Preparation and Characterization of a Novel Cassava Starch-Based Phosphorus Releasing Super-Absorbent Polymer, and Optimization of the Performance of Water Absorption and Phosphorus Release
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of CST-SAP
2.3. Preparation of CST-PRP-SAP and Optimization of Reaction Conditions
2.4. Characterization Techniques
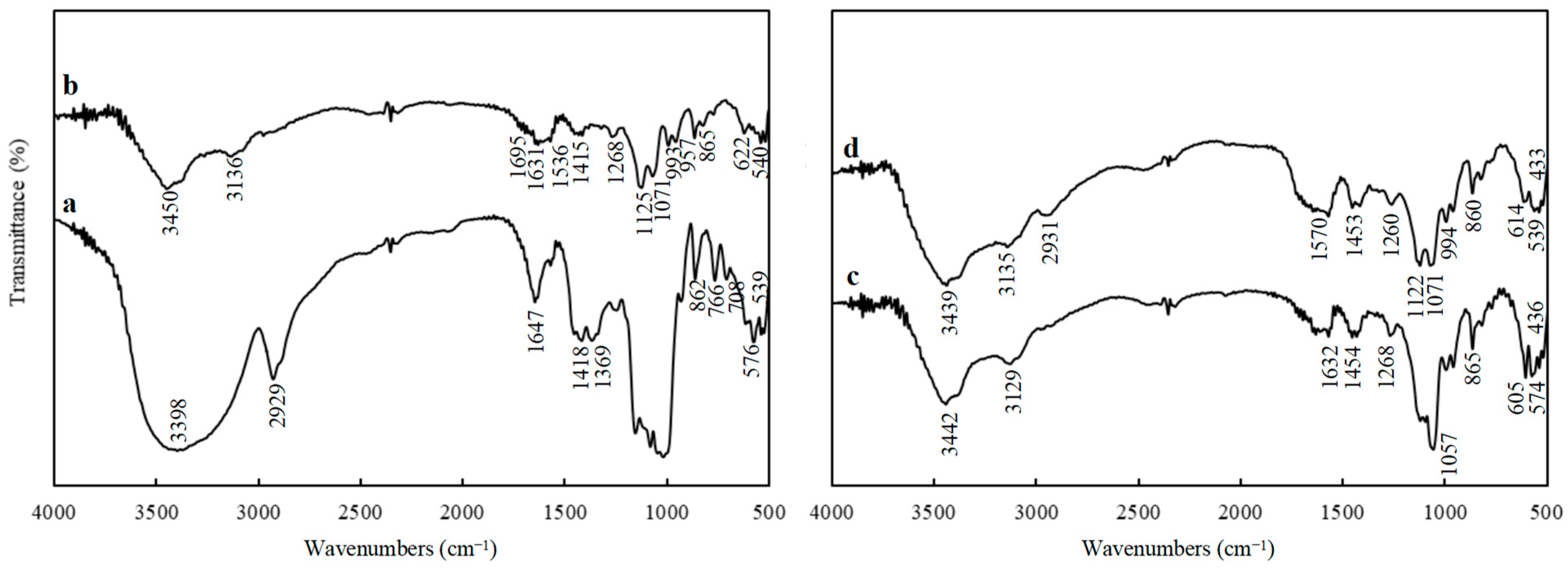
2.4.1. FTIR Analysis
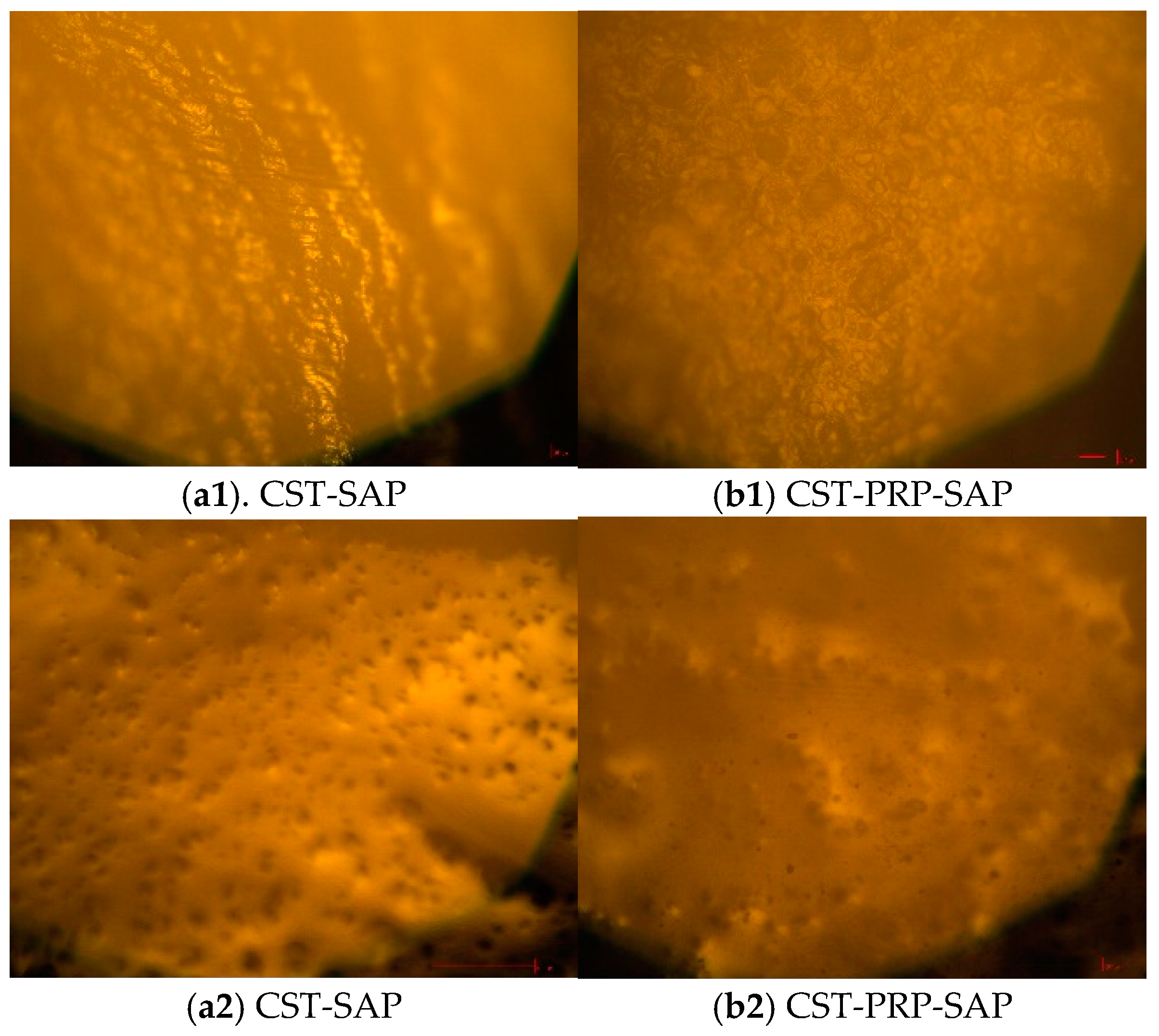
2.4.2. XRD and Optical Microscope Analysis
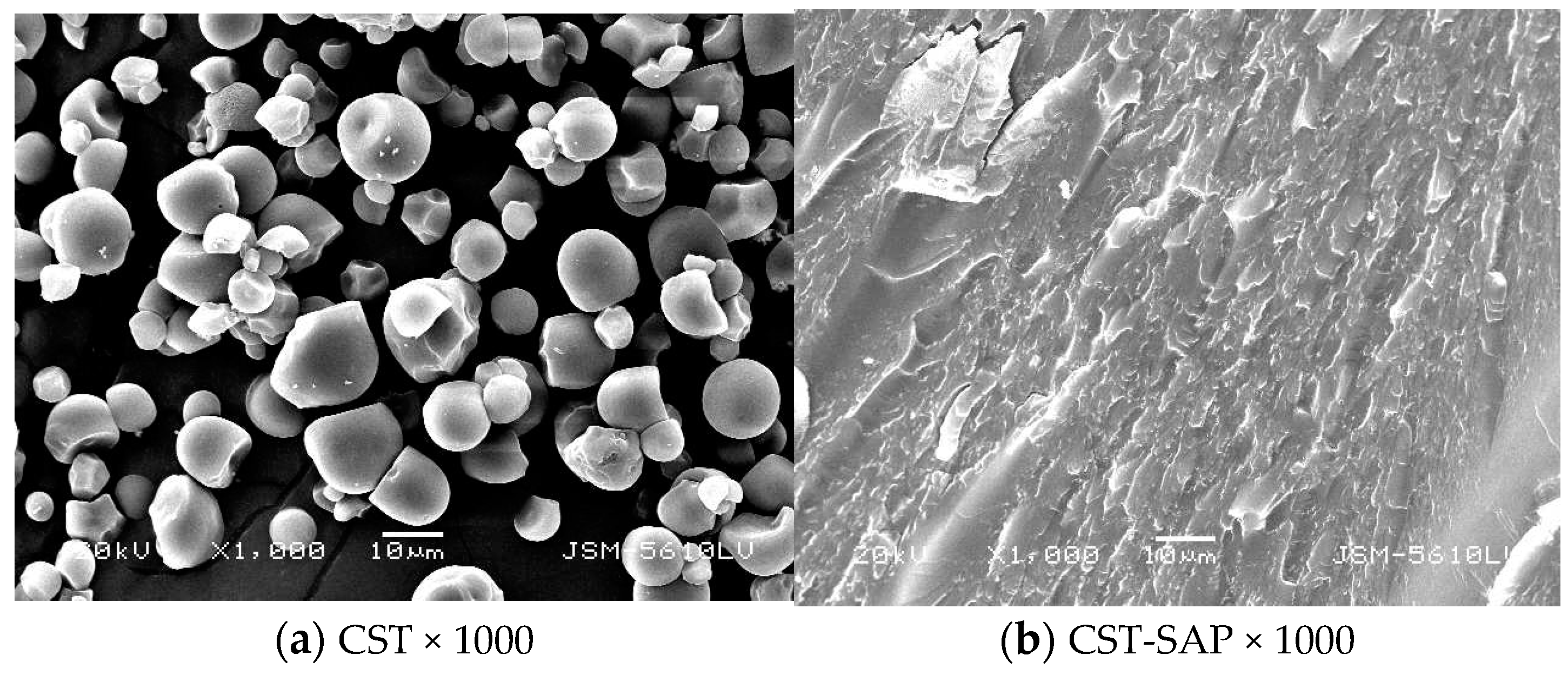
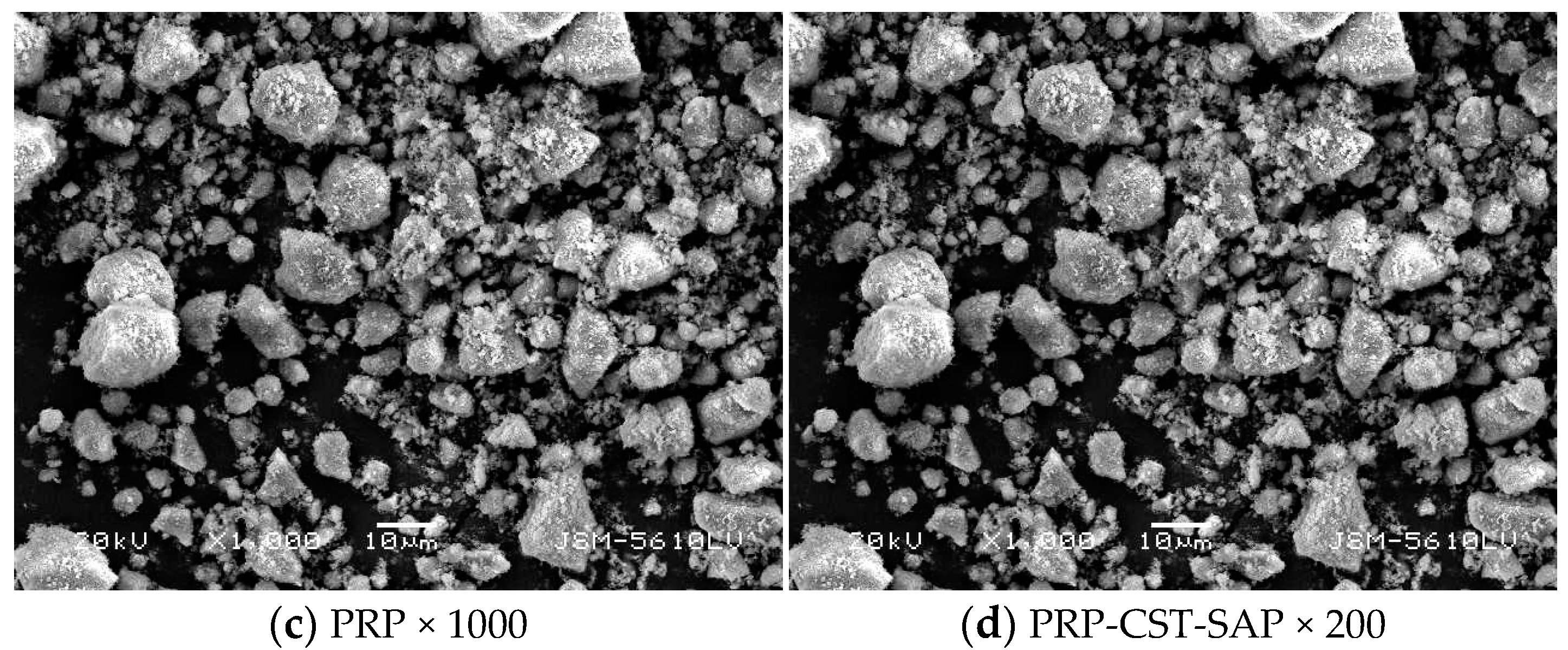
2.4.3. Morphological Studies
2.5. Index Measurement and Method
2.5.1. Water Absorbency and Water Retention Behaviors Studies
2.5.2. Phosphorus Release Behaviors Tests
2.5.3. Kinetic Model and Sugihara Kinetic Model
3. Results and Discussion
3.1. Valence Bond Structure and Morphological Characteristics
3.1.1. FTIR Spectra Analysis
3.1.2. XRD Analysis
3.1.3. SEM Analysis
3.1.4. Optical Microscope Analysis
3.2. Effects of Different Reaction Conditions on Absorption and Phosphorus Release Capacities of the CST-PRP-SAP Samples
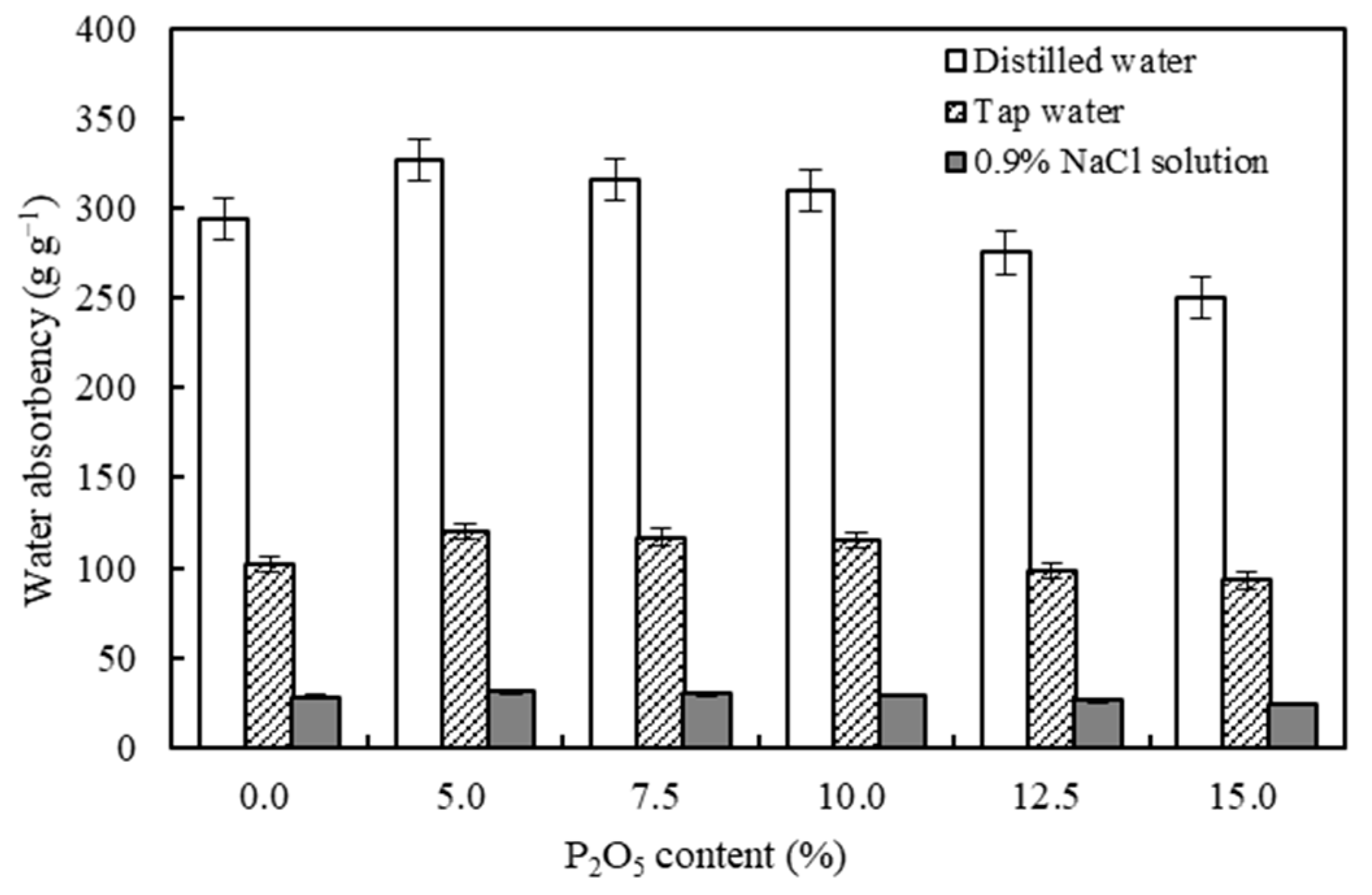
3.3. Effects of PRP Contents on the Water Absorption Capacities of the CST-PRP-SAP Samples
3.4. Water Absorption-Swelling Process of the CST-PRP-SAP Samples
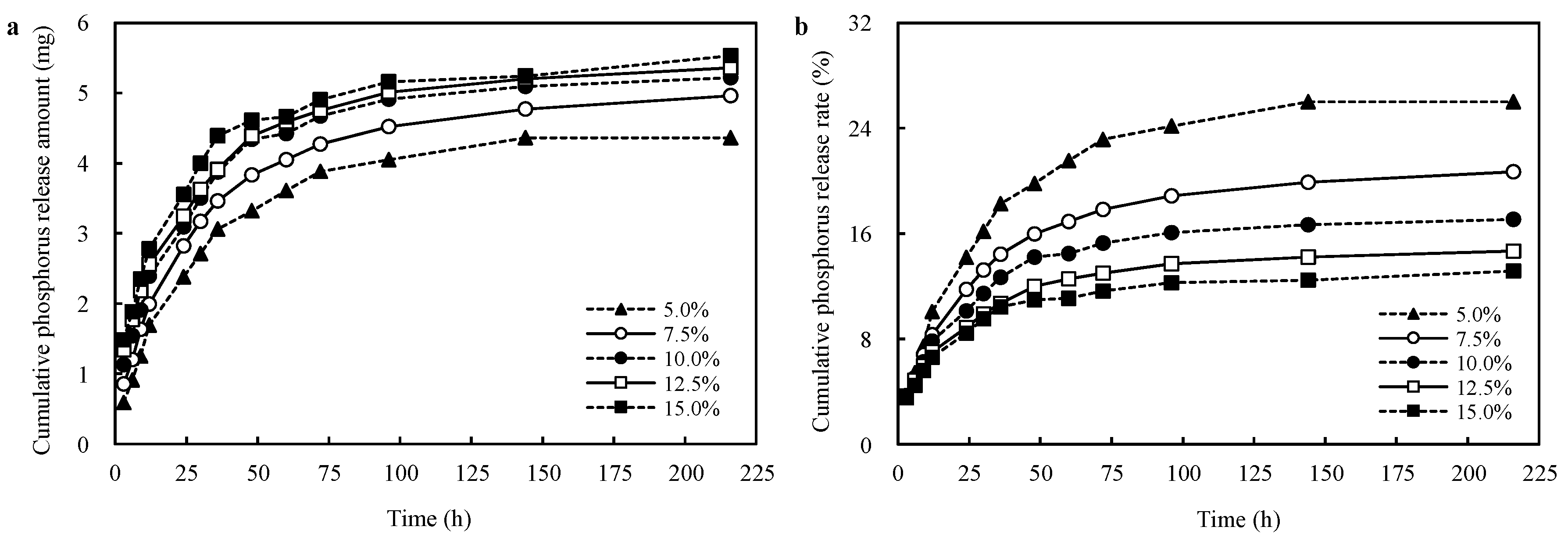
3.5. Effects of PRP Contents on the Phosphorus Release Capacity of the CST-PRP-SAP Samples
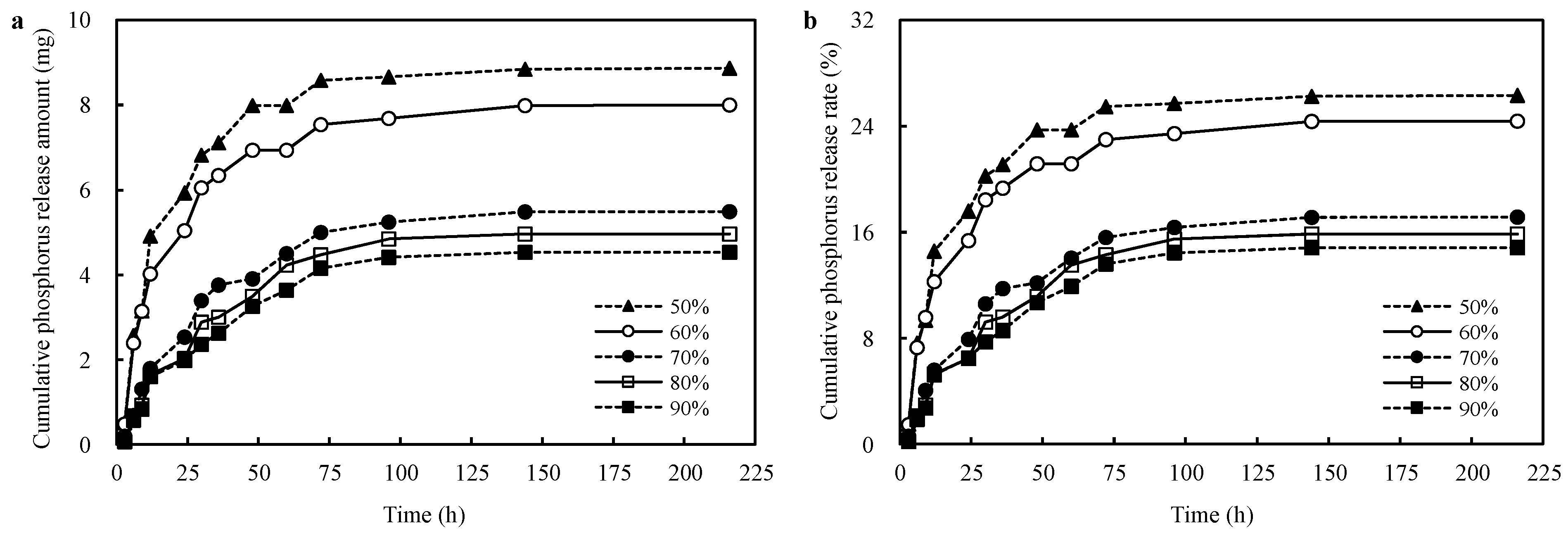
3.6. Effects of Different Neutralization Degrees on the Phosphorus Release Capacity of the CST-PRP-SAP Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ai, F.J.; Yin, X.Z.; Hu, R.C.; Ma, H.L.; Liu, W. Research into the super-absorbent polymers on agricultural water. Agric. Water Manag. 2021, 245, 106513. [Google Scholar] [CrossRef]
- Liao, R.K.; Yang, P.L.; Zhu, Y.H.; Wu, W.; Ren, S. Modeling soil water flow and quantification of root water extraction from different soil layers under multi-chemicals application in dry land field. Agric. Water Manag. 2018, 203, 75–86. [Google Scholar] [CrossRef]
- Ma, X.F.; Wen, G.H. Development history and synthesis of super-absorbent polymers: A review. J. Polym. Res. 2020, 27, 136. [Google Scholar] [CrossRef]
- Sharma, M.; Bajpai, A. Superabsorbent nanocomposite from sugarcane bagasse, chitin and clay: Synthesis, characterization and swelling behavior. Carbohydr. Polym. 2018, 193, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, S.; Eyni, H.; Bazaz, S.R.; Nazari, H.; Asl, L.S.; Zaferani, H.; Kiani, V.; Mehrizi, A.A.; Soleimain, M. Hydrogels based on cellulose and its derivatives: Applications, synthesis, and characteristics. Polym. Sci. Ser. A 2018, 60, 707–722. [Google Scholar] [CrossRef]
- Guo, J.Z.; Shi, W.J.; Li, J.K.; Zhai, Z.M. Effects of poly-γ-glutamic acid and poly-γ-glutamic acid super absorbent polymer on the sandy loam soil hydro-physical properties. PLoS ONE 2021, 16, e0245365. [Google Scholar] [CrossRef]
- Yang, Y.H.; Wu, J.C.; Zhao, S.W.; Gao, C.M.; Pan, X.Y.; Tang, D.M.S.; Vander, M.P. Effects of long-term super absorbent polymer and organic manure on soil structure and organic carbon distribution in different soil layers. Soil Tillage Res. 2021, 206, 104781. [Google Scholar] [CrossRef]
- Yang, W.; Li, P.F.; Guo, S.W.; Fan, B.Q.; Song, R.Q.; Zhang, J.S.; Yu, J. Compensating effect of fulvic acid and super-absorbent polymer on leaf gas exchange and water use efficiency of maize under moderate water deficit conditions. Plant Growth Regul. 2017, 83, 351–360. [Google Scholar] [CrossRef]
- Jahan, M.; Mahallati, M.N.; Amiri, M.B. The effect of humic acid and water super absorbent polymer application on sesame in an ecological cropping system: A new employment of structural equation modeling in agriculture. Chem. Biol. Technol. Agric. 2019, 6, 1. [Google Scholar] [CrossRef]
- Garbowski, M.; Brown, C.S.; Johnston, D.B. Soil amendment interacts with invasive grass and drought to uniquely influence aboveground versus belowground biomass in aridland restoration. Restor. Ecol. 2019, 28, A21–A23. [Google Scholar] [CrossRef]
- Liu, X.; Chan, Z.L. Application of potassium polyacrylate increases soil water status and improves growth of bermudagrass (Cynodon dactylon) under drought stress condition. Sci. Hortic. 2015, 197, 705–711. [Google Scholar] [CrossRef]
- Guo, J.Z.; Shi, W.J.; Wen, L.J.; Shi, X.X.; Li, J.K. Effects of a super-absorbent polymer derived from poly-γ-glutamic acid on water infiltration, field water capacity, soil evaporation, and soil water-stable aggregates. Arch. Agron. Soil Sci. 2020, 66, 1627–1638. [Google Scholar] [CrossRef]
- Tian, X.M.; Fan, H.; Wang, J.Q.; Ippolito, J.; Li, Y.B.; Feng, S.S.; An, M.J.; Zhang, F.H.; Wang, K.Y. Effect of polymer materials on soil structure and organic carbon under drip Irrigation. Geoderma 2019, 340, 94–103. [Google Scholar] [CrossRef]
- Li, X.; He, J.Z.; Hughes, J.M.; Liu, Y.R.; Zheng, Y.M. Effects of super-absorbent polymers on a soil-wheat (Triticum aestivum L.) system in the field. Appl. Soil Ecol. 2014, 73, 58–63. [Google Scholar] [CrossRef]
- Liang, J.P.; Shi, W.J.; He, Z.J.; Pang, L.N.; Zhang, Y.C. Effects of poly-γ-glutamic acid on water use efficiency, cotton yield, and fiber quality in the sandy soil of southern Xinjing, China. Agric. Water Manag. 2019, 218, 48–59. [Google Scholar] [CrossRef]
- Liu, X.M.; Feng, T.; Ding, W.B.; Zeng, W.; Wang, N.; Yang, F.H.; Yang, C.L.; Yang, S.H.; Kong, Y.R.; Lei, Z.Q. Synthesis of tamarind seed gum-based semi-IPN hydrogelswith integration of fertilizer retention and anti-evaporation. J. Appl. Polym. Sci. 2023, 140, e53325. [Google Scholar]
- Lan, G.H.; Zhang, M.; Liu, Y.Q.; Qiu, H.Y.; Xue, S.S.; Zhang, T.L.; Xu, Q.X. Synthesis and swelling behavior of super-absorbent soluble starch-g-poly(AM-co-NaAMC14S) through graft copolymerization and hydrolysis. Starch 2019, 71, 1800272. [Google Scholar] [CrossRef]
- Lee, J.; Park, S.; Rou, H.G.; Oh, S.; Kim, S.; Kim, M.; Kim, D.; Park, J. Preparation and characterization of superabsorbent polymers based on starch aldehydes and carboxymethyl cellulose. Polymers 2018, 10, 605. [Google Scholar] [CrossRef]
- Bai, W.B.; Fan, L.R.; Zhou, Y.; Zhang, Y.; Shi, J.Y.; Lv, G.H.; Wu, Y.F.; Liu, Q.; Song, J.Q. Removal of Cd2+ ions from aqueous solution using cassava starch-based superabsorbent polymers. J. Appl. Polym. Sci. 2017, 134, 44758. [Google Scholar] [CrossRef]
- Yadav, H.; Fatima, R.; Sharma, A.; Mathur, S. Enhancement of applicability of rock phosphate in alkaline soils by organic compost. Appl. Soil Ecol. 2017, 113, 80–85. [Google Scholar] [CrossRef]
- Yaseen, M.; Malhi, S.S. Differential growth response of wheat genotypes to ammonium phosphate and rock phosphate phosphorus source. J. Plant Nutr. 2009, 32, 410–432. [Google Scholar] [CrossRef]
- Mohammadi-Khoo, P.; Moghadam, P.N.; Fareghi, A.R.; Movagharnezhad, N. Synthesis of a cellulose-based hydrogel network: Characterization and study of urea fertilizer slow release. J. App. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Xiang, Y.; Ru, X.D.; Shi, J.G.; Song, J.; Zhao, H.D.; Liu, Y.Q.; Guo, D.D.; Lu, X. Preparation and properties of a novel semi-IPN slow-release fertilizer with the function of water retention. J. Agric. Food Chem. 2017, 65, 10851–10858. [Google Scholar] [CrossRef]
- Song, J.; Zhao, H.D.; Zhao, G.Z.; Xiang, Y.; Liu, Y.Q. Novel semi-IPN nanocomposites with functions of both nutrient slow-release and water retention, microscopic structure, water absorbency, and degradation performance. J. Agric. Food Chem. 2019, 67, 7587–7597. [Google Scholar] [CrossRef]
- Parvathy, P.C.; Jyothi, A.N. Synthesis, characterization and swelling behavior of superabsorbent polymers from cassava starch-g-poly (acrylamide). Starch 2012, 64, 207. [Google Scholar] [CrossRef]
- Wang, F.Y.; Cai, M.T.; Yan, L. Effect of poly(acrylamide-acrylic acid) on the fire resistance and anti-aging properties of transparent flame-retardant hydrogel applied in fireproof glass. Polymers 2021, 13, 3668. [Google Scholar] [CrossRef]
- Tan, T.; Zhou, J.L.; Gao, X.; Tang, X.N.; Zhang, H. Synthesis, characterization and water-absorption behavior of tartaric acid-modified cellulose gel from corn stalk pith. Ind. Crops Prod. 2021, 169, 113641. [Google Scholar] [CrossRef]
- Bai, W.B.; Song, J.Q.; Zhuang, H.Z. Repeated water absorbency of super-absorbent polymers in agricultural field applications: A simulation study. Acta Agric. Scand. Sect. B-Soil Plant Sci. 2013, 64, 433–441. [Google Scholar] [CrossRef]
- Zhao, G.Z.; Liu, Y.Q.; Tian, Y.; Sun, Y.Y.; Cao, Y. Preparation and properties of macromolecular slow-release fertilizer containing nitrogen, phosphorus and potassium. J. Polym. Res. 2010, 17, 119–125. [Google Scholar] [CrossRef]
- Franson, N.M.; Peppas, N.A. Influence of copolymer composition on non-Fickian water transport through glassy copolymers. J. Appl. Polym. Sci. 1983, 28, 1299–1310. [Google Scholar] [CrossRef]
- Kobayashi, A.; Fujisawa, E.; Hanyuu, T.A. Mechanism of nutrient release from resin-coated fertilizers and its estimation by kinetic methods, 1: Effect of water vapor pressure on nutrient release. Jpn. J. Soil Sci. Plant Nutr. 1997, 68, 8–13. [Google Scholar]
- Rafikov, A.S.; Khodjaeva, S.A.; Karimov, S.K.; Ibragimov, A.T. Molecular characteristics, mechanism of synthesis, adhesive properties of graft copolymer of chloroprene rubber with acrylic acid. Polym. Eng. Sci. 2022, 62, 2909–2921. [Google Scholar] [CrossRef]
- Sarmah, D.; Karak, N. Biodegradable superabsorbent hydrogel for water holding in soil and controlled-release fertilizer. J. Appl. Polym. Sci. 2020, 137, 48495. [Google Scholar] [CrossRef]
- Rashidzadeh, A.; Olad, A.; Reyhanitabar, A. Hydrogel/clinoptilolite nanocomposite-coated fertilizer: Swelling, water-retention and slow-release fertilizer properties. Polym. Bull. 2015, 72, 2667–2684. [Google Scholar] [CrossRef]
- Bai, W.B.; Zhang, H.Z.; Liu, B.C.; Wu, Y.F.; Song, J.Q. Effects of super-absorbent polymers on the physical and chemical properties of soil following different wetting and drying cycles. Soil Use Manag. 2010, 26, 253–260. [Google Scholar] [CrossRef]
- Ji, B.Y.; Zhao, C.P.; Wu, Y.; Han, W.; Song, J.Q.; Bai, W.B. Effects of different concentrations of super-absorbent polymers on soil structure and hydro-physical properties following continuous wetting and drying cycles. J. Integr. Agric. 2022, 21, 3368–3381. [Google Scholar] [CrossRef]
- Rashidzadeh, A.; Olad, A.; Salari, D.; Reyhanitabar, A. On the preparation and swelling properties of hydrogel nanocomposite based on Sodium alginate-g-poly (acrylic acid-co-acrylamide)/clinoptilolite and its application as slow release fertilizer. J. Polym. Res. 2014, 21, 344. [Google Scholar] [CrossRef]
- Enscore, D.J.; Hopfraberg, H.B.; Stannett, V.T. Effect of particle size on the mechanism controlling n-hexane sorption in glassy polystyrene microspheres. Polymers 1977, 18, 793–800. [Google Scholar] [CrossRef]
- Zhang, X.Z.; Zhou, R.X. Synthesis of temperature-sensitive poly (N-isopropylacrylamide) hydrogel with improved surface property 2000. J. Colloid Interface Sci. 2000, 223, 311–314. [Google Scholar] [CrossRef]
- Zhang, X.Z.; Zhou, R.X.; Yan, G.Y. Using mixed solvent to synthesize temperature sensitive poly (N-isopropylacrylamide) gel with rapid dynamics properties. Biomaterials 2002, 23, 313–318. [Google Scholar] [CrossRef]
- Li, J.Z.; Feng, Y.Y.; Huang, Z.B.; Chen, W. Interactive effects of super absorbent polymer and nitrate nitrogen. Adv. Mater. Res. 2012, 598, 323–327. [Google Scholar]
- Ni, B.L.; Liu, M.Z.; Lv, S.Y. Multifunctional slow-release urea fertilizer from methylcellulose and superabsorbent coated formulations. Chem. Eng. J. 2009, 155, 892–898. [Google Scholar] [CrossRef]


| Number | Reaction Factors a (% w/w) | Water Absorbency (g g−1) | Phosphorus Release Amount (mg) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | Distilled Water | Tap Water | 0.9% NaCl Solution | ||
| 1 | 7.5 | 0.02 | 0.4 | 60 | 10 | 204 | 76 | 23 | 5.87 |
| 2 | 7.5 | 0.04 | 0.6 | 70 | 15 | 225 | 80 | 22 | 6.04 |
| 3 | 7.5 | 0.06 | 0.8 | 80 | 20 | 128 | 71 | 21 | 5.22 |
| 4 | 7.5 | 0.08 | 1.0 | 90 | 25 | 127 | 67 | 20 | 4.41 |
| 5 | 10.0 | 0.02 | 0.6 | 80 | 25 | 275 | 102 | 27 | 5.11 |
| 6 | 10.0 | 0.04 | 0.4 | 90 | 20 | 264 | 95 | 29 | 4.94 |
| 7 | 10.0 | 0.06 | 1.0 | 60 | 15 | 186 | 70 | 22 | 6.25 |
| 8 | 10.0 | 0.08 | 0.8 | 70 | 10 | 145 | 83 | 21 | 5.98 |
| 9 | 12.5 | 0.02 | 0.8 | 90 | 15 | 204 | 86 | 22 | 5.29 |
| 10 | 12.5 | 0.04 | 1.0 | 80 | 10 | 170 | 71 | 21 | 5.80 |
| 11 | 12.5 | 0.06 | 0.4 | 70 | 25 | 121 | 55 | 18 | 5.91 |
| 12 | 12.5 | 0.08 | 0.6 | 60 | 20 | 131 | 51 | 17 | 6.76 |
| 13 | 15.0 | 0.02 | 1.0 | 70 | 20 | 160 | 67 | 18 | 6.18 |
| 14 | 15.0 | 0.04 | 0.8 | 60 | 25 | 126 | 46 | 16 | 6.03 |
| 15 | 15.0 | 0.06 | 0.6 | 90 | 10 | 174 | 84 | 20 | 5.51 |
| 16 | 15.0 | 0.08 | 0.4 | 80 | 15 | 135 | 61 | 17 | 6.08 |
| Mean 1 | 171.00 | 210.75 | 181.00 | 161.75 | 173.25 | Water absorbency in distilled water (g g−1) | |||
| Mean 2 | 217.50 | 196.25 | 201.25 | 172.75 | 187.50 | ||||
| Mean 3 | 156.50 | 152.25 | 150.75 | 177.00 | 170.75 | ||||
| Mean 4 | 148.75 | 134.50 | 160.75 | 192.25 | 162.25 | ||||
| Range | 68.75 | 76.25 | 50.50 | 30.50 | 25.25 | ||||
| Primary and secondary factors | B > A > C > D > E | ||||||||
| Optimal level | A2B1C2D4E2 | ||||||||
| Mean 1 | 73.50 | 82.75 | 71.75 | 60.75 | 78.50 | Water absorbency in tap water (g g−1) | |||
| Mean 2 | 87.50 | 73.00 | 79.25 | 71.25 | 74.25 | ||||
| Mean 3 | 65.75 | 70.00 | 71.50 | 76.25 | 71.00 | ||||
| Mean 4 | 64.50 | 65.50 | 68.75 | 83.00 | 67.50 | ||||
| Range | 23.00 | 17.25 | 10.50 | 22.25 | 11.00 | ||||
| Primary and secondary factors | A > D > B > E > C | ||||||||
| Optimal level | A2B1C2D4E1 | ||||||||
| Mean 1 | 21.50 | 22.50 | 21.75 | 19.50 | 21.25 | Water absorbency in 0.9% NaCl solution (g g−1) | |||
| Mean 2 | 24.75 | 22.00 | 21.50 | 19.75 | 20.75 | ||||
| Mean 3 | 19.50 | 20.25 | 20.00 | 21.50 | 21.25 | ||||
| Mean 4 | 17.75 | 18.75 | 20.25 | 22.75 | 20.25 | ||||
| Range | 7.00 | 3.75 | 1.75 | 3.25 | 1.00 | ||||
| Primary and secondary factors | A > B > D > C > E | ||||||||
| Optimal level | A2B1C1D4E1 | ||||||||
| Mean 1 | 5.39 | 5.61 | 5.70 | 6.23 | 5.79 | Phosphorus release amount (mg) | |||
| Mean 2 | 5.57 | 5.70 | 5.86 | 6.03 | 5.92 | ||||
| Mean 3 | 5.94 | 5.72 | 5.63 | 5.55 | 5.78 | ||||
| Mean 4 | 5.95 | 5.81 | 5.66 | 5.04 | 5.37 | ||||
| Range | 0.57 | 0.20 | 0.23 | 1.19 | 0.55 | ||||
| Primary and secondary factors | D > A > E > C > B | ||||||||
| Optimal level | A4B4C2D1E2 | ||||||||
| P2O5 Content (%) | Linear Fitting Equation a | n b | Lnk c | R2 d |
|---|---|---|---|---|
| 0.0 | y = 0.823x − 5.052 | 0.823 | −5.052 | 0.970 |
| 5.0 | y = 0.806x − 4.943 | 0.806 | −4.943 | 0.972 |
| 7.5 | y = 0.770x − 4.724 | 0.770 | −4.724 | 0.972 |
| 10.0 | y = 0.760x − 4.637 | 0.760 | −4.637 | 0.966 |
| 12.5 | y = 0.729x − 4.430 | 0.729 | −4.430 | 0.961 |
| 15.0 | y = 0.675x − 4.107 | 0.675 | −4.107 | 0.961 |
| P2O5 Content (%). | a a | b b | k c | R2 d |
|---|---|---|---|---|
| 5.0 | 4.259 | 25.394 | 0.035 | 0.991 |
| 7.5 | 4.666 | 19.465 | 0.040 | 0.980 |
| 10.0 | 4.932 | 16.141 | 0.047 | 0.965 |
| 12.5 | 4.971 | 13.596 | 0.052 | 0.937 |
| 15.0 | 5.051 | 12.003 | 0.062 | 0.943 |
| Neutralization Degree (%) | a | b | k | R2 |
|---|---|---|---|---|
| 50 | 8.702 | 25.823 | 0.052 | 0.980 |
| 60 | 7.740 | 23.590 | 0.050 | 0.979 |
| 70 | 5.562 | 17.365 | 0.029 | 0.991 |
| 80 | 5.139 | 16.429 | 0.026 | 0.986 |
| 90 | 4.698 | 15.373 | 0.025 | 0.985 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, W.; Ji, B.; Fan, L.; Peng, Q.; Liu, Q.; Song, J. Preparation and Characterization of a Novel Cassava Starch-Based Phosphorus Releasing Super-Absorbent Polymer, and Optimization of the Performance of Water Absorption and Phosphorus Release. Polymers 2023, 15, 1233. https://doi.org/10.3390/polym15051233
Bai W, Ji B, Fan L, Peng Q, Liu Q, Song J. Preparation and Characterization of a Novel Cassava Starch-Based Phosphorus Releasing Super-Absorbent Polymer, and Optimization of the Performance of Water Absorption and Phosphorus Release. Polymers. 2023; 15(5):1233. https://doi.org/10.3390/polym15051233
Chicago/Turabian StyleBai, Wenbo, Bingyi Ji, Liren Fan, Qin Peng, Qi Liu, and Jiqing Song. 2023. "Preparation and Characterization of a Novel Cassava Starch-Based Phosphorus Releasing Super-Absorbent Polymer, and Optimization of the Performance of Water Absorption and Phosphorus Release" Polymers 15, no. 5: 1233. https://doi.org/10.3390/polym15051233
APA StyleBai, W., Ji, B., Fan, L., Peng, Q., Liu, Q., & Song, J. (2023). Preparation and Characterization of a Novel Cassava Starch-Based Phosphorus Releasing Super-Absorbent Polymer, and Optimization of the Performance of Water Absorption and Phosphorus Release. Polymers, 15(5), 1233. https://doi.org/10.3390/polym15051233







