Abstract
The lactic acid (LA) production from corn stover using Lewis acid catalysts was optimized. Initially, an equimolar mixture of Al(III)/Sn(II) was used as a catalytic system at 190 °C with 5 wt% biomass. Increasing the catalyst concentration led to higher LA production, showing the optimal results at 16 mM. A low catalyst concentration mainly produced furfural and HMF, dehydration products from the corn stover sugars. Higher catalyst concentration increased LA yield but also produced the degradation of the glucose dehydration products into levulinic and formic acids, reducing LA selectivity. Al(III) was essential for LA formation, while Sn(II) was less effective due to its lower solubility, shown by the presence of Sn(II) in the solid residue after treatments. A total of 16 mM Al(III) yielded the highest LA levels at 190 °C, 7.4 g/L, and 20.7% yield. Increasing the temperature to 210 °C accelerated the LA production while also achieving the lowest energy consumption, which was 0.47 kWh/g LA at the highest LA production point. However, longer treatments at this temperature caused LA degradation. AlCl3 has been identified as an ideal catalyst for biomass conversion to LA, being inexpensive and low in toxicity.
1. Introduction
In order to decrease the dependence on fossil fuels, different strategies are being proposed to create biorefineries that transform renewable biomass into various valuable bioproducts, such as energy, fuels, and certain platform chemicals. Biomass can be considered the total amount of material derived from living organisms within a given space at a given time [1]. Biomass sources can vary based on their composition, with lignocellulosic biomass being the most abundant on Earth, with a global yield of approximately 200 billion tons [2]. Lignocellulosic biomass is composed of three different biopolymers: cellulose, hemicellulose, and lignin. In 2023, the global market for lignocellulosic biomass was valued at USD 5.68 billion and is expected to grow to USD 9.18 billion by the end of 2030 [3].
Agricultural harvesting residues, an example of lignocellulosic biomass, have shown potential as carbon sources for the production of a variety of chemicals. A recent study evaluating the potential of agroforestry residues for the bioeconomy in the European Union identified wheat straw as the most promising lignocellulosic material, followed by corn stover [4]. The residue-to-crop ratio of corn stover, averaging 1.13, along with its chemical composition (17 ± 3 wt% of lignin, 37 ± 3 wt% of cellulose, and 26 ± 4 wt% of hemicellulose), makes a raw material that is of great interest in modern biorefineries [4].
This study focused on the conversion of lignocellulosic biomass into valuable organic acids, which serve as platform chemicals. This conversion properly aligns with the principle of high atomic economy since elements such as carbon and oxygen are retained in the acids [5,6]. In contrast to current petroleum methods that form C–O bonds by adding oxygen atoms, breaking down oxygen-rich natural biomass into organic acids offers a sustainable production approach [7]. Lactic acid (LA) stands out among organic acids as a highly adaptable bio-based chemical, finding extensive use across the food, chemical, and pharmaceutical sectors. It is the monomer for the production of biodegradable lactic acid polymers (PLA). Furthermore, LA can be converted to value-added products, which are also widely used in different industries The global market size of LA in 2023 was USD 3.37 billion and is expected to grow at an average rate of 8.0% from 2024 to 2030 [8].
LA is currently produced through chemical synthesis or fermentation, with 90% of industrial LA production currently achieved via biological fermentation [9]. As a drawback, fermentation requires a long operation time (2–8 days), resulting in low productivity. Furthermore, industrial fermentation typically uses hexose sugars or easily hydrolyzable polysaccharides from starch [5], which leads to competition for food resources.
Biorefineries face a major challenge due to the structural complexity of the raw materials, which complicates processing. To break down the structure of lignocellulosic biomass, different pretreatments are necessary. Compared to starch-based biomass sources like corn kernels, lignocellulosic biomass generally demands a more rigorous treatment to achieve the extraction of sugars [10].
Hydrothermal treatments, or subcritical water (subW), have emerged as a sustainable and green alternative to produce a variety of second-generation chemicals from lignocellulosic materials. This technology offers a promising approach to decomposing the solid biopolymeric structures of biomass into liquid components in a hot and pressurized aqueous environment. Under subcritical conditions, water remains in the liquid state above its boiling point by increasing pressure, and it exhibits unique properties, becoming an effective catalyst and reaction medium. Hydrothermal treatment of pure sugar monosaccharides, such as xylose and glucose, without any external catalyst, mainly yields the dehydration products of these sugars, furfural and hydroxymethyl furfural (HMF), respectively [11]. Adding an external catalyst can lead to other monosaccharide degradation routes that produce valuable chemicals, such as lactic acid. This approach could replace the current fermentation processes to produce organic acids, offering new processing opportunities, although it still faces several challenges.
Recently, Ca(OH)2 was explored as an affordable and readily available catalyst to optimize lactic acid production from corn stover. The “OH−/sugar monomer” molar ratio emerged as a key design parameter, with an optimum value of 2.61, resulting in 12.4 g LA/L and 32% yield produced during alkaline treatment of a 5 wt% corn stover aqueous suspension [12].
However, a different approach has been also studied in the literature based on the use of Lewis acid catalysts to obtain lactic acid from sugars derived from biomass. It has been reported that trioses, hexoses, and even cellulose can be effectively converted into LA at moderate temperatures by using different Lewis acid metal ions as catalysts. An outstanding yield of LA (68%) was attained in the presence of diluted Pb(II) with a concentration of 7 mM for the conversion of ball-milled cellulose [9]; however, the toxicity of Pb(II) is a concern. Deng et al. studied the catalytic performance of several readily available, cheap, and less toxic single metal cations, concluding that an equimolar combination of AlCl3 with SnCl2 highly facilitated the conversion of fructose, glucose, and ball-milled cellulose to LA, resulting in yields of 90%, 81%, and 65%, respectively, at 463 K for 2 h. The LA yield dropped to 36% when starting from microcrystalline cellulose [5].
Research has also shown that using lanthanides as Lewis acid catalysts significantly enhanced LA yields to 39.5 and 42.9% from xylose and glucose, respectively [13]. When corn stover was used as the feedstock, the LA yield was slightly reduced, reaching approximately 30.3% [13]. Despite these encouraging outcomes, the application of rare earth metals as catalysts remains constrained for large-scale use due to the energy and capital expenses involved [14].
Optimizing hydrothermal catalysis to produce LA not just from pure carbohydrates but directly from lignocellulosic materials like corn stover is essential, as lignocellulosic biomass is more cost-effective and more abundant compared to carbohydrates. As reported by Lai et al. [15], the cost of feedstocks decreases from trioses, pentose, hexoses, disaccharides, and polysaccharides to raw biomass. However, the complexity of reaction networks increases considerably with more complex substrates, with higher requirements on catalysts and harsher reaction conditions.
The main challenge of hydrothermal catalytic processes is the generation of undesired byproducts, and the yields from lignocellulosic materials are generally lower compared to those from pure model compounds due to their complex chemical composition. Different degradation routes have been described in the literature for sugar monomers in hydrothermal processes. Additionally, the cleavage of the C–C bond can take place at various locations within the sugar monomers released from the cellulose and hemicellulose fractions. Consequently, the yield of LA can be reduced by the formation of numerous side-products [10]. HMF can be formed through the dehydration of glucose by acid catalysis, and it is known to degrade into levulinic and formic acids. For example, Kohler et al. [10] reported similar yields of lactic acid and levulinic acid using an Sn-Beta zeolite as a catalyst from glucose at 200 °C and a 2:1 catalyst-to-sugar ratio. In any case, levulinic acid is also an important building block with greater versatility to be upgraded to various value-added products, growing its demand [2].
Based on the high LA yields from pure saccharides reported by Deng et al. [5] using a catalyst mixture of AlCl3/SnCl2, further studies were performed in this work for the optimization of LA production from corn stover. Several effects, including the molar concentration of the catalyst, the ratio of AlCl3/SnCl2 cation metals, the reaction temperature, and the concentration of biomass, were analyzed on LA concentration and yield.
2. Results and Discussion
2.1. Effect of Molar Concentration of an Equimolar Al(III)/Sn(II) Mixture
The effect of the molar concentration of the catalyst was tested in the range from 5 mM to 32 mM for an equimolar mixture of AlCl3/SnCl2, and from now on, Al(III)/Sn(II). Corn stover at 5 wt%.% concentration was treated at 190 °C in subW. Different product distributions were obtained by changing the catalyst molar concentration (Figure 1). By increasing the molar concentration of the catalyst system, an increase in LA concentration and yield was observed. This increase was remarkable in the low concentration range of the catalyst, with values of 1.20 and 2.2 gLA/L at 5 and 9 mM, respectively, while values among 2.9–4.5 gLA/L were obtained at catalyst concentrations higher than 12 mM and up to 32 mM, with yields in the range of 8.2 to 12.3%.

Figure 1.
Kinetic profile of different compounds. (a) Lactic acid, (b) acetic acid, (c) furfural, (d) HMF, (e) levulinic acid, and (f) formic acid in the conversion of corn stover (5 wt%) by using Al(III) /Sn(II) (1/1) at different molar concentrations: ▲ 5 mM, ▲ 9 mM, ▲ 12 mM, ▲ 16 mM, ▲ 24 mM, and ▲ 32 mM at 190 °C.
In the hydrothermal transformation of the polysaccharide fraction of the biomass, a complex network has been described, including dehydration, isomerization, and retro-aldol condensations.
When performing the reaction at 190 °C in the absence of an external catalyst (Figure 2), 49.4% of the initial corn stover was solubilized, mainly the hemicellulose fraction and some of the glucan fraction. This can be clearly observed in the product distribution since the acetyl groups from the hemicellulose are released, yielding a high concentration of acetic acid in the reaction medium, up to 2.5 g/L after 140 min of isothermal treatment time. On the other hand, the dehydration of the pentoses produced furfural in an increasing concentration with time, up to 2.4 g/L. HMF, as the dehydration product from hexoses, was also found in the reaction medium but in a much lower concentration since the solid residue still contained 62% glucans. Therefore, in the absence of a catalyst, the dehydration route is the predominant route. Glyceraldehyde (triose), as a precursor in the retroaldol route, was the predominant triose. Lower amounts of glycolaldehyde (diose) were identified, being also an intermediate in the LA production route through dehydration with a concentration value of 0.37 g/L after treatment.
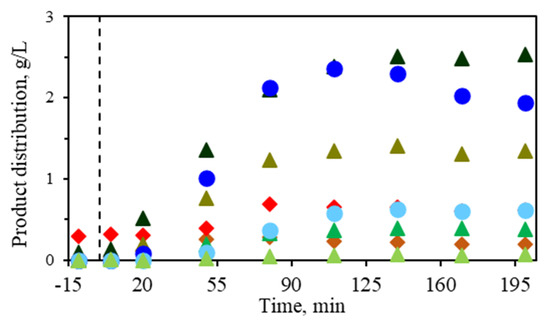
Figure 2.
Corn stover treatment (5 wt%) at 190 °C in the absence of catalyst: furfural (●), HMF (●), acetic acid (▲), formic acid (▲), lactic acid (▲), levulinic acid (▲), glyceraldehyde (◆), and glycolaldehyde (◆).
Rasrendra et al. [16] also indicated that when reacting glyceraldehyde or dihydroxyacetone in the absence of salt catalysts or with Bronsted acids like HCl, lactic acid was not detected, while the formation of the isomerization products (dihydroxyacetone or glyceraldehyde), the dehydration product (pyruvaldehyde) and brown insoluble products were observed instead.
It can be concluded that the presence of inorganic salts acting as Lewis acidic metal centers is crucial in the production of lactic acid. To check the neat effect of subW as a reaction medium, a study was conducted using glucose as the initial sugar monomer and an equimolar mixture of Al(III)/Sn(II) with water at 90 °C. After 4 h, no glucose breakdown was observed, keeping its concentration constant. This suggests a positive effect between the subW and the Lewis acid catalyst mixture to produce lactic acid from sugar.
When using the equimolar mixture Al(III)/Sn(II), the production of furfural is promoted at low concentration (Figure 1c), reaching a maximum of 4.3 g/L at 5 mM. Lower concentrations of furfural were observed when increasing catalyst concentration, probably due to its degradation to humins and other products, such as formic acid, and the promotion of the retro aldol route to produce LA. In fact, different chloride metals have been proven as optimum catalysts in the formation of furfural from lignocellulosic biomass, with furfural yields slightly higher than 50% from a xylose solution of 73 mM at 180 °C when using metal salt chloride in the range of 0.83 mM to 0.91 mM of CrCl3 and AlCl3, respectively [17]. A similar behavior was observed for HMF, but the highest concentration of HMF was lower than furfural, with values of 1.2 g/L and concentrations lower than 0.1 g/L for concentrations of catalyst higher than 16 mM. By increasing metal concentration, the concentration of the glyceraldehyde and glycolaldehyde decreased dramatically, limiting the LA production.
Therefore, high concentrations of Lewis acid promote the degradation of dehydration products and LA formation. In this regard, it is well known that water can rehydrate HMF to form levulinic and formic acids [18]. At low molar catalyst concentrations (5–9 mM), the levulinic acid concentration was low, from 0 to 0.3 g/L. A higher concentration of formic acid was obtained, probably from the degradation of other components present in the reaction medium, such as furfural or xylose, as has been described in the literature [19]. By increasing the catalyst molar concentration, the levulinic acid formation increased, similar to formic acid.
To support the statement that Lewis acids catalyze the conversion of HMF into levulinic and formic acids, two separate subW experiments (initial concentration of HMF was 114 mM) were carried out at 190 °C using two different molar concentrations of the catalyst equimolar mixture, 24 mM and 5 mM, to check its effect on the degradation of HMF (Figure 3a).
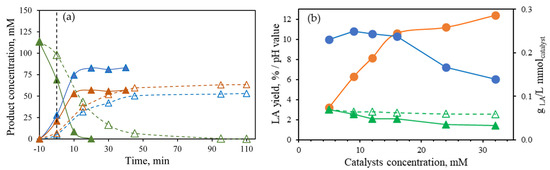
Figure 3.
(a) HMF degradation at 190 °C at different molar concentrations by using an equimolar mixture Al(III)/Sn(II). Filled symbols 24 mM, open symbols 5 mM: (△, ▲) HMF, (△, ▲) formic acid, and (△, ▲) levulinic acid. (b) Final lactic acid yield (●), lactic acid productivity per mmol of catalyst (●), initial pH value of water and catalyst (△), and medium pH values during treatment of corn stover (5 wt%) at different molar concentrations of the equimolar mixture Al(III)/Sn(II) (▲).
It can be clearly observed that an increase in the catalyst concentration led to a faster increase in the levulinic and formic acid formation. At 5 mM, a slightly higher formic acid concentration was obtained (63.2 mM, 9.3% yield) than levulinic acid (52.4 mM, 39% yield). However, at 24 mM, after 20 min of isothermal time, HMF was completely degraded, and the corresponding final concentrations of levulinic and formic acids were 83 mM (61.6% yield) and 57 mM (8.4% yield), respectively, highlighting that no equimolar concentrations were obtained. Rasrendra et al. [16] also reported that when an aqueous solution of HMF (0.1 M) and AlCl3 (5 mM) were heated to 180 °C for 1 h, 90% HMF conversion was observed, and levulinic acid was obtained in 50 mol% yield. These authors also observed that in the conversion of D-glucose using AlCl3 (Cglucose = 0.1 mol/L, Csalt = 5 mM) at 140 °C, levulinic and formic acids were not formed in a 1:1 molar ratio, with the amount of formic acid being slightly higher than that of levulinic acid, similar to the results presented in Figure 3a. These authors did not propose a sound explanation for this observation, suggesting that additional formic acid is formed by yet unknown pathways. In the present work, when treating corn stover at 190 °C at different molar concentrations of the equimolar mixture of Al(III)/Sn(II), a higher molar concentration of formic acid was obtained than for levulinic acid. For instance, at 24 mM of the equimolar mixture of Al(III) /Sn(II), formic acid molar concentration was 69.5 mM (3.2 g/L), while levulinic acid concentration was 43.1 mM (5 g/L). As previously published by Illera et al. [19], formic acid could be formed as a degradation product of furfural and also from xylose. According to Lange et al. [20], protonation at C3-OH of xylose would result in decomposition to formic acid and a C4 fragment, which degrades very rapidly, and it is not observed experimentally, according to these authors.
Figure 3b plots the final LA yield achieved using the different catalyst molar concentrations and the LA productivity per mmol of catalyst used after the treatment of corn stover at 190 °C. As previously described, LA yield significantly increased when the catalyst concentration ranged from 5 to 16 mM. Beyond 16 mM, the increase in LA yield became less significant. When plotting productivity in terms of LA produced per mmol of catalyst, relatively consistent productivity is noted up to 16 mM. At higher concentrations, productivity declines. Therefore, 16 mM was considered the optimum catalyst concentration to maintain high selectivity for LA and also high LA productivity.
Figure 3b also shows the initial pH of the water and catalyst mixtures and the medium pH during the corn stover treatments. As the catalyst molar concentration increased, the final pH decreased, which can be attributed to the higher organic acid concentrations by increasing the catalyst molar concentration.
The values of LA yields contrast with the results previously reported by Deng et al. [5]. These authors reported that the combination of equimolar AlCl3/SnCl2 enabled highly effective conversion of fructose, glucose, and cellulose to LA, affording yields of 90%, 81%, and 65%, respectively. However, other results reported in the literature were lower than the values reported by Deng et al. [5] for pure sugar monomers or polysaccharides. Chambón et al. [21] showed that cellulose could yield 27% and 18.5 mol% of lactic acid by using tungstated zirconia (ZrW) and tungstated alumina (AlW) as Lewis acids. Other authors [10] reported that an LA yield of 13% (graphical lecture) and 17% of levulinic acid (7.5% of formic acid and 2% of acetic acid) were obtained from glucose when using a Sn-Beta zeolite as a catalyst at 200 °C and a 2:1 catalyst-to-sugar ratio (graphical lecture at 3 h, where maximum of LA was reached, then decreased down to 11% after 5 h).
In any case, as has been highlighted by Mäki-Arvela et al. [22], it is more challenging to produce lactic acid from lignocellulosic biomass than to produce it directly from sugar.
2.2. Effect of the Ratio of Al(III)/Sn(II)
Optimizing catalyst properties is an important issue in the conversion of biomass to LA. To evaluate the effect of each metal cation, various Al(III)/Sn(II) molar ratios were tested at a constant concentration of 16 mM (the optimum one), including 1/3, 1/1, 3/1, 5/1, and solely Al(III) at 190 °C (Figure 4).
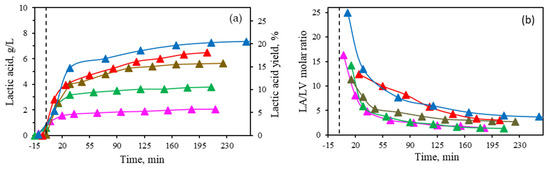
Figure 4.
(a) Lactic acid concentration and yield. (b) Molar ratio of lactic acid to levulinic acid (LA/LV) at different ratios of Al(III)/Sn(II) at 190 °C in the conversion of corn stover (5 wt%): 1/3 (▲), 1/1 (▲), 3/1 (▲), 5/1 (▲), and solely Al (III) (▲).
By increasing the proportion of Al(III), both the concentration and yield of LA improved, with the highest levels achieved using only Al(III) as a catalyst, resulting in 7.4 gLA/L and a yield of 20.7%. In this work, the effect of Sn(II) was found to be not so effective in the conversion of biomass to LA. These findings contrast with those of Deng et al. [5], who reported that an equimolar mixture of Al(III)/Sn(II) worked synergistically, achieving high lactic acid yields.
Supporting the results observed in this work, other studies have indicated that tin ions show low activity and selectivity for LA synthesis in the aqueous phase [23]. Bayu et al. [23] suggested that the low catalytic activity of SnCl2 in pure water is likely due to the hydrolysis of Sn(II) ions, which form insoluble tin (hydr)oxide species. This was evidenced by the formation of a white precipitate of Sn(OH)Cl, as confirmed by X-ray analysis, which removes the active tin species from the aqueous reaction media. These authors observed that the addition of choline chloride (ChCl) into water increased the catalytic activity and the selectivity towards LA in the conversion of trioses, monosaccharides, and disaccharides [23]. At ChCl concentrations of 40–50%, SnCl2 was fully dissolved, and the selectivity towards LA achieved 40–77% under moderate conditions (155–190 °C for 0.1–1.5 h). In contrast, LA selectivity was only ~7% in pure water under the same conditions when SnCl2 was used as a catalyst.
In this work, to account for the lower solubility of Sn(II) species compared to Al(III), these metals were measured using IPC-MS in the solid residue generated after treatment with Al(III)/Sn(II) (5/1) and solely Al(III) as the catalyst. The insoluble salts resulting from the hydrolysis of metal salts (e.g., SnCl2, AlCl3) in water present different solubility. It was determined that in the 5/1 Al(III)/Sn(II) experiment, 70 ± 6% of the initial Sn (II) species added to the medium were found in the solid residue, while only 38 ± 4% of the Al(III) species remained in the solid residue. When only Al(III) was used as the catalyst, 24 ± 6% of the Al(III) species were in the solid residue, supporting the less effective treatment when using Sn(II) in water.
The low activity of Sn(II) was also described in the literature by Lai et al. [15], noting that SnCl2 and SnSO4 result in poor LA selectivity, with the formation of significant quantities of dark brown insoluble products. This suggests that Sn(II) salts will contribute to uncontrollable condensation reactions.
As previously described (Figure 1), other organic acids are present in the reaction medium, with levulinic acid being one of the major compounds formed. The effect of Sn(II) on the molar ratio of lactic acid/levulinic acid is plotted in Figure 4b. It can be observed that by increasing the ratio of Al(III), the molar ratio LA/LV also increased (final values in the range of 1.4 to 3.7 at 1/3 Al(III)/Sn(II) ratio and only Al(III), respectively) since the presence of Sn(II) induced a faster degradation of HMF into levulinic and formic acids. Thus, the presence of Al (III) was found essential for the LA formation.
Rasrendra et al. [16] also reported that the use of aluminum salts results in the formation of considerable amounts of lactic acid, 19% yield, using glucose as raw material (Cglucose = 0.1 M) with a 5 mM Al(III) at 140 °C. Besides the lower solubility of Sn(II) species compared to Al(III) species, according to Azlan et al. [2], Al(III) ions have a small ionic radius, determined as 54 pm [24], which, along with their high charge density and strong electrostatic force, allows them to effectively interact with the substrate, facilitating the cleavage of glycosidic bonds. In contrast, Sn(II) ions have a significantly larger ionic radius of 118 pm [24], which may hinder their ability to attach to the O atoms for breaking glycosidic bonds, as suggested for Sn(IV), with a 69 pm ionic radius, by Azlan et al. [2].
To explore the increased concentration and yield of LA with Al(III), further experiments were conducted using AlCl3 as the sole catalyst, with the Al (III) concentration increased to 32 mM (Figure 5a). However, increasing the Al(III) concentration up to this level did not lead to a higher LA concentration in the medium with the biomass concentration tested in this study, and 16 mM Al (III) was still considered the optimum catalyst concentration. Figure 5a also shows the lactic acid profile obtained with 32 mM of the equimolar mixture Al(III)/Sn(II), equivalent to 16 mM of Al(III), observing lower LA yields. Therefore, it is confirmed that the presence of Sn(II) lowered LA production.
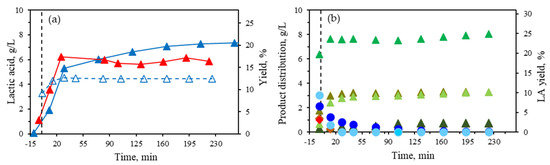
Figure 5.
(a) Lactic acid concentration and yield after corn stover treatment (5 wt%, 190 °C): 32 mM Al (III) (▲), 16 mM Al (III) (▲), and 32 mM equimolar mixture Al(III)/Sn(II) equivalent to 16 mM Al(III) (△). (b) Glucose (20.5 g/L) + xylose (13.1 g/L) feed treated at 190 °C 16 mM Al (III): furfural (●), HMF (●), acetic acid (▲), formic acid (▲), lactic acid (▲), levulinic acid (▲), glyceraldehyde (◆), and glycoaldehyde (◆).
A further kinetic study was performed using pure sugar monomers derived from biomass to compare it with the results obtained from the corn stover treatments using 16 mM Al (III). The feedstock comprised a blend of pure glucose and xylose. The concentrations were selected based on the assumption that the entire glucan and xylan fractions of corn stover were present as free sugar monomers in the reaction medium (glucose 20.5 g/L + xylose 13.1 g/L). The kinetic profile of the identified components has been plotted in Figure 5b. The main component released into the medium was LA, similar to corn stover, with concentrations slightly higher than those obtained from corn stover at 8.1 g/L (24.9% yield). However, the production of LA was significantly faster when starting from pure sugars, as less than 15 min of isothermal treatment was sufficient to achieve stable concentrations of LA. In the case of lignocellulosic biomass, the polysaccharide fraction must first undergo hydrolysis to release sugar monomers, which are then converted into LA, thereby slowing the LA formation rate. Due to the rapid kinetic process, the sugar dehydration product concentrations continuously decreased during the isothermal process when starting from the glucose + xylose mixture. Formic and levulinic acids were found in relatively high concentrations due to the degradation of HMF, as previously demonstrated, although formic acid could also result from the degradation of furfural and xylose. The main difference between the component profiles from corn stover and pure sugars lies in the acetic acid profile. Up to 2.7 g/L were obtained from corn stover due to the hydrolysis of the acetyl groups from hemicellulose, while only 0.75 g/L of acetic was formed from the glucose + xylose mixture.
Therefore, AlCl3 has been found as an ideal catalyst for biomass conversion into LA. Furthermore, as pointed out by Guo et al. [25], Al(III) in chloride form, AlCl3, is inexpensive and has low toxicity.
2.3. Effect of the Corn Stover Concentration
To assess the effect of biomass concentration, two additional biomass loadings were tested besides the 5 wt% previously reported: 0.5 wt% and 2.5 wt%. Experiments were conducted at 190 °C with 16 mM Al(III). Higher biomass concentrations were not attempted due to stirring difficulties, as reported in the study on the alkaline treatment of corn stover to produce LA [12]. During the treatment, the percentage of corn stover that was hydrolyzed was similar across all three biomass loadings, with values of 71%, 69%, and 72% for 0.5 wt%, 2.5 wt%, and 5 wt%, respectively. LA production parameters after 220 min of isothermal treatment at 190 °C are presented in Figure 6. The LA yield showed little variation across the three different biomass loadings tested, in the range from 20 to 21%. However, the LA concentration rose with increasing biomass concentration, reaching 0.7 gLA/L, 3.7 gLA/L, and 7.4 gLA/L at 0.5 wt%, 2.5 wt%, and 5 wt%, respectively. As the LA concentration increased, the molar ratio of lactic acid to levulinic acid (LA/LV) also rose (Figure 6).
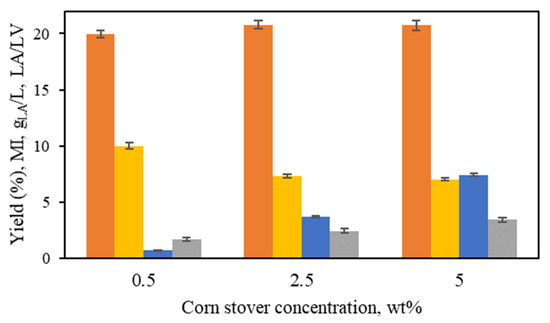
Figure 6.
Lactic acid yield (%, ■), mass intensity (MI, ■), LA concentration (g/L, ■), and molar ratio of lactic acid/levulinic acid (LA/LV, ■).
To evaluate the impact of increased biomass loading and the resulting LA production, mass intensity (MI) was assessed to compare the outcomes at various corn stover loadings in the reactor [26]:
In the ideal situation, MI would approach 1. The total mass includes all components introduced into the reaction vessel, such as solvents, catalysts, and reagents, but excludes water, as water typically does not have a significant environmental impact on its own. Therefore, in the current process, the mass of corn stover and the mass of the Al(III) as catalysts were included.
For a concentration of 0.5 wt%, the MI reached a value of 10.0 ± 0.3, but it decreased to similar values for 2.5 wt% (7.3 ± 0.2) and 5 wt% (7.0 ± 0.1). Considering the higher LA concentration, a biomass loading of 5 wt% was considered as the optimal.
2.4. Effect of Treatment Temperature
Temperature plays a crucial role in determining reaction pathways [15]. The breakage of the C–C bond requires high temperatures. To analyze how temperature affects lactic acid production from corn stover, experiments were conducted at three different temperatures, 190 °C, 210 °C, and 260 °C, with 16 mM Al(III) as the catalyst. Figure 7a illustrates the LA profiles observed in the reaction medium at these three temperatures.
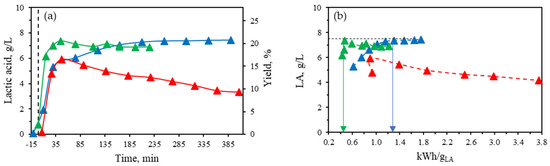
Figure 7.
(a) Lactic acid concentration and yield profile during corn stover treatment (5 wt%) with 16 mM Al(III) at different temperatures. (b) Lactic acid concentration as a function of energy consumption per g of LA produced at different temperatures: (▲) 190 °C, (▲) 210 °C, and (▲) 260 °C.
At 190 °C, a maximum in LA concentration at 7.3–7.4 g/L was reached after 210 min of isothermal treatment time, with no further degradation observed. To further confirm the thermal stability of LA at this temperature, an aqueous LA solution was prepared at a concentration of 5.8 g/L. This concentration remained stable throughout the isothermal treatment at 190 °C for 225 min, maintaining a level of 5.8 ± 0.1 g/L. Previous research using Yb3+ (a Lewis lanthanide) as a catalyst under subcritical water conditions showed that LA suffered degradation at temperatures above 220 °C and isothermal times longer than 20 min, corresponding to severity factors above 5.5 [13]. The severity factor, Ro, is a common parameter that considers the combined effect of temperature and reaction time according to [27]:
At a constant temperature of 190 °C throughout the isothermal treatment, the highest severity factor recorded was 5.2, which is below the threshold of 5.5, where LA degradation begins [13]. By increasing the temperature up to 210 °C, the initial reaction rate of LA formation increased, allowing the maximum LA concentration of 7.4 g/L to be achieved faster after 45 min of isothermal treatment. Longer treatment resulted in LA degradation due to increasing severity factor over time, reaching values of 5.6 by the end of the treatment at 210 °C (225 min). Nonetheless, the degradation curve was smooth, as the concentration decreased from 7.4 to 6.9 g/L after treatment at 210 °C.
At the highest temperature tested in this study, 260 °C, the maximum LA concentration was lower than the maximum levels achieved at 190 °C and 210 °C, measuring 5.9 g/L compared to 7.4 g/L. This suggests that LA degradation began early in the process, as the severity factor after just 7 min of isothermal treatment at 260 °C was already 5.6, surpassing the previously reported threshold of 5.5, beyond which degradation occurs. After reaching this maximum, the rate of degradation exceeded that of production, resulting in a rapid decline.
During the catalytic hydrothermal treatment with Al (III) as the catalyst, the total energy consumption was continuously registered. Figure 7b illustrates the relationship between lactic acid concentration in the reaction medium and the energy consumption per g of LA produced at the three tested temperatures: 190 °C, 210 °C, and 260 °C. As previously described, a comparable maximum concentration of LA was attained at both 190 °C and 210 °C, although this peak was reached more rapidly at 210 °C. Despite the higher energy consumption recorded at 210 °C compared to 190 °C, the earlier attainment of the lactic acid peak resulted in lower energy consumption per gram of lactic acid at the maximum concentration, with values of 0.47 kWh/gLA at 210 °C and 1.2–1.3 kWh/gLA at 190 °C for the same maximum lactic acid concentration achieved in these treatments. Therefore, 210 °C appears to be an optimal temperature for lactic acid production, yielding high concentrations with minimal energy consumption at the peak. Nonetheless, it is advisable to expedite cooling after reaching this maximum, as a slow but continuous degradation of lactic acid was observed at 210 °C, whereas no degradation occurred at 190 °C. At the highest temperature tested, 260 °C, the maximum concentration was lower, accompanied by increased energy consumption per gram of lactic acid due to ongoing degradation.
3. Materials and Methods
3.1. Raw Materials
Corn stover consisting of leaves and stems was used as lignocellulosic raw material, kindly provided by a local farmer from Saldaña (Palencia, Spain). Its moisture content was determined gravimetrically to be 6.2 ± 0.6 wt% by weighing before and after drying in an oven at 105 °C until constant weight was reached. The corn stover was milled using a Retsch SM100 mill (Retsch GmbH, Düsseldorf, Germany) with a 2 mm aperture size for subsequent use. The composition of the corn stover was analyzed following the National Renewable Energy Laboratory (NREL, “Protocols for lignocellulosic biomass characterization”) [28]. The chemical composition of the corn stover used in this work, expressed as a weight percentage on a dry basis (6.0 wt% of moisture content), has been recently reported [12]. The total carbohydrate portion comprised 67.9 wt% (not including acetyl groups in the hemicellulose fraction), with glucan at 39.2 ± 0.9 wt%, xylan at 24.5 ± 0.7 wt%, and arabinan at 4.2 ± 0.2 wt%. Corn stover also contains a considerable amount of acid-insoluble lignin, 15.3 ± 0.4 wt%. Small quantities of protein, lipids, and ash were also detected.
3.2. Lewis Catalysis in Subcritical Water Reaction Medium
The experiments were conducted using a custom-built batch system with a reactor capacity of 0.5 L (maximum pressure: 70 bar). To reach and maintain the required operating temperature, the reactor was covered with a ceramic heating jacket (230 V, 4000 W, diameter 95 mm, height 160 mm). Nitrogen was used to pressurize the system, maintaining a constant pressure of 55 bar. Temperature regulation and monitoring throughout the process were achieved via a Pt100 sensor (TC-direct, Madrid, Spain) inside the reactor, connected to a PID system. A magnetic stirrer at the reactor’s base ensured uniformity in the reaction mixture. To follow the reaction’s progress, samples were withdrawn at selected time intervals through a needle valve (Autoclave Engineers) linked to a cooling mechanism. Zero time was defined as the time when the selected temperature was reached. Once the designated reaction time elapsed, the vessel was cooled and depressurized after the temperature dropped below 90 °C.
In a typical experiment, the selected quantity of corn stover was suspended in 200 mL of distilled water with the required amount of catalyst and then charged into the reactor. A magnetic stirring bar was placed at the bottom of the reactor to enhance homogeneity, with the stirring rate fixed at 700 rpm. Two catalysts were used in this study: AlCl3 (98.5%) from Fisher Scientific and SnCl2 (98%) from Alfa Aesar. The study initially examined organic acid production using an equimolar mixture of Al(III)/Sn(II) at various molar concentrations ranging from 5 mM to 32 mM. These studies were conducted at 190 °C with a 5 wt% biomass concentration, aiming to maximize lactic acid yield. Additionally, an HMF aqueous solution was treated with selected Al(III)/Sn(II) concentrations to explore its degradation into formic and levulinic acids. The study then explored how different Al(III)/Sn(II) affected the selectivity toward lactic acid formation. To further analyze the results, a mixture of the primary sugar monomers glucose and xylose from the polysaccharide fraction of the corn stover was subjected to treatment to investigate the reaction equilibrium and conversion achieved and subsequently compared with the results obtained from corn stover. Finally, the influence of biomass concentration was also evaluated by varying the biomass load in the reactor from 0.5 wt% to 5 wt%, and operation temperature was varied in the range from 190 °C to 260 °C. A summary of all the treatments carried out in this work is collected in Table 1.

Table 1.
Hydrothermal treatments performed to study lactic acid production.
3.3. Analytical Methods
Quantification of Organic Acids, Sugars, and Other Degradation Products
HPLC analysis was employed to determine monomeric sugars, lactic acid, and other sugar-derived compounds, following the method outlined by Alonso-Riaño et al. [29]. The HPLC setup incorporated a Biorad (Hercules, California, USA.) Aminex-HPX-87 H column with its associated pre-column, along with two detection systems: a variable wavelength detector (VWD) set at 210 nm and a refractive index detector (RID). Both the column and the refractive index detector were maintained at 40 °C. Prior to injection, samples (10 μL) were passed through a 0.2 μm syringe filter. The mobile phase consisted of 0.005 M sulfuric acid, flowing at a rate of 0.6 mL/min. Pure standards were used for calibration purposes.
Standards for L(+)-lactic acid solution (40 wt% in water), glucose (99%), xylose (99%), furfural (99%), glyceraldehyde (98%), and glycolaldehyde (98%) were purchased from Sigma-Aldrich (Burlington, Massachusetts). Manose was purchased from Apollo Scientific (Stockport, England) (99.5%). Standards for 5-HMF (97%) were obtained from Alfa Aesar (Ward Hill, Massachusetts, USA), formic acid (98%) from Fluka (Honeywell Research Chemicals, Seelze, Germany), acetic acid (99.8%) from VWR (Avantor, Center Valley, Pennsylvania, USA), glycolic acid (98%) and propionic acid (99%) from TCI (Tokyo, Japan), 1,3-dihydroxyacetone (95%) from Fluorochem (Derbyshire, UK), and hydroxyacetone (95%) from Thermoscientific (Waltham, Massachusetts, USA). The identification of peaks was performed by comparing the retention times of peaks in the sample with those of pure standard compounds.
3.4. Parameters in Lactic Acid Production
The C-yield of LA was assessed by considering the initial carbon content in the feed solution coming from the saccharide fraction and the carbon content in the lactic acid produced:
The carbon yield was used to compensate for the fact that not all products have a similar carbon number as the starting material, following a procedure published by Bicker et al. [30]. The molar ratio of LA to LV was also assessed to evaluate the selectivity of the process toward LA production.
3.5. pH Measurements
The pH values of all collected samples were measured using a GLP 21 pH meter (Crison Instruments S.A., Alella, Barcelona, Spain) after collection and before HPLC analysis.
4. Conclusions
The production of lactic acid from corn stover was investigated through hydrothermal catalysis using Al(III) and Sn(II) chlorides as Lewis acid catalysts. It was observed that Sn(II) was less effective than Al(III) in promoting lactic acid formation from the polysaccharide fraction of lignocellulosic biomass. This reduced efficacy was attributed to the low solubility of Sn(II) species in aqueous solutions, as evidenced by the presence of Sn(II) in the solid residue after treatment. An increase in the proportion of Al(III) in the binary catalyst mixture resulted in a higher molar ratio of lactic acid to levulinic acid, as the presence of Sn(II) accelerated the degradation of the dehydration-sugar compound, HMF, into levulinic and formic acids.
A biomass loading of 5 wt% was considered optimal, as it yielded similar lactic acid results compared to lower biomass loadings, while higher lactic acid concentrations were achieved with increased biomass concentrations. Temperatures ranging from 190 to 210 °C produced high lactic acid yields, with lower energy consumption per gram of lactic acid at 210 °C evaluated at the maximum concentration. However, a slight decline of lactic acid concentration with time due to thermal degradation was noted at 210 °C, which was not observed at 190 °C.
Although the Al(III)/Sn(II) catalytic system was optimized for lactic production from corn stover, it is important to note that if another lignocellulosic biomass is selected, optimum conditions could somehow vary. A difference in the cellulose and hemicellulose content and their configuration in the substrate structure can change the availability of monosaccharides and, therefore, their conversion into lactic acid and other products.
Overall, the production of lactic acid from lignocellulosic biomass remains challenging, with ongoing efforts needed to enhance lactic acid production and selectivity.
Author Contributions
Conceptualization, M.T.S. and A.E.I.; methodology, H.C.; formal analysis, M.T.S., A.E.I., and S.B.; investigation, H.C. and P.B.; data curation, all authors; writing—original draft preparation, M.T.S. and A.E.I.; writing—review and editing, all authors.; supervision, M.T.S. and A.E.I.; funding acquisition, M.T.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Agencia Estatal de Investigación, grant numbers PID2022-136385OB-I00 and TED2021-129311B-I00, and the Junta de Castilla y León (JCyL) and the European Regional Development Fund (ERDF), grant numbers BU027P23. A.E. Illera’s post-doctoral contract was funded by BU027P23. H. Candela’s pre-doctoral contract was funded by JCyL and the European Social Fund [ORDEN EDU/1009/2024]. P. Barea’s pre-doctoral contract is funded by JCyL and the European Social Fund (ESF) [ORDEN EDU/1868/2022].
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Segers, B.; Nimmegeers, P.; Spiller, M.; Tofani, G.; Jasiukaityte-Grojzdek, E.; Dace, E.; Kikas, T.; Marchetti, J.M.; Rajic, M.; Yildiz, G.; et al. Lignocellulosic Biomass Valorisation: A Review of Feedstocks, Processes and Potential Value Chains and Their Implications for the Decision-Making Process. RSC Sustain. 2024, 2, 3730–3749. [Google Scholar] [CrossRef]
- Azlan, N.S.M.; Yap, C.L.; Gan, S.; Rahman, M.B.A. Recent Advances in the Conversion of Lignocellulosic Biomass and Its Degraded Products to Levulinic Acid: A Synergy of Brønsted-Lowry Acid and Lewis Acid. Ind. Crops Prod. 2022, 181, 114778. [Google Scholar] [CrossRef]
- Available online: https://virtuemarketresearch.com/report/lignocellulosic-biomass-market (accessed on 16 April 2025).
- Thorenz, A.; Wietschel, L.; Stindt, D.; Tuma, A. Assessment of Agroforestry Residue Potentials for the Bioeconomy in the European Union. J. Clean. Prod. 2018, 176, 348–359. [Google Scholar] [CrossRef]
- Deng, W.; Wang, P.; Wang, B.; Wang, Y.; Yan, L.; Li, Y.; Zhang, Q.; Cao, Z.; Wang, Y. Transformation of Cellulose and Related Carbohydrates into Lactic Acid with Bifunctional Al(III)-Sn(II) Catalysts. Green Chem. 2018, 20, 735–744. [Google Scholar] [CrossRef]
- Yan, W.; Guan, Q.; Jin, F. Catalytic Conversion of Cellulosic Biomass to Harvest High-Valued Organic Acids. iScience 2023, 26, 107933. [Google Scholar] [CrossRef]
- Jyoti; Pandey, N.; Negi, P.; Singh, M.; Mishra, B.B. Hydrothermal Depolymerization of Spent Biomass for Production of Lactic Acid and Small Aromatics. Clean. Chem. Eng. 2024, 9, 100116. [Google Scholar] [CrossRef]
- MarketsandMarketsTM. Lactic Acid and Polylactic Acid Market; Global Forecast to 2028, Report Code: FB2647, 2023.
- Wang, Y.; Deng, W.; Wang, B.; Zhang, Q.; Wan, X.; Tang, Z.; Wang, Y.; Zhu, C.; Cao, Z.; Wang, G.; et al. Chemical Synthesis of Lactic Acid from Cellulose Catalysed by Lead(II) Ions in Water. Nat. Commun. 2013, 4, 2141. [Google Scholar] [CrossRef] [PubMed]
- Kohler, A.; Seames, W.; Foerster, I.; Kadrmas, C. Catalytic Formation of Lactic and Levulinic Acids from Biomass Derived Monosaccarides through Snbeta Formed by Impregnation. Catalysts 2020, 10, 1219. [Google Scholar] [CrossRef]
- Alonso-Riaño, P.; Illera, A.E.; Benito-Román, O.; Melgosa, R.; Bermejo-López, A.; Beltrán, S.; Sanz, M.T. Degradation Kinetics of Sugars (Glucose and Xylose), Amino Acids (Proline and Aspartic Acid) and Their Binary Mixtures in Subcritical Water: Effect of Maillard Reaction. Food Chem. 2024, 442, 138421. [Google Scholar] [CrossRef]
- Candela, H.; Illera, A.E.; Barea, P.; Ruiz, M.O.; Beltrán, S.; Sanz, M.T. Optimization of Second-generation Lactic Acid from Corn Stover by Alkaline Catalysis in Subcritical Water Reaction Medium. Biofuels Bioprod. Biorefining 2025, 1–14. [Google Scholar] [CrossRef]
- Bermejo-López, A.; Illera, A.E.; Melgosa, R.; Beltrán, S.; Sanz, M.T. Comparative Selective Conversion of Biomass-Derived Mono- and Polysaccharides into Lactic Acid with Lanthanide Lewis Acid Catalysts. Food Bioproc. Tech. 2024, 17, 4851–4867. [Google Scholar] [CrossRef]
- Saviano, L.; Brouziotis, A.A.; Suarez, E.G.P.; Siciliano, A.; Spampinato, M.; Guida, M.; Trifuoggi, M.; Del Bianco, D.; Carotenuto, M.; Spica, V.R.; et al. Catalytic Activity of Rare Earth Elements (REEs) in Advanced Oxidation Processes of Wastewater Pollutants: A Review. Molecules 2023, 28, 6185. [Google Scholar] [CrossRef]
- Lai, R.; Qu, F.; Ju, M.; Xie, C.; Qian, H.; Xia, T.; Wang, C.; Yu, G.; Tang, Y.; Bai, X.; et al. Review on Synthesis of Lactic Acid and Lactates from Biomass Derived Carbohydrates via Chemocatalysis Routes. Bioresour. Technol. 2025, 419, 132031. [Google Scholar] [CrossRef]
- Rasrendra, C.B.; Makertihartha, I.G.B.N.; Adisasmito, S.; Heeres, H.J. Green Chemicals from D-Glucose: Systematic Studies on Catalytic Effects of Inorganic Salts on the Chemo-Selectivity and Yield in Aqueous Solutions. Proc. Top. Catal. 2010, 53, 1241–1247. [Google Scholar] [CrossRef]
- Illera, A.E.; Candela, H.; Bermejo-López, A.; Barea, P.; Alonso-Riaño, P.; Benito-Román, Ó.; Beltrán, S.; Sanz, M.T. Evaluation of Homogeneous and Heterogeneous Catalytic Strategies for Furfural Production from Sugar-Derived Biomass in a Solvent-Free Green Pressurized Reaction Media (Subcritical Water-CO2). Biomass Bioenergy 2024, 187, 107304. [Google Scholar] [CrossRef]
- Choudhary, V.; Mushrif, S.H.; Ho, C.; Anderko, A.; Nikolakis, V.; Marinkovic, N.S.; Frenkel, A.I.; Sandler, S.I.; Vlachos, D.G. Insights into the Interplay of Lewis and Brønsted Acid Catalysts in Glucose and Fructose Conversion to 5-(Hydroxymethyl)Furfural and Levulinic Acid in Aqueous Media. J. Am. Chem. Soc. 2013, 135, 3997–4006. [Google Scholar] [CrossRef]
- Illera, A.E.; Candela, H.; Beltrán, S.; Sanz, M.T. Insights into the Kinetics of Furfural Production from Different Monomers and Polymers Derived from Biomass in a Subcritial Water Reaction Medium Intensified by CO2 as Pressurization Agent. Biomass Bioenergy 2025, 193, 107550. [Google Scholar] [CrossRef]
- Lange, J.P.; Van Der Heide, E.; Van Buijtenen, J.; Price, R. Furfural-A Promising Platform for Lignocellulosic Biofuels. ChemSusChem 2012, 5, 150–166. [Google Scholar] [CrossRef]
- Chambon, F.; Rataboul, F.; Pinel, C.; Cabiac, A.; Guillon, E.; Essayem, N. Cellulose Hydrothermal Conversion Promoted by Heterogeneous Brønsted and Lewis Acids: Remarkable Efficiency of Solid Lewis Acids to Produce Lactic Acid. Appl. Catal. B 2011, 105, 171–181. [Google Scholar] [CrossRef]
- Mäki-Arvela, P.; Simakova, I.L.; Salmi, T.; Murzin, D.Y. Production of Lactic Acid/Lactates from Biomass and Their Catalytic Transformations to Commodities. Chem. Rev. 2014, 114, 1909–1971. [Google Scholar] [CrossRef]
- Bayu, A.; Yoshida, A.; Karnjanakom, S.; Kusakabe, K.; Hao, X.; Prakoso, T.; Abudula, A.; Guan, G. Catalytic Conversion of Biomass Derivatives to Lactic Acid with Increased Selectivity in an Aqueous Tin(Ii) Chloride/Choline Chloride System. Green Chem. 2018, 20, 4112–4119. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised Effective Ionic Radii and Systematic Studies of Interatomie Distances in Halides and Chaleogenides. Acta Cryst. 1976, A32, 751–767. [Google Scholar] [CrossRef]
- Guo, W.; Heeres, H.J.; Yue, J. Continuous Synthesis of 5-Hydroxymethylfurfural from Glucose Using a Combination of AlCl3 and HCl as Catalyst in a Biphasic Slug Flow Capillary Microreactor. Chem. Eng. J. 2020, 381, 122754. [Google Scholar] [CrossRef]
- Jiménez-González, C.; Constable, D.J.C. Green Chemistry and Engineering: A Practical Design Approach; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011. [Google Scholar]
- Alonso-Riaño, P.; Ramos, C.; Trigueros, E.; Beltrán, S.; Sanz, M.T. Study of Subcritical Water Scale-up from Laboratory to Pilot System for Brewer’s Spent Grain Valorization. Ind. Crops. Prod. 2023, 191, 115927. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass: Laboratory Analytical Procedure (LAP) (Revised July 2011); 2008. [Google Scholar]
- Alonso-Riaño, P.; Sanz, M.T.; Benito-Román, O.; Beltrán, S.; Trigueros, E. Subcritical Water as Hydrolytic Medium to Recover and Fractionate the Protein Fraction and Phenolic Compounds from Craft Brewer’s Spent Grain. Food Chem. 2021, 351, 129264, 1–10. [Google Scholar] [CrossRef]
- Bicker, M.; Endres, S.; Ott, L.; Vogel, H. Catalytical Conversion of Carbohydrates in Subcritical Water: A New Chemical Process for Lactic Acid Production. J. Mol. Catal. A Chem. 2005, 239, 151–157. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).