Development and Characterization of Drug Loaded PVA/PCL Fibres for Wound Dressing Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
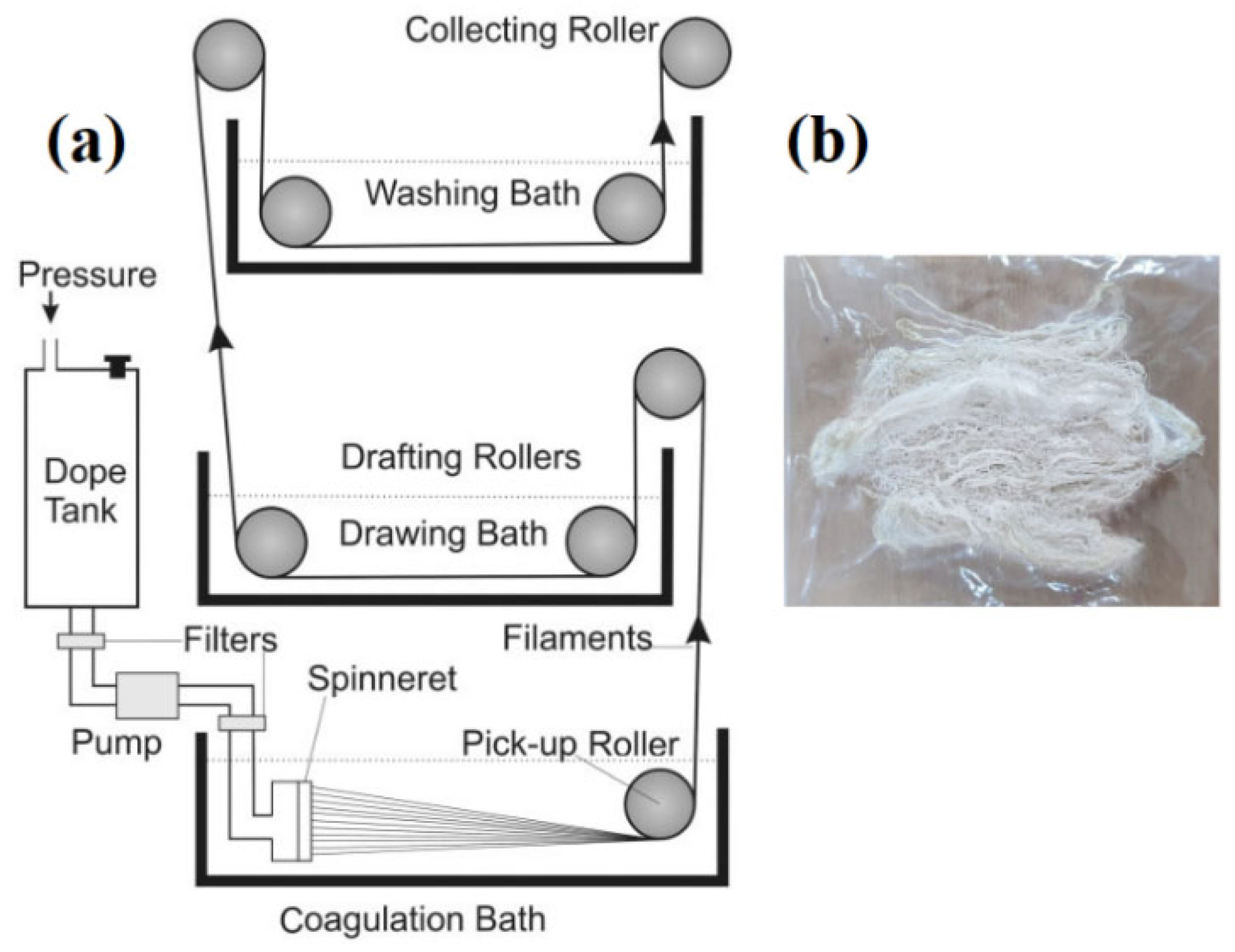
Dope Solution Preparation and Fibre Development
2.3. Characterization
2.3.1. Linear Density
2.3.2. Tensile Properties
2.3.3. Liquid Absorption
2.3.4. Swelling Behaviour
2.3.5. Degradation Test
2.3.6. FTIR
2.3.7. Scanning Electron Microscopy (SEM)
2.3.8. Antimicrobial Efficiency
2.3.9. In Vitro Drug Release
3. Results and Discussion
3.1. Optimization of the Wet Spinning Process
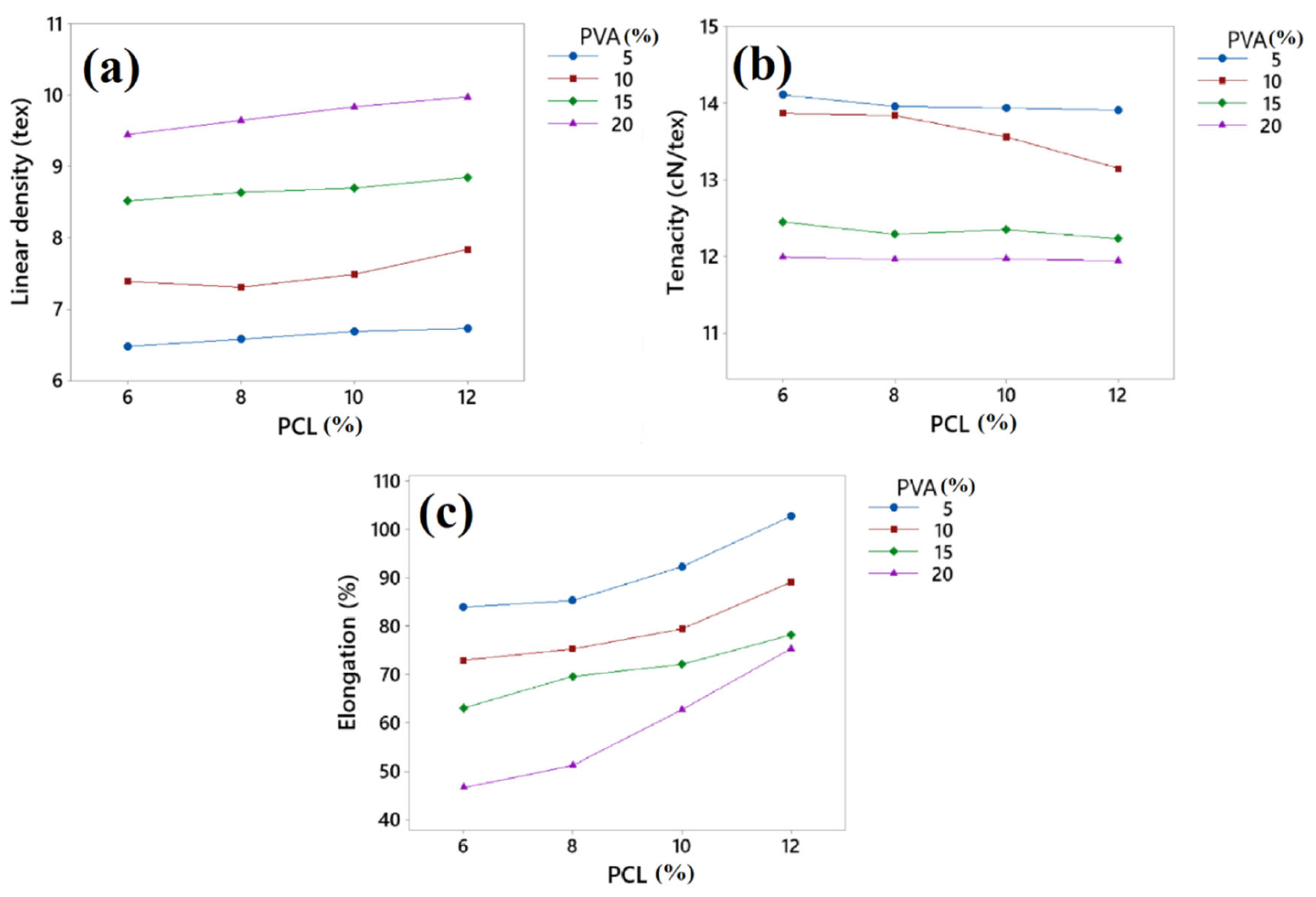
3.2. Linear Density, Tenacity, and Elongation
3.3. Liquid Absorption (g/g)
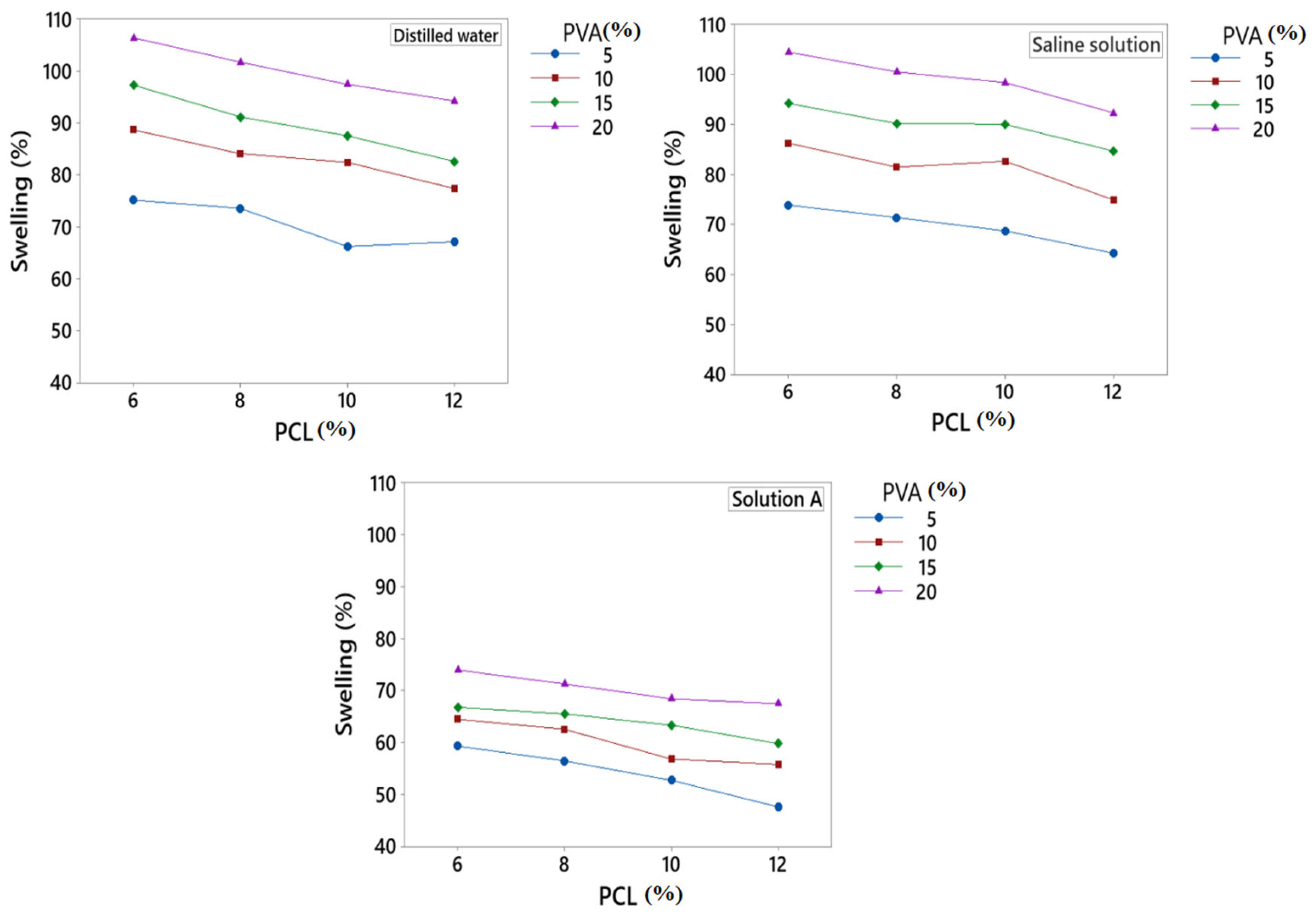
3.4. Swelling %
3.5. Degradation Test
3.6. FTIR
3.7. Scanning Electron Microscopy
3.8. Antimicrobial Activity
3.9. In Vitro Drug Release
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wan, W.; Cai, F.; Huang, J.; Chen, S.; Liao, Q. A skin-inspired 3D bilayer scaffold enhances granulation tissue formation and anti-infection for diabetic wound healing. J. Mater. Chem. B 2019, 7, 2954–2961. [Google Scholar] [CrossRef]
- Wojtowicz, A.M.; Oliveira, S.; Carlson, M.W.; Zawadzka, A.; Rousseau, C.F.; Baksh, D. The importance of both fibroblasts and keratinocytes in a bilayered living cellular construct used in wound healing. Wound Repair Regen. 2014, 22, 246–255. [Google Scholar] [CrossRef]
- Noor, A.; Afzal, A.; Masood, R.; Khaliq, Z.; Ahmad, S.; Ahmad, F.; Qadir, M.-B.; Irfan, M. Dressings for burn wound: A review. J. Mater. Sci. 2022, 57, 6536–6572. [Google Scholar] [CrossRef]
- Sen, C.K.; Gordillo, G.M.; Roy, S.; Kirsner, R.; Lambert, L.; Hunt, T.K.; Gottrup, F.; Gurtner, G.C.; Longaker, M.T. Human skin wounds: A major and snowballing threat to public health and the economy. Wound Repair Regen. 2009, 17, 763–771. [Google Scholar] [CrossRef]
- Abdel-Sayed, P.; Tornay, D.; Hirt-Burri, N.; de Buys Roessingh, A.; Raffoul, W.; Applegate, L.A. Implications of chlorhexidine use in burn units for wound healing. Burns 2020, 46, 1150–1156. [Google Scholar] [CrossRef] [PubMed]
- Afzal, A.; Azam, F.; Ahmad, S.; Khaliq, Z.; Shahzad, A.; Qadir, M.B.; Hai, A.M. Development and characterization of biodegradable starch-based fibre by wet extrusion. Cellulose 2021, 28, 2039–2051. [Google Scholar] [CrossRef]
- Peck, M.D. Epidemiology of burns throughout the world. Part I: Distribution and risk factors. Burns 2011, 37, 1087–1100. [Google Scholar] [CrossRef]
- Hu, S.; Hui, Z.; Lirussi, F.; Garrido, C.; Ye, X.-Y.; Xie, T. Small molecule DNA-PK inhibitors as potential cancer therapy: A patent review (2010–present). Expert Opin. Ther. Pat. 2021, 31, 435–452. [Google Scholar] [CrossRef] [PubMed]
- Dhivya, S.; Padma, V.V.; Santhini, E. Wound dressings—A review. Biomedicine 2015, 5, 24–28. [Google Scholar] [CrossRef]
- Lu, J.; Chen, Y.; Ding, M.; Fan, X.; Hu, J.; Chen, Y.; Li, J.; Li, Z.; Liu, W. A 4arm-PEG macromolecule crosslinked chitosan hydrogels as antibacterial wound dressing. Carbohydr. Polym. 2022, 277, 118871. [Google Scholar] [CrossRef]
- Pan, Z.-Y.; Zhong, H.-J.; Huang, D.-N.; Wu, L.-H.; He, X.-X. Beneficial Effects of Repeated Washed Microbiota Transplantation in Children With Autism. Front. Pediatr. 2022, 10, 928785. [Google Scholar] [CrossRef] [PubMed]
- Afzal, A.; Khaliq, Z.; Ahmad, S.; Ahmad, F.; Noor, A.; Qadir, M.B. Development and characterization of biodegradable composite film. Environ. Technol. Innov. 2021, 23, 101664. [Google Scholar] [CrossRef]
- Shi, G.; Rouabhia, M.; Wang, Z.; Dao, L.H.; Zhang, Z. A novel electrically conductive and biodegradable composite made of polypyrrole nanoparticles and polylactide. Biomaterials 2004, 25, 2477–2488. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Roberge, C.; Wan, Y.; Dao, L.H.; Guidoin, R.; Zhang, Z. A biodegradable electrical bioconductor made of polypyrrole nanoparticle/poly(D,L-lactide) composite: A preliminary in vitro biostability study. J. Biomed. Mater. Res. A 2003, 66A, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Shende, P.; Gupta, H. Formulation and comparative characterization of nanoparticles of curcumin using natural, synthetic and semi-synthetic polymers for wound healing. Life Sci. 2020, 253, 117588. [Google Scholar] [CrossRef] [PubMed]
- Tahir, R.; Albargi, H.B.; Ahmad, A.; Qadir, M.B.; Khaliq, Z.; Nazir, A.; Khalid, T.; Batool, M.; Arshad, S.N.; Jalalah, M.; et al. Development of Sustainable Hydrophilic Azadirachta indica loaded PVA Nanomembranes for Cosmetic Facemask Applications. Membranes 2023, 13, 156. [Google Scholar] [CrossRef]
- Zhang, S.D.; Zhai, Y.C.; Zhang, Z.F. Study on Medical Polyvinyl Alcohol (PVA) / Polyvinyl Pyrrolidone (PVP) Hydrogel Wound Dressing. Adv. Mater. Res. 2011, 287–290, 1925–1928. [Google Scholar] [CrossRef]
- Teodorescu, M.; Bercea, M.; Morariu, S. Biomaterials of PVA and PVP in medical and pharmaceutical applications: Perspectives and challenges. Biotechnol. Adv. 2019, 37, 109–131. [Google Scholar] [CrossRef]
- Zhou, X.; Hou, C.; Chang, T.-L.; Zhang, Q.; Liang, J.F. Controlled released of drug from doubled-walled PVA hydrogel/PCL microspheres prepared by single needle electrospraying method. Colloids Surf. B 2020, 187, 110645. [Google Scholar] [CrossRef]
- Chen, Y.; Li, J.; Lu, J.; Ding, M.; Chen, Y. Synthesis and properties of Poly(vinyl alcohol) hydrogels with high strength and toughness. Polym. Test. 2022, 108, 107516. [Google Scholar] [CrossRef]
- Bourke, S.L.; Al-Khalili, M.; Briggs, T.; Michniak, B.B.; Kohn, J.; Poole-Warren, L.A. A photo-crosslinked poly(vinyl alcohol) hydrogel growth factor release vehicle for wound healing applications. AAPS PharmSci 2003, 5, 101–111. [Google Scholar] [CrossRef]
- Fadaie, M.; Mirzaei, E.; Geramizadeh, B.; Asvar, Z. Incorporation of nanofibrillated chitosan into electrospun PCL nanofibers makes scaffolds with enhanced mechanical and biological properties. Carbohydr. Polym. 2018, 199, 628–640. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.; Park, H.; Kim, M.; Lee, D. Preparation of PCL/(+)-catechin/gelatin film for wound healing using air-jet spinning. Appl. Surf. Sci. 2020, 509, 145033. [Google Scholar] [CrossRef]
- Ng, K.W.; Achuth, H.N.; Moochhala, S.; Lim, T.C.; Hutmacher, D.W. In vivo evaluation of an ultra-thin polycaprolactone film as a wound dressing. J. Biomater. Sci. Polym. Ed. 2007, 18, 925–938. [Google Scholar] [CrossRef]
- Jones, D.S.; McLaughlin, D.W.J.; McCoy, C.P.; Gorman, S.P. Physicochemical characterisation and biological evaluation of hydrogel-poly(ε-caprolactone) interpenetrating polymer networks as novel urinary biomaterials. Biomaterials 2005, 26, 1761–1770. [Google Scholar] [CrossRef]
- Dhanaraju, M.D.; Gopinath, D.; Ahmed, M.R.; Jayakumar, R.; Vamsadhara, C. Characterization of polymeric poly (epsilon-caprolactone) injectable implant delivery system for the controlled delivery of contraceptive steroids. J. Biomed. Mater. Res. Part A 2006, 76, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Nair, L.S.; Laurencin, C.T. Polymers as Biomaterials for Tissue Engineering and Controlled Drug Delivery. In Tissue Engineering I; Lee, K., Kaplan, D., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 47–90. [Google Scholar]
- Mehteroğlu, E.; Çakmen, A.B.; Aksoy, B.; Balcıoğlu, S.; Köytepe, S.; Ateş, B.; Yılmaz, İ. Preparation of hybrid PU/PCL fibers from steviol glycosides via electrospinning as a potential wound dressing materials. J. Appl. Polym. Sci. 2020, 137, 49217. [Google Scholar] [CrossRef]
- Cipitria, A.; Skelton, A.; Dargaville, T.R.; Dalton, P.D.; Hutmacher, D.W. Design, fabrication and characterization of PCL electrospun scaffolds—A review. J. Mater. Chem. 2011, 21, 9419–9453. [Google Scholar] [CrossRef]
- Borzacchiello, A.; Gloria, A.; Mayol, L.; Dickinson, S.; Miot, S.; Martin, I.; Ambrosio, L. Natural/synthetic porous scaffold designs and properties for fibro-cartilaginous tissue engineering. J. Bioact. Compat. Polym. 2011, 26, 437–451. [Google Scholar] [CrossRef]
- Chiari, C.; Koller, U.; Dorotka, R.; Eder, C.; Plasenzotti, R.; Lang, S.; Ambrosio, L.; Tognana, E.; Kon, E.; Salter, D.; et al. A tissue engineering approach to meniscus regeneration in a sheep model. Osteoarthr. Cartil. 2006, 14, 1056–1065. [Google Scholar] [CrossRef]
- Sinha, V.R.; Bansal, K.; Kaushik, R.; Kumria, R.; Trehan, A. Poly-ϵ-caprolactone microspheres and nanospheres: An overview. Int. J. Pharm. 2004, 278, 1–23. [Google Scholar] [CrossRef]
- Peng, Y.; Shi, C.; Zhu, Y.; Gu, M.; Zhuang, S. Terahertz spectroscopy in biomedical field: A review on signal-to-noise ratio improvement. PhotoniX 2020, 1, 12. [Google Scholar] [CrossRef]
- Wan, Y.; Cao, X.; Zhang, S.; Wang, S.; Wu, Q. Fibrous poly(chitosan-g-dl-lactic acid) scaffolds prepared via electro-wet-spinning. Acta Biomater. 2008, 4, 876–886. [Google Scholar] [CrossRef]
- Javaid, A.; Jalalah, M.; Safdar, R.; Khaliq, Z.; Qadir, M.B.; Zulfiqar, S.; Ahmad, A.; Satti, A.N.; Ali, A.; Faisal, M.; et al. Ginger Loaded Polyethylene Oxide Electrospun Nanomembrane: Rheological and Antimicrobial Attributes. Membranes 2022, 12, 1148. [Google Scholar] [CrossRef] [PubMed]
- Puppi, D.; Chiellini, F. Wet-spinning of biomedical polymers: From single-fibre production to additive manufacturing of three-dimensional scaffolds. Polym. Int. 2017, 66, 1690–1696. [Google Scholar] [CrossRef]
- Zheng, H.; Du, Y.; Yu, J.; Huang, R.; Zhang, L. Preparation and characterization of chitosan/poly(vinyl alcohol) blend fibers. J. Appl. Polym. Sci. 2001, 80, 2558–2565. [Google Scholar] [CrossRef]
- Puppi, D.; Piras, A.M.; Chiellini, F.; Chiellini, E.; Martins, A.; Leonor, I.B.; Neves, N.; Reis, R. Optimized electro- and wet-spinning techniques for the production of polymeric fibrous scaffolds loaded with bisphosphonate and hydroxyapatite. J. Tissue Eng. Regen. Med. 2011, 5, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Miraftab, M.; Barnabas, J.; Kennedy, J.F.; Masood, R. Antimicrobial Properties of Alginate-Chitosan (Alchite) Fibers Developed for Wound Care Applications. J. Ind. Text. 2010, 40, 345–360. [Google Scholar] [CrossRef]
- Hussain, T.; Masood, R.; Umar, M.; Areeb, T.; Ullah, A. Development and characterization of alginate-chitosan-hyaluronic acid (ACH) composite fibers for medical applications. Fiber. Polym. 2016, 17, 1749–1756. [Google Scholar] [CrossRef]
- Ullah, A.; Ullah, S.; Areeb, T.; Umar, M.; Phan, D.-N.; Masood, R.; Park, S.; Kim, I.-S. An Experimental Study on Modelling the Physical Properties of Composite Psyllium, Alginate and Chitosan Fibers Using Box-Behnken Technique. Fiber. Polym. 2020, 21, 2494–2504. [Google Scholar] [CrossRef]
- Masood, R.; Hussain, T.; Miraftab, M.; Ali Raza, Z.; Ullah, A.; Areeb, T.; Umar, M.; Riaz, R. Development of tri-component antibacterial hybrid fibres for potential use in wound care. J. Wound Care 2018, 27, 394–402. [Google Scholar] [CrossRef]
- Masood, R.; Miraftab, M.; Hussain, T.; Edward-Jones, V. Development of slow release silver-containing biomaterial for wound care applications. J. Ind. Text. 2013, 44, 699–708. [Google Scholar] [CrossRef]
- Zhang, S.; Li, F.-X.; Yu, J.-Y. Preparation of cellulose/chitin blend bio-fibers via direct dissolution. Cellul. Chem. Technol. 2009, 43, 393–398. [Google Scholar]
- Gupta, B.; Revagade, N.; Hilborn, J. Poly(lactic acid) fiber: An overview. Prog. Polym. Sci. 2007, 32, 455–482. [Google Scholar] [CrossRef]
- Um, I.C.; Kweon, H.; Lee, K.G.; Ihm, D.W.; Lee, J.-H.; Park, Y.H. Wet spinning of silk polymer: I. Effect of coagulation conditions on the morphological feature of filament. Int. J. Biol. Macromol. 2004, 34, 89–105. [Google Scholar] [CrossRef]
- Foroughi, J.; Spinks, G.M.; Wallace, G.G.; Whitten, P.G. Production of polypyrrole fibres by wet spinning. Synth. Met. 2008, 158, 104–107. [Google Scholar] [CrossRef]
- Hirano, S.; Zhang, M.; Nakagawa, M.; Miyata, T. Wet spun chitosan–collagen fibers, their chemical N-modifications, and blood compatibility. Biomaterials 2000, 21, 997–1003. [Google Scholar] [CrossRef]
- Meyer, M.; Baltzer, H.; Schwikal, K. Collagen fibres by thermoplastic and wet spinning. Mater. Sci. Eng. C 2010, 30, 1266–1271. [Google Scholar] [CrossRef]
- Vega-Cázarez, C.A.; López-Cervantes, J.; Sánchez-Machado, D.I.; Madera-Santana, T.J.; Soto-Cota, A.; Ramírez-Wong, B. Preparation and Properties of Chitosan–PVA Fibers Produced by Wet Spinning. J. Polym. Env. 2018, 26, 946–958. [Google Scholar] [CrossRef]
- DeMerlis, C.C.; Schoneker, D.R. Review of the oral toxicity of polyvinyl alcohol (PVA). Food Chem. Toxicol. 2003, 41, 319–326. [Google Scholar] [CrossRef]
- Zhang, C.; Li, J.; Xiao, M.; Wang, D.; Qu, Y.; Zou, L.; Zheng, C.; Zhang, J. Oral colon-targeted mucoadhesive micelles with enzyme-responsive controlled release of curcumin for ulcerative colitis therapy. Chin. Chem. Lett. 2022, 33, 4924–4929. [Google Scholar] [CrossRef]
- Mohamady Hussein, M.A.; Guler, E.; Rayaman, E.; Cam, M.E.; Sahin, A.; Grinholc, M.; Sezgin Mansuroglu, D.; Sahin, Y.M.; Gunduz, O.; Muhammed, M.; et al. Dual-drug delivery of Ag-chitosan nanoparticles and phenytoin via core-shell PVA/PCL electrospun nanofibers. Carbohydr. Polym. 2021, 270, 118373. [Google Scholar] [CrossRef]
- Ren, W.; Yu, X.; Chen, L.; Shi, T.; Bou-Akl, T.; Markel, D.C. Osteoblastic differentiation and bactericidal activity are enhanced by erythromycin released from PCL/PLGA-PVA coaxial nanofibers. J. Biomater. Appl. 2022, 37, 712–723. [Google Scholar] [CrossRef]
- Lan, X.; Liu, Y.; Wang, Y.; Tian, F.; Miao, X.; Wang, H.; Tang, Y. Coaxial electrospun PVA/PCL nanofibers with dual release of tea polyphenols and ε-poly (L-lysine) as antioxidant and antibacterial wound dressing materials. Int. J. Pharm. 2021, 601, 120525. [Google Scholar] [CrossRef] [PubMed]
- Jin, K.; Huang, X.; Zhou, J.; Li, Y.; Yan, Y.; Sun, Y.; Zhang, Q.; Wang, Y.; Ye, J. FIVES: A Fundus Image Dataset for Artificial Intelligence based Vessel Segmentation. Sci. Data 2022, 9, 475. [Google Scholar] [CrossRef]
- Yang, R.; Hou, E.; Cheng, W.; Yan, X.; Zhang, T.; Li, S.; Yao, H.; Liu, J.; Guo, Y. Membrane-Targeting Neolignan-Antimicrobial Peptide Mimic Conjugates to Combat Methicillin-Resistant Staphylococcus aureus (MRSA) Infections. J. Med. Chem. 2022, 65, 16879–16892. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhang, H.; Lirussi, F.; Garrido, C.; Ye, X.-Y.; Xie, T. Dual inhibitors of histone deacetylases and other cancer-related targets: A pharmacological perspective. Biochem. Pharmacol. 2020, 182, 114224. [Google Scholar] [CrossRef]
- Klicova, M.; Klapstova, A.; Chvojka, J.; Koprivova, B.; Jencova, V.; Horakova, J. Novel double-layered planar scaffold combining electrospun PCL fibers and PVA hydrogels with high shape integrity and water stability. Mater. Lett. 2020, 263, 127281. [Google Scholar] [CrossRef]
- Sweeney, I.R.; Miraftab, M.; Collyer, G. Absorbent alginate fibres modified with hydrolysed chitosan for wound care dressings–II. Pilot scale development. Carbohydr. Polym. 2014, 102, 920–927. [Google Scholar] [CrossRef]
- Masood, R.; Miraftab, M. Novel materials for moist wound management: Alginate-psyllium hybrid fibres. J. Wound Care 2014, 23, 153–159. [Google Scholar] [CrossRef]
- Xu, Y.; Huang, C.; Li, L.; Yu, X.; Wang, X.; Peng, H.; Gu, Z.; Wang, Y. In vitro enzymatic degradation of a biological tissue fixed by alginate dialdehyde. Carbohydr. Polym. 2013, 95, 148–154. [Google Scholar] [CrossRef]
- Chee, W.K.; Ibrahim, N.A.; Zainuddin, N.; Abd Rahman, M.F.; Chieng, B.W. Impact Toughness and Ductility Enhancement of Biodegradable Poly(lactic acid)/Poly(ε-caprolactone) Blends via Addition of Glycidyl Methacrylate. Adv. Mater. Sci. Eng. 2013, 2013, 976373. [Google Scholar] [CrossRef]
- Abdullah, Z.W.; Dong, Y.; Davies, I.J.; Barbhuiya, S. PVA, PVA Blends, and Their Nanocomposites for Biodegradable Packaging Application. Polym. Plast. Technol. Eng. 2017, 56, 1307–1344. [Google Scholar] [CrossRef]
- Krewulak, K.D.; Vogel, H.J. Structural biology of bacterial iron uptake. Biochim. Biophys. Acta (BBA)-Biomembr. 2008, 1778, 1781–1804. [Google Scholar] [CrossRef] [PubMed]
- Herget, S.; Toukach, P.V.; Ranzinger, R.; Hull, W.E.; Knirel, Y.A.; von der Lieth, C.-W. Statistical analysis of the Bacterial Carbohydrate Structure Data Base (BCSDB): Characteristics and diversity of bacterial carbohydrates in comparison with mammalian glycans. BMC Struct. Biol. 2008, 8, 35. [Google Scholar] [CrossRef]








| Sr. No. | PVA Concentration % (w/v) | PCL Concentration % (w/v) | Sample Code |
|---|---|---|---|
| 1 | 5 | 6 | F1 |
| 2 | 8 | F2 | |
| 3 | 10 | F3 | |
| 4 | 12 | F4 | |
| 5 | 10 | 6 | F5 |
| 6 | 8 | F6 | |
| 7 | 10 | F7 | |
| 8 | 12 | F8 | |
| 9 | 15 | 6 | F9 |
| 10 | 8 | F10 | |
| 11 | 10 | F11 | |
| 12 | 12 | F12 | |
| 13 | 20 | 6 | F13 |
| 14 | 8 | F14 | |
| 15 | 10 | F15 | |
| 16 | 12 | F16 |
| Sr. No. | PVA/PCL Conc. % (w/v) | NaSD % (w/v) | Drug Containing Fibres |
|---|---|---|---|
| 1 | PVA 20 + PCL 6 | 1 | OF1 |
| 2 | PVA 20 + PCL 6 | 1.5 | OF2 |
| 3 | PVA 20 + PCL 6 | 2 | OF3 |
| Sr. No. | Spinning Parameters | ||
|---|---|---|---|
| Bath 1 | Bath 2 | ||
| 1 | Spinning bath | 100% Ethanol | Deionized water |
| 2 | Temperature (°C) | 4 | 25 |
| 3 | Speed of rollers (rpm) | 8 | 20 |
| 4 | Pump speed (rpm) | 5 | |
| Sample Type | PVA/PCL Conc. % (w/v) | Viscosity (Pa.s) |
|---|---|---|
| F7 | PVA10 + PCL 10 | 2.52 |
| F10 | PVA15 + PCL 8 | 8.56 |
| F11 | PVA15 + PCL 10 | 10.61 |
| F12 | PVA15 + PCL 12 | 13.99 |
| F15 | PVA20 + PCL 10 | 24.99 |
| Sample Type | Zone of Inhibition (mm) |
|---|---|
| S. aureus | |
| F13 (without drug) | Not formed |
| OF1 | 5.33 |
| OF2 | 7.57 |
| OF3 | 8.29 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Afzal, A.; Jalalah, M.; Noor, A.; Khaliq, Z.; Qadir, M.B.; Masood, R.; Nazir, A.; Ahmad, S.; Ahmad, F.; Irfan, M.; et al. Development and Characterization of Drug Loaded PVA/PCL Fibres for Wound Dressing Applications. Polymers 2023, 15, 1355. https://doi.org/10.3390/polym15061355
Afzal A, Jalalah M, Noor A, Khaliq Z, Qadir MB, Masood R, Nazir A, Ahmad S, Ahmad F, Irfan M, et al. Development and Characterization of Drug Loaded PVA/PCL Fibres for Wound Dressing Applications. Polymers. 2023; 15(6):1355. https://doi.org/10.3390/polym15061355
Chicago/Turabian StyleAfzal, Ali, Mohammed Jalalah, Abid Noor, Zubair Khaliq, Muhammad Bilal Qadir, Rashid Masood, Ahsan Nazir, Sheraz Ahmad, Faheem Ahmad, Muhammad Irfan, and et al. 2023. "Development and Characterization of Drug Loaded PVA/PCL Fibres for Wound Dressing Applications" Polymers 15, no. 6: 1355. https://doi.org/10.3390/polym15061355
APA StyleAfzal, A., Jalalah, M., Noor, A., Khaliq, Z., Qadir, M. B., Masood, R., Nazir, A., Ahmad, S., Ahmad, F., Irfan, M., Afzal, M., Faisal, M., Alsareii, S. A., & Harraz, F. A. (2023). Development and Characterization of Drug Loaded PVA/PCL Fibres for Wound Dressing Applications. Polymers, 15(6), 1355. https://doi.org/10.3390/polym15061355