HIPK2 as a Novel Regulator of Fibrosis
Abstract
Simple Summary
Abstract
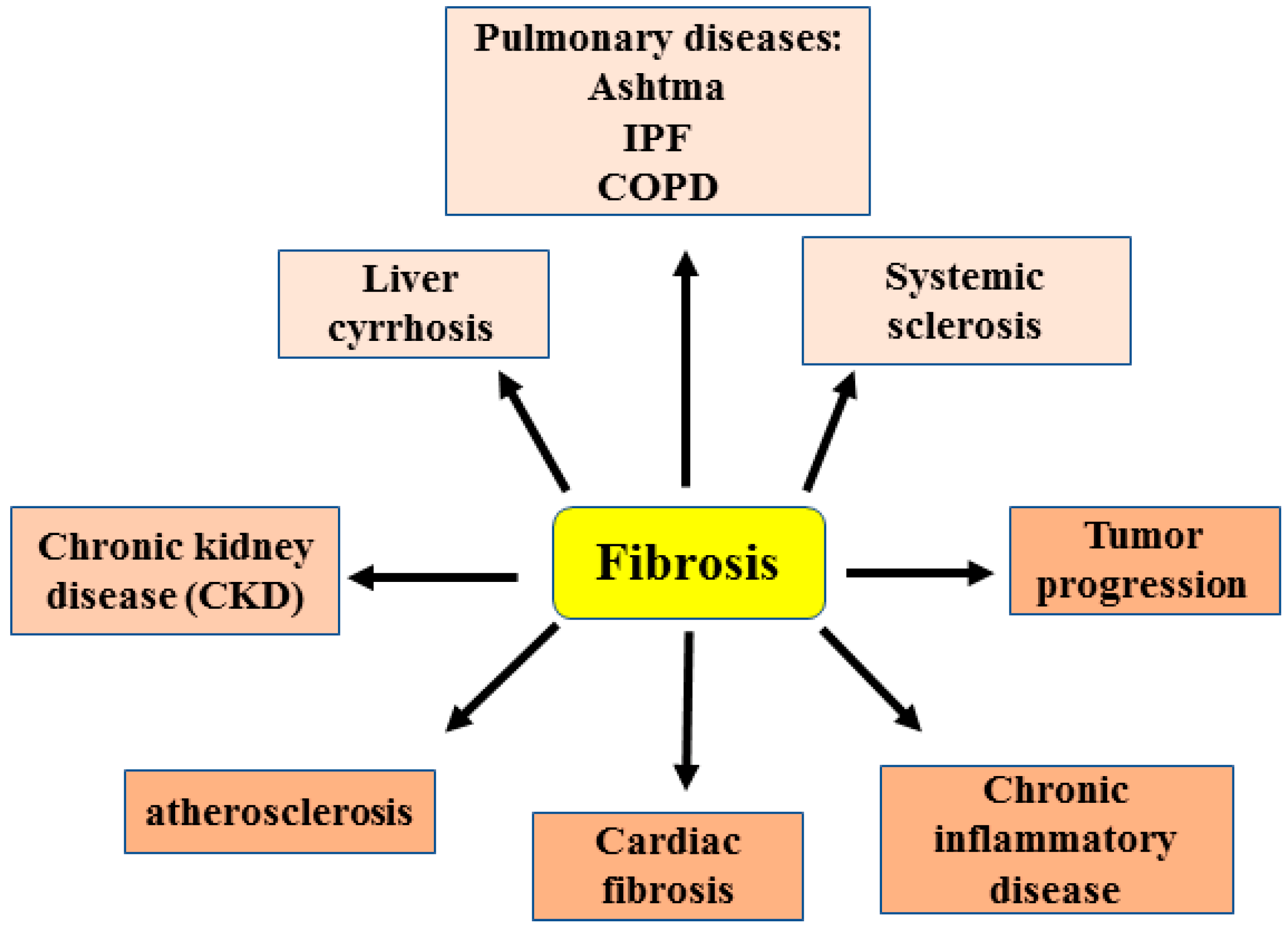
1. Introduction
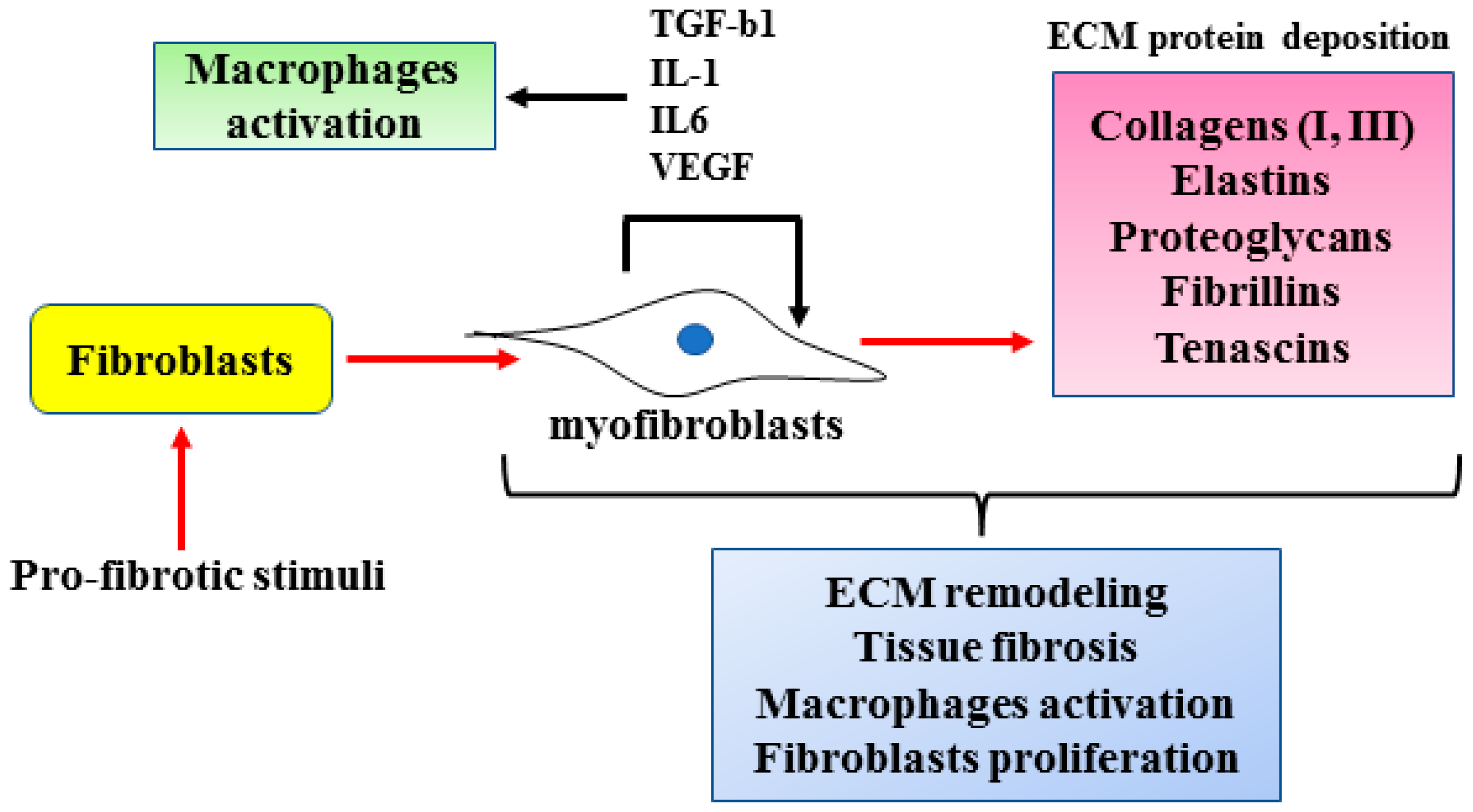
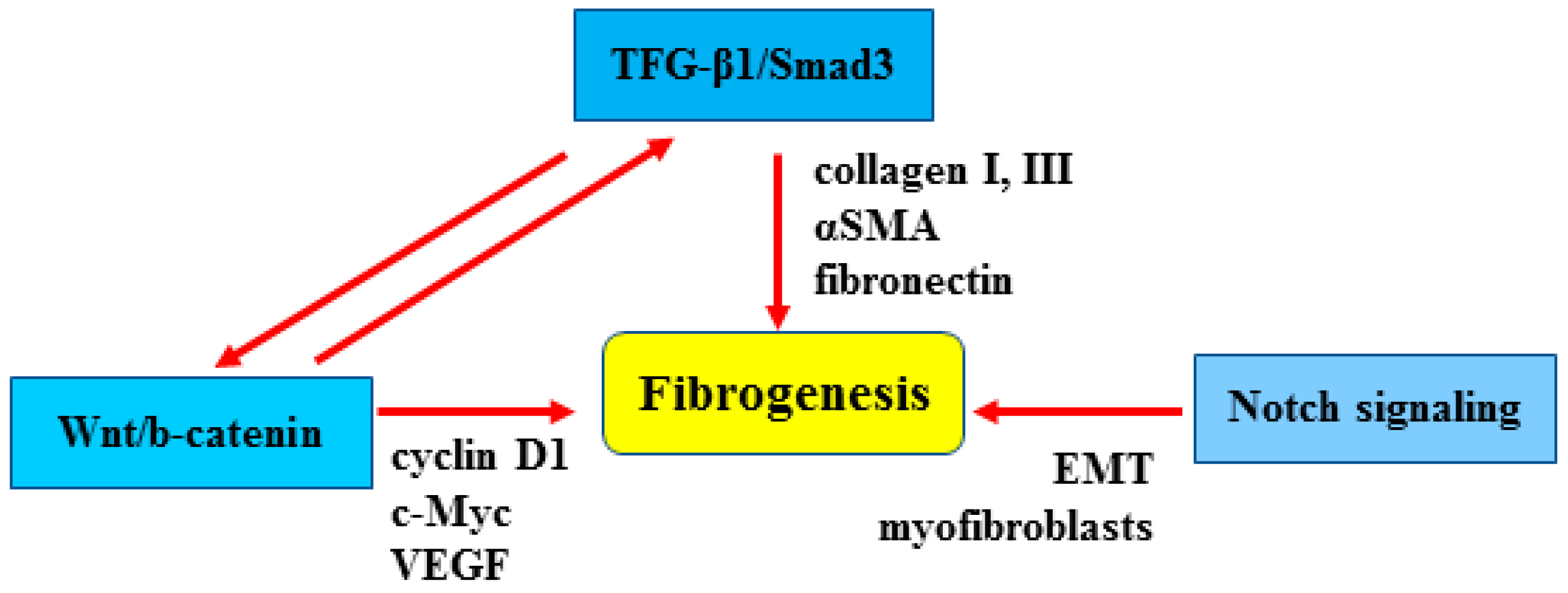
2. Cellular and Molecular Mechanisms of Fibrosis
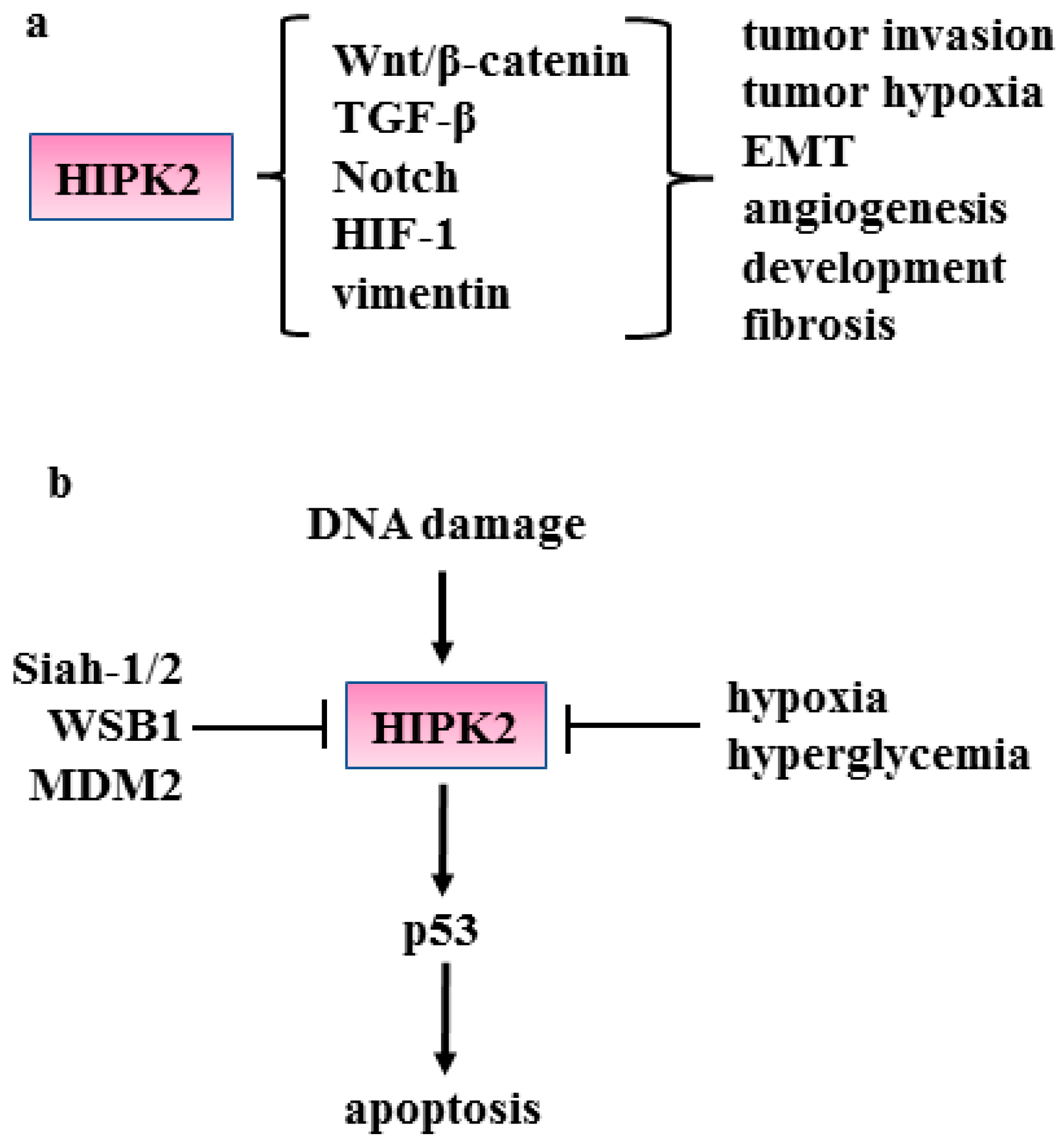
3. HIPK2 Function
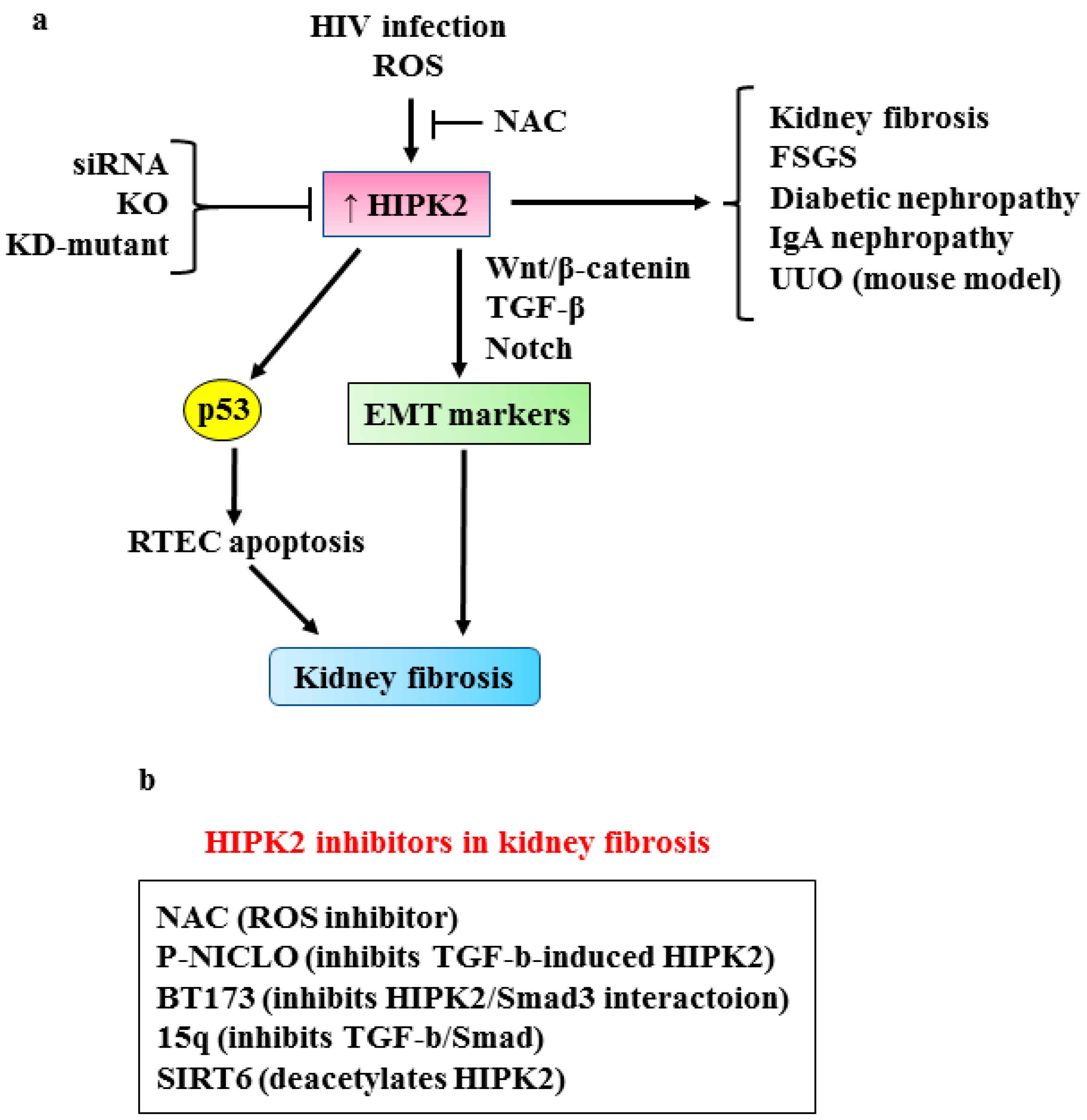
3.1. HIPK2 in Renal Fibrosis
3.2. HIPK2 in Pulmonary Fibrosis
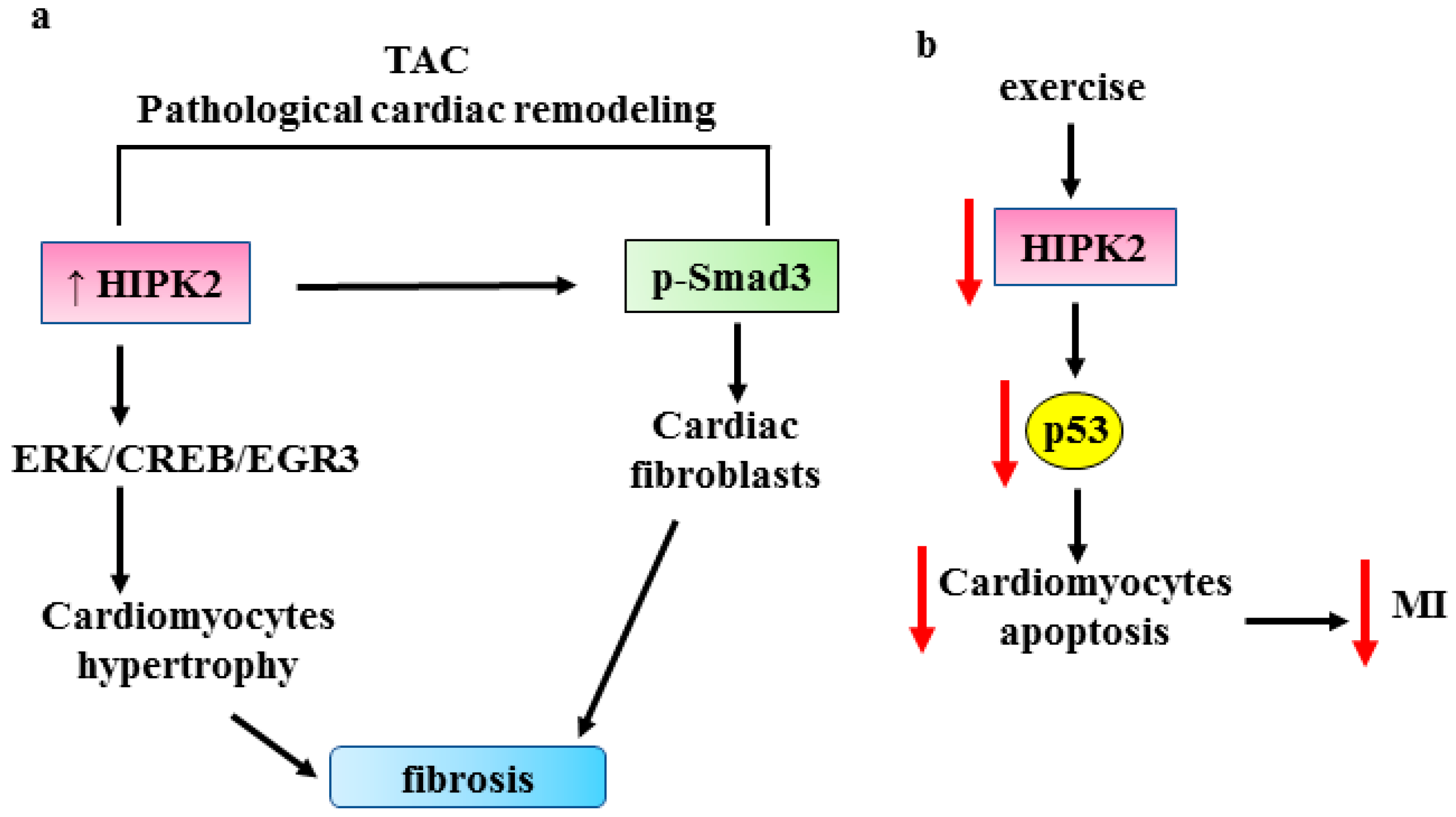
3.3. HIPK2 in Cardiac Fibrosis
3.4. HIPK2 in Liver Fibrosis
3.5. HIPK2 in Other Fibrotic Disorders
3.6. HIPK2 and Cancer-Associated Fibroblasts
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Thannickal, V.J.; Zhou, Y.; Gaggar, A.; Duncan, S.R. Fibrosis: Ultimate and proximate causes. J. Clin. Investig. 2014, 124, 4673–4677. [Google Scholar] [CrossRef] [PubMed]
- Varga, J.; Abraham, D. Systemic sclerosis: A prototypic multisystem fibrotic disorder. J. Clin. Investig. 2007, 117, 557–567. [Google Scholar] [CrossRef] [PubMed]
- Rosenbloom, J.; Macarak, E.; Piera-Velazquez, S.; Jimenez, S.A. Human Fibrotic Diseases: Current Challenges in Fibrosis Research. Methods Mol. Biol. 2017, 2017, 1627. [Google Scholar] [CrossRef]
- Piersma, B.; Hayward, M.K.; Weaver, V.M. Fibrosis and cancer: A strained relationship. BBA Rev. Cancer 2020, 1873, 188356. [Google Scholar] [CrossRef]
- Kendall, R.T.; Feghali-Bostwick, C.A. Fibroblasts in fibrosis: Novel roles and mediators. Front. Pharmacol. 2014, 5, 123. [Google Scholar] [CrossRef]
- Gyorfi, A.H.; Matei, A.E.; Distler, J.H.W. Targeting TGF-β signaling for the treatment of fibrosis. Matrix Biol. 2018, 68–69, 8–27. [Google Scholar] [CrossRef]
- Lam, A.P.; Gottardi, C.J. β-catenin signaling: A novel mediator of fibrosis and potential therapeutic target. Curr. Opin. Rheumatol. 2011, 23, 562–567. [Google Scholar] [CrossRef]
- Hu, B.; Phan, S.H. Notch in fibrosis and as a target of anti-fibrotic therapy. Pharmacol. Res. 2016, 108, 57–64. [Google Scholar] [CrossRef]
- Duffield, J.S.; Lupher, M.; Thannickal, V.J.; Wynn, T.A. Host responses in tissue repair and fibrosis. Annu. Rev. Pathol. 2013, 8, 241–276. [Google Scholar] [CrossRef]
- Dees, C.; Chakraborty, D.; Distler, J.H.-W. Cellular and molecular mechanisms in fibrosis. Exp. Dermatol. 2021, 30, 121–131. [Google Scholar] [CrossRef]
- Conte, A.; Valente, V.; Paladino, S.; Pierantoni, G.M. HIPK2 in cancer biology and therapy: Recent findings and future perspectives. Cell. Signal. 2023, 101, 110491. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Wang, N.; Chuang, P.; He, J.C. Role of HIPK2 in kidney fibrosis. Kidney Int. Suppl. 2014, 4, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Nugent, M.M.; Lee, K.; He, J.C. HIPK2 is a new drug target for anti-fibrosis therapy in kidney disease. Front. Physiol. 2015, 6, 132. [Google Scholar] [CrossRef] [PubMed]
- McAnulty, R.J. Fibroblasts and myofibroblasts: Their source, function and role in disease. Int. J. Biochem. Cell Biol. 2007, 39, 666–671. [Google Scholar] [CrossRef]
- Klingberg, F.; Hinz, B.; White, E.S. The myofibroblast matrix: Implications for tissue repair and fibrosis. J. Pathol. 2013, 229, 298–309. [Google Scholar] [CrossRef]
- Borthwick, L.A.; Wynn, T.A.; Fisher, A.J. Cytokine mediated tissue fibrosis. Biochim. Biophys. Acta 2013, 1832, 1049–1060. [Google Scholar] [CrossRef]
- Tao, L.; Huang, G.; Song, H.; Chen, Y.; Chen, L. Cancer associated fibroblasts: An essential role in the tumor microenvironment. Oncol. Lett. 2017, 14, 2611–2620. [Google Scholar] [CrossRef]
- Wynn, T.A.; Vannella, K.M. Macrophages in tissue repair, regeneration, and fibrosis. Immunity 2016, 44, 450–462. [Google Scholar] [CrossRef]
- Biernacka, A.; Dobaczewski, M.; Frangogiannis, N.G. TGF-β signaling in fibrosis. Growth Factors 2011, 29, 196–202. [Google Scholar] [CrossRef]
- Li, M.; Krishnaveni, M.S.; Li, C.; Zhou, B.; Xing, Y.; Banfalvi, A.; Li, A.; Lombardi, V.; Akbari, O.; Borok, Z.; et al. Epithelium-specific deletion of TGF-β receptor type II protects mice from bleomycin-induced pulmonary fibrosis. J. Clin. Investig. 2011, 121, 277–287. [Google Scholar] [CrossRef]
- Lafyatis, R. Transforming growth factor β at the center of systemic sclerosis. Nat. Rev. Rheumatol. 2014, 10, 706–719. [Google Scholar] [CrossRef]
- Lodyga, M.; Hinz, B. TGF-beta1—A truly transforming growth factor in fibrosis and immunity. Semin. Cell Dev. Biol. 2020, 101, 123–139. [Google Scholar] [CrossRef] [PubMed]
- de Streel, G.; Lucas, S. Targeting immunosuppression by TGF-beta1 for cancer immunotherapy. Biochem. Pharmacol. 2021, 192, 114697. [Google Scholar] [CrossRef] [PubMed]
- Clevers, H. Wnt/beta-catenin signaling in development and disease. Cell 2006, 127, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Lecarpentier, Y.; Schussler, O.; Henert, J.L.; Vallé, A. Multiple targets of the canonical WNT/b-catenin signaling in cancers. Front. Oncol. 2019, 9, 1248. [Google Scholar] [CrossRef] [PubMed]
- Piersma, B.; Bamk, R.A.; Boersema, M. Signaling in Fibrosis: TGF-b, WNT, and YAP/TAZ converge. Front. Med. 2015, 3, 59. [Google Scholar] [CrossRef] [PubMed]
- Kopan, R.; Ilagan, M.X.G. The canonical Notch signaling pathway: Unfolding the activation mechanism. Cell 2009, 137, 216–233. [Google Scholar] [CrossRef]
- Gonzalez, D.M.; Medici, D. Signaling mechanisms of the epithelial-mesenchymal transition. Sci. Signal. 2014, 7, re8. [Google Scholar] [CrossRef]
- Liu, F.; Zhuang, S. New therapies for the treatment of renal fibrosis. Adv. Exp. Med. Biol. 2019, 1165, 625–629. [Google Scholar] [CrossRef]
- Blaquiere, J.A.; Verheyen, E.M. Homeodomani-interacting protein kinases: Diverse and complex roles in development and diseases. Curr. Top. Dev. Biol. 2017, 123, 73–103. [Google Scholar] [CrossRef]
- D’Orazi, G.; Rinaldo, C.; Soddu, S. Updates on HIPK2: A resourceful oncosuppressor for clearing cancer. J. Exp. Clin. Cancer Res. 2012, 31, 63. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Zhou, L.; Sun, X.; Li, Q. Homeodomain-interacting protein kinase 2 (HIPK2): A promising target for anti-cancer therapies. Oncotarget 2017, 8, 20452–20461. [Google Scholar] [CrossRef] [PubMed]
- Calzado, M.A.; Renner, F.; Roscic, A.; Schitz, M.L. HIPK2: A versatile switchboard regulating the transcription machinery and cell death. Cell Cycle 2014, 6, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Puca, R.; Nardinocchi, L.; Givol, D.; D’Orazi, G. Regulation of p53 activity by HIPK2: Molecular mechanisms and therapeutical implications in human cancer cells. Oncogene 2010, 29, 4378–4387. [Google Scholar] [CrossRef]
- Puca, R.; Nardinocchi, L.; Gal, H.; Rechavi, G.; Amariglio, N.; Domany, E.; Notterman, D.A.; Scarsella, M.; Leonetti, C.; Sacchi, A.; et al. Reversible dysfunction of wild-type p53 following homeodomain interacting protein kinase-2 knockdown. Cancer Res. 2008, 15, 3707–3714. [Google Scholar] [CrossRef]
- Puca, R.; Nardinocchi, L.; Bossi, G.; Sacchi, A.; Rechavi, G.; Givol, D.; D’Orazi, G. Restoring wtp53 activity in HIPK2 depleted MCF7 cells by modulating metallothionein and zinc. Exp. Cell Res. 2009, 315, 67–75. [Google Scholar] [CrossRef]
- Wei, G.; Ku, S.; Ma, G.K.; Saito, S.; Tang, A.A.; Zhang, J.; Mail, J.H.; Appella, E.; Balmain, A.; HIang, E.J. HIPK2 represses beta-catenin-mediated transcription, epidermal stem cell expansion, and skin tumorigenesis. Proc. Natl. Acad. Sci. USA 2007, 104, 13040–13045. [Google Scholar] [CrossRef]
- Kim, E.A.; Kim, J.E.; Sung, K.S.; Choi, D.W.; Lee, B.J.; Choi, C.Y. Homeodomain-interacting protein kinase 2 (HIPK2) targets beta-catenin for phosphorylation and proteasomal degradation. Biochem. Biophys. Res. Commun. 2010, 394, 966–971. [Google Scholar] [CrossRef]
- Puca, R.; Nardinocchi, L.; D’Orazi, G. Regulation of vascular endothelial growth factor expression by homeodomain-interacting protein kinase-2. J. Exp. Clin. Cancer Res. 2008, 27, 22. [Google Scholar] [CrossRef]
- Lee, W.; Swarup, S.; Chen, J.; Ishitani, T.; Verheyen, E.M. Homeodomain-interacting protein kinases (Hipks) promote Wnt/Wg signaling through stabilization of b-catenin/Arm and stimulation of target gene expression. Development 2009, 136, 241–251. [Google Scholar] [CrossRef]
- Lee, W.; Andrews, B.C.; Faust, M.; Walldorf, U.; Verheyen, E.M. Hipk is an essential protein that promotes Notch signal transduction in the Drosophila eye by inhibition of the global co-repressor Groucho. Dev. Biol. 2009, 325, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Ann, E.J.; Kim, M.Y.; Yoon, J.H.; Ahn, J.S.; Jo, E.H.; Lee, H.J.; Lee, H.W.; Kang, H.G.; Choi, D.W.; Chun, K.H. Tumor suppressor HIPK2 regulates malignant growth via phosphorylation of Notch1. Cancer Res. 2016, 76, 4728–4740. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, T.G.; Stolberg, N.; Schmitz, M.L.; Will, H. HIPK2 regulates transforming growth factor-beta-induced c-Jun NH(2)-terminal kinase activation and apoptotis in human hepatoma cells. Cancer Res. 2003, 63, 8271–8277. [Google Scholar]
- Zhang, J.; Pho, V.; Bonasera, S.J.; Holtzman, J.; Tang, A.T.; Hellmuth, J.; Tang, S.; Janak, P.H.; Tecott, L.H.; Huang, E.J. Essential function of HIPK2 in TGFbeta-dependent survival of midbrain dopamine neurons. Nat. Neurosci. 2007, 10, 77–86. [Google Scholar] [CrossRef]
- Shang, Y.; Doan, C.N.; Arnold, T.D.; Lee, S.; Tang, A.A.; Reichardt, L.F.; Huang, E.J. Transcriptional corepressors HIPK2 and HIPK2 control angiogenesis via TGF-β-TAK1-dependent mechanism. PLoS Biol. 2013, 11, e1001527. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wen, P.; Li, F.; Yao, C.; Wang, T.; Liang, B.; Yang, Q.; Ma, L.; He, L. HIPK2 inhibits cell metastasis and improves chemosensitivity in esophageal squamous cell carcinoma. Exp. Ther. Med. 2018, 15, 1113–1118. [Google Scholar] [CrossRef] [PubMed]
- Nodale, C.; Sheffer, M.; Jacob-Hirsch, J.; Folgiero, V.; Falcioni, R.; Aiello, A.; Garufi, A.; Rechavi, G.; Givol, D.; D’Orazi, G. NHIPK2 downregulates vimentin and inhibits breast cancer cell invasion. Cancer Biol. Ther. 2012, 13, 198–205. [Google Scholar] [CrossRef]
- Nardinocchi, L.; Puca, R.; D’Orazi, G. HIF-1α antagonizes p53-mediated apoptosis by triggering HIPK2 degradation. Aging 2011, 3, 33–43. [Google Scholar] [CrossRef]
- Bon, G.; Di Carlo, S.E.; Folgiero, V.; Avetrani, P.; Lazzari, C.; D’Orazi, G.; Brizzi, M.F.; Sacchi, A.; Soddu, S.; Blandino, G.; et al. Negative regulation of beta(β) integrin transcription by homeodomain-interacting protein kinase e and p53 impairs tumor progression. Cancer Res. 2009, 69, 5978–5986. [Google Scholar] [CrossRef]
- Garufi, A.; Pistritto, G.; Ceci, C.; Di Renzo, L.; Santarelli, R.; Faggioni, A.; Cirone, M.; D’Orazi, G. Targeting COX-2/PGE(2) pathway in HIPK2 knockdown cancer cells: Impact on dendritic cell maturation. PLoS ONE 2012, 7, e48342. [Google Scholar] [CrossRef]
- Cirone, M.; Garufi, A.; Di Renzo, L.; Granato, M.; Faggioni, A.; D’Orazi, G. Zinc supplementation is required for the cytotoxic and immunogenic effects of chemotherapy in chemoresistant p53-functionally deficient cells. Oncoimmunology 2013, 2, e26198. [Google Scholar] [CrossRef] [PubMed]
- Lanni, C.; Nardinocchi, L.; Puca, R.; Stanga, S.; Uberti, D.; Memo, M.; Govono, S.; D’Orazi, G.; Racchi, M. Homeodomain interacting protein kinase 2: A target for Alzheimer’s beta amyloid leading to misfolded p53 and inappropriate cell survival. PLoS ONE 2010, 5, e10171. [Google Scholar] [CrossRef] [PubMed]
- Baldari, S.; Garufi, A.; Granato, M.; Pistritto, G.; Cuomo, L.; Pistritto, G.; Cirone, M.; D’Orazi, G. Hyperglicemia triggers HIPK2 protein degradation. Oncotarget 2017, 8, 1190–1203. [Google Scholar] [CrossRef] [PubMed]
- Ryu, T.Y.; Park, J.; Scherer, P.E. Hyperglycemia as a risk factor for cancer progression. Diabetes Metab. J. 2014, 38, 330–336. [Google Scholar] [CrossRef]
- Petrova, V.; Annichiarico-Petruzzelli, M.; Melino, G.; Amelio, I. The hypoxic tumor microenvironment. Oncogene 2018, 7, 10. [Google Scholar] [CrossRef]
- Wang, D.; Ma, Y.; Tong, X.; Zhang, Y.; Fan, H. Diabetes mellitus contributes to idiophatic pulmonary fibrosis: A review from clinical appearance to possible pathogenesis. Front. Public Health 2020, 8, 196. [Google Scholar] [CrossRef]
- Kim, J.A.; Aquino-Galvez, A. Hypoxia in cancer and fibrosis: Part of the problem and part of the solution. Int. J. Mol. Sci. 2021, 22, 8335. [Google Scholar] [CrossRef]
- Liu, Y. Cellular and molecular mechanisms of renal fibrosis. Nat. Rev. Nephrol. 2011, 7, 684–696. [Google Scholar] [CrossRef]
- Lan, H.Y.; Nikolic-Peterson, D.J. Advances in mechanisms of renal fibrosis. Front. Physiol. 2018, 9, 284. [Google Scholar] [CrossRef]
- Schlondorff, D.O. Overview of factors contributing to the pathophysiology of progressive renal disease. Kidney Int. 2008, 74, 860–866. [Google Scholar] [CrossRef]
- He, W.; Dai, C.; Li, Y.; Zeng, G.; Monga, S.P.; Liu, Y. Wnt/beta-catenin signaling promotes renal interstitial fibrosis. J. Am. Soc. Nephrol. 2009, 20, 765–776. [Google Scholar] [CrossRef] [PubMed]
- Bielesz, B.; Sirin, Y.; Si, H.; Nianjan, T.; Gruenwald, A.; Kato, S.A.; Pullman, J.; Gessler, M.; Haase, V.H.; Susztak, K. Epithelial Notch signaling regulates interstitial fibrosis development in the kidneys of mice and humans. J. Clin. Investig. 2010, 120, 4040–4054. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Xiao, L.; Sun, L.; Liu, F. Wnt/beta-catenin signaling: A promising new target for fibrosis diseases. Physiol. Res. 2012, 61, 337–346. [Google Scholar] [CrossRef]
- Meng, X.M.; Tang, P.M.K.; Li, J.; Lan, H.J. TGF-β/Smad signaling in renal fibrosis. Front. Physiol. 2015, 6, 82. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, M.; Fogarty, E.; Janga, M.; Surendran, K. Notch signaling in kidney development, maintenance and disease. Biomolecules 2019, 9, 692. [Google Scholar] [CrossRef]
- Bozic, M.; Caus, M.; Rodrigues-Diez, R.R.; Pedraza, N.; Ruiz-Ortega, M.; Garí, E.; Gallel, P.; Panadés, M.J.; Martinez, A.; Fernández, E.; et al. Protective role of renal proximal tubular alpha-synuclein in the pathogenesis of kidney fibrosis. Nat. Commun. 2020, 11, 1943. [Google Scholar] [CrossRef]
- Jin, Y.; Ratnam, K.; Chuang, P.Y.; Fan, Y.; Zhong, Y.; Dai, Y.; Mazloom, A.R.; Chen, E.Y.; D’Agati, V.; Xiong, H.; et al. Systems approach identifies HIPK2 as a key regulator of kidney fibrosis. Nat. Med. 2012, 18, 580–588. [Google Scholar] [CrossRef]
- Rosenstiel, P.; Gharavi, A.; Dand’Agati, V.; Klotman, P. Transgenic and infectious animal models of HIV-associated nephropathy. J. Am. Soc. Nephrol. 2009, 20, 2296–2304. [Google Scholar] [CrossRef]
- Winter, M.; Soembroek, D.; Dauth, I.; Moehlenbrink, J.; Scheuermann, K.; Crone, J.; Hofman, T.G. Control of HIPK2 stability by ubiquitin ligase Siah-1 and checkpoint kinases ATM and ATR. Nat. Cell. Biol. 2008, 10, 812–824. [Google Scholar] [CrossRef]
- Xiao, W.E.J.; Bao, L.; Fan, Y.; Jin, Y.; Wang, A.; Bauman, D.; Li, Z.; Zheng, Y.-L.; Liu, R.; Lee, K.; et al. Tubular HIPK2 is a key contributor to renal fibrosis. J. Clin. Investig. 2020, 5, e136004. [Google Scholar] [CrossRef]
- Chen, W.; Mook, R.A., Jr.; Premont, R.T.; Wang, J. Niclosamide: Beyond an antihelminthic drug. Cell. Signal. 2018, 41, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Zhen, X.; Liu, J.; Ren, X.; Hu, Z.; Zhou, Z.; Zhu, F.; Ding, K.; Nie, J. The antihelmenthic phosphate niclosamide impedes renal fibrosis by inhibiting homeodomain-interacting protein kinase 2 expression. Kidney Int. 2017, 92, 612–624. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Das, B.; Xiao, W.; Li, Z.; Li, H.; Lee, K.; He, J.C. A novel inhibitor of homeodomain interacting protein kinase 2 mitigates kidney fibrosis through inhibition of the TGF-β1/Smad3 pathway. J. Am. Soc. Nephrol. 2017, 28, 2133–2143. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Wang, G.; Zhao, G.; Peng, Z.; Tao, L.; Chen, Z.; Hu, G.; Li, Q. Identification of selective homeodomain interacting protein kinase 2 inhibitors, a potential treatment for renal fibrosis. Bioorg. Chem. 2022, 126, 105866. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, W.; Zhang, Z.; Wang, W.; Huang, H. SIRT6 overexpression retards renal interstitial fibrosis through targeting HIPK2 in chronic kidney disease. Front. Pharmacol. 2022, 13, 1007168. [Google Scholar] [CrossRef]
- Chang, A.; Ferrer, C.; Mostoslavsky, R. SIRT6, a mammalian deacylase with multitasking abilities. Physiol. Rev. 2020, 100, 145–169. [Google Scholar] [CrossRef]
- Li, Z.; Xu, K.; Zhang, N.; Amador, G.; Wang, Y.; Zhao, S.; Li, L.; Qiu, Y.; Wang, Z. Overexpressed SIRT6 attenuates cisplatin-induced acute kidney injury by inhibiting ERK1/2 signaling. Kidney Int. 2018, 93, 881–892. [Google Scholar] [CrossRef]
- Cai, J.; Liu, Z.; Huang, X.; Shu, S.; Hu, X.; Zheng, M. The deacetylase sirtuin 6 protects against kidney fibrosis by epigenetically blocking β-catenin target gene expression. Kidney Int. 2022, 101, 422. [Google Scholar] [CrossRef]
- Maity, S.; Muhamed, J.; Sarikhani, M.; Kumar, S.; Ahamed, F.; Spurthi, K.; Ravi, V.; Jain, A.; Khan, D.; Arathi, B.P.; et al. Sirtuin 6 deficiency transcriptionally up-regulates TGF-β signaling and induces fibrosis in mice. J. Biol. Chem. 2020, 295, 415–434. [Google Scholar] [CrossRef]
- Jin, J.; Li, W.; Wang, T.; Park, B.H.; Park, S.K.; Kang, K.P. Loss of proximal tubular Sirtuin 6 aggravates unilateral ureteral obstruction-induced tubulointerstitial inflammation and fibrosis by regulation of β-catenin acetylation. Cells 2022, 11, 1477. [Google Scholar] [CrossRef]
- de la Vega, L.; Grishina, I.; Moreno, R.; Kruger, M.; Braun, T.; Schmitz, M.L. A redox-regulated SUMO/acetylation switch of HIPK2 controls the survival threshold to oxidative stress. Mol. Cell 2012, 46, 472–483. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.W.; Choi, Y.C. HIPK2 modification code for cell death and survival. Mol. Cell. Oncol. 2014, 1, e955999. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Li, X.; Zhang, F.; Wu, L.; Dong, Z.; Zhang, D. EGFR drives the progression of AKI to CDK through HIPK2 overexpression. Theranostics 2019, 9, 2712–2726. [Google Scholar] [CrossRef] [PubMed]
- Ray, A.S.; Haikal, A.; Hammoud, K.A.; Yu, A.S. Vancomycin and the Risk of AKI: A Systematic Review and Meta-Analysis. Clin. J. Am. Soc. Nephrol. 2016, 11, 2132–2140. [Google Scholar] [CrossRef]
- Chen, J.; Wang, J.; Li, H.; Wang, S.; Xiang, X.; Zhang, D. P53 activates miR-192-5p to mediate vancomycin-induced AKI. Sci. Rep. 2016, 6, 38868. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Ma, Z.; Zhu, J.; Liu, Z.; Liu, Y.; Liu, Y.; Cai, J.; Dong, Z. P53 in kidney injury and repair: Mechanisms and therapeutic potentials. Pharmacol. Ther. 2019, 195, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Zhang, K.; Jiang, S.; Fu, L.; Shi, Y.; Tan, R.; Cui, J.; Zhou, Y. P53: A key protein that regulates pulmonary fibrosis. Oxidative Med. Cell. Longev. 2020, 2020, 6635794. [Google Scholar] [CrossRef]
- Yu, S.; Ji, G.; Zhang, L. The role of p53 in liver fibrosis. Front. Pharmacol. 2022, 12, 1057829. [Google Scholar] [CrossRef]
- Huang, Y.; Tong, J.; He, F.; Yu, X.; Fan, L.; Hu, J.; Tan, J.; Chen, Z. miR-141 regulates TGF-β1-induced epithelial-mesenchymal transition through repression of HIPK2 expression in renal tubular epithelial cells. Int. J. Mol. Med. 2015, 35, 311–318. [Google Scholar] [CrossRef]
- Noble, P.W.; Barkauskas, C.E.; Jiang, D. Pulmonary fibrosis: Patterns and penetrators. J. Clin. Investig. 2012, 122, 2756–2762. [Google Scholar] [CrossRef]
- Spagnolo, P.; Kropski, J.A.; Jones, M.G.; Lee, J.S.; Rossi, G.; Karampitsakos, T.; Maher, T.M.; Tzouvelekis, A.; Ryerson, C.J. Idiopathic pulmonary fibrosis: Disease mechanisms and drug development. Pharmacol. Ther. 2021, 222, 107798. [Google Scholar] [CrossRef] [PubMed]
- Richeldi, L.; Wilson, K.C.; Raghu, G. Diagnosing idiopathic pulmonary fibrosis in 2018: Bridging recommendations made by experts serving different societies. Eur. Respir. J. 2018, 52, 1801485. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhang, Y.; Ren, J.; Yu, W. HIPK2 attenuates pulmonary fibrosis by suppression the Wnt/β-catenin signaling pathway. Folia Histochem. Cytobiol. 2022, 60, 247–259. [Google Scholar] [CrossRef] [PubMed]
- Lam, A.P.; Flozak, A.S.; Russell, S.; Wei, J.; Jain, M.; Mutlu, G.M.; Budinger, G.R.S.; Feghali-Bostwick, C.A.; Varga, J.; Gottardi, C. Nuclear β-catenin is increased in systemic sclerosis pulmonary fibrosis and promotes lung fibroblast migration and proliferation. Am. J. Respir. Cell Mol. Biol. 2011, 45, 915–922. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Kim, S.H.; Seo, J.Y.; Chung, H.; Kwak, H.J.; Lee, S.K.; Yoon, H.J.; Shin, D.H.; Park, S.S.; Sohn, J.W. Blockade of the Wnt/beta-catenin pathway attenuates bleomycin-induced pulmonary fibrosis. Tohoku J. Exp. Med. 2011, 223, 45–54. [Google Scholar] [CrossRef]
- Cao, H.; Wang, C.; Chen, X.; Hou, J.; Xiang, Z.; Shen, Y.; Han, X. Inhibition of Wnt/β-catenin signaling suppresses myofibroblast differentiation of lung resident mesenchymal stem cells and pulmonary fibrosis. Sci. Rep. 2018, 81, 13644. [Google Scholar] [CrossRef]
- Ricci, A.; Cherubini, E.; Ulivieri, A.; Lavra, L.; Sciacchitano, S.; Scozzi, D.; Mancini, R.; Ciliberto, G.; Bartolazzi, A.; Bruno, P.; et al. Homeodomain-interacting protein kinase2 in human idiopathic pulmonary fibrosis. J. Cell. Physiol. 2013, 228, 235–241. [Google Scholar] [CrossRef]
- Cao, Z.; Xiao, Q.; Dai, X.; Zhou, Z.; Jiang, R.; Cheng, Y.; Yang, X.; Guo, H.; Wang, J.; Xi, Z.; et al. circHIPK2-mediated σ-IR promotes endoplasmic reticulum stress in human pulmonary fibroblasts exposed to silica. Cell Death Dis. 2017, 8, 3212. [Google Scholar] [CrossRef]
- Yu, C.Y.; Kuo, H.C. The emerging roles and functions of circular RNAs and their generation. J. Biomed. Sci. 2019, 26, 29. [Google Scholar] [CrossRef]
- Jeck, W.R.; Sorrentino, J.A.; Wang, K.; Slevin, M.K.; Burd, C.E.; Liu, J.; Marzluff, W.F.; Sharpless, N.E. Circular RNAs are abundant, conserved, and associated with ALU repeats. RNA 2013, 19, 141–157. [Google Scholar] [CrossRef]
- Yao, J.; Dai, Q.; Liu, Z.; Zhou, L.; Xu, J. Circular RNAs in organ fibrosis. Adv. Exp. Med. Biol. 2018, 1087, 259–273. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Bao, C.; Guo, W.; Li, S.; Chen, J.; Chen, B.; Luo, Y.; Lyu, D.; Li, Y.; Shi, G.; et al. Circular RNA profiling revelas an abundant circHIPK3 that regulates cell growth by sponging multiple miRNAs. Nat. Commun. 2016, 7, 11215. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Yang, L.; Yi, M.X.; Liu, B.; Yuan, X.L. Impact of genetic variant of HIPK2 on the risk of severe radiation pneumonitis in lung cancer patients treated with radiation. Radiat. Oncol. 2020, 15, 9. [Google Scholar] [CrossRef] [PubMed]
- Hinderer, S.; Schenke-Layland, K. Cardiac fibrosis—A short review of causes and therapeutic strategies. Adv. Drug Deliv. Rev. 2019, 146, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Meng, D.; Li, F.; Zhang, X.; Liu, L.; Zhu, Y.; Liu, S.; Xu, M.; Deng, J.; Lei, Z.; et al. Inhibition of HIPK2 protects stress-induced pathological cardiac remolding. Ebiomedicine 2022, 85, 104274. [Google Scholar] [CrossRef] [PubMed]
- Kong, P.; Shinde, A.V.; Su, Y.; Russo, I.; Chen, B.; Saxene, A.; Conway, S.J.; Graff, J.M.; Frangogiannis, N.G. Opposing actions of fibroblast and cardiomyocyte smad3 signaling in the infarcted myocardium. Circulation 2018, 137, 707–724. [Google Scholar] [CrossRef]
- Xu, F.; Mao, B.; Li, Y.; Zhao, Y. Knockdown of HIPK2 attenuates angiotensin II-induced cardiac fibrosis in cardiac fibroblasts. J. Cardiovasc. Pharmacol. 2022, 80, 125–131. [Google Scholar] [CrossRef]
- Zhou, Q.; Deng, J.; Yao, J.; Song, J.; Meng, D.; Zhu, Y.; Xu, M.; Liang, Y.; Xu, J.; Sluijter, J.P.; et al. Exercise downregulates HIPK2 and HIPK2 inhibition protects against myocardial infarction. Ebiomedicine 2021, 74, 103713. [Google Scholar] [CrossRef]
- Liu, X.; Xiao, J.; Zhu, H.; Wei, X.; Platt, C.; Damilano, F.; Xiao, C.; Bezzerides, V.; Bostrom, P.; Che, L.; et al. miR-222 is necessary for exercise-induced cardiac growth and protects against pathological cardiac remodeling. Cell Metab. 2015, 21, 584–595. [Google Scholar] [CrossRef]
- Dang, X.; Zhang, R.; Peng, Z.; Qin, Y.; Sun, J.; Niu, Z.; Pei, H. HIPK2 overexpression relieves hypoxia/reoxygenation-induced apoptosis and oxidative damage of cardiomyocytes through enhancement of the Nrf2/ARE signaling pathway. Chem. Biol. Interact. 2020, 316, 108922. [Google Scholar] [CrossRef]
- Mata, A.; Cadenas, S. The antioxidant transcription factor Nrf2 in cardiac ischemia-reperfusion injury. Int. J. Mol. Sci. 2021, 22, 11939. [Google Scholar] [CrossRef] [PubMed]
- Garufi, A.; Pistritto, G.; D’Orazi, V.; Cirone, M.; D’Orazi, G. The impact of NRF2 inhibition on drug-induced colon cancer cell death and p53 activity: A pilot study. Biomolecules 2022, 12, 461. [Google Scholar] [CrossRef] [PubMed]
- Torrente, L.; Sanchez, C.; Moreno, R.; Chowdhry, S.; Cabello, P.; Isono, K.; Koseki, H.; Honda, T.; Hayes, J.D.; Dinkova-Kostova, A.T.; et al. Crosstalk between NRF2 and HIPK2 shapes cytoprotective responses. Oncogene 2017, 36, 6204–6212. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Sui, J.Y.; Kim, K.; Zhang, Z.; Qu, X.A.; Nam, Y.-J.; Willette, R.N.; Barnett, J.V.; Knollmann, B.C.; Force, T.; et al. Cardiomyocyte homeodomain-interacting protein kinase 2 maintains basal cardiac function via extracellular signal-regulated kinase signaling. Circulation 2019, 140, 1820–1833. [Google Scholar] [CrossRef]
- Bataller, R.; Brenner, D.A. Liver fibrosis. J. Clin. Investig. 2005, 115, 209–218. [Google Scholar] [CrossRef]
- Marcellin, P.; Kutala, B.K. Liver diseases: A major neglected global public health problem requiring urgent actions and large-scale screening. Liver Int. 2018, 38, 2–6. [Google Scholar] [CrossRef]
- Puche, J.E.; Saiman, Y.; Friedman, S.L. Hepatic stellate cells and liver fibrosis. Compr. Physiol. 2013, 3, 1473–1492. [Google Scholar] [CrossRef]
- He, P.; Yu, Z.J.; Sun, C.Y.; Jiao, S.J.; Jiang, H.Q. Knockdown of HIPK2 attenuates the pro-fibrogenic response of hepatic stellate cells induced by TGF-β1. Biomed. Pharm. 2017, 85, 575–581. [Google Scholar] [CrossRef]
- Jiang, Z.; Bo, L.; Meng, Y.; Wang, C.; Chen, T.; Wang, C.; Yu, X.; Deng, X. Overexpression of homeodomain-interacting protein kinase 2 (HIPK2) attenuates sepsis-mediated liver injury by restoring autophagy. Cell Death Dis. 2018, 9, 847. [Google Scholar] [CrossRef]
- Zhong, X.; Huang, M.; Kim, H.; Zhang, Y.; Chowdhury, K.; Cai, W.; Saxena, R.; Schwabe, R.F.; Liangpunsakul, S.; Dong, X.C. SIRT6 protect against liver fibrosis by deacetylation and suppression of SMAD3 in hepatic stellate cells. Cell. Mol. Gastroenterol. Hepatol. 2020, 10, 341–364. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Y.; Liu, Q.; Huang, Y.; Li, R.; Wu, T.; Zhan, Z.; Zhou, J.; Huang, H.; Tang, Q.; et al. Sirt6 alleviated liver fibrosis by deacetylating conserved lysine 54 on Smad2 in hepatic stellate cells. Hepatology 2021, 73, 1140–1157. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.L.; Sun, Z.L.; Feng, Y.; Liu, S.Y.; Du, Y.; Yu, S.; Yang, M.L.; Lv, G.Z. Epithelial mesenchymal transition in the formation of hypertrophyc scars and keloids. J. Cell. Physiol. 2019, 234, 21662–21669. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.X.; Zhang, G.Y.; Wang, A.Y.; Chen, Y.H.; Lin, D.M.; Li, Q.F. Role of homeodomain-interacting protein kinase 2 in the pathogenesis of tissue fibrosis in keloid-derived keratinocytes. Ann. Plast. Surg. 2017, 79, 546–551. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhang, M.Z.; Wen, S.H.; Sun, Y.F.; Jiang, P.H.; Wang, L.L.; Zhao, R.; Wang, C.L.; Jiang, S.K.; Guan, D.W. The distribution and time-dependent expression of HIPK2 during the repair of contused skeletal muscle in mice. Histol. Histopathol. 2019, 34, 745–753. [Google Scholar] [CrossRef]
- Shen, T.; Zheng, Q.; Luo, H.; Li, X.; Chen, Z.; Song, Z.; Zhou, G.; Hong, C. Exosomal miR-19a from adipose-derived stem cells suppresses differentiation of corneal keratocytes into myofibroblasts. Aging 2020, 12, 4093–4110. [Google Scholar] [CrossRef]
- Wang, M.; Zhao, J.; Zhang, L.; Wei, F.; Lian, Y.; Wu, Y.; Gong, Z.; Zhang, S.; Zhou, J.; Cao, K.; et al. Role of tumor microenvironment in tumorigenesis. J. Cancer 2017, 8, 761–773. [Google Scholar] [CrossRef]
- Lopez-Novoa, J.M.; Nieto, M.A. Inflammation and EMT: An alliance towards organ fibrosis and cancer progression. EMBO Mol. Med. 2009, 1, 303–314. [Google Scholar] [CrossRef]
- Dvorak, H.F. Tumors: Wounds that do not heal: An old hypothesis revisited. Cancer Immunol. Res. 2015, 3, 1–11. [Google Scholar] [CrossRef]
- Garufi, A.; Traversi, G.; Cirone, M.; D’Orazi, G. HIPK2 role in the tumor-host interaction: Impact of fibroblasts transdifferentiation CAF-like. IUBMB Life 2019, 71, 2055–2061. [Google Scholar] [CrossRef]
- Bar, J.; Feniger-Barish, R.; Lukashchuk, N.; Shaham, H.; Moskovits, N.; Goldfinger, N.; Simansky, D.; Perlman, M.; Papa, M.; Yosepovich, A.; et al. Cancer cells suppress p53 in adjacent fibroblasts. Oncogene 2009, 28, 933–936. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garufi, A.; Pistritto, G.; D’Orazi, G. HIPK2 as a Novel Regulator of Fibrosis. Cancers 2023, 15, 1059. https://doi.org/10.3390/cancers15041059
Garufi A, Pistritto G, D’Orazi G. HIPK2 as a Novel Regulator of Fibrosis. Cancers. 2023; 15(4):1059. https://doi.org/10.3390/cancers15041059
Chicago/Turabian StyleGarufi, Alessia, Giuseppa Pistritto, and Gabriella D’Orazi. 2023. "HIPK2 as a Novel Regulator of Fibrosis" Cancers 15, no. 4: 1059. https://doi.org/10.3390/cancers15041059
APA StyleGarufi, A., Pistritto, G., & D’Orazi, G. (2023). HIPK2 as a Novel Regulator of Fibrosis. Cancers, 15(4), 1059. https://doi.org/10.3390/cancers15041059





