Influence of Nanoparticles on Thermal and Electrical Conductivity of Composites
Abstract
1. Introduction
2. Nanoparticles for Improving Thermal Conductivity
2.1. Hydrogel PCM Nanoparticle Systems
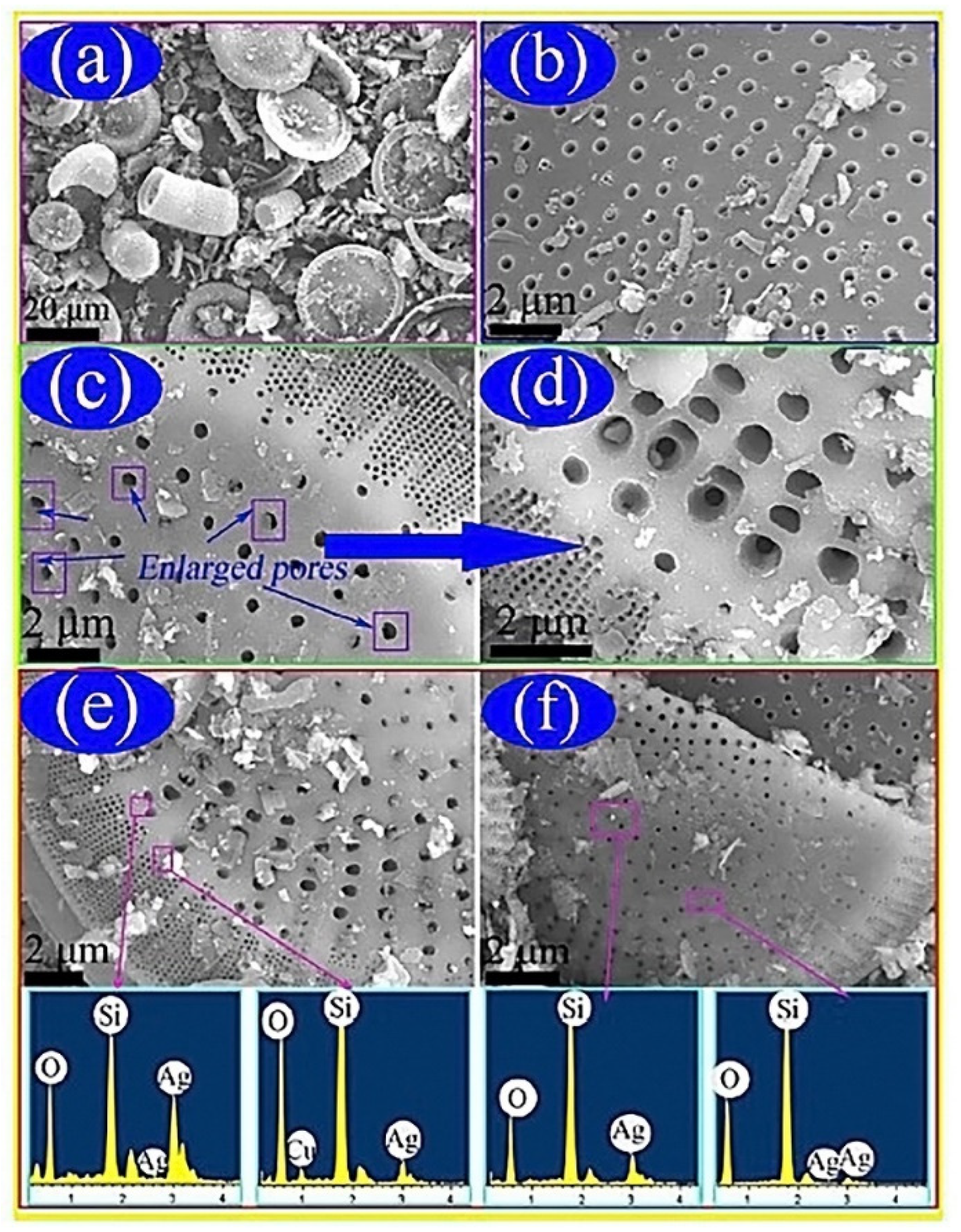
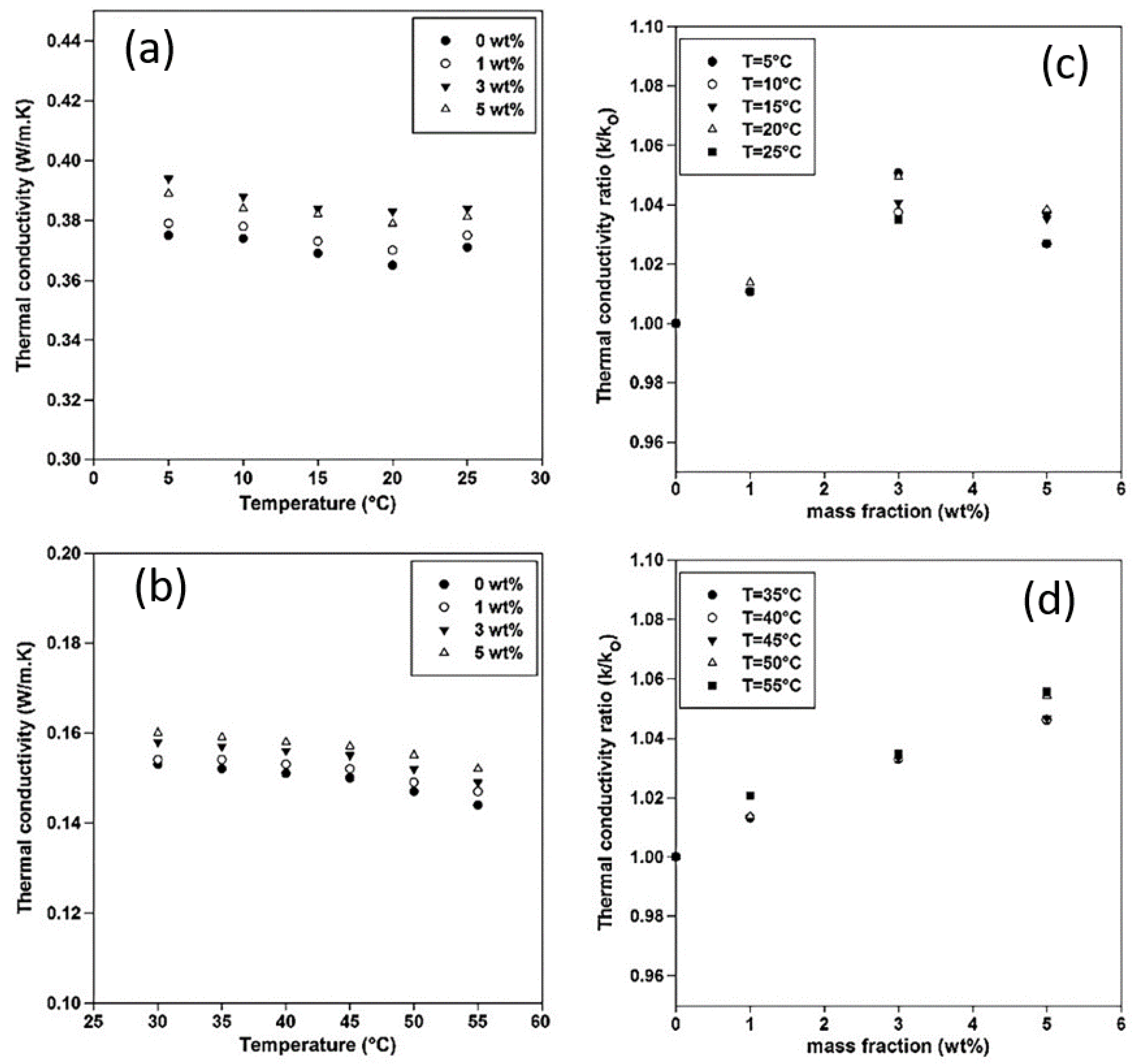
2.2. Silver/Diatomite Nanoparticles for PCMs
2.3. Thermal Interference Composites (TICs) Using Carbon Nanotubes
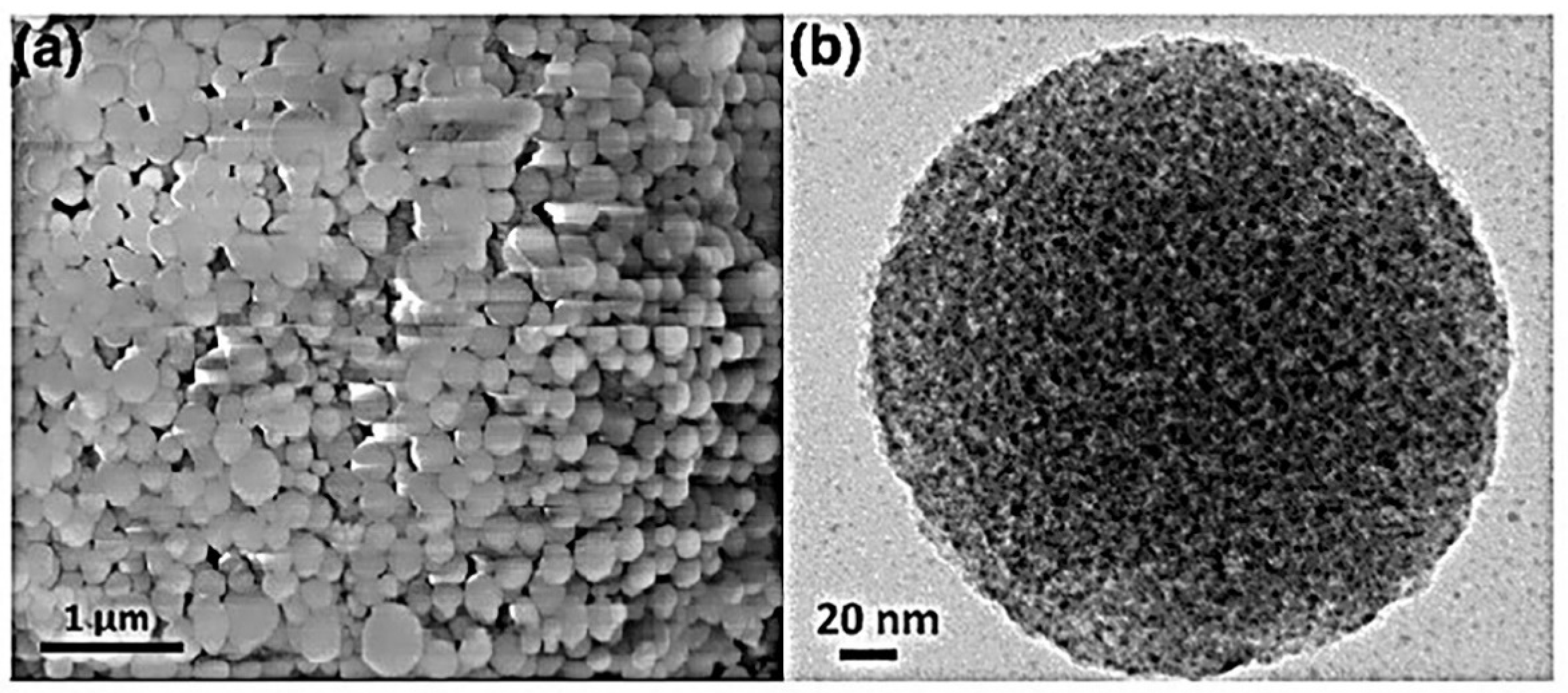
2.4. Mesoporous Silica MPSiO2 Nanoparticles
2.5. Disperse Alumina Nanoparticles (Al2O3)
2.6. Microencapsulated PCM Nanoparticles
3. Nanoparticles for Improving Electrical Conductivity
3.1. Increasing the Seebeck Coefficient with Conductive Nanoparticles
3.1.1. Bi2Te3 Nanoparticle Alloys
3.1.2. CoSb3 Nanoparticles
3.1.3. Ag1−xPb18SbTe20 Nanoparticles
3.1.4. Metal Oxide Nanoparticles
3.2. Silver Nanoparticles
3.3. Conductive Carbon Nanoparticles
3.4. Electromagnetic Interference (EMI) Materials
3.4.1. Nickel Nanoparticles
3.4.2. Fe3O4 Nanoparticles
3.4.3. Reduced Graphene Oxide (GO) Nanoparticles
4. Recovery and Disposal of Nanoparticles in Composites
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Vivek, P. Yashwant Polymer nanocomposites drive opportunities in the automotive sector. Nanotech Insights 2011, 2, 17–24. [Google Scholar]
- Motahar, S.; Nikkam, N.; Alemrajabi, A.A.; Khodabandeh, R.; Toprak, M.S.; Muhammed, M. A novel phase change material containing mesoporous silica nanoparticles for thermal storage: A study on thermal conductivity and viscosity. Int. Commun. Heat Mass Transf. 2014, 56, 114–120. [Google Scholar] [CrossRef]
- Goyal, R.; Sharma, M.; Amberiya, U.K. Innovative Nano Composite Materials and Applications in Automobiles. Int. J. Eng. Res. Technol. 2014, 3, 3001–3009. [Google Scholar]
- Hanemann, T.; Szabó, D.V. Polymer-nanoparticle composites: From synthesis to modern applications. Materials 2010, 3, 3468–3517. [Google Scholar] [CrossRef]
- Camargo, P.H.C.; Satyanarayana, K.G.; Wypych, F. Nanocomposites: Synthesis, structure, properties and new application opportunities. Mater. Res. 2009, 12, 1–39. [Google Scholar] [CrossRef]
- Choi, J.; Shin, H.; Yang, S.; Cho, M. The influence of nanoparticle size on the mechanical properties of polymer nanocomposites and the associated interphase region: A multiscale approach. Compos. Struct. 2014, 119, 365–376. [Google Scholar] [CrossRef]
- Li, X. Modeling the size- and shape-dependent cohesive energy of nanomaterials and its applications in heterogeneous systems. Nanotechnology 2014, 25, 185702. [Google Scholar] [CrossRef]
- Dhole, S.G.; Dake, S.A.; Prajapati, T.A.; Helambe, S.N. Effect of ZnO Filler on Structural and Optical Properties of Polyaniline-ZnO Nanocomposites. Procedia Manuf. 2018, 20, 127–134. [Google Scholar] [CrossRef]
- Jiang, J.; Oberdörster, G.; Biswas, P. Characterization of size, surface charge, and agglomeration state of nanoparticle dispersions for toxicological studies. J. Nanopart. Res. 2009, 11, 77–89. [Google Scholar] [CrossRef]
- Krishnamoorti, R. Strategies for dispersing nanoparticles in polymers. MRS Bull. 2007, 32, 341–347. [Google Scholar] [CrossRef]
- Khosla, A. Nanoparticle-doped electrically-conducting polymers for flexible Nano-micro systems. Electrochem. Soc. Interface 2012, 21, 67–70. [Google Scholar] [CrossRef]
- Baheti, V.; Abbasi, R.; Melitky, J. Optimization of Ball Milling Parameters for refinement of Waste Jute Fibres to Nano/Micro Scale in Dry Conditions. J. Text. Eng. 2013, 59, 87–92. [Google Scholar] [CrossRef][Green Version]
- Militký, J.; Baheti, V.; Mishra, R. Reinforcement of Wet Milled Jute Nano/Micro Particles in Polyvinyl Alcohol Films. Fibers Polym. 2013, 14, 133–137. [Google Scholar]
- Yu, W.; Xie, H.; Yin, L.; Zhao, J.; Xia, L.; Chen, L. Exceptionally high thermal conductivity of thermal grease: Synergistic effects of graphene and alumina. Int. J. Therm. Sci. 2015, 91, 76–82. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, L.D. Thermoelectric materials: Energy conversion between heat and electricity. J. Mater. 2015, 1, 92–105. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Liu, Y.; Wang, H.; Huang, Z.; Peijs, T.; Bilotti, E. Synergistic effects of spray-coated hybrid carbon nanoparticles for enhanced electrical and thermal surface conductivity of CFRP laminates. Compos. Part A Appl. Sci. Manuf. 2018, 105, 9–18. [Google Scholar] [CrossRef]
- Shahzad, F.; Alhabeb, M.; Hatter, C.B.; Anasori, B.; Hong, S.M.; Koo, C.M.; Gogotsi, Y. Electromagnetic interference shielding with 2D transition metal carbides (MXenes). Science 2016, 353, 1137–1140. [Google Scholar] [CrossRef]
- Pawar, S.P.; Marathe, D.A.; Pattabhi, K.; Bose, S. Electromagnetic interference shielding through MWNT grafted Fe3O4nanoparticles in PC/SAN blends. J. Mater. Chem. A 2015, 3, 656–669. [Google Scholar] [CrossRef]
- Pawar, S.P.; Biswas, S.; Kar, G.P.; Bose, S. High frequency millimetre wave absorbers derived from polymeric nanocomposites. Polymer 2016, 84, 398–419. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, G.; Tang, M.; Zhou, L.; Li, J.; Fan, X.; Shi, X.; Qin, J. Synergistic effect of carbon nanotube and graphene nanoplates on the mechanical, electrical and electromagnetic interference shielding properties of polymer composites and polymer composite foams. Chem. Eng. J. 2018, 353, 381–393. [Google Scholar] [CrossRef]
- Kasgoz, A.; Akin, D.; Durmus, A. Effects of size and shape originated synergism of carbon nano fillers on the electrical and mechanical properties of conductive polymer composites. J. Appl. Polym. Sci. 2015, 132, 1–11. [Google Scholar] [CrossRef]
- Si, P.; Trinidad, J.; Chen, L.; Lee, B.; Chen, A.; Persic, J.; Lyn, R.; Leonenko, Z.; Zhao, B. PEDOT:PSS nano-gels for highly electrically conductive silver/epoxy composite adhesives. J. Mater. Sci. Mater. Electron. 2018, 29, 1837–1846. [Google Scholar] [CrossRef]
- Nezakati, T.; Seifalian, A.; Tan, A.; Seifalian, A.M. Conductive Polymers: Opportunities and Challenges in Biomedical Applications. Chem. Rev. 2018, 118, 6766–6843. [Google Scholar] [CrossRef] [PubMed]
- Tonelli, L.; Refat, M.; Toschi, S.; Ahmed, M.M.Z.; Ahmed, E.; Morri, A.; El-Mahallawi, I.; Ceschini, L. Production of AlSi12CuNiMg/Al2O3 Micro/Nanodispersed Surface Composites Using Friction Stir Processing for Automotive Applications; Springer International Publishing: Berlin/Heidelberg, Germany, 2019; ISBN 9783030057510. [Google Scholar]
- Chai, F.; Zhang, D.; Li, Y.; Zhang, W. High strain rate superplasticity of a fine-grained AZ91 magnesium alloy prepared by submerged friction stir processing. Mater. Sci. Eng. A 2013, 568, 40–48. [Google Scholar] [CrossRef]
- Njuguna, J.; Silva, F.; Sachse, S. Nanocomposites for Vehicle Structural Applications. In Nanofibers—Production, Properties and Functional Applications; ResearchGate: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Liao, J.; Blok, S.; Van Der Molen, S.J.; Diefenbach, S.; Holleitner, A.W.; Schönenberger, C.; Vladyka, A.; Calame, M. Ordered nanoparticle arrays interconnected by molecular linkers: Electronic and optoelectronic properties. Chem. Soc. Rev. 2015, 44, 999–1014. [Google Scholar] [CrossRef]
- Garcés, J.M.; Moll, D.J.; Bicerano, J.; Fibiger, R.; McLeod, D.G. Polymeric nanocomposites for automotive applications. Adv. Mater. 2000, 12, 1835–1839. [Google Scholar] [CrossRef]
- Elsaesser, A.; Howard, C.V. Toxicology of nanoparticles. Adv. Drug Deliv. Rev. 2012, 64, 129–137. [Google Scholar] [CrossRef]
- Los, P.; Lukomska, A.; Jeziorska, R. Metal-polymer composites for electromagnetic interference shielding applications. Polimery/Polymers 2016, 61, 663–669. [Google Scholar] [CrossRef]
- Fratoddi, I.; Matassa, R.; Fontana, L.; Venditti, I.; Familiari, G.; Battocchio, C.; Magnano, E.; Nappini, S.; Leahu, G.; Belardini, A.; et al. Electronic Properties of a Functionalized Noble Metal Nanoparticles Covalent Network. J. Phys. Chem. C 2017, 121, 18110–18119. [Google Scholar] [CrossRef]
- Sadanand, V.; Rajini, N.; Satyanarayana, B.; Rajulu, A.V. Preparation and properties of cellulose/silver nanoparticle composites with in situ-generated silver nanoparticles using Ocimum sanctum leaf extract. Int. J. Polym. Anal. Charact. 2016, 21, 408–416. [Google Scholar] [CrossRef]
- Venditti, I. Morphologies and functionalities of polymeric nanocarriers as chemical tools for drug delivery: A review. J. King Saud Univ. Sci. 2019, 31, 398–411. [Google Scholar] [CrossRef]
- Liu, G.; Yuan, Q.; Hollett, G.; Zhao, W.; Kang, Y.; Wu, J. Cyclodextrin-based host-guest supramolecular hydrogel and its application in biomedical fields. Polym. Chem. 2018, 9, 3436–3449. [Google Scholar] [CrossRef]
- Scordo, G.; Bertana, V.; Scaltrito, L.; Ferrero, S.; Cocuzza, M.; Marasso, S.L.; Romano, S.; Sesana, R.; Catania, F.; Pirri, C.F. A novel highly electrically conductive composite resin for stereolithography. Mater. Today Commun. 2019, 19, 12–17. [Google Scholar] [CrossRef]
- D’Angelo, P.; Tarabella, G.; Romeo, A.; Giodice, A.; Marasso, S.; Cocuzza, M.; Ravanetti, F.; Cacchioli, A.; Petronini, P.G.; Iannotta, S. Monitoring the adaptive cell response to hyperosmotic stress by organic devices. MRS Commun. 2017, 7, 229–235. [Google Scholar] [CrossRef]
- Tiwary, C.S.; Kishore, S.; Vasireddi, R.; Mahapatra, D.R.; Ajayan, P.M.; Chattopadhyay, K. Electronic waste recycling via cryo-milling and nanoparticle beneficiation. Mater. Today 2017, 20, 67–73. [Google Scholar] [CrossRef]
- Khodadadi, J.M.; Hosseinizadeh, S.F. Nanoparticle-enhanced phase change materials (NEPCM) with great potential for improved thermal energy storage. Int. Commun. Heat Mass Transf. 2007, 34, 534–543. [Google Scholar] [CrossRef]
- Esapour, M.; Hamzehnezhad, A.; Rabienataj Darzi, A.A.; Jourabian, M. Melting and solidification of PCM embedded in porous metal foam in horizontal multi-tube heat storage system. Energy Convers. Manag. 2018, 171, 398–410. [Google Scholar] [CrossRef]
- Thoniyot, P.; Tan, M.J.; Karim, A.A.; Young, D.J.; Loh, X.J. Nanoparticle–Hydrogel Composites: Concept, Design, and Applications of These Promising, Multi-Functional Materials. Adv. Sci. 2015, 2, 1–13. [Google Scholar] [CrossRef]
- Qian, T.; Li, J.; Min, X.; Guan, W.; Deng, Y.; Ning, L. Enhanced thermal conductivity of PEG/diatomite shape-stabilized phase change materials with Ag nanoparticles for thermal energy storage. J. Mater. Chem. A 2015, 3, 8526–8536. [Google Scholar] [CrossRef]
- Tonapi, S.S.; Zhong, H.; Simone, D.L.; Fillion, R.A. Thermal Conductive Material Utilizing Electrically Conductive Nanoparticles. U.S. Patent No. 7,550,097, 23 June 2009. [Google Scholar]
- Nurul, M.S.; Mariatti, M. Effect of thermal conductive fillers on the properties of polypropylene composites. J. Thermoplast. Compos. Mater. 2013, 26, 627–639. [Google Scholar] [CrossRef]
- Ho, C.J.; Gao, J.Y. Preparation and thermophysical properties of nanoparticle-in-paraffin emulsion as phase change material. Int. Commun. Heat Mass Transf. 2009, 36, 467–470. [Google Scholar] [CrossRef]
- Kang, H.U.; Kim, S.H.; Oh, J.M. Estimation of thermal conductivity of nanofluid using experimental effective particle volume. Exp. Heat Transf. 2006, 19, 181–191. [Google Scholar] [CrossRef]
- Salaün, F.; Devaux, E.; Bourbigot, S.; Rumeau, P.; Chapuis, P.O.; Saha, S.K.; Volz, S. Polymer nanoparticles to decrease thermal conductivity of phase change materials. Thermochim. Acta 2008, 477, 25–31. [Google Scholar] [CrossRef]
- Silakhori, M.; Metselaar, H.S.C.; Mahlia, T.M.I.; Fauzi, H. Preparation and characterisation of microencapsulated paraffin wax with polyaniline-based polymer shells for thermal energy storage. Mater. Res. Innov. 2014, 18, S6-480. [Google Scholar] [CrossRef]
- Park, M.; Im, J.; Shin, M.; Min, Y.; Park, J.; Cho, H.; Park, S.; Shim, M.B.; Jeon, S.; Chung, D.Y.; et al. Highly stretchable electric circuits from a composite material of silver nanoparticles and elastomeric fibres. Nat. Nanotechnol. 2012, 7, 803–809. [Google Scholar] [CrossRef]
- Li, J.F.; Liu, W.S.; Zhao, L.D.; Zhou, M. High-performance nanostructured thermoelectric materials. NPG Asia Mater. 2010, 2, 152–158. [Google Scholar] [CrossRef]
- Kasap, S. Thermoelectric Effects in Metals. Dep. Electr. Eng. Univ. 2001, 1–11. Available online: http://courses.nus.edu.sg/course/elengv/ee3406/seebeck%20and%20thermocouple.pdf (accessed on 24 March 2020).
- Singh, M.; Hlabana, K.K.; Singhal, S.; Devlal, K. Grain-size effects on the thermal conductivity of nanosolids. J. Taibah Univ. Sci. 2016, 10, 375–380. [Google Scholar] [CrossRef]
- Green, M.; Liu, Z.; Xiang, P.; Liu, Y.; Zhou, M.; Tan, X.; Huang, F.; Liu, L.; Chen, X. Doped, conductive SiO2 nanoparticles for large microwave absorption. Light Sci. Appl. 2018, 7, 1–9. [Google Scholar] [CrossRef]
- Dermanaki Farahani, R.; Gagne, M.; Klemberg-Sapieha, J.E.; Therriault, D. Electrically Conductive Silver Nanoparticles-Filled Nanocomposite Materials as Surface Coatings of Composite Structures. Adv. Eng. Mater. 2016, 18, 1189–1199. [Google Scholar] [CrossRef]
- Xue, C.H.; Chen, J.; Yin, W.; Jia, S.T.; Ma, J.Z. Superhydrophobic conductive textiles with antibacterial property by coating fibers with silver nanoparticles. Appl. Surf. Sci. 2012, 258, 2468–2472. [Google Scholar] [CrossRef]
- Böger, L.; Wichmann, M.H.G.; Meyer, L.O.; Schulte, K. Load and health monitoring in glass fibre reinforced composites with an electrically conductive nanocomposite epoxy matrix. Compos. Sci. Technol. 2008, 68, 1886–1894. [Google Scholar] [CrossRef]
- Ferreira, L.M.P.; Bayraktar, E.; Robert, M.H. Magnetic and electrical properties of aluminium matrix composite reinforced with magnetic nano iron oxide (Fe 3 O 4 ). Adv. Mater. Process. Technol. 2016, 2, 165–173. [Google Scholar]
- Pawar, S.P.; Stephen, S.; Bose, S.; Mittal, V. Tailored electrical conductivity, electromagnetic shielding and thermal transport in polymeric blends with graphene sheets decorated with nickel nanoparticles. Phys. Chem. Chem. Phys. 2015, 17, 14922–14930. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Zhao, Z.; Wu, K.; Huang, R.; Liu, T.; Jiang, H.; Chen, F.; Fu, Q. Ultrathin flexible reduced graphene oxide/cellulose nanofiber composite films with strongly anisotropic thermal conductivity and efficient electromagnetic interference shielding. J. Mater. Chem. C 2017, 5, 3748–3756. [Google Scholar] [CrossRef]
- Fang, M.; Li, D.; Lin, H.; Luo, C.; Qi, R.; Peng, H. Flexible and recyclable conductive composite based on few-layered graphene with excellent self-healing capability. Eur. Polym. J. 2018, 108, 536–541. [Google Scholar] [CrossRef]
- Ohde, M.; Ohde, H.; Wai, C.M. Recycling nanoparticles stabilized in water-in-CO 2 microemulsions for catalytic hydrogenations. Langmuir 2005, 21, 1738–1744. [Google Scholar] [CrossRef]
- Huang, Y.; Kormakov, S.; He, X.; Gao, X.; Zheng, X.; Liu, Y.; Sun, J.; Wu, D. Conductive Polymer Composites from Renewable Resources: An Overview of Preparation, Properties, and Applications. Polymers 2019, 11, 187. [Google Scholar] [CrossRef]
- Lee, H.; Song, C.; Hong, Y.S.; Kim, M.S.; Cho, H.R.; Kang, T.; Shin, K.; Choi, S.H.; Hyeon, T.; Kim, D.H. Wearable/disposable sweat-based glucose monitoring device with multistage transdermal drug delivery module. Sci. Adv. 2017, 3, 1–9. [Google Scholar] [CrossRef]
- Magu, T.O.; Agobi, A.U.; Hitler, L.; Dass, P.M. A Review on Conducting Polymers-Based Composites for Energy Storage Application. J. Chem. Rev. 2019, 1, 19–34. [Google Scholar]
- Lonjon, A.; Demont, P.; Dantras, E.; Lacabanne, C. Low filled conductive P(VDF-TrFE) composites: Influence of silver particles aspect ratio on percolation threshold from spheres to nanowires. J. Non-Cryst. Solids 2012, 358, 3074–3078. [Google Scholar] [CrossRef]
- Roy, A. Effect of Multi-Walled Carbon Nanotubes on Automotive and Aerospace Applications—Case Study. Int. J. Emerg. Trends Sci. Technol. 2017, 5, 5102–5113. [Google Scholar] [CrossRef]
- Pradhan, S.; Hedberg, J.; Blomberg, E.; Wold, S.; Odnevall Wallinder, I. Effect of sonication on particle dispersion, administered dose and metal release of non-functionalized, non-inert metal nanoparticles. J. Nanopart. Res. 2016, 18, 285. [Google Scholar] [CrossRef] [PubMed]
- Müller, K.; Bugnicourt, E.; Latorre, M.; Jorda, M.; Sanz, Y.E.; Lagaron, J.M.; Miesbauer, O.; Bianchin, A.; Hankin, S.; Bölz, U.; et al. Review on the processing and properties of polymer nanocomposites and nanocoatings and their applications in the packaging, automotive and solar energy fields. Nanomaterials 2017, 7, 74. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhu, C.; Pfattner, R.; Yan, H.; Jin, L.; Chen, S.; Molina-Lopez, F.; Lissel, F.; Liu, J.; Rabiah, N.I.; et al. A highly stretchable, transparent, and conductive polymer. Sci. Adv. 2017, 3, 1–11. [Google Scholar] [CrossRef] [PubMed]
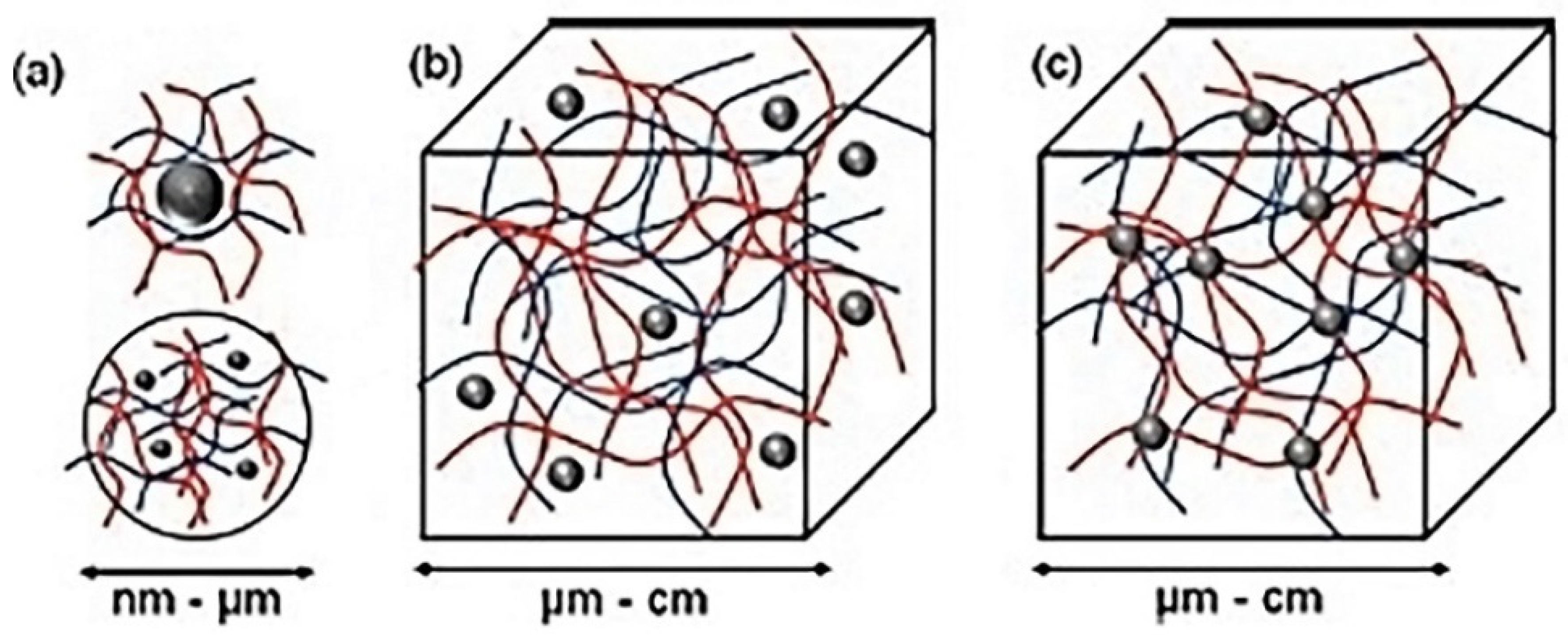



| Properties | Feature Sizes (nm) at Which Changes Might Be Expected |
|---|---|
| Catalytic activity | <5 |
| Making hard magnetic materials soft | <20 |
| Producing refractive index changes | <50 |
| Producing super paramagnetism and others electromagnetic phenomena | <100 |
| Producing strengthening and toughening | <100 |
| Modifying hardness and plasticity | <100 |
| Samples | PEG Mass Ratio (wt %) | Melting Process | Solidifying Process | ||
|---|---|---|---|---|---|
| HM (J/g) | TM (°C) | HS (J/g) | TS (°C) | ||
| PEG PCM | 100 | 180.3 | 60.51 | 164.6 | 38.5 |
| ss-PCM1 | 30 | 51.5 | 59.21 | 46.1 | 38.63 |
| ss-PCM2 | 40 | 70.2 | 58.86 | 64.1 | 39.21 |
| ss-PCM3 | 50 | 88.4 | 59.13 | 80.3 | 40.54 |
| ss-PCM4 | 63 | 111.3 | 59.45 | 102.4 | 41.02 |
| ss-PCM5 | 63 | 110.7 | 59.83 | 103.3 | 39.54 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coetzee, D.; Venkataraman, M.; Militky, J.; Petru, M. Influence of Nanoparticles on Thermal and Electrical Conductivity of Composites. Polymers 2020, 12, 742. https://doi.org/10.3390/polym12040742
Coetzee D, Venkataraman M, Militky J, Petru M. Influence of Nanoparticles on Thermal and Electrical Conductivity of Composites. Polymers. 2020; 12(4):742. https://doi.org/10.3390/polym12040742
Chicago/Turabian StyleCoetzee, Divan, Mohanapriya Venkataraman, Jiri Militky, and Michal Petru. 2020. "Influence of Nanoparticles on Thermal and Electrical Conductivity of Composites" Polymers 12, no. 4: 742. https://doi.org/10.3390/polym12040742
APA StyleCoetzee, D., Venkataraman, M., Militky, J., & Petru, M. (2020). Influence of Nanoparticles on Thermal and Electrical Conductivity of Composites. Polymers, 12(4), 742. https://doi.org/10.3390/polym12040742








