Ion-Imprinted Polymer Modified with Carbon Quantum Dots as a Highly Sensitive Copper(II) Ion Probe
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
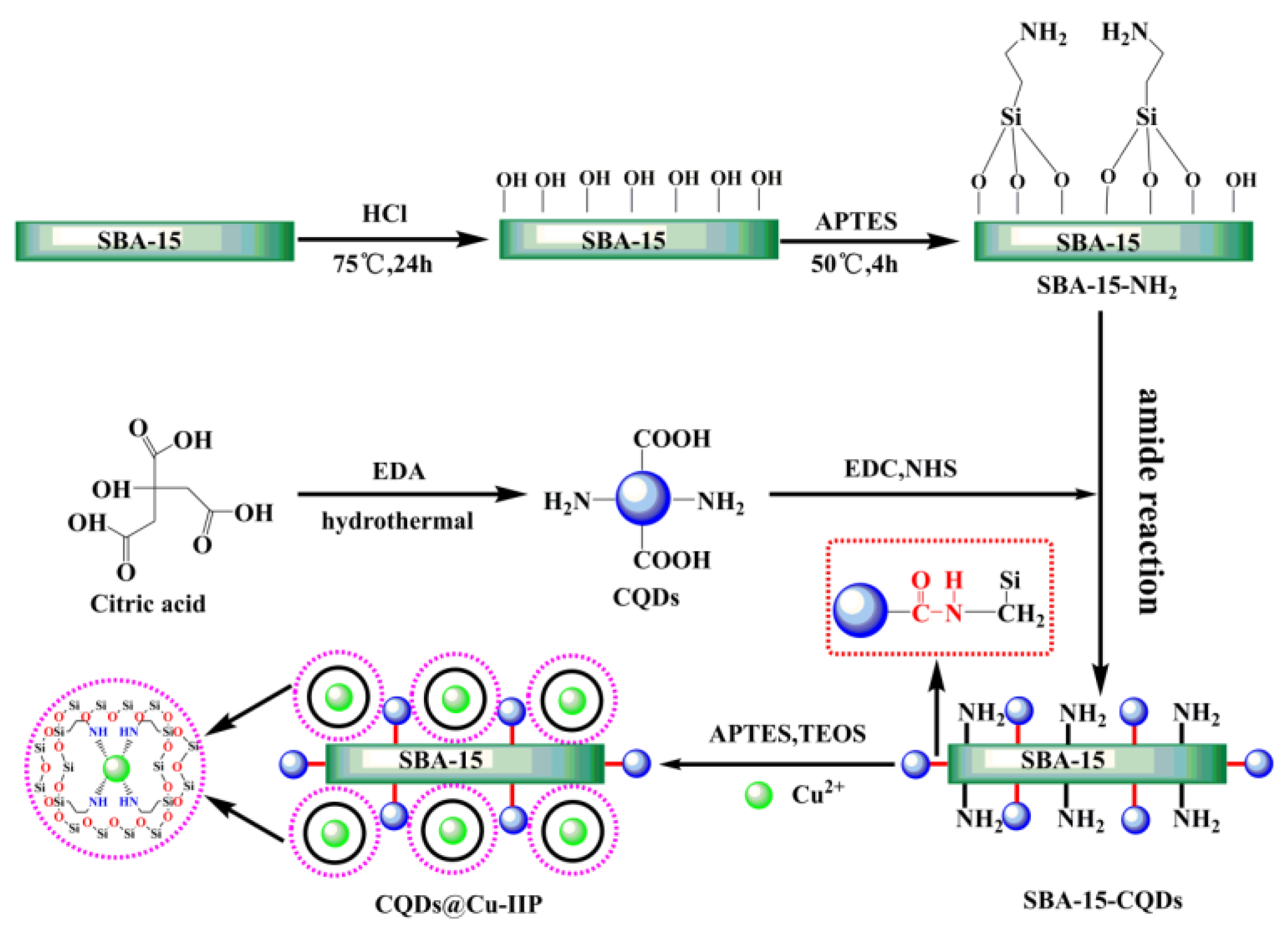
2.2. Synthesis of CQDs
2.3. Preparation of Amino-Functionalized SBA-15
2.4. Synthesis of SBA-15-CQDs
2.5. Synthesis of CQDs@Cu-IIP
2.6. Material Characterization
2.7. Determination of the Fluorescence Quantum Yield
3. Results and Discussion
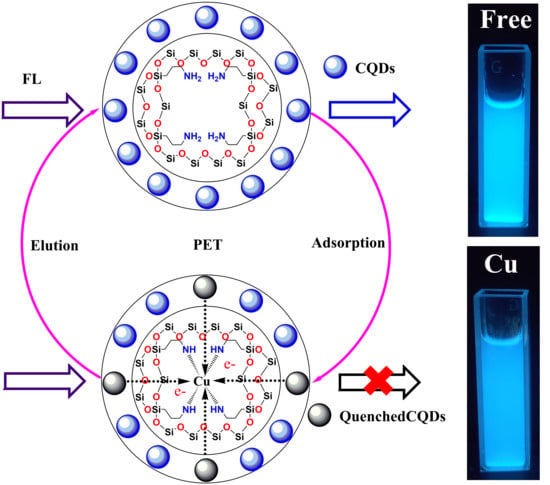
3.1. Synthesis of CQDs and CQDs@Cu-IIP
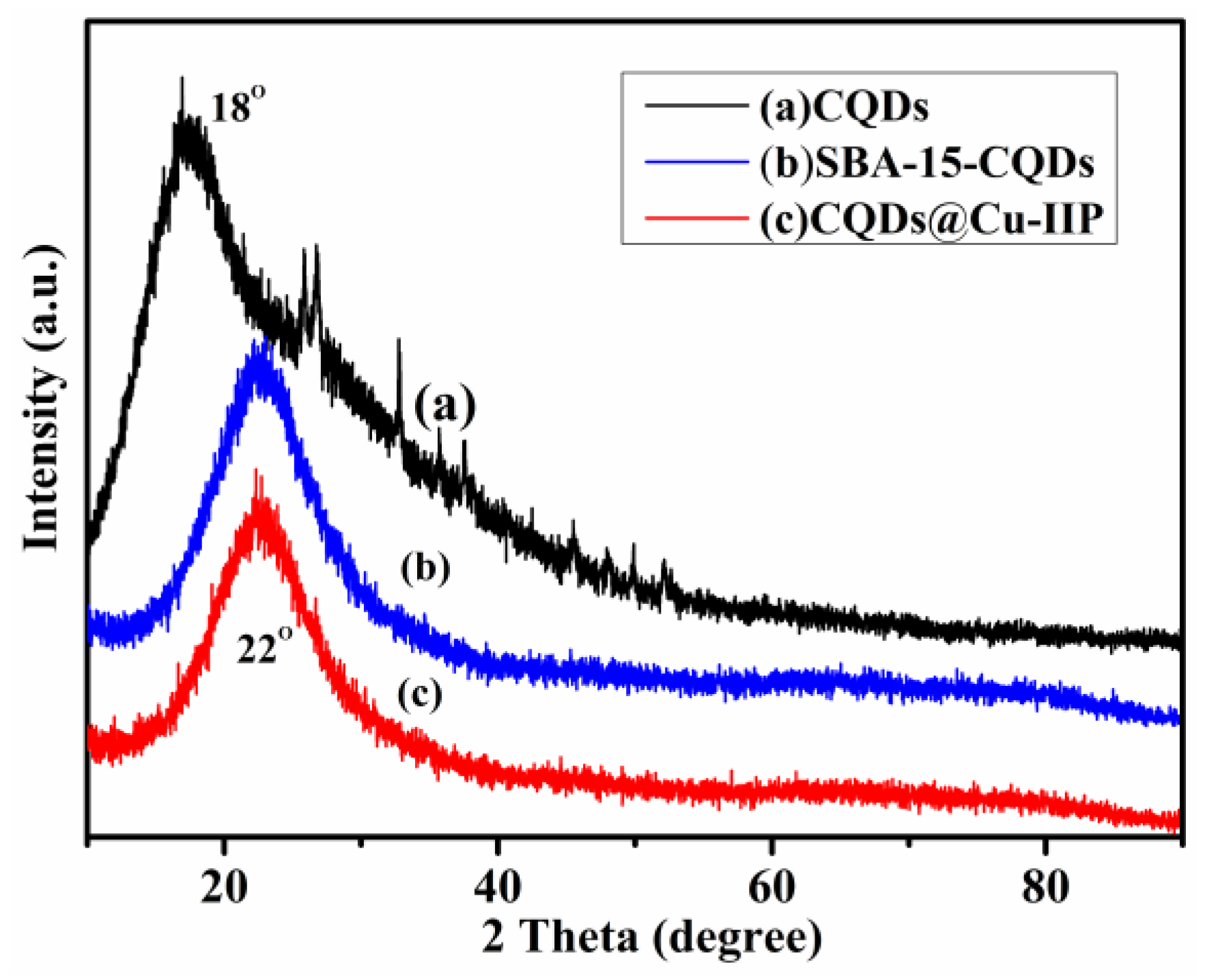
3.2. Physical and Optical Properties of CQDs and CQDs@Cu-IIP
3.3. CQDs@Cu-IIP Sensing Properties
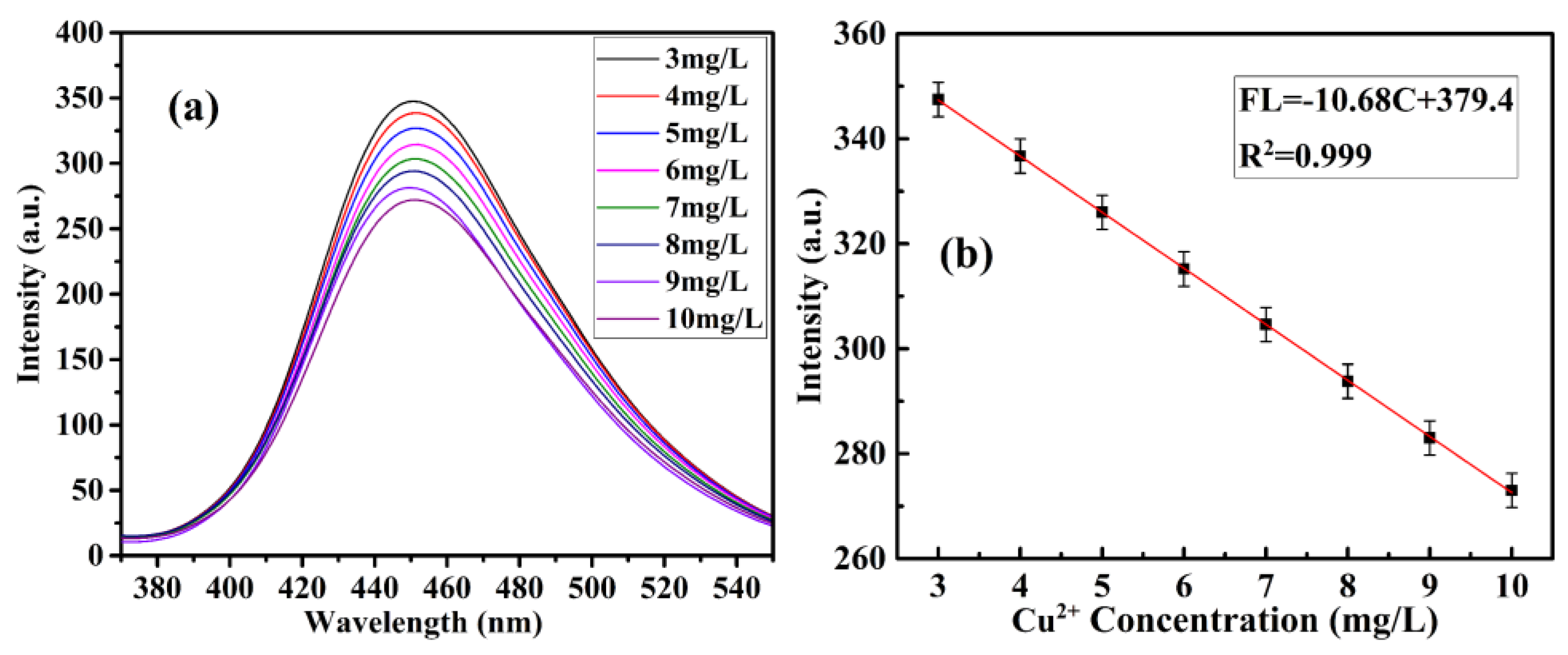
3.4. Fluorescence Detection of Cu(II) ion by CQDs@Cu-IIP
3.5. CQDs@Cu-IIP for Analysis of Tap Water Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pichel, N.; Vivar, M.; Fuentes, M. The problem of drinking water access: A review of disinfection technologies with an emphasis on solar treatment methods. Chemosphere 2019, 218, 1014–1030. [Google Scholar] [CrossRef]
- Sarkar, S.; Chatti, M.; Venkata, N.K.B.; Adusumalli, V. Mahaling-am, Highly selective and sensitive detection of Cu2+ ions using Ce(III)/Tb(III)-doped SrF2 nanocrystals as fluorescent probe. ACS Appl. Mater. 2015, 7, 25702. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Sa, R. Formation of Cu3,4(TCA), making the TCA complex a highly selective probe for Cu2+ detection: A TDDFT study. J. Mater. Chem. C 2019, 7, 2443–2456. [Google Scholar] [CrossRef]
- Georgopoulos, P.G.; Roy, A.; Yonone-Lioy, M.J.; Opiekun, R.E.; Lioy, P.J. Environmental copper: Its dynamics and human exposure issues. J. Toxicol. Environ. Health Part B 2001, 4, 341–394. [Google Scholar] [CrossRef] [PubMed]
- Mei, M.; Pang, J.; Huang, X.; Luo, Q. Magnetism-reinforced in-tube solid phase microextraction for the online determination of trace heavy metal ions in complex samples. Anal. Chim. Acta 2019, 1090, 82–90. [Google Scholar] [CrossRef]
- Becker, J.S.; Matusch, A.; Depboylu, C.; Dobrowolska, J.; Zoriy, M.V. Quantitative Imaging of Selenium, Copper, and Zinc in Thin Sections of Biological Tissues (Slugs−Genus Arion) Measured by Laser Ablation Inductively Coupled Plasma Mass Spectrometry. Anal. Chem. 2007, 79, 6074–6080. [Google Scholar] [CrossRef]
- Lin, T.; Huan, S. Direct and Simultaneous Determination of Copper, Chromium, Aluminum, and Manganese in Urine with a Multielement Graphite Furnace Atomic Absorption Spectrometer. Anal. Chem. 2001, 73, 4319–4325. [Google Scholar] [CrossRef]
- Otero-Romaní, J.; Moreda-Piñeiro, A.; Bermejo-Barrera, A.; Bermejo-Barrera, P. Evaluation of commercial C18 cartridges for trace elements solid phase extraction from seawater followed by inductively coupled plasma-optical emission spectrometry determination. Anal. Chim. Acta 2005, 536, 213–218. [Google Scholar] [CrossRef]
- Yang, W.; Jaramillo, D.; Gooding, J.J.; Hibbert, D.B.; Zhang, R.; Willett, G.D.; Fisher, K.J. Sub-ppt detection limits for copper ions with Gly-Gly-His modified electrodes. Chem. Commun. 2001, 19, 1982–1983. [Google Scholar] [CrossRef]
- Wen, T.; Qu, F.; Li, N.B.; Luo, H.Q. A facile, sensitive, and rapid spectrophotometric method for copper(II) ion detection in aqueous media using polyethyleneimine. Arab. J. Chem. 2017, 10, S1680–S1685. [Google Scholar] [CrossRef]
- Wang, X.-R.; Li, B.-W.; You, H.-Y.; Chen, L.-X. An Ion Imprinted Polymers Grafted Paper-based Fluorescent Sensor Based on Quantum Dots for Detection of Cu2+ Ions. Chin. J. Anal. Chem. 2015, 43, 1499–1504. [Google Scholar] [CrossRef]
- Feng, J.; Tao, Y.; Shen, X.; Jin, H.; Zhou, T.; Zhou, Y.; Hu, L.; Luo, D.; Mei, S.; Lee, Y.-I. Highly sensitive and selective fluorescent sensor for tetrabromobisphenol-A in electronic waste samples using molecularly imprinted polymer coated quantum dots. Microchem. J. 2019, 144, 93–101. [Google Scholar] [CrossRef]
- Wang, B.; Li, J.; Tang, Z.; Yang, B.; Lu, S. Near-infrared emissive carbon dots with 33.96% emission in aqueous solution for cellular sensing and light-emitting diodes. Sci. Bull. 2019, 64, 1285–1292. [Google Scholar] [CrossRef]
- Liu, Y.; Li, X.; Zhang, Q.; Li, W.; Xie, Y.; Liu, H.; Shang, L.; Liu, Z.; Chen, Z.; Gu, L.; et al. A General Route to Prepare Low-Ruthenium-Content Bimetallic Electrocatalysts for pH-Universal Hydrogen Evolution Reaction by Using Carbon Quantum Dots. Angew. Chem. Int. Ed. Engl. 2020, 59, 1718–1726. [Google Scholar] [CrossRef]
- Lu, S.; Xiao, G.; Sui, L.; Feng, T.; Yong, X.; Zhu, S.; Li, B.; Liu, Z.; Zou, B.; Jin, M.; et al. Piezochromic Car-bon Dots with Two-photon Fluorescence. Angew. Chem. Int. Ed. Engl. 2017, 56, 6187–6191. [Google Scholar] [CrossRef] [PubMed]
- Molaei, M.J. A review on nanostructured carbon quantum dots and their applications in biotechnology, sensors, and chemiluminescence. Talanta 2019, 196, 456–478. [Google Scholar] [CrossRef]
- Khan, Z.M.; Rahman, R.S.; Shumaila Islam, S.; Zulfequar, M. Hydrothermal treatment of red lentils for the synthesis of fluorescent carbon quantum dots and its application for sensing Fe3+. Opt. Mater. 2019, 91, 386–395. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Y.; Shi, H.; Yan, Y.; Liu, E.; Hu, X.; Fan, J. A strong blue fluorescent nanoprobe for highly sensitive and selective detection of mercury(II) based on sulfur doped carbon quantum dots. Mater. Chem. Phys. 2019, 232, 145–151. [Google Scholar] [CrossRef]
- Feng, S.; Gao, Z.; Liu, H.; Huang, J.; Li, X.; Yang, Y. Feasibility of detection valence speciation of Cr(III) and Cr(VI) in envi-ronmental samples by spectrofluorimetric method with fluorescent carbon quantum dots. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 212, 286–292. [Google Scholar] [CrossRef]
- Shakerian, F.; Kim, K.-H.; Kwon, E.; Szulejko, J.E.; Kumar, P.; Dadfarnia, S.; Shabani, A.M.H. Advanced polymeric materials: Synthesis and analytical application of ion imprinted polymers as selective sorbents for solid phase extraction of metal ions. TrAC Trends Anal. Chem. 2016, 83, 55–69. [Google Scholar] [CrossRef]
- Qi, J.; Li, B.; Wang, X.; Zhang, Z.; Wang, Z.; Han, J.; Chen, L. Three-dimensional paper-based microfluidic chip device for multiplexed fluorescence detection of Cu2+ and Hg2+ ions based on ion imprinting technology. Sens. Actuators B Chem. 2017, 251, 224–233. [Google Scholar] [CrossRef]
- Shao, H.; Li, C.; Ma, C.; Sun, L.; Chen, R.; Cheng, R.; Liu, Y.; Yan, Y.; Sun, Q.; Wu, C. An ion-imprinted material embedded carbon quantum dots for selective fluorometric determination of lithium ion in water samples. Microchim. Acta 2017, 184, 4861–4868. [Google Scholar] [CrossRef]
- Dhaneswara, D.; Sofyan, N. Effect of Different Pluronic P123 Triblock Copolymer Surfactant Concentrations on SBA-15 Pore Formation. Int. J. Technol. 2016, 7, 1009. [Google Scholar] [CrossRef]
- He, L.; Xu, Q.; Liu, Y.; Wei, H.; Tang, Y.; Lin, W. Coumarin-Based Turn-On Fluorescence Probe for Specific Detection of Glutathione over Cysteine and Homocysteine. ACS Appl. Mater. Interfaces 2015, 7, 12809–12813. [Google Scholar] [CrossRef]
- Razym, G.; Bakhshpour, M.; Yavuz, H.; Kip, C.; Tuncel, A.; Denizli, A. Surface-imprinted silica particles for Concanavalin A purification from Canavalia ensiformis. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2020, 1136, 121852. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Shang, X.; Chao, D.; Zhou, L.; Zhang, H. Highly sensitive and selective detection of Fe3+ by utilizing carbon quantum dots as fluorescent probes. Chem. Phys. Lett. 2018, 705, 1–6. [Google Scholar] [CrossRef]
- Mohapatra, S.; Sahu, S.; Sinha, N.; Bhutia, S.K. Synthesis of a carbon-dot-based photoluminescent probe for selective and ultrasensitive detection of Hg2+ in water and living cells. Analyst 2014, 140, 1221–1228. [Google Scholar] [CrossRef]
- Park, S.Y.; Lee, H.U.; Park, E.S.; Lee, S.C.; Lee, J.-W.; Jeong, S.W.; Kim, C.H.; Lee, Y.-C.; Huh, Y.S.; Lee, J. Photoluminescent Green Carbon Nanodots from Food-Waste-Derived Sources: Large-Scale Synthesis, Properties, and Biomedical Applications. ACS Appl. Mater. Interfaces 2014, 6, 3365–3370. [Google Scholar] [CrossRef]
- Yang, Z.; Xu, M.; Liu, Y.; He, F.; Gao, F.; Su, Y.; Wei, H.; Zhang, Y. Nitrogen-doped, carbon-rich, highly photoluminescent carbon dots from ammonium citrate. Nanoscale 2014, 6, 1890–1895. [Google Scholar] [CrossRef]
- Jing, S.; Zhao, Y.; Sun, R.-C.; Zhong, L.; Peng, X. Facile and High-Yield Synthesis of Carbon Quantum Dots from Biomass-Derived Carbons at Mild Condition. ACS Sustain. Chem. Eng. 2019, 7, 7833–7843. [Google Scholar] [CrossRef]
- Riaz, R.; Ali, M.; Maiyalagan, T.; Anjum, A.S.; Lee, S.Y.; Ko, M.J.; Jeong, S.H. Dye-sensitized solar cell (DSSC) coated with energy down shift layer of nitrogen-doped carbon quantum dots (N-CQDs) for enhanced current density and stability. Appl. Surf. Sci. 2019, 483, 425–431. [Google Scholar] [CrossRef]
- Kang, J.-K.; Lee, S.-C.; Kim, S.-B. Enhancement of selective Cu(II) sorption through preparation of surface-imprinted mesoporous silica SBA-15 under high molar concentration ratios of chloride and copper ions. Microporous Mesoporous Mater. 2018, 272, 193–201. [Google Scholar] [CrossRef]
- Çanakçı, D. Thermal stability, degradation kinetic and structural characterization of novel aromatic amide compounds. J. Mol. Struct. 2020, 1205, 127645. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, Y.; Cheng, H.; Hu, Y.; Shi, G.; Dai, L.; Qu, L. Nitrogen-Doped Graphene Quantum Dots with Oxygen-Rich Functional Groups. J. Am. Chem. Soc. 2012, 134, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Cheng, D.; Liu, X.; Han, A. Green Hydrothermal Synthesis of N-doped Carbon Dots from Biomass Highland Barley for the Detection of Hg2+. Sensors 2014, 58, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Ju, J.; Chen, W. Synthesis of highly fluorescent nitrogen-doped graphene quantum dots for sensitive, label-free detection of Fe (III) in aqueous media. Biosens. Bioelectron. 2014, 58, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhou, H.S. Green Synthesis of Luminescent Nitrogen-Doped Carbon Dots from Milk and Its Imaging Application. Anal. Chem. 2014, 86, 8902–8905. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Shi, C.; Bian, X.; Bai, Y. Microwave radiation hydrothermal synthesis and characterization of micro- and mesoporous composite molecular sieve Y/SBA-15. Arab. J. Chem. 2017, 10, S2773–S2777. [Google Scholar] [CrossRef]
- Xing, S.; Lv, P.; Fu, J.; Wang, J.; Fan, P.; Yang, L.; Yuan, Z. Direct synthesis and characterization of pore-broadened Al-SBA-15. Microporous Mesoporous Mater. 2017, 239, 316–327. [Google Scholar] [CrossRef]
- Wang, H.; Shang, H.; Sun, X.; Hou, L.; Wen, M.; Qiao, Y. Preparation of thermo-sensitive surface ion-imprinted polymers based on multi-walled carbon nanotube composites for selective adsorption of lead(II) ion. Colloids Surf. A Physicochem. Eng. Asp. 2020, 585, 124139. [Google Scholar] [CrossRef]
- Chen, J.; Li, Y.; Lv, K.; Zhong, W.; Wang, H.; Wu, Z.; Yi, P.; Jiang, J. Cyclam-functionalized carbon dots sensor for sensitive and selective detection of copper(II) ion and sulfide anion in aqueous media and its imaging in live cells. Sens. Actuators B Chem. 2016, 224, 298–306. [Google Scholar] [CrossRef]
- Shen, Q.; Tang, S.; Li, W.; Nie, Z.; Liu, Z.; Huang, Y.; Yao, S. A novel DNA-templated click chemistry strategy for fluorescent detection of copper(ii) ions. Chem. Commun. 2011, 48, 281–283. [Google Scholar] [CrossRef]
- Zong, J.; Yang, X.; Trinchi, A.; Hardin, S.; Cole, I.; Zhu, Y.; Li, C.; Muster, T.; Wei, G. Carbon dots as fluorescent probes for “off–on” detection of Cu2+ and l-cysteine in aqueous solution. Biosens. Bioelectron. 2014, 51, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wang, L.; Liu, Q.; Chen, M.; Chen, X. Facile synthesis of B,N-doped CQDs as versatile fluorescence probes for sensitive detection of cobalt ions in environmental water and biological samples. Microchem. J. 2021, 163, 105888. [Google Scholar] [CrossRef]
- Ng, Y.H.; Chin, S.F.; Pang, S.C.; Ng, S.M. Utilising the interface interaction on tris(hydroxymethy)aminomethane-capped carbon dots to enhance the sensitivity and selectivity towards the detection of Co(II) ions. Sens. Actuators B Chem. 2018, 273, 83–92. [Google Scholar] [CrossRef]

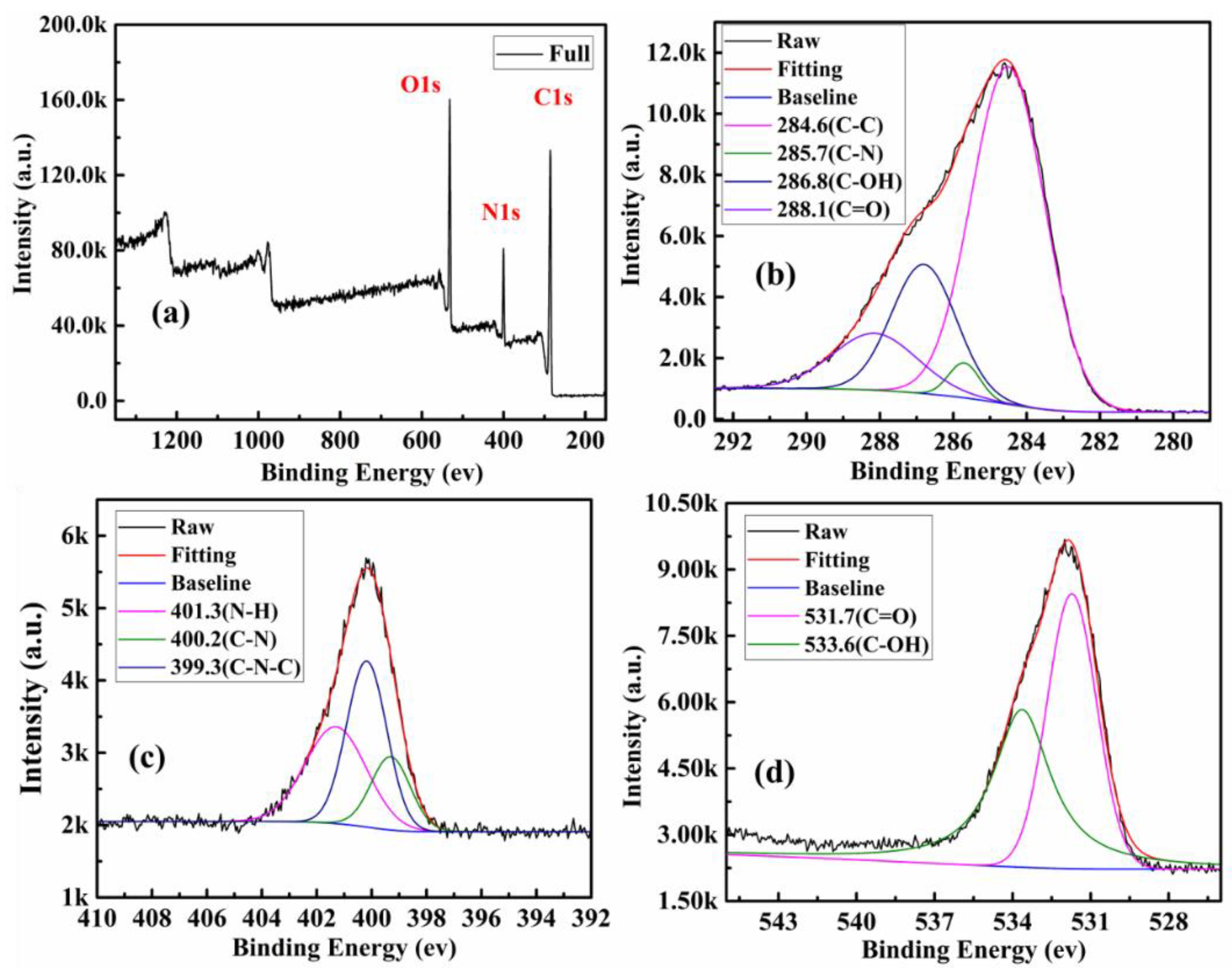

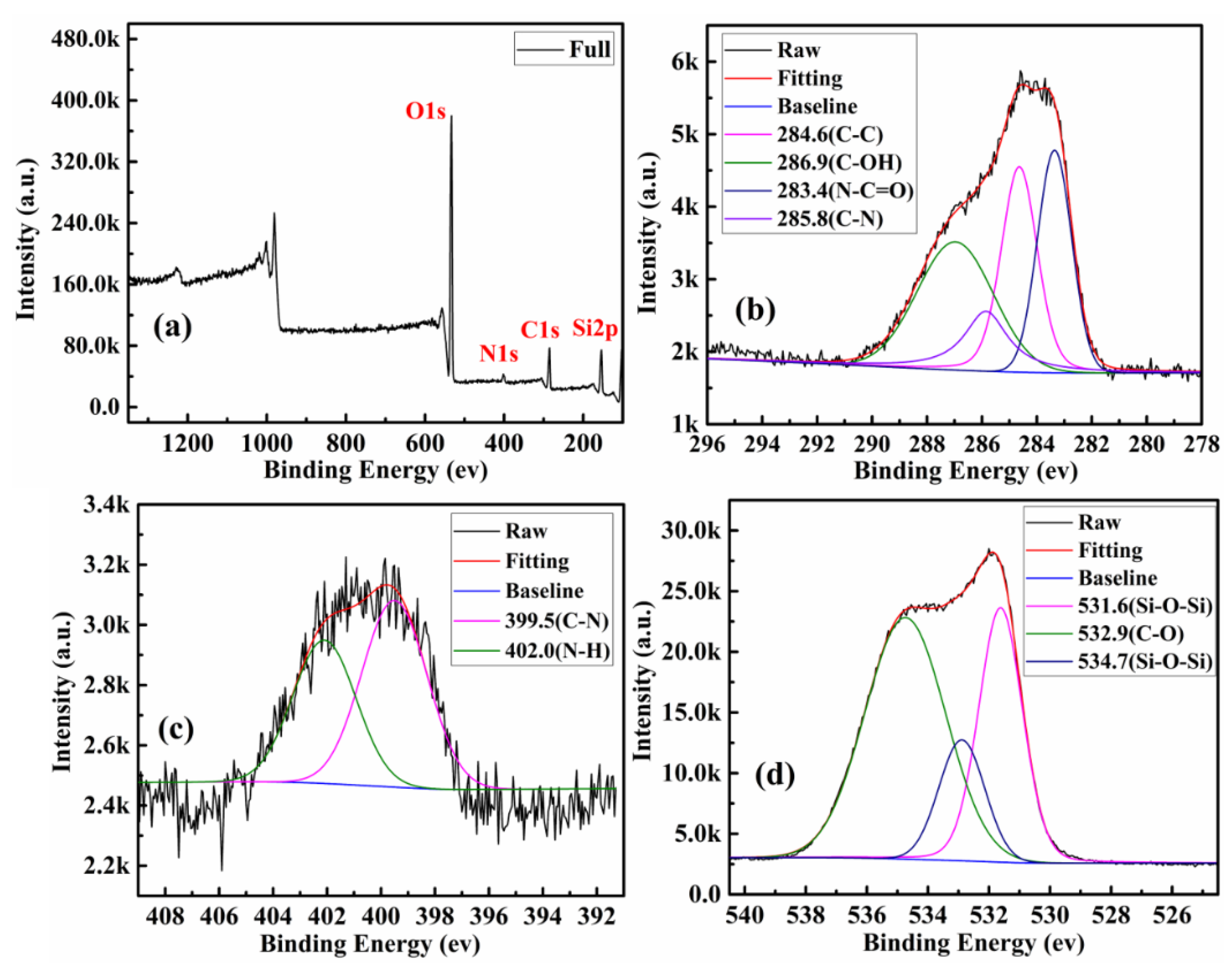
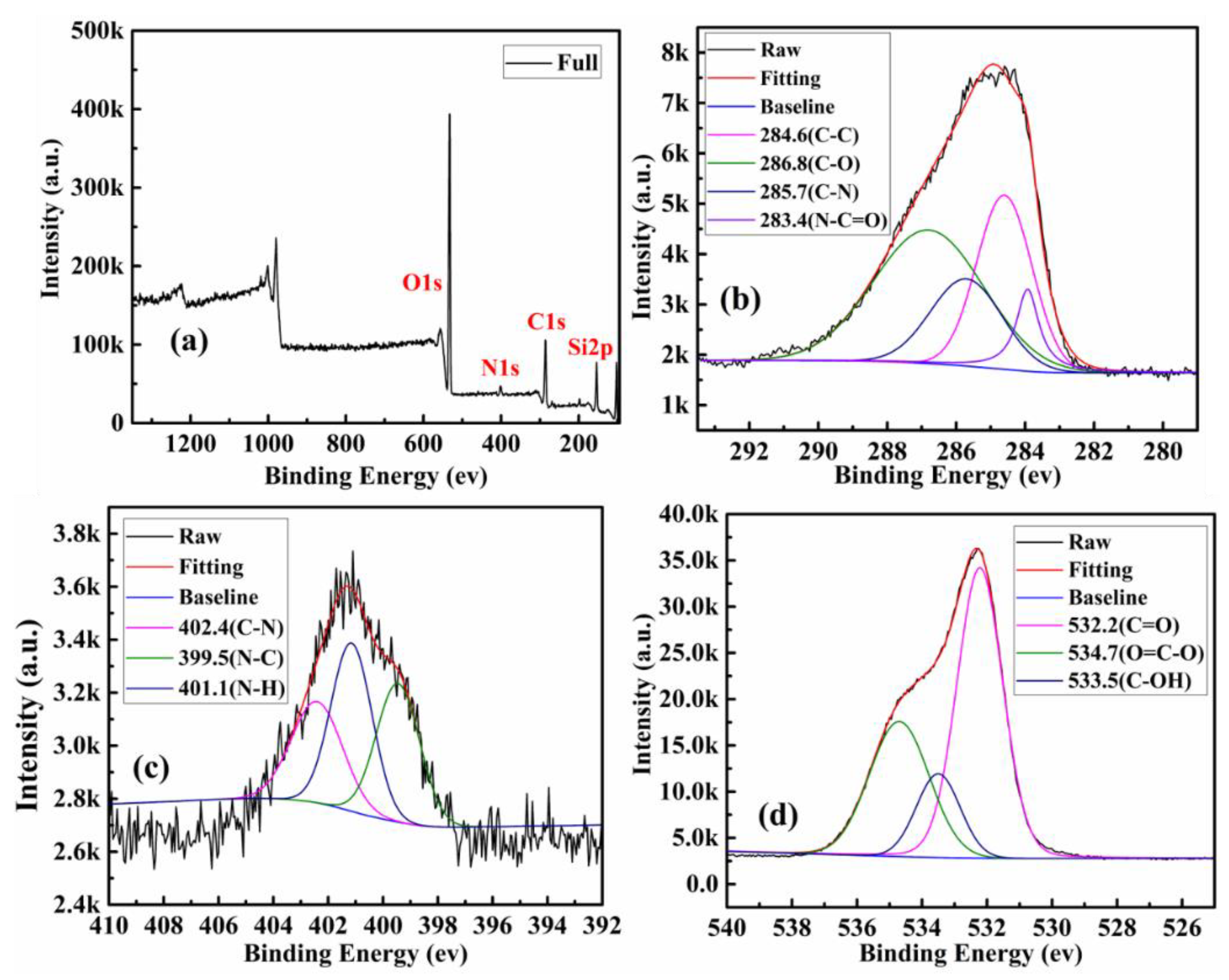

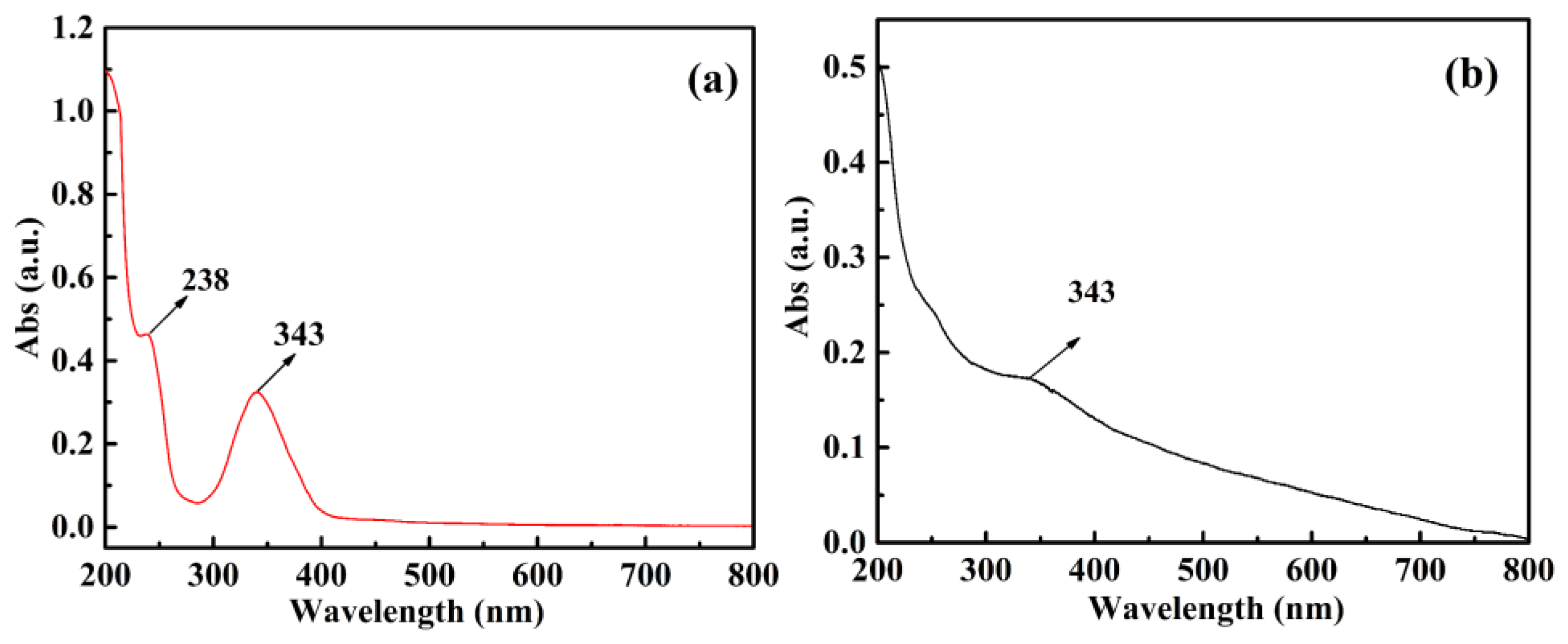


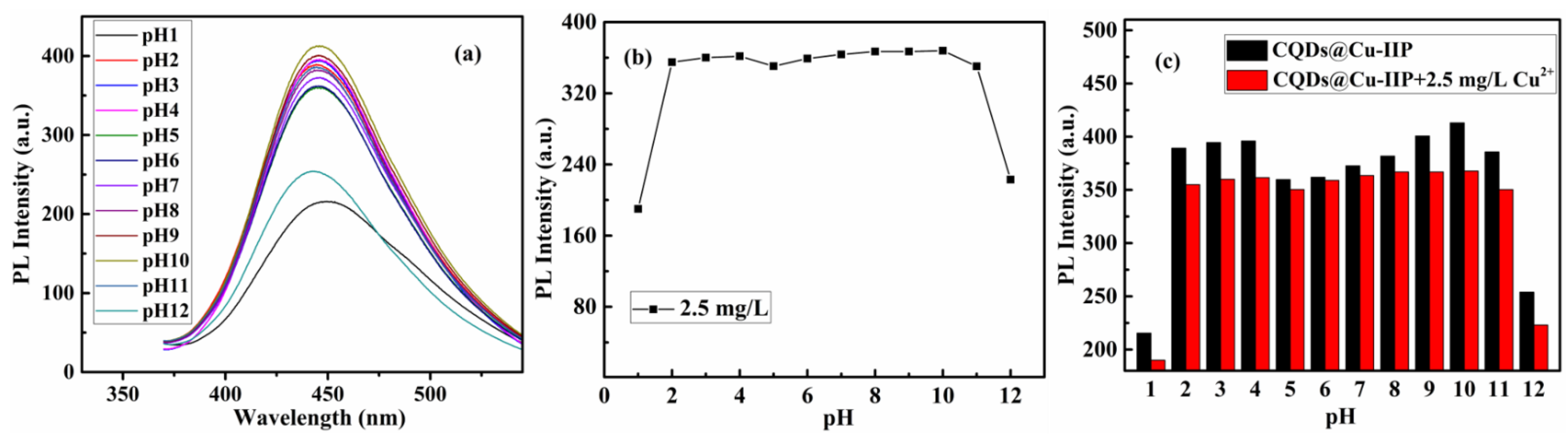
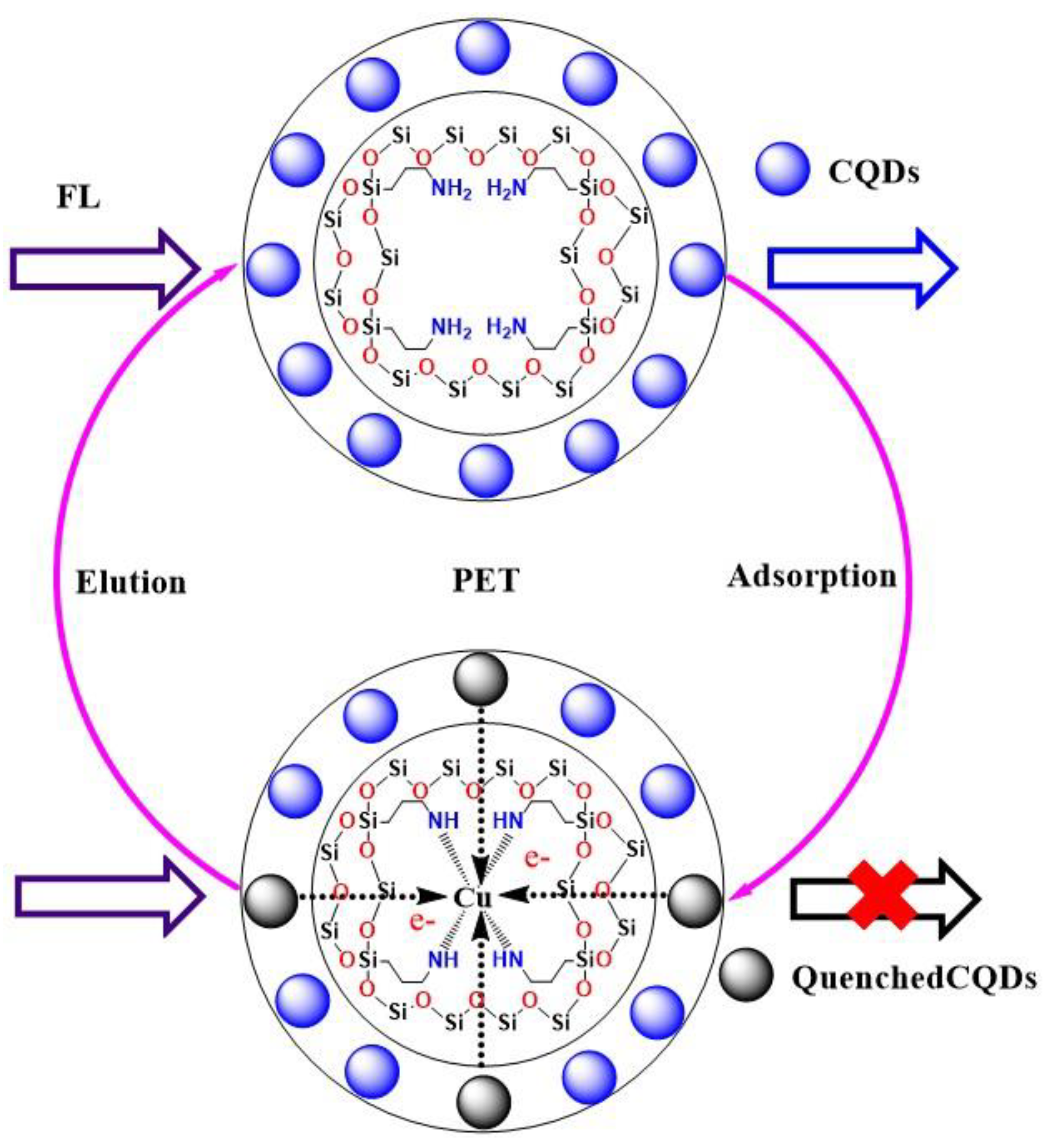



| Components | CQDs | SBA-15-NH2 | SBA-15-CQDs | CQDs@IIP | ||||
|---|---|---|---|---|---|---|---|---|
| BE(eV) | Atomic% | BE(eV) | Atomic% | BE(eV) | Atomic% | BE(eV) | Atomic% | |
| C | 285.93 | 67.77 | 285.3 | 34.86 | 285.73 | 20.22 | 285.71 | 30.40 |
| N | 400.28 | 11.94 | 400.45 | 4.18 | 401.16 | 2.85 | 401.59 | 3.42 |
| O | 531.92 | 20.29 | 532.84 | 43.31 | 533.55 | 53.89 | 532.95 | 46.46 |
| Si | - | - | 103.81 | 17.65 | 103.37 | 20.04 | 102.97 | 19.72 |
| Probe | Detection Limit | Detection Range | Reference |
|---|---|---|---|
| Ce(III)/Tb(III)-doped SrF2 | 0.11–0.14 µM | 0–70.0 µM | [2] |
| Carbon dots Sensor | 0.10µM | 0.7–4.0 µM | [41] |
| double-stranded DNA | 0.29µM | 0.5–10 µM | [42] |
| Carbon dots Sensor | 23 nM | 0–10 µM | [43] |
| B, N-CQDs | 0.52 nM | 0.5–10, 10–2000 nM | [44] |
| Carbon dots Sensor | 16.84 µM | 0–1870 µM | [45] |
| CQDs@Cu-IIP | 2.86 µM (0.0016 mg/L) | 3.93–31.47, 47.21–157.37 µM (0.25–2, 3–10 mg/L) | This work |
| Sample | Spike (mg/L) | CQDs@Cu-IIP Found (mg/L) | Recovery a (%) | AAS Found (mg/L) | Recovery a (%) |
|---|---|---|---|---|---|
| Tap Water | 0 | 0.6468 | - | 0.6421 | - |
| 1 | 1.6397 | 99.29 | 1.6303 | 98.82 | |
| 5 | 5.8278 | 103.62 | 5.6901 | 100.96 | |
| Beichuan River Water | 0 | 0.4592 | - | 0.4250 | - |
| 1 | 1.4766 | 101.74 | 1.4060 | 98.10 | |
| 5 | 5.7301 | 105.42 | 5.4970 | 101.44 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Zhou, C.; Wu, S.; Sun, C. Ion-Imprinted Polymer Modified with Carbon Quantum Dots as a Highly Sensitive Copper(II) Ion Probe. Polymers 2021, 13, 1376. https://doi.org/10.3390/polym13091376
Wang Z, Zhou C, Wu S, Sun C. Ion-Imprinted Polymer Modified with Carbon Quantum Dots as a Highly Sensitive Copper(II) Ion Probe. Polymers. 2021; 13(9):1376. https://doi.org/10.3390/polym13091376
Chicago/Turabian StyleWang, Zhiming, Cuo Zhou, Shunwei Wu, and Chunyan Sun. 2021. "Ion-Imprinted Polymer Modified with Carbon Quantum Dots as a Highly Sensitive Copper(II) Ion Probe" Polymers 13, no. 9: 1376. https://doi.org/10.3390/polym13091376
APA StyleWang, Z., Zhou, C., Wu, S., & Sun, C. (2021). Ion-Imprinted Polymer Modified with Carbon Quantum Dots as a Highly Sensitive Copper(II) Ion Probe. Polymers, 13(9), 1376. https://doi.org/10.3390/polym13091376