Enzymatic Production of p-Methoxycinnamate Monoglyceride Under Solventless Conditions: Kinetic Analysis and Product Characterization
Abstract
1. Introduction
2. Results
2.1. Preliminary Experiments
2.1.1. Solubility of Methoxycinnamic Acid in Glycerol/Water Mixtures
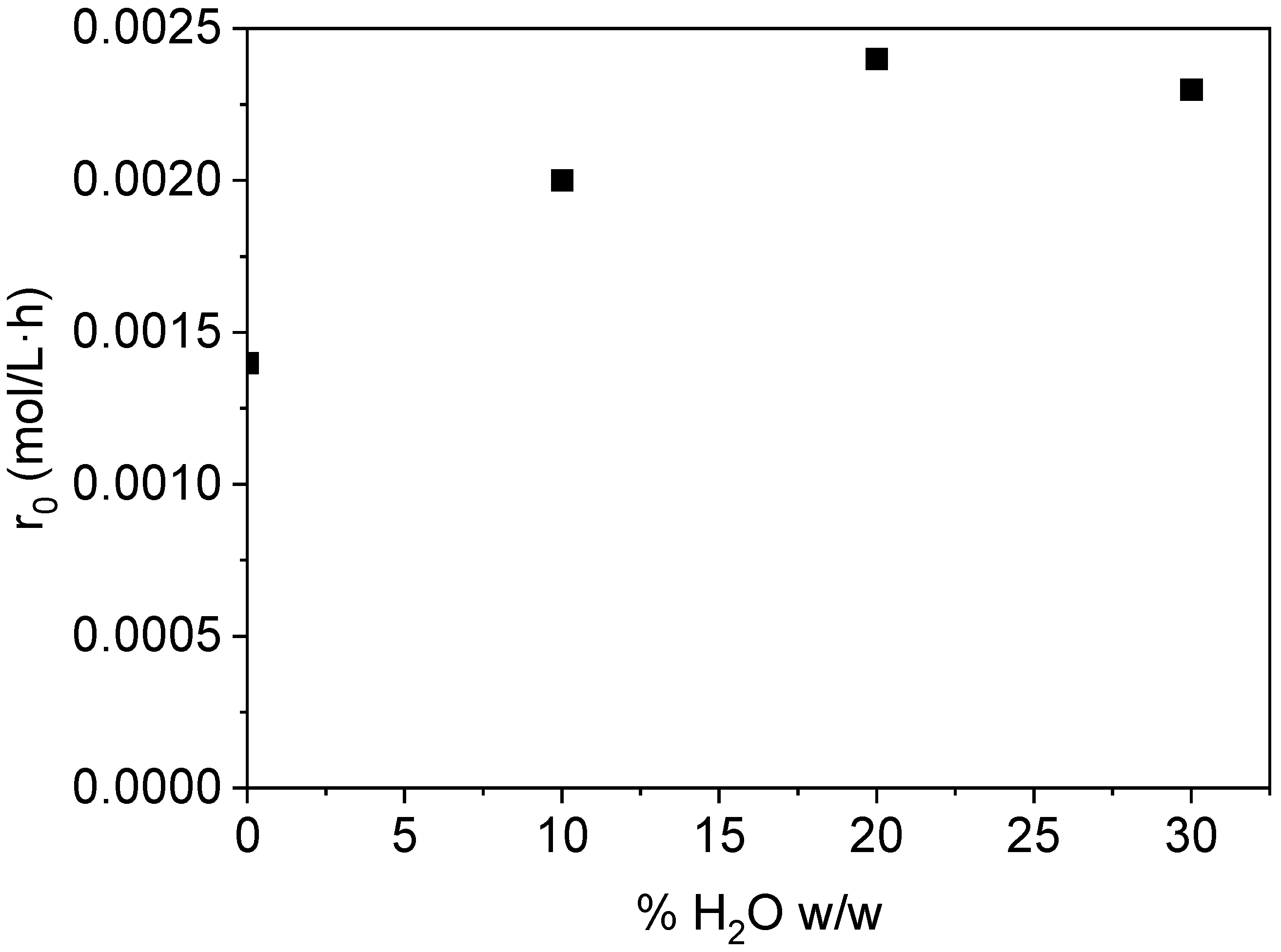
2.1.2. Internal Mass Transfer Assessment: Effect of Water Addition
2.1.3. Internal Mass Transfer Assessment: Effect of Particle Size
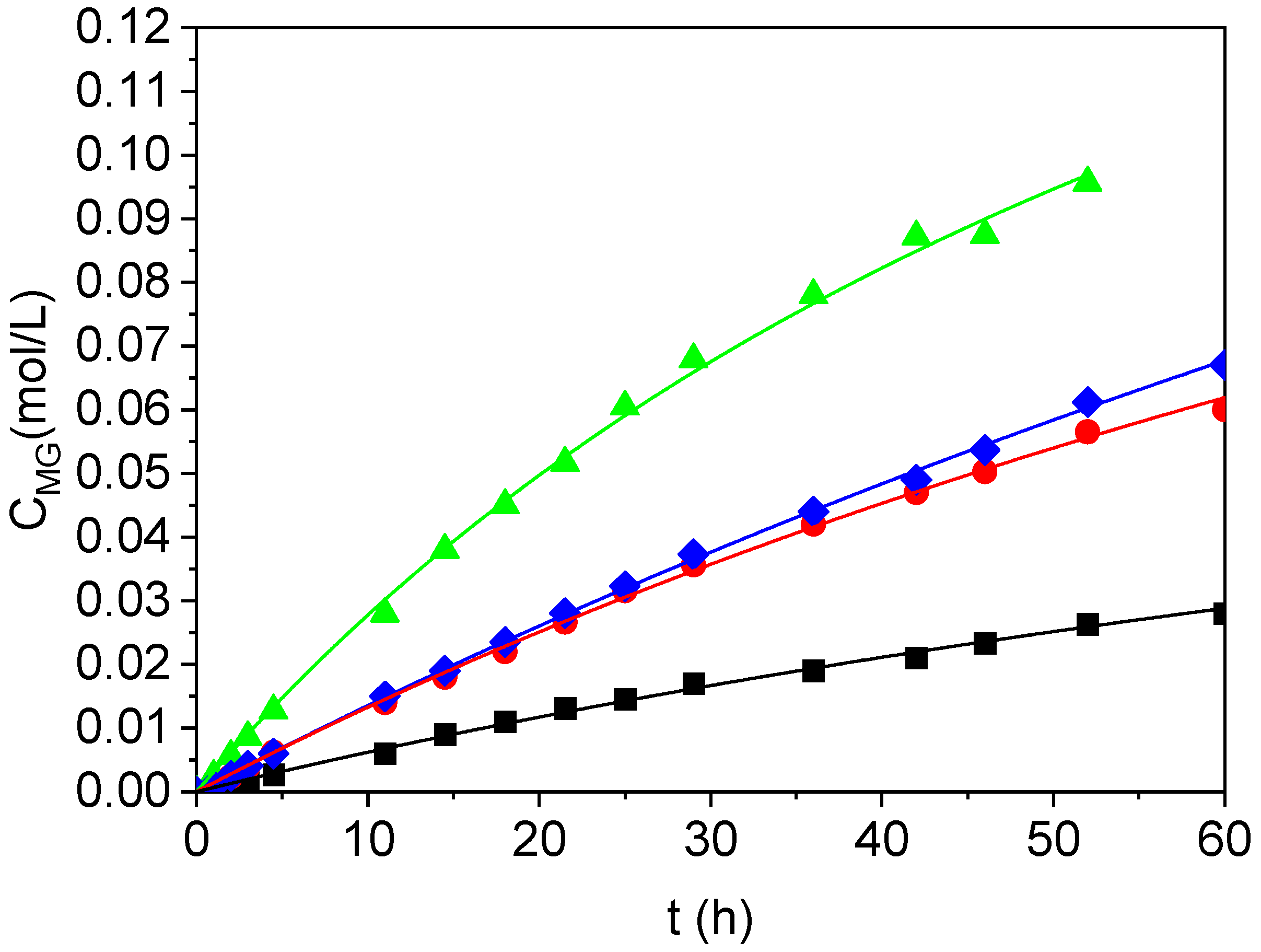
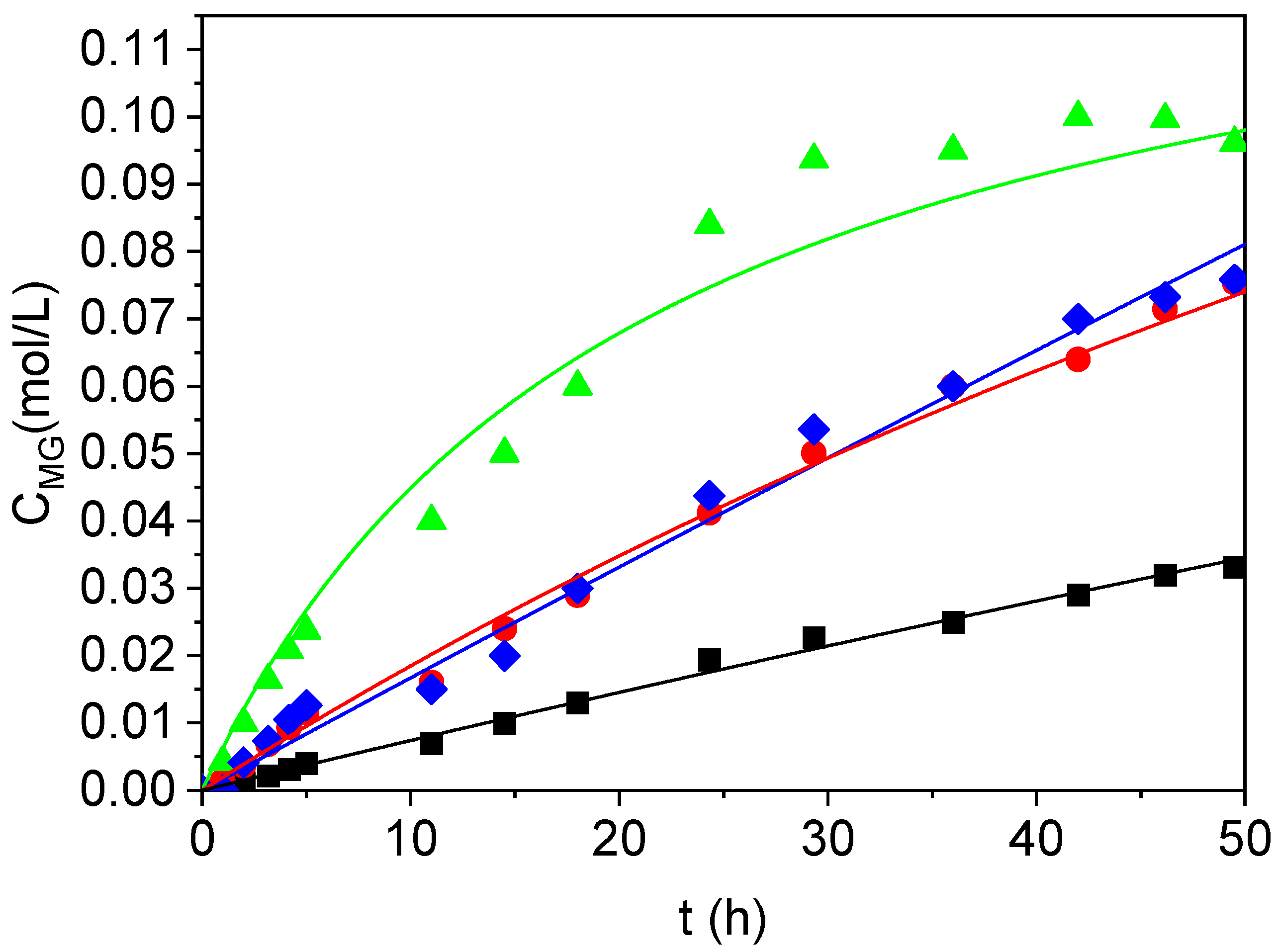
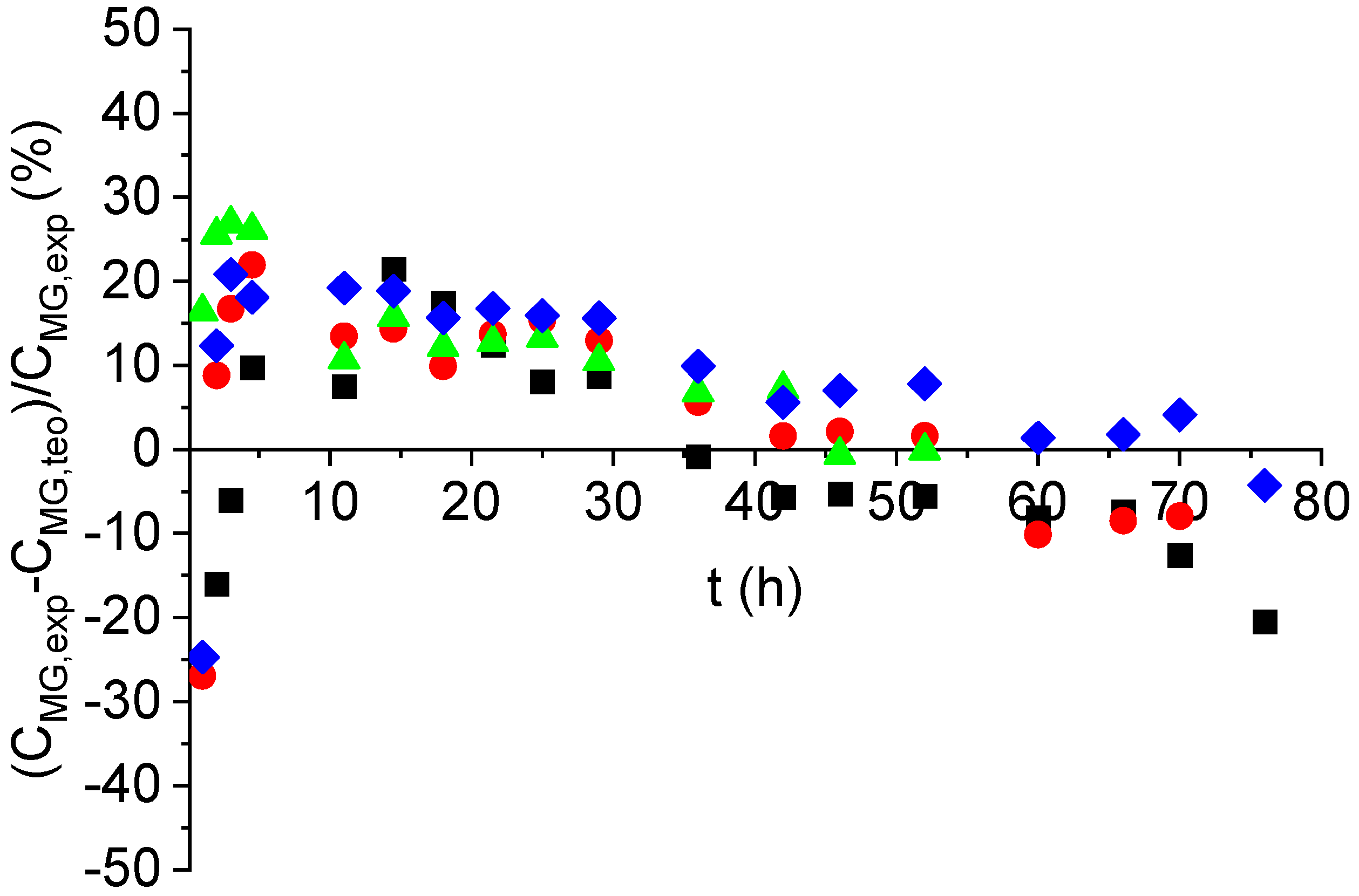
2.2. Kinetic Modeling
2.3. Product Characterization
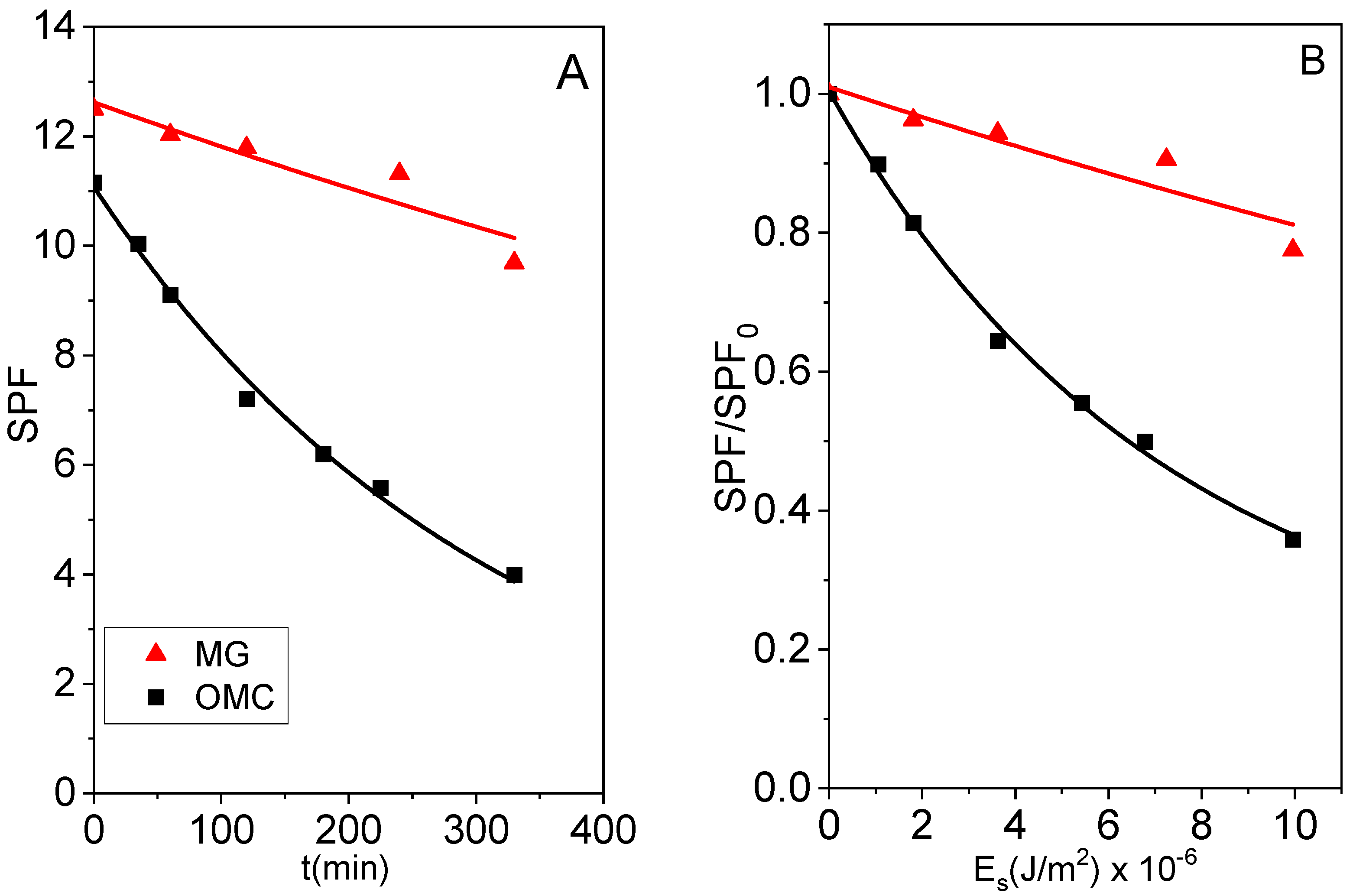
2.3.1. UV Filter Activity (SPF)
2.3.2. In Vitro Photostability
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Equipment
4.2.1. Experimental Facility
4.2.2. Analysis of Samples
4.3. Methods
4.3.1. Development of an Esterification Experiment
4.3.2. High Performance Liquid Chromatography (HPLC)
4.3.3. Mathematical Analysis: Kinetic Model Fitting
4.3.4. Determination of Product Properties
- Activity test: Measurement of sun protection factor (SPF)
- Photostability test in solar simulators
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Malozyomov, B.V.; Martyushev, N.V.; Kukartsev, V.V.; Tynchenko, V.S.; Bukhtoyarov, V.V.; Wu, X.; Tyncheko, Y.A.; Kukartsev, V.A. Overview of methods for enhanced oil recovery from conventional and unconventional reservoirs. Energies 2023, 16, 4907. [Google Scholar] [CrossRef]
- Wang, Z.; Fan, Z.; Chen, X.; Li, Y.; Fan, Z.; Wei, Q.; Peng, Y.; Liu, B.; Yue, W.; Wang, X.; et al. Global oil and gas development situation, trends and enlightenment in 2023. Petrol. Explor. Devel. 2024, 51, 1536–1555. [Google Scholar] [CrossRef]
- Reynolds, D.B. US shale oil production and trend estimation: Forecasting a Hubbert model. Econ. Inq. 2024, 62, 468–487. [Google Scholar] [CrossRef]
- Deming, D.M. King Hubbert and the rise and fall of peak oil theory. AAPG Bull. 2023, 107, 851–861. [Google Scholar] [CrossRef]
- Goswami, L.; Kayalvizhi, R.; Dikshit, P.K.; Sherpa, K.C.; Roy, S.; Kushwaha, A.; Kim, B.S.; Banerjee, R.; Jacob, S.; Rajak, R.C. A critical review on prospects of bio-refinery products from second and third generation biomasses. Chem. Eng. J. 2022, 448, 137677. [Google Scholar] [CrossRef]
- Mujtaba, M.; Fraceto, L.F.; Fazeli, M.; Mukherjee, S.; Savassa, S.M.; de Medeiros, G.A.; Pereira, A.D.E.S.; Mancini, S.D.; Lipponen, J.; Vilaplana, F. Lignocellulosic biomass from agricultural waste to the circular economy: A review with focus on biofuels, biocomposites and bioplastics. J. Clean. Prod. 2023, 402, 136815. [Google Scholar] [CrossRef]
- Singh, D.; Sharma, D.; Soni, S.L.; Sharma, S.; Sharma, P.K.; Jhalani, A. A review on feedstocks, production processes, and yield for different generations of biodiesel. Fuel 2020, 262, 116553. [Google Scholar] [CrossRef]
- Ziaei, S.M.; Szulczyk, K.R. Estimating the potential of algal biodiesel to improve the environment and mitigate palm oil mill effluents in Malaysia. J. Clean. Prod. 2022, 338, 130583. [Google Scholar] [CrossRef]
- Prasad, S.; Yadav, K.K.; Kumar, S.; Pandita, P.; Bhutto, J.K.; Alreshidi, M.A.; Ravindran, B.; Yaseen, Z.M.; Osman, S.M.; Cabral-Pinto, M.M. Review on biofuel production: Sustainable development scenario, environment, and climate change perspectives− A sustainable approach. J. Environ. Chem. Eng. 2024, 12, 111996. [Google Scholar] [CrossRef]
- Elsayed, M.; Eraky, M.; Osman, A.I.; Wang, J.; Farghali, M.; Rashwan, A.K.; Yacoub, I.H.; Hanelt, D.; Abomohra, A. Sustainable valorization of waste glycerol into bioethanol and biodiesel through biocircular approaches: A review. Environ. Chem. Lett. 2024, 22, 609–634. [Google Scholar] [CrossRef]
- Sandid, A.; Spallina, V.; Esteban, J. Glycerol to value-added chemicals: State of the art and advances in reaction engineering and kinetic modelling. Fuel Proc. Technol. 2024, 253, 108008. [Google Scholar] [CrossRef]
- Pogorzelska-Nowicka, E.; Hanula, M.; Pogorzelski, G. Extraction of polyphenols and essential oils from herbs with green extraction methods—An insightful review. Food Chem. 2024, 460, 140456. [Google Scholar] [CrossRef]
- Stanciauskaite, M.; Marksa, M.; Rimkiene, L.; Ramanauskiene, K. Evaluation of chemical composition, sun protection factor and antioxidant activity of Lithuanian propolis and its plant precursors. Plants 2022, 11, 3558. [Google Scholar] [CrossRef] [PubMed]
- Nunes, A.R.; Vieira, Í.G.P.; Queiroz, D.B.; Leal, A.L.A.B.; Morais, S.M.; Muniz, D.F.; Calixto-Junior, J.T.; Coutinho, H.D.M. Use of flavonoids and cinnamates, the main photoprotectors with natural origin. Adv. Pharmacol. Pharm. Sci. 2018, 2018, 5341487. [Google Scholar] [CrossRef]
- Olivieri, D.; Verboni, M.; Benedetti, S.; Paderni, D.; Carfagna, C.; Duranti, A.; Lucarini, S. New cinnamic acid sugar esters as potential UVB filters: Synthesis, cytotoxicity, and physicochemical properties. Carbohydr. Res. 2025, 550, 109405. [Google Scholar] [CrossRef] [PubMed]
- Milutinov, J.; Pavlović, N.; Ćirin, D.; Atanacković Krstonošić, M.; Krstonošić, V. The potential of natural compounds in UV protection products. Molecules 2024, 29, 5409. [Google Scholar] [CrossRef]
- National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Division on Earth and Life Studies; Board on Health Sciences Policy; Board on Environmental Studies and Toxicology; Ocean Studies Board; Committee on Environmental Impact of Currently Marketed Sunscreens and Potential Human Impacts of Changes in Sunscreen Usage. 2. Introduction to sunscreens and their UV filters. In Review of Fate, Exposure, and Effects of Sunscreens in Aquatic Environments and Implications for Sunscreen Usage and Human Health; National Academies Press: Washington, DC, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK587270/ (accessed on 21 April 2025).
- Lorigo, M.; Quintaneiro, C.; Breitenfeld, L.; Cairrao, E. Exposure to UV-B filter octylmethoxycinnamate and human health effects: Focus on endocrine disruptor actions. Chemosphere 2024, 358, 142218. [Google Scholar] [CrossRef]
- Widsten, P. Lignin-based sunscreens—State-of-the-art, prospects and challenges. Cosmetics 2020, 7, 85. [Google Scholar] [CrossRef]
- Peyrot, C.; Mention, M.M.; Brunissen, F.; Allais, F. Sinapic acid esters: Octinoxate substitutes combining suitable UV protection and antioxidant activity. Antioxidants 2020, 9, 782. [Google Scholar] [CrossRef]
- Zambrano, D.; Millán, D.; Guevara-Pulido, J. In silico design, synthesis and evaluation of a less toxic octinoxate alternative with suitable photoprotection properties. Eur. J. Pharm. Sci. 2023, 180, 106332. [Google Scholar] [CrossRef]
- Molinero, L.; Ladero, M.; Tamayo, J.J.; Esteban, J.; Garcia-Ochoa, F. Thermal esterification of cinnamic and p-methoxycinnamic acids with glycerol to cinnamate glycerides in solventless media: A kinetic model. Chem. Eng. J. 2013, 225, 710–719. [Google Scholar] [CrossRef]
- Molinero, L.; Ladero, M.; Tamayo, J.J.; García-Ochoa, F. Homogeneous catalytic esterification of glycerol with cinnamic and methoxycinnamic acids to cinnamate glycerides in solventless medium: Kinetic modeling. Chem. Eng. J. 2014, 247, 174–182. [Google Scholar] [CrossRef]
- Molinero, L.; Esteban, J.; Sánchez, F.; García-Ochoa, F.; Ladero, M. Solventless esterification of glycerol with p-methoxycinnamic acid catalyzed by a novel sulfonic acid mesoporous solid: Reaction kinetics. J. Ind. Eng. Chem. 2022, 109, 442–452. [Google Scholar] [CrossRef]
- Rychlicka, M.; Gliszczyńska, A. Interesterification of egg-yolk phosphatidylcholine with p-methoxycinnamic acid catalyzed by immobilized lipase B from Candida antarctica. Catalysts 2020, 10, 1181. [Google Scholar] [CrossRef]
- Tamayo, J.J.; Ladero, M.; Santos, V.E.; García-Ochoa, F. Esterification of benzoic acid and glycerol to α-monobenzoate glycerol in solventless media using an industrial free Candida antarctica lipase B. Proc. Biochem. 2012, 47, 243–250. [Google Scholar] [CrossRef]
- Chang, M.Y.; Chan, E.S.; Song, C.P. Biodiesel production catalysed by low-cost liquid enzyme Eversa® Transform 2.0: Effect of free fatty acid content on lipase methanol tolerance and kinetic model. Fuel 2021, 283, 119266. [Google Scholar] [CrossRef]
- Ortiz, C.; Ferreira, M.L.; Barbosa, O.; dos Santos, J.C.S.; Rodrigues, R.C.; Berenguer-Murcia, Á.; Briand, L.E.; Fernandez-Lafuente, R. Novozym 435: The “perfect” lipase immobilized biocatalyst? Catal. Sci. Technol. 2019, 9, 2380–2420. [Google Scholar] [CrossRef]
- Nieto, S.; Martinez-Mora, F.; Lozano, I.; Ruiz, F.J.; Villa, R.; Lozano, P. New sustainable biocatalytic approach for producing lipophilic (hydroxy) cinnamic esters based on deep eutectic mixtures. Catal. Today 2024, 431, 114500. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, Y.; Zhong, H.; Zhu, H.; Wang, H.; Goh, K.-L.; Zhang, J.; Zheng, M. Efficient and sustainable preparation of cinnamic acid flavor esters by immobilized lipase microarray. LWT 2023, 173, 114322. [Google Scholar] [CrossRef]
- Gunia-Krzyżak, A.; Słoczyńska, K.; Popiół, J.; Koczurkiewicz, P.; Marona, H.; Pękala, E. Cinnamic acid derivatives in cosmetics: Current use and future prospects. Int. J. Cosmet. Sci. 2018, 40, 356–366. [Google Scholar] [CrossRef]
- Płowuszyńska, A.; Gliszczyńska, A. Recent developments in therapeutic and nutraceutical applications of p-methoxycinnamic acid from plant origin. Molecules 2021, 26, 3827. [Google Scholar] [CrossRef] [PubMed]
- Korkut, A.; Özkaya Gül, S.; Aydemir, E.; Er, H.; Odabaş Köse, E. Cinnamic acid compounds (p-coumaric, ferulic, and p-methoxycinnamic acid) as effective antibacterial agents against colistin-resistant Acinetobacter baumannii. Antibiotics 2025, 14, 71. [Google Scholar] [CrossRef]
- Rychlicka, M.; Rot, A.; Gliszczyńska, A. Biological properties, health benefits and enzymatic modifications of dietary methoxylated derivatives of cinnamic acid. Foods 2021, 10, 1417. [Google Scholar] [CrossRef] [PubMed]
- Gallego-Villada, L.A.; Alarcón, E.A.; Ruiz, D.M.; Romanelli, G.P. Kinetic study of the esterification of p-cinnamic acid over Preyssler structure acid. Mol. Catal. 2022, 528, 112507. [Google Scholar] [CrossRef]
- Ravelo, M.; Wojtusik, M.; Ladero, M.; García-Ochoa, F. Synthesis of ibuprofen monoglyceride in solventless medium with Novozym® 435: Kinetic analysis. Catalysts 2020, 10, 76. [Google Scholar] [CrossRef]
- Sousa, R.R.; Silva, A.S.A.; Fernandez-Lafuente, R.; Ferreira-Leitão, V.S. Solvent-free esterifications mediated by immobilized lipases: A review from thermodynamic and kinetic perspectives. Catal. Sci. Technol. 2021, 11, 5696–5711. [Google Scholar] [CrossRef]
- Piva, G.S.; Fischer, B.; Peruzzolo, M.; Puton, B.M.S.; Weschenfelder, T.; Junges, A.; Steffens, C.; Cansian, R.L.; Paroul, N. Mathematical modeling of enzymatic esterification process between essential oil from Cymbopogon winterianus and cinnamic acid. Ind. Crops Prod. 2024, 222, 119919. [Google Scholar] [CrossRef]
- Hao, L.; Zou, Y.; Li, X.; Xin, X.; Zhang, M.; Zhao, G. Green biocatalytic synthesis of (hydroxy) cinnamic monoterpenyl esters in biomass solvent: Mechanism underlying different reaction efficiency. Food Biosci. 2024, 61, 104914. [Google Scholar] [CrossRef]
- Do Prado, A.H.; Araújo, V.H.S.; Eloy, J.O.; Fonseca-Santos, B.; Pereira-da-Silva, M.A.; Peccinini, R.G.; Chorilli, M. Synthesis and characterization of nanostructured lipid nanocarriers for enhanced sun protection factor of octyl p-methoxycinnamate. AAPS PharmSciTech 2020, 21, 125. [Google Scholar] [CrossRef]
- Berkey, C.; Oguchi, N.; Miyazawa, K.; Dauskardt, R. Role of sunscreen formulation and photostability to protect the biomechanical barrier function of skin. Biochem. Biophys. Rep. 2019, 19, 100657. [Google Scholar] [CrossRef]
- José, C.; Bonetto, R.D.; Gambaro, L.A.; Torres, M.D.P.G.; Foresti, M.L.; Ferreira, M.L.; Briand, L.E. Investigation of the causes of deactivation–degradation of the commercial biocatalyst Novozym® 435 in ethanol and ethanol–aqueous media. J. Mol. Catal. B Enzym. 2011, 71, 95–107. [Google Scholar] [CrossRef]
- Ravelo, M.; Fuente, E.; Blanco, Á.; Ladero, M.; García-Ochoa, F. Esterification of glycerol and ibuprofen in solventless media catalyzed by free CALB: Kinetic modelling. Biochem. Eng. J. 2015, 101, 228–236. [Google Scholar] [CrossRef]
- Foresti, M.L.; Pedernera, M.; Bucala, V.; Ferreira, M.L. Multiple effects of water on solvent-free enzymatic esterifications. Enz. Microb. Technol. 2007, 41, 62–70. [Google Scholar] [CrossRef]
- Gonzalez, H.; Tarras-Wahlberg, N.; Strömdahl, B.; Juzeniene, A.; Moan, J.; Larkö, O.; Rosén, A.; Wennberg, A.M. Photostability of commercial sunscreens upon sun exposure and irradiation by ultraviolet lamps. BMC Dermat. 2007, 7, 1. [Google Scholar] [CrossRef]
- Scarpin, M.S.; Kawakami, C.M.; Rangel, K.C.; Pereira, K.D.C.; Benevenuto, C.G.; Gaspar, L.R. Effects of UV-filter photostabilizers in the photostability and phototoxicity of vitamin A palmitate combined with avobenzone and octyl methoxycinnamate. Photochem. Photobiol. 2021, 97, 700–709. [Google Scholar] [CrossRef]
- Bendová, H.; Akrman, J.; Krejčí, A.; Kubáč, L.; Jírová, D.; Kejlová, K.; Kolářová, H.; Brabec, M.; Malý, M. In vitro approaches to evaluation of Sun Protection Factor. Toxicol. In Vitro 2007, 21, 1268–1275. [Google Scholar] [CrossRef]








| % w/w H2O | n | m | R2 |
|---|---|---|---|
| 0 | 1.84 × 10−3 ± 4.2 × 10−4 | −22.5 ± 1.6 | 0.9876 |
| 15 | 1.30 × 10−3 ± 2.6 × 10−4 | −23.0 ± 1.5 | 0.9897 |
| 30 | 1.1 × 10−3 ± 2.3 × 10−4 | −23.6 ± 1.6 | 0.9874 |
| Run | T (°C) | CB,0 (g/L) | CW,0 (% w/w) |
|---|---|---|---|
| M1 | 50 | 20 | 0 |
| M2 | 60 | 20 | 0 |
| M3 | 70 | 20 | 0 |
| M4 | 60 | 40 | 0 |
| M5 | 50 | 20 | 30 |
| M6 | 60 | 20 | 30 |
| M7 | 70 | 20 | 30 |
| M8 | 60 | 40 | 30 |
| Reaction media | ; | ||||
| Pure glycerol | Parameter | Value ± error | SQR | F95 | AICc |
| Eak (kJ/mol) | 25.7 ± 3.67 | 1.13 × 10−3 | 1230 | −470 | |
| lnk0 | 1.50 ± 1.32 | ||||
| K (mol/L) | 0.28 ± 0.002 | ||||
| Glycerol + 30% w/w water | Eak (kJ/mol) | 38.2 ± 3.21 | 7.70 × 10−4 | 4310 | −520 |
| lnk0 | 6.80 ± 1.16 | ||||
| K (mol/L) | 0.28 ± 0.004 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molinero, L.; Tamayo, J.J.; Gandia, J.J.; García-Ochoa, F.; Ladero, M. Enzymatic Production of p-Methoxycinnamate Monoglyceride Under Solventless Conditions: Kinetic Analysis and Product Characterization. Catalysts 2025, 15, 548. https://doi.org/10.3390/catal15060548
Molinero L, Tamayo JJ, Gandia JJ, García-Ochoa F, Ladero M. Enzymatic Production of p-Methoxycinnamate Monoglyceride Under Solventless Conditions: Kinetic Analysis and Product Characterization. Catalysts. 2025; 15(6):548. https://doi.org/10.3390/catal15060548
Chicago/Turabian StyleMolinero, Laura, Juan J. Tamayo, José J. Gandia, Félix García-Ochoa, and Miguel Ladero. 2025. "Enzymatic Production of p-Methoxycinnamate Monoglyceride Under Solventless Conditions: Kinetic Analysis and Product Characterization" Catalysts 15, no. 6: 548. https://doi.org/10.3390/catal15060548
APA StyleMolinero, L., Tamayo, J. J., Gandia, J. J., García-Ochoa, F., & Ladero, M. (2025). Enzymatic Production of p-Methoxycinnamate Monoglyceride Under Solventless Conditions: Kinetic Analysis and Product Characterization. Catalysts, 15(6), 548. https://doi.org/10.3390/catal15060548









