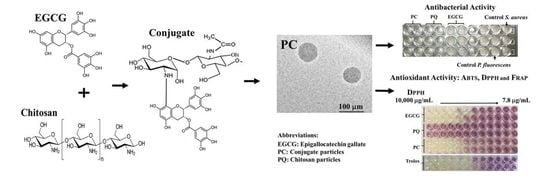
Characterization of Epigallocatechin-Gallate-Grafted Chitosan Nanoparticles and Evaluation of Their Antibacterial and Antioxidant Potential
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Bacteria Strains
2.3. Chemical Modification of Chitosan
2.4. Synthesis of Modified and Unmodified Chitosan Nanoparticles
2.5. FTIR Analysis
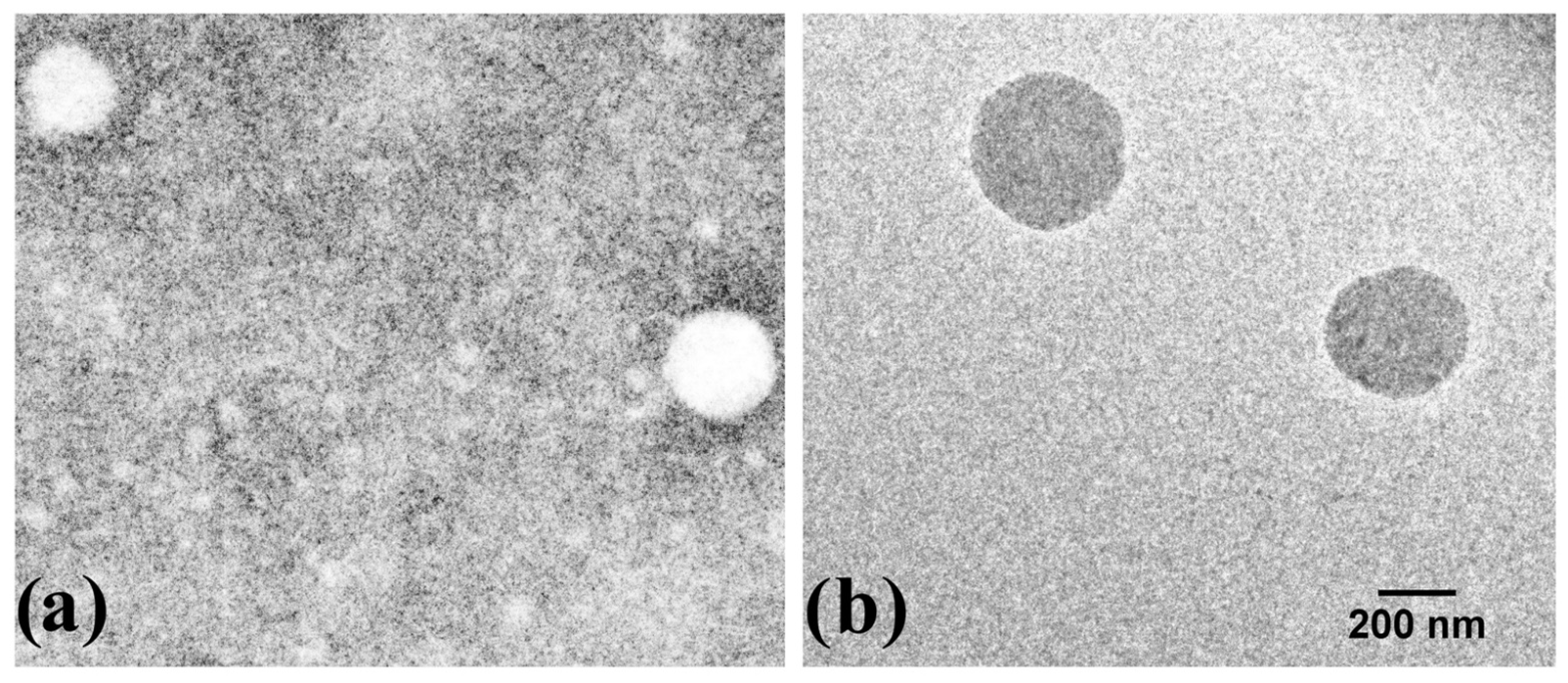
2.6. Morphological Characterization of Nanoparticles
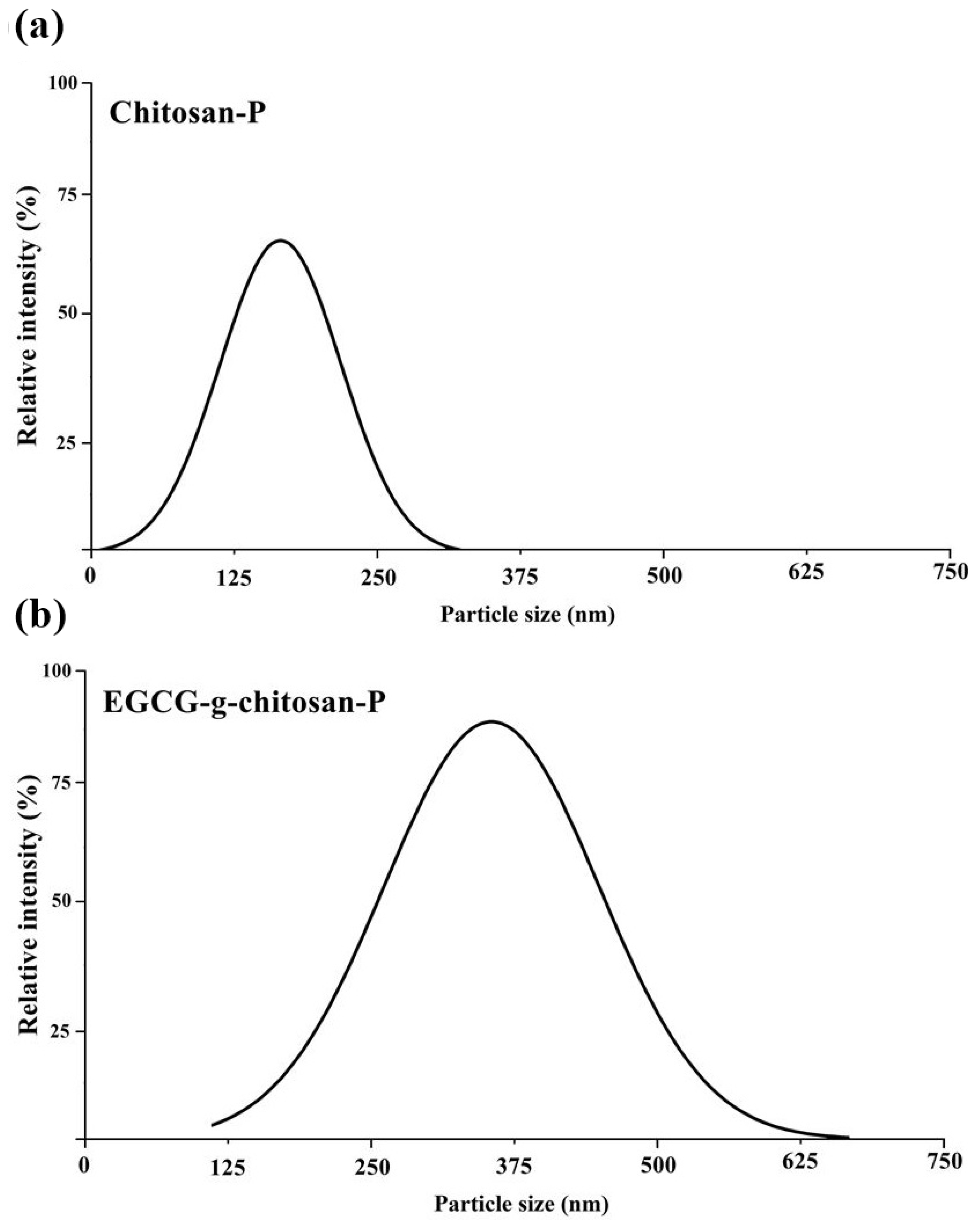
2.7. Particle Size and Polydispersity Index
2.8. Zeta Potential (ζ)
2.9. EGCG Quantification by the Folin—Ciocalteu Method
2.10. Determination of Antibacterial Activity In Vitro
2.10.1. Nanoparticles Stock Solutions
2.10.2. Bacteria and Culture Conditions
2.10.3. Bacterial Growth Inhibition (%)
2.10.4. Minimal Inhibitory Concentration (MIC) and Minimal Bactericidal Concentration (MBC)
2.10.5. Bacterial Morphometry
2.10.6. Determination of ROS
2.11. In Vitro Determination of the Antioxidant Activity of EGCG-grafted-Chitosan
2.11.1. ABTS Radical Scavenging Assay
2.11.2. DPPH Radical Scavenging Assay
2.11.3. FRAP Assay
2.12. Statistical Analysis
3. Results and Discussion
3.1. FTIR Analysis
3.2. Morphological Characterization of Nanoparticles
3.3. Particle Size and Polydispersity Index
3.4. Zeta Potential (ζ)
3.5. EGCG Content in the Nanoparticles
3.6. In Vitro Determination of Antibacterial Activity of Nanoparticles
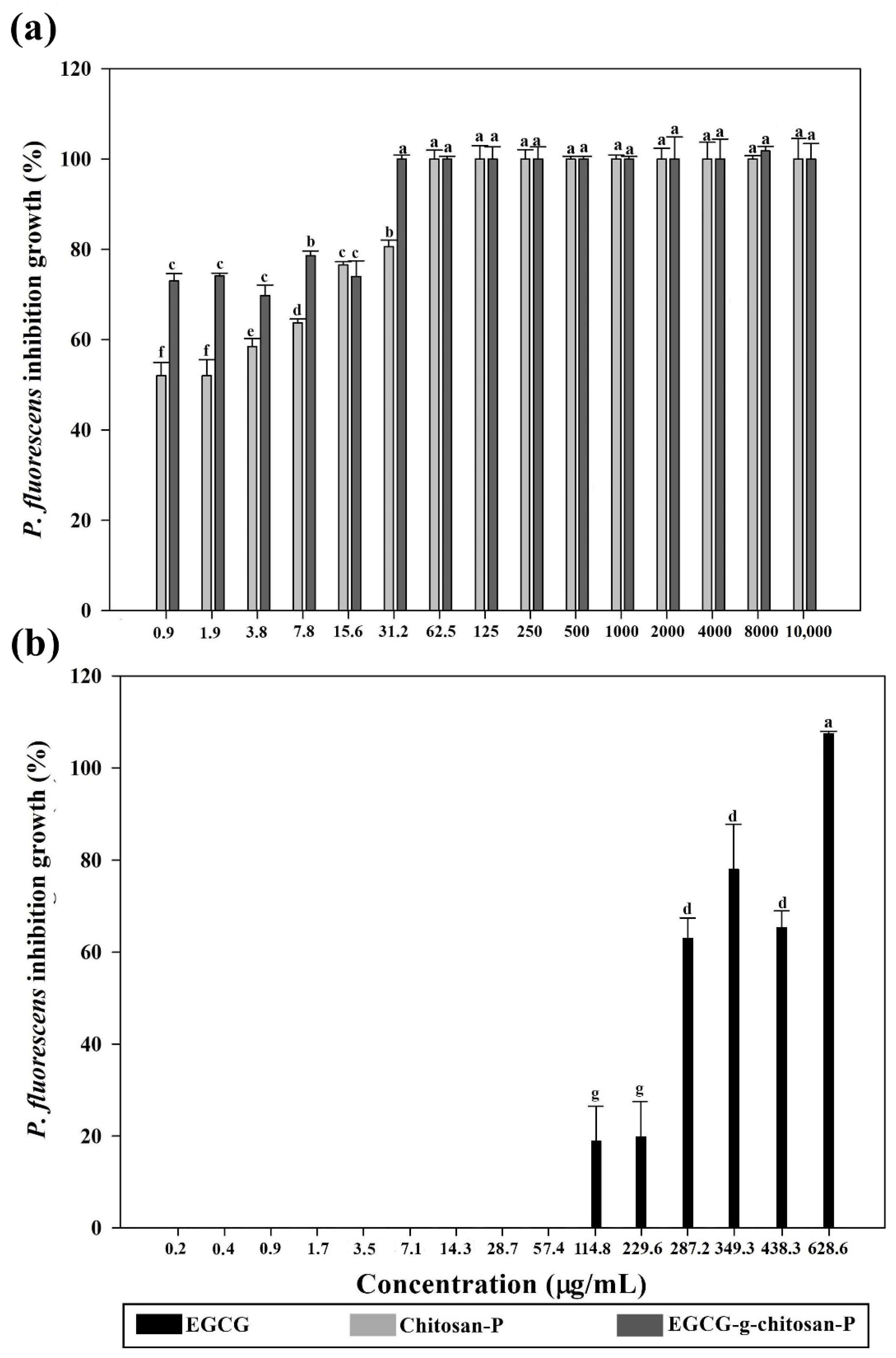
3.6.1. Bacterial Growth Inhibition (%)
3.6.2. MICs and MBCs
3.6.3. Bacterial Morphometry
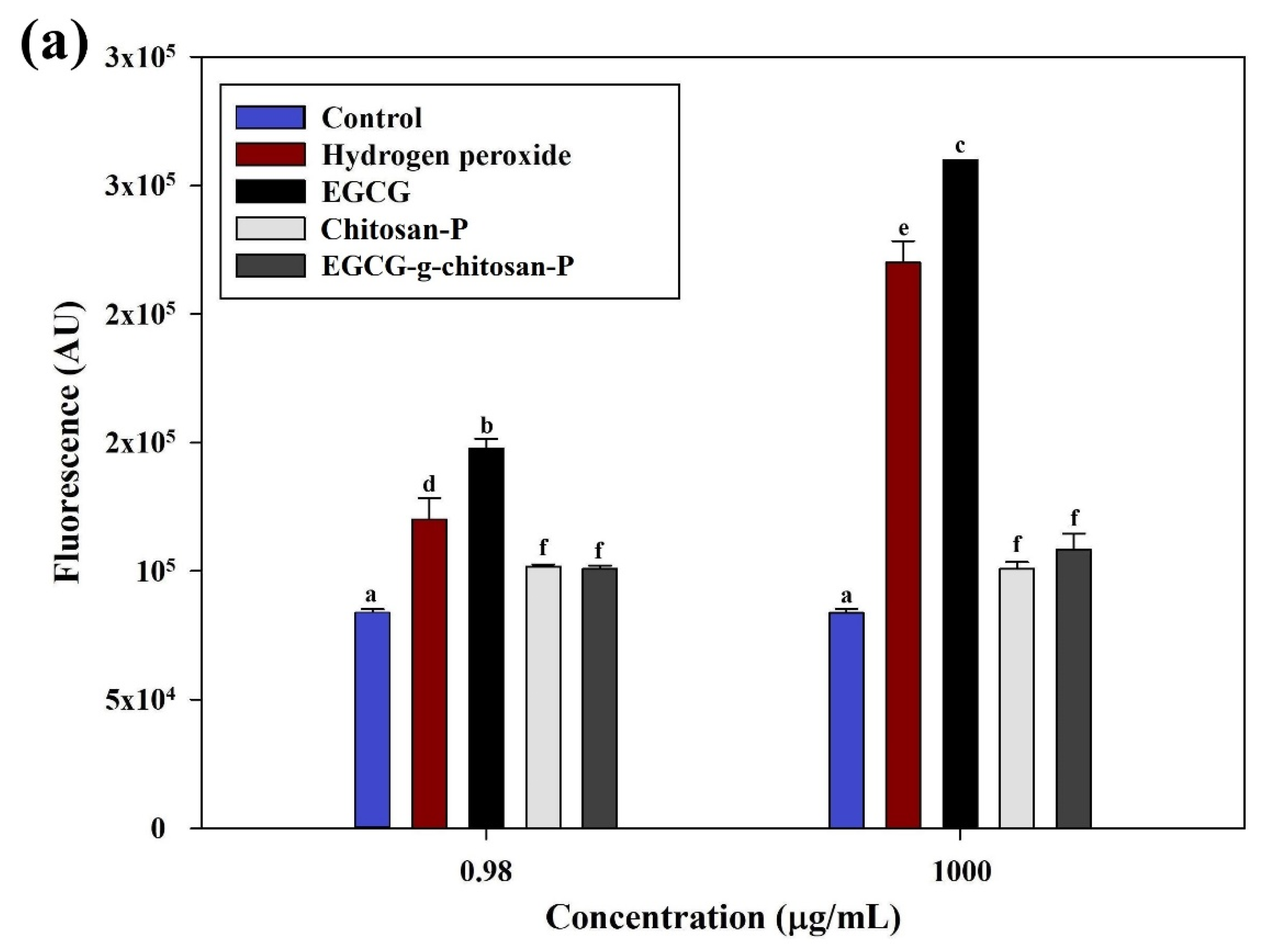
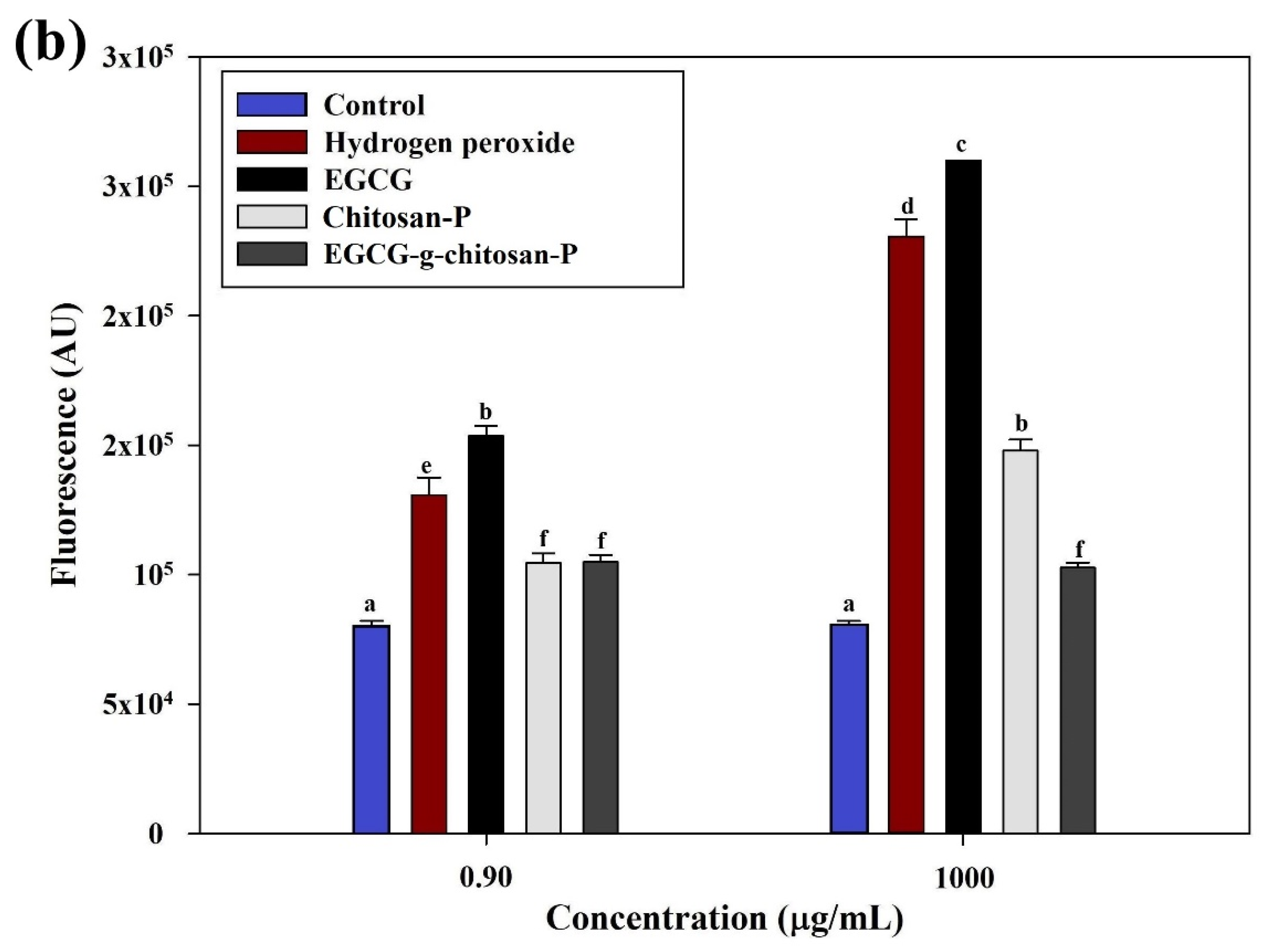
3.6.4. Reactive Oxygen Species (ROS)
3.7. In Vitro Determination of the Antioxidant Activity of EGCG-g-Chitosan-P
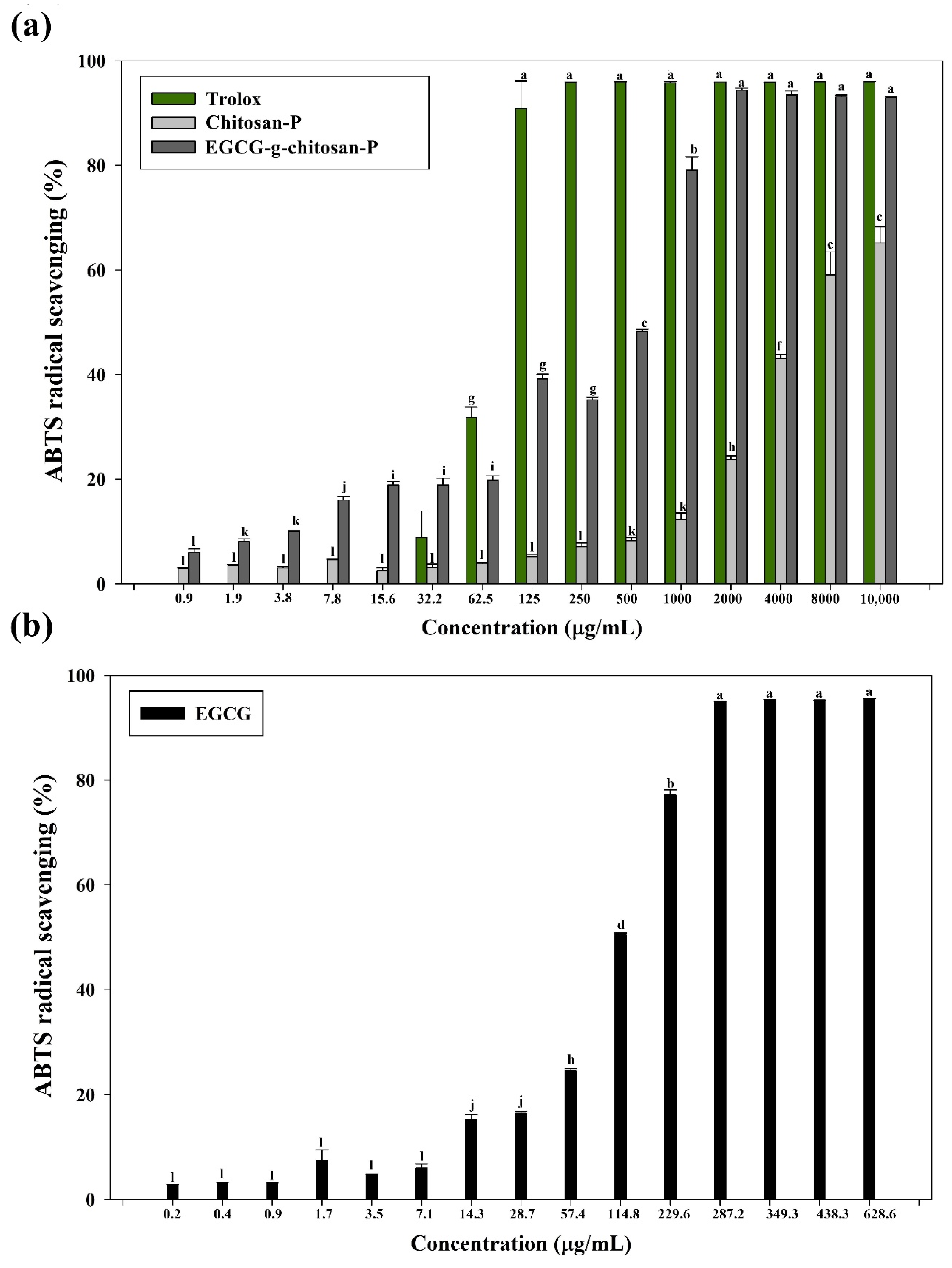
3.7.1. ABTS Radical Scavenging Assay
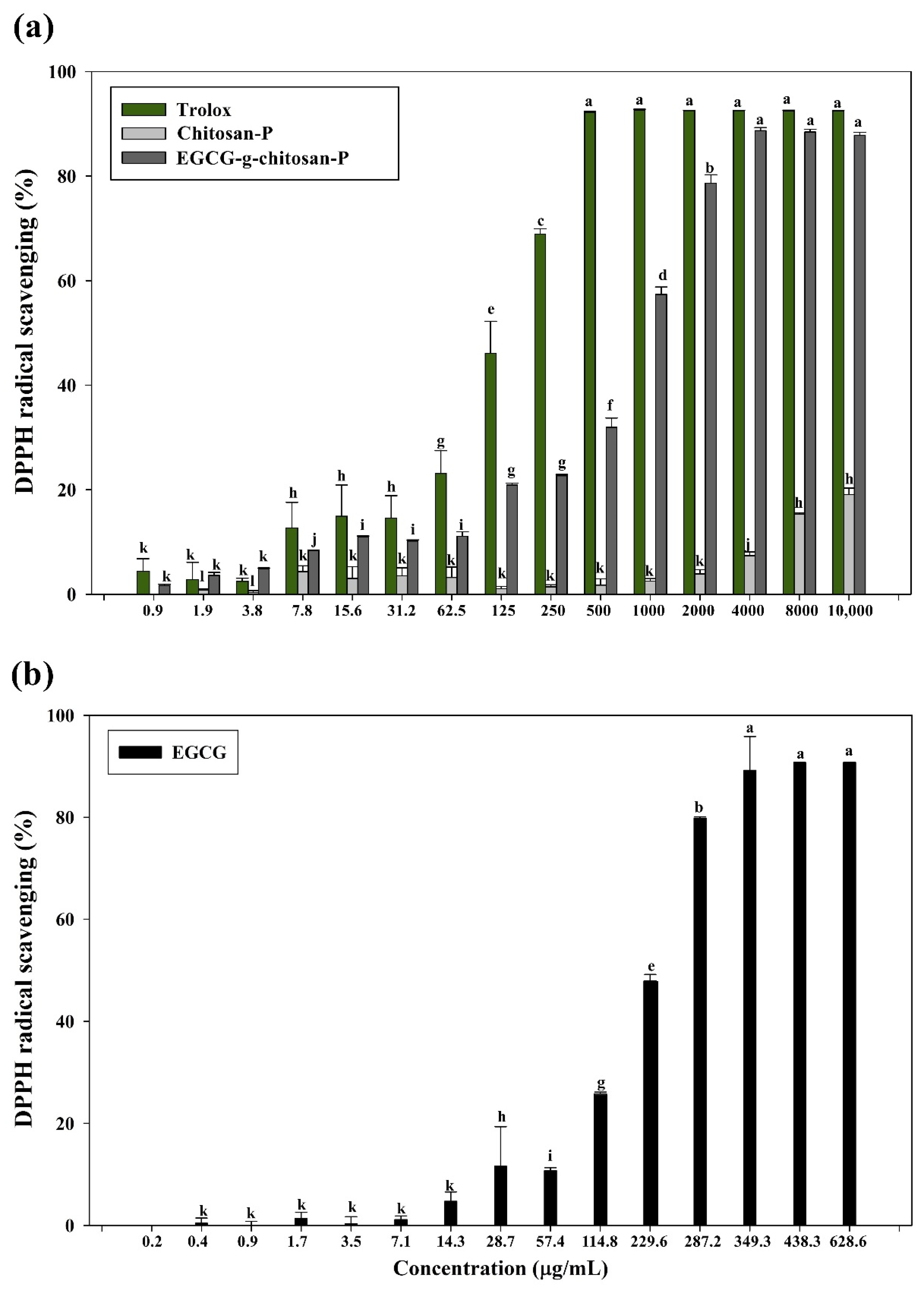
3.7.2. DPPH Radical Scavenging Assay
3.7.3. Ferric-Reducing Antioxidant Power (FRAP) Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lipinsk, B.; Hanson, C.; Lomax, J.; Kitinoja, L.; Waite, R.; Searchinger, T. Reducing Food Loss and Waste; Working Paper, Installment 2 of Creating a Sustainable Food Future; World Resources Institute: Washington, DC, USA, 2013; Available online: http://www.worldresourcesreport.org (accessed on 11 February 2020).
- Singh, T.K.; Drake, M.A.; Cadwallader, K.R. Flavor of Cheddar cheese: A chemical and sensory perspective. Compr. Rev. Food Sci. Food Saf. 2003, 2, 166–189. [Google Scholar] [CrossRef]
- Tajkarimi, M.M.; Ibrahim, S.A.; Cliver, D.O. Antimicrobial herb and spice compounds in food. Food Control 2010, 21, 1199–1218. [Google Scholar] [CrossRef]
- Nakayama, M.; Shimatani, K.; Ozawa, T.; Shigemune, N.; Tsugukuni, T.; Tomiyama, D.; Nonaka, A.; Miyamoto, T. A study of the antibacterial mechanism of catechins: Isolation and identification of Escherichia coli cell surface proteins that interact with epigallocatechin gallate. Food Control 2013, 33, 433–439. [Google Scholar] [CrossRef]
- Zhang, J.; Rui, X.; Wang, L.; Guan, Y.; Sun, X.; Dong, M. Polyphenolic extract from Rosa rugosa tea inhibits bacterial quorum sensing and biofilm formation. Food Control 2014, 42, 125–131. [Google Scholar] [CrossRef]
- Hu, J.; Du, X.; Huang, C.; Fu, D.; Ouyang, X.; Wang, Y. Antibacterial and physical properties of EGCG-containing glass ionomer cements. J. Dent. 2013, 41, 927–934. [Google Scholar] [CrossRef] [PubMed]
- Novy, P.; Rondevaldova, J.; Kourimska, L.; Kokoska, L. Synergistic interactions of epigallocatechin gallate and oxytetracycline against various drug resistant Staphylococcus aureus strains in vitro. Phytomedicine 2013, 20, 432–435. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M. Overview of antibacterial, antitoxin, antiviral, and antifungal activities of tea flavonoids and teas. Mol. Nutr. Food Res. 2007, 51, 116–134. [Google Scholar] [CrossRef] [PubMed]
- Yanagawa, Y.; Yamamoto, Y.; Hara, Y.; Shimamura, T. A combination effect of epigallocatechin gallate, a major compound of green tea catechins, with antibiotics on Helicobacter pylori growth in vitro. Curr. Microbiol. 2003, 47, 0244–0249. [Google Scholar]
- Maung, A.T.; Sato, J.; Sonoda, T.; Masuda, Y.; Honjoh, K.; Miyamoto, T. Mechanism for antibacterial action of epigallocatechin gallate and theaflavin-3,3′-digallate on Clostridium perfringens. J. Appl. 2018, 126, 633–640. [Google Scholar]
- Hu, C.; Kitts, D.D. Evaluation of antioxidant activity of epigallocatechin gallate in biphasic model systems in vitro. Mol. Cel. Biochem. 2001, 218, 147–155. [Google Scholar] [CrossRef]
- Banerjee, S. Inhibition of mackerel (Scomber scombrus) muscle lipoxygenase by green tea polyphenols. Food Res. 2006, 39, 486–491. [Google Scholar] [CrossRef]
- Hu, J.; Zhou, D.; Chen, Y. Preparation and antioxidant activity of green tea extract enriched in epigallocatechin (EGC) and epigallocatechin gallate (EGCG). J. Agric. Food Chem. 2009, 57, 1349–1353. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Bhandari, B. Encapsulation of polyphenols–A review. Trends Food Sci. Technol. 2010, 21, 510–523. [Google Scholar] [CrossRef]
- Hu, B.; Wang, Y.; Xie, M.; Hu, G.; Ma, F.; Zeng, X. Polymer nanoparticles composed with gallic acid grafted chitosan and bioactive peptides combined antioxidant, anticancer activities and improved delivery property for labile polyphenols. J. Funct. Foods 2015, 15, 593–603. [Google Scholar] [CrossRef]
- Souto, E.B.; Zielinska, A.; Souto, S.B.; Durazzo, A.; Lucarini, M.; Santini, A.; Silva, A.M.; Atasasov, A.G.; Maarquez, C.; Andrade, L.N.; et al. (+)-Limonene 1, 2-epoxide-loaded slns: Evaluation of drug release, antioxidant activity, and cytotoxicity in an HaCaT cell line. Int. J. Mol. Sci. 2020, 21, 1449. [Google Scholar] [CrossRef]
- Souto, E.B.; Souto, S.B.; Zielinska, A.; Durazzo, A.; Lucarini, M.; Santini, A.; Orbańczuk, O.K.; Atasasov, A.G.; Maarquez, C.; Andrade, L.N.; et al. Perillaldehyde 1, 2-epoxide loaded SLN-tailored mAb: Production, physicochemical characterization and in vitro cytotoxicity profile in MCF-7 cell lines. Pharmaceutics 2020, 12, 161. [Google Scholar] [CrossRef]
- Lee, D.S.; Woo, J.Y.; Ahn, C.B.; Je, J.Y. Chitosan-hydroxycinnamic acid conjugates: Preparation, antioxidant and antimicrobial activity. Food Chem. 2014, 148, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Benjakul, S.; Huda, N.; Xu, C.; Wu, P. Preparation and characterization of squid pen chitooligosaccharide–epigallocatechin gallate conjugates and their antioxidant and antimicrobial activities. RSC Adv. 2020, 10, 33196–33204. [Google Scholar] [CrossRef]
- Xie, M.H.; Hu, B.; Wang, Y.; Zeng, X. Grafting of gallic acid onto chitosan enhances antioxidant activities and alters rheological properties of the copolymer. J. Agric. Food Chem. 2014, 62, 9128–9136. [Google Scholar] [CrossRef]
- Choi, C.; Nam, J.P.; Nah, J.W. Application of chitosan and chitosan derivatives as biomaterials. J. Ind. Eng. Chem. 2016, 33, 1–10. [Google Scholar] [CrossRef]
- Moreno-Vásquez, M.J.; Valenzuela-Buitimea, E.L.; Plascencia-Jatomea, M.; Encinas-Encinas, J.C.; Rodríguez-Félix, F.; Sánchez-Valdes, S.; Rosas-Burgos, E.C.; Ocaño-Higuera, V.M.; Graciano-Verdugo, A.Z. Functionalization of chitosan by a free radical reaction: Characterization, antioxidant and antibacterial potential. Carbohyd. Polym. 2017, 155, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Barreras-Urbina, C.G.; Ramírez-Wong, B.; López-Ahumada, G.A.; Burruel-Ibarra, S.E.; Martínez-Cruz, O.; Tapia-Hernández, J.A.; Rodriguez Felix, F. Nano-and Micro-Particles by Nanoprecipitation: Possible Application in the Food and Agricultural Industries. Int. J. Food Prop. 2016, 19, 1912–1923. [Google Scholar] [CrossRef]
- Lammari, N.; Louaer, O.; Meniai, A.H.; Elaissari, A. Encapsulation of essential oils via nanoprecipitation process: Overview, progress, challenges and prospects. Pharmaceutics 2020, 12, 431. [Google Scholar] [CrossRef]
- Zhang, H.; Fu, Y.; Niu, F.; Li, Z.; Ba, C.; Jin, B.; Chena, G.; Li, X. Enhanced antioxidant activity and in vitro release of propolis by acid-induced aggregation using heat-denatured zein and carboxymethyl chitosan. Food Hydrocoll. 2018, 81, 104–112. [Google Scholar] [CrossRef]
- Liang, J.; Yan, H.; Wang, X.; Zhou, Y.; Gao, X.; Puligundla, P.; Wan, X. Encapsulation of epigallocatechin gallate in zein/chitosan nanoparticles for controlled applications in food systems. Food Chem. 2017, 231, 19–24. [Google Scholar] [CrossRef]
- Aluani, D.; Tzankova, V.; Kondeva-Burdina, M.; Yordanov, Y.; Nikolova, E.; Odzhakov, F.; Apostolov, A.; Markova, T.; Yoncheva, K. Evaluation of biocompatibility and antioxidant efficiency of chitosan-alginate nanoparticles loaded with quercetin. Int. J. Biol. Macromol. 2017, 103, 771–782. [Google Scholar] [CrossRef]
- Barzegar, M.; Ghaderi, M.; Sahari, M.A.; Azizi, M.H. Enhancement of thermal stability and antioxidant activity of thyme essential oil by encapsulation in chitosan nanoparticles. J. Agric. Sci. Technol. 2016, 18, 1781–1792. [Google Scholar]
- Aguiar, J.; Costa, R.; Rocha, F.; Estevinho, B.N.; Santos, L. Design of microparticles containing natural antioxidants: Preparation, characterization and controlled release studies. Powder Technol. 2017, 313, 287–292. [Google Scholar] [CrossRef]
- Ghodake, G.S.; Shinde, S.K.; Saratale, G.D.; Saratale, R.G.; Kim, M.; Jee, S.C.; Kadam, A.A. α-Cellulose Fibers of Paper-Waste Origin Surface-Modified with Fe3O4 and Thiolated-Chitosan for Efficacious Immobilization of Laccase. Polymers 2021, 13, 581. [Google Scholar] [CrossRef]
- Kadam, A.A.; Shinde, S.K.; Ghodake, G.S.; Saratale, G.D.; Saratale, R.G.; Sharma, B.; Sung, J.S. Chitosan-Grafted Halloysite Nanotubes-Fe3O4 Composite for Laccase-Immobilization and Sulfamethoxazole-Degradation. Polymer 2020, 12, 2221. [Google Scholar] [CrossRef]
- Chen, F.; Shi, Z.; Neoh, K.G.; Kang, E.T. Antioxidant and antibacterial activities of eugenol and carvacrol-grafted chitosan nanoparticles. Biotechnol. Bioeng. 2009, 104, 30–39. [Google Scholar] [CrossRef]
- Luque-Alcaraz, A.G.; Lizardi, J.; Goycoolea, F.M.; Valdez, M.A.; Acosta, A.L.; Iloki-Assanga, S.B.; Higuera-Ciapara, I.; Argüelles-Monal, W. Characterization and antiproliferative activity of nobiletin loaded chitosan nanoparticles. J. Nanomater. 2012, 100, 1–7. [Google Scholar] [CrossRef]
- Kumar, A.; Pandey, A.K.; Singh, S.S.; Shanker, R.; Dhawan, A. Cellular uptake and mutagenic potential of metal oxide nanoparticles in bacterial cells. Chemosphere 2011, 83, 1124–1132. [Google Scholar] [CrossRef]
- Aljawish, A.; Chevalot, I.; Jasniewski, J.; Revol-Junelles, A.M.; Scher, J.; Muniglia, L. Laccase-catalysed functionalisation of chitosan by ferulic acid and ethyl ferulate: Evaluation of physicochemical and biofunctional properties. Food Chem. 2014, 161, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Cueva, C.; Moreno-Arribas, M.V.; Martín-Álvarez, P.J.; Bills, G.; Vicente, M.F.; Basilio, A.; Rivas, C.L.; Requena, T.; Rodríguez, J.M.; Bartolomé, B. Antimicrobial activity of phenolic acids against commensal, probiotic and pathogenic bacteria. Res. Microbiol. 2010, 161, 372–382. [Google Scholar] [CrossRef]
- Martínez-Camacho, A.P.; Cortez-Rocha, M.O.; Ezquerra-Brauer, J.M.; Graciano-Verdugo, A.Z.; Rodriguez-Félix, F.; Castillo-Ortega, M.M.; Yépiz-Gómez, M.S.; Plascencia-Jatomea, M. Chitosan composite films: Thermal, structural, mechanical and antifungal properties. Carbohyd. Polym. 2010, 82, 305–315. [Google Scholar] [CrossRef]
- Dwivedi, S.; Wahab, R.; Khan, F.; Mishra, Y.K.; Musarrat, J.; Al-Khedhairy, A.A. Reactive oxygen species mediated bacterial biofilm inhibition via zinc oxide nanoparticles and their statistical determination. PLoS ONE 2014, 9, e111289. [Google Scholar] [CrossRef] [PubMed]
- Amorati, R.; Valgimigli, L. Methods to measure the antioxidant activity of phytochemicals and plant extracts. J. Agric. Food Chem. 2018, 66, 3324–3329. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Caro, C.A.; Cabello, G.; Landaeta, E.; Pérez, J.; Zagal, J.H.; Lillo, L. Synthesis and spectroscopic and electrochemical studies of chitosan Schiff base derivatives. Russ. J. Appl. Chem. 2013, 86, 1791–1797. [Google Scholar] [CrossRef]
- Lei, F.; Wang, X.; Liang, C.; Yuan, F.; Gao, Y. Preparation and functional evaluation of chitosan-EGCG conjugates. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Kumar, C.S. (Ed.) Transmission Electron Microscopy Characterization of Nanomaterials; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Decuzzi, P.; Ferrari, M. The adhesive strength of non-spherical particles mediated by specific interactions. Biomaterials 2006, 27, 5307–5314. [Google Scholar] [CrossRef]
- Alameh, M.; Lavertu, M.; Tran-Khanh, N.; Chang, C.Y.; Lesage, F.; Bail, M.; Buschmann, M.D. siRNA delivery with chitosan: Influence of chitosan molecular weight, degree of deacetylation, and amine to phosphate ratio on in vitro silencing efficiency, hemocompatibility, biodistribution, and in vivo efficacy. Biomacromolecules 2018, 19, 112–131. [Google Scholar] [CrossRef]
- Liu, F.; Sun, C.; Yang, W.; Yuan, F.; Gao, Y. Structural characterization and functional evaluation of lactoferrin–polyphenol conjugates formed by free-radical graft copolymerization. Chem. Sci. 2015, 5, 15641–15651. [Google Scholar] [CrossRef]
- Albanese, A.; Tang, P.S.; Chan, W.C. The effect of nanoparticle size, shape, and surface chemistry on biological systems. Annu. Rev. Biomed. Eng. 2012, 14, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Cerbelaud, M.; Videcoq, A.; Rossignol, F.; Piechowiak, M.A.; Bochicchio, D.; Ferrando, R. Heteroaggregation of ceramic colloids in suspensions. Adv. Phys. X 2017, 2, 35–53. [Google Scholar] [CrossRef]
- Shegokar, R.; Muller, R.H.; Ismail, M.; Gohla, S. Algal Nanosuspensions for Dermal and Oral Delivery. Curr. Nanomed. 2018, 8, 45–57. [Google Scholar] [CrossRef]
- Li, F.; Jin, H.; Xiao, J.; Yin, X.; Liu, X.; Li, D.; Huang, Q. The simultaneous loading of catechin and quercetin on chitosan-based nanoparticles as effective antioxidant and antibacterial agent. Food Res. Int. 2018, 111, 351–360. [Google Scholar] [CrossRef]
- Xing, K.; Chen, X.G.; Kong, M.; Liu, C.S.; Cha, D.S.; Park, H.J. Effect of oleoyl-chitosan nanoparticles as a novel antibacterial dispersion system on viability, membrane permeability and cell morphology of Escherichia coli and Staphylococcus aureus. Carbohyd. Polym. 2009, 76, 17–22. [Google Scholar] [CrossRef]
- Saratale, R.G.; Shin, H.S.; Kumar, G.; Benelli, G.; Ghodake, G.S.; Jiang, Y.Y.; Saratale, G.D. Exploiting fruit byproducts for eco-friendly nanosynthesis: Citrus× clementina peel extract mediated fabrication of silver nanoparticles with high efficacy against microbial pathogens and rat glial tumor C6 cells. Environ. Sci. Pollut. Res. Int. 2018, 25, 10250–10263. [Google Scholar] [CrossRef]
- Qi, L.; Xu, Z.; Jiang, X.; Hu, C.; Zou, X. Preparation and antibacterial activity of chitosan nanoparticles. Carbohydr. Res. 2004, 339, 2693–2700. [Google Scholar] [CrossRef]
- Ahmad, W.; Ijaz, B.; Shabbiri, K.; Ahmed, F.; Rehman, S. Oxidative toxicity in diabetes and Alzheimer’s disease: Mechanisms behind ROS/RNS generation. J. Biomed. Sci. 2017, 24, 76. [Google Scholar] [CrossRef] [PubMed]
- Angelé-Martínez, C.; Nguyen, K.V.T.; Ameer, F.S.; Anker, J.N.; Brumaghim, J.L. Reactive oxygen species generation by copper (II) oxide nanoparticles determined by DNA damage assays and EPR spectroscopy. Nanotoxicology 2017, 11, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Zhu, N.; Zhu, Y.; Zamir, S.M.; Wu, Y. Sustainable pollutant removal by periphytic biofilm via microbial composition shifts induced by uneven distribution of CeO2 nanoparticles. Bioresour. Technol. 2018, 248, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Zhong, Y. Measurement of antioxidant activity. J. Funct. Foods 2015, 18, 757–781. [Google Scholar] [CrossRef]
- Durazzo, A. Study Approach of Antioxidant Properties in Foods: Update and Considerations. Foods 2017, 6, 17. [Google Scholar] [CrossRef] [PubMed]
- Dulong, V.; Kouassi, M.C.; Labat, B.; Le Cerf, D.; Picton, L. Antioxidant properties and bioactivity of Carboxymethylpullulan grafted with ferulic acid and of their hydrogels obtained by enzymatic reaction. Food Chem. 2018, 262, 21–29. [Google Scholar] [CrossRef]
- Puoci, F.; Iemma, F.; Curcio, M.; Parisi, O.I.; Cirillo, G.; Spizzirri, U.G.; Picci, N. Synthesis of methacrylic-ferulic acid copolymer with antioxidant properties by single-step free radical polymerization. J. Agric. Food Chem. 2008, 56, 10646–10650. [Google Scholar] [CrossRef]
- Reznichenko, L.; Amit, T.; Zheng, H.; Avramovich-Tirosh, Y.; Youdim, M.B.H.; Weinreb, O.; Mandel, S. Reduction of iron-regulated amyloid precursor protein and β-amyloid peptide by (–)-epigallocatechin-3-gallate in cell cultures: Implications for iron chelation in Alzheimer’s disease. J. Neurochem. 2006, 97, 527–536. [Google Scholar] [CrossRef]
- Pérez-Jiménez, J.; Arranz, S.; Tabernero, M.; Díaz-Rubio, M.E.; Serrano, J.; Goñi, I. Updated methodology to determine antioxidant capacity in plant foods, oils and beverages: Extraction, measurement and expression of results. Food Res. Int. 2008, 41, 274–285. [Google Scholar] [CrossRef]
- Boulmokh, Y.; Belguidoum, K.; Meddour, F.; Amira-Guebaili, H. Investigation of antioxidant activity of epigallocatechin gallate and epicatechin as compared to resveratrol and ascorbic acid: Experimental and theoretical insights. Struct. Chem. 2021. [Google Scholar] [CrossRef]
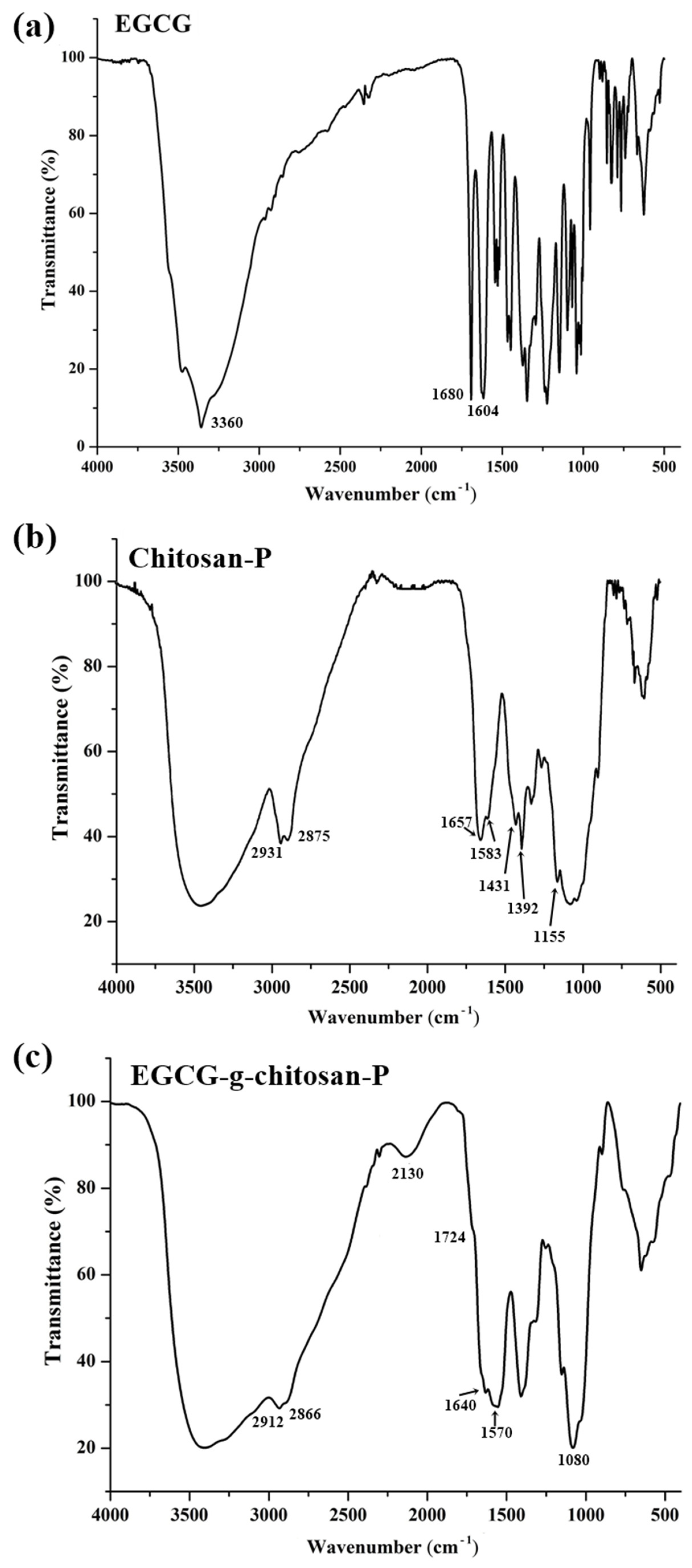


| Sample | Peak Position Wavenumber (cm−1) | Peak Assignment |
|---|---|---|
| EGCG | 3650–3200 | OH group (aromatic ring) |
| 1600–1450 | C=C group (aromatic ring) | |
| 1148 | C=O group (heterocyclic pyranose) | |
| Chitosan-P | 3600–3200 | OH group |
| 2931–2875 | CH group | |
| 1657 | C=O (amide I group) | |
| 1583 | N-H (amide II group) | |
| 1431 | C-N (amide III group) | |
| 1400–1200 | C–C–H, O–C–H, and C–O–H bending vibrations mode of mono or polysaccharides | |
| 1156–1030 | C-O-C (glycosidic bond) | |
| 1080 | C-O (saccharide structure) | |
| EGCG-g-chitosan-P | 3650–3200 | OH group |
| 2912–2866 | CH group | |
| 2130 | C=N (imine bond) | |
| 1640 | C=N (imine bond) | |
| 1600–1500 | C=C group (aromatic ring) | |
| 1570 | N-H (amide II group) | |
| 1416 | C-N (amide III group) | |
| 1080 | C-O (saccharide structure) | |
| 1060 | C-O- group |
| Antibacterial Activity | Bacteria | Concentration (μg/mL) | ||
|---|---|---|---|---|
| EGCG | Chitosan-P | EGCG-g-Chitosan-P | ||
| Minimal Inhibitory Concentration (MIC) | S. aureus | 62.5 ± 0.21 a | <0.98 b | <0.98 b |
| P. fluorescens | 500 ± 0.01 a | 31.2 ± 0.3 b | 15.6 ± 0.9 c | |
| Minimal Bactericide Concentration (MBC) | S. aureus | 125 ± 0.00 a | <0.98 b | <0.98 b |
| P. fluorescens | 1000 ± 0.01 a | 62.5 ± 0.51 b | 31.2 ± 0.9 c | |
| Samples | TEAC (mmol/L Trolox equiv/g Sample) | DPPH (EC50 mg/mL) | FRAP (μmol TE/g) |
|---|---|---|---|
| EGCG | 0.39 ± 0.01 a | 1.16 ± 0.01 a | 261.61 ± 14.81 a |
| Chitosan-P | 0.03 ± 0.01 b | >10 b | 39.56 ± 5.26 b |
| EGCG-g-chitosan-P | 0.40 ± 0.03 a | 1.00 ± 0.01 c | 310.75 ± 15.96 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreno-Vásquez, M.J.; Plascencia-Jatomea, M.; Sánchez-Valdes, S.; Tanori-Córdova, J.C.; Castillo-Yañez, F.J.; Quintero-Reyes, I.E.; Graciano-Verdugo, A.Z. Characterization of Epigallocatechin-Gallate-Grafted Chitosan Nanoparticles and Evaluation of Their Antibacterial and Antioxidant Potential. Polymers 2021, 13, 1375. https://doi.org/10.3390/polym13091375
Moreno-Vásquez MJ, Plascencia-Jatomea M, Sánchez-Valdes S, Tanori-Córdova JC, Castillo-Yañez FJ, Quintero-Reyes IE, Graciano-Verdugo AZ. Characterization of Epigallocatechin-Gallate-Grafted Chitosan Nanoparticles and Evaluation of Their Antibacterial and Antioxidant Potential. Polymers. 2021; 13(9):1375. https://doi.org/10.3390/polym13091375
Chicago/Turabian StyleMoreno-Vásquez, María J., Maribel Plascencia-Jatomea, Saúl Sánchez-Valdes, Judith C. Tanori-Córdova, Francisco J. Castillo-Yañez, Idania E. Quintero-Reyes, and Abril Z. Graciano-Verdugo. 2021. "Characterization of Epigallocatechin-Gallate-Grafted Chitosan Nanoparticles and Evaluation of Their Antibacterial and Antioxidant Potential" Polymers 13, no. 9: 1375. https://doi.org/10.3390/polym13091375
APA StyleMoreno-Vásquez, M. J., Plascencia-Jatomea, M., Sánchez-Valdes, S., Tanori-Córdova, J. C., Castillo-Yañez, F. J., Quintero-Reyes, I. E., & Graciano-Verdugo, A. Z. (2021). Characterization of Epigallocatechin-Gallate-Grafted Chitosan Nanoparticles and Evaluation of Their Antibacterial and Antioxidant Potential. Polymers, 13(9), 1375. https://doi.org/10.3390/polym13091375