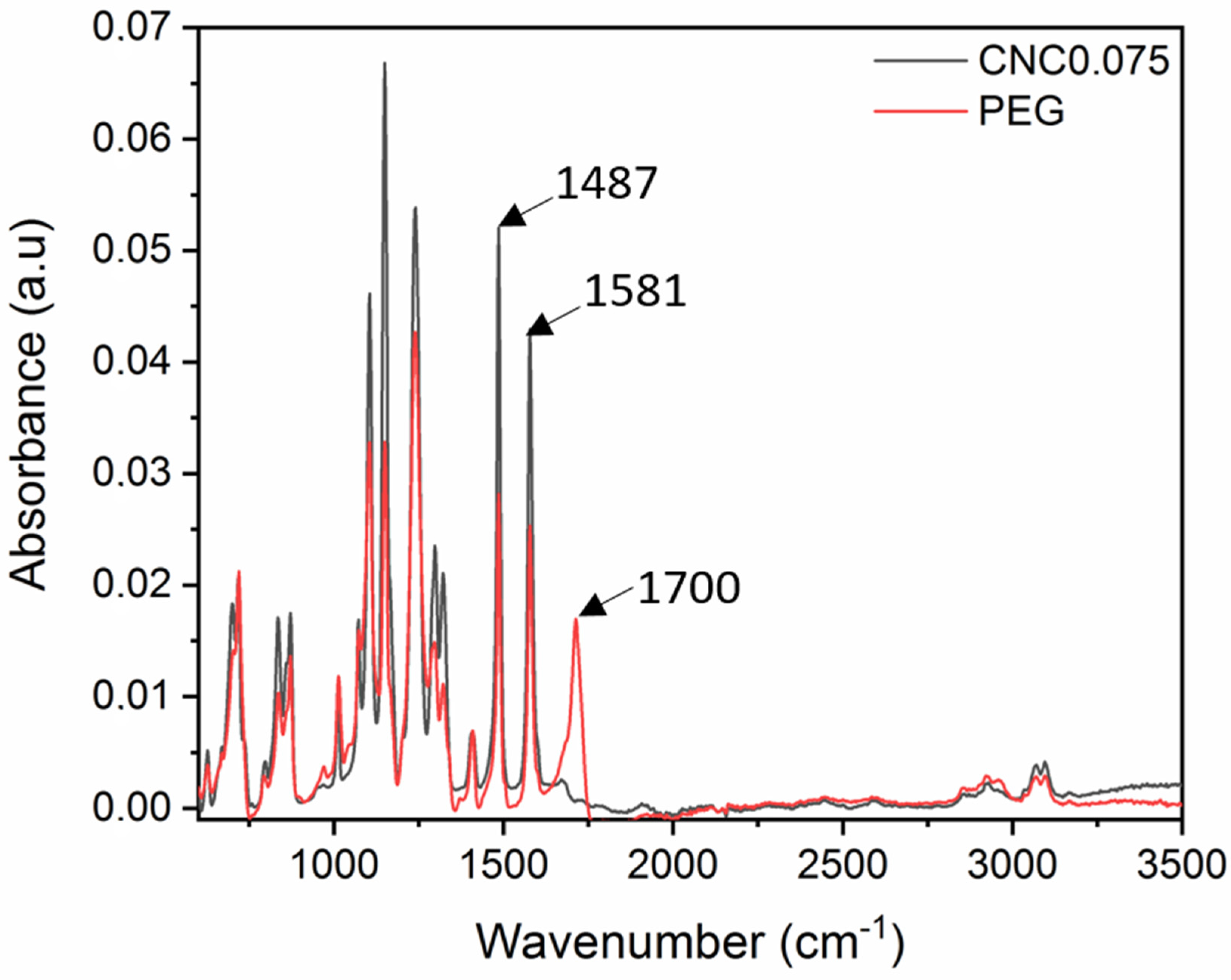
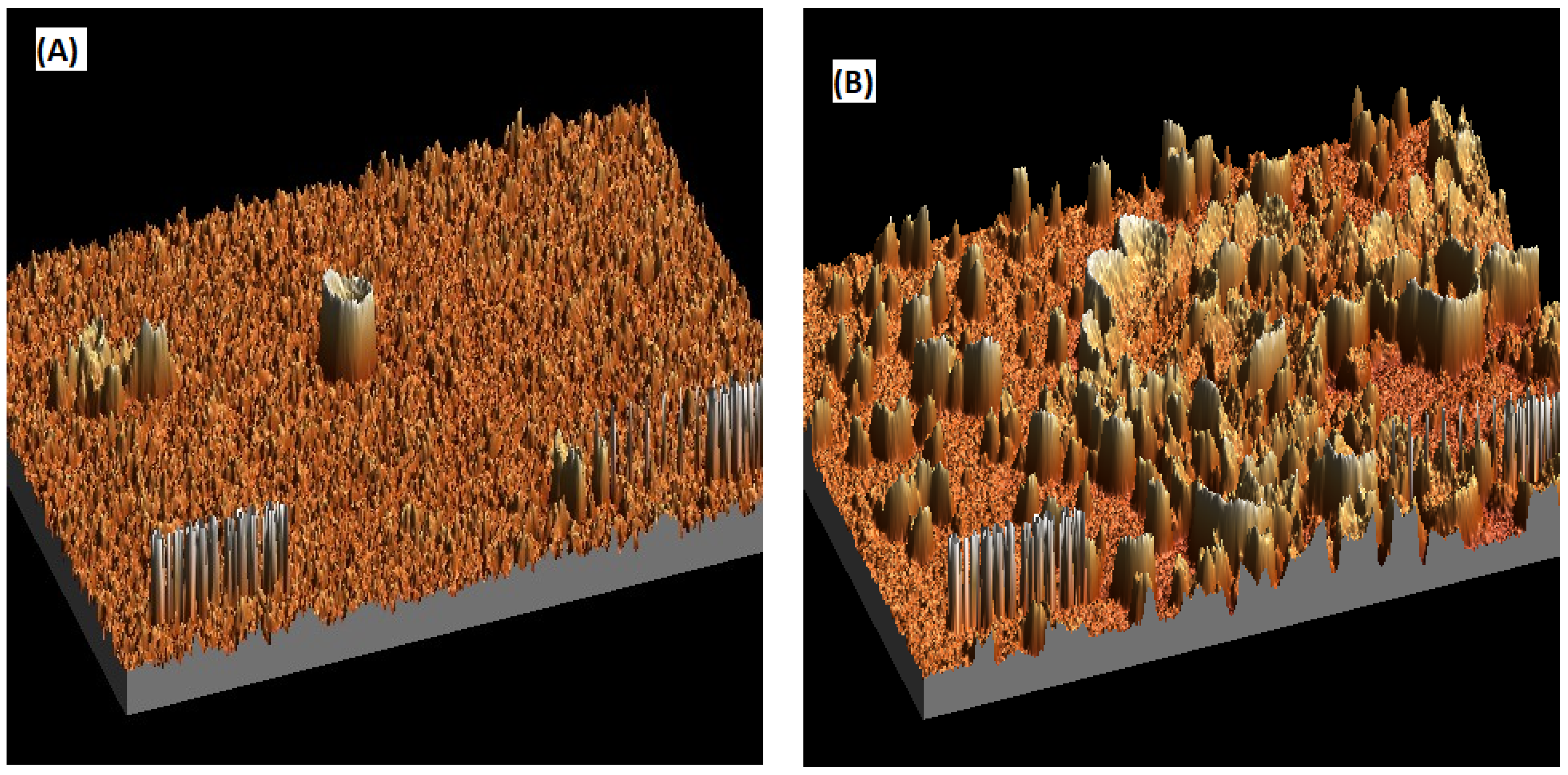
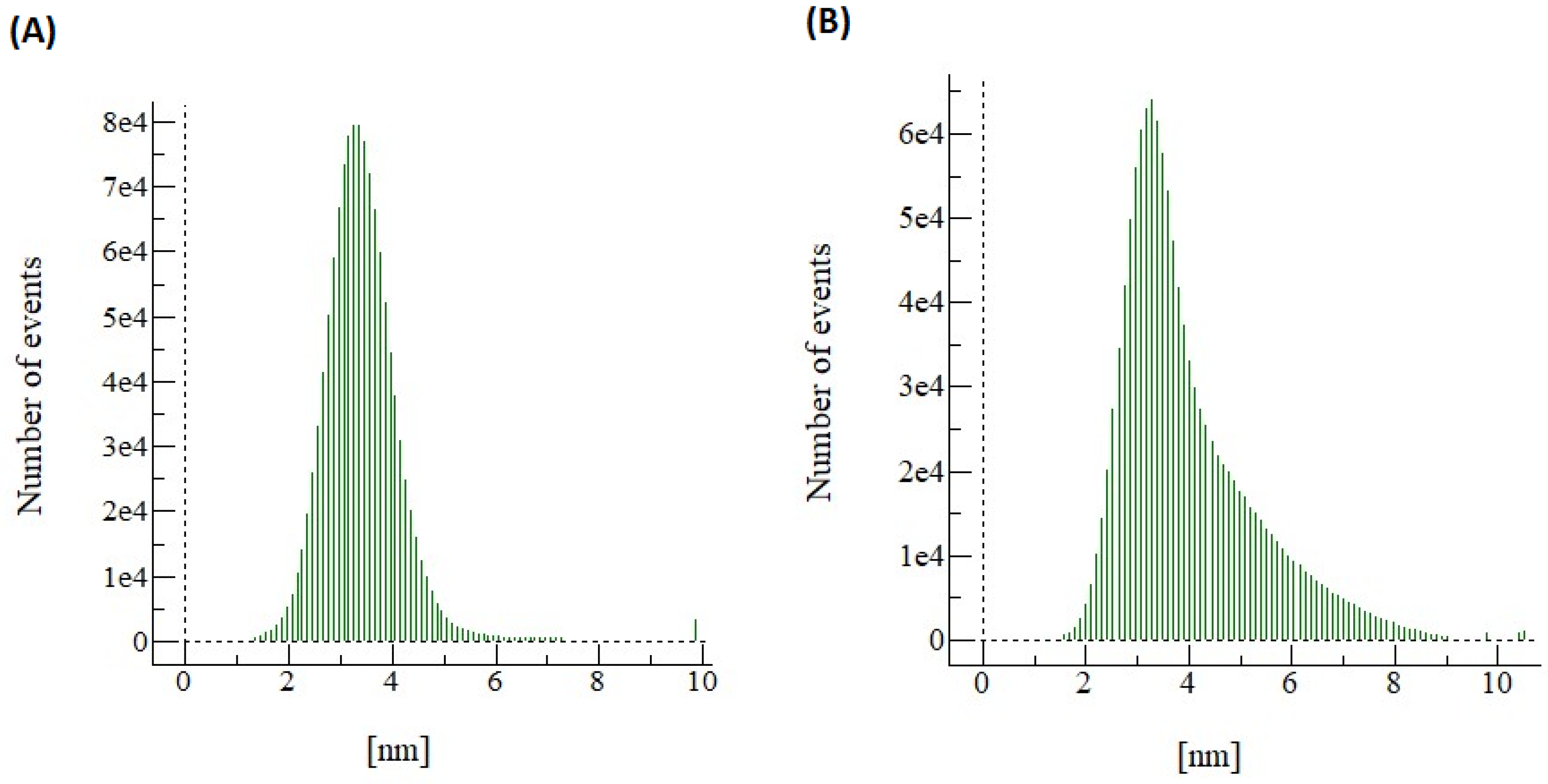
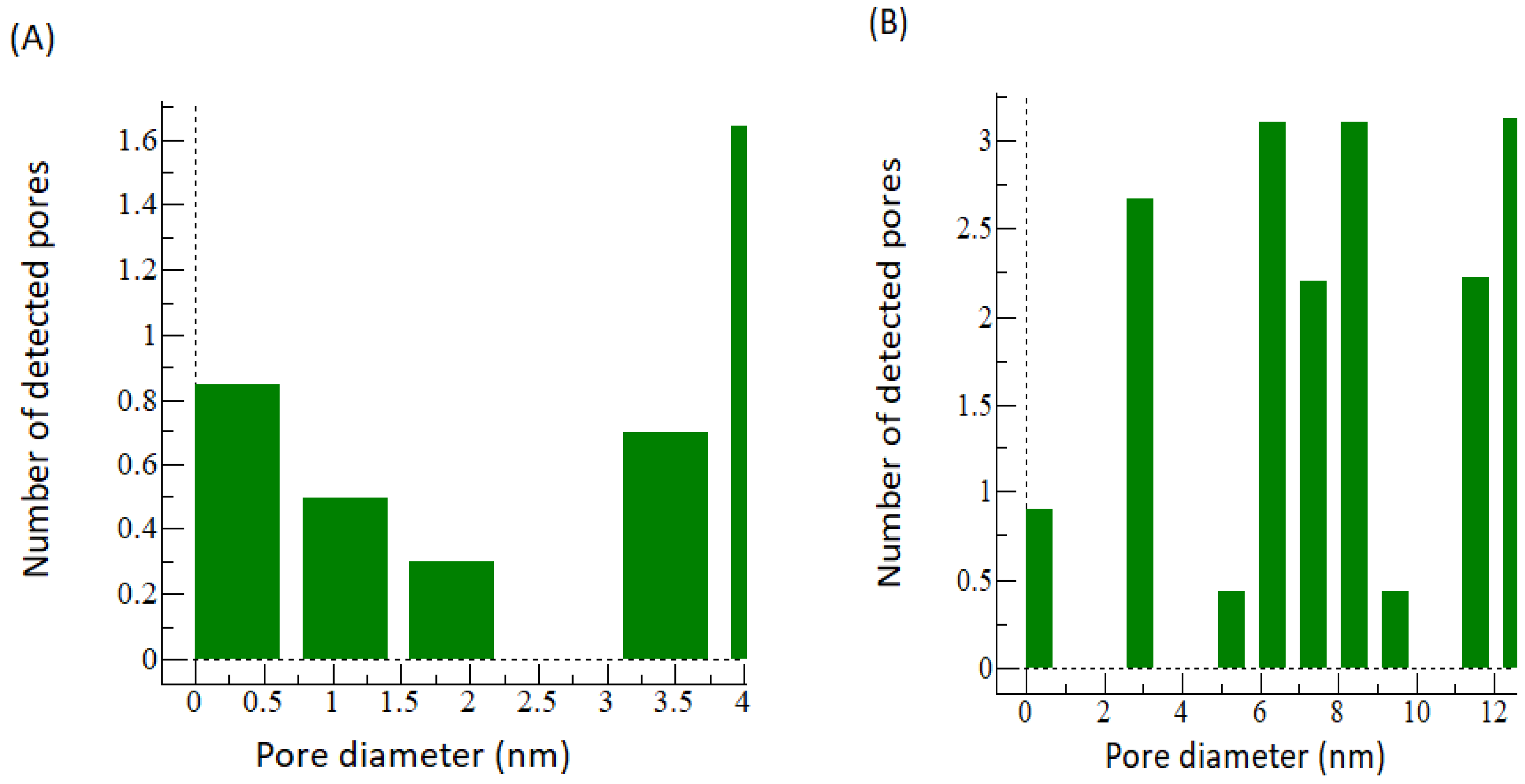
3.1. Comparing the Membrane Characteristics
3.2. Comparing the Membrane Performance
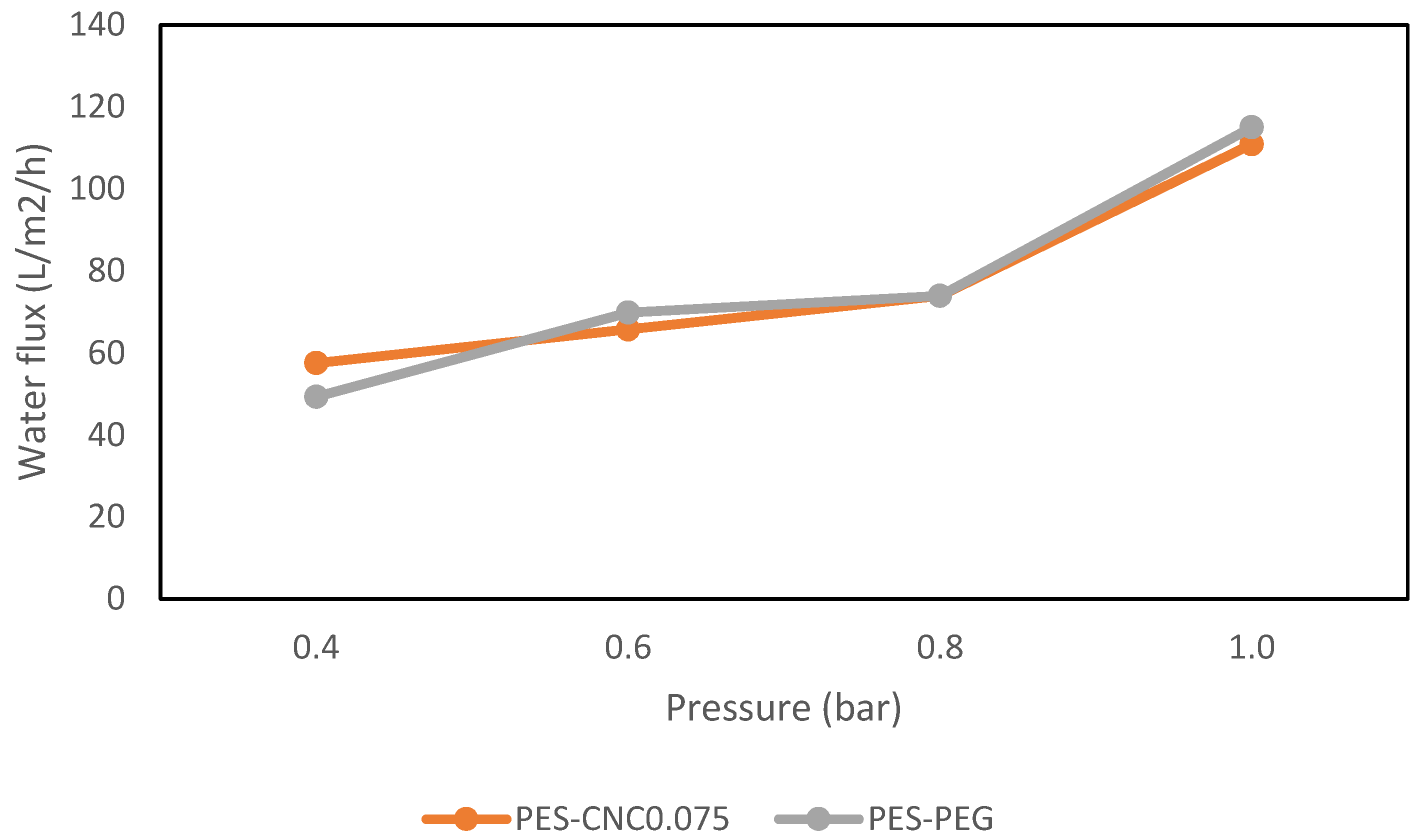
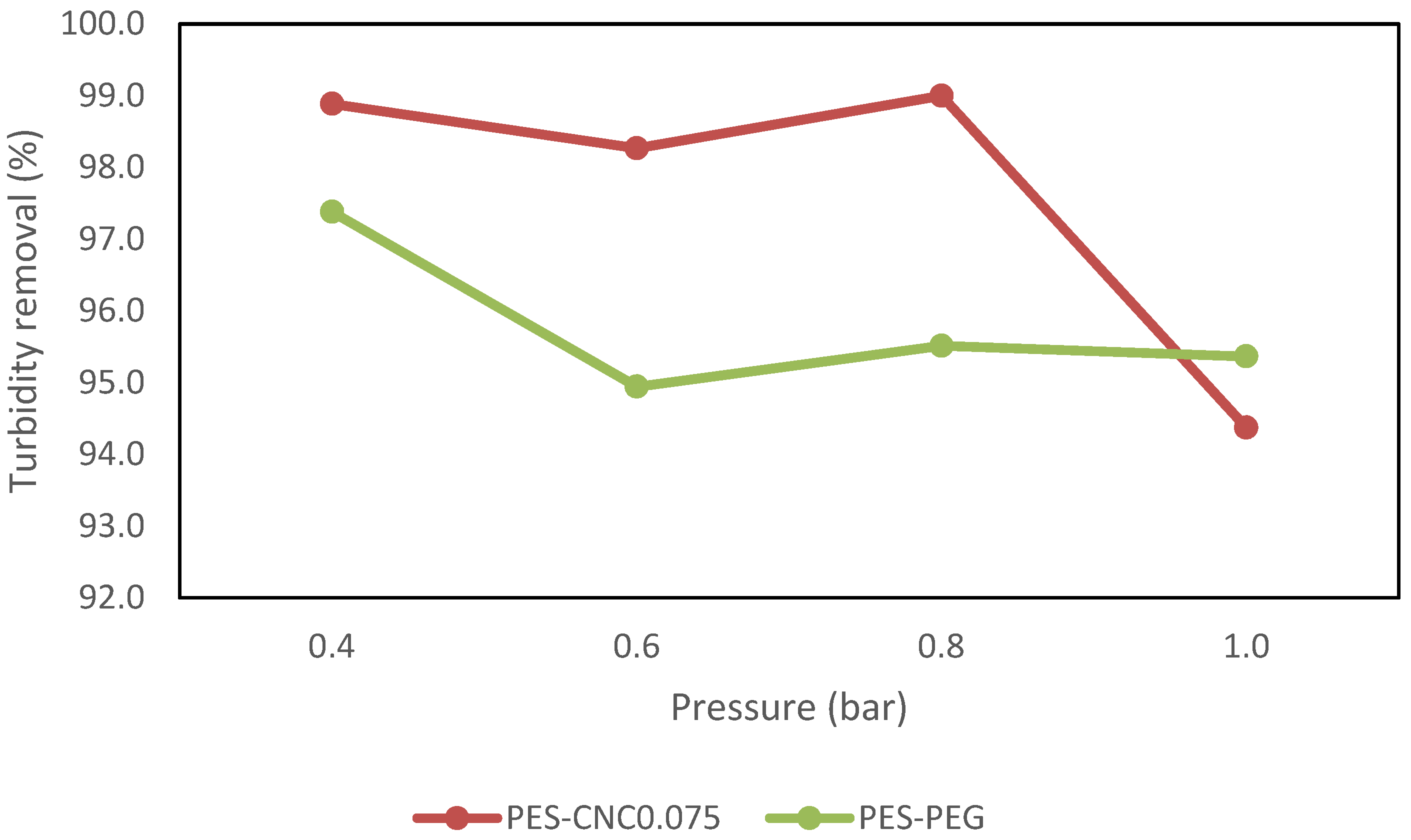
3.2.1. Performance with Synthetic Water
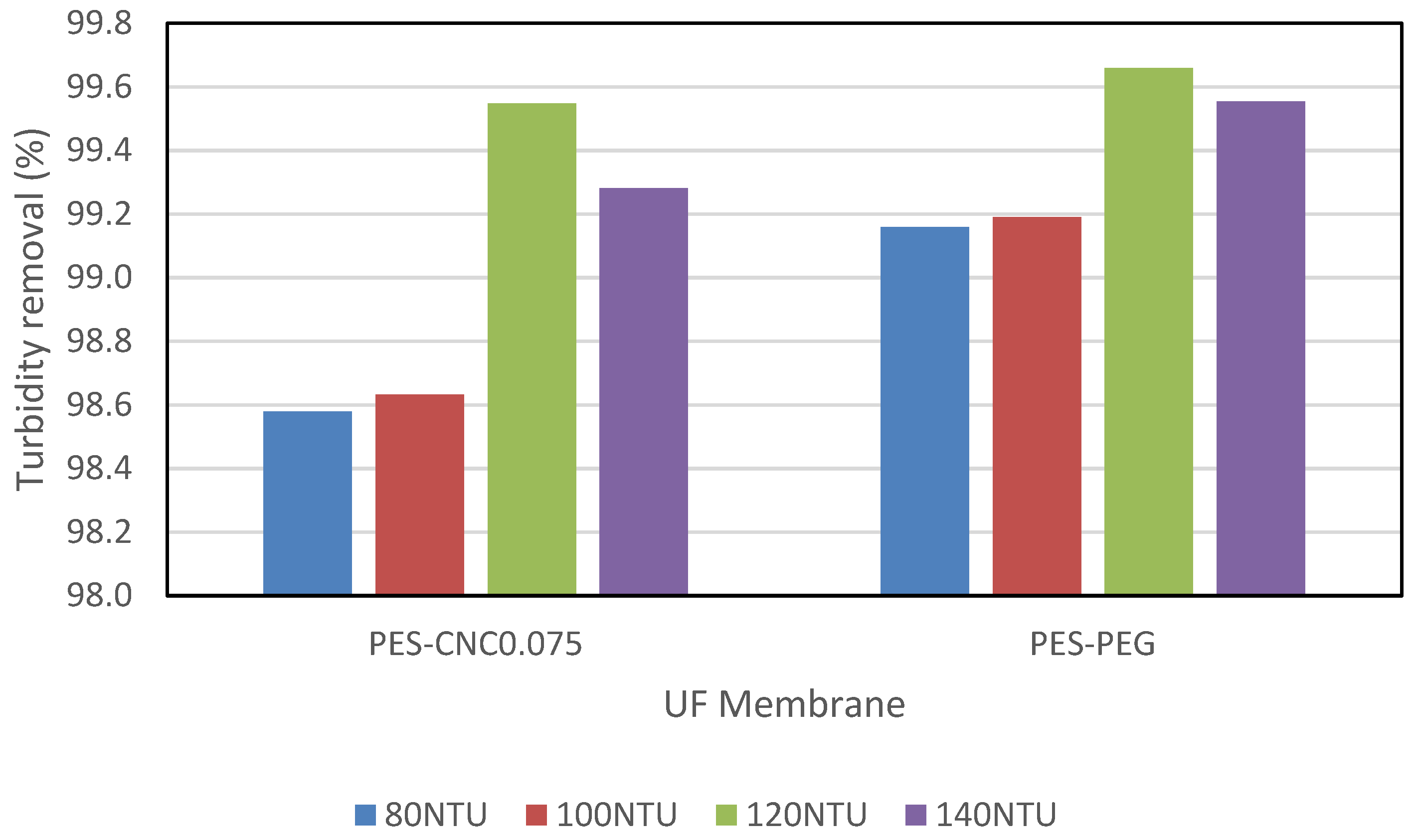
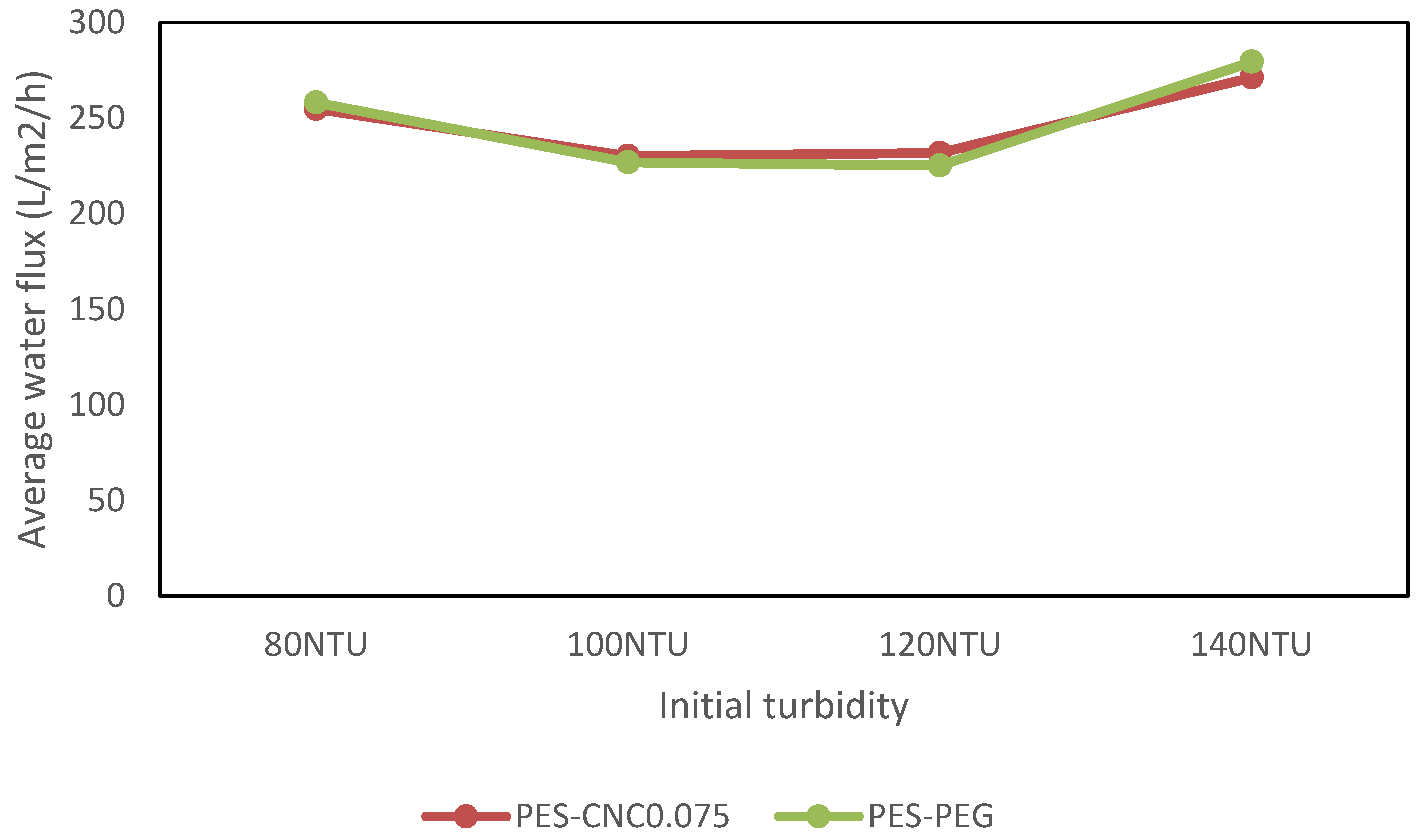
3.2.2. Performance with Raw Restaurant Water
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mishra, B.K.; Kumar, P.; Saraswat, C.; Chakraborty, S.; Gautam, A. Water security in a changing environment: Concept, challenges and solutions. Water 2021, 13, 490. [Google Scholar] [CrossRef]
- Thyagaraju, N. Water pollution and its impact on environment of society. Int. Res. J. Manag. IT Soc. Sci. 2016, 3, 1–7. [Google Scholar] [CrossRef]
- Beler-Baykal, B. An appraisal of domestic wastewater segregation from the perspective of recovery, recycling, and reuse. In Recycling and Reuse Approaches for Better Sustainability; Springer: Berlin/Heidelberg, Germany, 2019; pp. 11–20. [Google Scholar] [CrossRef]
- Salgot, M.; Folch, M. Wastewater treatment and water reuse. Curr. Opin. Environ. Sci. Health 2018, 2, 64–74. [Google Scholar] [CrossRef]
- Rodriguez-Narvaez, O.M.; Peralta-Hernandez, J.M.; Goonetilleke, A.; Bandala, E.R. Treatment technologies for emerging contaminants in water: A review. Chem. Eng. J. 2017, 323, 361–380. [Google Scholar] [CrossRef]
- Holkar, C.R.; Jadhav, A.J.; Pinjari, D.V.; Mahamuni, N.M.; Pandit, A.B. A critical review on textile wastewater treatments: Possible approaches. J. Environ. Manag. 2016, 182, 351–366. [Google Scholar] [CrossRef]
- Elsaid, K.; Sayed, E.T.; Abdelkareem, M.A.; Mahmoud, M.S.; Ramadan, M.; Olabi, A.G. Environmental impact of emerging desalination technologies: A preliminary evaluation. J. Environ. Chem. Eng. 2020, 8, 104099. [Google Scholar] [CrossRef]
- Kaner, P.; Rubakh, E.; Asatekin, A. Zwitterion-Containing polymer additives for fouling resistant ultrafiltration membranes. J. Membr. Sci. 2017, 533, 141–159. [Google Scholar] [CrossRef]
- Gohari, R.J.; Korminouri, F.; Lau, W.J.; Ismail, A.F.; Matsuura, T.; Chowdhury, M.N.K.; Halakoo, E.; Gohari, M.J. A novel super-hydrophilic PSf/HAO nanocomposite ultrafiltration membrane for efficient separation of oil/water emulsion. Sep. Purif. Technol. 2015, 150, 13–20. [Google Scholar] [CrossRef]
- Muppalla, R.; Jewrajka, S.K.; Reddy, A.V.R. Fouling resistant nanofiltration membranes for the separation of oil–water emulsion and micropollutants from water. Sep. Purif. Technol. 2015, 143, 125–134. [Google Scholar] [CrossRef]
- Tian, X.; Jin, H.; Sainio, J.; Ras, R.H.; Ikkala, O. Droplet and fluid gating by biomimetic Janus membranes. Adv. Funct. Mater. 2014, 24, 6023–6028. [Google Scholar] [CrossRef]
- Islam, M.S.; Sultana, A.; Saadat, A.H.M.; Shammi, M.; Uddin, M.K. Desalination technologies for developing countries: A review. J. Sci. Res. 2018, 10, 77–97. [Google Scholar] [CrossRef]
- Keskin, B.; Ormancı-Acar, T.; Türken, T.; Imer, D.Y.; Koyuncu, I. Effect of wetting agent on the dye filtration performance of ultrafiltration membrane. Water Sci. Technol. 2020, 82, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Grishkewich, N.; Mohammed, N.; Tang, J.; Tam, K.C. Recent advances in the application of cellulose nanocrystals. Curr. Opin. Colloid Interface Sci. 2017, 29, 32–45. [Google Scholar] [CrossRef]
- Lessan, F.; Karimi, M.; Bañuelos, J.L.; Foudazi, R. Phase separation and performance of polyethersulfone/cellulose nanocrystals membranes. Polymer 2020, 186, 121969. [Google Scholar] [CrossRef]
- Zhong, L.; Ding, Z.; Li, B.; Zhang, L. Preparation and characterization of polysulfone/sulfonated polysulfone/cellulose nanofibers ternary blend membranes. BioResources 2015, 10, 2936–2948. [Google Scholar] [CrossRef]
- Zhang, D.; Karkooti, A.; Liu, L.; Sadrzadeh, M.; Thundat, T.; Liu, Y.; Narain, R. Fabrication of antifouling and antibacterial polyethersulfone (PES)/cellulose nanocrystals (CNC) nanocomposite membranes. J. Membr. Sci. 2018, 549, 350–356. [Google Scholar] [CrossRef]
- Prasad, N.S.; Gayatri, N.L.; Sandhya, B.N.; Kalyani, S.; Bhargava, S.K.; Sridhar, S. Hydrophilized Ultrafiltration Membranes Synthesized from Acrylic Acid Grafted Polyethersulfone for Downstream Processing of Therapeutic Insulin and Cobalamin. Appl. Biochem. Biotechnol. 2022, 194, 3400–3418. [Google Scholar] [CrossRef]
- Goetz, L.; Mathew, A.; Oksman, K.; Gatenholm, P.; Ragauskas, A.J. A novel nanocomposite film prepared from crosslinked cellulosic whiskers. Carbohydr. Polym. 2009, 75, 85–89. [Google Scholar] [CrossRef]
- Asempour, F.; Emadzadeh, D.; Matsuura, T.; Kruczek, B. Synthesis and characterization of novel Cellulose Nanocrystals-based Thin Film Nanocomposite membranes for reverse osmosis applications. Desalination 2018, 439, 179–187. [Google Scholar] [CrossRef]
- Adeniyi, A.; Odo, G.O.; Gonzalez-Ortiz, D.; Pochat- Bohatier, C.; Onyango, M. COD and Turbidity Removal from Restaurant Wastewater Using Polyethersulfone Ultrafiltration Membrane Containing Sawdust-Derived Cellulose Nanocrystals; South African Chemical Engineering Congress: Randburg, South Africa, 2021; pp. 258–270. ISBN 978-1-991213-99-0. [Google Scholar]
- Parnian, P.; D’Amore, A. Fabrication of high-performance CNT reinforced polymer composite for additive manufacturing by phase inversion technique. Polymers 2021, 13, 4007. [Google Scholar] [CrossRef]
- Farjami, M.; Vatanpour, V.; Moghadassi, A. Effect of nanoboehmite/poly (ethylene glycol) on the performance and physiochemical attributes EPVC nano-composite membranes in protein separation. Chem. Eng. Res. Des. 2020, 156, 371–383. [Google Scholar] [CrossRef]
- Kusworo, T.D.; Aryanti, N.; Utomo, D.P. Oilfield produced water treatment to clean water using integrated activated carbon-bentonite adsorbent and double stages membrane process. Chem. Eng. J. 2018, 347, 462–471. [Google Scholar] [CrossRef]
- Adeniyi, A.; Gonzalez-Ortiz, D.; Pochat-Bohatier, C.; Oyewo, O.; Sithole, B.; Onyango, M. Incorporation of Cellulose Nanocrystals (CNC) derived from sawdust into polyamide thin-film composite membranes for enhanced water recovery. Alex. Eng. J. 2020, 59, 4201–4210. [Google Scholar] [CrossRef]
- Horcas, I.; Fernández, R.; Gomez-Rodriguez, J.M.; Colchero, J.; Gómez-Herrero, J.; Baro, A.M. WSXM: A software for scanning probe microscopy and a tool for nanotechnology. Rev. Sci. Instrum. 2007, 78, 013705. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Wu, Z.; Zhang, Y.; Wei, Y.; Wang, J. Effect of non-solvent additives on the morphology, pore structure, and direct contact membrane distillation performance of PVDF-CTFE hydrophobic membranes. J. Environ. Sci. 2016, 45, 28–39. [Google Scholar] [CrossRef]
- Mohamed, M.A.; Jaafar, J.; Ismail, A.F.; Othman, M.H.D.; Rahman, M.A. Fourier Transform Infrared (FTIR) Spectroscopy; InMembrane Characterization; Elsevier: Amsterdam, The Netherlands, 2017; pp. 3–29. [Google Scholar]
- Oh, S.Y.; Yoo, D.I.; Shin, Y.; Seo, G. FTIR analysis of cellulose treated with sodium hydroxide and carbon dioxide. Carbohydr. Res. 2005, 340, 417–428. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Gu, L.; Wu, S.; Dong, G.; Qiao, X.; Zhang, K.; Lu, X.; Wen, H.; Zhang, D. Hydrothermal carbon nanospheres assisted-fabrication of PVDF ultrafiltration membranes with improved hydrophilicity and antifouling performance. Sep. Purif. Technol. 2020, 247, 116889. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Y.; Liu, T.; Xu, X.; Hu, Y. Improving the perm-selectivity and anti-fouling property of UF membrane through the micro-phase separation of PSf-b-PEG block copolymers. J. Membr. Sci. 2020, 599, 117851. [Google Scholar] [CrossRef]
- Lv, J.; Zhang, G.; Zhang, H.; Zhao, C.; Yang, F. Improvement of antifouling performances for modified PVDF ultrafiltration membrane with hydrophilic cellulose nanocrystal. Appl. Surf. Sci. 2018, 440, 91–100. [Google Scholar] [CrossRef]
- Arthanareeswaran, G.; Starov, V.M. Effect of solvents on performance of polyethersulfone ultrafiltration membranes: Investigation of metal ion separations. Desalination 2011, 267, 57–63. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, Y.; Wang, Y. Fly ash-based zeolite-complexed polyethylene-glycol on an interdigitated electrode surface for high-performance determination of diabetes mellitus. Int. J. Nanomed. 2020, 66, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.Y.; Tsai, W.T.; Ing, C.H.; Chang, C.F. Adsorption of polyethylene glycol (PEG) from aqueous solution onto hydrophobic zeolite. J. Colloid Interface Sci. 2020, 260, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Zhou, J.; Liu, F.; Xu, B.M.; Pan, Y. Recent Progress of Adsorptive Ultrafiltration Membranes in Water Treatment—A Mini Review. Membranes 2022, 12, 519. [Google Scholar] [CrossRef]
- Lee, J.J.; Woo, Y.C.; Kim, H.S. Effect of driving pressure and recovery rate on the performance of nanofiltration and reverse osmosis membranes for the treatment of the effluent from MBR. Desalination Water Treat. 2015, 54, 89–95. [Google Scholar] [CrossRef]
- Mulyati, S.; Aprilia, S.; Armando, M.A.; Mawardi, H. The effect of poly ethylene glycol additive on the characteristics and performance of cellulose acetate ultrafiltration membrane for removal of Cr (III) from aqueous solution. InIOP Conf. Ser. Mater. Sci. Eng. 2018, 352, 012051. [Google Scholar] [CrossRef]




| Mass Composition | Atomic Composition | |||
|---|---|---|---|---|
| PEG | CNC0.075 | PEG | CNC0.075 | |
| Carbon | 68.79% | 68.71% | 77.04% | 77.81% |
| Oxygen | 23.45% | 19.72% | 20.92% | 17.78% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adeniyi, A.; Odo, G.O.; Gonzalez-Ortiz, D.; Pochat-Bohatier, C.; Mbakop, S.; Onyango, M.S. A Comparison of the Effect of Cellulose Nanocrystals (CNCs) and Polyethylene Glycol (PEG) as Additives in Ultrafiltration Membranes (PES-UF): Characterization and Performance. Polymers 2023, 15, 2636. https://doi.org/10.3390/polym15122636
Adeniyi A, Odo GO, Gonzalez-Ortiz D, Pochat-Bohatier C, Mbakop S, Onyango MS. A Comparison of the Effect of Cellulose Nanocrystals (CNCs) and Polyethylene Glycol (PEG) as Additives in Ultrafiltration Membranes (PES-UF): Characterization and Performance. Polymers. 2023; 15(12):2636. https://doi.org/10.3390/polym15122636
Chicago/Turabian StyleAdeniyi, Amos, Gerald Oke Odo, Danae Gonzalez-Ortiz, Celine Pochat-Bohatier, Sandrine Mbakop, and Maurice Stephen Onyango. 2023. "A Comparison of the Effect of Cellulose Nanocrystals (CNCs) and Polyethylene Glycol (PEG) as Additives in Ultrafiltration Membranes (PES-UF): Characterization and Performance" Polymers 15, no. 12: 2636. https://doi.org/10.3390/polym15122636
APA StyleAdeniyi, A., Odo, G. O., Gonzalez-Ortiz, D., Pochat-Bohatier, C., Mbakop, S., & Onyango, M. S. (2023). A Comparison of the Effect of Cellulose Nanocrystals (CNCs) and Polyethylene Glycol (PEG) as Additives in Ultrafiltration Membranes (PES-UF): Characterization and Performance. Polymers, 15(12), 2636. https://doi.org/10.3390/polym15122636






