Abstract
The construction of multicomponent transition metal oxide catalysts can effectively increase the surface defects of catalysts, and bring a synergistic effect from different components, thus enhancing the generation of reactive oxygen species and improving the catalytic activity of catalysts for volatile organic compounds (VOCs) oxidation. In this article, CuO/Co3O4 catalysts with abundant oxygen vacancies for the degradation of ethyl acetate was prepared by a simple impregnation method. The effect of the ratio of Co/Cu on the redox capacity, oxygen vacancy, active oxygen species and catalytic oxidation activity of ethyl acetate were studied. The 90% conversion and mineralization temperatures of ethyl acetate for the optimal catalyst Co3O4-20Cu are 211 and 214 °C (WHSV = 60,000 mL/(g·h), 1000 ppm ethyl acetate), which also shows good stability and excellent water vapor resistance. Compared with pure Co3O4, the CuO/Co3O4 catalysts have more oxygen vacancies, provide more reactive oxygen species, allowing the catalyst better low-temperature reduction. Through in situ DRIFTS study, the intermediates of ethyl acetate decomposition were analyzed, then a possible catalytic oxidation mechanism of ethyl acetate on the Co3O4-20Cu catalyst was proposed. In addition, we prepared a Co3O4-20Cu/cordierite monolithic catalyst on the basis of Co3O4-20Cu, exhibiting a good catalytic activity in degradation of ethyl acetate.
1. Introduction
Volatile organic compounds (VOCs) not only threaten the ecological environment but also bring serious health hazards to humans. Their emissions are instrumental in the formation of ozone and particle matters, which may bring secondary pollution. Therefore, VOCs control and elimination has become the key issue of air pollution control [1,2,3]. Ethyl acetate (EA), a kind of VOCs, is known for its excellent solubility and rapid evaporation characteristics. It is widely used in industry, employed in chemical synthesis, serving as a solvent, an extraction agent, and an additive. This widespread utilization entails a substantial release of ethyl acetate into the environment [4,5]. Therefore, the exploration of degradation technologies for ethyl acetate assumes critical practical significance.
At present, there are many methods of eliminating ethyl acetate. Including biodegradation, plasma catalysis, photocatalysis, thermal catalysis and other technologies. Among these, catalytic oxidation technology can effectively reduce the activation energy at high temperatures, enhance the pyrolysis kinetics of VOCs, and enable the efficient removal of pollutants under reduced energy consumption [6,7]. Due to the relatively low toxicity and lesser research focus on ester-containing VOCs, the elimination of ethyl acetate faces more challenges. In general, VOCs with higher molar mass tend to be more difficult to eliminate and the order of oxidation difficulty is alkanes > acetates > ketones > aromatics > aldehydes > alcohols [8]. Ethyl acetate possesses a high molecular mass and strong C=O bonds [9], results in the catalysts capable of efficiently degrading ethyl acetate is even more difficult to obtain. Currently, the commonly used catalysts for ethyl acetate degradation can be divided into noble metal catalysts and transition metal oxide catalysts. Among them, noble metal catalysts possess excellent catalytic performance, but they have a higher cost. However, transition metal oxide catalysts have stable catalytic activity, they are cheap and widely available, have high oxygen storage capacity, which has garnered extensive attention [10,11].
Cobalt oxides demonstrate effective activity in the catalytic oxidation of ethyl acetate. Zhu et al. prepared four different morphologies of Co3O4 and found that the NP-Co3O4 catalyst exhibited the best catalytic activity for the degradation of ethyl acetate. Compared with Co3O4 possessing the other morphologies, NP-Co3O4 had a high abundance of Co3+ species exposed on the (220) crystal plane, along with a sufficient number of active surface oxygen species. At 210 °C, NP-Co3O4 completed the degradation of 1000 ppm of ethyl acetate at WHSV = 30,000 mL/(g·h) [12]. Wang et al. prepared LaCoO3 by different methods, and the LCO-E prepared by selective etching exhibited more oxygen vacancies and stronger oxidizing capabilities. The catalyst achieved 90% degradation of 1000 ppm ethyl acetate and 2000 ppm NO at 205 °C (WHSV = 50,000 mL/(g·h)) [13]. Zhang et al. successfully prepared Co3O4@MnOx catalysts on Ni foam by in situ growth. The addition of KMnO4 enhanced the reducibility of the catalyst, increased the amount of adsorbed oxygen (Oads) and Co3+ species, and improved the catalytic performance for ethyl acetate oxidation. At WHSV = 17,000 mL/(g·h), the best catalyst Co3O4@MnOx-NF achieved 90% mineralization of 1000 ppm ethyl acetate at 205 °C [14].
Oxygen vacancies play a significant role in enhancing the catalytic oxidation performance of catalysts by activating oxygen molecules. According to the MvK mechanism, oxygen from the environment can be adsorbed onto the surface oxygen vacancies of catalysts, participate in the reaction and enhance the oxidation capacity of catalyst and facilitating the reaction of VOCs degradation [15,16]. Jiang et al. synthesized MnOxCeO2-s catalysts by the impregnation method based on Ce-MOFs, exhibiting excellent low temperature activity. Frankel oxygen vacancies (F-OVs) were identified as critical sites in the reaction process and the distinctive cross channel architecture of Ce-MOFs enabled it to exhibit a high specific surface area and a remarkable potential for the recovery of F-OVs even at elevated temperatures [17]. Ye et al. improved the dipping method to prepare a 5-MI-500 catalyst by dipping the soluble copper salt into cerium acetate. The incorporation of Cu species into the CeO2 lattice leaded to the formation of a Cu-O-Ce structure in the catalysts, which made the catalyst with abundant oxygen vacancies, demonstrating excellent catalytic activity of ethyl acetate oxidation [18]. Lv et al. prepared Mn-Ce catalysts derived from Ce-MOF and found it has a wealth of oxygen vacancies, high specific surface area, and numerous lattice defects, contributing to the augmented catalytic performance [19].
In this work, we used a traditional impregnation method to introduce CuO into Co3O4, resulting in a significant number of oxygen vacancies, which were further applied in the degradation of ethyl acetate. According to the results, when the loading amount is 20%, Co3O4-20Cu exhibits good catalytic performance with 90% conversion and mineralization at 211 and 214 °C, and demonstrates an excellent resistance to water vapor. The interaction between Co and Cu oxides allows the catalyst to have a greater SBET, giving the material more oxygen vacancies and better low-temperature reducibility, thus achieves a more efficient catalytic oxidation of ethyl acetate. In addition, the corresponding cordierite monolithic catalyst was prepared by a simple method, and its catalytic activity was evaluated. This work affords a reference for the practical application of the Co/Cu oxides catalysts in ethyl acetate degradation.
2. Results and Discussion
2.1. Characterization of the Catalysts
The XRD patterns of Co3O4 and Co3O4-XCu (X = 10, 15, 20, 30) samples with different molar fractions of CuO are shown in Figure 1. The pure Co3O4 sample displays peaks at 19.1°, 31.4°, 36.9°, 44.9°, 55.7°, 59.5° and 65.3° attributed to (1 1 1), (2 2 0), (3 1 1), (4 0 0), (3 3 1), (5 1 1), and (4 4 0) crystal planes of the spinel phase of Co3O4 (JCPDS no. 42-1467), respectively. The sample Co3O4-10Cu also exhibits the same diffraction patterns like pure Co3O4 without any additional peaks belong to CuO, it is shown that Cu enters the lattice of Co3O4 and forms the structure of CuCo2O4 spinel. However, a new weak peak at 35.5° assigned to the (−1 1 1) planes of CuO (JCPDS no. 89-5895) is present for the samples Co3O4-XCu (X = 15, 20, 30), it is shown that in addition to part of the Cu enters the lattice of Co3O4, the excess Cu accumulates on the surface of the material. Through the application of the Scherrer equation to the (3 1 1) plane of Co3O4, the particle sizes of the catalysts are calculated as 21.5, 17.7, 17.3, 15.8 and 13.7 nm for Co3O4, Co3O4-10Cu, Co3O4-15Cu, Co3O4-20Cu, Co3O4-30Cu, respectively (Supplementary Table S1). This indicates that the addition of CuO suppresses the growth of Co3O4 crystals and makes the Co3O4 crystal size smaller [20]. Figure 1a shows that with the CuO content increase, the intensity of the Co3O4 diffraction peaks gradually decreases, and the peak widths also broaden, while the intensity of the CuO diffraction peaks progressively increases. Obviously, the introduction of CuO into Co3O4 leads to the structure change of Co3O4. The interaction between Co3O4 and CuO may change the physicochemical properties of the catalysts, thereby influencing the catalytic activities of samples.
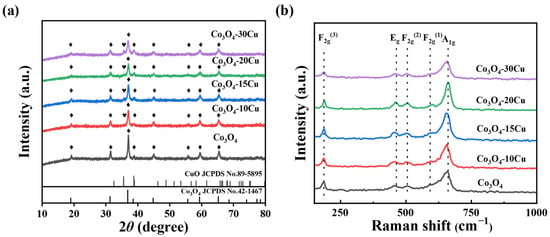
Figure 1.
(a) XRD patterns and (b) Raman spectra of CuO and Co3O4-XCu.
Raman spectroscopy was utilized to examine the structural distortions. Figure 1b shows the Raman spectra of Co3O4 and Co3O4-XCu samples with varying CuO contents. For Co3O4, five different Raman bands (A1g, Eg, 3F2g) can be observed at 186, 462, 504, 591 and 660 cm−1. Among these, the peak observed at 660 cm−1 is associated with the octahedral CoO6 site, exhibiting A1g symmetry pattern [21], and the peaks at 462, 504 and 186 cm−1 belong to the Eg, F2g(2) and F3g(3) patterns [22,23], while the peak at 591 cm−1 is attributed to the tetrahedral CoO4 site with an F2g(1) pattern [21]. CuO exhibits a characteristic peak at 282 cm−1 [24]; however, it is not observed in the spectra of Co3O4-XCu, perhaps due to the relatively low contents and high dispersity. When CuO is doped, the Co3O4 structure changes due to residual stress or lattice deformation (including lattice defects). And the strong interaction between Co3O4 and CuO will lead to a large degree of lattice defects [25], which subsequently enhances the formation of oxygen vacancies and also facilitates the catalytic oxidation reaction. This was combined with the phenomenon in XRD that the intensity of the signal peak decreased and the half-peak width increased with the doping of Cu (Supplementary Table S2).
Elemental compositions of the samples were analyzed using ICP-OES. The molar ratios of Co and Cu in the samples are shown in Table 1, and it is found that the molar proportion of Cu were 0.10, 0.15, 0.20, and 0.29, respectively. It means that Cu was successfully added to the Co3O4 catalysts, as required. The structural properties of the catalysts were analyzed through N2 adsorption–desorption isotherms as shown in Figure 2. All the samples display type III isotherms and H3-type hysteresis loop [26]. This indicates that the series of catalysts possess mesoporous structures; however, their pore structures are not highly ordered or uniform. The specific surface area of pure Co3O4 is only 15.86 m2/g. When CuO is introduced, the specific surface area and pore volume of the samples become larger, especially when the contents of CuO is 20%, the sample has the largest specific surface area of 27.49 m2/g, which enable the samples adsorb more gas phase molecules to make the catalytic oxidation reaction occur [27].

Table 1.
Elemental compositions and physical properties of the catalysts.
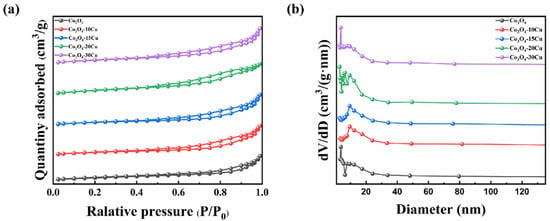
Figure 2.
(a) N2 adsorption–desorption isotherms and (b) pore−size distribution of CuO and Co3O4−XCu.
Supplementary Figure S2 shows the SEM images of Co3O4 and Co3O4-XCu with different concentrations of CuO. The pure Co3O4 sample shows an irregular block-like structure formed by the aggregation of small particles with some pore channels on its surface. After introducing CuO, no significant morphology change is observed, the sample still keeps an irregular texture and possesses noticeable pore channels. The irregularity of the sample and the presence of pore channels in it may be attributed to the decomposition of precursors and released gas molecules during the high-temperature calcination. The photos of Co3O4-XCu/cordierite monolithic catalysts are shown in Supplementary Figures S3 and S4a,b show the SEM images of the pure cordierite substrates, we can observe that the material exhibits an irregular porous structure, composed of many small particles interconnected to form a network-like structure. Supplementary Figure S4c,d shows the SEM images of the untested Co3O4-20Cu/cordierite. It is found that the material is loaded with many tiny particles on the cordierite substrates, and some particles are stacked together to form larger particles distributed on the surface of the monolithic catalyst. Supplementary Figure S4e,f show the SEM images of the used Co3O4-20Cu/cordierite catalyst. It is found that the small particles are evenly distributed on the surface of the catalyst, and the original large particles disappear. The reason for this change is large number of active components adsorbed onto the surface of the support during the preparation of the catalyst. After calcination, these components accumulated on the surface of the catalyst. Some particles were directly loaded onto the framework of the support, while others were stacked on the surfaces of other particles, with weaker interactions between them and the support framework. During testing, at higher temperatures and gas flow rates, these loosely loaded particles spread by the degradation products such as ethanol, acetic acid and water, resulting in a surface with uniformly distributed small particles.
The structure of the sample was further analyzed by TEM. Figure 3a–e are the TEM images of the catalysts. The catalysts comprise irregular block structures formed by small particles, consistent with the results investigated by SEM. Supplementary Figure S5 shows the enlarged image, demonstrating that all samples show a stacked particle morphology. The particle size of the pure Co3O4 is larger than that of the other Co3O4-XCu samples. Figure 3f–j display the HRTEM images that illustrate the lattice parameters of Co3O4 and Co3O4-XCu catalysts. The lattice streaks of the five catalysts are clearly visible in the HRTEM images, indicating that each of these catalysts has a high crystallinity. Figure 3f shows a HRTEM image of pure Co3O4, displaying a lattice spacing of 0.243 nm, which corresponds to the (3 1 1) crystal plane of Co3O4 (JCPDS no. 42-1467). Co3O4-10Cu (Figure 3g) shows the 0.285 nm lattice spacing belonging to the (2 2 0) crystal plane of Co3O4 and the 0.275 nm lattice streak of the (1 1 0) crystal plane of CuO. Figure 3h,i show the HRTEM images of Co3O4-15Cu and Co3O4-20Cu, respectively. They have the same lattice streaks of 0.243 and 0.252 nm, corresponding to the (3 1 1) crystal plane of Co3O4 and the (−1 1 1) crystal plane of CuO. Co3O4-30Cu (Figure 3j) shows the lattice spacing of 0.243, 0.285 and 0.252 nm corresponds to the (3 1 1), (2 2 0) surfaces of Co3O4, and the (−1 1 1) surface of CuO. The analysis of the HRTEM images shows that CuO lattice fringes can be observed, indicating that Cu does not enter the Co3O4 lattice but disperses in Co3O4 in the form of small highly dispersed particles. The elemental composition of the catalyst was analyzed by the elemental mapping characterization. In Figure 3k–o, all the elements are evenly distributed on the sample surfaces. Among them, the content of Co elements is the highest, and the elements dots are the densest. The element reflection of Cu is affected by the addition amount in the preparation. When the amount is 30%, the Cu element is the most densely reflected, which is consistent with the ICP results.
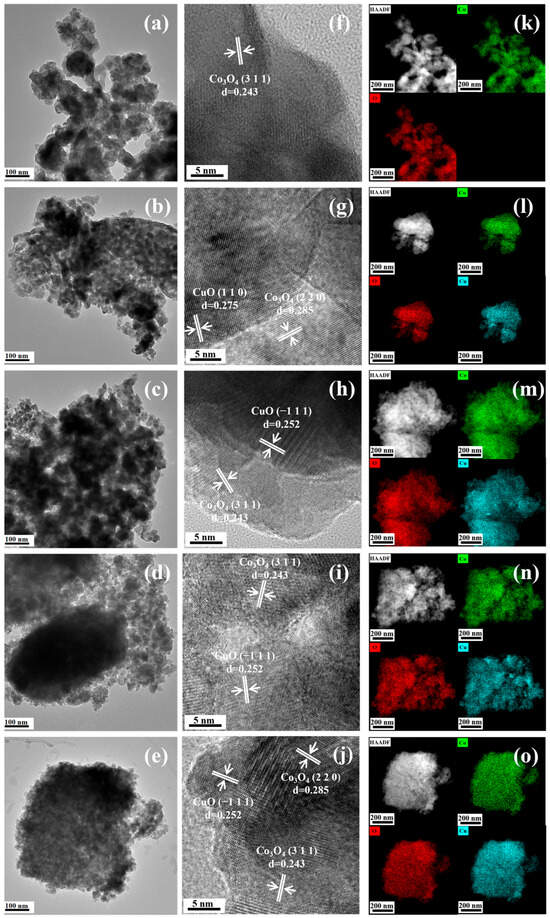
Figure 3.
TEM images of (a) Co3O4, (b) Co3O4-10Cu, (c) Co3O4-15Cu, (d) Co3O4-20Cu, (e) Co3O4-30Cu; HRTEM images of (f) Co3O4, (g) Co3O4-10Cu, (h) Co3O4-15Cu, (i) Co3O4-20Cu, (j) Co3O4-30Cu; element mapping images of (k) Co3O4, (l) Co3O4-10Cu, (m) Co3O4-15Cu, (n) Co3O4-20Cu, (o) Co3O4-30Cu.
The chemical state of the surface of Co3O4 and Co3O4-XCu samples were further analyzed by XPS. The survey spectra and the Co 2p, Cu 2p and O 1s spectra of the catalysts are displayed in Supplementary Figure S6. The Co 2p spectra of Co3O4 and Co3O4-XCu contain two separated signals, and every peak can be split into two spin orbitals, belonging to Co 2p3/2 and Co 2p1/2 [28] (Figure 4a). Among these, the deconvoluted peaks at 781.2 and 796.7 eV belong to Co2+, while those peaks located at 779.8 and 794.9 eV assigned to Co3+. The peak integral areas of the simulated Co3+ and Co2+ signals can determine the ratios of Co2+/(Co2+ + Co3+). The ratio of Co2+/(Co2+ + Co3+) in Co3O4-20Cu is obviously higher than that of the others (Table 2), revealing that Co3O4-20Cu contains more Co2+ [29]. There is a positive correlation between the presence of Co2+ ions and the number of oxygen vacancies in the catalysts. The Cu 2p spectra of the Co3O4-XCu are presented in Figure 4b. The two characteristic peaks of the catalysts at 934.1 and 953.8 eV belong to Cu 2p3/2 and Cu 2p1/2, respectively, in which peaks at 932.4 and 952.7 eV belong to Cu+, and peaks at 934.4 and 955.0 eV attribute to Cu2+. And the peaks at 941.6, 943.6 and 962.3 eV belong to the satellite peaks of Cu, among which S1 and S2 belong to Cu2+ [30], and the strength of S1 and S2 increases with the increase in Cu2+ content [31]. The ratios of Cu2+/(Cu+ + Cu2+) are determined by calculating the peak areas of the simulated Cu+ and Cu2+ (Table 2). Compared to the other Co3O4-XCu, the Co3O4-20Cu surface has more Cu2+, and the intensity of the corresponding satellite peaks S1 and S2 is also stronger. Figure 4c displays the O 1s spectrum of the Co3O4 and Co3O4-XCu samples. The peak of Co3O4-XCu can be deconvoluted into three peaks at 529.6, 531.8 and 533.2 eV, belong to the lattice oxygen (Olatt), chemically adsorbed oxygen (Oads), physically adsorbed water/hydroxyl group (OOH) [32,33]. In Co3O4, lattice oxygen occupies most of the oxygen species, but with the addition of CuO, the content of adsorbed oxygen begins to increase. According to Table 2, the Oads/Otot ratio of Co3O4-20Cu is the highest in the all samples, and this indicates that the addition of CuO promotes the production of oxygen vacancies and facilitates the participation of more active oxygen in the catalytic oxidation reaction.
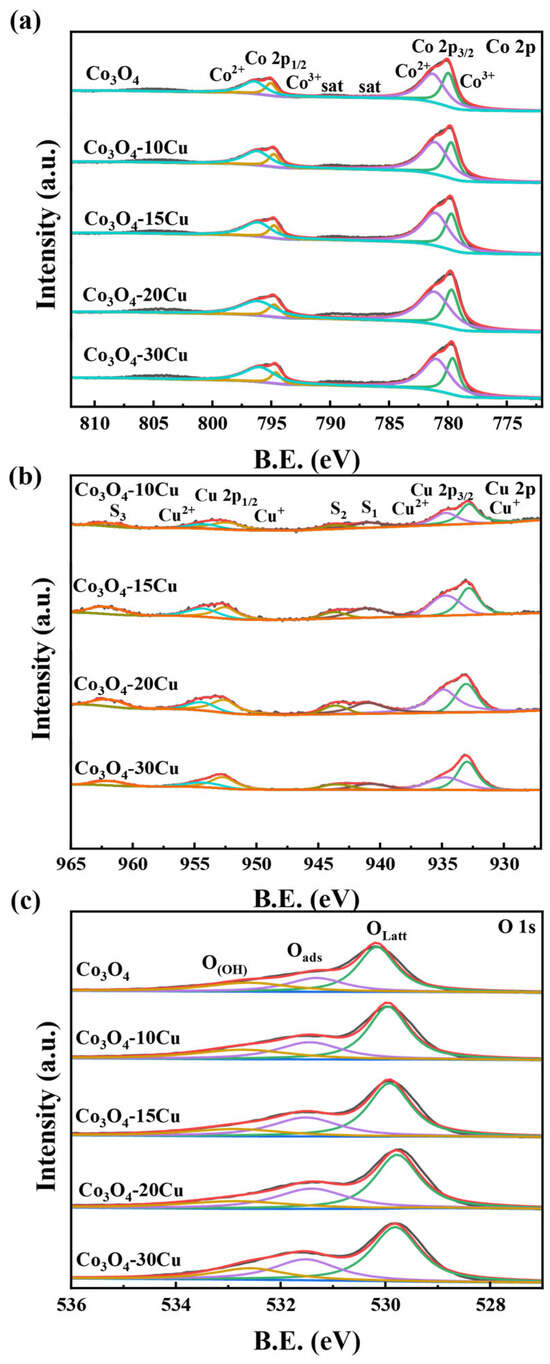
Figure 4.
XPS spectra of CuO and Co3O4-XCu: (a) Co 2p, (b) Cu 2p and (c) O 1s.

Table 2.
The XPS and O2-TPD results of all catalysts.
O2-TPD analysis was used to study the ability of CuO and Co3O4-XCu catalysts to desorb oxygen. As illustrated in Figure 5a, three peaks were present in the all samples in the range of 20–800 °C. Among these, the peaks below 150 °C represent the peaks of physically adsorbed molecular oxygen on catalysts surface, which is weakly bound to the catalyst surface. The peaks occurring between 150 and 400 °C represent the peaks of surface adsorbed oxygen ions (Oads). The peaks between 400 and 700 °C are the peaks of the surface lattice oxygen (Olatt, s). Peaks higher than 700 °C belong to the peaks of lattice oxygen in bulk (Olatt, b) [34]. From Figure 5a, we can see the addition of CuO shifts these peaks to lower temperatures, indicating that lattice oxygen desorption occurs more easily in Co3O4-XCu compared to the pure Co3O4 sample. According to Table 2, the order of O2 total consumption is: Co3O4-10Cu > Co3O4-20Cu > Co3O4-15Cu > Co3O4 > Co3O4-30Cu, suggesting that after CuO doping, the oxygen desorption capability of Co3O4-XCu is significantly enhanced, improving the oxygen mobility of the catalyst [35]. This would be beneficial for the deep oxidation of ethyl acetate at lower temperatures. When the doping amount of Cu is 10%, all the Cu dopes into the Co3O4 lattice, corresponding to XRD. The interaction between Cu and Co enhances the oxygen desorption capacity of Co3O4-10Cu [36]. When the doping amount of Cu exceeds 10%, some Cu enters the Co3O4 lattice, while another part accumulates on the surface of the catalyst, covering some active sites, leading to a decrease in oxygen desorption capacity [37].
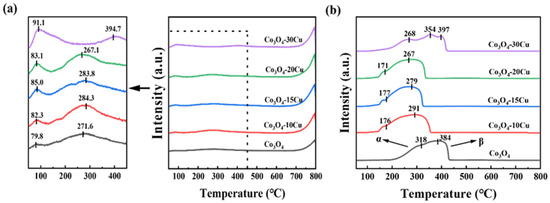
Figure 5.
(a) O2-TPD and (b) H2-TPR profiles of CuO and Co3O4-XCu.
The reducibility performance of the catalyst is a key factor affecting the catalytic oxidation properties of ethyl acetate, and in order to further understand the redox capacity of the catalyst, Co3O4-XCu were tested for H2-TPR. As displayed in Figure 5b, H2-TPR peaks of pure Co3O4 appear at 318 and 384 °C, representing the reduction in Co3+→Co2+ and Co2+→Co0 [38,39]. According to the literature, pure CuO shows a peak at around 310 °C, corresponding to the reduction in CuO [40]. Compared with pure Co3O4 and CuO, when the appropriate amount of CuO is added into Co3O4, the reduction peaks (α and β) of Co3O4-XCu is moved to lower temperature, and there is an overlap between the reduction peaks. It is shown that the appropriate introduction of CuO can enhance the low-temperature reducibility of the catalyst. Compared with the other samples, the initial reduction temperature is the lowest for Co3O4-20Cu. It is suggested that the proper introduction of Cu can help to form more structural defects and enhance the interaction between Co and Cu, which reaches the strongest when the amount of Cu is 20%. The existence of Cu+ and Cu2+ also promotes the production of more Co2+ in the catalyst and enhances the reduction ability [41]. Moreover, the improvement of low-temperature reduction and oxygen mobility facilitates the cyclic replenishment of oxygen vacancies throughout the reaction [42]. Considering the oxygen mobility and reduction at low temperature, Co3O4-20Cu exhibits superior catalytic performance for ethyl acetate degradation among all samples [36].
We verified the abundance of oxygen vacancies in the samples by performing EPR on Co3O4 and Co3O4-XCu. As displayed in Figure 6, all the samples have a signal with a g-value of 2.003 [43]. Compared to the other Co3O4-XCu samples, Co3O4-20Cu exhibits much higher signal intensity, which indicates more oxygen vacancies existing in it. Furthermore, the oxygen vacancies concentration follows the order: Co3O4-20Cu > Co3O4-30Cu > Co3O4-15Cu > Co3O4-10Cu > Co3O4. The rich oxygen vacancy of Co3O4-20Cu is possible one of the reasons for its excellent catalytic activities [44].
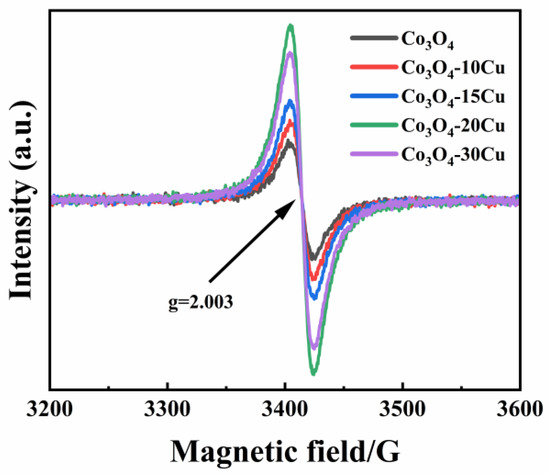
Figure 6.
EPR spectra of CuO and Co3O4-XCu.
2.2. Catalytic Activity of the Catalysts
The catalytic performance of catalysts for ethyl acetate degradation was tested, and the results are shown in Figure 7. Co3O4 exhibits T50 and T90 for ethyl acetate conversion at 229 and 250 °C, and the corresponding temperature for 90% mineralization is 253 °C. When CuO is introduced into Co3O4, the catalysts show much better activity. From Table 3, it can be observed that the catalytic activity for the oxidation of ethyl acetate follows the order: Co3O4-20Cu > Co3O4-15Cu > Co3O4-30Cu > Co3O4-10Cu > Co3O4. Among these, Co3O4-20Cu exhibits the best catalytic performance, achieving T50 and T90 for conversion temperatures of 193 and 211 °C, respectively, and T90 for mineralization at 214 °C. The results demonstrate that, appropriate addition of CuO can significantly enhance the catalytic activity of the catalyst. Figure 7 illustrates that the catalytic activity first increases and subsequently decreases with the rise in CuO content. The optimal CuO doping amount was determined to be 20%. Supplementary Table S3 shows the activity of catalysts for EA degradation, from that we can see, our catalyst Co3O4-20Cu exhibits certain advantages in comparison to the noble metal free catalysts in the similar conditions. The stability of catalytic activity is a crucial metric for assessing the catalytic performance of catalysts. Among them, the stability of Co3O4-20Cu in ethyl acetate oxidation was evaluated by long-term stability test. As shown in Figure 7c, at WHSV = 60,000 mL/(g·h) and 1000 ppm concentration of ethyl acetate gas, the conversion rate of ethyl acetate at 218 °C (~90%) is not change over 24 h, which indicates the good stability of Co3O4-20Cu. Furthermore, to judge the stability of the catalyst Co3O4-20Cu, an 80-h stability test was performed, during which 3% water vapor was introduced into the reaction gas to evaluate the catalyst’s resistance to water vapor. As shown in Figure 7c, at 218 °C, the reaction is initially maintained at 90% conversion for 24 h, then an additional 3% water vapor is added into the ethyl acetate gas flow. Subsequently, the conversion drops to 70% and remains stable, after additional 24 h, the water vapor is removed, the conversion rate of the catalyst recovered to 90%. This suggests that the decrease in catalyst activity by the introduction of water vapor may be attributed to competitive adsorption of water on the catalyst surface, significantly, this effect is reversible. The results show that the Co3O4-20Cu catalyst demonstrates resilience against the adverse effects of water vapor. The catalytic activity may be reactive after the water vapor was removed.
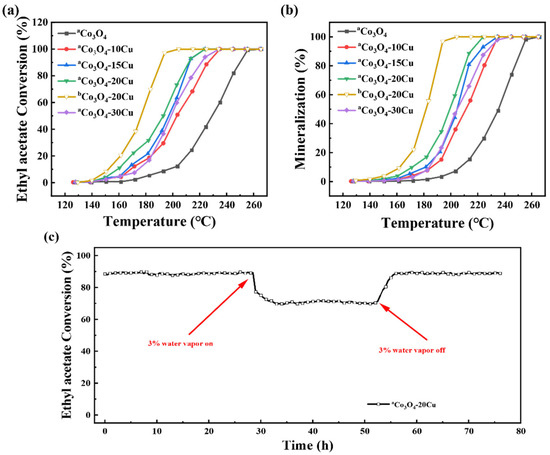
Figure 7.
Catalytic oxidation performance of Co3O4-20Cu for ethyl acetate: (a) conversion, (b) CO2 yield and (c) water vapor resistance.

Table 3.
The catalytic activities of samples for EA oxidation.
We also tested the catalytic activity of the cordierite monolithic catalyst. During the test, WHSV = 10,000 mL/(g·h) and the concentration of ethyl acetate was 1000 ppm. Firstly, the active component Co3O4-20Cu powder was tested. The temperature for 90% ethyl acetate conversion and mineralization (T90) of the powder were 191 and 193 °C, respectively, indicating that reducing the space velocity can improve the catalytic oxidation performance of Co3O4-20Cu. Supplementary Figure S7 shows the catalytic activity of the fragments and the Co3O4-20Cu/cordierite monolithic catalyst. The excess fragments cut off from the monolithic catalyst were collected for performance testing, which was conducted on the fixed-bed reactor. The T90 conversion and mineralization of ethyl acetate are 254 °C and 257 °C, respectively. Compared with the powder samples, the active components of monolithic catalyst are less, resulting in a decrease in performance. Then, the performance of Co3O4-20Cu/cordierite module was tested on the self-made equipment, and T90 for the conversion and mineralization are 263 °C and 265 °C, respectively. And at 340 °C the ethyl acetate nearly degradation completely (99.95%, ~100%). The performance is slightly lower than the fragments, the air resistance of fragments and monolithic catalyst module are different, resulting in a tiny difference in the activities. In conclusion, the Co3O4-20Cu/cordierite monolithic catalyst was successfully prepared by impregnation method, which can achieve high activity in ethyl acetate degradation and provide a reference for practical application.
2.3. Degradation Mechanism for Ethyl Acetate Oxidation
To further investigate the mechanism of ethyl acetate catalytic oxidation, we conducted in situ DRIFTS tests on Co3O4-20Cu. Figure 8a shows the analysis of the intermediates produced during the catalytic oxidation of ethyl acetate on the surface of the Co3O4-20Cu catalyst from 40 to 240 °C. At 40 °C in air, ethyl acetate is firstly introduced for 10 min, four absorption bands at 1770, 1378, 1240 and 1053 cm−1 bands belong to the bending vibration of C=O, CH3(CO), C=O in C-O group and C-O-C on the samples present, which are the characteristic peak of ethyl acetate. [45]. Then, the samples are gradually heated up to 240 °C. As the temperature increases, the intensity of characteristic peaks decreases or the peaks disappear obviously. Other signals appear simultaneously, in which the 2400–2300 cm−1 band is the C-O stretching vibration of CO2 [46], the 2200–2000 cm−1 band is the C-O stretching vibration of CO [47], the 1463 cm−1 band is the COO- stretching vibration [18], the 1175 cm−1 band is the characteristic peak of acetic acid [48], and the 1097 m−1 band is the C-O stretching vibration of ethanol [49]. According to Figure 8a, it can be speculated that after ethyl acetate participates in the decomposition of the reaction, the intermediate product ethanol and acetic acid produce, which subsequently react to produce CO2 and H2O.
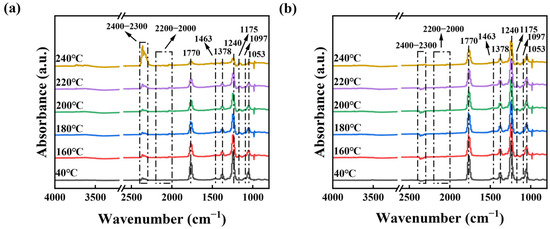
Figure 8.
In situ DRIFTS spectra for Co3O4-20Cu with ethyl acetate in (a) air and (b) N2.
In order to explore how lattice oxygen contributes to the catalytic oxidation of ethyl acetate on Co3O4-20Cu, we need to pretreat Co3O4-20Cu to remove surface-adsorbed oxygen and then conduct the tests in pure N2 condition, as shown in Figure 8b. It can be found that the characteristic peaks of ethyl acetate at 1770, 1378, 1240 and 1053 cm−1 band decrease with increasing temperature, but the intensity of the characteristic peaks is more intense than that in air. Only weak CO2 characteristic peaks appear in the 2400–2300 cm−1. It can be speculated that the lattice oxygen is involved in the catalytic oxidation reaction of ethyl acetate; however, the high content of oxygen vacancies in the catalyst results in a reduced amount of lattice oxygen, and the reaction can only be carried out in trace without the supplement of O2. However, the participation of lattice oxygen in the ethyl acetate catalytic oxidation reaction, indicating that the catalytic oxidation of ethyl acetate on Co3O4-20Cu follows the Mars van Krevelen mechanism.
3. Materials and Methods
3.1. Chemicals
All the reagents including cobalt acetate (Co (CH3COO)2·4H2O, 99%, Adamas, Shanghai, China), cupric nitrate (Cu(NO3)2·3H2O, 99% Adamas, Shanghai, China), Polyvinylpyrrolidone ((C6H9NO)n, A.R., Aladdin, Shanghai, China), triethanolamine ((HOCH2CH2)3N, 99%, Greagent, Shanghai, China) and cordierite honeycomb ceramic carrier were used directly from commercial sources without any further purification. The deionized water was obtained from GZY-P10-W ultrapure water machine (Sichuan Zhuoyue Water Treatment Equipment Co., Ltd., Sichuan, China).
3.2. Preparation of the Catalysts
3.2.1. Synthesis of the Powder Catalysts
We prepared CuO/Co3O4 (Co3O4-XCu) catalysts by the impregnation method, where X represents the molar percentage of CuO in the CuO/Co3O4 samples and the values are, respectively, 5%, 10%, 15%, 20%, and 30% (X = 5, 10, 15, 20, 30). The sample Co3O4-20Cu is taken as an example to describe the synthesis procedure. Co (CH3COO)2·4H2O (2.491 g, 10 mmol) and Cu(NO3)2·3H2O (0.602 g, 2.5 mmol) were dispersed in 50 mL of deionized water and subsequently stirred at room temperature for 6 h. Following this, the solvent is evaporated under stirring at 80 °C. Then, the result solid samples were collected and further dried under vacuum at 80 °C. Finally, the products were calcined at 450 °C for 6 h to obtain the catalysts.
3.2.2. Preparation of the Cordierite Monolithic Catalyst
We prepared Co3O4-20Cu/cordierite monolithic catalyst by the impregnation method. Co (CH3COO)2·4H2O (9.964 g, 40 mmol) and Cu(NO3)2·3H2O (2.408 g, 10 mmol) were dispersed in 100 mL of deionized water, and then 8 g polyvinylpyrrolidone was added. The mixture was stirred at room temperature for 1 h to completely dissolve the materials. Subsequently, the solution was heated in an 80 °C oil bath while 6 mL of triethanolamine was added during the heating process. After removing excess water and the solution becomes viscous, it was used as the original slurry. The cubic cordierite was cut into blocks and ultrasonicated for 0.5 h to remove surface impurities. After ultrasonic treatment, the cordierite was added into the original slurry and impregnated for 12 h. Then, the cordierite was removed, and excess slurry was drained. Then, cordierite was dried under vacuum at 80 °C. Finally, it is calcined at 450 °C for 6 h to produce Co3O4-20Cu/cordierite monolithic catalyst.
3.3. Characterizations
XRD data were collected on X’pert Pro X-ray diffractometer Rigaku MiniFlex 600 X-ray diffractometer (Rigaku Corporation, Tokyo, Japan) with Cu-Kα radiation with operating conditions of 40 kV and 40 mA, scanning from 10 to 80°. The concentrations of metallic elements in the samples were analyzed using inductively coupled plasma optical emission spectroscopy (ICP-OES). The N2 static adsorption–desorption study was conducted using an Autosorb-iQ instrument (Quantachrome Instrument, Boynton Beach, FL, USA) at −196 °C. The specific surface area and pore size distribution were determined by Brunauer–Emmet–Teller (BET) and Barrett–Joyner–Halenda (BJH) methods, respectively. The scanning electron microscopy (SEM) characterizations were recorded on an S4800 SEM (Hitachi High-Technologies Corporation, Tokyo, Japan) with accelerating voltage of 20 kV. Transmission electron microscopy (TEM) characterizations were obtained on the machine FEI Talos F200S (Thermo Fisher Scientific, Waltham, MA, USA) with an accelerating voltage of 200 kV. The temperature-programmed desorption of oxygen (O2-TPD) were tested on chemical sorbent instrument Micromeritics AutoChem II 2920 (Micromeritics Instrument Corporation, Norcross, GE, USA). 100 mg samples were dried at 300 °C, pursed in He (50 mL/min) for 1 h, and then cooled to 50 °C. It was then raised to 800 °C for desorption at a ramp rate of 10 °C/min in He. The hydrogen temperature programmed reduction (H2-TPR) were tested on a chemical adsorption apparatus Micromeritics AutoChem II 2920 (Micromeritics Instrument Corporation, Norcross, GE, USA). Samples of 100 mg were dried at 300 °C, pursed in He (50 mL/min) flow for 1 h, cooled to 50 °C, and finally increased to 800 °C at a 10 °C/min ramp rate in 10% H2/Ar. The elements valence distribution of the sample surface was analyzed on the X-ray photoelectron spectroscopy (XPS) Thermo Scientific K-Alpha+ (Thermo Fisher Scientific, Waltham, MA, USA). The Raman data were obtained by a 532 nm wavelength laser source on the HORIBA HR Evolution. The electron paramagnetic resonance (EPR) data were obtained on a Bruker A300 spectrometer (Bruker Corporation, Billerica, MA, USA) tested at 77 K in nitrogen. In situ diffuse reflectance infrared Fourier transform spectroscopy (in situ DRIFTS) was performed on a Thermo Fisher iS50 (Thermo Fisher Scientific, Waltham, MA, USA) instrument equipped with Harrick cell and infrared spectroscopy Nicolet Nexus 760 (Thermo Fisher Scientific, Waltham, MA, USA) to study mechanism for ethyl acetate oxidation.
3.4. Catalytic Activity Measurements
To assess the catalytic performance of the synthesized catalysts in ethyl acetate combustion, a fixed-bed reactor (i. d. = 6.0 mm, length = 415 mm) was used. The test sample consists of 60 mg catalysts and 500 mg quartz sand. The height of the reaction bed was maintained at 2 to 2.5 cm. A thermocouple was inserted to the bottom of the reaction bed. During the reaction, this thermocouple served to measure the temperature of the flowing gas. The setting for the catalytic oxidation of ethyl acetate entailed an air stream containing 1000 ppm of ethyl acetate, with WHSV of 60,000 mL/(g·h). While keeping all other conditions constant, 3 vol. % water vapor was added to test the catalyst’s resistance to water vapor. The exhaust gas passing through the fixed bed entered into a gas chromatograph, where the components of the gas were analyzed by FID.
The catalytic activity test for the cordierite monolithic catalyst was performed on the self-made equipment (Supplementary Figure S1). During the test, the cordierite monolithic catalyst was cut into a cylinder with a radius of 2.3 cm and a height of 5 cm, and placed at the rear end of the catalytic chamber. Ethyl acetate was pushed into a preheater and vaporized there. Then, ethyl acetate gas was blown into the catalytic chamber by a fan, and the reaction temperature of the catalytic chamber was controlled by the temperature control system to degrade ethyl acetate. The specific experimental setup diagram was shown in Supplementary Figure S1. The test condition for the catalytic oxidation of ethyl acetate includes an air stream containing 1000 ppm of ethyl acetate, with WHSV of 10,000 mL/(g·h).
The conversion of ethyl acetate (XEA) was determined using the following Equation (1). In Equation (1), CEA(in) and CEA(out) represent the concentration of ethyl acetate at the inlet and outlet. The CO2 yield is calculated by using Equation (2). Here, denotes the actual production of CO2 at the outlet, whereas represents the theoretical production of CO2.
4. Conclusions
In this study, CuO/Co3O4 catalysts with abundant oxygen vacancies were synthesized using a straightforward impregnation method. The introduction of CuO into Co3O4 promotes the catalytic activity for ethyl acetate oxidation due to the increased oxygen vacancies and lattice defects, enlarged specific surface area, enhanced low temperature reducibility, and increased active oxygen species of the composite catalysts. The optimal catalyst Co3O4-20Cu exhibits T90 of ethyl acetate conversion and mineralization at 211 and 224 °C, respectively, which is about 40 °C lower than that of the pure Co3O4. Moreover, it exhibits good catalytic stability and excellent resistance to water vapor. In addition, the degradation of ethyl acetate on Co3O4-20Cu in air or N2 were analyzed by in situ DRIFTS. We found that ethanol and acetic acid were the main intermediates for ethyl acetate catalytic oxidation and the reaction follows the Mars-van Krevelen mechanism. This work develops a new idea for the preparation of catalysts with abound oxygen vacancies for the efficient degradation of ethyl acetate, and successfully applied it on monolithic catalyst for future application.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/catal15060538/s1, Figure S1: Photo of the self-made equipment for the catalytic oxidation performance estimation.; Figure S2: SEM images of (a) Co3O4, (b) Co3O4-10Cu, (c) Co3O4-15Cu, (d) Co3O4-20Cu, and (e) Co3O4-30Cu; Figure S3: The photos of the Co3O4-20Cu/cordierite catalyst; Figure S4: SEM images of (a,b) cordierite carrier, (c,d) untested Co3O4-20Cu/cordierite, (e,f) tested Co3O4-20Cu/cordierite; Figure S5: TEM images of (a) Co3O4, (b) Co3O4-10Cu, (c) Co3O4-15Cu, (d) Co3O4-20Cu, and (e) Co3O4-30Cu; Figure S6: XPS survey spectra of the samples; Figure S7: The catalytic oxidation activity for ethyl acetate oxidation on the Co3O4-20Cu cordierite monolithic catalysts. Table S1: The particle size of the catalysts; Table S2: Peak height and half peak width of catalyst (3 1 1) crystal plane; Table S3: Activity of cobalt-based catalysts to degrade ethyl acetate. References [12,13,14,50,51,52,53,54,55] are cited in the Supplementary Materials.
Author Contributions
Conceptualization, H.J.; methodology, H.J.; software, Z.H.; formal analysis, J.W. and Z.H.; investigation, J.W. and Z.H.; resources, H.J. and C.-Z.L.; data curation, J.W.; writing—original draft, J.W.; writing—review and editing, J.C.; supervision, J.C. and C.-Z.L.; project administration, J.C.; funding acquisition, J.C. and C.-Z.L. All authors have read and agreed to the published version of the manuscript.
Funding
The authors thanks for the funding support from the National Natural Science Foundation of China (22476195, 22275185) and the Natural Science Foundation of Fujian Province (2022Y0071), the XMIREM autonomously deployment project (2023CX07, 2023GG01).
Data Availability Statement
Data will be available when someone request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wang, R.; Duan, W.; Cheng, S.; Wang, X. Nonlinear and lagged effects of VOCs on SOA and O3 and multi-model validated control strategy for VOC sources. Sci. Total Environ. 2023, 887, 164113. [Google Scholar] [CrossRef]
- Pye, H.O.T.; Appel, K.W.; Seltzer, K.M.; Ward-Caviness, C.K.; Murphy, B.N. Human-health impacts of controlling secondary air pollution precursors. Environ. Sci. Technol. Lett. 2022, 9, 96–101. [Google Scholar] [CrossRef]
- Ren, Y.; Guan, X.; Peng, Y.; Gong, A.; Xie, H.; Chen, S.; Zhang, Q.; Zhang, X.; Wang, W.; Wang, Q. Characterization of VOC emissions and health risk assessment in the plastic manufacturing industry. J. Environ. Manag. 2024, 357, 120730. [Google Scholar] [CrossRef]
- He, C.; Cheng, J.; Zhang, X.; Douthwaite, M.; Pattisson, S.; Hao, Z. Recent advances in the catalytic oxidation of volatile organic compounds: A review based on pollutant sorts and sources. Chem. Rev. 2019, 119, 4471–4568. [Google Scholar] [CrossRef]
- Wen, M.; Song, S.; Zhao, W.; Liu, Q.; Chen, J.; Li, G.; An, T. Atomically dispersed Pd sites on Ti-SBA-15 for efficient catalytic combustion of typical gaseous VOCs. Environ. Sci. Nano 2021, 8, 3735–3745. [Google Scholar] [CrossRef]
- Li, K.; Luo, X. Research progress on catalytic combustion of volatile organic compounds in industrial waste gas. Catalysts 2023, 13, 268. [Google Scholar] [CrossRef]
- Xiao, M.; Zhao, T.; Li, Y.; Zhu, B.; Yu, T.; Liu, W.; Zhao, M.; Cui, B. Co3O4-based batalysts for the low-temperature catalytic oxidation of VOCs. ChemCatChem 2024, 16, e202301524. [Google Scholar] [CrossRef]
- Bao, M.; Liu, Y.; Deng, J.; Jing, L.; Hou, Z.; Wang, Z.; Wei, L.; Yu, X.; Dai, H. Catalytic performance and reaction mechanisms of ethyl acetate oxidation over the Au–Pd/TiO2 catalysts. Catalysts 2023, 13, 643. [Google Scholar] [CrossRef]
- Tílvez, E.; Cárdenas-Jirón, G.I.; Menéndez, M.I.; López, R. Understanding the hydrolysis mechanism of ethyl acetate catalyzed by an aqueous molybdocene: A computational chemistry investigation. Inorg. Chem. 2015, 54, 1223–1231. [Google Scholar] [CrossRef]
- Tang, H.; Wu, S.; Ding, L.; Fang, N.; Zhang, Q.; Chu, Y. Catalytic oxidation and mixed oxidation of ethyl acetate: A review. Sep. Purif. Technol. 2024, 343, 126980. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, H.; Yan, Y. Catalytic oxidation of ethyl acetate over CuO/ZSM-5 catalysts: Effect of preparation method. J. Taiwan Inst. Chem. Eng. 2018, 84, 162–172. [Google Scholar] [CrossRef]
- Zhu, X.; Bai, B.; Zhou, B.; Ji, S. Co3O4 nanoparticles with different morphologies for catalytic removal of ethyl acetate. Catal. Commun. 2021, 156, 106320. [Google Scholar] [CrossRef]
- Wang, S.; Zhu, J.; Yang, J.; Li, M.; Zhu, Y. Influence of LaCoO3 perovskite oxides prepared by different method on the catalytic combustion of ethyl acetate in the presence of NO. Appl. Surf. Sci. 2023, 623, 157045. [Google Scholar] [CrossRef]
- Zhao, Q.; Zheng, Y.; Song, C.; Liu, Q.; Ji, N.; Ma, D.; Lu, X. Novel monolithic catalysts derived from in-situ decoration of Co3O4 and hierarchical Co3O4@MnOx on Ni foam for VOC oxidation. Appl. Catal. B Environ. 2020, 265, 118552. [Google Scholar] [CrossRef]
- Zheng, Y.; Fu, K.; Yu, Z.; Su, Y.; Han, R.; Liu, Q. Oxygen vacancies in a catalyst for VOCs oxidation: Synthesis, characterization, and catalytic effects. J. Mater. Chem. A 2022, 10, 14171–14186. [Google Scholar] [CrossRef]
- Feng, X.; Chen, H.; Xue, Q.; Su, C.; Zhou, Y. Construction of core–shell structured CrOx/NiCoxO4 catalyst for low temperature catalytic oxidation of toluene: Understanding the reactivity promotion mechanism of the core–shell interactions. Chem. Eng. J. 2024, 490, 151464. [Google Scholar] [CrossRef]
- Jiang, Y.; Gao, J.; Zhang, Q.; Liu, Z.; Fu, M.; Wu, J.; Hu, Y.; Ye, D. Enhanced oxygen vacancies to improve ethyl acetate oxidation over MnOx-CeO2 catalyst derived from MOF template. Chem. Eng. J. 2019, 371, 78–87. [Google Scholar] [CrossRef]
- Ye, Y.; Xu, J.; Gao, L.; Zang, S.; Chen, L.; Wang, L.; Mo, L. CuO/CeO2 catalysts prepared by modified impregnation method for ethyl acetate oxidation. Chem. Eng. J. 2023, 471, 144667. [Google Scholar] [CrossRef]
- Lv, L.; Zhang, Z.; Wang, S.; Shan, Y.; Wang, L.; Xu, T.; He, P. Catalytic oxidation of ethyl acetate over Y (Y = Cu, Mn, Co)-modified CeO2 derived from Ce-MOF. Catal. Commun. 2024, 186, 106832. [Google Scholar] [CrossRef]
- Feng, X.; Guo, J.; Wen, X.; Xu, M.; Chu, Y.; Yuan, S. Enhancing performance of Co/CeO2 catalyst by Sr doping for catalytic combustion of toluene. Appl. Surf. Sci. 2018, 445, 145–153. [Google Scholar] [CrossRef]
- Bao, L.; Zhu, S.; Chen, Y.; Wang, Y.; Meng, W.; Xu, S.; Lin, Z.; Li, X.; Sun, M.; Guo, L. Anionic defects engineering of Co3O4 catalyst for toluene oxidation. Fuel 2022, 314, 122774. [Google Scholar] [CrossRef]
- Sun, L.; Liang, X.; Liu, H.; Cao, H.; Liu, X.; Jin, Y.; Li, X.; Chen, S.; Wu, X. Activation of Co-O bond in (110) facet exposed Co3O4 by Cu doping for the boost of propane catalytic oxidation. J. Hazard. Mater. 2023, 452, 131319. [Google Scholar] [CrossRef] [PubMed]
- Ren, Q.; Mo, S.; Peng, R.; Feng, Z.; Zhang, M.; Chen, L.; Fu, M.; Wu, J.; Ye, D. Controllable synthesis of 3D hierarchical Co3O4 nanocatalysts with various morphologies for the catalytic oxidation of toluene. J. Mater. Chem. A 2018, 6, 498–509. [Google Scholar] [CrossRef]
- Gao, H.; Lv, X.; Zhang, M.; Li, Q.; Chen, J.; Hu, Z.; Jia, H. Copper-cobalt strong interaction to improve photothermocatalytic performance of cobalt-copper oxides supported on copper foam for toluene oxidation. Chem. Eng. J. 2022, 434, 134618. [Google Scholar] [CrossRef]
- Chen, G.; Zhao, Y.; Fu, G.; Duchesne, P.N.; Gu, L.; Zheng, Y.; Weng, X.; Chen, M.; Zhang, P.; Pao, C.W.; et al. Interfacial effects in iron-nickel hydroxide-platinum nanoparticles enhance catalytic oxidation. Science 2014, 344, 495–499. [Google Scholar] [CrossRef]
- Chen, X.; Chen, X.; Cai, S.; Chen, J.; Xu, W.; Jia, H.; Chen, J. Catalytic combustion of toluene over mesoporous Cr2O3-supported platinum catalysts prepared by in situ pyrolysis of MOFs. Chem. Eng. J. 2018, 334, 768–779. [Google Scholar] [CrossRef]
- Jin, M.; Li, Z.; Piao, W.; Chen, J.; Jin, L.Y.; Kim, J.M. Highly ordered mesoporous cobalt-copper composite oxides for preferential CO oxidation. Catal. Surv. Asia 2017, 21, 45–52. [Google Scholar] [CrossRef]
- Jian, Y.; Tian, M.; He, C.; Xiong, J.; Jiang, Z.; Jin, H.; Zheng, L.; Albilali, R.; Shi, J.-W. Efficient propane low-temperature destruction by Co3O4 crystal facets engineering: Unveiling the decisive role of lattice and oxygen defects and surface acid-base pairs. Appl. Catal. B Environ. 2021, 283, 119657. [Google Scholar] [CrossRef]
- Meng, W.; Sun, S.; Xie, D.; Dai, S.; Shao, W.; Zhang, Q.; Qin, C.; Liang, G.; Li, X. Engineering defective Co3O4 containing both metal doping and vacancy in octahedral cobalt site as high performance catalyst for methane oxidation. Mol. Catal. 2024, 553, 113768. [Google Scholar] [CrossRef]
- Ye, Z.; Wang, G.; Giraudon, J.M.; Nikiforov, A.; Chen, J.; Zhao, L.; Zhang, X.; Wang, J. Investigation of Cu-Mn catalytic ozonation of toluene: Crystal phase, intermediates and mechanism. J. Hazard. Mater. 2022, 424, 127321. [Google Scholar] [CrossRef]
- Zagaynov, I.V.; Naumkin, A.V.; Konovalov, A.A. CuxCe1-xO2 solid solutions: Effect of low-content dopant. Ceram. Int. 2024, 50, 14513–14519. [Google Scholar] [CrossRef]
- Ren, J.T.; Zheng, Y.L.; Yuan, K.; Zhou, L.; Wu, K.; Zhang, Y.W. Self-templated synthesis of Co3O4 hierarchical nanosheets from a metal-organic framework for efficient visible-light photocatalytic CO2 reduction. Nanoscale 2020, 12, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, Y.; Liu, J.; Tian, Z.; Jing, Y. Regulation of oxygen vacancies in cobalt-cerium oxide catalyst for boosting decontamination of VOCs by catalytic oxidation. Sep. Purif. Technol. 2021, 277, 119505. [Google Scholar] [CrossRef]
- Li, Y.; Chen, T.; Zhao, S.; Wu, P.; Chong, Y.; Li, A.; Zhao, Y.; Chen, G.; Jin, X.; Qiu, Y.; et al. Engineering cobalt oxide with coexisting cobalt defects and oxygen vacancies for enhanced catalytic oxidation of toluene. ACS Catal. 2022, 12, 4906–4917. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, C.; Hua, W.; Guo, Y.; Lu, G.; Gil, S.; Giroir-Fendler, A. Catalytic oxidation of vinyl chloride emissions over Co-Ce composite oxide catalysts. Chem. Eng. J. 2017, 315, 392–402. [Google Scholar] [CrossRef]
- Wang, P.; Wang, J.; An, X.; Shi, J.; Shangguan, W.; Hao, X.; Xu, G.; Tang, B.; Abudula, A.; Guan, G. Generation of abundant defects in Mn-Co mixed oxides by a facile agar-gel method for highly efficient catalysis of total toluene oxidation. Appl. Catal. B Environ. 2021, 282, 119560. [Google Scholar] [CrossRef]
- Wan, C.; Liao, Y.; Huang, H.; Li, D.; Zhan, Y.; Xiao, Y.; Jiang, L. Novel preparation of copper–cobalt spinel oxides using Cu2+-Co2+-Co3+ hydrotalcite-like compounds as active catalysts for methane combustion. Chem. Eng. J. 2025, 513, 162813. [Google Scholar] [CrossRef]
- Li, X.; Li, X.; Zeng, X.; Zhu, T. Correlation between the physicochemical properties and catalytic performances of micro/mesoporous CoCeO mixed oxides for propane combustion. Appl. Catal. A Gen. 2019, 572, 61–70. [Google Scholar] [CrossRef]
- Mo, S.; Li, S.; Li, J.; Deng, Y.; Peng, S.; Chen, J.; Chen, Y. Rich surface Co(III) ions-enhanced Co nanocatalyst benzene/toluene oxidation performance derived from CoIICoIII layered double hydroxide. Nanoscale 2016, 8, 15763–15773. [Google Scholar] [CrossRef]
- Sun, N.; Zhang, Y.; Wang, L.; Cao, Z.; Sun, J. Catalytic oxidation of toluene over Co-Cu bimetallic oxides derived from CoyCu3-y-MOF-74. J. Alloys Compd. 2022, 928, 167105. [Google Scholar] [CrossRef]
- Wu, N.; Zhang, C.; Li, J.; Shi, Y.; Wang, Q.; Wu, S.; Yao, S.; Wu, Z.; Gao, E.; Wang, W.; et al. In situ synthesis of MOF-derived CuCoOx with enhanced catalytic activity and moisture resistance for aromatic VOCs combustion: The role of bimetallic oxide interactions on the catalytic mechanism. Sep. Purif. Technol. 2024, 341, 126947. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, D.; Zhao, G.; Liu, D.; Kuvarega, A.T.; Mamba, B.B.; Gui, J. Homogeneous CoCeOx nanocomposites with rich oxygen vacancies for effective catalytic oxidation of toluene. Sep. Purif. Technol. 2023, 320, 124130. [Google Scholar] [CrossRef]
- Yang, Y.; Si, W.; Peng, Y.; Wang, Y.; Liu, H.; Su, Z.; Li, J. Defect engineering on CuMn2O4 spinel surface: A new path to high-performance oxidation catalysts. Environ. Sci. Technol. 2022, 56, 16249–16258. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Lou, L.L.; Liu, S.; Zhou, W. Asymmetric oxygen vacancies: The intrinsic redox active sites in metal oxide catalysts. Adv. Sci. 2020, 7, 1901970. [Google Scholar] [CrossRef]
- Ma, M.; Yang, R.; He, C.; Jiang, Z.; Shi, J.W.; Albilali, R.; Fayaz, K.; Liu, B. Pd-based catalysts promoted by hierarchical porous Al2O3 and ZnO microsphere supports/coatings for ethyl acetate highly active and stable destruction. J. Hazard. Mater. 2021, 401, 123281. [Google Scholar] [CrossRef]
- Ma, X.; Wang, W.; Zhang, X.; Li, H.; Sun, J.; Liu, X.; Sun, C. MOF-derived FeOx with highly dispersed active sites as an efficient catalyst for enchaning catalytic oxidation of VOCs. J. Environ. Chem. Eng. 2024, 12, 111966. [Google Scholar] [CrossRef]
- Wang, X.; Wu, L.; Wang, Z.; Feng, Y.; Liu, Y.; Dai, H.; Wang, Z.; Deng, J. Photothermal synergistic catalytic oxidation of ethyl acetate over MOFs-derived mesoporous N-TiO2 supported Pd catalysts. Appl. Catal. B Environ. 2023, 322, 122075. [Google Scholar] [CrossRef]
- Wang, H.; Chen, S.; Wang, Z.; Zhou, Y.; Wu, Z. A novel hybrid Bi2MoO6-MnO2 catalysts with the superior plasma induced pseudo photocatalytic-catalytic performance for ethyl acetate degradation. Appl. Catal. B Environ. 2019, 254, 339–350. [Google Scholar] [CrossRef]
- Liu, L.; Zheng, Y.; Gong, P.; Shao, G.; Ma, C.; Wang, G.; Wang, J.; Mi, J. Enhanced plasma-catalytic decomposition of ethyl acetate with ordered three-dimensional multi-mesoporous bimetallic cobalt oxides. Chem. Eng. J. 2024, 483, 149351. [Google Scholar] [CrossRef]
- Xie, H.; Zhao, X.; Zhou, G.; He, X.; Lan, H.; Jiang, Z. Investigating the performance of CoxOy/activated carbon catalysts for ethyl acetate catalytic combustion. Appl. Surf. Sci. 2015, 326, 119–123. [Google Scholar] [CrossRef]
- Akram, S.; Wang, Z.; Chen, L.; Wang, Q.; Shen, G.; Han, N.; Chen, Y.; Ge, G. Low-temperature efficient degradation of ethyl acetate catalyzed by lattice-doped CeO2-CoOx nanocomposites. Catal. Commun. 2016, 73, 123–127. [Google Scholar] [CrossRef]
- Konsolakis, M.; Carabineiro, S.A.C.; Marnellos, G.E.; Asad, M.F.; Soares, O.S.G.P.; Pereira, M.F.R.; Órfão, J.J.M.; Figueiredo, J.L. Effect of cobalt loading on the solid state properties and ethyl acetate oxidation performance of cobalt-cerium mixed oxides. J. Colloid Interface Sci. 2017, 496, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Wu, X.; Li, S.; Li, W.; Chen, Y. Porous Mn-Co mixed oxide nanorod as a novel catalyst with enhanced catalytic activity for removal of VOCs. Catal. Commun. 2014, 56, 134–138. [Google Scholar] [CrossRef]
- Xu, Z.; Li, J.; Wang, X.; Wang, T.; Li, D.; Ao, Z. Pt-Co bimetals supported on UiO-66 as efficient and stable catalysts for the catalytic oxidation of various volatile organic compounds. Mater. Today Chem. 2023, 29, 101403. [Google Scholar] [CrossRef]
- Qin, Y.; Shen, F.; Zhu, T.; Hong, W.; Liu, X. Catalytic oxidation of ethyl acetate over LaBO3 (B = Co, Mn, Ni, Fe) perovskites supported silver catalysts. RSC Adv. 2018, 8, 33425–33431. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).