Autonomous and In Situ Ocean Environmental Monitoring on Optofluidic Platform
Abstract
:1. Introduction
2. Nutrients
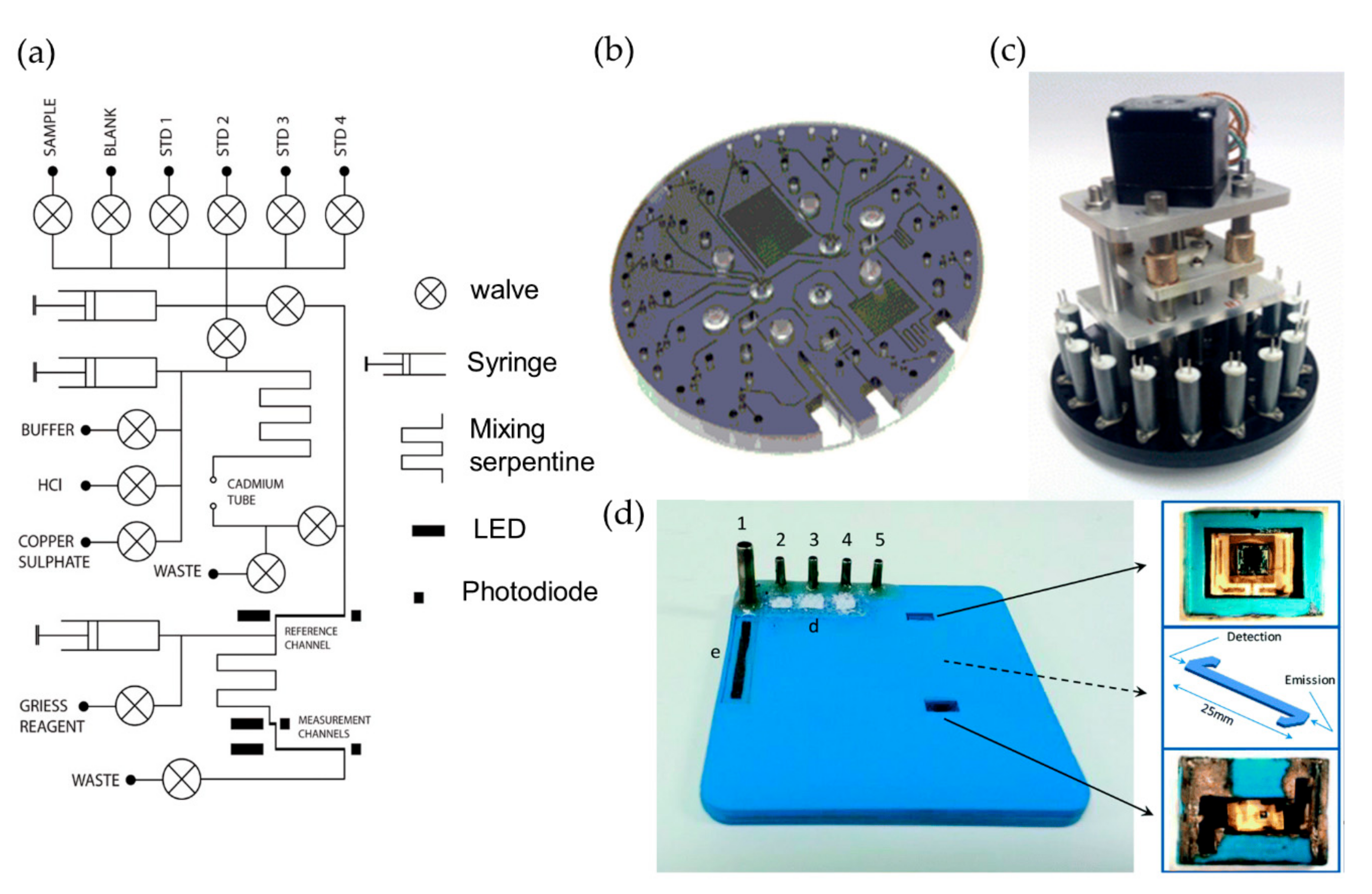
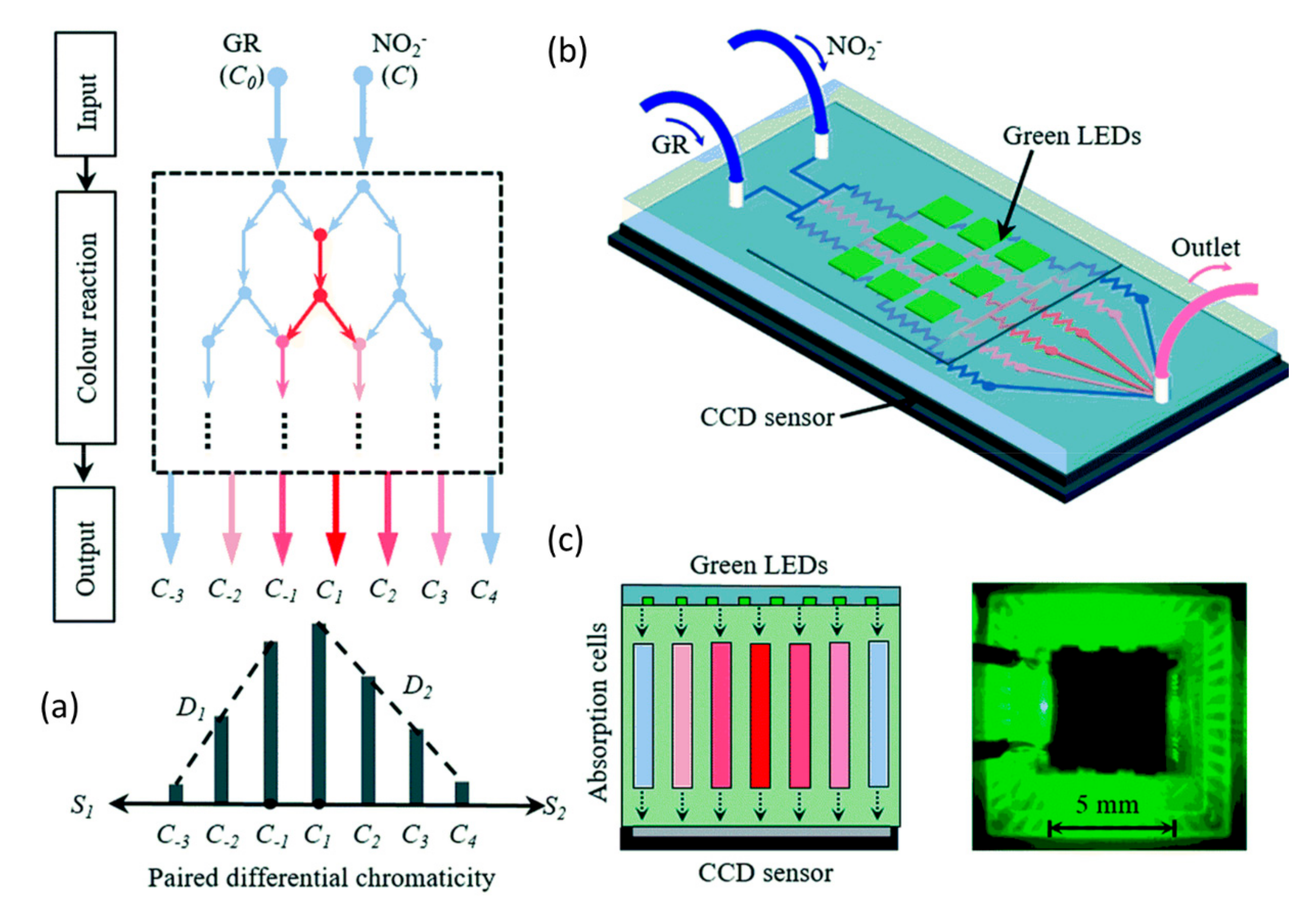
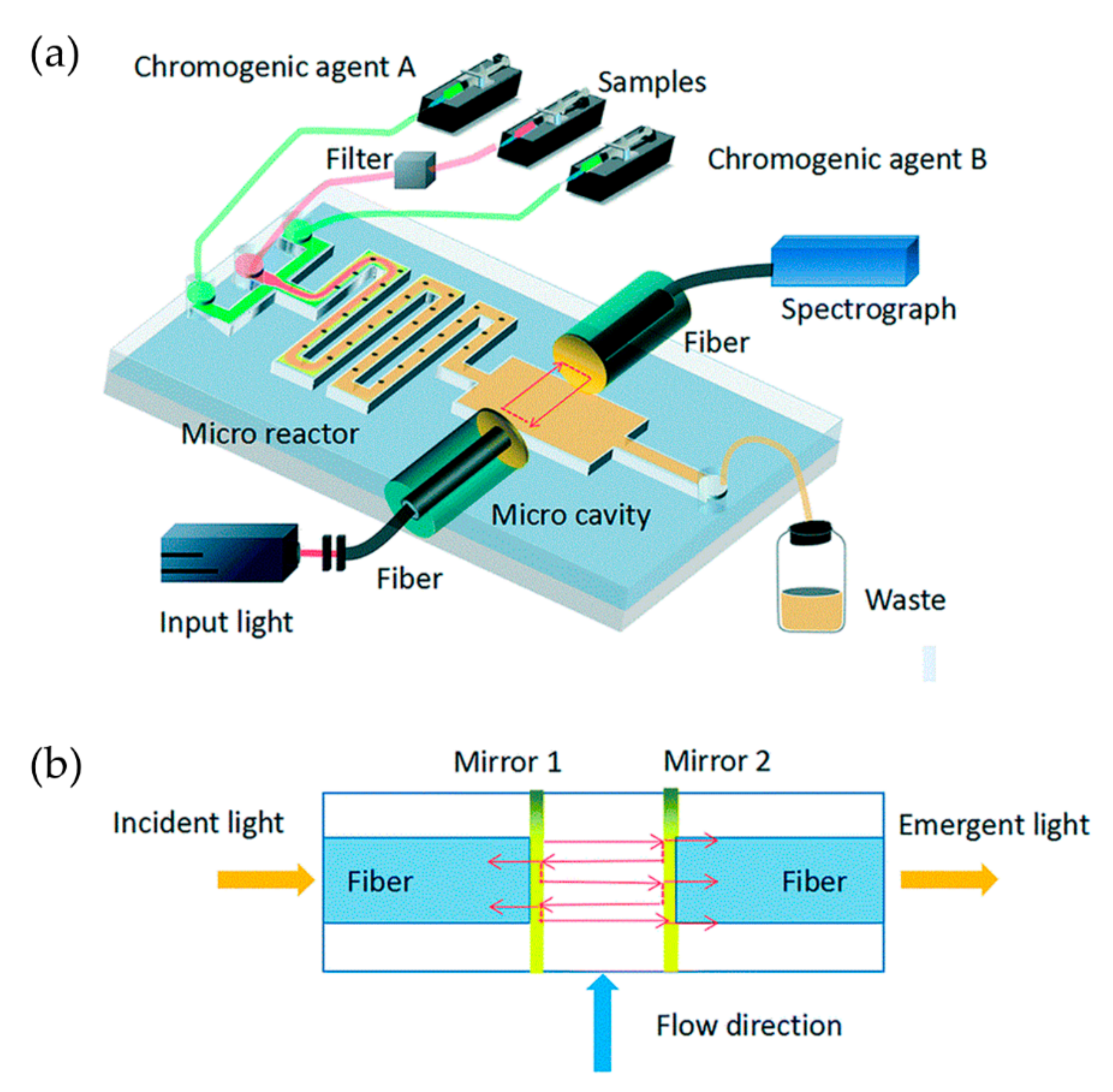
2.1. Nitrate and Nitrite
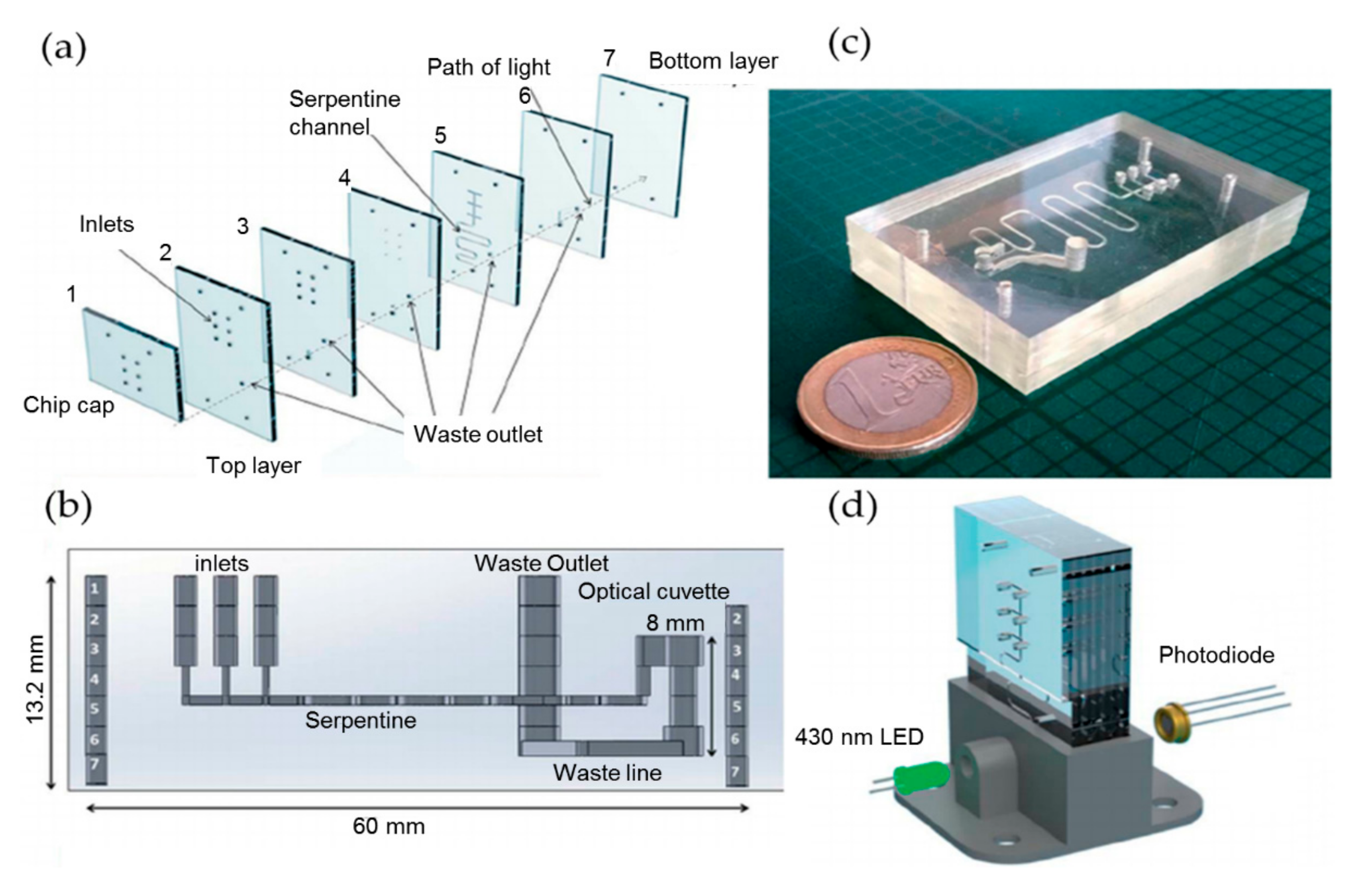
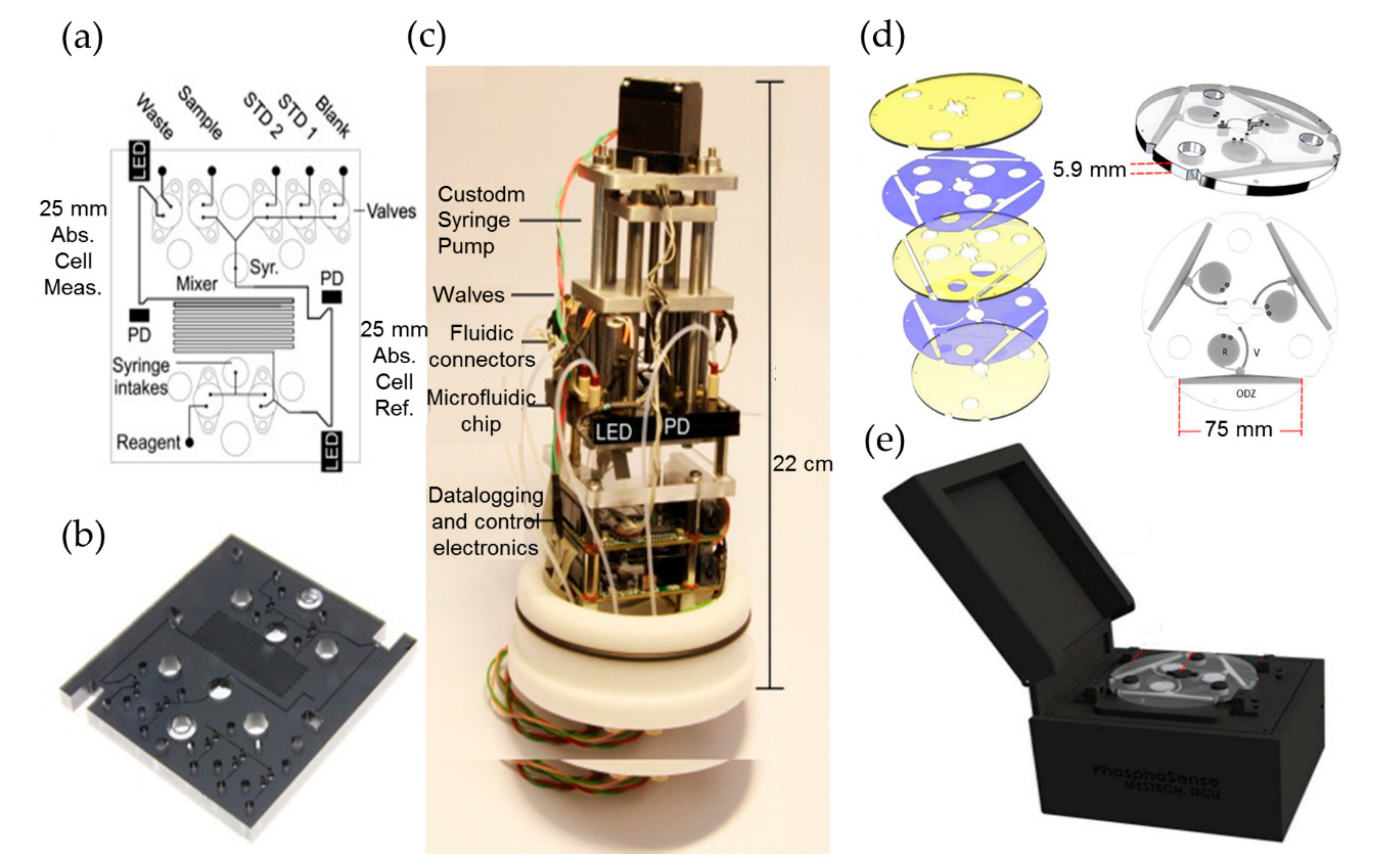
2.2. Phosphate
2.3. Silicate
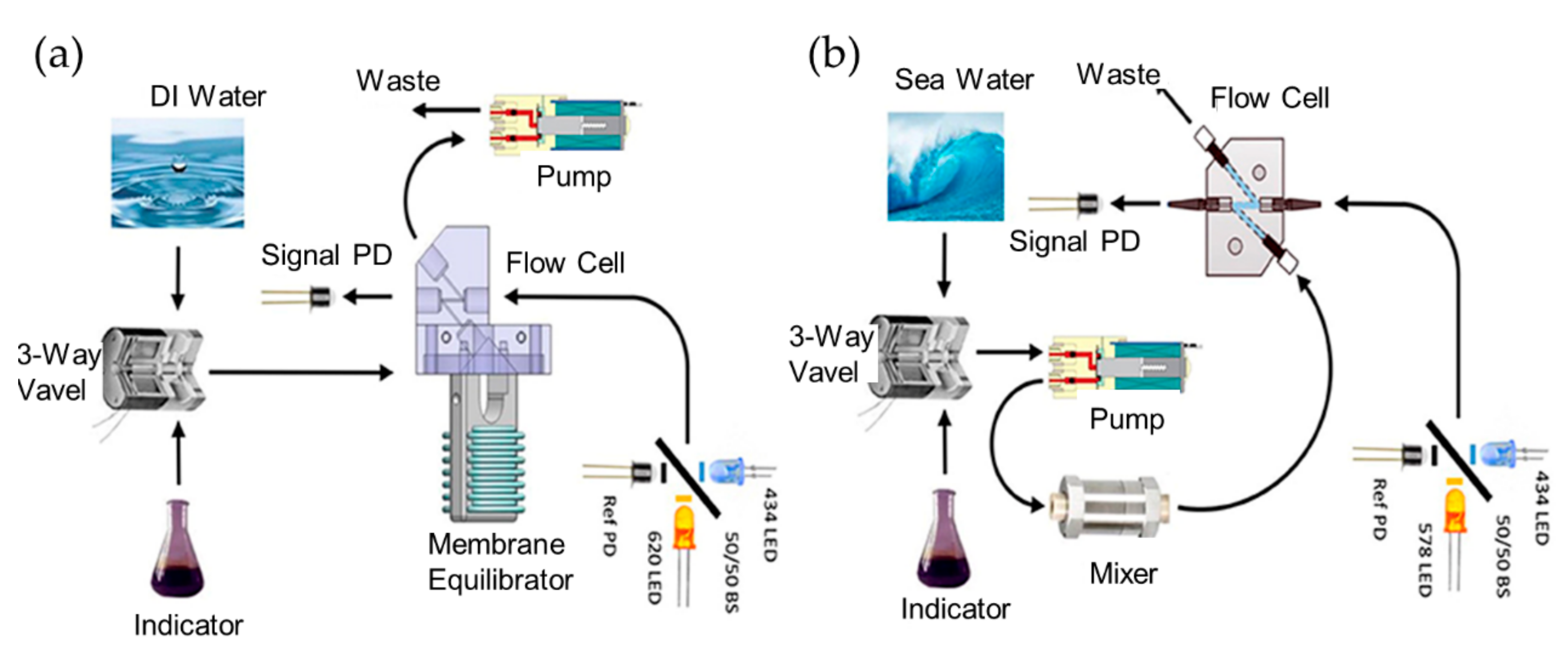
3. pH
4. DO
5. Heavy Metals
6. Discuss and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Anthony, K.R.; Kline, D.I.; Diaz-Pulido, G.; Dove, S.; Hoegh-Guldberg, O. Ocean acidification causes bleaching and productivity loss in coral reef builders. Proc. Natl. Acad. Sci. USA 2008, 105, 17442–17446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicholson, D.P.; Michel, A.P.M.; Wankel, S.D.; Manganini, K.; Sugrue, R.A.; Sandwith, Z.O.; Monk, S.A. Rapid mapping of dissolved methane and carbon dioxide in coastal ecosystems using the ChemYak Autonomous surface vehicle. Environ. Sci. Technol. 2018, 52, 13314–13324. [Google Scholar] [CrossRef] [PubMed]
- Nightingale, A.M.; Beaton, A.D.; Mowlem, M.C. Trends in Microfluidic Systems for In Situ Chemical Analysis of Natural Waters. Sens. Actuators B 2015, 221, 1398–1405. [Google Scholar] [CrossRef] [Green Version]
- Fukuba, T.; Imhof, A.; Matsunaga, A.; Takagi, N.; Yamamoto, T.; Okamura, K.; Naganuma, T.; Fujii, T. Development of miniaturized in situ analysis devices for biological and chemical oceanograph. In Proceedings of the 2005 3rd IEEE/EMBS Special Topic Conference on Microtechnology in Medicine and Biology, Oahu, HI, USA, 12–15 May 2005; pp. 56–59. [Google Scholar]
- Zhu, J.M.; Shi, Y.; Zhu, X.Q.; Yang, Y.; Jiang, F.H.; Sun, C.J.; Zhao, W.H.; Han, X.T. Optofluidic marine phosphate detection with enhanced absorption using a Fabry–Pérot resonator. Lab Chip 2017, 17, 4025–4030. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Cisneros, C.; Rocha, Z.D.; Seabra, A.; Valdés, F.; Alonso-Chamarro, J. Highly integrated autonomous lab-on-a-chip device for on-line and in situ determination of environmental chemical parameters. Lab Chip 2018, 18, 1884–1890. [Google Scholar] [CrossRef] [PubMed]
- Fang, T.Y.; Li, P.C.; Lin, K.N.; Chen, N.W.; Jiang, Y.Y.; Chen, J.X.; Yuan, D.X.; Ma, J. Simultaneous underway analysis of nitrate and nitrite in estuarine and coastal waters using an automated integrated syringe-pump-based environmental-water analyser. Anal. Chim. Acta 2019, 1076, 100–109. [Google Scholar] [CrossRef]
- Clarke, J.S.; Achterberg, E.P.; Connelly, D.P. Developments in marine pCO2 measurement technology; towards sustained in situ observations. TrAC, Trends Anal. Chem. 2017, 88, 53–61. [Google Scholar] [CrossRef] [Green Version]
- Gumpu, M.B.; Sethuraman, S.; Krishnan, U.M. A review on detection of heavy metal ions in water – An electrochemical approach. Sens. Actuators B 2015, 213, 515–533. [Google Scholar] [CrossRef]
- Psaltis, D.; Quake, S.R.; Yang, C. Developing optofluidic technology through the fusion of microfluidics and optics. Nature 2006, 442, 381–386. [Google Scholar] [CrossRef]
- Monat, C.; Domachuk, P.; Eggleton, B.J. Integrated optofluidics: A new river of light. Nat. Photonics 2007, 1, 106–114. [Google Scholar] [CrossRef]
- Fan, X.D.; White, I.M. Optofluidic microsystems for chemical and biological analysis. Nat. Photonics 2011, 5, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.N.; Zhao, Y.; Zhou, T.; Wu, Q. Applications and developments of on-chip biochemical sensors based on optofluidic photonic crystal cavities. Lab Chip 2018, 18, 57–74. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.M.; Zhu, X.Q.; Zuo, Y.F.; Hu, X.J.; Shi, Y.; Liang, L.; Yang, Y. Optofluidics: The interaction between light and flowing liquids in integrated devices. Opto-Electron. Adv. 2019, 2, 190007. [Google Scholar] [CrossRef]
- Fujii, T. PDMS-based microfluidic devices for biomedical applications. Microelectron. Eng. 2002, 61–62, 907–914. [Google Scholar] [CrossRef]
- Persichetti, G.; Grimaldi, I.A.; Testa, G.; Bernini, R. Multifunctional optofluidic lab-on-chip platform for Raman and fluorescence spectroscopic microfluidic analysis. Lab Chip 2017, 17, 2631–2639. [Google Scholar] [CrossRef]
- Lai, C.Z.; DeGrandpre, M.D.; Darlington, R.C. Autonomous Optofluidic Chemical Analysers for Marine Applications: Insights from the Submersible Autonomous Moored Instruments (SAMI) for pH and pCO2. Front. Mar. Sci. 2018, 4, 1–11. [Google Scholar] [CrossRef]
- Prien, R.D. The future of chemical in situ sensors. Mar. Chem. 2007, 107, 422–432. [Google Scholar] [CrossRef]
- Robidart, J.; Magasin, J.D.; Shilova, I.N.; Turk-Kubo, K.A.; Wilson, S.T.; Karl, D.M.; Scholin, C.A.; Zehr, J.P. Effects of nutrient enrichment on surface microbial community gene expression in the oligotrophic North Pacific Subtropical Gyre. ISME J. 2019, 13, 374–387. [Google Scholar] [CrossRef]
- Bricker, S.B.; Ferreira, J.G.; Simas, T. An integrated methodology for assessment of estuarine trophic status. Ecol. Modell. 2003, 169, 39–60. [Google Scholar] [CrossRef] [Green Version]
- Perez-Ruzafa, A.; Campillo, S.; Fernandez-Palacios, J.M.; Garcialacunza, A.; Garciaoliv, M.; Ibanez, H.; Navarromartinez, P.C.; Perezmarcos, M.; Perezruzafa, I.; Quispebecerra, J.I.; et al. Long-Term Dynamic in Nutrients, Chlorophyll a, and Water Quality Parameters in a Coastal Lagoon During a Process of Eutrophication for Decades, a Sudden Break and a Relatively Rapid Recovery. Front. Mar. Sci. 2019, 6, 26. [Google Scholar] [CrossRef] [Green Version]
- Tsikas, D. Analysis of nitrite and nitrate in biological fluids by assays based on the Griess reaction: Appraisal of the Griess reaction in the l-arginine/nitric oxide area of research. J. Chromatogr. B Biomed. Sci. Appl. 2007, 851, 51–70. [Google Scholar] [CrossRef] [PubMed]
- Nóbrega, L.N.N.; de O. Magalhaes, L.; Fonseca, A. A urethane-acrylate microflow-analyser with an integrated cadmium column. Microchem. J. 2013, 110, 553–557. [Google Scholar]
- Shi, Y.; Liu, H.L.; Zhu, X.Q.; Zhu, J.M.; Zuo, Y.F.; Yang, Y.; Jiang, F.H.; Sun, C.J.; Zhao, W.H. Optofluidic differential colorimetry for rapid nitrite determination. Lab Chip 2018, 18, 2994–3002. [Google Scholar] [CrossRef] [PubMed]
- Ellis, P.S.; Shabani, A.M.H.; Gentle, B.S.; McKelvie, I.D. Field measurement of nitrate in marine and estuarine waters with a flow analysis system utilizing on-line zinc reduction. Talanta 2011, 84, 98–103. [Google Scholar] [CrossRef]
- Cardoso, T.M.; Garcia, P.T.; Coltro, W.K. Colorimetric determination of nitrite in clinical, food and environmental samples using microfluidic devices stamped in paper platforms. Anal. Methods 2015, 7, 7311–7317. [Google Scholar] [CrossRef]
- Liu, R.; Ishimatsu, R.; Yahiro, M.; Adachi, C.; Nakano, K.; Imato, T. Photometric flow injection determination of phosphate on a PDMS microchip using an optical detection system assembled with an organic light emitting diode and an organic photodiode. Talanta 2015, 132, 96–105. [Google Scholar] [CrossRef]
- Nightingale, A.M.; Hassan, S.; Evans, G.W. Nitrate measurement in droplet flow: Gas-mediated crosstalk and correction. Lab Chip 2018, 18, 1903–1913. [Google Scholar] [CrossRef]
- Sieben, V.J.; Floquet, C.F.; Ogilvie, I.R.; Mowlem, M.C.; Morgan, H. Microfluidic colourimetric chemical analysis system: Application to nitrite detection. Anal. Methods 2010, 2, 484–491. [Google Scholar] [CrossRef]
- Cogan, D.; Fay, C.; Boyle, D.; Osborne, C.; Kent, N.; Cleary, J.; Diamond, D. Development of a low cost microfluidic sensor for the direct determination of nitrate using chromotropic acid in natural waters. Anal. Methods 2015, 7, 5396–5405. [Google Scholar] [CrossRef]
- Yang, K.; Peretzsoroka, H.; Liu, Y.; Lin, F. Novel developments in mobile sensing based on the integration of microfluidic devices and smartphones. Lab Chip 2016, 16, 943–958. [Google Scholar] [CrossRef] [Green Version]
- Beaton, A.D.; Sieben, V.J.; Floquet, C.F.; Waugh, E.M.; Ogilvie, I.R.; Mowlem, M.C.; Morgan, H. An automated microfluidic colourimetric sensor applied in situ to determine nitrite concentration. Sens. Actuators B 2011, 156, 1009–1014. [Google Scholar] [CrossRef]
- Beaton, A.D.; Cardwell, C.L.; Thomas, R.S.; Sieben, V.J.; Legiret, F.E.; Waugh, E.M.; Statham, P.J.; Mowlem, M.C.; Morgan, H. Lab-on-Chip Measurement of Nitrate and Nitrite for In Situ Analysis of Natural Waters. Environ. Sci. Technol. 2012, 46, 9548–9556. [Google Scholar] [CrossRef] [PubMed]
- Nightingale, A.M.; Hassan, S.; Warren, B.M.; Warren, K.; Makris, K.; Evans, G.W. A Droplet Microfluidic-Based Sensor for Simultaneous in Situ Monitoring of Nitrate and Nitrite in Natural Waters. Environ. Sci. Technol. 2019, 53, 9677–9685. [Google Scholar] [CrossRef] [PubMed]
- Gary, H. Chemiluminescent and bioluminescent methods in analytical chemistry. A review. Analyst 1983, 108, 1274–1296. [Google Scholar]
- Yaqoob, M.; Biot, B.F.; Nabi, A.; Worsfold, P.J. Determination of nitrate and nitrite in freshwaters using flow injection with luminol chemiluminescence de tection. Luminescence 2012, 27, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Dombrowsky, L.J.; Pratt, E.J. Fluorimetric method for determining nanogram quantities of nitrite ion. Anal. Chem. 1972, 44, 2268–2272. [Google Scholar]
- Guo, Y.X.; Zhang, Q.F.; Shang, X. Spectrofluorimetric determination of trace nitrite with o-phenylenediamine enhanced by hydroxypropyl-β-cyclodextrin. Spectrochim. Acta Part A 2013, 101, 107–111. [Google Scholar] [CrossRef]
- Lu, L.; Chen, C.; Zhao, D.; Yang, F.; Yang, X. A simple and sensitive assay for the determination of nitrite using folic acid as the fluorescent probe. Anal. Methods 2015, 7, 1543–1548. [Google Scholar] [CrossRef]
- Wang, X.; Adams, E.; Schepdael, A.V. A fast and sensitive method for the determination of nitrite in human plasma by capillary electrophoresis with fluorescence detection. Talanta 2012, 97, 142–144. [Google Scholar] [CrossRef]
- Armijo, F.; Goya, M.C.; Reina, M.; Canales, M.J.; Arévalo, M.C.; Aguirre, M.J. Electrocatalytic oxidation of nitrite to nitrate mediated by Fe (III) poly-3- aminophenyl porphyrin grown on five different electrode surfaces. J. Mol. Catal. A Chem. 2007, 268, 148–154. [Google Scholar] [CrossRef]
- Wang, X.; Li, H.; Wu, M.; Ge, S.L.; Zhu, Y.; Wang, Q.J.; He, P.G.; Fang, Y.Z. Simultaneous Electrochemical Determination of Sulphite and Nitrite by a Gold Nanoparticle/Graphene-Chitosan Modified Electrode. Chin. J. Anal. Chem. 2013, 41, 1232–1237. [Google Scholar] [CrossRef]
- Jońca, J.; Fernández, V.L.; Thouron, D. Phosphate determination in seawater: Toward an autonomous electrochemical method. Talanta 2011, 87, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Dahlloef, I.; Svensson, O.; Torstensson, C. Optimising the determination of nitrate and phosphate in sea water with ion chromatography using experimental design. J. Chromatogr. A 1997, 771, 163–168. [Google Scholar] [CrossRef]
- Carrozzino, S.; Righini, F. Ion chromatographic determination of nutrients in sea water. J. Chromatogr. A 1995, 706, 277–280. [Google Scholar] [CrossRef]
- He, L.; Zhang, K.; Wang, C.; Luo, X.; Zhang, S. Effective indirect enrichment and determination of nitrite ion in water and biological samples using ionic liquid dispersive liquid–liquid microextraction combined with high-performance liquid chromatography. J. Chromatogr. A 2011, 1218, 3595–3600. [Google Scholar] [CrossRef]
- Campos, C.D.; Silva, J.A. Applications of autonomous microfluidic systems in environmental monitoring. RSC Adv. 2013, 3, 18216–18227. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Lin, K.; Chen, N.; Yuan, D.; Ma, J. Automated determination of nitrate plus nitrite in aqueous samples with flow injection analysis using vanadium (III) chloride as reductant. Talanta 2016, 146, 744–748. [Google Scholar] [CrossRef]
- Khongpet, W.; Pencharee, S.; Puangpila, C.; Hartwell, S.K.; Lapanantnoppakhun, S.; Jakmunee, J. Exploiting an automated microfluidic hydrodynamic sequential injection system for determination of phosphate. Talanta 2017, 177, 77–85. [Google Scholar] [CrossRef]
- Duffy, G.; Regan, F. Recent developments in sensing methods for eutrophying nutrients with a focus on automation for environmental applications. Analyst 2017, 142, 4355–4372. [Google Scholar] [CrossRef]
- Legiret, F.E.; Sieben, V.J.; Woodward, E.M.S.; Bey, S.K.A.K.; Mowlem, M.C.; Connelly, D.P.; Achterberg, E.P. A high performance microfluidic analyser for phosphate measurements in marine waters using the vanadomolybdate method. Talanta 2013, 116, 382–387. [Google Scholar] [CrossRef]
- Duffy, G.; Maguire, I.; Heery, B.; Nwankire, C.; Ducree, J.; Regan, F. PhosphaSense: A fully integrated, portable lab-on-a-disc device for phosphate determination in water. Sens. Actuators B 2017, 246, 1085–1091. [Google Scholar] [CrossRef]
- Clinton-Bailey, G.; Beaton, A.; Nightingale, A.; Owsianka, D.; Slavik, G. A lab-on-chip analyser for in situ measurement of soluble reactive phosphate: Improved phosphate blue assay and application to fluvial monitoring. Environ. Sci. Technol. 2017, 51, 9989–9995. [Google Scholar] [CrossRef] [PubMed]
- Ruzicka, J.; Hansen, E.H. Flow injection analyses Part I. A new concept of fast continuous flow analysis. Anal. Chim. Acta 1975, 78, 145–157. [Google Scholar]
- Kennedy, J.F.; Weetman, D.A. A modified spectrophotometric method for the simple rapid determination of phosphate. Anal. Chim. Acta 1971, 55, 418–449. [Google Scholar] [CrossRef]
- Grand, M.M.; Clinton-Bailey, G.S.; Beaton, A.D.; Schaap, A.M.; Johengen, T.H.; Tamburri, M.N.; Connelly, D.P.; Mowlem, M.C.; Achterberg, E.P. A Lab-On-Chip Phosphate Analyser for Long-term In Situ Monitoring at Fixed Observatories: Optimization and Performance Evaluation in Estuarine and Oligotrophic Coastal Waters. Front. Mar. Sci. 2017, 4, 255. [Google Scholar] [CrossRef] [Green Version]
- Cao, X.; Zhang, S.W.; Chu, D.Z.; Wu, N.; Ma, H.K.; Liu, Y. A design of spectrophotometric microfluidic chip sensor for analyzing silicate in seawater. IOP Conf. Ser.: Earth Environ. Sci. 2017, 82, 012080. [Google Scholar] [CrossRef] [Green Version]
- Feely, R.A.; Sabine, C.L.; Lee, K.; Berelson, W.; Klepas, J.; Fabry, V.J.; Millero, F.J. Impact of anthropogenic CO2 on the CaCO3 system in the oceans. Science 2004, 305, 362–366. [Google Scholar] [CrossRef] [Green Version]
- Doney, S.C.; Fabry, V.J.; Feely, R.A.; Kleypas, J.A. Ocean acidifification: The other CO2 problem. Annu. Rev. Mar. Sci. 2009, 1, 169–192. [Google Scholar] [CrossRef] [Green Version]
- Mostofa, K.M.; Liu, C.; Zhai, W. Reviews and Syntheses: Ocean acidification and its potential impacts, on marine ecosystems. Biogeosciences 2016, 13, 1767–1786. [Google Scholar] [CrossRef] [Green Version]
- Orr, J.C.; Fabry, V.J.; Aumont, O. Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms. Nature 2005, 437, 681–686. [Google Scholar] [CrossRef]
- Botelho, R.G.; Tornisielo, V.L.; de Olinda, R.A.; Maranho, L.A.; Machado-Neto, L.; Botelho, R.G.; Tornisielo, V.L.; De Olinda, R.A. Acute toxicity of sugarcane vinasse to aquatic organisms before and after pH adjustment. Toxicol. Environ. Chem. 2012, 94, 2035–2045. [Google Scholar] [CrossRef]
- Wencel, D.; Abel, T.; McDonagh, C. Optical Chemical pH Sensors. Anal. Chem. 2014, 86, 15–29. [Google Scholar] [CrossRef] [PubMed]
- DeGrandpre, M.D.; Spaulding, R.S.; Newton, J.O.; Jaqueth, E.J.; Hamblock, S.E.; Umansky, A.A.; Harris, K.E. Considerations for the measurement of spectrophotometric pH for ocean acidification and other studies. Limnol. Oceanogr. Methods 2014, 12, 830–839. [Google Scholar]
- Zhang, H.; Byrne, R.H. Spectrophotometric pH measurements of surface seawater at in-situ conditions: Absorbance and protonation behavior of thymol blue. Mar. Chem. 1996, 52, 17–25. [Google Scholar] [CrossRef]
- Rérolle, V.M.C.; Floquet, C.F.A.; Harris, A.J.K.; Mowlem, M.C.; Bellerby, R.R.G.J.; Achterberg, E.P. Development of a colorimetric microfluidic pH sensor for autonomous seawater measurements. Anal. Chim. Acta 2013, 786, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Robert-Baldo, G.L.; Morris, M.J.; Byrne, R.H. Spectrophotometric determination of seawater pH using phenol red. Anal. Chim. 1985, 57, 2564–2567. [Google Scholar] [CrossRef]
- Byrne, R.H.; Breland, J.A. High precision multiwavelength pH determinations in seawater using cresol red. Deep-Sea Res. Part A 1989, 36, 803–810. [Google Scholar] [CrossRef]
- Clayton, T.D.; Byrne, R.H. Spectrophotometric seawater pH measurements: Total hydrogen ion concentration scale calibration of m-cresol purple and at-sea results. Deep-Sea Res. Part A 1993, 40, 2115–2129. [Google Scholar] [CrossRef]
- Florea, L.; Fay, C.; Lahiff, E.; Phelan, T.; O’Connor, N.E.; Corcoran, B.; Diamond, D.; Benito-Lopez, F. Dynamic pH mapping in microfluidic devices by integrating adaptive coatings based on polyaniline with colorimetric imaging techniques. Lab Chip 2013, 13, 1079–1085. [Google Scholar] [CrossRef] [Green Version]
- Sumriddetchkajorn, S.; Chaitavon, K.; Intaravanne, Y. Mobile device-based self-referencing colorimeter for monitoring chlorine concentration in water. Sens. Actuators B 2013, 182, 592–597. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.; Chen, P. Smartphone spectrometer for colorimetric biosensing. Analyst 2016, 141, 3233–3238. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Yuan, D.; Lin, K.; Feng, S.; Zhou, T.; Li, Q. Applications of flow techniques in seawater analysis: A review. Trends Environ. Anal. Chem. 2016, 10, 1–10. [Google Scholar]
- Tran-Ngoc, K.T.; Dinh, N.T.; Nguyen, T.H.; Roem, A.J.; Schrama, J.W.; Verreth, J.A. Interaction between dissolved oxygen concentration and diet composition on growth, digestibility and intestinal health of Niletilapia (Oreochromis niloticus). Aquaculture 2016, 462, 101–108. [Google Scholar] [CrossRef]
- Wu, S.; Wu, S.; Yi, Z.; Zeng, F.; Wu, W.; Qiao, Y.; Tian, Y. Hydrogel-based fluorescent dual pH and oxygen sensors loaded in 96-well plates for high-throughput cell metabolism studies. Sensors 2018, 18, 564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carpenter, J.H. The accuracy of the winkler method for dissolved oxygen analysis. Limnol. Oceanogr. 1965, 10, 135–140. [Google Scholar] [CrossRef]
- Wilkin, R.T.; Mcneil, M.S.; Adair, C.J.; Wilson, J.T. Field measurement of dissolved oxygen: A comparison of methods. Groundw. Monit. Remediat. 2001, 21, 124–132. [Google Scholar] [CrossRef]
- Trivellin, N.; Barbisan, D.; Badocco, D.; Pastore, P.; Meneghesso, G.; Meneghini, M.; Zanoni, E.; Belgioioso, G.; Cenedese, A. Study and development of a fluorescence based sensor system for monitoring oxygen in wine production: The WOW project. Sensors 2018, 18, 1130. [Google Scholar] [CrossRef] [Green Version]
- Mahoney, E.J.; Hsu, H.L.; Du, F.; Xiong, B.; Selvaganapathy, P.R.; Fang, Q. Optofluidic Dissolved Oxygen Sensing With Sensitivity Enhancement Through Multiple Reflections. IEEE Sens. J. 2019, 19, 10452–10460. [Google Scholar] [CrossRef]
- Sakai, T.; Piao, S.; Teshima, N.; Kuroishi, T.; Grudpan, K. Flow injection system with in-line Winkler’ sprocedure using 16-way valve and spectrofluorimetric determination of dissolved oxygen in environmental waters. Talanta 2004, 63, 893–898. [Google Scholar] [CrossRef]
- Zheng, G.; Xu, Z. A new design of high-precision dissolved oxygen sensor. Transducer Microsyst. Technol. 2012, 2, 112–114. [Google Scholar]
- Norvell, W.A.; Welc, R.M. Biological Monitoring of Heavy Metal Pollution: Land and Air. J. Environ. Qual. 1984, 13, 173–174. [Google Scholar] [CrossRef]
- Rainbow, P.S. Trace metal accumulation in marine invertebrates: Marine biology or marine chemistry. J. Mar. Biol. Assoc. UK 1997, 77, 195–210. [Google Scholar] [CrossRef]
- Fonseca, A.; Raimundo, I.M.; Rohwedder, J.J.R.; Lima, R.S.; Araújo, M. A microfluidic device with integrated fluorimetric detection for flow injection analysis. Anal. Bioanal. Chem. 2010, 396, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, K.; Sujatha, L. Fluorometric Sensor for Mercury Ion Detection in a Fluidic MEMS Device. IEEE Sens. J. 2018, 18, 5225–5231. [Google Scholar] [CrossRef]
- Lin, M.; Hu, X.; Pan, D.; Han, H.T. Determination of iron in seawater: From the laboratory to in situ measurements. Talanta 2018, 188, 135–144. [Google Scholar] [CrossRef]
- Heller, A.; Ronitz, O.; Barkleit, A. Complexation of Europium(III) with the Zwitterionic Form of Amino Acids Studied with Ultraviolet-Visible and Time-Resolved Laser-Induced Fluorescence Spectroscopy. Caryologia 2010, 69, 285–294. [Google Scholar] [CrossRef]
- Milani, A.; Statham, P.J.; Mowlem, M.C. Development and application of a microfluidic in-situ analyser for dissolved Fe and Mn in natural waters. Talanta 2015, 136, 15–22. [Google Scholar] [CrossRef]
- Provin, C.; Fukuba, T.; Okamura, K.; Fujii, T. An Integrated Microfluidic System for Manganese Anomaly Detection Based on Chemiluminescence: Description and Practical Use to Discover Hydrothermal Plumes Near the Okinawa Trough. IEEE J. Ocean. Eng. 2013, 38, 178–185. [Google Scholar] [CrossRef]
- Lace, A.; Ryan, D.; Bowkett, M.; Cleary, J. Chromium Monitoring in Water by Colorimetry Using Optimised 1,5Diphenylcarbazide Method. Int. J. Environ. Res. Public Health 2019, 16, 1803. [Google Scholar] [CrossRef] [Green Version]
- Lace, A.; Ryan, D.; Bowkett, M.; Cleary, J. Arsenic detection in water using microfluidic detection systems based on the leucomalachite green method. Anal. Methods 2019, 11, 5431–5438. [Google Scholar] [CrossRef]
- Nguyen, H.L.; Naresh, L.; Jean-Frederic, A.; Ghasemi, R.; Lefevre, J.P.; Ha-Thi, M.H.; Mongin, C.; Leray, I. Water-soluble aluminium fluorescent sensor based on aggregation-induced emission enhancement. New J. Chem. 2019, 43, 15302–15310. [Google Scholar] [CrossRef]
- Zhang, H.; Faye, D.; Jean-Pierre Lefèvre, J.; Delaire, J.A.; Leray, I. Selective fluorimetric detection of cadmium in a microfluidic device. Microchem. J. 2013, 106, 167–173. [Google Scholar] [CrossRef]
- Kim, H.N.; Ren, W.X.; Kim, J.S.; Yoon, J. Fluorescent and colorimetric sensors for detection of lead, cadmium, and mercury ions. Chem. Soc. Rev. 2012, 41, 3210–3244. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.Y.; Yan, B. Eu(III) functionalized Zr-based metal-organic framework as excellent flourescent probe for Cd2+ detection in aqueous environment. Sens. Actuators B 2016, 222, 347–353. [Google Scholar] [CrossRef]
- Siangproh, W.; Chailapakul, O.; Songsrirote, K. Simple and fast colorimetric detection of inorganic arsenic selectively adsorbed onto ferrihydrite-coated silica gel using silver nanoplates. Talanta 2016, 153, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Sikorowska, C.; Wichrowska, B. Comparative studies on the determination of zinc in water by the colorimetric method. Roczniki Państwowego Zakadu Higieny 1971, 22, 17–24. [Google Scholar]
- Liu, H.; Liu, T.; Li, J.; Zhang, Y.; Li, J.; Song, J.; Qu, J.; Wong, W.-Y. A simple Schiff base as dual-responsive fluorescent sensor for bioimaging recognition of Zn2+ and Al3+ in living cells. J. Mater. Chem. 2018, 6, 5435–5442. [Google Scholar] [CrossRef]
- Jo, T.G.; Bok, K.H.; Han, J.; Lim, M.H.; Kim, C. Colorimetric detection of Fe3+ and Fe2+ and sequential fluorescent detection of Al3+ and pyrophosphate by an imidazole-based chemosensor in a near-perfect aqueous solution. Dyes Pigments 2017, 139, 136–147. [Google Scholar] [CrossRef]
- Saini, A.K.; Natarajan, K.; Mobin, S.M. A new multitalented azine ligand: Elastic bending, single-crystal-to-single-crystal transformation and a fluorescence turn-on Al(III) sensor. Chem. Commun. 2017, 53, 9870–9873. [Google Scholar] [CrossRef]
- Neupane, L.N.; Mehta, P.K.; Oh, S.; Park, S.H.; Lee, K.H. Ratiometric red-emission fluorescence detection of Al3+ in pure aqueous solution and live cells by a fluorescent peptidyl probe using aggregation-induced emission. Analyst 2018, 143, 5285–5294. [Google Scholar] [CrossRef]
- Zhao, L.; Wu, T.; Lefèvre, J.; Leray, I.; Delaire, J.A. Fluorimetric lead detection in a microfluidic device. Lab Chip 2009, 9, 2818–2823. [Google Scholar] [CrossRef] [PubMed]
- Fukuba, T.; Noguchi, T.; Okamura, K.; Fujii, T. Adenosine Triphosphate Measurement in Deep Sea Using a Microfluidic Device. Micromachines 2018, 9, 370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamahara, K.M.; Preston, C.M.; Birch, J.M.; Walz, K.; Marin, R.; Jensen, S.; Pargett, D.; Roman, B.; Ussler, W.; Zhang, Y.W.; et al. In situ Autonomous Acquisition and Preservation of Marine Environmental DNA Using an Autonomous Underwater Vehicle. Front. Mar. Sci. 2019, 6, 373. [Google Scholar] [CrossRef] [Green Version]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, F.; Zhu, J.; Chen, L.; Zuo, Y.; Hu, X.; Yang, Y. Autonomous and In Situ Ocean Environmental Monitoring on Optofluidic Platform. Micromachines 2020, 11, 69. https://doi.org/10.3390/mi11010069
Wang F, Zhu J, Chen L, Zuo Y, Hu X, Yang Y. Autonomous and In Situ Ocean Environmental Monitoring on Optofluidic Platform. Micromachines. 2020; 11(1):69. https://doi.org/10.3390/mi11010069
Chicago/Turabian StyleWang, Fang, Jiaomeng Zhu, Longfei Chen, Yunfeng Zuo, Xuejia Hu, and Yi Yang. 2020. "Autonomous and In Situ Ocean Environmental Monitoring on Optofluidic Platform" Micromachines 11, no. 1: 69. https://doi.org/10.3390/mi11010069
APA StyleWang, F., Zhu, J., Chen, L., Zuo, Y., Hu, X., & Yang, Y. (2020). Autonomous and In Situ Ocean Environmental Monitoring on Optofluidic Platform. Micromachines, 11(1), 69. https://doi.org/10.3390/mi11010069



