The Clinical Value of Measuring Circulating HPV DNA during Chemo-Radiotherapy in Squamous Cell Carcinoma of the Anus
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Patient Characteristics
3.2. Plasma HPV Sample Collection
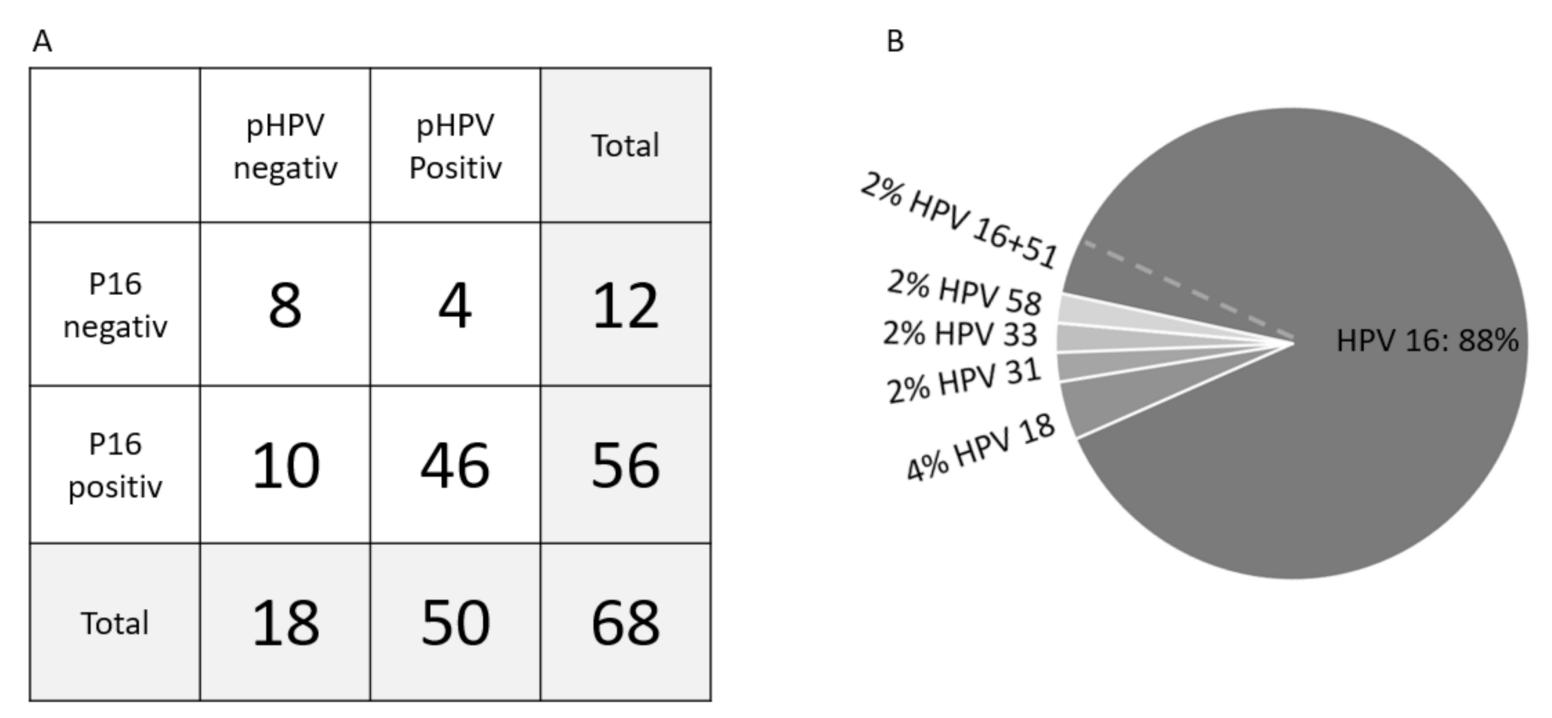
3.3. P16 Status and pHPV Subtypes
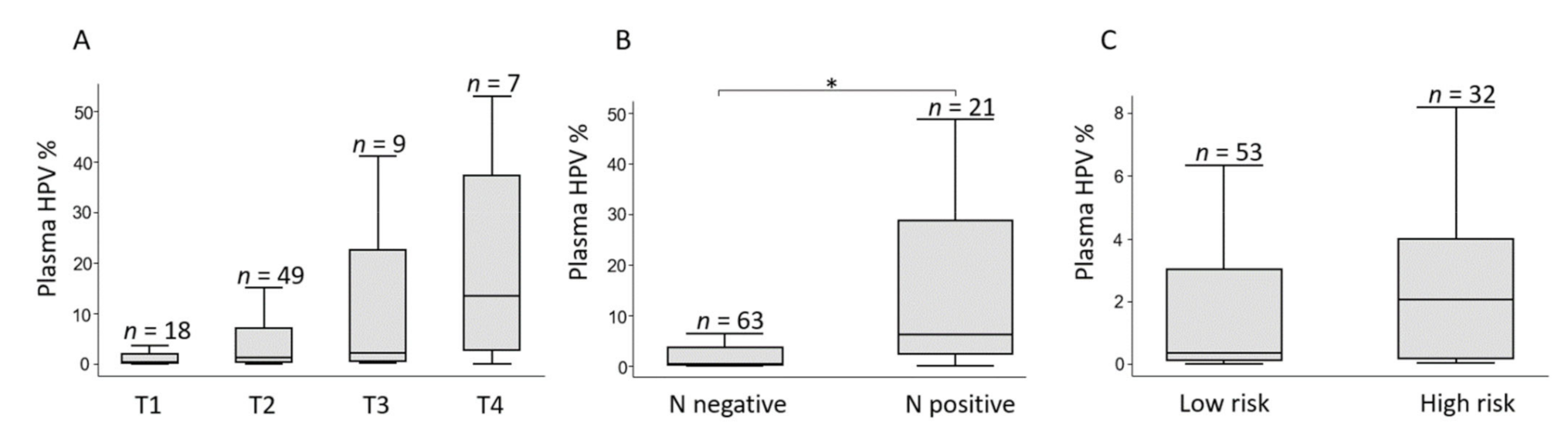
3.4. Circulating HPV Levels and Pre-Treatment Characteristics
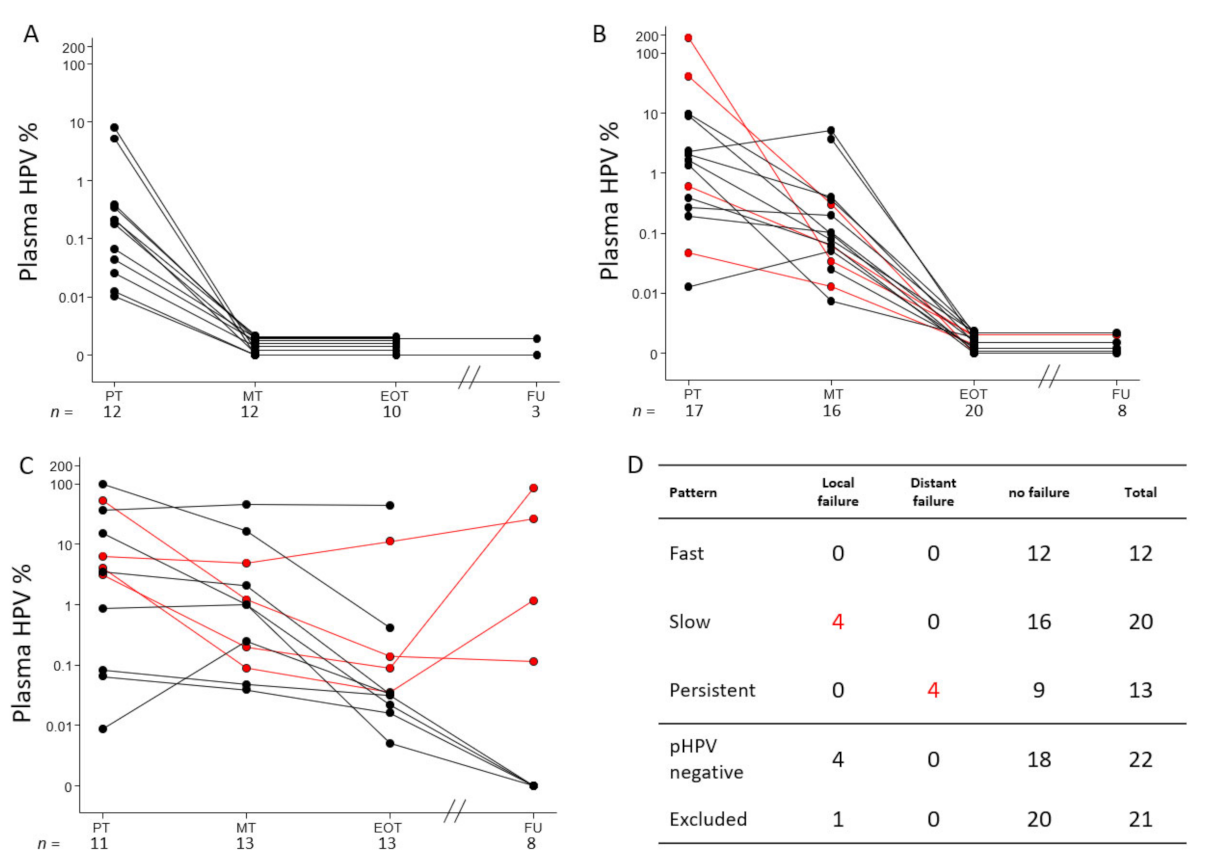
3.5. Elimination Patterns of pHPV during CRT
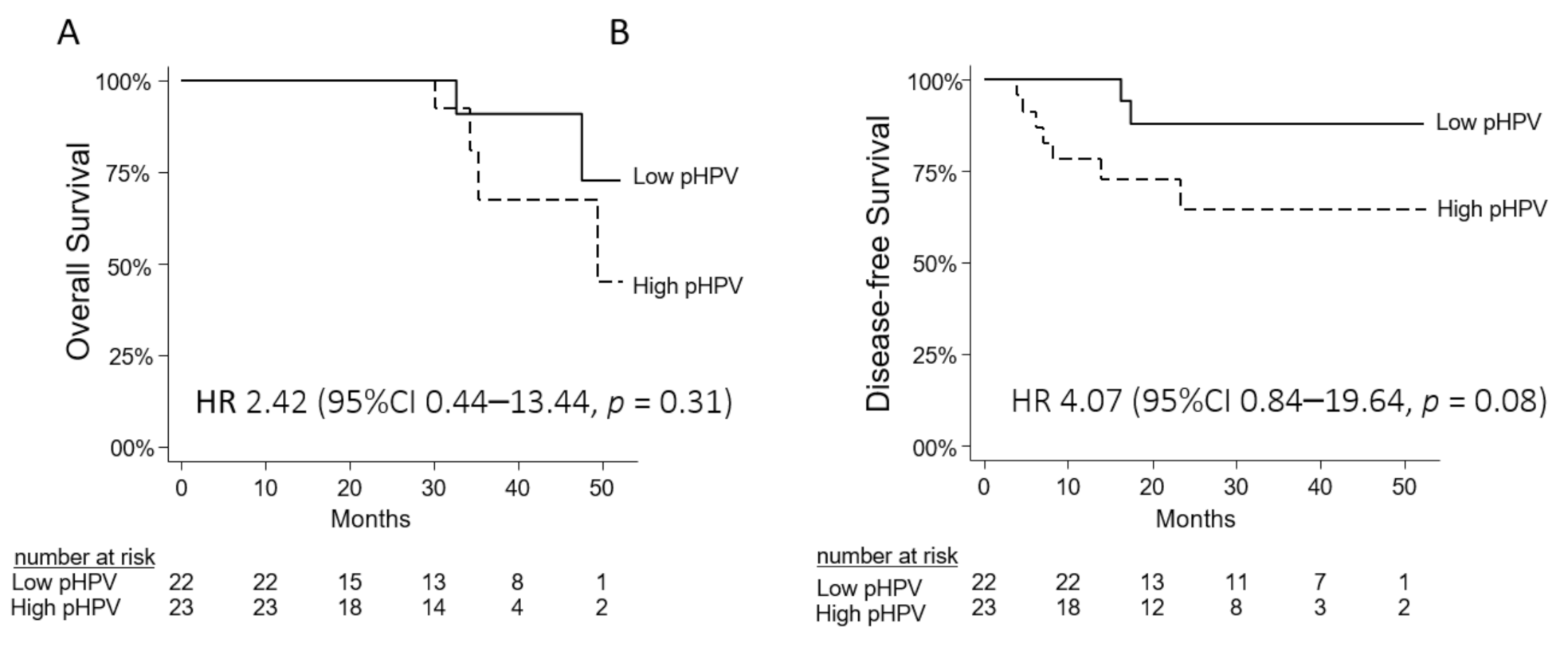
3.6. Prognostic Value of pHPV
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Deshmukh, A.; Suk, R.; Shiels, M.S.; Sonawane, K.; Nyitray, A.G.; Liu, Y.; Gaisa, M.M.; Palefsky, J.M.; Sigel, K. Recent Trends in Squamous Cell Carcinoma of the Anus Incidence and Mortality in the United States, 2001–2015. J. Natl. Cancer Inst. 2020, 112, 829–838. [Google Scholar] [CrossRef] [PubMed]
- Guren, M.G.; Aagnes, B.; Nygård, M.; Dahl, O.; Møller, B. Rising Incidence and Improved Survival of Anal Squamous Cell Carcinoma in Norway, 1987–2016. Clin. Color. Cancer 2019, 18, e96–e103. [Google Scholar] [CrossRef] [PubMed]
- Serup-Hansen, E.; Linnemann, D.; Skovrider-Ruminski, W.; Høgdall, E.; Geertsen, P.F.; Havsteen, H. Human papillomavirus genotyping and p16 expression as prognostic factors for patients with American Joint Committee on Cancer stages I to III carcinoma of the anal canal. J. Clin. Oncol. 2014, 32, 1812–1817. [Google Scholar] [CrossRef] [PubMed]
- Abramowitz, L.; Jacquard, A.-C.; Jaroud, F.; Haesebaert, J.; Siproudhis, L.; Pradat, P.; Aynaud, O.; Leocmach, Y.; Soubeyrand, B.; Dachez, R.; et al. Human papillomavirus genotype distribution in anal cancer in France: The EDiTH V study. Int. J. Cancer 2011, 129, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Lassen, P. The role of Human papillomavirus in head and neck cancer and the impact on radiotherapy outcome. Radiother. Oncol. 2010, 95, 371–380. [Google Scholar] [CrossRef]
- Shakir, R.; Adams, R.; Cooper, R.; Downing, A.; Geh, I.; Gilbert, D.; Jacobs, C.; Jones, C.; Lorimer, C.; Namelo, W.C.; et al. Patterns and Predictors of Relapse Following Radical Chemoradiation Therapy Delivered Using Intensity Modulated Radiation Therapy with a Simultaneous Integrated Boost in Anal Squamous Cell Carcinoma. Int. J. Radiat. Oncol. 2020, 106, 329–339. [Google Scholar] [CrossRef]
- Welzel, G.; Hägele, V.; Wenz, F.; Mai, S.K. Quality of life outcomes in patients with anal cancer after combined radiochemotherapy. Strahlentherapie Onkol. 2011, 187, 175–182. [Google Scholar] [CrossRef]
- Fish, R.; Sanders, C.; Ryan, N.; Van Der Veer, S.; Renehan, A.G.; Williamson, P.R. Systematic review of outcome measures following chemoradiotherapy for the treatment of anal cancer (CORMAC). Color. Dis. 2018, 20, 371–382. [Google Scholar] [CrossRef]
- Kronborg, C.; Serup-Hansen, E.; Lefevre, A.; Wilken, E.E.; Petersen, J.B.; Hansen, J.; Schouboe, A.; Nyvang, L.; Spindler, K.-L.G. Prospective evaluation of acute toxicity and patient reported outcomes in anal cancer and plan optimization. Radiother. Oncol. 2018, 128, 375–379. [Google Scholar] [CrossRef]
- Sunesen, K.G.; Nørgaard, M.; Lundby, L.; Havsteen, H.; Buntzen, S.; Laurberg, S.; Thorlacius-Ussing, O. Long-term anorectal, urinary and sexual dysfunction causing distress after radiotherapy for anal cancer: A Danish multicentre cross-sectional questionnaire study. Color. Dis. 2015, 17, O230–O239. [Google Scholar] [CrossRef]
- Rao, S.; Sclafani, F.; Eng, C.; Guren, M.G.; Adams, R.; Benson, A.; Sebag-Montefiore, D.; Segelov, E.; Bryant, A.; Peckitt, C.; et al. InterAACT: A multicentre open label randomised phase II advanced anal cancer trial of cisplatin (CDDP) plus 5-fluorouracil (5-FU) vs carboplatin (C) plus weekly paclitaxel (P) in patients (pts) with inoperable locally recurrent (ILR) or metastatic treatme. Ann. Oncol. 2018, 29, viii715–viii716. [Google Scholar] [CrossRef]
- Spindler, K.-L.G. Methodological, biological and clinical aspects of circulating free DNA in metastatic colorectal cancer. Acta Oncol. 2016, 56, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Lefèvre, A.C.; Kronborg, C.; Sørensen, B.S.; Krag, S.R.P.; Serup-Hansen, E.; Spindler, K.-L.G. Measurement of circulating free DNA in squamous cell carcinoma of the anus and relation to risk factors and recurrence. Radiother. Oncol. 2020, 150, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Dasari, A.; Morris, V.K.; Allegra, C.J.; Atreya, C.; Benson, A.B.; Boland, P.; Chung, K.; Copur, M.S.; Corcoran, R.B.; Deming, D.A.; et al. ctDNA applications and integration in colorectal cancer: An NCI Colon and Rectal–Anal Task Forces whitepaper. Nat. Rev. Clin. Oncol. 2020, 17, 757–770. [Google Scholar] [CrossRef]
- Cicchillitti, L.; Corrado, G.; De Angeli, M.; Mancini, E.; Baiocco, E.; Patrizi, L.; Zampa, A.; Merola, R.; Martayan, A.; Conti, L.; et al. Circulating cell-free DNA content as blood based biomarker in endometrial cancer. Oncotarget 2017, 8, 115230–115243. [Google Scholar] [CrossRef]
- Corcoran, R.B.; Chabner, B.A. Application of cell-free DNA analysis to cancer treatment. N. Engl. J. Med. 2018, 379, 1754–1765. [Google Scholar] [CrossRef]
- Boysen, A.K.; Pallisgaard, N.; Andersen, C.S.A.; Spindler, K.-L.G. Circulating tumor DNA as a marker of minimal residual disease following local treatment of metastases from colorectal cancer. Acta Oncol. 2020, 59, 1424–1429. [Google Scholar] [CrossRef]
- Bernard-Tessier, A.; Jeannot, E.; Guenat, D.; Debernardi, A.; Michel, M.; Proudhon, C.; Vincent-Salomon, A.; Bièche, I.; Pierga, J.-Y.; Buecher, B.; et al. Clinical validity of HPV circulating tumor DNA in advanced anal carcinoma: An ancillary study to the EPITOPES-HPV02 trial. Clin. Cancer Res. 2019, 25, 2109–2115. [Google Scholar] [CrossRef]
- Chera, B.S.; Kumar, S.; Shen, C.; Amdur, R.; Dagan, R.; Green, R.; Goldman, E.; Weiss, J.; Grilley-Olson, J.; Patel, S.; et al. Plasma Circulating Tumor HPV DNA for the Surveillance of Cancer Recurrence in HPV-Associated Oropharyngeal Cancer. J. Clin. Oncol. 2020, 38, 1050–1058. [Google Scholar] [CrossRef]
- Chera, B.S.; Kumar, S.; Beaty, B.T.; Marron, D.; Jefferys, S.; Green, R.; Goldman, E.C.; Amdur, R.; Sheets, N.; Dagan, R.; et al. Rapid clearance profile of plasma circulating tumor HPV type 16 DNA during chemoradiotherapy correlates with disease control in HPV-associated oropharyngeal cancer. Clin. Cancer Res. 2019, 25, 4682–4690. [Google Scholar] [CrossRef]
- Hanna, G.; Supplee, J.; Kuang, Y.; Mahmood, U.; Lau, C.; Haddad, R.; Jänne, P.; Paweletz, C. Plasma HPV cell-free DNA monitoring in advanced HPV-associated oropharyngeal cancer. Ann. Oncol. 2018, 29, 1980–1986. [Google Scholar] [CrossRef]
- Jeannot, E.; Becette, V.; Campitelli, M.; Calméjane, M.; Lappartient, E.; Ruff, E.; Saada, S.; Holmes, A.; Bellet, D.; Sastre-Garau, X. Circulating human papillomavirus DNA detected using droplet digital PCR in the serum of patients diagnosed with early stage human papillomavirus-associated invasive carcinoma. J. Pathol. Clin. Res. 2016, 2, 201–209. [Google Scholar] [CrossRef]
- Cabel, L.; Jeannot, E.; Bieche, I.; Vacher, S.; Callens, C.; Bazire, L.; Morel, A.; Bernard-Tessier, A.; Chemlali, W.; Schnitzler, A.; et al. Prognostic Impact of Residual HPV ctDNA Detection after Chemoradiotherapy for Anal Squamous Cell Carcinoma. Clin. Cancer Res. 2018, 24, 5767–5771. [Google Scholar] [CrossRef]
- Damerla, R.R.; Lee, N.Y.; You, D.; Soni, R.; Shah, R.; Reyngold, M. Original report abstract Detection of Early Human Papillomavirus—Associated Cancers by Liquid Biopsy. JCO Precis. Oncol. 2019, 3, 1–17. [Google Scholar]
- Lee, J.Y.; Cutts, R.J.; White, I.; Augustin, Y.; Garcia-Murillas, I.; Fenwick, K.; Matthews, N.; Turner, N.C.; Harrington, K.; Gilbert, D.C.; et al. Next Generation Sequencing Assay for Detection of Circulating HPV DNA (cHPV-DNA) in Patients Undergoing Radical (Chemo)Radiotherapy in Anal Squamous Cell Carcinoma (ASCC). Front. Oncol. 2020, 10, 505. [Google Scholar] [CrossRef]
- Lee, J.Y.; Garcia-Murillas, I.; Cutts, R.J.; De Castro, D.G.; Grove, L.; Hurley, T.; Wang, F.; Nutting, C.; Newbold, K.; Harrington, K.; et al. Predicting response to radical (chemo)radiotherapy with circulating HPV DNA in locally advanced head and neck squamous carcinoma. Br. J. Cancer 2017, 117, 876–883. [Google Scholar] [CrossRef]
- Available online: www.regioner.dk/rbgben (accessed on 17 May 2021).
- Pallisgaard, N.; Spindler, K.-L.G.; Andersen, R.F.; Brandslund, I.; Jakobsen, A. Controls to validate plasma samples for cell free DNA quantification. Clin. Chim. Acta 2015, 446, 141–146. [Google Scholar] [CrossRef]
- Lassen, P.; Overgaard, J. Scoring and classification of oropharyngeal carcinoma based on HPV-related p16-expression. Radiother. Oncol. 2012, 105, 269–270. [Google Scholar] [CrossRef]
- Conesa-Zamora, P.; Doménech-Peris, A.; Orantes-Casado, F.J.; Ortiz-Reina, S.; Sahuquillo-Frías, L.; Acosta-Ortega, J.; García-Solano, J.; Pérez-Guillermo, M. Effect of human papillomavirus on cell cycle-related proteins p16, Ki-67, cyclin D1, p53, and ProEx C in precursor lesions of cervical carcinoma: A tissue microarray study. Am. J. Clin. Pathol. 2009, 132, 378–390. [Google Scholar] [CrossRef]
- Larsen, C.G.; Gyldenløve, M.; Jensen, D.H.; Therkildsen, M.H.; Kiss, K.; Norrild, B.; Konge, L.; Von Buchwald, C. Correlation between human papillomavirus and p16 overexpression in oropharyngeal tumours: A systematic review. Br. J. Cancer 2014, 110, 1587–1594. [Google Scholar] [CrossRef]
- Wong, K.S.; Krane, J.F.; Jo, V.Y. Heterogeneity of p16 immunohistochemistry and increased sensitivity of RNA in situ hybridization in cytology specimens of HPV-related head and neck squamous cell carcinoma. Cancer Cytopathol. 2019, 127, 632–642. [Google Scholar] [CrossRef]
- Sun, G.; Dong, X.; Tang, X.; Qu, H.; Zhang, H.; Zhao, E. The prognostic value of HPV combined p16 status in patients with anal squamous cell carcinoma: A meta-analysis. Oncotarget 2018, 9, 8081–8088. [Google Scholar] [CrossRef]
- Zhang, X.C.; Xu, C.; Mitchell, R.M.; Zhang, B.; Zhao, D.; Li, Y.; Huang, X.; Fan, W.; Wang, H.; Lerma, L.A.; et al. Tumor evolution and intratumor heterogeneity of an oropharyngeal squamous cell carcinoma revealed by whole-genome sequencing. Neoplasia 2013, 15, 1371–1378. [Google Scholar] [CrossRef]
- Cho, M.-S.; Park, C.H.; Lee, S.; Park, H.S. Clinicopathological parameters for circulating tumor DNA shedding in surgically resected non-small cell lung cancer with EGFR or KRAS mutation. PLoS ONE 2020, 15, e0230622. [Google Scholar] [CrossRef]
- McBride, A.A.; Warburton, A. The role of integration in oncogenic progression of HPV-associated cancers. PLoS Pathog. 2017, 13, e1006211. [Google Scholar] [CrossRef]
- Anayannis, N.V.; Schlecht, N.F.; Ben-Dayan, M.; Smith, R.V.; Belbin, T.J.; Ow, T.J.; Blakaj, D.M.; Burk, R.D.; Leonard, S.M.; Woodman, C.B.; et al. Association of an intact E2 gene with higher HPV viral load, higher viral oncogene expression, and improved clinical outcome in HPV16 positive head and neck squamous cell carcinoma. PLoS ONE. 2018, 13, e0191581. [Google Scholar] [CrossRef]
- Valmary-Degano, S.; Jacquin, E.; Prétet, J.-L.; Monnien, F.; Girardo, B.; Arbez-Gindre, F.; Joly, M.; Bosset, J.-F.; Kantelip, B.; Mougin, C. Signature patterns of human papillomavirus type 16 in invasive anal carcinoma. Hum. Pathol. 2013, 44, 992–1002. [Google Scholar] [CrossRef]
| Patient Characteristics | Total Cohort n = 88 (%) | Measurable pHPV n = 45 (%) | Median pHPV in % of Total cfDNA (95% CI) | p-Value |
|---|---|---|---|---|
| Age (years) | ||||
| Median, range | 62, 26–84 | |||
| <62 | 44 (50) | 26 (58) | 1.10 (0.28–4.15) | |
| >62 | 44 (50) | 19 (42) | 2.05 (0.17–10.91) | 0.87 |
| Sex | ||||
| Female | 65 (74) | 36 (80) | 1.46 (0.28–4.93) | |
| Male | 23 (26) | 9 (20) | 1.34 (0.04–3.90) | 0.35 |
| Performance status (PS) | ||||
| PS = 0 | 62 (70) | 32 (71) | 1.85 (0.21–4.00) | |
| PS > 0 | 26 (30) | 13 (29) | 0.86 (0.05–39.20) | 0.84 |
| P16 status | ||||
| Positive | 72 (82) | 39 (87) | 1.66 (0.27–4.07) | |
| Negative | 13 (15) | 4 (9) | 0.19 (0.02–53.03) | 0.40 |
| Unknown | 3 (3) | 2 (4) | 2.41 (1.34–3.48) | 0.80 |
| Stage | 0.39 * | |||
| T1 | 19 (22) | 9 (20) | 0.35 (0.04–3.51) | |
| T2 | 53 (60) | 28 (62) | 1.26 (0.23–4.36) | |
| T3 | 9 (10) | 4 (9) | 2.29 (0.18–41.24) | |
| T4 | 7 (8) | 4 (9) | 13.46 (0.03–53.03) | |
| N negative | 67 (76) | 33 (73) | 0.39 (0.18–2.59) | |
| N positive | 21 (24) | 12 (27) | 6.09 (2.08–34.55) | 0.02 |
| M negative | 87 (99) | 44 (98) | 1.50 (0.29–3.63) | |
| M positive | 1 (1) | 1 (2) | 0.08 | 0.25 |
| Risk | ||||
| Low | 56 (64) | 27 (60) | 0.39 (0.19–3.05) | |
| High | 32 (36) | 18 (40) | 3.74 (0.37–19.75) | 0.09 |
| Number of Available and Excluded Blood Samples | Number |
|---|---|
| Total: | n = 88 |
| Pre-Treatment (PT) (n = 63) + Pilot (n = 10) | 73 |
| Mid Treatment (MT) | 72 |
| End of Treatment (EOT) | 64 |
| Follow-up (FU) | 41 |
| Plasma HPV (pHPV) detection: | n = 88 |
| Detectable pHPV (PT or MT + Pilot) | 52 |
| Not measurable pHPV (PT + pilot) * | 23 |
| Missing PT and undetectable pHPV in remaining samples | 13 |
| P16 status and pHPV subtypes ** | n = 68 |
| PT (43 pHPV positive, 18 pHPV negative) | 61 |
| Pilot pHPV positive | 5 |
| MT pHPV positive if missing PT sample | 2 |
| Pre-treatment characteristics and survival analysis | n = 45 |
| PT pHPV positive | 45 |
| Elimination patterns *** | n = 67 |
| PT + Pilot positive | 65 |
| MT | 60 |
| EOT | 59 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lefèvre, A.C.; Pallisgaard, N.; Kronborg, C.; Wind, K.L.; Krag, S.R.P.; Spindler, K.-L.G. The Clinical Value of Measuring Circulating HPV DNA during Chemo-Radiotherapy in Squamous Cell Carcinoma of the Anus. Cancers 2021, 13, 2451. https://doi.org/10.3390/cancers13102451
Lefèvre AC, Pallisgaard N, Kronborg C, Wind KL, Krag SRP, Spindler K-LG. The Clinical Value of Measuring Circulating HPV DNA during Chemo-Radiotherapy in Squamous Cell Carcinoma of the Anus. Cancers. 2021; 13(10):2451. https://doi.org/10.3390/cancers13102451
Chicago/Turabian StyleLefèvre, Anna C., Niels Pallisgaard, Camilla Kronborg, Karen L. Wind, Søren R. P. Krag, and Karen-Lise G. Spindler. 2021. "The Clinical Value of Measuring Circulating HPV DNA during Chemo-Radiotherapy in Squamous Cell Carcinoma of the Anus" Cancers 13, no. 10: 2451. https://doi.org/10.3390/cancers13102451
APA StyleLefèvre, A. C., Pallisgaard, N., Kronborg, C., Wind, K. L., Krag, S. R. P., & Spindler, K.-L. G. (2021). The Clinical Value of Measuring Circulating HPV DNA during Chemo-Radiotherapy in Squamous Cell Carcinoma of the Anus. Cancers, 13(10), 2451. https://doi.org/10.3390/cancers13102451






