Synergy in Sonogashira Cross-Coupling Reactions with a Magnetic Janus-Type Catalyst
Abstract
1. Introduction
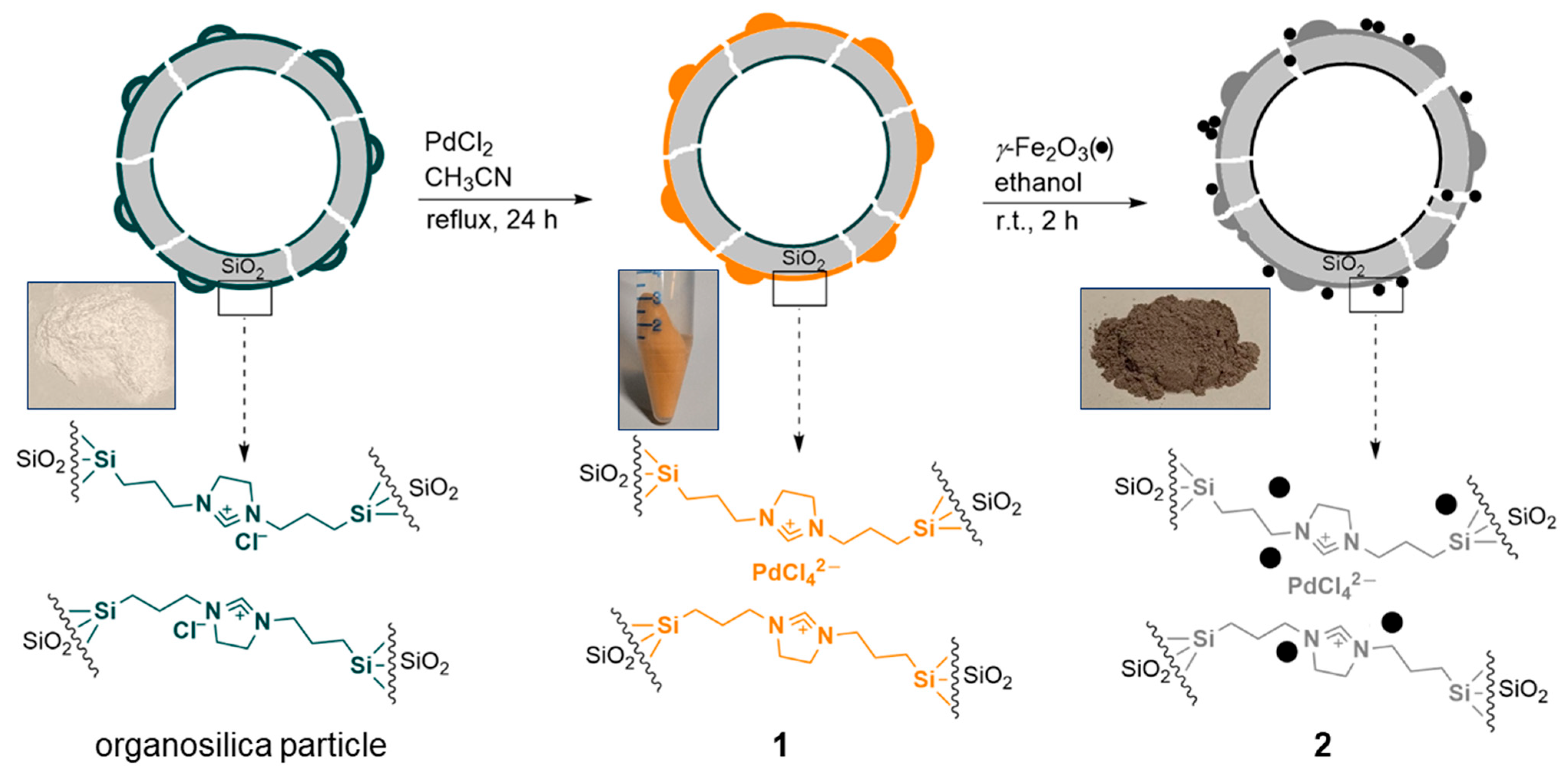
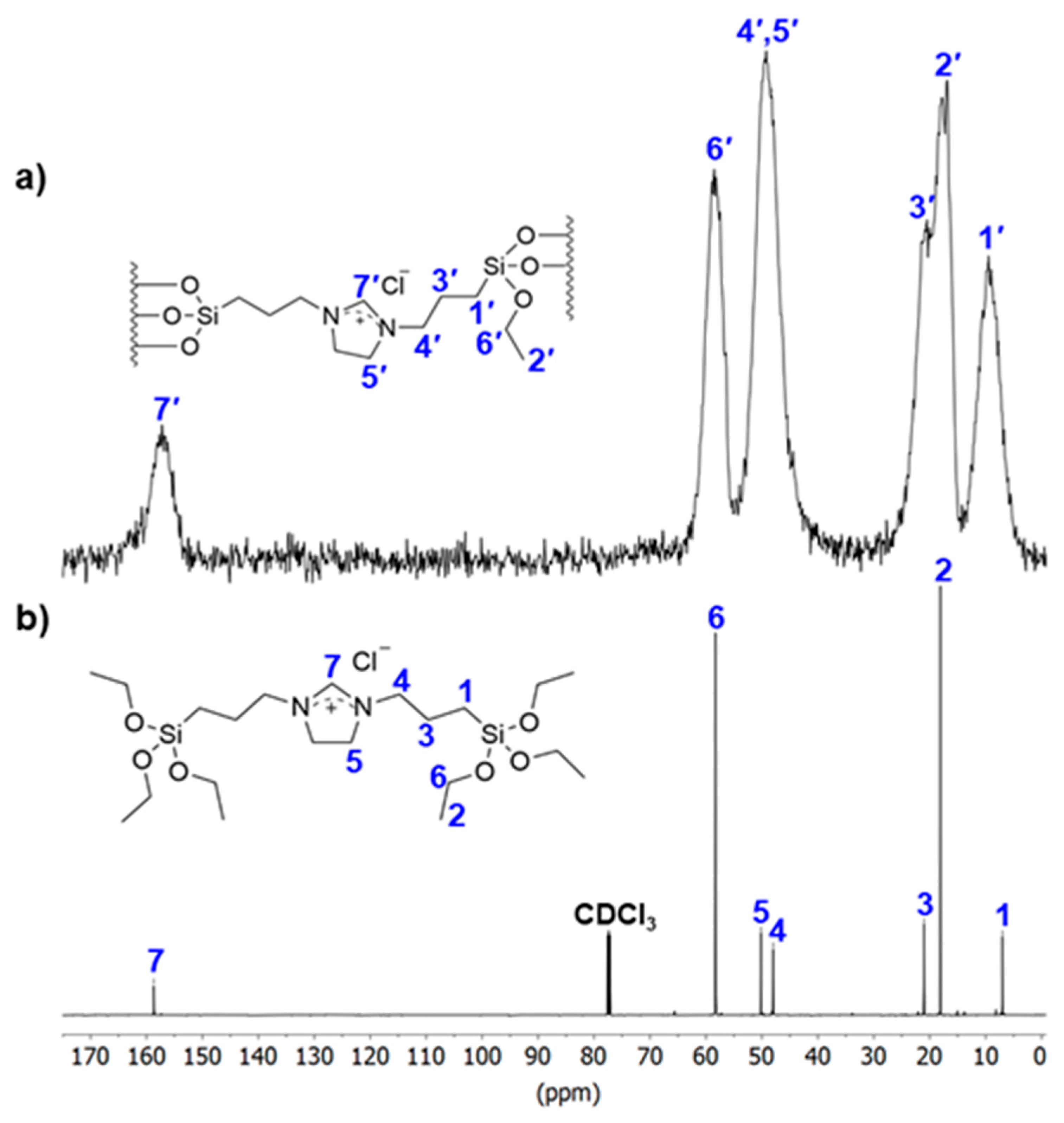
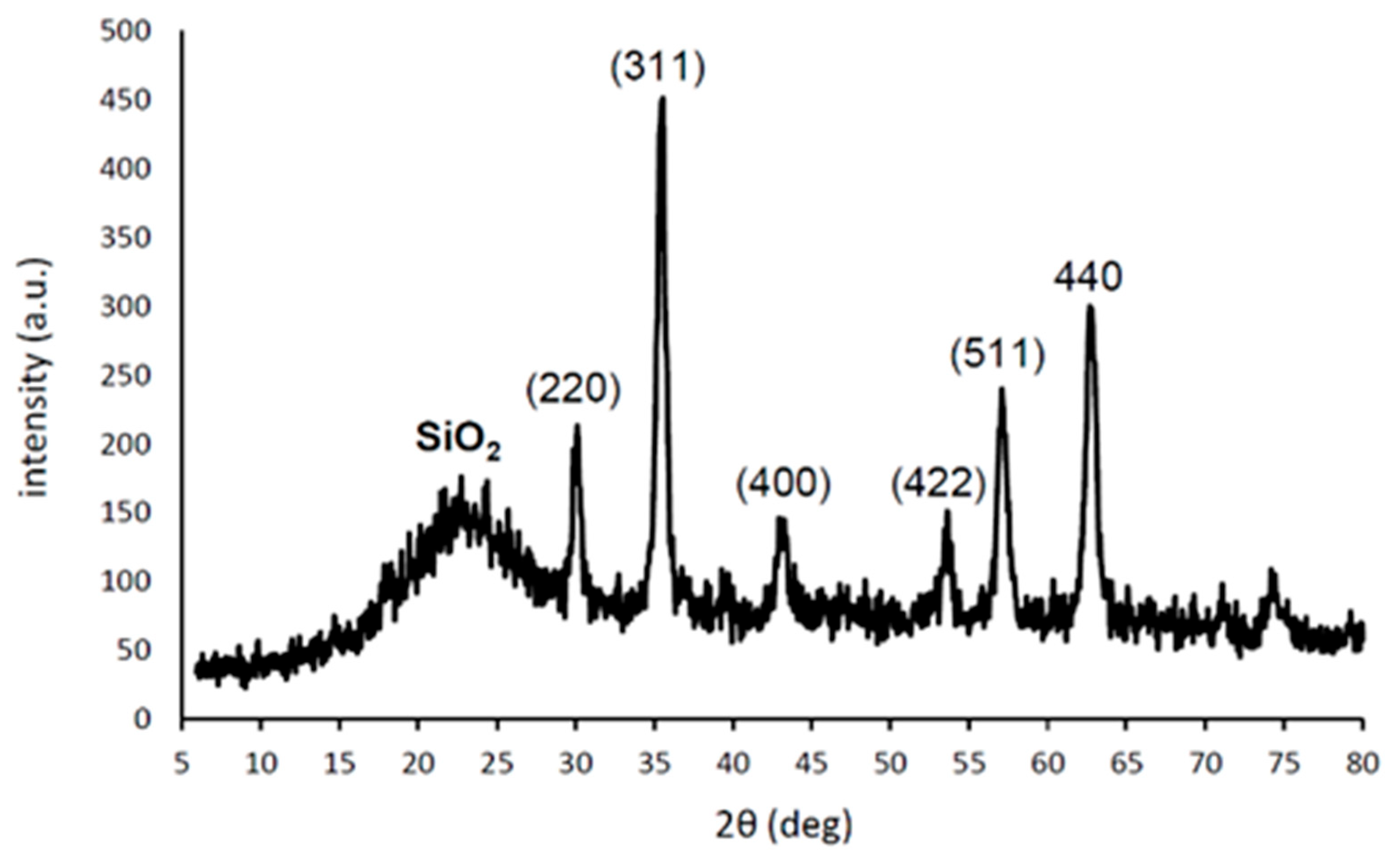
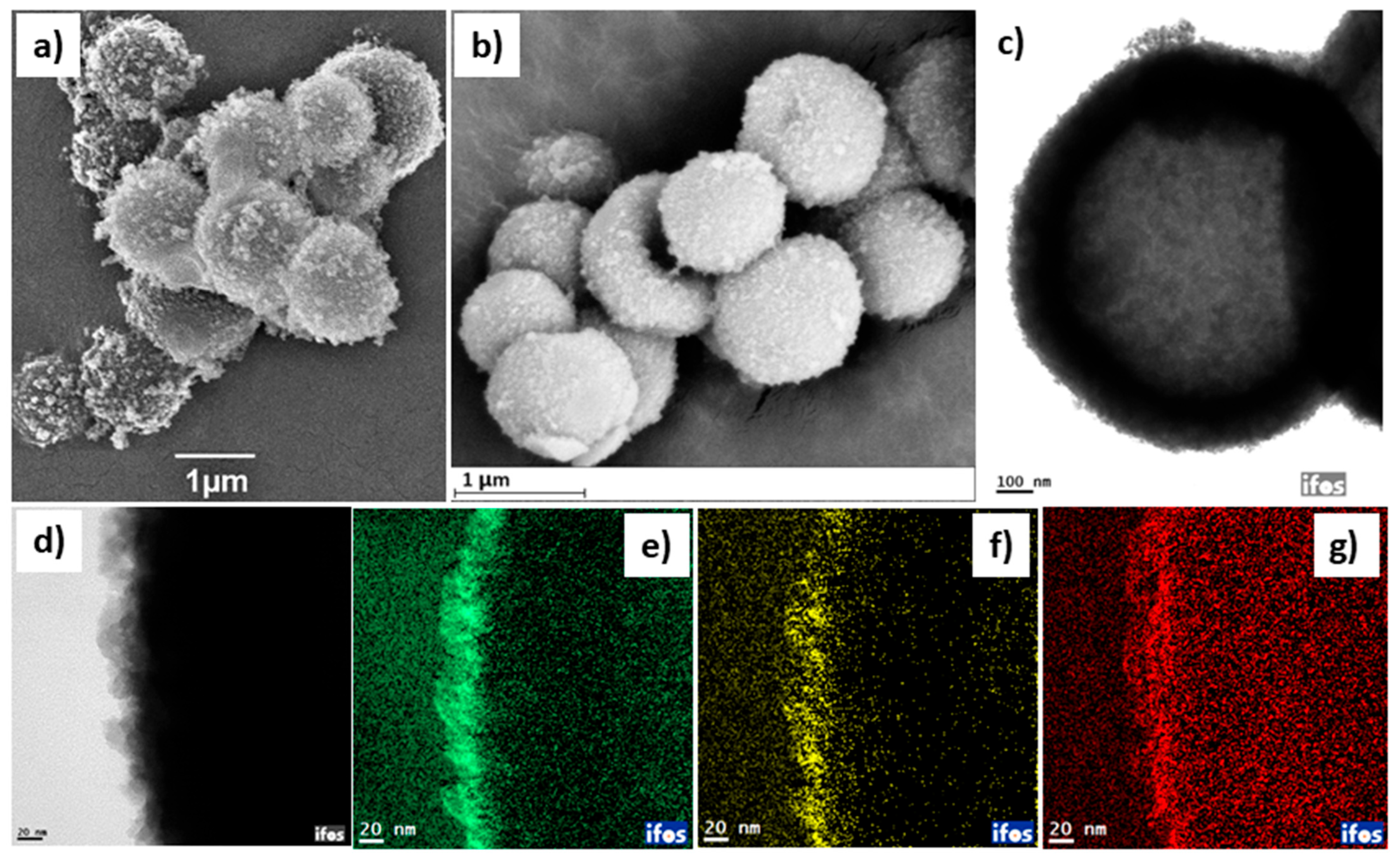
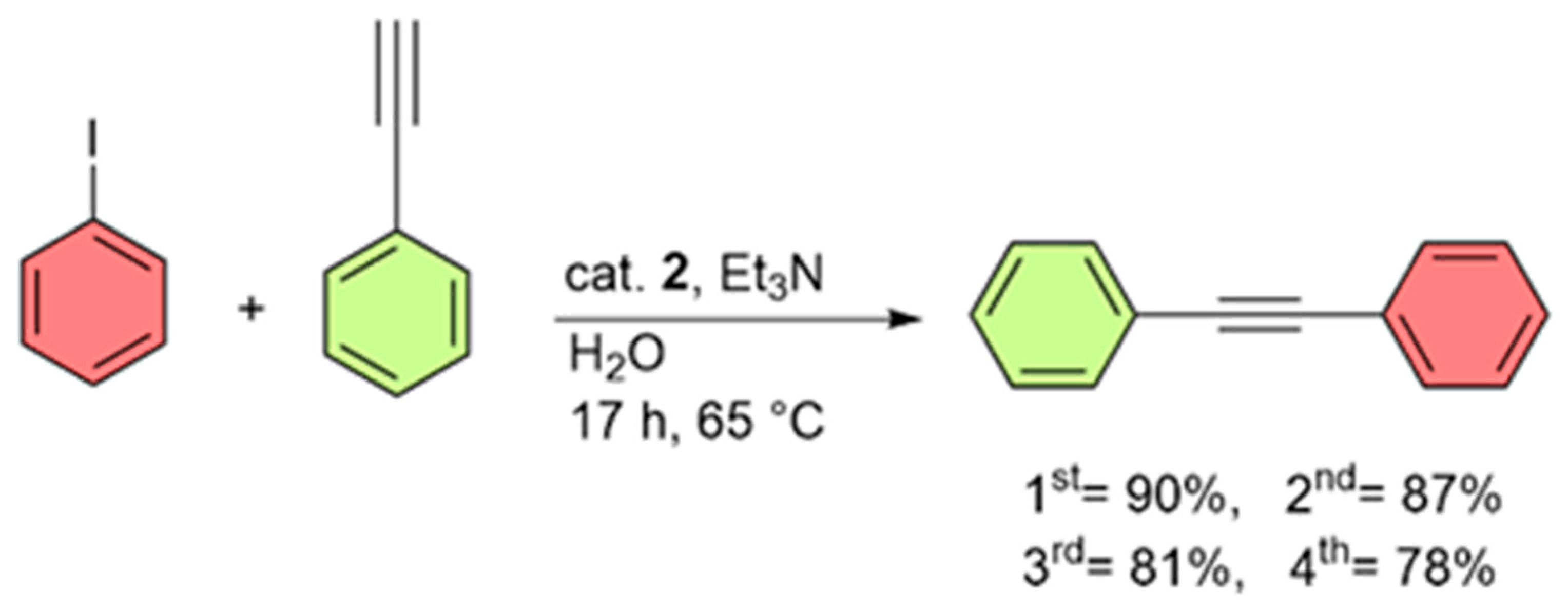
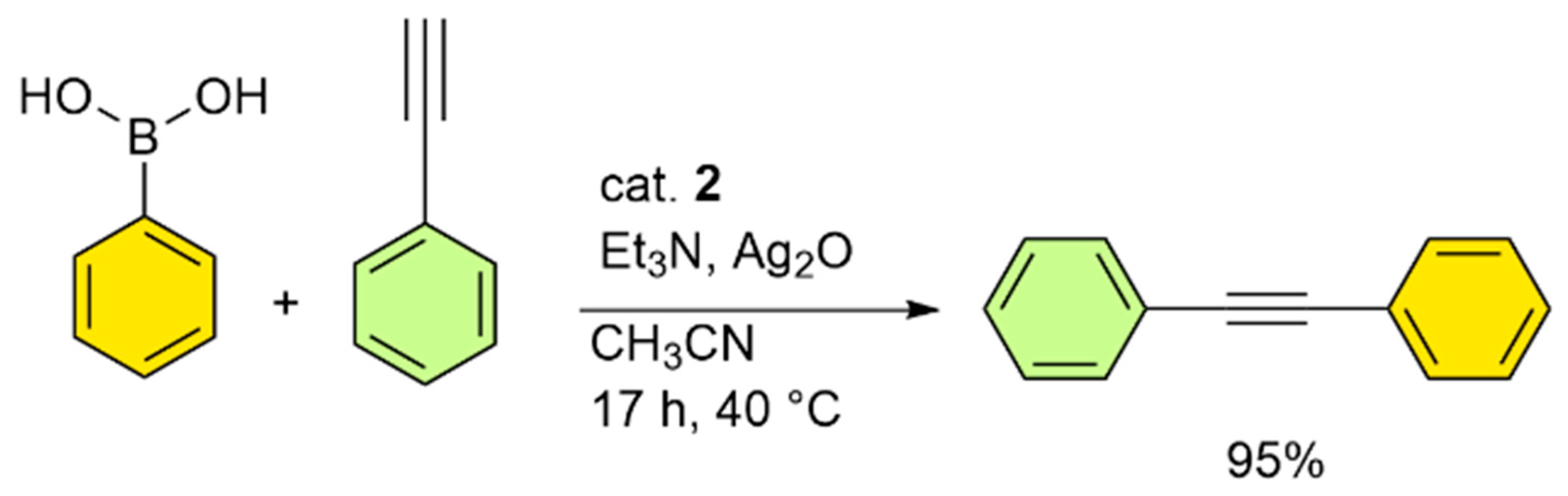
2. Results
3. Materials and Methods
3.1. General Information and Instruments
3.2. Synthesis of the Functionalized Silica Particles
3.3. Procedure for the Preparation of Material 1 [94]
3.4. Synthesis of Catalyst 2
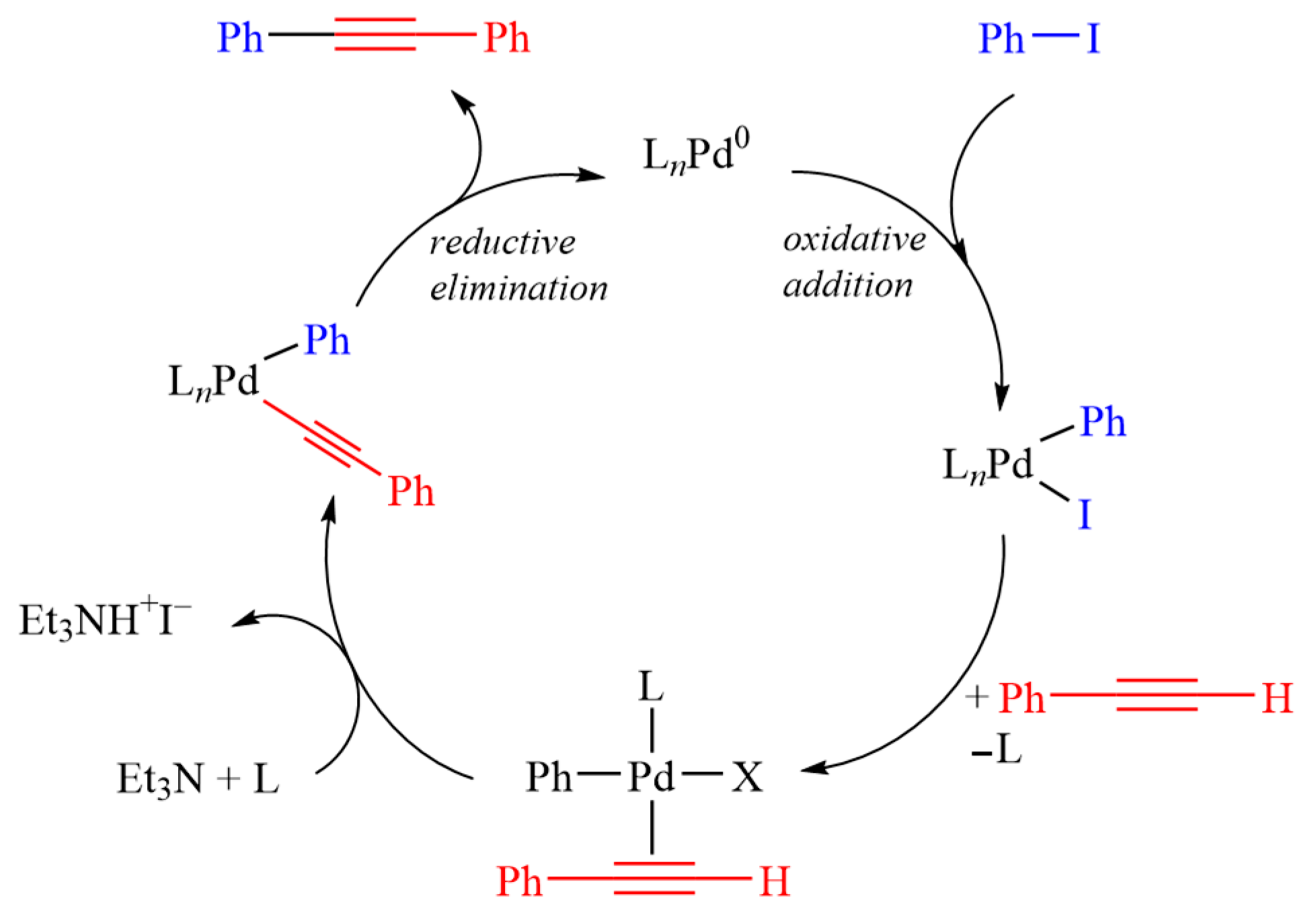
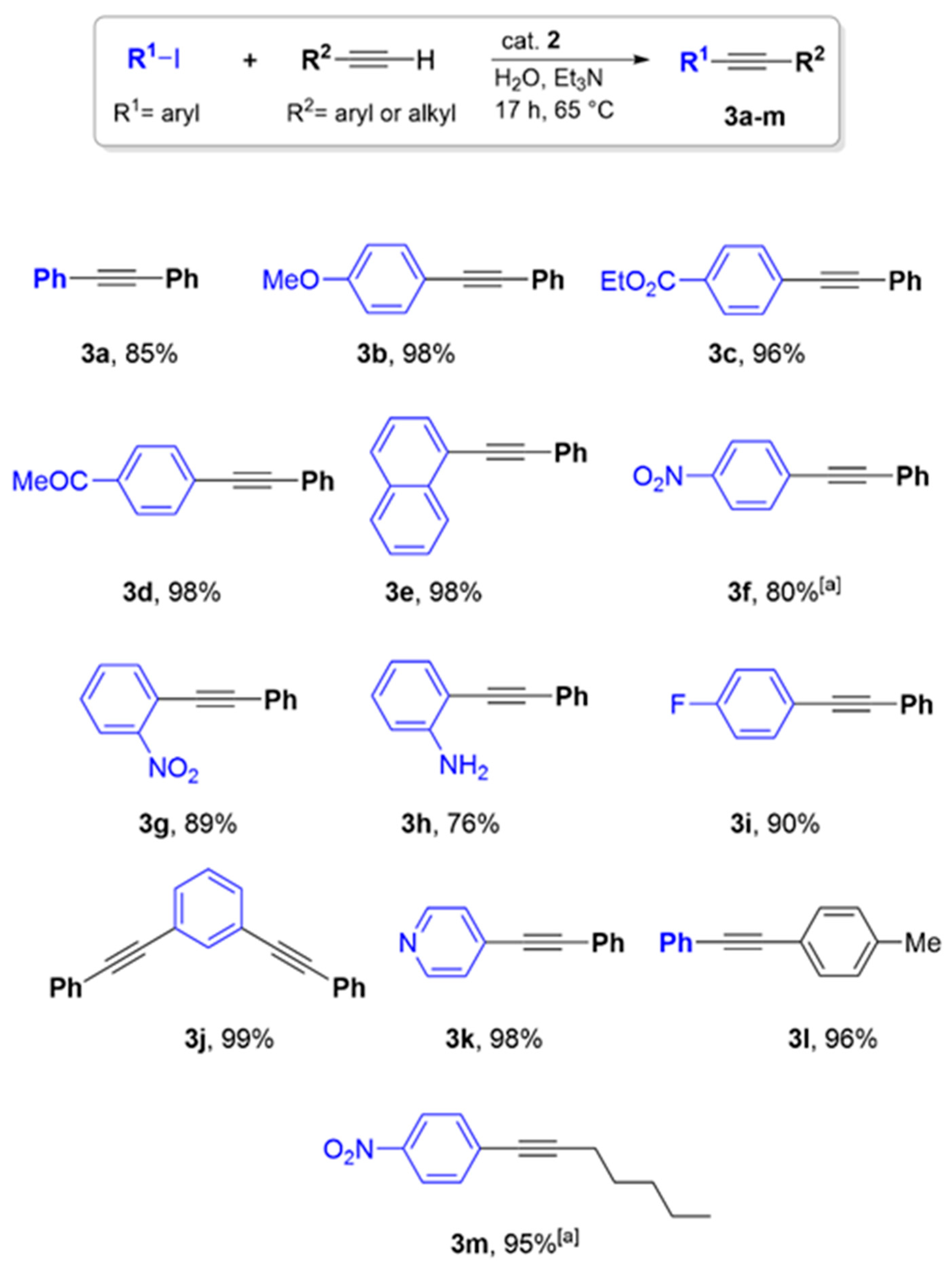
3.5. Procedure for the Sonogashira Cross-Coupling Reaction
3.6. Procedure for the Oxidative Sonogashira Cross-Coupling Reaction
3.7. Recycling Experiment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Dedication
References
- Shelke, Y.G.; Yashmeen, A.; Gholap, A.V.A.; Gharpure, S.J.; Kapdi, A.R. Homogeneous Catalysis: A Powerful Technology for the Modification of Important Biomolecules. Chem. Asian J. 2018, 13, 2991–3013. [Google Scholar] [CrossRef]
- Bender, T.A.; Dabrowski, J.A.; Gagné, M.R. Homogeneous Catalysis for the Production of Low-Volume, High-Value Chemicals from Biomass. Nat. Rev. Chem. 2018, 2, 35–46. [Google Scholar] [CrossRef]
- Kumar, A.; Daw, P.; Milstein, D. Homogeneous Catalysis for Sustainable Energy: Hydrogen and Methanol Economies, Fuels from Biomass, and Related Topics. Chem. Rev. 2022, 122, 385–441. [Google Scholar] [CrossRef]
- Shylesh, S.; Schünemann, V.; Thiel, W.R. Magnetically Separable Nanocatalysts: Bridges Between Homogeneous and Heterogeneous Catalysis. Angew. Chem. Int. Ed. 2010, 49, 3428–3459. [Google Scholar] [CrossRef] [PubMed]
- Shylesh, S.; Wang, L.; Demeshko, S.; Thiel, W.R. Facile Synthesis of Mesoporous Magnetic Nanocomposites and Their Catalytic Application in Carbon–Carbon Coupling Reactions. ChemCatChem 2010, 2, 1543–1547. [Google Scholar] [CrossRef]
- Shylesh, S.; Wang, L.; Thiel, W.R. Palladium(II)-Phosphine Complexes Supported on Magnetic Nanoparticles: Filtration-Free, Recyclable Catalysts for Suzuki–Miyaura Cross-Coupling Reactions. Adv. Synth. Catal. 2010, 352, 425–432. [Google Scholar] [CrossRef]
- Lindner, E.; Schneller, T.; Auer, F.; Mayer, H.A. Chemistry in Interphases—A New Approach to Organometallic Syntheses and Catalysis. Angew. Chem. Int. Ed. 1999, 38, 2154–2174. [Google Scholar] [CrossRef]
- Vafaeezadeh, M.; Thiel, W.R. Janus Interphase Catalysts for Interfacial Organic Reactions. J. Mol. Liq. 2020, 315, 113735. [Google Scholar] [CrossRef]
- Benaglia, M.; Puglisi, A.; Cozzi, F. Polymer-Supported Organic Catalysts. Chem. Rev. 2003, 103, 3401–3430. [Google Scholar] [CrossRef]
- Howard, I.C.; Hammond, C.; Buchard, A. Polymer-supported metal catalysts for the heterogeneous polymerisation of lactones. Polym. Chem. 2019, 10, 5894–5904. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Motahharifar, N.; Ghorbannezhad, F.; Bidgolia, N.S.S.; Baran, T.; Varma, R.S. Recent advances in polymer supported palladium complexes as (nano)catalysts for Sonogashira coupling reaction. Mol. Catal. 2020, 480, 110645. [Google Scholar] [CrossRef]
- Karimi, B.; Vafaeezadeh, M.; Akhavan, P.F. N-Heterocyclic Carbene–Pd Polymers as Reusable Precatalysts for Cyanation and Ullmann Homocoupling of Aryl Halides: The Role of Solvent in Product Distribution. ChemCatChem 2015, 7, 2248–2254. [Google Scholar] [CrossRef]
- Liang, Y. Recent advanced development of metal-loaded mesoporous organosilicas as catalytic nanoreactors. Nanoscale Adv. 2021, 3, 6827–6868. [Google Scholar] [CrossRef]
- Vafaeezadeh, M.; Hashemi, M.M. Efficient fatty acid esterification using silica supported Brønsted acidic ionic liquid catalyst: Experimental study and DFT modeling. Chem. Eng. J. 2014, 250, 35–41. [Google Scholar] [CrossRef]
- Karimi, B.; Vafaeezadeh, M. SBA-15 functionalized sulfonic acid containing a confined hydrophobic and acidic ionic liquid: A highly efficient catalyst for solvent-free thioacetalization of carbonyl compounds at room temperature. RSC Adv. 2013, 3, 23207–23211. [Google Scholar] [CrossRef]
- Karimi, B.; Vafaeezadeh, M. SBA-15-functionalized sulfonic acid confined acidic ionic liquid: A powerful and water-tolerant catalyst for solvent-free esterifications. Chem. Commun. 2012, 48, 3327–3329. [Google Scholar] [CrossRef] [PubMed]
- Díaz, U.; Brunel, D.; Corma, A. Catalysis using multifunctional organosiliceous hybrid materials. Chem. Soc. Rev. 2013, 42, 4083–4097. [Google Scholar] [CrossRef] [PubMed]
- Corma, A.; Das, D.; García, H.; Leyva, A. A periodic mesoporous organosilica containing a carbapalladacycle complex as heterogeneous catalyst for Suzuki cross-coupling. J. Catal. 2005, 229, 322–331. [Google Scholar] [CrossRef]
- Baleizão, C.; Gigante, B.; Das, D.; Álvaro, M.; Garcia, H.; Corma, A. Periodic mesoporous organosilica incorporating a catalytically active vanadyl Schiff base complex in the framework. J. Catal. 2004, 223, 106–113. [Google Scholar] [CrossRef]
- Yu, X.; Williams, C.T. Recent advances in the applications of mesoporous silica in heterogeneous catalysis. Catal. Sci. Technol. 2022, 12, 5765–5794. [Google Scholar] [CrossRef]
- Maschmeyer, T.; Rey, F.; Sankar, G.; Thomas, J.M. Heterogeneous catalysts obtained by grafting metallocene complexes onto mesoporous silica. Nature 1995, 378, 159–162. [Google Scholar] [CrossRef]
- Minakata, S.; Komatsu, M. Organic Reactions on Silica in Water. Chem. Rev. 2009, 109, 711–724. [Google Scholar] [CrossRef]
- Karimi, B.; Ganji, N.; Pourshiani, O.; Thiel, W.R. Periodic mesoporous organosilicas (PMOs): From synthesis strategies to applications. Prog. Mater. Sci. 2022, 125, 100896. [Google Scholar] [CrossRef]
- Jia, M.; Seifert, A.; Thiel, W.R. Mesoporous MCM-41 Materials Modified with Oxodiperoxo Molybdenum Complexes: Efficient Catalysts for the Epoxidation of Cyclooctene. Chem. Mater. 2003, 15, 2174–2180. [Google Scholar] [CrossRef]
- Shylesh, S.; Wagner, A.; Seifert, A.; Ernst, S.; Thiel, W.R. Cooperative Acid–Base Effects with Functionalized Mesoporous Silica Nanoparticles: Applications in Carbon–Carbon Bond-Formation Reactions. Chem. Eur. J. 2009, 15, 7052–7062. [Google Scholar] [CrossRef]
- Shylesh, S.; Wagener, A.; Seifert, A.; Ernst, S.; Thiel, W.R. Mesoporous Organosilicas with Acidic Frameworks and Basic Sites in the Pores: An Approach to Cooperative Catalytic Reactions. Angew. Chem. Int. Ed. 2010, 49, 184–187. [Google Scholar] [CrossRef]
- Vafaeezadeh, M.; Thiel, W.R. Task-Specific Janus Materials in Heterogeneous Catalysis. Angew. Chem. Int. Ed. 2022, 61, e202206403. [Google Scholar] [CrossRef]
- Pang, X.; Wan, C.; Wang, M.; Lin, Z. Strictly Biphasic Soft and Hard Janus Structures: Synthesis, Properties, and Applications. Angew. Chem. Int. Ed. 2014, 53, 5524–5538. [Google Scholar] [CrossRef]
- Yang, J.; Wang, J.; Liu, Y.; Li, H.; Lin, Z. Stimuli-responsive Janus mesoporous nanosheets towards robust interfacial emulsification and catalysis. Mater. Horiz. 2020, 7, 3242–3249. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J.; Shao, Y.; Deng, R.; Zhu, J.; Yang, Z. Recent advances in scalable synthesis and performance of Janus polymer/inorganic nanocomposites. Prog. Mater. Sci. 2022, 124, 100888. [Google Scholar] [CrossRef]
- Li, C.; Hu, J.; Tang, C.; Liu, Z.; Li, X.; Liu, S.; Tan, R. Thermo-Switchable Pickering Emulsion Stabilized by Smart Janus Nanosheets for Controllable Interfacial Catalysis. ACS Sustain. Chem. Eng. 2023, 11, 14144–14157. [Google Scholar] [CrossRef]
- Li, C.; Liu, S.; Pi, Y.; Feng, J.; Liu, Z.; Li, S.; Tan, R. Ionic liquid-functionalized amphiphilic Janus nanosheets afford highly accessible interface for asymmetric catalysis in water. J. Catal. 2021, 395, 236–245. [Google Scholar] [CrossRef]
- Zhang, M.; Tang, Z.; Fu, W.; Wang, W.; Tan, R.; Yin, D. An ionic liquid-functionalized amphiphilic Janus material as a Pickering interfacial catalyst for asymmetric sulfoxidation in water. Chem. Commun. 2019, 55, 592–595. [Google Scholar] [CrossRef]
- Vafaeezadeh, M.; Breuninger, P.; Lösch, P.; Wilhelm, C.; Ernst, S.; Antonyuk, S.; Thiel, W.R. Janus Interphase Organic-Inorganic Hybrid Materials: Novel Water-Friendly Heterogeneous Catalysts. ChemCatChem 2019, 11, 2304–2312. [Google Scholar] [CrossRef]
- Vafaeezadeh, M.; Wilhelm, C.; Breuninger, P.; Ernst, S.; Antonyuk, S.; Thiel, W.R. A Janus-type Heterogeneous Surfactant for Adipic Acid Synthesis. ChemCatChem 2020, 12, 2695–2701. [Google Scholar] [CrossRef]
- Liang, F.; Shen, K.; Qu, X.; Zhang, C.; Wang, Q.; Li, J.; Liu, J.; Yang, Z. Inorganic Janus Nanosheets. Angew. Chem. Int. Ed. 2011, 50, 2379–2382. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, L.; Wang, N.; Sun, D.; Yang, Z. Janus Composite Particles and Interfacial Catalysis Thereby. Macromol. Rapid Commun. 2023, 44, 2300280. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Hou, Y.; Meng, Q.B.; Zhang, G.; Liang, F.; Song, X. Heteropoly acids-functionalized Janus particles as catalytic emulsifier for heterogeneous acylation in flow ionic liquid-in-oil Pickering emulsion. Colloids Surf. A Physicochem. Eng. Asp. 2019, 570, 191–198. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, J.; Yu, X.; Xu, X.; Gao, Y.; Li, H.; Liang, F. Preparation of Janus-type catalysts and their catalytic performance at emulsion interface. J. Colloid Interface Sci. 2017, 490, 357–364. [Google Scholar] [CrossRef]
- Vafaeezadeh, M.; Saynisch, R.; Lösch, A.; Kleist, W.; Thiel, W.R. Fast and Selective Aqueous-Phase Oxidation of Styrene to Acetophenone Using a Mesoporous Janus-Type Palladium Catalyst. Molecules 2021, 26, 6450. [Google Scholar] [CrossRef]
- Vafaeezadeh, M.; Schaumloffel, J.; Lösch, A.; De Cuyper, A.; Thiel, W.R. Dinuclear Copper Complex Immobilized on a Janus-Type Material as an Interfacial Heterogeneous Catalyst for Green Synthesis. ACS Appl. Mater. Interfaces 2021, 13, 33091–33101. [Google Scholar] [CrossRef]
- Vafaeezadeh, M.; Thiel, W.R. Atomically-Dispersed Metal Heterocatalysts: A Practical Step Toward Sustainability. ChemNanoMat 2023, 9, e202300399. [Google Scholar] [CrossRef]
- Mitchell, S.; Vorobyeva, E.; Pérez-Ramírez, J. The Multifaceted Reactivity of Single-Atom Heterogeneous Catalysts. Angew. Chem. Int. Ed. 2018, 57, 15316–15329. [Google Scholar] [CrossRef]
- Fu, X.; Zhao, X.; Lu, T.-B.; Yuan, M.; Wang, M. Graphdiyne-Based Single-Atom Catalysts with Different Coordination Environments. Angew. Chem. Int. Ed. 2023, 62, e202219242. [Google Scholar] [CrossRef]
- Saptal, V.B.; Ruta, V.; Bajada, M.A.; Vilé, G. Single-Atom Catalysis in Organic Synthesis. Angew. Chem. Int. Ed. 2023, 62, e202219306. [Google Scholar] [CrossRef]
- Dedovets, D.; Li, Q.; Leclercq, L.; Nardello-Rataj, V.; Leng, J.; Zhao, S.; Pera-Titus, M. Multiphase Microreactors Based on Liquid–Liquid and Gas–Liquid Dispersions Stabilized by Colloidal Catalytic Particles. Angew. Chem. Int. Ed. 2022, 61, e202107537. [Google Scholar] [CrossRef]
- Ni, L.; Yu, C.; Wei, Q.; Liu, D.; Qiu, J. Pickering Emulsion Catalysis: Interfacial Chemistry, Catalyst Design, Challenges, and Perspectives. Angew. Chem. Int. Ed. 2022, 61, e202115885. [Google Scholar] [CrossRef]
- Prieto, G.; Tüysüz, H.; Duyckaerts, N.; Knossalla, J.; Wang, G.-H.; Schüth, F. Hollow Nano- and Microstructures as Catalysts. Chem. Rev. 2016, 116, 14056–14119. [Google Scholar] [CrossRef] [PubMed]
- Vafaeezadeh, M.; Weber, K.; Demchenko, A.; Lösch, P.; Breuninger, P.; Lösch, A.; Kopnarski, M.; Antonyuk, S.; Kleist, W.; Thiel, W.R. Janus Bifunctional Periodic Mesoporous Organosilica. Chem. Commun. 2022, 58, 112–115. [Google Scholar] [CrossRef]
- Wichaita, W.; Polpanich, D.; Tangboriboonrat, P. Review on Synthesis of Colloidal Hollow Particles and Their Applications. Ind. Eng. Chem. Res. 2019, 58, 20880–20901. [Google Scholar] [CrossRef]
- Vafaeezadeh, M.; Thiel, W.R. Periodic Mesoporous Organosilica Nanomaterials with Unconventional Structures and Properties. Chem. Eur. J. 2023, 29, e202204005. [Google Scholar] [CrossRef]
- Zhang, R.; Ahmed, A.; Yu, B.; Cong, H.; Shen, Y. Preparation, application and development of poly(ionic liquid) microspheres. J. Mol. Liq. 2022, 362, 119706. [Google Scholar] [CrossRef]
- Nakamura, R.; Tokuda, M.; Suzuki, T.; Minami, H. Preparation of Poly(ionic liquid) Hollow Particles with Switchable Permeability. Langmuir 2016, 32, 2331–2337. [Google Scholar] [CrossRef]
- An, S.; Wang, Z.; Zhang, H.; Miras, H.N.; Song, Y. Self-Organization of Ionic Liquid-Modified Organosilica Hollow Nanospheres and Heteropolyacids: Efficient Preparation of 5-HMF Under Mild Conditions. ChemCatChem 2019, 11, 2526–2536. [Google Scholar] [CrossRef]
- Salih, K.S.M.; Mamone, P.; Dörr, G.; Bauer, T.O.; Brodyanski, A.; Wagner, C.; Kopnarski, M.; Taylor, R.N.K.; Demeshko, S.; Meyer, F.; et al. Facile Synthesis of Monodisperse Maghemite and Ferrite Nanocrystals from Metal Powder and Octanoic Acid. J. Chem. Mater. 2013, 25, 1430–1435. [Google Scholar] [CrossRef]
- Marquez-Medina, M.D.; Prinsen, P.; Li, H.; Shih, K.; Romero, A.A.; Luque, R. Continuous-Flow Synthesis of Supported Magnetic Iron Oxide Nanoparticles for Efficient Isoeugenol Conversion into Vanillin. ChemSusChem 2018, 11, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Yang, X.; Guan, J. Applications of Magnetic Nanomaterials in Heterogeneous Catalysis. ACS Appl. Nano Mater. 2019, 2, 4681–4697. [Google Scholar] [CrossRef]
- Dai, J.; Zou, H.; Wang, R.; Wang, Y.; Shia, Z.; Qiu, S. Yolk–shell Fe3O4@SiO2@PMO: Amphiphilic magnetic nanocomposites as an adsorbent and a catalyst with high efficiency and recyclability. Green Chem. 2017, 19, 1336–1344. [Google Scholar] [CrossRef]
- Al-Abdallat, Y.; Jum’h, I.; Al Bsoul, A.; Jumah, R.; Telfah, A. Photocatalytic Degradation Dynamics of Methyl Orange Using Coprecipitation Synthesized Fe3O4 Nanoparticles. Water Air Soil Pollut. 2019, 230, 277. [Google Scholar] [CrossRef]
- Sonogashira, K. Development of Pd–Cu catalyzed cross-coupling of terminal acetylenes with sp2-carbon halides. J. Organomet. Chem. 2002, 653, 46–49. [Google Scholar] [CrossRef]
- Sonogashira, K.; Tohda, Y.; Hagihara, N. A convenient synthesis of acetylenes: Catalytic substitutions of acetylenic hydrogen with bromoalkenes, iodoarenes and bromopyridines. Tetrahedron Lett. 1975, 16, 4467–4470. [Google Scholar] [CrossRef]
- Chinchilla, R.; Nájera, C. The Sonogashira Reaction: A Booming Methodology in Synthetic Organic Chemistry. Chem. Rev. 2007, 107, 874–922. [Google Scholar] [CrossRef]
- Kanwal, I.; Mujahid, A.; Rasool, N.; Rizwan, K.; Malik, A.; Ahmad, G.; Shah, S.A.A.; Rashid, U. Palladium and Copper Catalyzed Sonogashira cross Coupling an Excellent Methodology for C-C Bond Formation over 17 Years: A Review. Catalysts 2020, 10, 443. [Google Scholar] [CrossRef]
- Doucet, H.; Hierso, J.-C. Palladium-Based Catalytic Systems for the Synthesis of Conjugated Enynes by Sonogashira Reactions and Related Alkynylations. Angew. Chem. Int. Ed. 2007, 46, 834–871. [Google Scholar] [CrossRef]
- Tukhani, M.; Panahi, F.; Khalafi-Nezhad, A. Supported Palladium on Magnetic Nanoparticles–Starch Substrate (Pd-MNPSS): Highly Efficient Magnetic Reusable Catalyst for C–C Coupling Reactions in Water. ACS Sustain. Chem. Eng. 2018, 6, 1456–1467. [Google Scholar] [CrossRef]
- Sobhani, S.; Zeraatkar, Z.; Zarifi, F. Pd complex of an NNN pincer ligand supported on γ-Fe2O3@SiO2 magnetic nanoparticles: A new catalyst for Heck, Suzuki and Sonogashira coupling reactions. New J. Chem. 2015, 39, 7076–7085. [Google Scholar] [CrossRef]
- Nath, I.; Chakraborty, J.; Khan, A.; Arshad, M.N.; Azum, N.; Rab, M.A.; Asiri, A.M.; Alamry, K.A.; Verpoort, F. Conjugated mesoporous polyazobenzene–Pd(II) composite: A potential catalyst for visible-light-induced Sonogashira coupling. J. Catal. 2019, 377, 183–189. [Google Scholar] [CrossRef]
- Zhao, L.; Yang, H.; Li, R.; Tao, Y.; Guo, X.; Anderson, E.A.; Whiting, A.; Wu, N. Synthesis of Sulfonamide-Based Ynamides and Ynamines in Water. J. Org. Chem. 2021, 86, 1938–1947. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Chen, Y.; Jv, J.; Li, Y.; Li, W.; Dong, Y. Porous organic polymer with in situ generated palladium nanoparticles as a phase-transfer catalyst for Sonogashira cross-coupling reaction in water. RSC Adv. 2019, 9, 21671–21678. [Google Scholar] [CrossRef]
- Islam, M.; Mondal, P.; Roy, A.S.; Tuhina, K. Suzuki and Sonogashira Cross-Coupling Reactions in Water Medium with a Reusable Poly(N-vinylcarbazole)-Anchored Palladium(II) Complex. Synthesis 2010, 2010, 2399–2406. [Google Scholar] [CrossRef]
- Handa, S.; Smith, J.D.; Zhang, Y.; Takale, B.S.; Gallou, F.; Lipshutz, B.H. Sustainable HandaPhos-ppm Palladium Technology for Copper-Free Sonogashira Couplings in Water under Mild Conditions. Org. Lett. 2018, 20, 542–545. [Google Scholar] [CrossRef]
- Lipshutz, B.H.; Chung, D.W.; Rich, B. Sonogashira Couplings of Aryl Bromides: Room Temperature, Water Only, No Copper. Org. Lett. 2008, 10, 3793–3796. [Google Scholar] [CrossRef]
- Liang, B.; Dai, M.; Chen, J.; Yang, Z. Copper-Free Sonogashira Coupling Reaction with PdCl2 in Water under Aerobic Conditions. J. Org. Chem. 2005, 70, 391–393. [Google Scholar] [CrossRef] [PubMed]
- Struwe, J.; Ackermann, L.; Gallou, F. Recent progress in copper-free Sonogashira-Hagihara cross-couplings in water. Chem Catal. 2023, 3, 100485. [Google Scholar] [CrossRef]
- Mohajer, F.; Heravi, M.M.; Zadsirjan, V.; Poormohammad, N. Copper-free Sonogashira cross-coupling reactions: An overview. RSC Adv. 2021, 11, 6885–6925. [Google Scholar] [CrossRef]
- Chinchilla, R.; Nájera, C. Recent advances in Sonogashira reactions. Chem. Soc. Rev. 2011, 40, 5084–5121. [Google Scholar] [CrossRef]
- Glaser, C. Beiträge zur Kenntniss des Acetenylbenzols. Ber. Dtsch. Chem. Ges. 1869, 2, 422–424. [Google Scholar] [CrossRef]
- Glaser, C. Untersuchungen über einige Derivate der Zimmtsäure. Justus Liebigs Ann. Chem. 1870, 154, 137–171. [Google Scholar] [CrossRef]
- Siemsen, P.; Livingston, R.C.; Diederich, F. Acetylenic Coupling: A Powerful Tool in Molecular Construction. Angew. Chem. Int. Ed. 2000, 39, 2632–2657. [Google Scholar] [CrossRef]
- Shekarrao, K.; Kaishap, P.P.; Gogoi, S.; Boruaha, R.C. Palladium-Catalyzed One-Pot Sonogashira Coupling, exo-dig Cyclization and Hydride Transfer Reaction: Synthesis of Pyridine-Substituted Pyrroles. Adv. Synth. Catal. 2015, 357, 1187–1192. [Google Scholar] [CrossRef]
- Platonova, Y.B.; Volov, A.N.; Tomilova, L.G. Palladium(II) phthalocyanines efficiently promote phosphine-free Sonogashira cross-coupling reaction at room temperature. J. Catal. 2020, 391, 224–228. [Google Scholar] [CrossRef]
- Platonova, Y.B.; Volov, A.N.; Tomilova, L.G. Palladium(II) octaalkoxy- and octaphenoxyphthalocyanines: Synthesis and evaluation as catalysts in the Sonogashira reaction. J. Catal. 2019, 373, 222–227. [Google Scholar] [CrossRef]
- Martek, B.A.; Gazvoda, M.; Urankar, D.; Košmrlj, J. Designing Homogeneous Copper-Free Sonogashira Reaction through a Prism of Pd–Pd Transmetalation. Org. Lett. 2020, 22, 4938–4943. [Google Scholar] [CrossRef] [PubMed]
- Urgaonkar, S.; Verkade, J.G. Ligand-, Copper-, and Amine-Free Sonogashira Reaction of Aryl Iodides and Bromides with Terminal Alkynes. J. Org. Chem. 2004, 69, 5752–5755. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Sun, Y.; Wang, F.; Guo, M.; Xu, J.; Pan, Y.; Zhang, Z. A Copper- and Amine-Free Sonogashira Reaction Employing Aminophosphines as Ligands. J. Org. Chem. 2004, 69, 5428–5432. [Google Scholar] [CrossRef]
- Gholinejad, M.; Bahrami, M.; Nájera, C.; Pullithadathil, B. Magnesium oxide supported bimetallic Pd/Cu nanoparticles as an efficient catalyst for Sonogashira reaction. J. Catal. 2018, 363, 81–91. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, C.; Xu, G.; Yuan, J.; Ye, S.; Chen, L.; Lv, Q.; Luo, G.; Yang, J.; Li, M.; et al. An efficient nanocluster catalyst for Sonogashira reaction. J. Catal. 2021, 401, 206–213. [Google Scholar] [CrossRef]
- Heidenreich, R.G.; Köhler, K.; Krauter, J.G.E.; Pietsch, J. Pd/C as a Highly Active Catalyst for Heck, Suzuki and Sonogashira Reactions. Synlett 2002, 2002, 1118–1122. [Google Scholar] [CrossRef]
- Carril, M.; Correa, A.; Bolm, C. Iron-Catalyzed Sonogashira Reactions. Angew. Chem. Int. Ed. 2008, 47, 4862–4865. [Google Scholar] [CrossRef]
- Zou, L.; Johansson, A.J.; Zuidema, E.; Bolm, C. Mechanistic Insights into Copper-Catalyzed Sonogashira–Hagihara-Type Cross-Coupling Reactions: Sub-Mol% Catalyst Loadings and Ligand Effects. Chem. Eur. J. 2013, 19, 8144–8152. [Google Scholar] [CrossRef]
- Gao, R.; Xu, J.; Wang, J.; Lim, J.; Peng, C.; Pan, L.; Zhang, X.; Yang, H.; Zou, J. Pd/Fe2O3 with Electronic Coupling Single-Site Pd–Fe Pair Sites for Low-Temperature Semihydrogenation of Alkynes. J. Am. Chem. Soc. 2022, 144, 573–581. [Google Scholar] [CrossRef]
- Hensley, A.J.R.; Hong, Y.; Zhang, R.; Zhang, H.; Sun, J.; Wang, Y.; McEwen, J. Enhanced Fe2O3 Reducibility via Surface Modification with Pd: Characterizing the Synergy within Pd/Fe Catalysts for Hydrodeoxygenation Reactions. ACS Catal. 2014, 4, 3381–3392. [Google Scholar] [CrossRef]
- Hong, Y.; Zhang, H.; Sun, J.; Ayman, K.M.; Hensley, A.J.R.; Gu, M.; Engelhard, M.H.; McEwen, J.; Wang, Y. Synergistic Catalysis between Pd and Fe in Gas Phase Hydrodeoxygenation of m-Cresol. ACS Catal. 2014, 4, 3335–3345. [Google Scholar] [CrossRef]
- Sasaki, T.; Zhong, C.; Tada, M.; Iwasawa, Y. Immobilized metal ion-containing ionic liquids: Preparation, structure and catalytic performance in Kharasch addition reaction. Chem. Commun. 2005, 2005, 2506–2508. [Google Scholar] [CrossRef] [PubMed]
- Vafaeezadeh, M.; Hashemi, M.M. Simple and green oxidation of cyclohexene to adipic acid with an efficient and durable silica-functionalized ammonium tungstate catalyst. Catal. Commun. 2014, 43, 169–172. [Google Scholar] [CrossRef]
- Yurdakal, S.; Garlisi, C.; Özcan, L.; Bellardita, M.; Palmisano, G. (Photo)catalyst Characterization Techniques: Adsorption Isotherms and BET, SEM, FTIR, UV–Vis, Photoluminescence, and Electrochemical Characterizations. In Heterogeneous Photocatalysis; Marcì, G., Palmisano, L., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 87–152, Chapter 4. [Google Scholar]
- Greenwood, N.N.; Gibb, T.C. Mössbauer Spectroscopy; Chapman and Hall: London, UK, 1971. [Google Scholar]
- Kataria, M.; Pramanik, S.; Kaur, N.; Kumar, M.; Bhalla, V. Ferromagnetic α-Fe2O3 NPs: A potential catalyst in Sonogashira–Hagihara cross coupling and hetero-Diels–Alder reactions. Green Chem. 2016, 18, 1495–1505. [Google Scholar] [CrossRef]
- Jian, W.; Wang, S.; Zhang, H.; Bai, F. Disentangling the role of oxygen vacancies on the surface of Fe3O4 and γ-Fe2O3. Inorg. Chem. Front. 2019, 6, 2660–2666. [Google Scholar] [CrossRef]
- Liu, S.; Li, T.; Shi, F.; Ma, H.; Wang, B.; Dai, X.; Cui, X. Constructing multiple active sites in iron oxide catalysts for improving carbonylation reactions. Nat. Commun. 2023, 14, 4973. [Google Scholar] [CrossRef]
- Ivančič, A.; Košmrlj, J.; Gazvoda, M. Elucidating the reaction mechanism of a palladium-palladium dual catalytic process through kinetic studies of proposed elementary steps. Commun. Chem. 2023, 6, 51. [Google Scholar] [CrossRef]
- He, C.; Ke, J.; Xu, H.; Lei, A. Synergistic Catalysis in the Sonogashira Coupling Reaction: Quantitative Kinetic Investigation of Transmetalation. Angew. Chem. Int. Ed. 2013, 52, 1527–1530. [Google Scholar] [CrossRef]
- Chan, K.; Ta, L.T.; Huang, Y.; Su, H.; Lin, Z. Incorporating Domain Knowledge and Structure-Based Descriptors for Machine Learning: A Case Study of Pd-Catalyzed Sonogashira Reactions. Molecules 2023, 28, 4730. [Google Scholar] [CrossRef]
- Gazvoda, M.; Virant, M.; Pinter, B.; Košmrlj, J. Mechanism of copper-free Sonogashira reaction operates through palladium-palladium transmetallation. Nat. Commun. 2018, 9, 4814. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, W.; Li, D.; Yang, R.; Xia, Z. Base-assisted transmetalation enables gold-catalyzed oxidative Sonogashira coupling reaction. iScience 2024, 27, 108531. [Google Scholar] [CrossRef]
- Zhang, G.; Peng, Y.; Cui, L.; Zhang, L. Gold-Catalyzed Homogeneous Oxidative Cross-Coupling Reactions. Angew. Chem. Int. Ed. 2009, 48, 3112–3115. [Google Scholar] [CrossRef]
- Zhou, M.; Wei, W.; Xie, Y.; Lei, Y.; Li, J. Palladium-Catalyzed Cross-Coupling of Electron-Poor Terminal Alkynes with Arylboronic Acids under Ligand-Free and Aerobic Conditions. J. Org. Chem. 2010, 75, 5635–5642. [Google Scholar] [CrossRef]
- Qian, D.; Zhang, J. Au(I)/Au(III)-catalyzed Sonogashira-type reactions of functionalized terminal alkynes with arylboronic acids under mild conditions. Beilstein J. Org. Chem. 2011, 7, 808–812. [Google Scholar] [CrossRef] [PubMed]
- Basha, K.S.; Balamurugan, R. Gold(I)-Catalyzed Regioselective Hydroarylation of Propiolic Acid with Arylboronic Acids. Org. Lett. 2023, 25, 4803–4807. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhang, H.; Shi, W.; Lei, A. Bond Formations between Two Nucleophiles: Transition Metal Catalyzed Oxidative Cross-Coupling Reactions. Chem. Rev. 2011, 111, 1780–1824. [Google Scholar] [CrossRef] [PubMed]
- Gunnlaugsson, H.P. Spreadsheet based analysis of Mössbauer spectra. Hyperfine Interact. 2016, 237, 79. [Google Scholar] [CrossRef]
- Liu, M.; Ye, M.; Xue, Y.; Yin, G.; Wang, D.; Huang, J. Sonogashira coupling catalyzed by the Cu(Xantphos)I–Pd(OAc)2 system. Tetrahedron Lett. 2016, 57, 3137–3139. [Google Scholar] [CrossRef]
- Likhar, P.R.; Subhas, M.S.; Roy, M.; Roy, S.; Kantam, M.L. Copper-Free Sonogashira Coupling of Acid Chlorides with Terminal Alkynes in the Presence of a Reusable Palladium Catalyst: An Improved Synthesis of 3-Iodochromenones (=3-Iodo-4H-1-benzopyran-4-ones). Helv. Chim. Acta 2008, 91, 259–264. [Google Scholar] [CrossRef]
- Handa, S.; Jin, B.; Bora, P.P.; Wang, Y.; Zhang, X.; Gallou, F.; Reilly, J.; Lipshutz, B.H. Sonogashira Couplings Catalyzed by Fe Nanoparticles Containing ppm Levels of Reusable Pd, under Mild Aqueous Micellar Conditions. ACS Catal. 2019, 9, 2423–2431. [Google Scholar] [CrossRef]
- Islam, K.; Bhunia, B.K.; Mandal, G.; Nag, B.; Jaiswal, C.; Mandal, B.B.; Kumar, A. Room-Temperature, Copper-Free, and Amine-Free Sonogashira Reaction in a Green Solvent: Synthesis of Tetraalkynylated Anthracenes and In Vitro Assessment of Their Cytotoxic Potentials. ACS Omega 2023, 8, 16907–16926. [Google Scholar] [CrossRef] [PubMed]


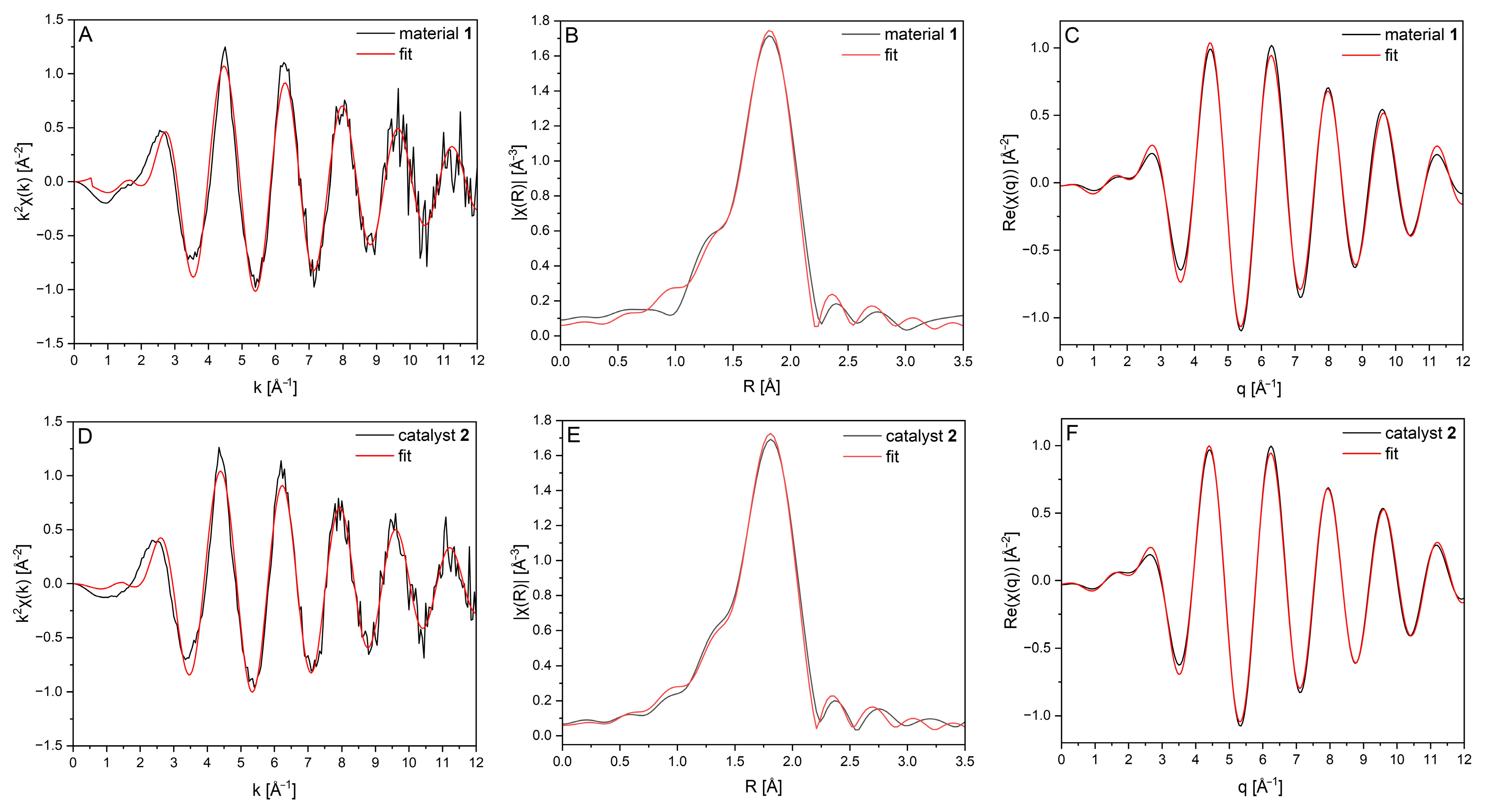
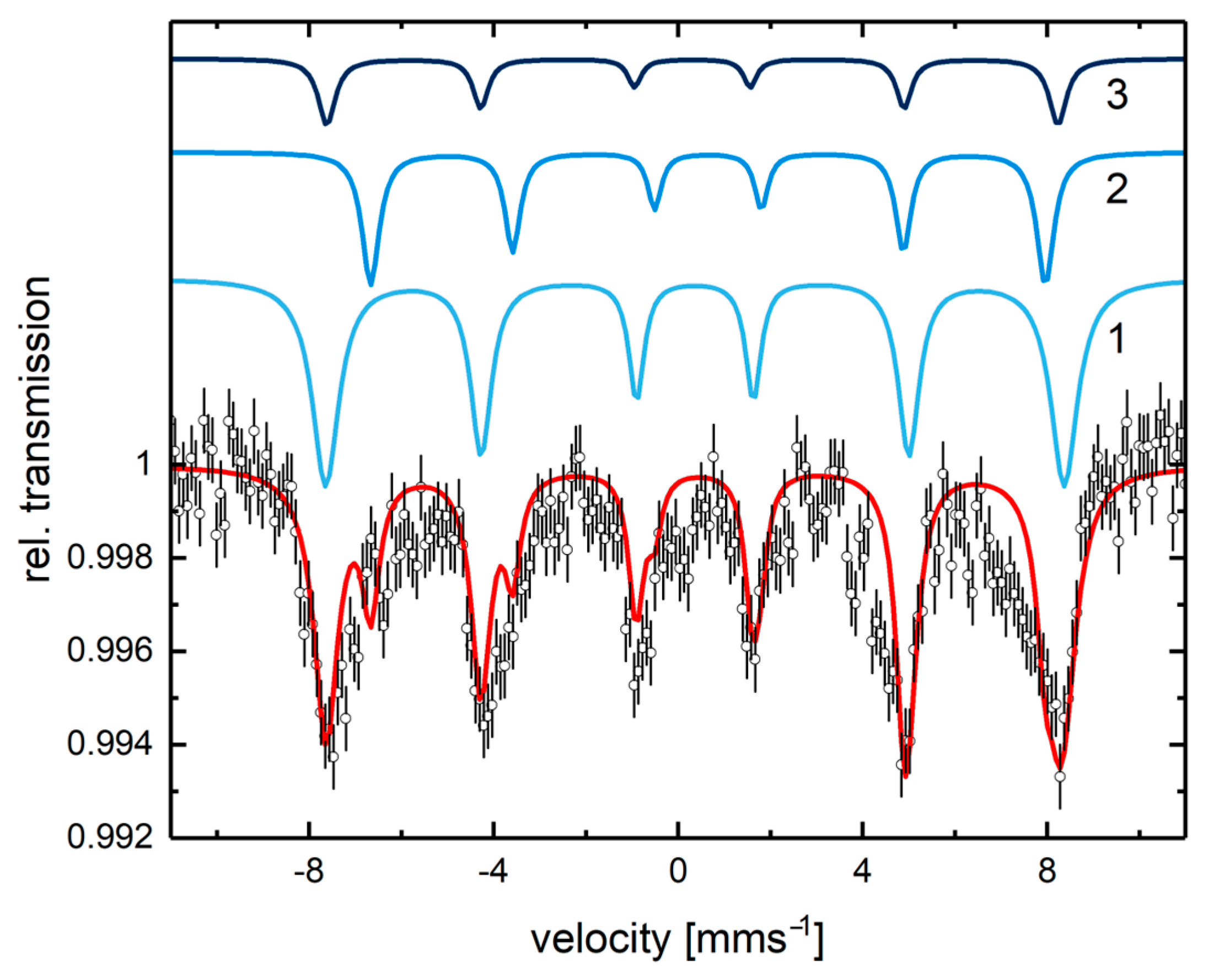
| Sample | Scattering Path | Number of Backscatterers (NBS) | Rexpected [Å] | Rexperimental [Å] | σ2 |
|---|---|---|---|---|---|
| Material 1 [a] | Pd–Cl | 3.79 ± 0.26 | 2.32 | 2.31 ± 0.001 | 0.0040 ± 0.0004 |
| Catalyst 2 [b] | Pd–Cl | 3.84 ± 0.21 | 2.32 | 2.31 ± 0.005 | 0.0038 ± 0.0003 |
| Entry | Catalyst | Yield (%) [a, b] |
|---|---|---|
| 1 | 2 | 85 |
| 2 | 1 [c] | 68 |
| 3 | Silica particles/γ-Fe2O3 | Trace |
| 4 | SiO2-IL/Pd-γ-Fe2O3 [d] | 54 |
| 5 | Crushed 2 | 87 |
| 6 | Janus nanosheets/Pd-γ-Fe2O3 [e] | 88 |
| Entry | Catalyst | Substrates | Additives | Solvent/Base | Temp. (°C) | Time (h) | Yield (%) | Ref. |
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | phenylboronic acid/ phenylacetylene | Ag2O | CH3CN/Et3N | 40 | 17 | 97 | this work |
| 2 | Ph3PAuCl | phenylboronic acid/ 4-fluorophenylacetylene | Selectfluor® | CH3OH/K2CO3 | r.t. | 18 | 87 | [104] |
| 3 | Ph3PAuCl | phenylboronic acid/ propargyl tosylamide | Selectfluor®/ AgBF4 | CH3CN/Et3N | r.t. | 12 | 75 | [107] |
| 4 | Pd(OAc)2 | phenylboronic acid/ ethyl propiolate | Ag2O | CH3CN/K2CO3 | 70 | 12 | 71 | [106] |
| 5 | JohnPhos(AuCl)[a] | phenylboronic acid/ propiolic acid | AgSbF6 | dichloroethane/– | r.t. | 14 | 61 | [108] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vafaeezadeh, M.; Rajabi, F.; Qiu, X.; Tummeley, M.A.M.; Hausbrandt, P.; Schaefer, S.; Ouissa, A.; Demchenko, A.; L’huillier, J.; Schünemann, V.; et al. Synergy in Sonogashira Cross-Coupling Reactions with a Magnetic Janus-Type Catalyst. Catalysts 2025, 15, 1123. https://doi.org/10.3390/catal15121123
Vafaeezadeh M, Rajabi F, Qiu X, Tummeley MAM, Hausbrandt P, Schaefer S, Ouissa A, Demchenko A, L’huillier J, Schünemann V, et al. Synergy in Sonogashira Cross-Coupling Reactions with a Magnetic Janus-Type Catalyst. Catalysts. 2025; 15(12):1123. https://doi.org/10.3390/catal15121123
Chicago/Turabian StyleVafaeezadeh, Majid, Fatemeh Rajabi, Xuanya Qiu, Marco A. M. Tummeley, Paul Hausbrandt, Sven Schaefer, Alina Ouissa, Anna Demchenko, Johannes L’huillier, Volker Schünemann, and et al. 2025. "Synergy in Sonogashira Cross-Coupling Reactions with a Magnetic Janus-Type Catalyst" Catalysts 15, no. 12: 1123. https://doi.org/10.3390/catal15121123
APA StyleVafaeezadeh, M., Rajabi, F., Qiu, X., Tummeley, M. A. M., Hausbrandt, P., Schaefer, S., Ouissa, A., Demchenko, A., L’huillier, J., Schünemann, V., Kleist, W., & Thiel, W. R. (2025). Synergy in Sonogashira Cross-Coupling Reactions with a Magnetic Janus-Type Catalyst. Catalysts, 15(12), 1123. https://doi.org/10.3390/catal15121123







