Mechanical Properties of 3D Nanostructures Obtained by Focused Electron/Ion Beam-Induced Deposition: A Review
Abstract
1. Introduction
2. Methods and Materials
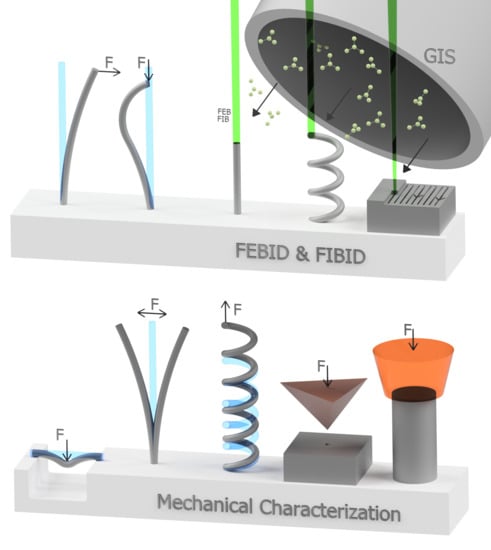
2.1. 3D Nanoprinting via FEBID & FIBID
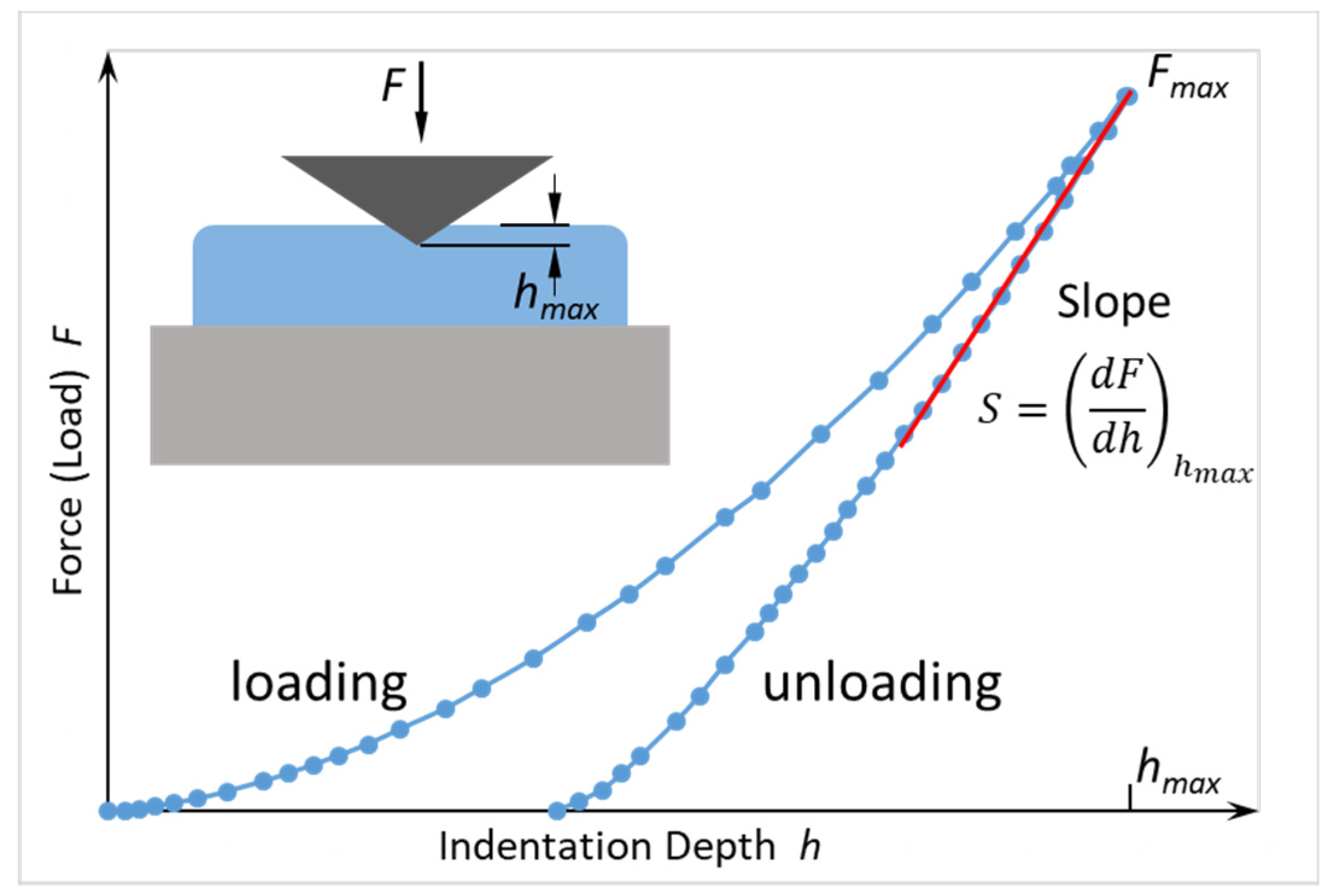
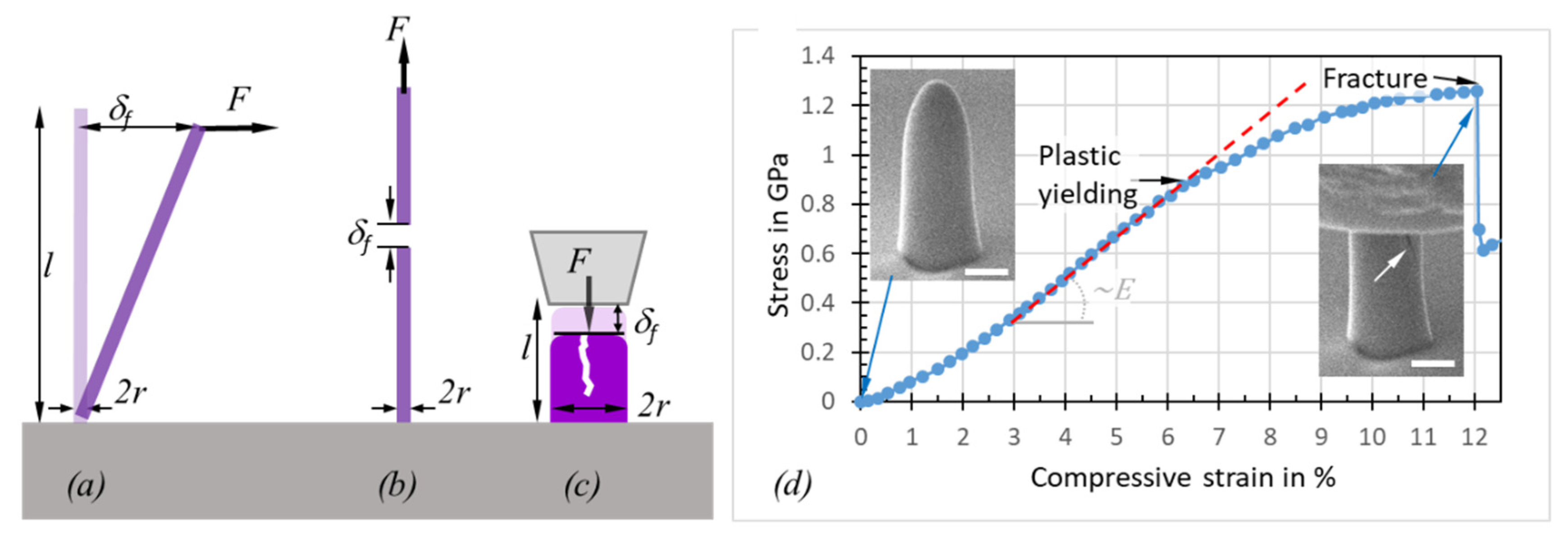
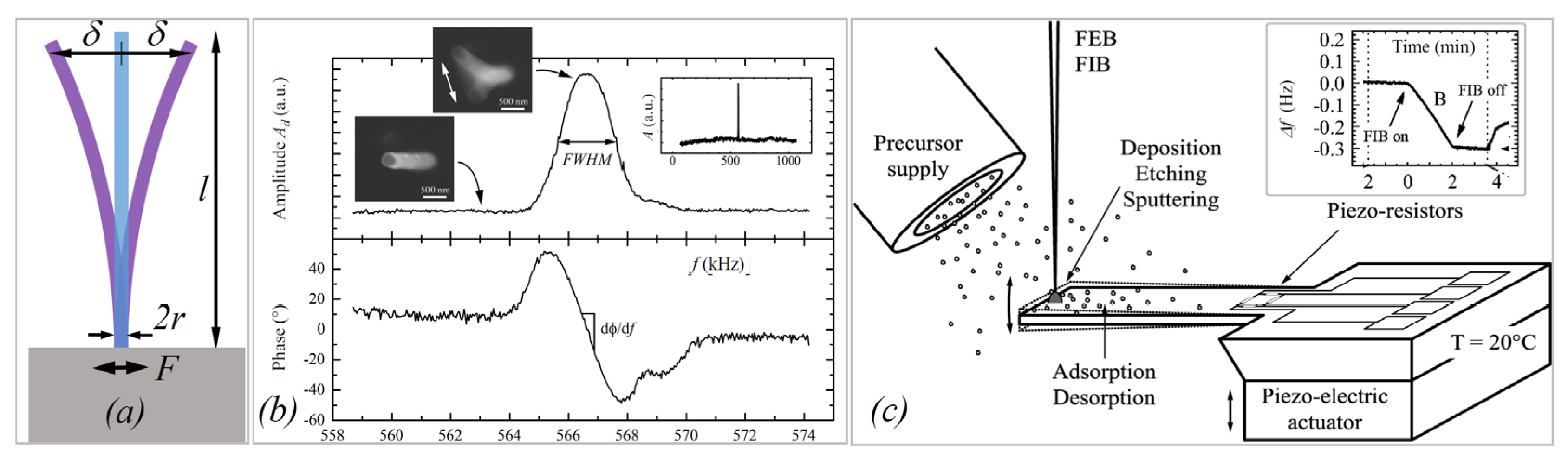
2.2. Mechanical Characterization
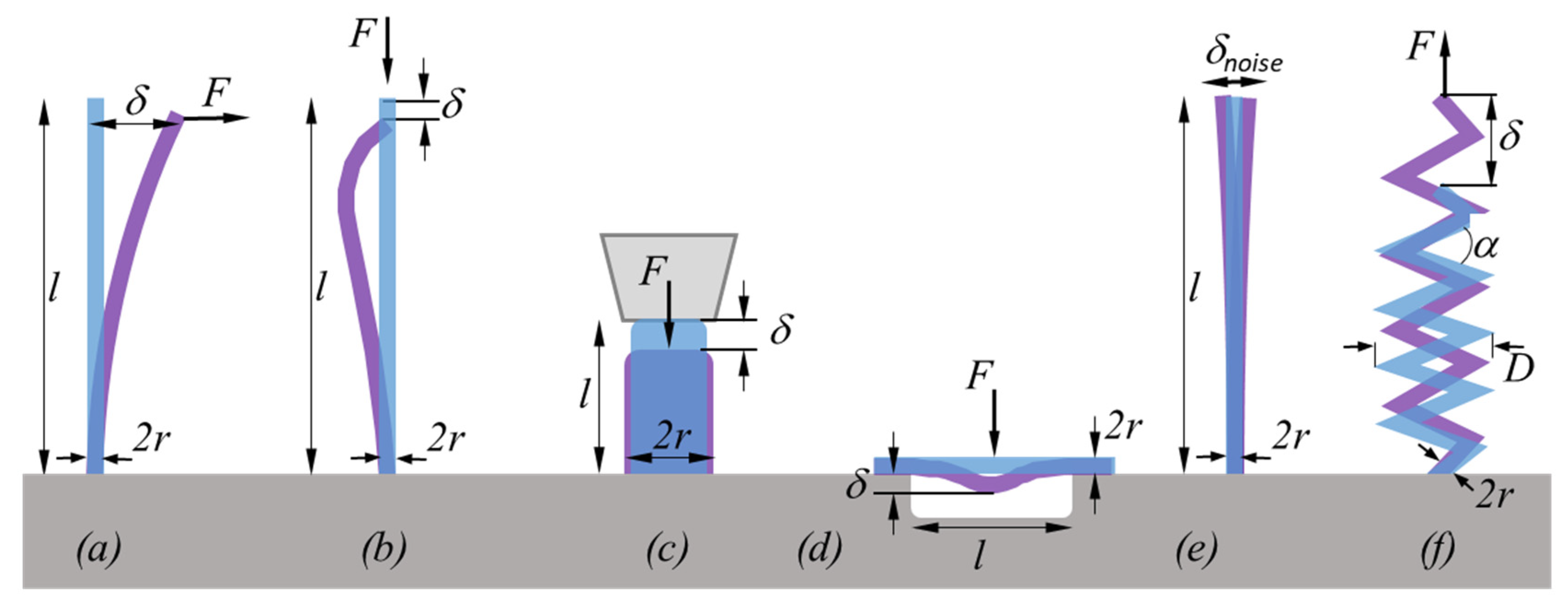
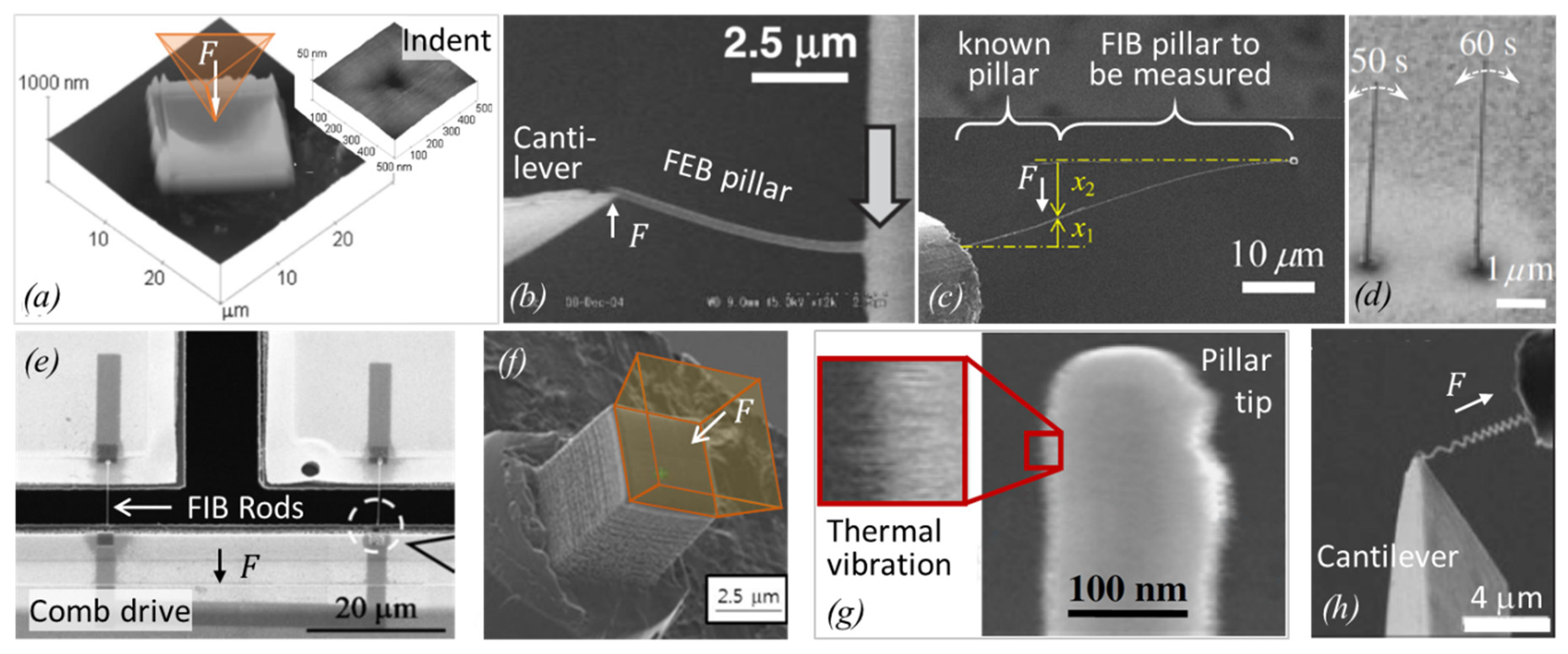
2.2.1. Elastic Modulus
2.2.2. Hardness
2.2.3. Plasticity and Fracture
2.2.4. Density of FEBID and FIBID Nanostructures
3. Mechanical Properties of FEBID and FIBID Materials
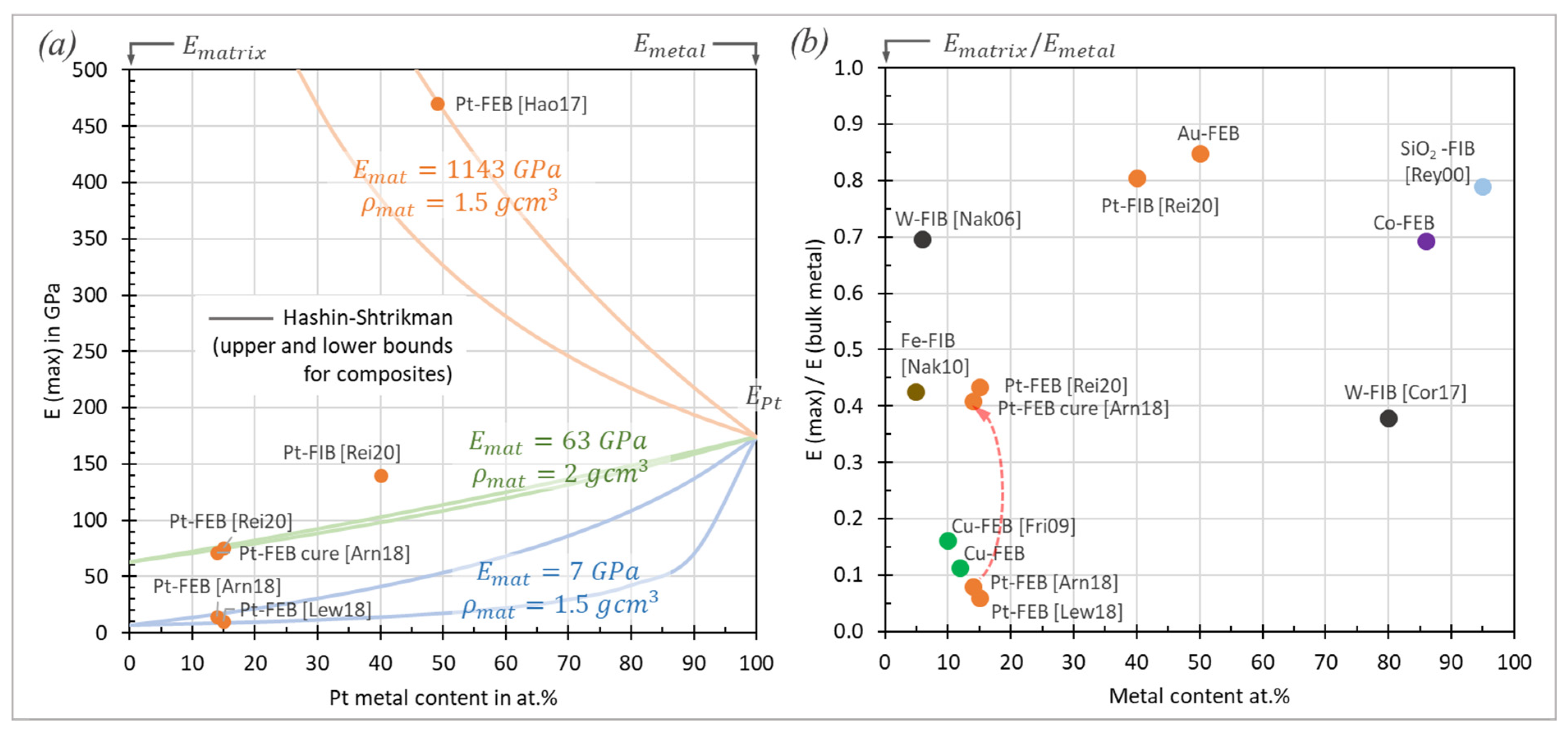
3.1. Elastic Modulus
3.1.1. Carbon FEBID and Carbon:Gallium FIBID Materials
3.1.2. Metal–Carbon Materials
Platinum FEBID and FIBID Material
W–, Co–, Au–, and Cu–Carbon FEBID Materials:
Pt–Carbon, Fe–Carbon, W–Carbon, and Si–Carbon FIBID Materials
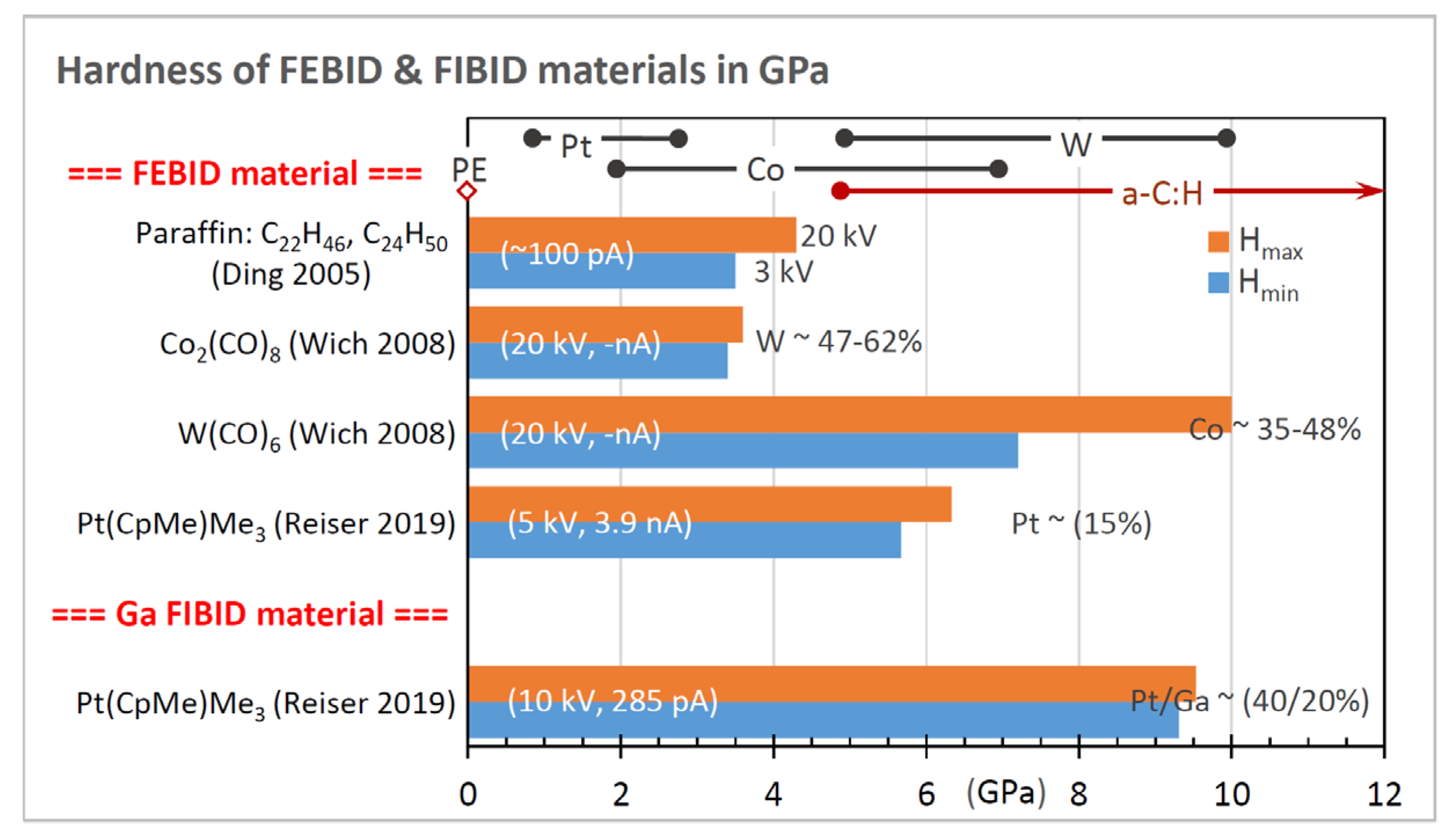
3.2. Hardness
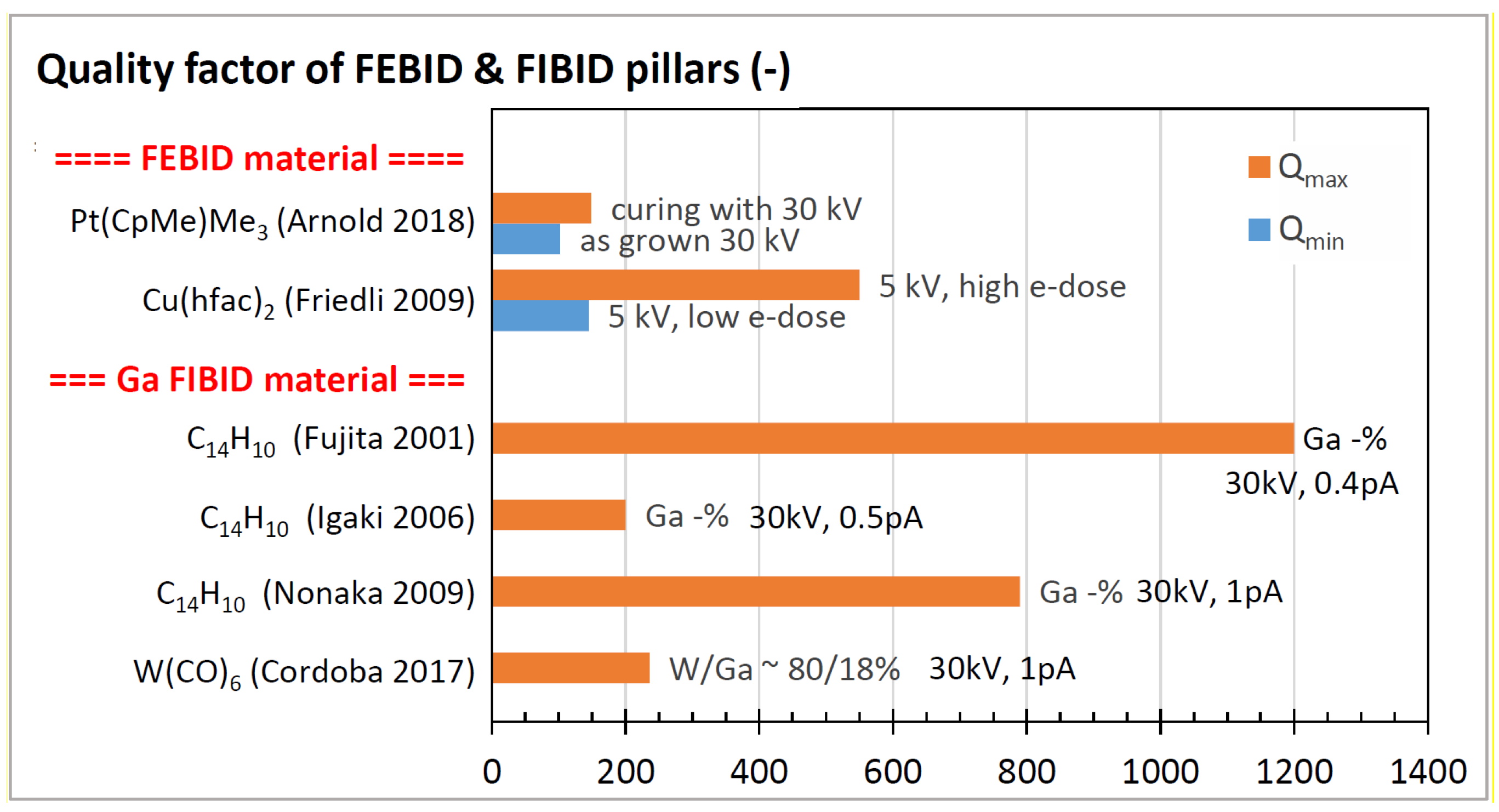
3.3. Quality Factors
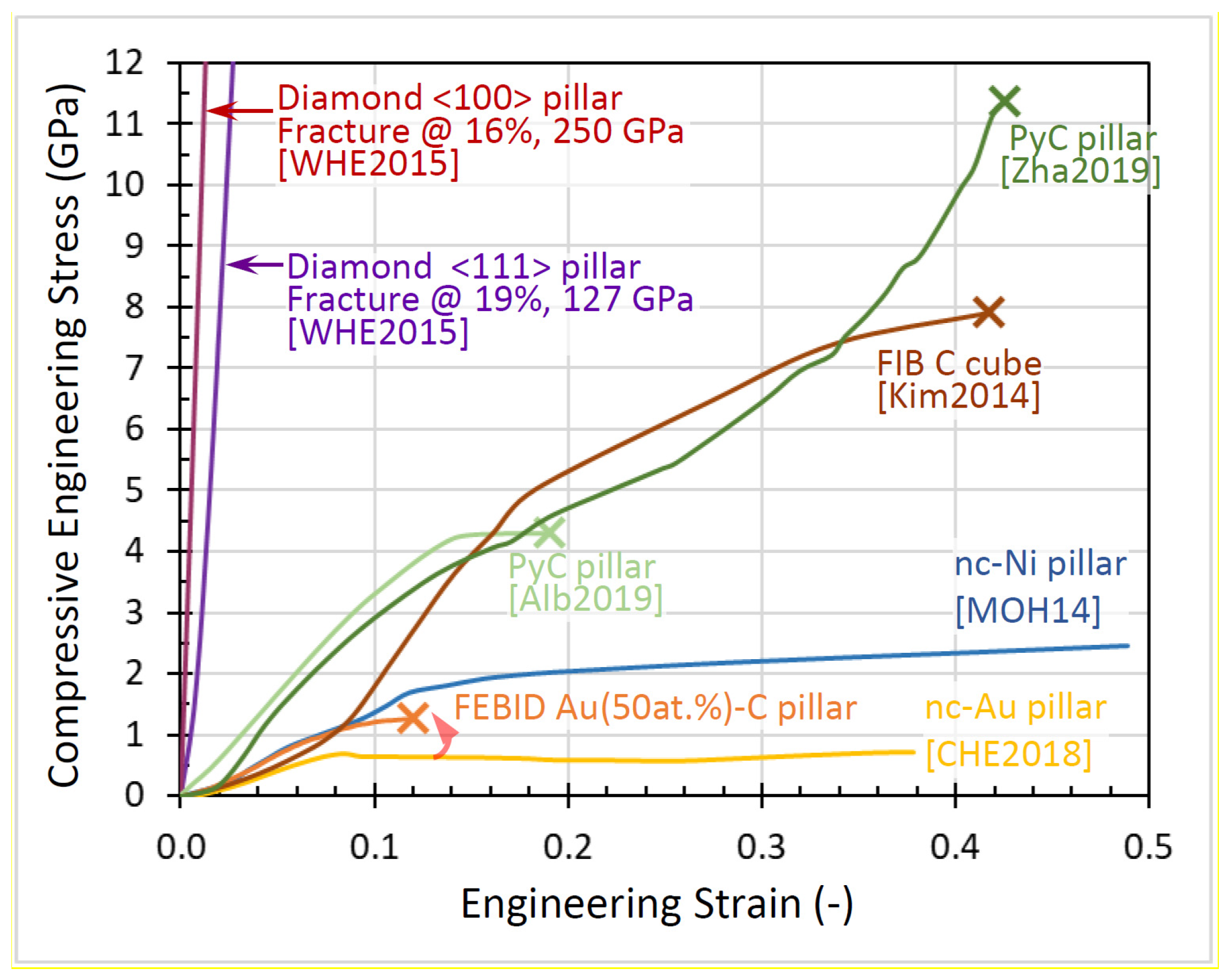
3.4. Yield and Fracture Strength
3.5. Density
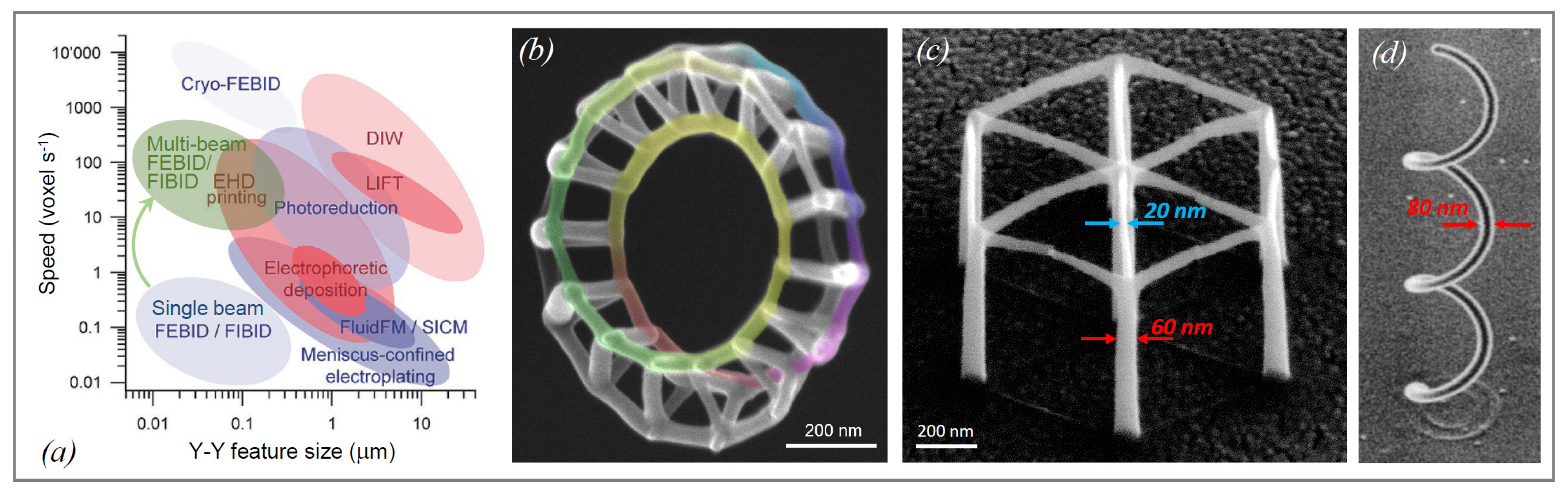
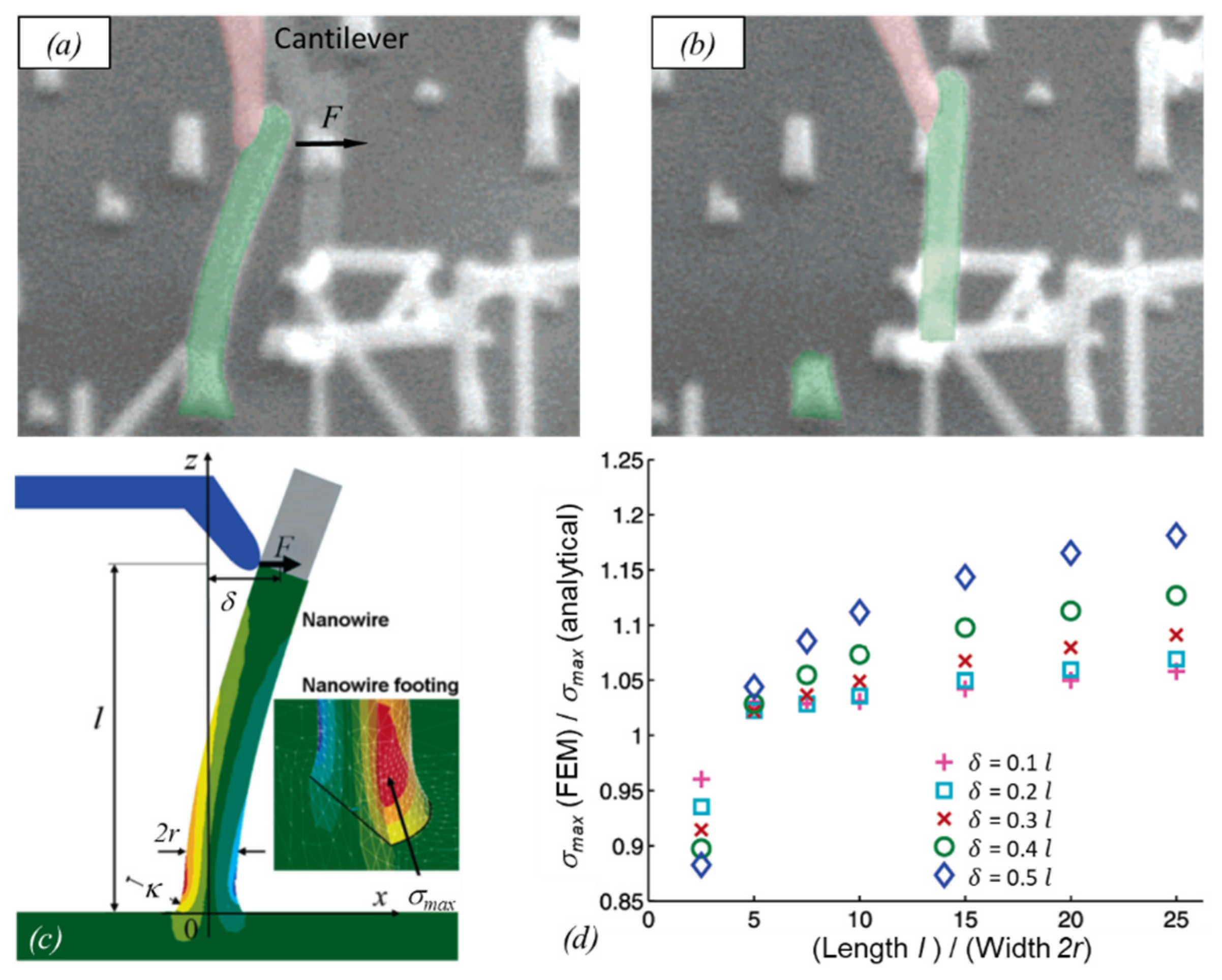
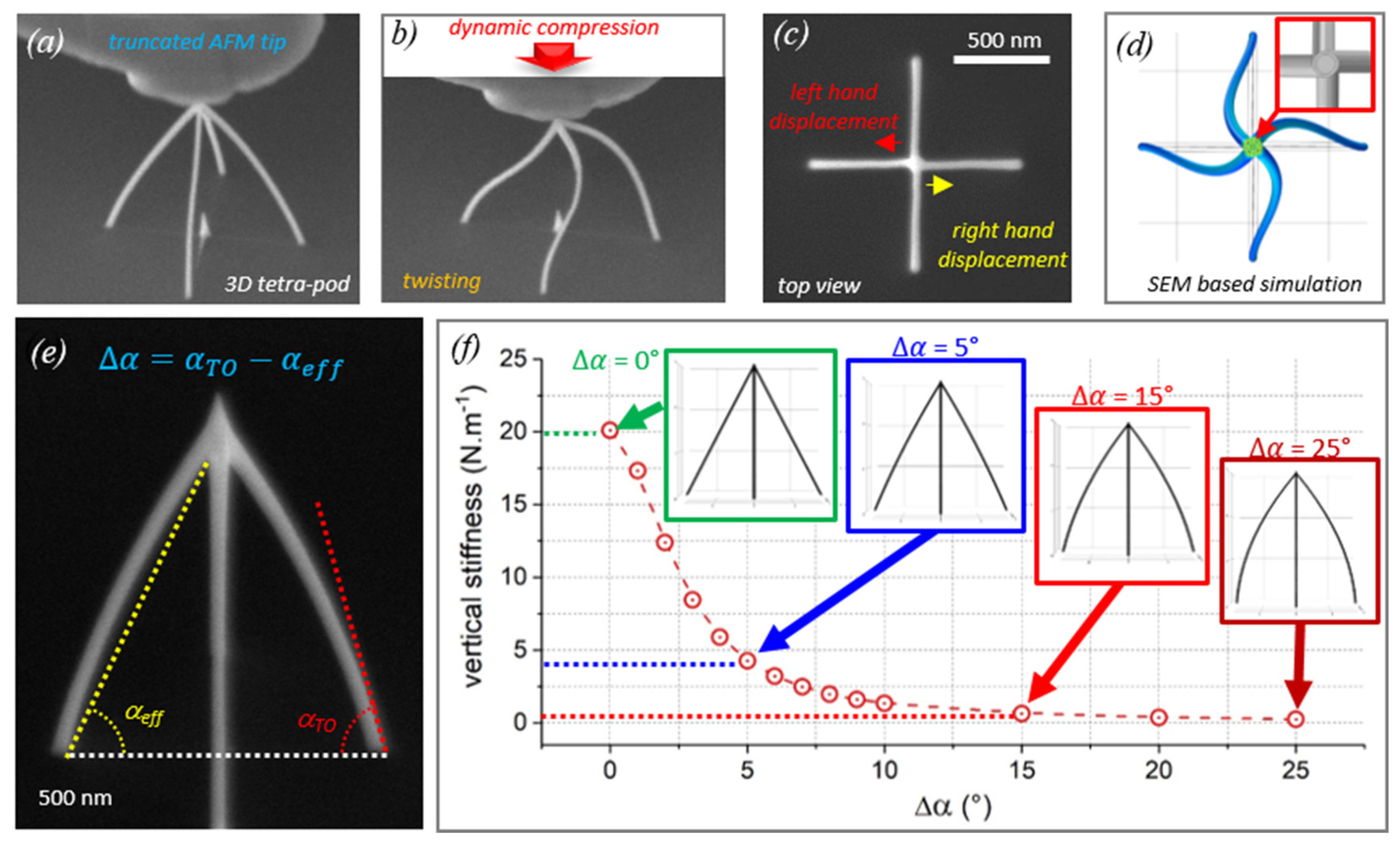
4. 3D Structures in Mechanical Experiments
5. Irradiation Parameter Influence on Mechanical Properties
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hirt, L.; Reiser, A.; Spolenak, R.; Zambelli, T. Additive Manufacturing of Metal Structures at the Micrometer Scale. Adv. Mater. 2017, 29, 1604211. [Google Scholar] [CrossRef] [PubMed]
- Post, P.C.; Mohammadi-Gheidari, A.; Hagen, C.; Kruit, P. Parallel electron-beam-induced deposition using a multi-beam scanning electron microscope. J. Vac. Sci. Technol. B 2011, 29, 06F310. [Google Scholar] [CrossRef]
- Klein, C.; Loeschner, H.; Platzgummer, E. Performance of the proof-of-concept multi-beam mask writer (MBMW POC). SPIE Photomask Technology 2013, 8880, 88801. [Google Scholar]
- Koeck, A.; Bruck, R.; Wellenzohn, M.; Hainberger, R.; Platzgummer, E.; Loeschner, H.; Joechl, P.; Eder-Kapl, S.; Ebm, C.; Czepl, P.; et al. Argon ion multibeam nanopatterning of Ni–Cu inserts for injection molding. J. Vac. Sci. Technol. B 2010, 28, 1–6. [Google Scholar] [CrossRef]
- Saha, S.K.; Wang, D.; Nguyen, V.H.; Chang, Y.; Oakdale, J.S.; Chen, S.-C. Scalable submicrometer additive manufacturing. Sci. 2019, 366, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Fischer, J.; Wegener, M. Three-dimensional direct laser writing inspired by stimulated-emission-depletion microscopy [Invited]. Opt. Mater. Express 2011, 1, 614. [Google Scholar] [CrossRef]
- Zhu, Y.; Inada, H.; Hartschuh, A.; Shi, L.; Della Pia, A.; Costantini, G.; De Parga, A.L.V.; Miranda, R.; Barbier, A.; Mocuta, C.; et al. Synthesis of Graphene. In Encyclopedia of Nanotechnology; Springer Science and Business Media LLC: Berlin, Germany, 2012; pp. 2630–2638. [Google Scholar]
- Matsui, S.; Kaito, T.; Fujita, J.-I.; Komuro, M.; Kanda, K.; Haruyama, Y. Three-dimensional nanostructure fabrication by focused-ion-beam chemical vapor deposition. J. Vac. Sci. Technol. B: Microelectron. Nanometer Struct. 2000, 18, 3181. [Google Scholar] [CrossRef]
- Utke, I.; Gölzhäuser, A. Small, Minimally Invasive, Direct: Electrons Induce Local Reactions of Adsorbed Functional Molecules on the Nanoscale. Angew. Chem. Int. Ed. 2010, 49, 9328–9330. [Google Scholar] [CrossRef]
- Utke, I.; Hoffmann, P.; Melngailis, J. Gas-assisted focused electron beam and ion beam processing and fabrication. J. Vac. Sci. Technol. B: Microelectron. Nanometer Struct. 2008, 26, 1197. [Google Scholar] [CrossRef]
- Utke, I.; Moshkalev, S.; Russell, P.E. Nanofabrication Using Focused ion and Electron Beams: Principles and Applications; Utke, I., Moshkalev, S., Russels, P., Eds.; Oxford University Press: Oxford, UK, 2012. [Google Scholar]
- Ingolfsson, O. Low energy electrons: Fundamentals & Applications; Pan Stanford Publishing: Singapore, 2019. [Google Scholar]
- Jones, A.C.; Hitchman, M.L. Chemical Vapour Deposition: Precursors, Processes and Applications; Royal Society of Chemistry: London, UK, 2009. [Google Scholar] [CrossRef]
- Bachmann, J. Front Matter. Atomic Layer Depos. Energy Convers. Appl. 2017, 46. [Google Scholar]
- Ziegler, J.F.; Biersack, J.P.; Ziegler, M.D. The stopping and range of ions in matter. 2015. Available online: www.srim.org (accessed on 3 March 2020).
- Shorubalko, I.; Pillatsch, L.; Utke, I. Direct–Write Milling and Deposition with Noble Gases; Springer Science and Business Media LLC: Berlin, Germany, 2016; pp. 355–393. [Google Scholar]
- Alkemade, P.F.A.; Miro, H. Focused helium-ion-beam-induced deposition. Appl. Phys. A 2014, 117, 1727–1747. [Google Scholar] [CrossRef]
- Spencer, J.A.; Rosenberg, S.G.; Barclay, M.; Wu, Y.-C.; McElwee-White, L.; Fairbrother, D.H. Understanding the electron-stimulated surface reactions of organometallic complexes to enable design of precursors for electron beam-induced deposition. Appl. Phys. A 2014, 117, 1631–1644. [Google Scholar] [CrossRef]
- Wnuk, J.; Rosenberg, S.; Gorham, J.; Van Dorp, W.; Hagen, C.; Fairbrother, D.H. Electron beam deposition for nanofabrication: Insights from surface science. Surf. Sci. 2011, 605, 257–266. [Google Scholar] [CrossRef]
- Porrati, F.; Pohlit, M.; Muller, J.; Barth, S.; Biegger, F.; Gspan, C.; Plank, H.; Huth, M. Direct writing of CoFe alloy nanostructures by focused electron beam induced deposition from a heteronuclear precursor. Nanotechnology 2015, 26, 475701. [Google Scholar] [CrossRef] [PubMed]
- De Teresa, J.M.; Fernández-Pacheco, A.; Córdoba, R.; Serrano-Ramón, L.; Sangiao, S.; Ibarra, M.R. Review of magnetic nanostructures grown by focused electron beam induced deposition (FEBID). J. Phys. D: Appl. Phys. 2016, 49, 243003. [Google Scholar] [CrossRef]
- Walz, M.-M.; Schirmer, M.; Vollnhals, F.; Lukasczyk, T.; Steinrück, H.-P.; Marbach, H. Electrons as “Invisible Ink”: Fabrication of Nanostructures by Local Electron Beam Induced Activation of SiOx. Angew. Chem. Int. Ed. 2010, 49, 4669–4673. [Google Scholar] [CrossRef]
- Gavagnin, M.; Wanzenböck, H.; Wachter, S.; Shawrav, M.M.; Persson, A.; Gunnarsson, K.; Svedlindh, P.; Stöger-Pollach, M.; Bertagnolli, E. Free-Standing Magnetic Nanopillars for 3D Nanomagnet Logic. ACS Appl. Mater. Interfaces 2014, 6, 20254–20260. [Google Scholar] [CrossRef]
- Utke, I.; Hoffmann, P.; Dwir, B.; Leifer, K.; Kapon, E.; Doppelt, P. Focused electron beam induced deposition of gold. J. Vac. Sci. Technol. B: Microelectron. Nanometer Struct. 2000, 18, 3168. [Google Scholar] [CrossRef]
- Höflich, K.; Jurczyk, J.; Zhang, Y.; Dos Santos, M.V.P.; Götz, M.; Guerra-Nuñez, C.; Best, J.P.; Kapusta, C.; Utke, I. Direct Electron Beam Writing of Silver-Based Nanostructures. ACS Appl. Mater. Interfaces 2017, 9, 24071–24077. [Google Scholar] [CrossRef]
- Geier, B.; Gspan, C.; Winkler, R.; Schmied, R.; Fowlkes, J.D.; Fitzek, H.; Rauch, S.; Rattenberger, J.; Rack, P.D.; Plank, H. Rapid and Highly Compact Purification for Focused Electron Beam Induced Deposits: A Low Temperature Approach Using Electron Stimulated H2O Reactions. J. Phys. Chem. C 2014, 118, 14009–14016. [Google Scholar] [CrossRef]
- Plank, H.; Noh, J.H.; Fowlkes, J.D.; Lester, K.; Lewis, B.B.; Rack, P.D. Electron-Beam-Assisted Oxygen Purification at Low Temperatures for Electron-Beam-Induced Pt Deposits: Towards Pure and High-Fidelity Nanostructures. ACS Appl. Mater. Interfaces 2014, 6, 1018–1024. [Google Scholar] [CrossRef] [PubMed]
- Begun, E.; Dobrovolskiy, O.V.; Kompaniiets, M.; Sachser, R.; Gspan, C.; Plank, H.; Huth, M. Post-growth purification of Co nanostructures prepared by focused electron beam induced deposition. Nanotechnol. 2015, 26, 75301. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, M.V.P.; Barth, S.; Beron, F.; Pirota, K.R.; Pinto, A.L.; Sinnecker, J.P.; Moshkalev, S.; Diniz, J.A.; Utke, I.; Dos Santos, M.V.P. Magnetoelectrical Transport Improvements of Postgrowth Annealed Iron–Cobalt Nanocomposites: A Possible Route for Future Room-Temperature Spintronics. ACS Appl. Nano Mater. 2018, 1, 3364–3374. [Google Scholar] [CrossRef]
- Dos Santos, M.V.P.; Velo, M.F.; Domingos, R.D.; Zhang, Y.; Maeder, X.; Guerra-Nuñez, C.; Best, J.P.; Beron, F.; Pirota, K.R.; Moshkalev, S.; et al. Annealing-Based Electrical Tuning of Cobalt–Carbon Deposits Grown by Focused-Electron-Beam-Induced Deposition. ACS Appl. Mater. Interfaces 2016, 8, 32496–32503. [Google Scholar] [CrossRef] [PubMed]
- Stanford, M.G.; Lewis, B.B.; Noh, J.H.; Fowlkes, J.D.; Roberts, N.; Plank, H.; Rack, P.D. Purification of Nanoscale Electron-Beam-Induced Platinum Deposits via a Pulsed Laser-Induced Oxidation Reaction. ACS Appl. Mater. Interfaces 2014, 6, 21256–21263. [Google Scholar] [CrossRef]
- Shawrav, M.M.; Taus, P.; Wanzenböck, H.; Schinnerl, M.; Stöger-Pollach, M.; Schwarz, S.; Steiger-Thirsfeld, A.; Bertagnolli, E. Highly conductive and pure gold nanostructures grown by electron beam induced deposition. Sci. Rep. 2016, 6, 34003. [Google Scholar] [CrossRef]
- Jurczyk, J.; Brewer, C.; Hawkins, O.M.; Polyakov, M.; Kapusta, C.; McElwee-White, L.; Utke, I. Focused Electron Beam-Induced Deposition and Post-Growth Purification Using the Heteroleptic Ru Complex (η3-C3H5)Ru(CO)3Br. ACS Appl. Mater. Interfaces 2019, 11, 28164–28171. [Google Scholar] [CrossRef]
- Perentes, A.; Hoffmann, P. Focused Electron Beam Induced Deposition of Si-Based Materials From SiOxCy to Stoichiometric SiO2: Chemical Compositions, Chemical-Etch Rates, and Deep Ultraviolet Optical Transmissions. Chem. Vap. Depos. 2007, 13, 176–184. [Google Scholar] [CrossRef]
- Fowlkes, J.D.; Geier, B.; Lewis, B.B.; Rack, P.D.; Stanford, M.G.; Winkler, R.; Plank, H. Electron nanoprobe induced oxidation: a simulation of direct-write purification. Phys. Chem. Chem. Phys. 2015, 17, 18294–18304. [Google Scholar] [CrossRef]
- Okada, S.; Mukawa, T.; Kobayashi, R.; Ishida, M.; Ochiai, Y.; Kaito, T.; Matsui, S.; Fujita, J.-I. Comparison of Young’s Modulus Dependency on Beam Accelerating Voltage between Electron-Beam- and Focused Ion-Beam-Induced Chemical Vapor Deposition Pillars. Jpn. J. Appl. Phys. 2006, 45, 5556–5559. [Google Scholar] [CrossRef]
- Guo, D.; Warisawa, S.; Ishihara, S.; Kometani, R. Mechanical characteristics of ultra-long horizontal nanocantilevers grown by real-time feedback control on focused-ion-beam chemical vapour deposition. J. Micromechanics Microengineering 2015, 25, 125028. [Google Scholar] [CrossRef]
- Kometani, R.; Warisawa, S.; Ishihara, S. Non-Core–Shell Nanostructure Deposition on Focused-Ion-Beam Chemical Vapor Deposition. Jpn. J. Appl. Phys. 2010, 49, 6. [Google Scholar] [CrossRef]
- Kiuchi, M.; Matsui, S.; Isono, Y. The piezoresistance effect of FIB-deposited carbon nanowires under severe strain. J. Micromechanics Microengineering 2008, 18, 65011. [Google Scholar] [CrossRef]
- Igaki, J.-Y.; Kanda, K.; Haruyama, Y.; Ishida, M.; Ochiai, Y.; Fujita, J.-I.; Kaito, T.; Matsui, S. Comparison of FIB-CVD and EB-CVD growth characteristics. Microelectron. Eng. 2006, 83, 1225–1228. [Google Scholar] [CrossRef]
- Kim, C.-S.; Ahn, S.-H. Mechanical behavior of microscale carbon pillar fabricated by focused ion beam induced deposition. Int. J. Precis. Eng. Manuf. 2014, 15, 1485–1488. [Google Scholar] [CrossRef]
- Nonaka, K.; Tamaru, K.; Nagase, M.; Yamaguchi, H.; Warisawa, S.; Ishihara, S. Evaluation of Thermal–Mechanical Vibration Amplitude and Mechanical Properties of Carbon Nanopillars Using Scanning Electron Microscopy. Jpn. J. Appl. Phys. 2009, 48, 06FG07. [Google Scholar] [CrossRef]
- Nakamatsu, K.-I.; Ichihashi, T.; Kanda, K.; Haruyama, Y.; Kaito, T.; Matsui, S. Nanostructure Analysis of Nanosprings Fabricated by Focused-Ion-Beam Chemical Vapor Deposition. Jpn. J. Appl. Phys. 2009, 48, 105001. [Google Scholar] [CrossRef]
- Fujita, J.-I.; Okada, S.; Ueki, R.; Ishida, M.; Kaito, T.; Matsui, S. Elastic Double Structure of Amorphous Carbon Pillar Grown by Focused-Ion-Beam Chemical Vapor Deposition. Jpn. J. Appl. Phys. 2007, 46, 6286–6289. [Google Scholar] [CrossRef]
- Ding, W.; Dikin, D.A.; Chen, X.; Piner, R.D.; Ruoff, R.S.; Zussman, E.; Wang, X.; Li, X. Mechanics of hydrogenated amorphous carbon deposits from electron-beam-induced deposition of a paraffin precursor. J. Appl. Phys. 2005, 98, 14905. [Google Scholar] [CrossRef]
- Friedli, V.; Santschi, C.; Michler, J.; Hoffmann, P.; Utke, I. Mass sensor for in situ monitoring of focused ion and electron beam induced processes. Appl. Phys. Lett. 2007, 90, 053106. [Google Scholar] [CrossRef]
- Arnold, G.; Winkler, R.; Stermitz, M.; Orthacker, A.; Noh, J.-H.; Fowlkes, J.D.; Kothleitner, G.; Huth, M.; Rack, P.D.; Plank, H. Tunable 3D Nanoresonators for Gas-Sensing Applications. Adv. Funct. Mater. 2018, 28, 1707387. [Google Scholar] [CrossRef]
- Lewis, B.B.; Srijanto, B.R.; Fowlkes, J.D.; Pharr, G.M.; Rack, P.D.; Mound, B. Growth and nanomechanical characterization of nanoscale 3D architectures grown via focused electron beam induced deposition. Nanoscale 2017, 9, 16349–16356. [Google Scholar] [CrossRef] [PubMed]
- Reiser, A.; Koch, L.; Dunn, K.A.; Matsuura, T.; Iwata, F.; Fogel, O.; Kotler, Z.; Zhou, N.; Charipar, K.; Pique, A.; et al. Metals by micro-scale additive manufacturing: comparison of microstructure and mechanical properties. arXiv 2020, arXiv:1912.03581v1. [Google Scholar]
- Langford, R.; Wang, T.-X.; Ozkaya, D. Reducing the resistivity of electron and ion beam assisted deposited Pt. Microelectron. Eng. 2007, 84, 784–788. [Google Scholar] [CrossRef]
- Córdoba, R.; Lorenzoni, M.; Pablo-Navarro, J.; Dominguez, C.M.; Perez-Murano, F.; De Teresa, J.M. Suspended tungsten-based nanowires with enhanced mechanical properties grown by focused ion beam induced deposition. Nanotechnol. 2017, 28, 445301. [Google Scholar] [CrossRef] [PubMed]
- Wich, T.; Luttermann, T.; Mircea, I. Hardness determination of EBiD-layers containing tungsten and cobalt. DEBRIS FLOW 08 2007, 57, 73. [Google Scholar]
- Friedli, V.; Utke, I.; Mølhave, K.; Michler, J. Dose and energy dependence of mechanical properties of focused electron-beam-induced pillar deposits from Cu(C5HF6O2)2. Nanotechnol. 2009, 20, 385304. [Google Scholar] [CrossRef] [PubMed]
- Szkudlarek, A.; Vaz, A.R.; Zhang, Y.; Rudkowski, A.; Kapusta, C.; Erni, R.; Moshkalev, S.; Utke, I. Formation of pure Cu nanocrystals upon post-growth annealing of Cu–C material obtained from focused electron beam induced deposition: comparison of different methods. Beilstein J. Nanotechnol. 2015, 6, 1508–1517. [Google Scholar] [CrossRef]
- Utke, I.; Friedli, V.; Michler, J.; Bret, T.; Multone, X.; Hoffmann, P. Density determination of focused-electron-beam-induced deposits with simple cantilever-based method. Appl. Phys. Lett. 2006, 88, 031906. [Google Scholar] [CrossRef]
- Luisier, A.; Utke, I.; Bret, T.; Cicoira, F.; Hauert, R.; Rhee, S.-W.; Doppelt, P.; Hoffmann, P. Comparative Study of Cu Precursors for 3D Focused Electron Beam Induced Deposition. J. Electrochem. Soc. 2004, 151, C535–C537. [Google Scholar] [CrossRef][Green Version]
- Utke, I.; Luisier, A.; Hoffmann, P.; Laub, D.; Buffat, P.A. Focused-electron-beam-induced deposition of freestanding three-dimensional nanostructures of pure coalesced copper crystals. Appl. Phys. Lett. 2002, 81, 3245–3247. [Google Scholar] [CrossRef]
- Della Ratta, A.D. Focused-ion beam induced deposition of copper. J. Vac. Sci. Technol. B: Microelectron. Nanometer Struct. 1993, 11, 2195. [Google Scholar] [CrossRef]
- Nakai, Y.; Kang, Y.; Okada, M.; Haruyama, Y.; Kanda, K.; Ichihashi, T.; Matsui, S. Mechanical Characteristics of Nanosprings Fabricated by Focused-Ion-Beam Chemical Vapor Deposition Using Ferrocene Source Gas. Jpn. J. Appl. Phys. 2010, 49, 6. [Google Scholar] [CrossRef]
- Lukasczyk, T.; Schirmer, M.; Steinrück, H.-P.; Marbach, H. Electron-Beam-Induced Deposition in Ultrahigh Vacuum: Lithographic Fabrication of Clean Iron Nanostructures. Small 2008, 4, 841–846. [Google Scholar] [CrossRef] [PubMed]
- Gavagnin, M.; Wanzenböck, H.; Shawrav, M.M.; Belić, D.; Wachter, S.; Waid, S.; Stöger-Pollach, M.; Bertagnolli, E. Focused Electron Beam-Induced CVD of Iron: a Practical Guide for Direct Writing. Chem. Vap. Depos. 2014, 20, 243–250. [Google Scholar] [CrossRef]
- Reyntjens, S.; Puers, R. Focused ion beam induced deposition: fabrication of three-dimensional microstructures and Young’s modulus of the deposited material. J. Micromechanics Microengineering 2000, 10, 181–188. [Google Scholar] [CrossRef]
- Komano, H.; Nakamura, H.; Kariya, M.; Ogasawara, M. Silicon oxide deposition using a gallium-focused ion beam. SPIE’s 1996 International Symposium on Microlithography 1996, 2723, 46–53. [Google Scholar]
- Cicoira, F.; Leifer, K.; Hoffmann, P.; Utke, I.; Dwir, B.; Laub, D.; Buffat, P.; Kapon, E.; Doppelt, P. Electron beam induced deposition of rhodium from the precursor [RhCl(PF3)2]2: morphology, structure and chemical composition. J. Cryst. Growth 2004, 265, 619–626. [Google Scholar] [CrossRef]
- Winkler, R.; Fowlkes, J.D.; Rack, P.D.; Plank, H. 3D nanoprinting via focused electron beams. J. Appl. Phys. 2019, 125, 210901. [Google Scholar] [CrossRef]
- Demers, H.; Poirier-Demers, N.; Couture, A.R.; Joly, D.; Guilmain, M.; De Jonge, N.; Drouin, D. Three-dimensional electron microscopy simulation with the CASINO Monte Carlo software. Scanning 2011, 33, 135–146. [Google Scholar] [CrossRef]
- Plank, H.; Winkler, R.; Schwalb, C.H.; Hütner, J.; Fowlkes, J.D.; Rack, P.D.; Utke, I.; Huth, M. Focused Electron Beam-Based 3D Nanoprinting for Scanning Probe Microscopy: A Review. Micromachines 2019, 11, 48. [Google Scholar] [CrossRef] [PubMed]
- Wendel, M.; Lorenz, H.; Kotthaus, J.P. Sharpened electron beam deposited tips for high resolution atomic force microscope lithography and imaging. Appl. Phys. Lett. 1995, 67, 3732–3734. [Google Scholar] [CrossRef]
- Matsui, S.; Kometani, R. Three-Dimensional Nanostructure Fabrication by Focused-Ion-Beam Chemical Vapor Deposition and Its Applications. IEICE Trans. Electron. 2007, E90C, 25–35. [Google Scholar] [CrossRef]
- Bethe, H. Zur Theorie des Durchgangs schneller Korpuskularstrahlen durch Materie. Ann. der Phys. 1930, 397, 325–400. [Google Scholar] [CrossRef]
- Reimer, L.; Reimer, P.D.L. Scanning Electron Microscopy; Springer Science and Business Media LLC, 1998; Vol. 45; In Scanning Electron Microscopy; Springer Science and Business Media LLC: Berlin, Germany, 1998; Volume 45. [Google Scholar]
- Bullen, A.J.; O’Hara, K.E.; Cahill, D.G.; Monteiro, O.; Von Keudell, A. Thermal conductivity of amorphous carbon thin films. J. Appl. Phys. 2000, 88, 6317–6320. [Google Scholar] [CrossRef]
- Winkler, R.; Lewis, B.B.; Fowlkes, J.D.; Rack, P.D.; Plank, H. High-Fidelity 3D-Nanoprinting via Focused Electron Beams: Growth Fundamentals. ACS Appl. Nano Mater. 2018, 1, 1014–1027. [Google Scholar] [CrossRef]
- Fowlkes, J.D.; Winkler, R.; Lewis, B.B.; Fernandez-Pacheco, A.; Skoric, L.; Sanz-Hernández, D.; Stanford, M.G.; Mutunga, E.; Rack, P.D.; Plank, H. High-Fidelity 3D-Nanoprinting via Focused Electron Beams: Computer-Aided Design (3BID). ACS Appl. Nano Mater. 2018, 1, 1028–1041. [Google Scholar] [CrossRef]
- Winkler, R.; Schmidt, F.-P.; Haselmann, U.; Fowlkes, J.D.; Lewis, B.B.; Kothleitner, G.; Rack, P.D.; Plank, H. Direct-Write 3D Nanoprinting of Plasmonic Structures. ACS Appl. Mater. Interfaces 2016, 9, 8233–8240. [Google Scholar] [CrossRef]
- Mutunga, E.; Winkler, R.; Sattelkow, J.; Rack, P.D.; Plank, H.; Fowlkes, J.D. Impact of Electron-Beam Heating during 3D Nanoprinting. ACS Nano 2019, 13, 5198–5213. [Google Scholar] [CrossRef]
- Fowlkes, J.D.; Winkler, R.; Mutunga, E.; Rack, P.D.; Plank, H. Simulation Informed CAD for 3D Nanoprinting. Micromachines 2019, 11, 8. [Google Scholar] [CrossRef]
- Keller, L.; Al Mamoori, M.K.I.; Pieper, J.; Gspan, C.; Stockem, I.; Schröder, C.; Barth, S.; Winkler, R.; Plank, H.; Pohlit, M.; et al. Direct-write of free-form building blocks for artificial magnetic 3D lattices. Sci. Rep. 2018, 8, 6160. [Google Scholar] [CrossRef] [PubMed]
- Toth, M.; Lobo, C.; Friedli, V.; Szkudlarek, A.; Utke, I. Continuum models of focused electron beam induced processing. Beilstein J. Nanotechnol. 2015, 6, 1518–1540. [Google Scholar] [CrossRef] [PubMed]
- Fowlkes, J.D.; Randolph, S.J.; Rack, P.D. Growth and simulation of high-aspect ratio nanopillars by primary and secondary electron-induced deposition. J. Vac. Sci. Technol. B: Microelectron. Nanometer Struct. 2005, 23, 2825. [Google Scholar] [CrossRef]
- Timoshenko, S.P.; Goodier, J.N. Theory of Elasticity, 3rd ed. (23rd print ed.); McGraw-Hill Book: Auckland, New Zealand, 1988. [Google Scholar]
- Timoshenko, S.P.; Young, D.H.; Weaver, W. Vibration Problems in Engineering, 4th ed.; Wiley: New York, NY, USA, 1974. [Google Scholar]
- Timoshenko, S.P.; Gere, J.M.; Prager, W. Theory of Elastic Stability, Second Edition. J. Appl. Mech. 1962, 29, 220–221. [Google Scholar] [CrossRef]
- Gere, G.M.; Goodno, B.J. Mechanics of Materials, 8th ed.; Cengage Learning: Boston, MA, USA, 2012. [Google Scholar]
- Friedli, V.; Hoffmann, S.; Michler, J.; Utke, I. AFM Sensors in Scanning Electron and Ion Microscopes: Tools for Nanomechanics, Nanoanalytics, and Nanofabrication. In Nano Science and Technolgy; Springer Science and Business Media LLC: Berlin, Germany, 2007; pp. 247–287. [Google Scholar]
- Igaki, J.-Y.; Nakamatsu, K.-I.; Kanda, K.; Haruyama, Y.; Kometani, R.; Kaito, T.; Matsui, S. Mechanical characteristics and applications of diamondlike-carbon cantilevers fabricated by focused-ion-beam chemical vapor deposition. J. Vac. Sci. Technol. B: Microelectron. Nanometer Struct. 2006, 24, 2911. [Google Scholar] [CrossRef]
- Nonaka, K.; Tamaru, K.; Nagase, M.; Yamaguchi, H.; Warisawa, S.; Ishihara, S. Height Dependence of Young’s Modulus for Carbon Nanopillars Grown by Focused-Ion-Beam-Induced Chemical Vapor Deposition. Jpn. J. Appl. Phys. 2008, 47, 5116–5119. [Google Scholar] [CrossRef]
- Oliver, W.; Pharr, G. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- Hoffmann, S.; Utke, I.; Moser, B.; Michler, J.; Christiansen, S.; Schmidt, V.; Senz, S.; Werner, P.; Gösele, U.; Ballif, C. Measurement of the Bending Strength of Vapor−Liquid−Solid Grown Silicon Nanowires. Nano Lett. 2006, 6, 622–625. [Google Scholar] [CrossRef]
- Hao, T.; Shen, T.H.; Li, W.; Song, C.; Xu, Z.; Jin, A.; Jin, L.; Li, J.; Bai, X.; Gu, C. Platinum composite nanowires for ultrasensitive mass detection. Appl. Phys. Lett. 2017, 110, 143102. [Google Scholar] [CrossRef]
- Jacob, W.; Möller, W. On the structure of thin hydrocarbon films. Appl. Phys. Lett. 1993, 63, 1771–1773. [Google Scholar] [CrossRef]
- Robertson, J. Diamond-like amorphous carbon. Mater. Sci. Eng. R: Rep. 2002, 37, 129–281. [Google Scholar] [CrossRef]
- Bret, T.; Mauron, S.; Utke, I.; Hoffmann, P. Characterization of focused electron beam induced carbon deposits from organic precursors. Microelectron. Eng. 2005, 78, 300–306. [Google Scholar] [CrossRef]
- Hoole, A.C.F.; E Welland, M.; Broers, A.N. Negative PMMA as a high-resolution resist - the limits and possibilities. Semicond. Sci. Technol. 1997, 12, 1166–1170. [Google Scholar] [CrossRef]
- Porrati, F.; Sachser, R.; Schwalb, C.H.; Frangakis, A.S.; Huth, M. Tuning the electrical conductivity of Pt-containing granular metals by postgrowth electron irradiation. J. Appl. Phys. 2011, 109, 63715. [Google Scholar] [CrossRef]
- Battu, A.K.; Ramana, C.V. Mechanical Properties of Nanocrystalline and Amorphous Gallium Oxide Thin Films. Adv. Eng. Mater. 2018, 20, 1701033. [Google Scholar] [CrossRef]
- Gallium Wikipedia. Available online: https://en.wikipedia.org/wiki/Gallium (accessed on 28 March 2020).
- Klein, C.A.; Cardinale, G.F. Young’s modulus and Poisson’s ratio of CVD diamond. Diam. Relat. Mater. 1993, 2, 918–923. [Google Scholar] [CrossRef]
- Jänchen, G.; Hoffmann, P.; Kriele, A.; Lorenz, H.; Kulik, A.J.; Dietler, G. Mechanical properties of high-aspect-ratio atomic-force microscope tips. Appl. Phys. Lett. 2002, 80, 4623–4625. [Google Scholar] [CrossRef]
- Ishida, M.; Fujita, J.; Ichihashi, T.; Ochiai, Y.; Kaito, T.; Matsui, S. Focused ion beam-induced fabrication of tungsten structures. J. Vac. Sci. Technol. B: Microelectron. Nanometer Struct. 2003, 21, 2728. [Google Scholar] [CrossRef]
- Winkler, R.; Fowlkes, J.; Szkudlarek, A.; Utke, I.; Rack, P.D.; Plank, H. The Nanoscale Implications of a Molecular Gas Beam during Electron Beam Induced Deposition. ACS Appl. Mater. Interfaces 2014, 6, 2987–2995. [Google Scholar] [CrossRef]
- Trummer, C.; Winkler, R.; Plank, H.; Kothleitner, G.; Haberfehlner, G. Analyzing the Nanogranularity of Focused-Electron-Beam-Induced-Deposited Materials by Electron Tomography. ACS Appl. Nano Mater. 2019, 2, 5356–5359. [Google Scholar] [CrossRef]
- Utke, I.; Michler, J.; Gasser, P.; Santschi, C.; Laub, D.; Cantoni, M.; Buffat, P.A.; Jiao, C.; Hoffmann, P. Cross Section Investigations of Compositions and Sub-Structures of Tips Obtained by Focused Electron Beam Induced Deposition. Adv. Eng. Mater. 2005, 7, 323–331. [Google Scholar] [CrossRef]
- Gerstl, S.; Morrone, A.; Kvitek, R. Three-Dimensional Nanoscale Characterization of Pt Deposition from an Organometallic Precursor Induced by a Focused Ion Beam. Microsc. Microanal. 2006, 12, 1252–1253. [Google Scholar] [CrossRef][Green Version]
- Köster, W.; Franz, H. POISSON’S RATIO FOR METALS AND ALLOYS. Met. Rev. 1961, 6, 1–56. [Google Scholar] [CrossRef]
- Nakamatsu, K.; Igaki, J.; Nagase, M.; Ichihashi, T.; Matsui, S. Mechanical characteristics of tungsten-containing carbon nanosprings grown by FIB-CVD. Microelectron. Eng. 2006, 83, 808–810. [Google Scholar] [CrossRef]
- Hashin, Z.; Shtrikman, S. A variational approach to the theory of the elastic behaviour of multiphase materials. J. Mech. Phys. Solids 1963, 11, 127–140. [Google Scholar] [CrossRef]
- Maughan, M.; Zbib, H.; Bahr, D.F. Variation in the nanoindentation hardness of platinum. J. Mater. Res. 2013, 28, 2819–2828. [Google Scholar] [CrossRef]
- Durst, K.; Backes, B.; Franke, O.; Göken, M. Indentation size effect in metallic materials: Modeling strength from pop-in to macroscopic hardness using geometrically necessary dislocations. Acta Mater. 2006, 54, 2547–2555. [Google Scholar] [CrossRef]
- Graca, S.; Colaço, R.; Vilar, R. Indentation size effect in nickel and cobalt laser clad coatings. Surf. Coatings Technol. 2007, 202, 538–548. [Google Scholar] [CrossRef]
- Grashchenko, A.S.; Kukushkin, S.A.; Nikolaev, V.I.; Osipov, A.V.; Osipova, E.V.; Soshnikov, I.P. Study of the Anisotropic Elastoplastic Properties of β-Ga2O3 Films Synthesized on SiC/Si Substrates. Phys. Solid State 2018, 60, 852–857. [Google Scholar] [CrossRef]
- Kleber, R.; Dworschak, W.; Gerber, J.; Fuchs, A.; Putz, T.; Scherer, J.; Jung, K.; Ehrhardt, H. Mechanical properties of hard a-C:H films. Vac. 1990, 41, 1378–1380. [Google Scholar] [CrossRef]
- Jee, A.-Y.; Lee, M. Comparative analysis on the nanoindentation of polymers using atomic force microscopy. Polym. Test. 2010, 29, 95–99. [Google Scholar] [CrossRef]
- Xu, S.; Flynn, D.; Tay, B.K.; Prawer, S.; Nugent, K.W.; Silva, S.R.P.; Lifshitz, Y.; Milne, W.I. Mechanical properties and Raman spectra of tetrahedral amorphous carbon films with high sp3 fraction deposited using a filtered cathodic arc. Philos. Mag. B 1997, 76, 351–361. [Google Scholar] [CrossRef]
- Friedmann, T.A.; Mccarty, K.; Barbour, J.C.; Siegal, M.P.; Dibble, D.C. Thermal stability of amorphous carbon films grown by pulsed laser deposition. Appl. Phys. Lett. 1996, 68, 1643–1645. [Google Scholar] [CrossRef]
- Tao, Y.; Degen, C.L. Facile Fabrication of Single-Crystal-Diamond Nanostructures with Ultrahigh Aspect Ratio. Adv. Mater. 2013, 25, 3962–3967. [Google Scholar] [CrossRef] [PubMed]
- Najar, H.; Chan, M.-L.; Yang, H.-A.; Lin, L.; Cahill, D.G.; Horsley, D.A. High quality factor nanocrystalline diamond micromechanical resonators limited by thermoelastic damping. Appl. Phys. Lett. 2014, 104, 151903. [Google Scholar] [CrossRef]
- Zhang, X.; Zhong, L.; Mateos, A.; Kudo, A.; Vyatskikh, A.; Gao, H.; Greer, J.R.; Li, X. Theoretical strength and rubber-like behaviour in micro-sized pyrolytic carbon. Nat. Nanotechnol. 2019, 14, 762–769. [Google Scholar] [CrossRef]
- Albiez, A.; Schwaiger, R. Size Effect on the Strength and Deformation Behavior of Glassy Carbon Nanopillars. MRS Adv. 2019, 4, 133–138. [Google Scholar] [CrossRef]
- Wheeler, J.M.; Raghavan, R.; Wehrs, J.; Zhang, Y.; Erni, R.; Michler, J. Approaching the Limits of Strength: Measuring the Uniaxial Compressive Strength of Diamond at Small Scales. Nano Lett. 2015, 16, 812–816. [Google Scholar] [CrossRef]
- Novel Metal Electrodeposition and the Recent Application. In Novel Metal Electrodeposition and the Recent Application; IntechOpen, 2019; p. 10 5772 73382.
- Mohanty, G.; Wheeler, J.M.; Raghavan, R.; Wehrs, J.; Hasegawa, M.; Mischler, S.; Philippe, L.; Michler, J. Elevated temperature, strain rate jump microcompression of nanocrystalline nickel. Philos. Mag. 2014, 95, 1–18. [Google Scholar] [CrossRef]
- Naik, S.N.; Walley, S.M. The Hall–Petch and inverse Hall–Petch relations and the hardness of nanocrystalline metals. J. Mater. Sci. 2019, 55, 2661–2681. [Google Scholar] [CrossRef]
- Tabor, D. The hardness of solids. Rev. Phys. Technol. 1970, 1, 145–179. [Google Scholar] [CrossRef]
- Fowlkes, J.D.; Winkler, R.; Lewis, B.B.; Stanford, M.G.; Plank, H.; Rack, P.D. Simulation-Guided 3D Nanomanufacturing via Focused Electron Beam Induced Deposition. ACS Nano 2016, 10, 6163–6172. [Google Scholar] [CrossRef] [PubMed]
- Ashby, M.F.; Cebon, D. Materials Selection in Mechanical Design; EDP Sciences: Julius, France, 1993; Volume 3, pp. C7-1–C7-9. [Google Scholar]
- Bauer, J.; Schröer, A.; Schwaiger, R.; Kraft, O. Approaching theoretical strength in glassy carbon nanolattices. Nat. Mater. 2016, 15, 438–443. [Google Scholar] [CrossRef]
- Sattelkow, J.; Fröch, J.E.; Winkler, R.; Hummel, S.; Schwalb, C.H.; Plank, H. Three-Dimensional Nanothermistors for Thermal Probing. ACS Appl. Mater. Interfaces 2019, 11, 22655–22667. [Google Scholar] [CrossRef] [PubMed]
- Bond dissociation energy Wikipedia. Available online: https://en.wikipedia.org/wiki/Bond-dissociation_energy (accessed on 28 March 2020).

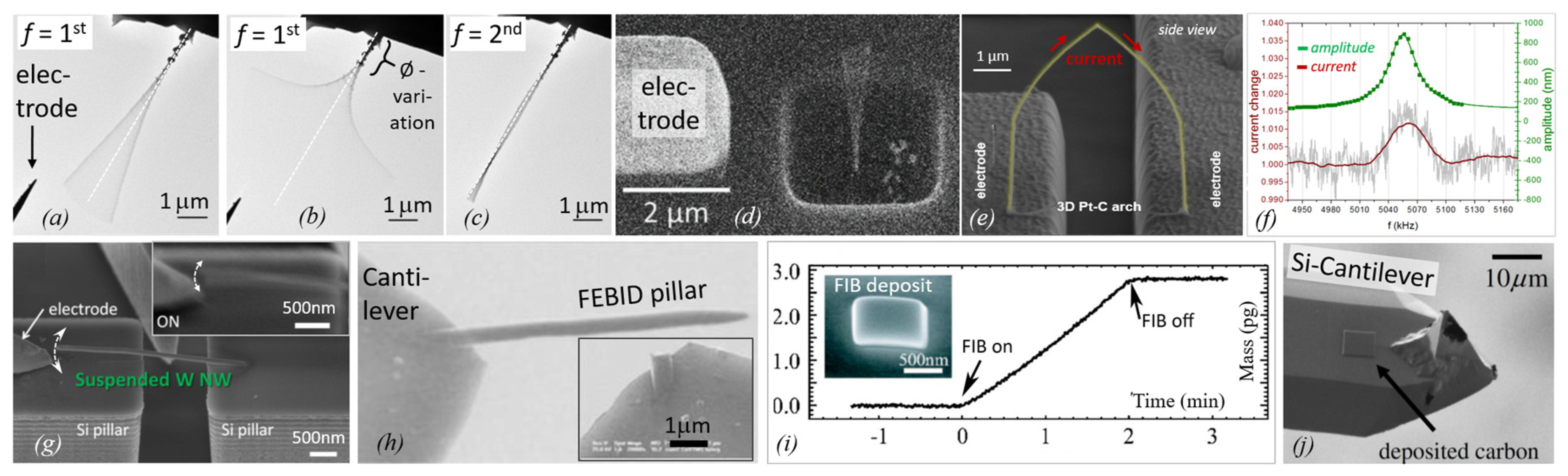
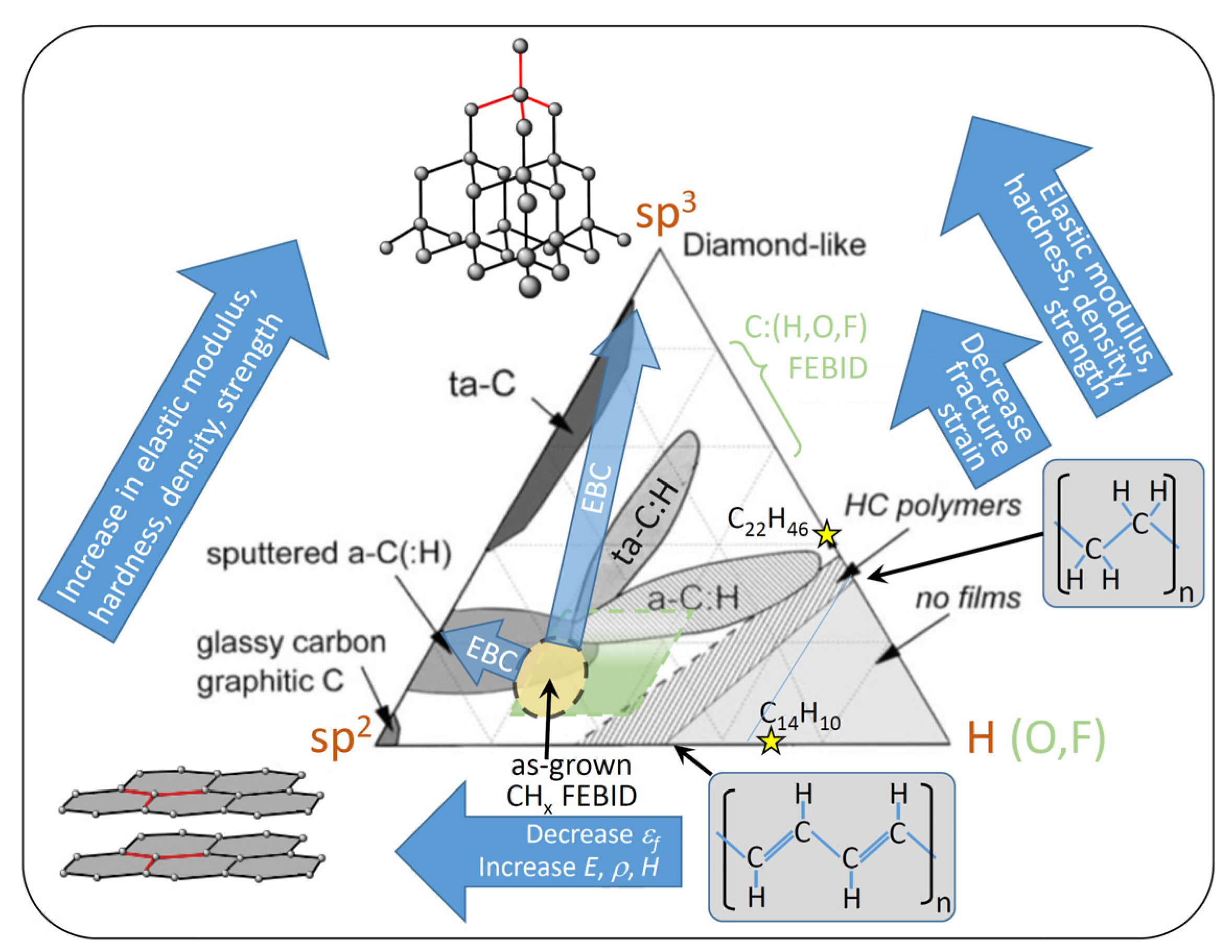
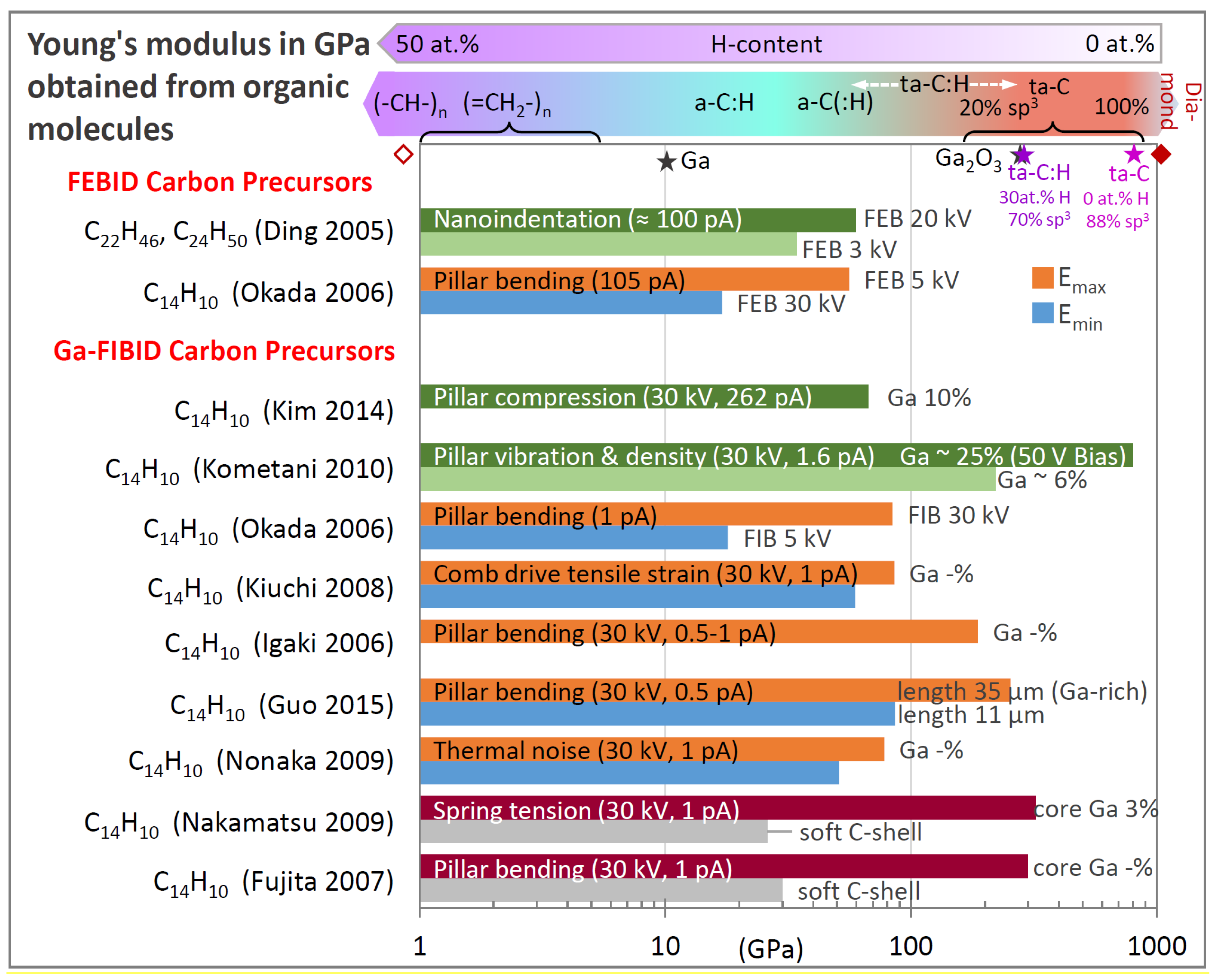
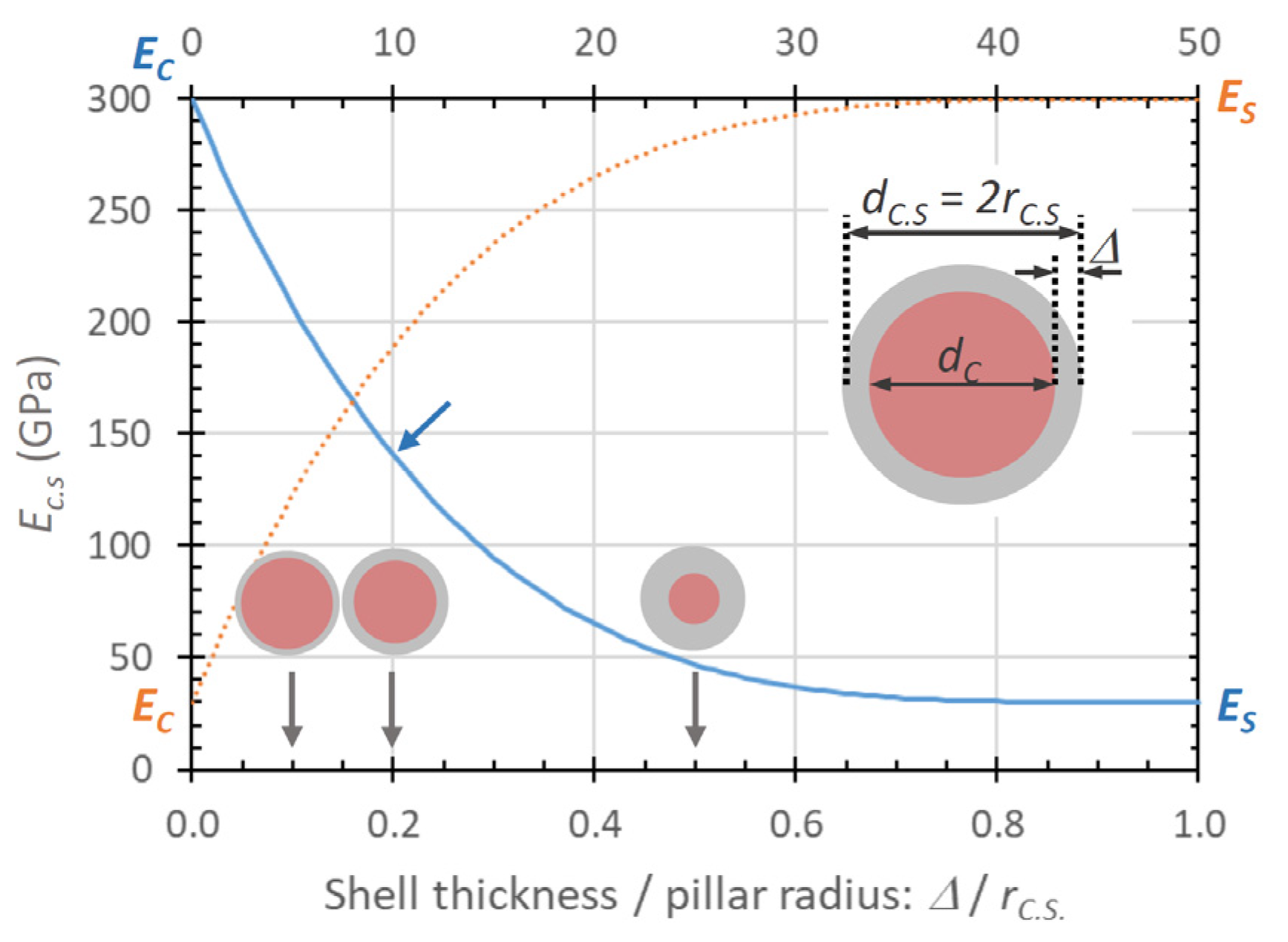
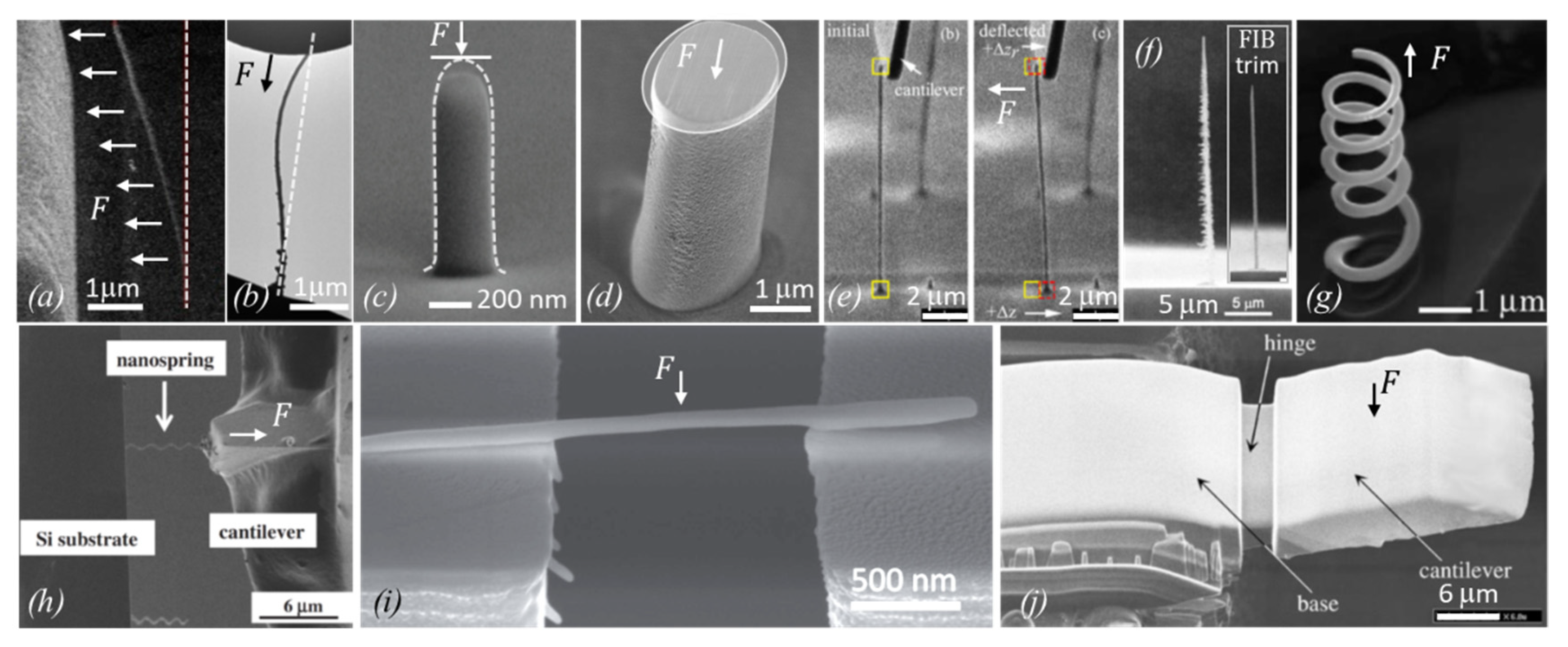
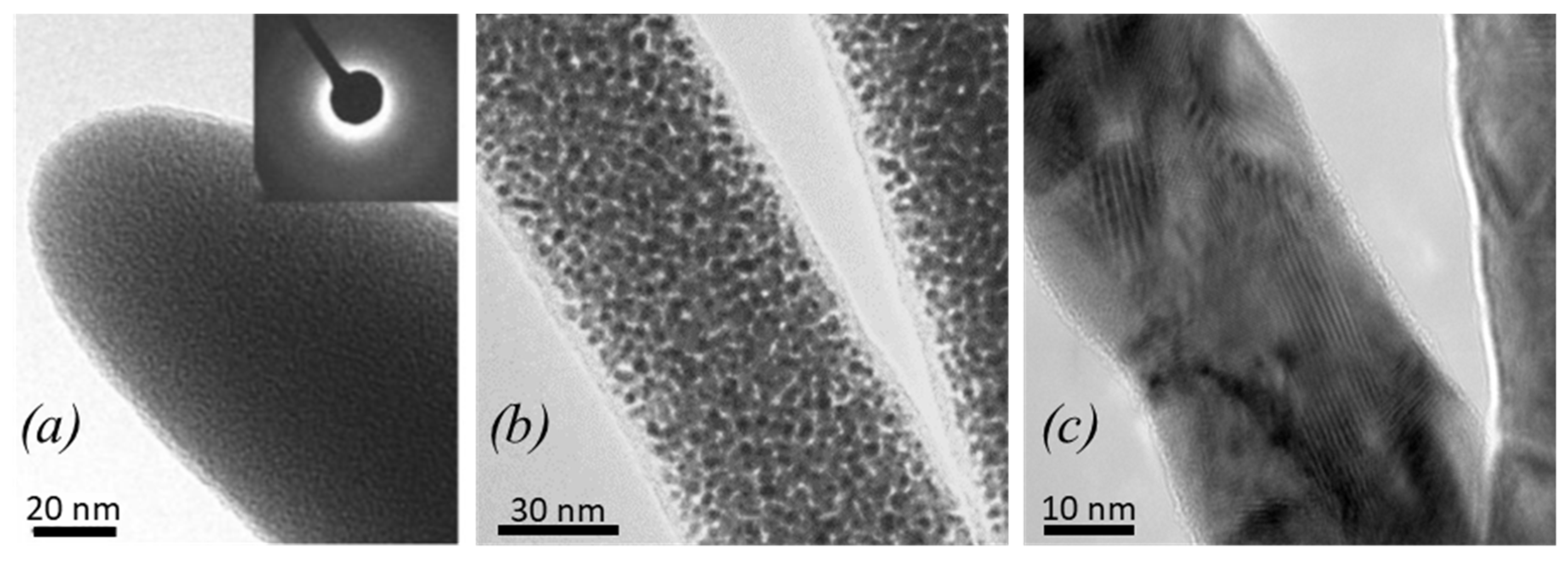
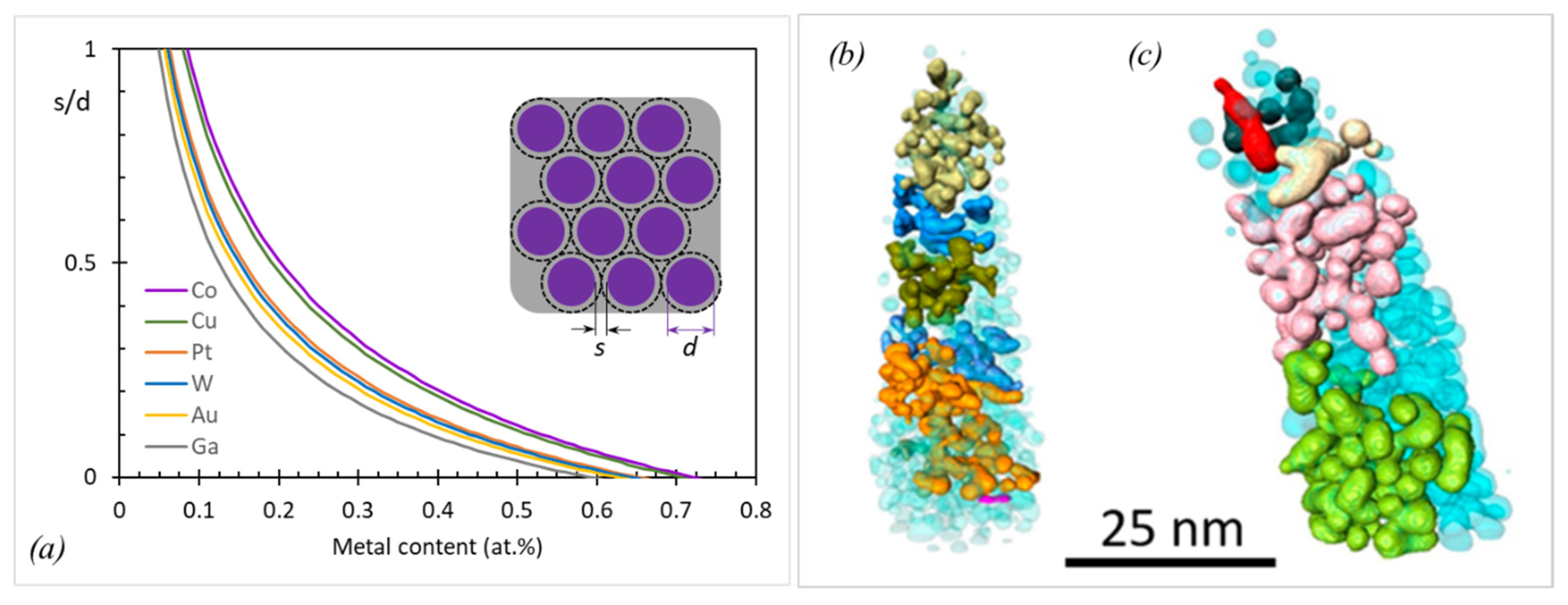
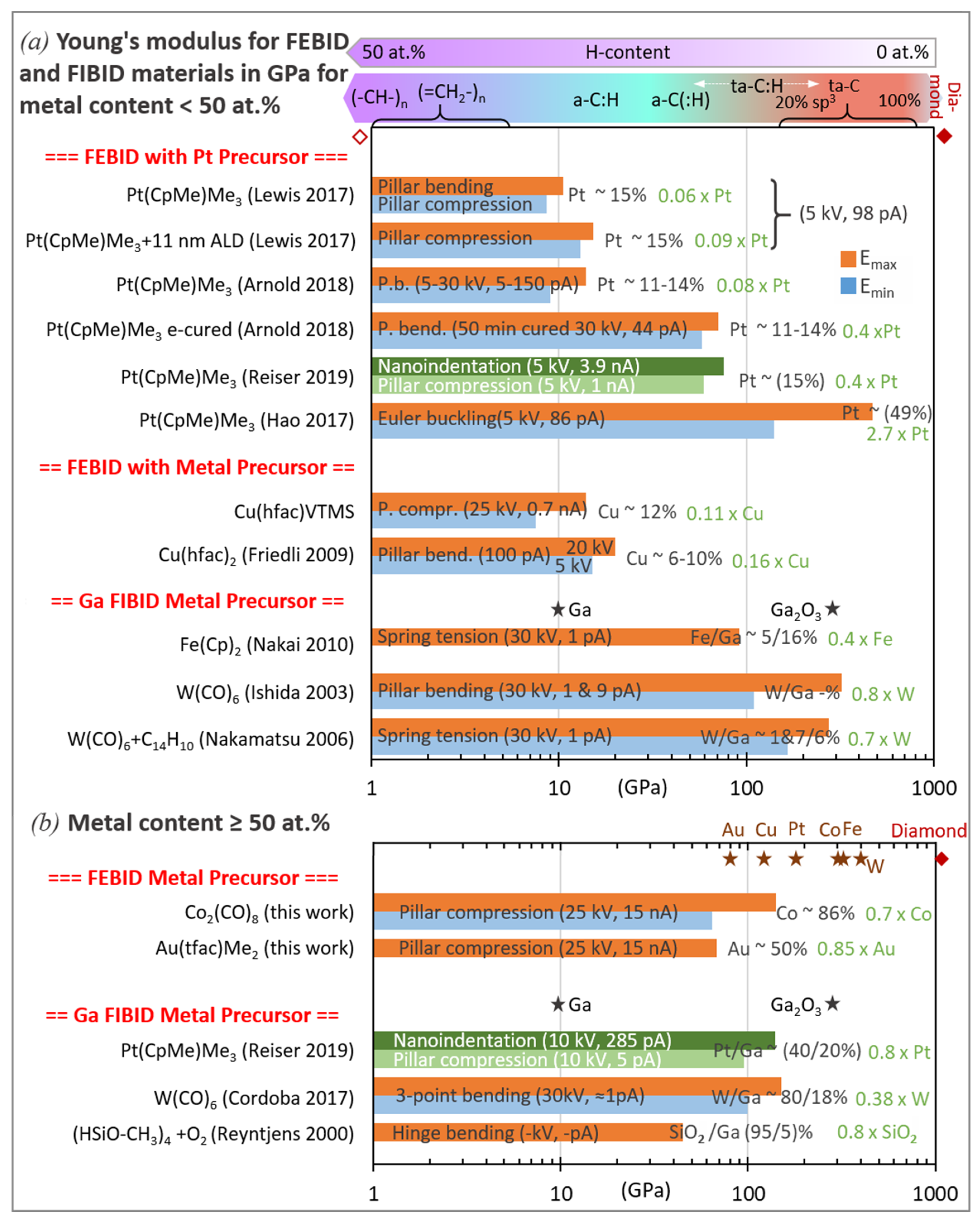
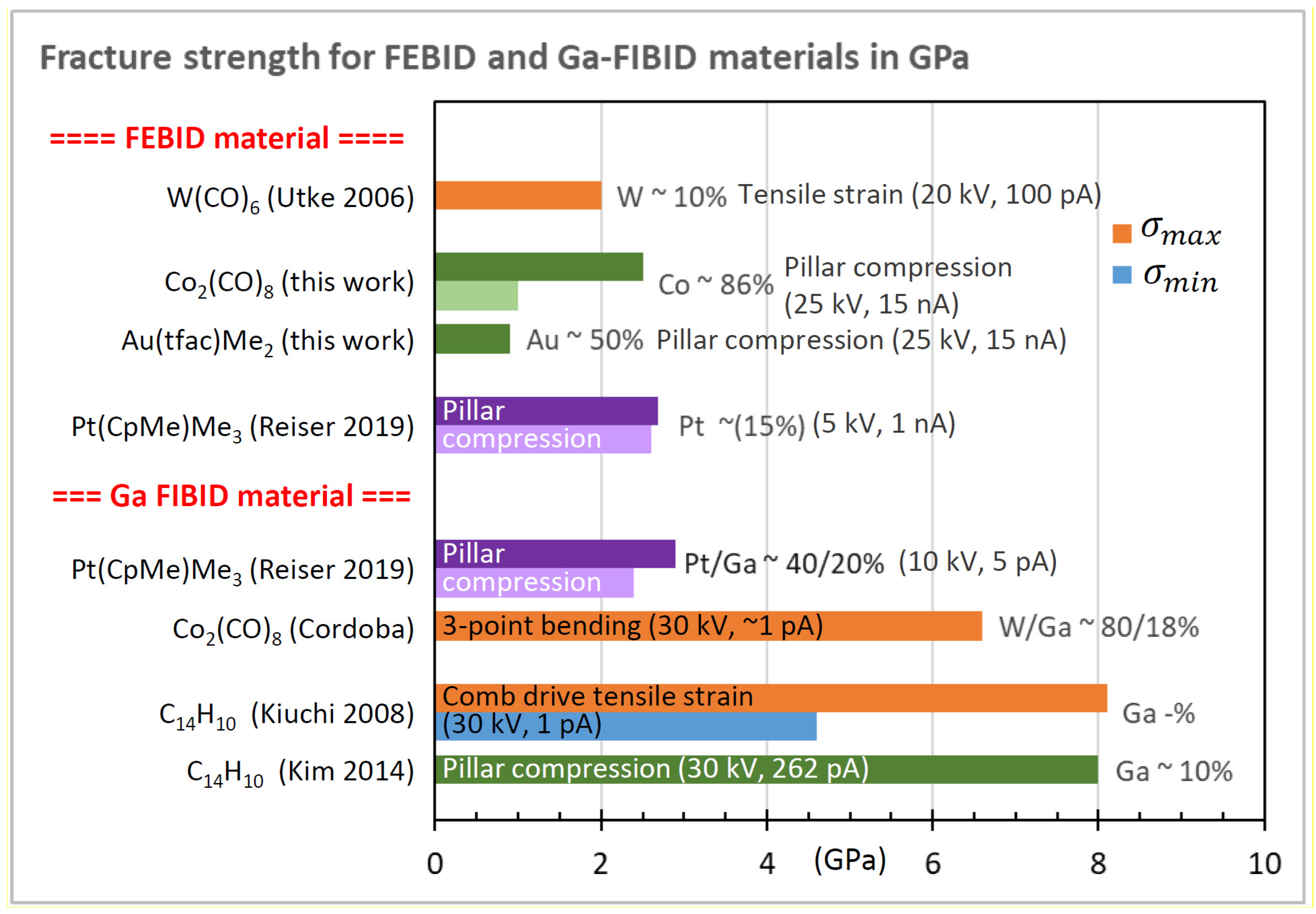
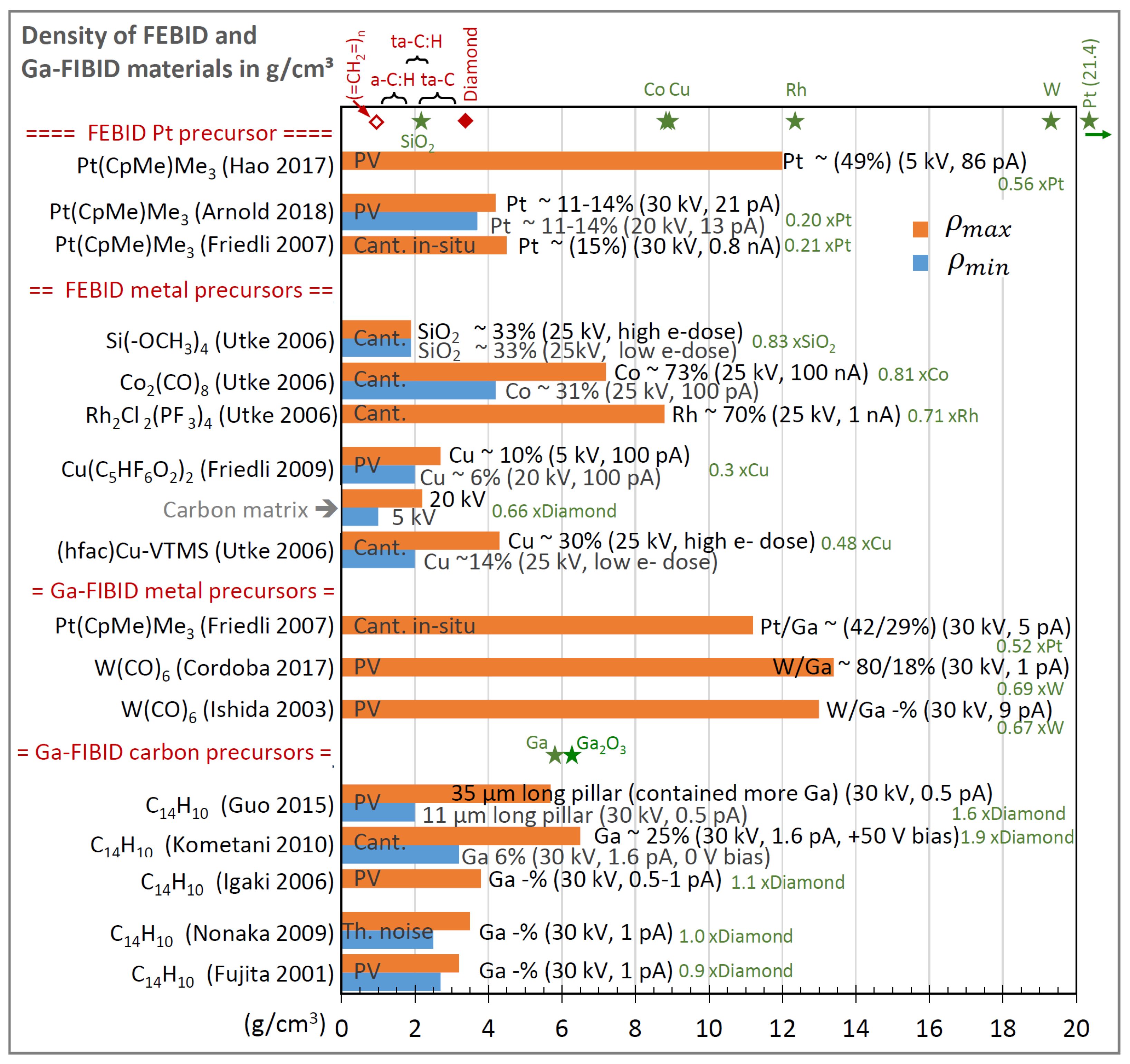
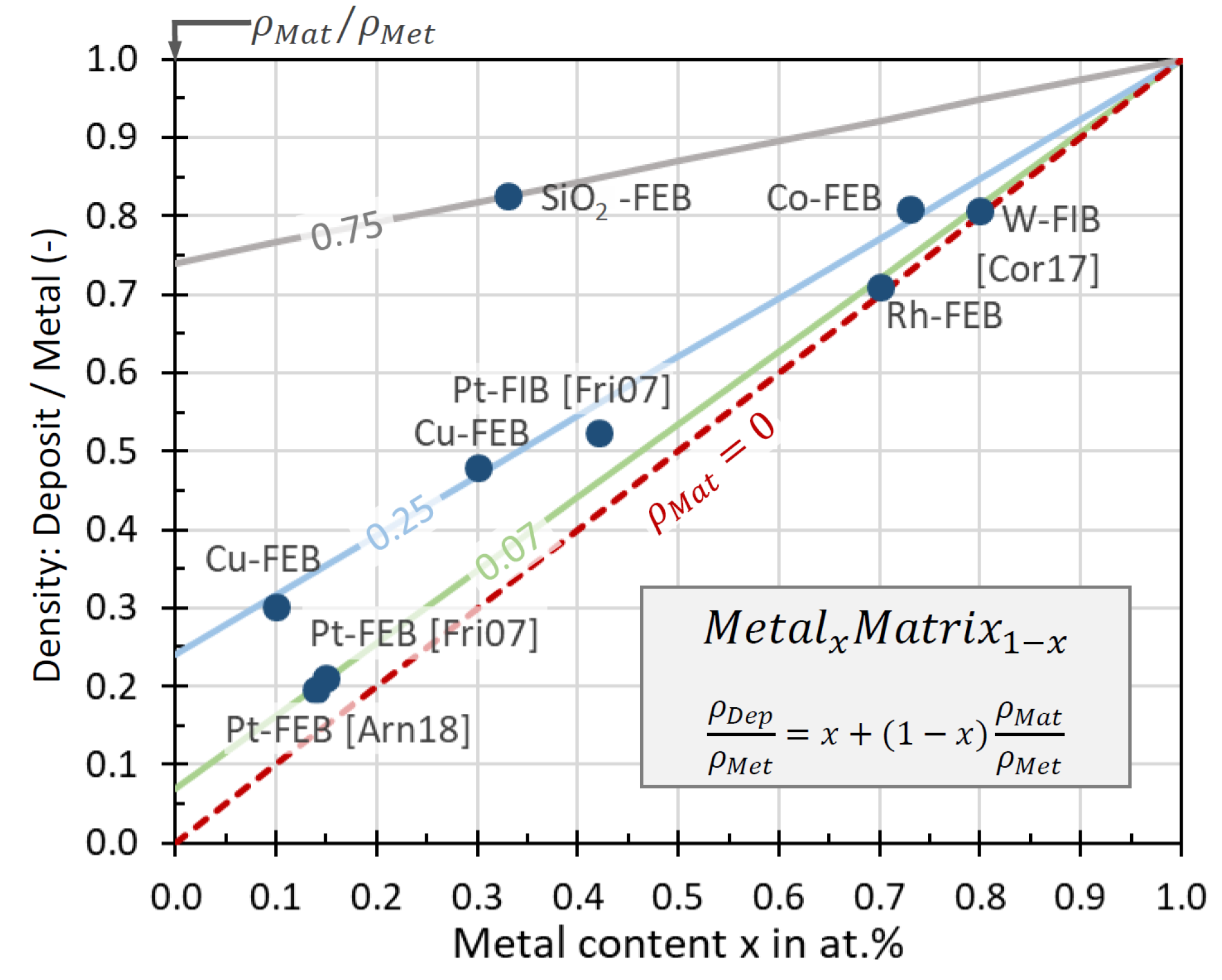
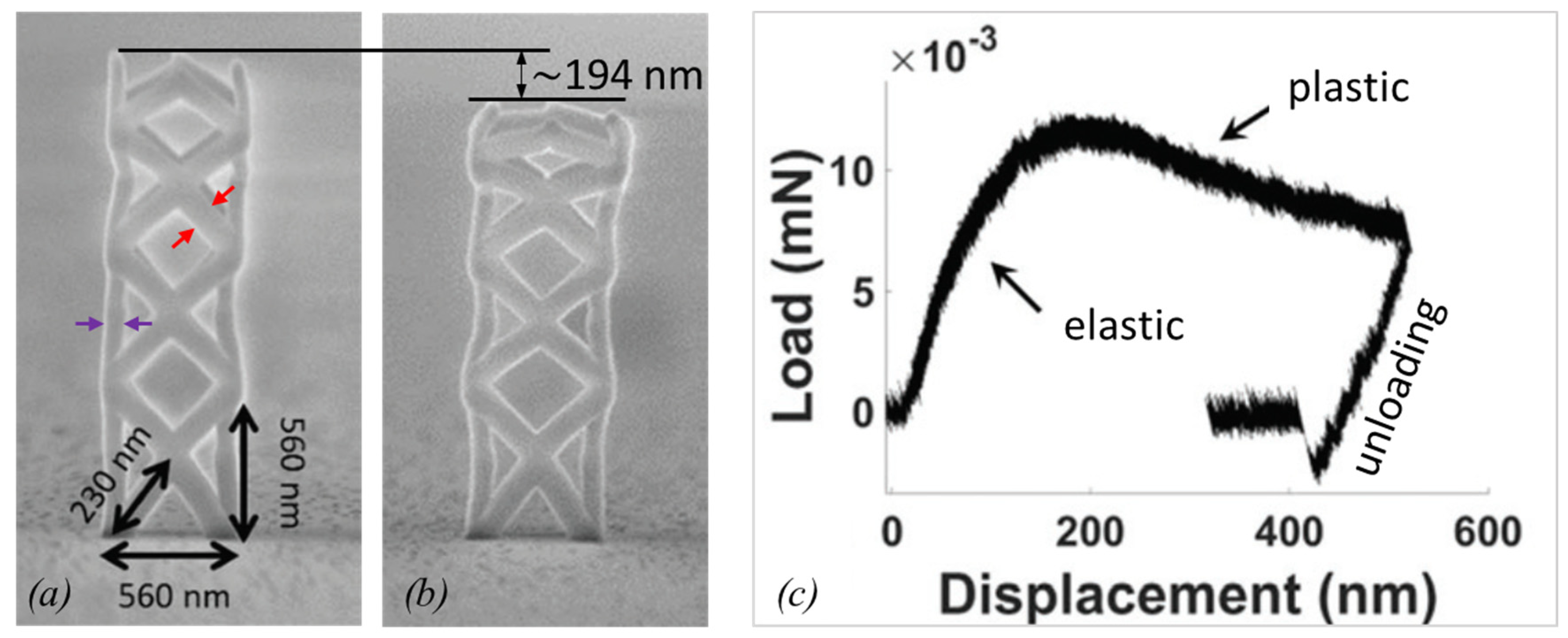

| Element with Precursor, Linear Formula, Sum Formula | Abbreviation | Metal (at.%) * | Matrix * | Max Metal Content Reported so far |
|---|---|---|---|---|
| C-FIBID with phenanthrene [36,37,38,39,40,41,42,43,44], C14H10 | C14H10 | 3–25 Ga | a-C:Hx | Up to 25 at% Ga contamination depending on deposition parameters |
| C-FEBID with paraffin (CnH2n+2) [45] : n-docesane C22H46; n-tetracosane, C24H50 | C22H46, C24H50 | 0 | a-C:Hx | Cleanest—no metal in deposit when using FEBID |
| Pt-FEBID and FIBID with Trimethyl- (methylcyclopentadienyl)–platinum [46,47,48,49], (CH3)3Pt(C5H4CH3); PtC9H13 | Pt- (CpMe)Me3 | 10–15 (FEB) Pt/Ga: 42/20 (FIB) [46] | a-C:Hx | FEB: Pt ≈100 at.% (+H2O) [26] FIB: ≈ 45 at% (5 at.% Ga) [50] |
| W-FEBID and FIBID with Tungsten-hexacarbonyl [51,52], W(CO)6 | W(CO)6 | W/Ga: ≈ 80/18 (FIB) [51] W: 47–62 (FEB) [52] | FIB: a-C:O, ≈ C85O15 [51] FEB: a-C:O, ≈ C80O20 [52] | FIB: ≈ 80 at.% W [51] FEB: ≈ 62 at.% [52] |
| Co-FEBID with Dicobaltoctacarbonyl Co2(CO)8 (This work and [52]) | Co2(CO)8 | ≈ 72 (This work) ≈ 42 [52] | a-C:O, ≈ C67O33 (This work)a-C:O, ≈ C80O20 [52] | Co ≈ 95 at.% (FEB) [21] |
| Au-FEBID with Dimethyl(trifluoro- acetylacetonate)-gold (This work), (CH3)2Au(O2C5H4F3); C7H10AuF3O2 | Au(tfac)Me2 | 50 (FEB) | a-C:O,F,H ≈ C85O20Hx | FEB: Au 100 at.% (+H2O) [32] |
| Cu-FEB: Copper(II) hexafluoroacetyl- acetonate [53], Cu(C5HF6O2)2; CuC10H2F12O4 | Cu(hfac)2 | 6–10 (FEB) | a-C:O,F,H ≈ C60...70O20..22F4..8Hx | FEB: ≈ 10 at.% [54] No FIB data |
| Cu-FEBID with hexafluoropentane- dionate-Copper–vinyltrimethylsilane [55], C5H12Si-Cu-C5HF6O2; CuC10H13F6O2Si | (hfac)Cu- VTMS | 14–30 (FEB) | a-C:O,F,H,Si≈ C70O14Si10Hx [56] | FEB: ≈ 95 at.% [57]FIB: ≈ 95 at.% Cu (5 at.% Ga) [58] Both achieved with heating |
| Fe-FIBID with Ferrocene [59], Fe(C5H5)2; FeC10H10 | Fe(Cp)2 | 5 at% Fe (FIB) | a-C:Hx | FEB: 95 at.% with Fe(CO)5 [60,61] |
| SiO2-FIBID with Tetramethylcyclotetra- siloxane [62], (HSiCH3O)4; Si4C4H12O4 | (HSiCH3O)4 | 5 at% Ga [63] | C < AES noise level [63] | FIB: 95 at.% SiO2, 5at.% Ga (+traces of O2) [63] |
| SiO2-FEBID with Tetramethoxysilane (This work), Si(OCH3)4; SiO4C4H12 | Si(OCH3)4 | 33 (FEB) | a-C:H,O ≈ C56O44Hx [34] | FEB 100 at.% SiO2 (+H2O) [34] |
| Rh-FEBID with Rhodium-tetrakis- chlorotrifluorophosphine (This work), [RhCl(PF3)2]2; Rh2Cl2P4F12 | Rh2Cl2(PF3)4 | 70 (FEB) | a-P:Cl ≈ P77Cl23F0 [64] | FEB: 60–70 at.% (This work) No FIB data |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Utke, I.; Michler, J.; Winkler, R.; Plank, H. Mechanical Properties of 3D Nanostructures Obtained by Focused Electron/Ion Beam-Induced Deposition: A Review. Micromachines 2020, 11, 397. https://doi.org/10.3390/mi11040397
Utke I, Michler J, Winkler R, Plank H. Mechanical Properties of 3D Nanostructures Obtained by Focused Electron/Ion Beam-Induced Deposition: A Review. Micromachines. 2020; 11(4):397. https://doi.org/10.3390/mi11040397
Chicago/Turabian StyleUtke, Ivo, Johann Michler, Robert Winkler, and Harald Plank. 2020. "Mechanical Properties of 3D Nanostructures Obtained by Focused Electron/Ion Beam-Induced Deposition: A Review" Micromachines 11, no. 4: 397. https://doi.org/10.3390/mi11040397
APA StyleUtke, I., Michler, J., Winkler, R., & Plank, H. (2020). Mechanical Properties of 3D Nanostructures Obtained by Focused Electron/Ion Beam-Induced Deposition: A Review. Micromachines, 11(4), 397. https://doi.org/10.3390/mi11040397