Electrothermally Driven Hydrogel-on-Flex-Circuit Actuator for Smart Steerable Catheters
Abstract
1. Introduction
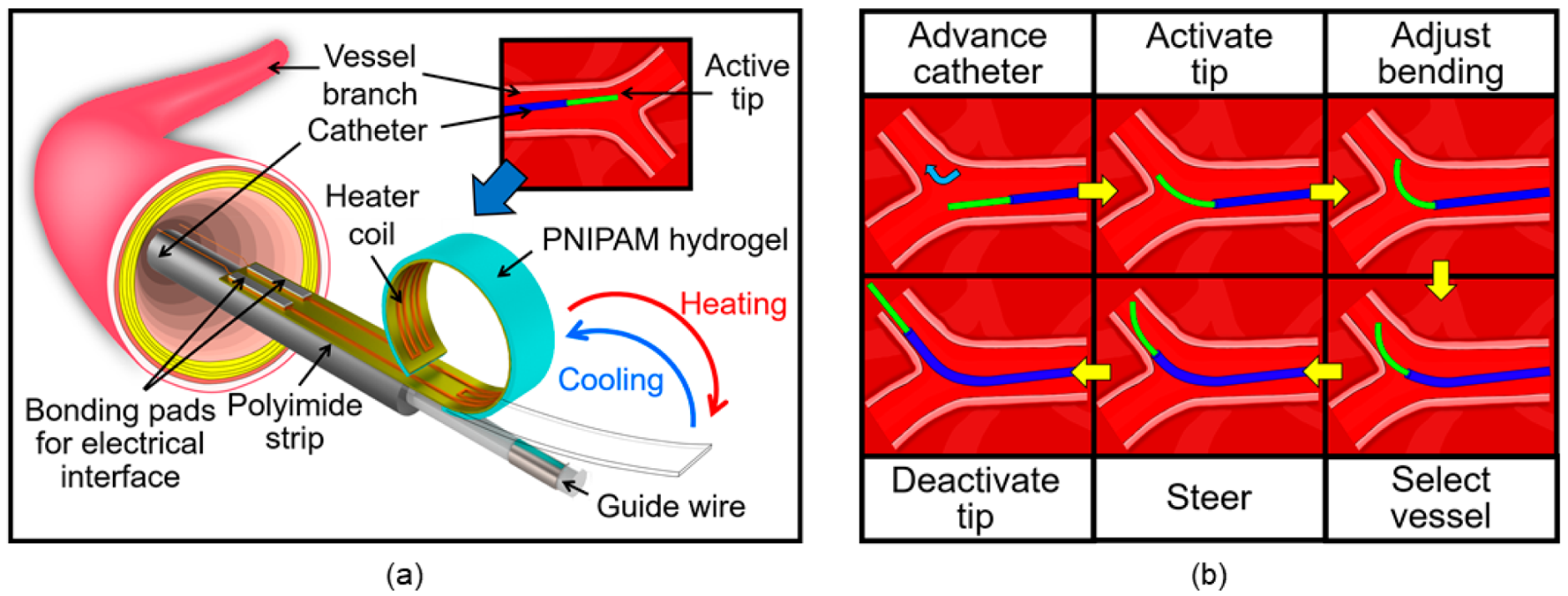
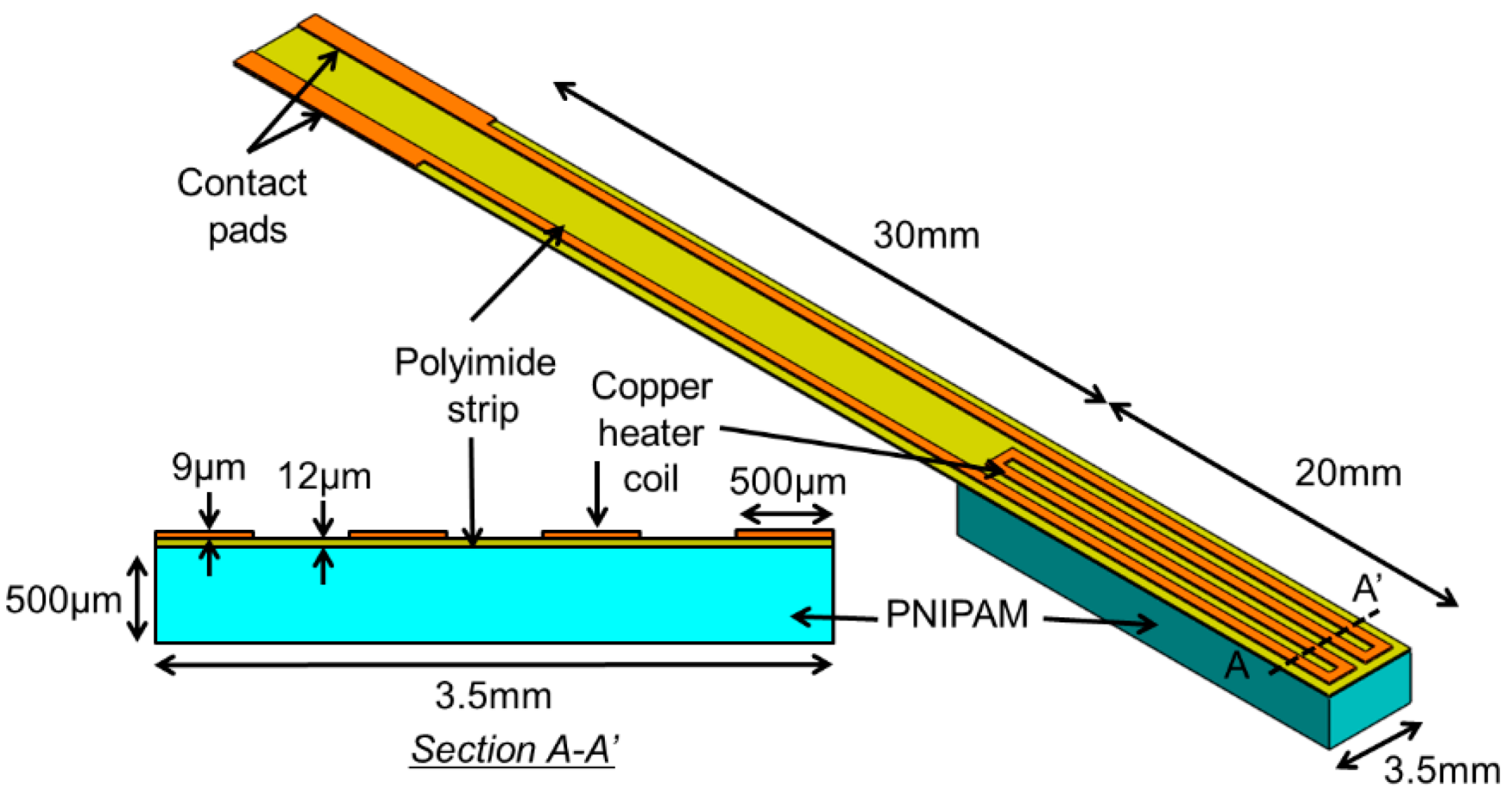
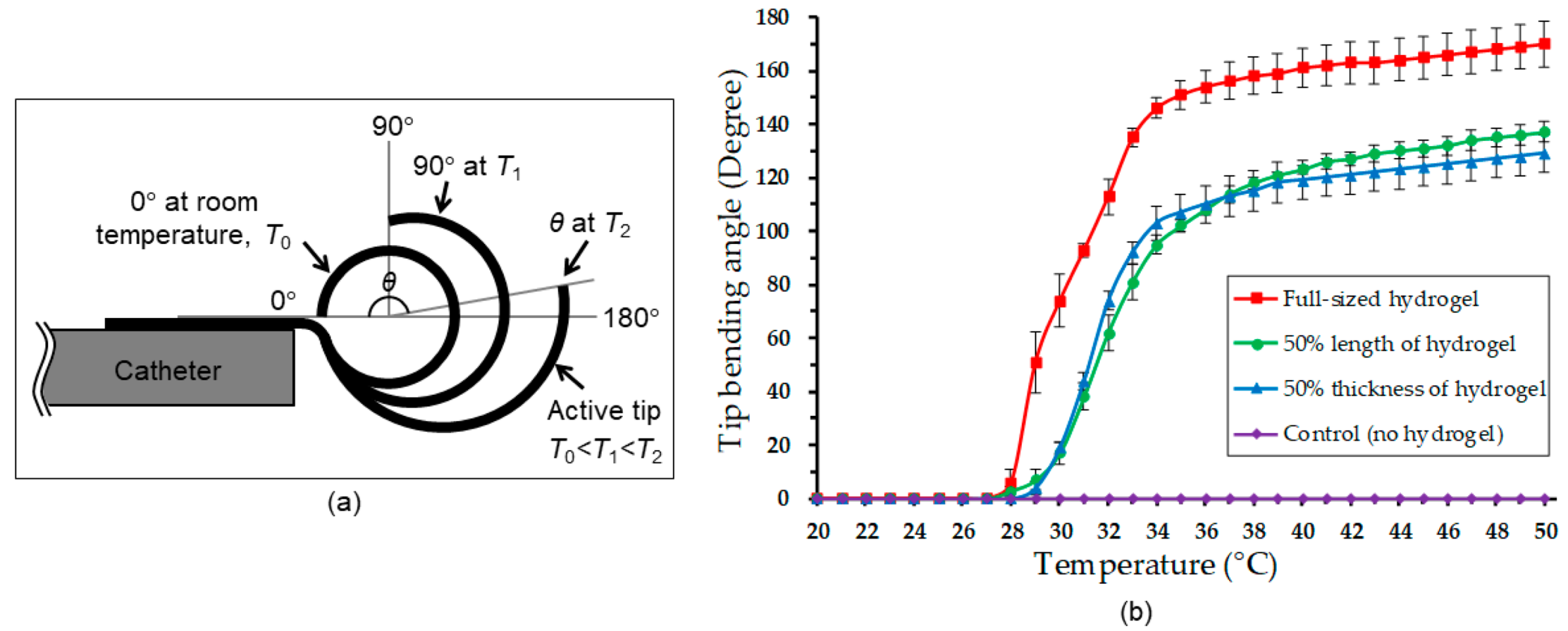
2. Principle and Design
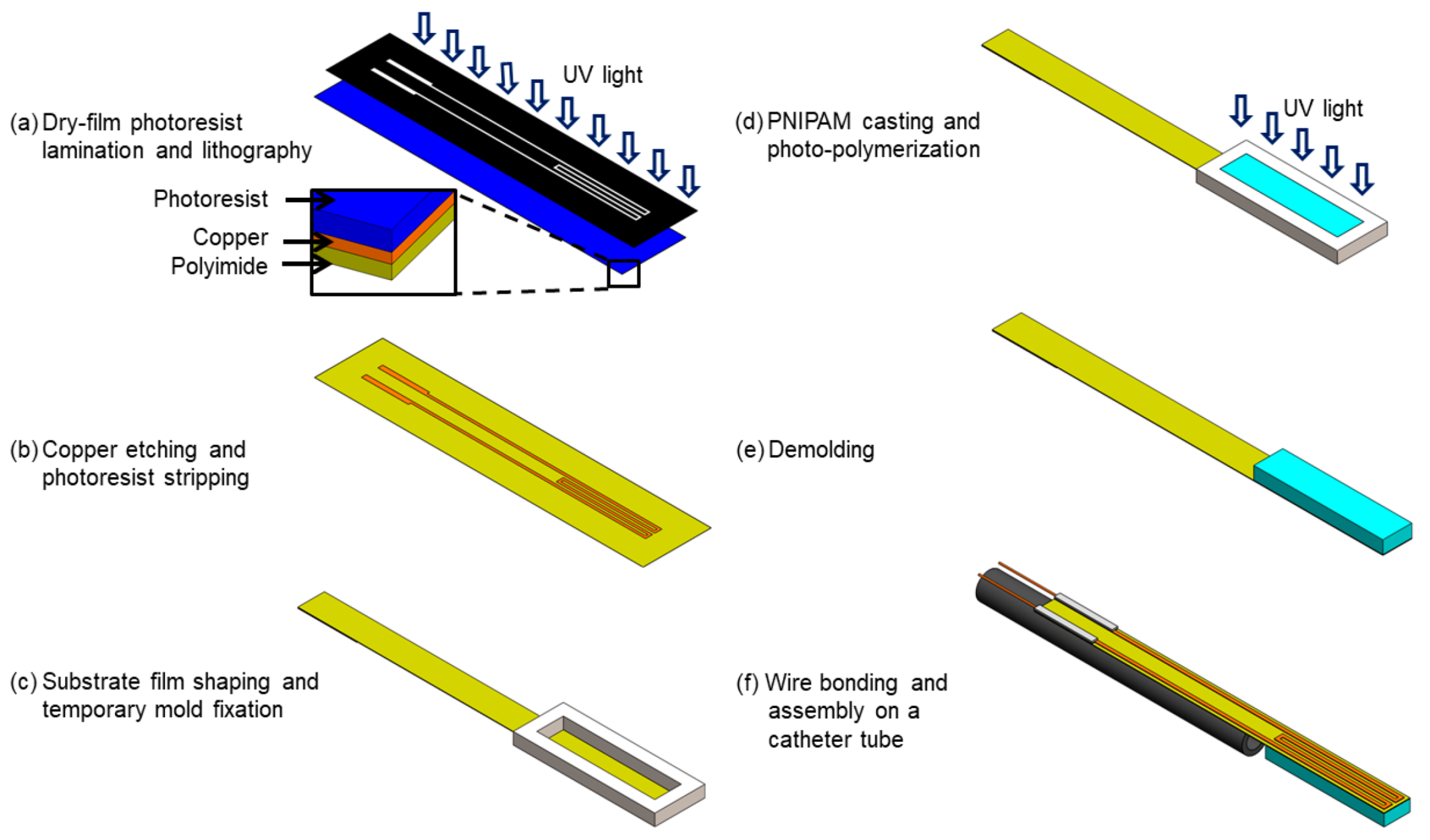
3. Device Fabrication
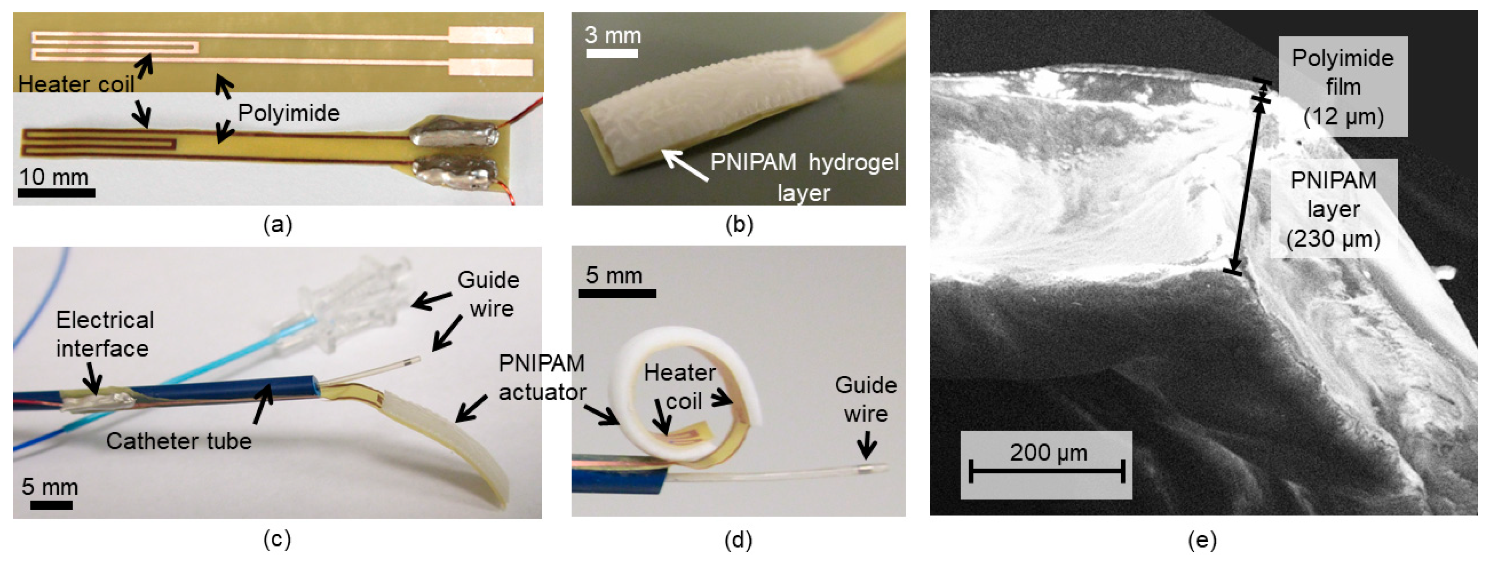
4. Results and Discussion
4.1. Characterization of PNIPAM Synthesized on the Flexible Strip
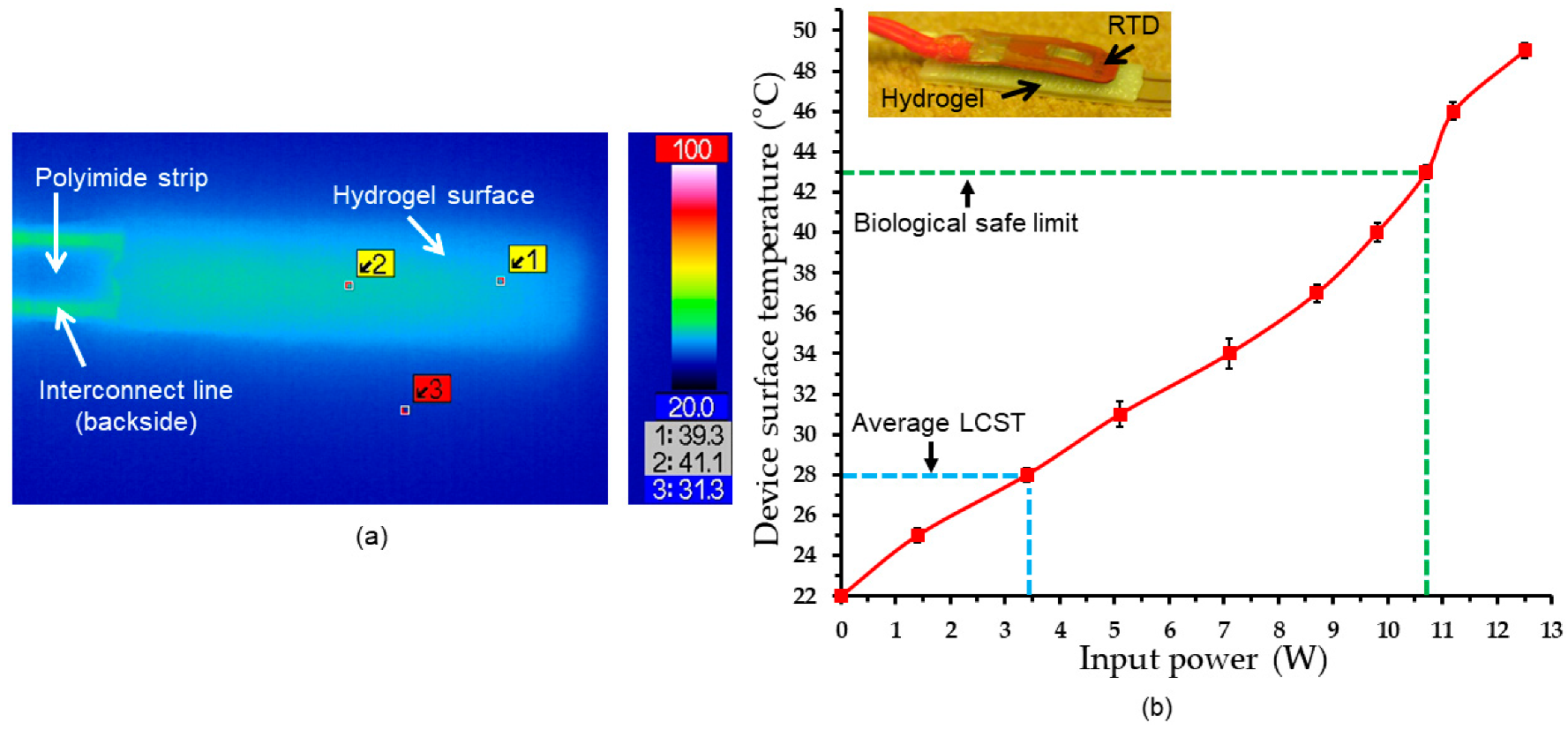
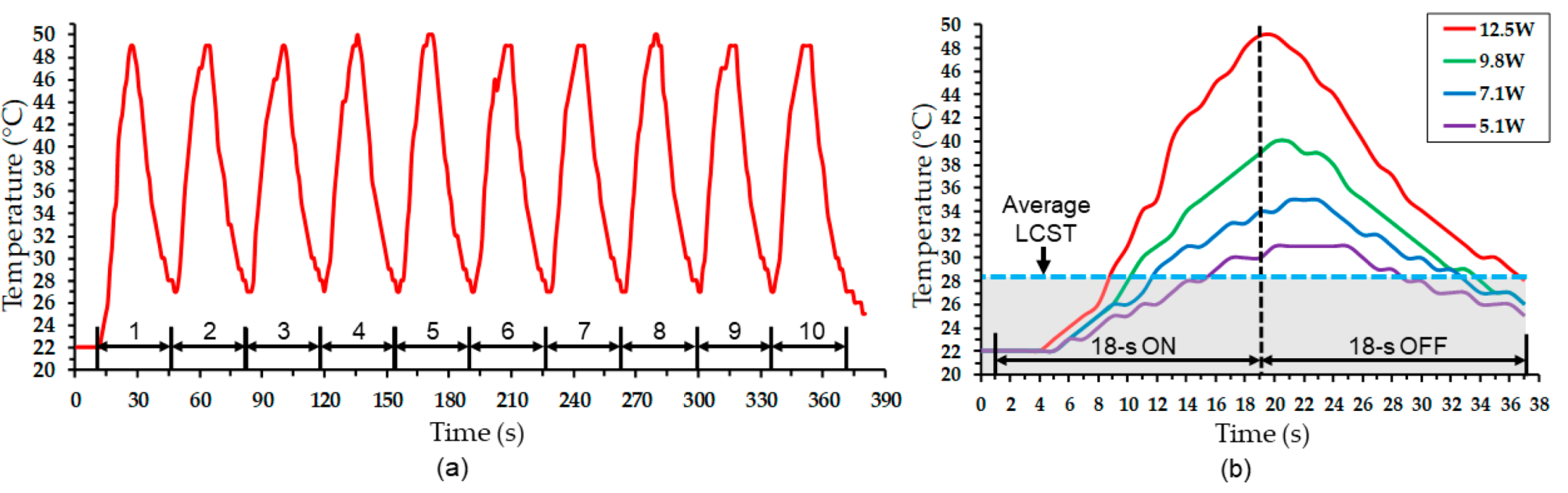
4.2. Electrothermal Response
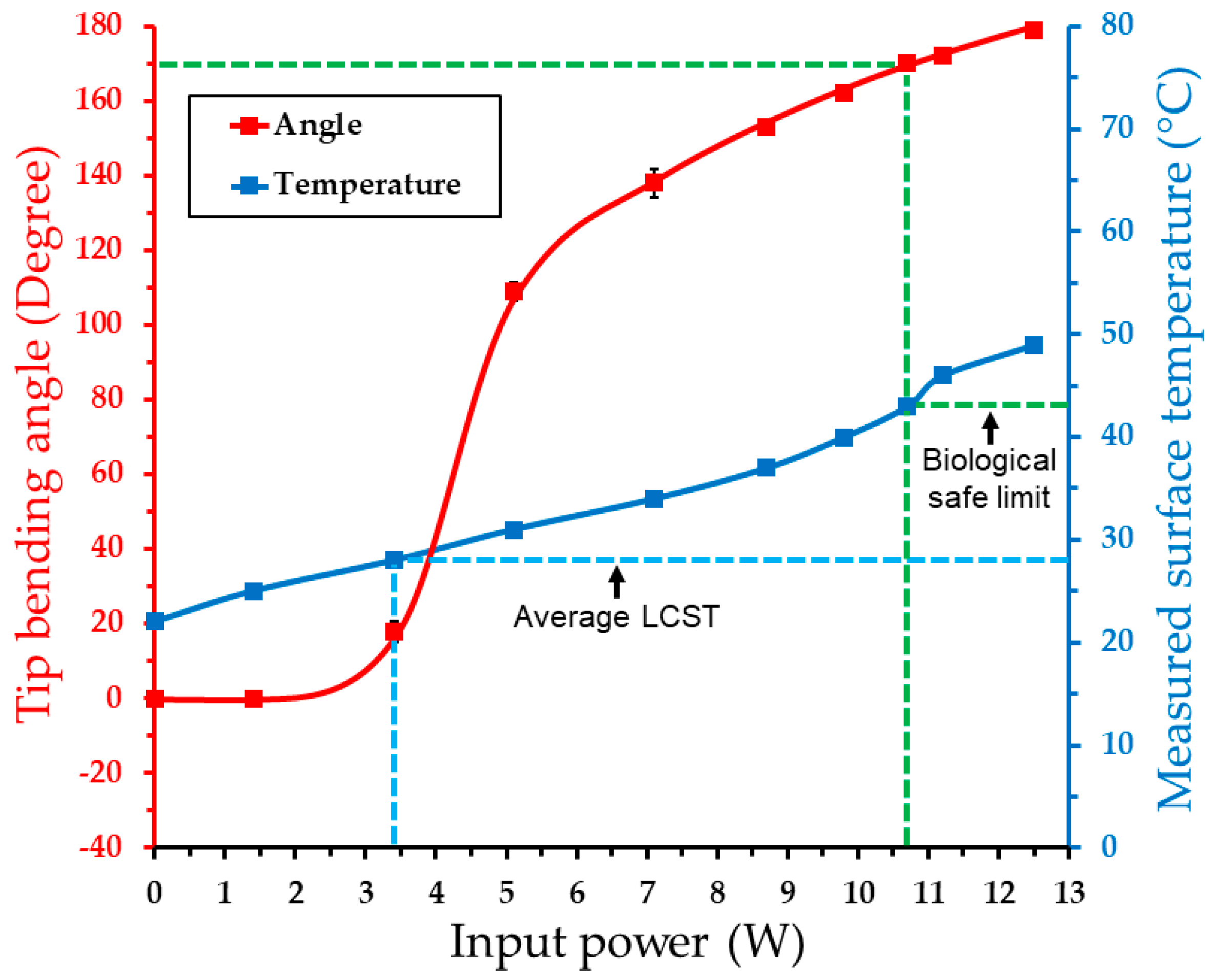
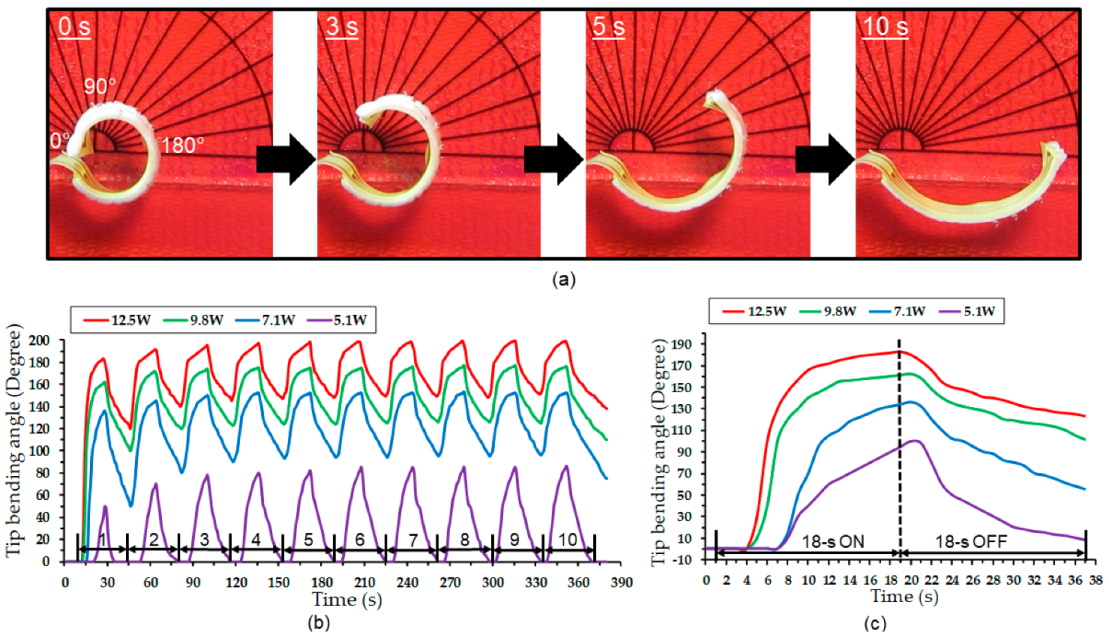
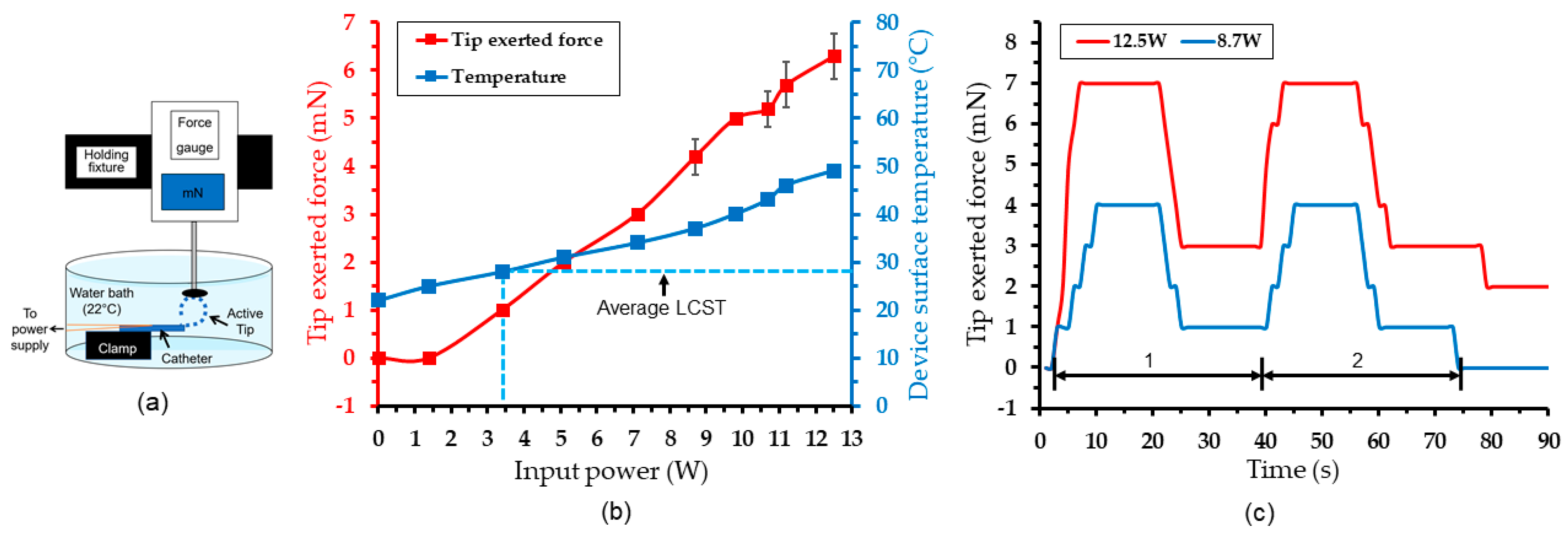
4.3. Actuation Test Results
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mao, H.; Wang, F.; Bao, J.; Chen, Z. Conventional Endovascular Devices. In Endovascular Surgery and Devices; Jing, Z., Mao, H., Dai, W., Eds.; Springer: Singapore, 2018; pp. 9–28. [Google Scholar]
- Manda, Y.R.; Baradhi, K.M. Cardiac Catheterization, Risks and Complications. Available online: https://www.ncbi.nlm.nih.gov/books/NBK531461/ (accessed on 1 December 2019).
- Moftakhar, P.; Lillaney, P.; Losey, A.D.; Cooke, D.L.; Martin, A.J.; Thorne, B.R.; Arenson, R.L.; Saeed, M.; Wilson, M.W.; Hetts, S.W. New-Generation Laser-lithographed Dual-Axis Magnetically Assisted Remote-controlled Endovascular Catheter for Interventional MR Imaging: In Vitro Multiplanar Navigation at 1.5 T and 3 T versus X-ray Fluoroscopy. Radiology 2015, 277, 842–852. [Google Scholar] [CrossRef] [PubMed]
- Heunis, C.; Sikorski, J.; Misra, S. Flexible Instruments for Endovascular Interventions: Improved Magnetic Steering, Actuation, and Image-Guided Surgical Instruments. IEEE Robot. Autom. Mag. 2018, 25, 71–82. [Google Scholar] [CrossRef]
- Correia, M.; Isabel, T.D. (Eds.) 13 Catheters. In The Practical Handbook of Perioperative Metabolic and Nutritional Care; Academic Press: Cambridge, MA, USA, 2019; pp. 157–167. [Google Scholar]
- Clogenson, H.C.M.; Dankelman, J.; van den Dobbelsteen, J.J. Time-Action Analysis of Catheter Manipulation during Navigation Tasks in Bifurcations. ASME J. Med. Devices 2013, 7, 044501. [Google Scholar] [CrossRef]
- Sinceri, S.; Carbone, M.; Marconi, M.; Moglia, A.; Ferrari, M.; Ferrari, V. Basic Endovascular Skills Trainer: A surgical simulator for the training of novice practitioners of endovascular procedures. In Proceedings of the 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Milan, Italy, 25–29 August 2015; pp. 5102–5105. [Google Scholar]
- Mazomenos, E.B.; Chang, P.L.; Rippel, R.A.; Rolls, A.; Hawke, D.J.; Bicknell, C.D.; Desjardins, A.; Riga, C.V.; Stoyanov, D. Catheter manipulation analysis for objective performance and technical skills assessment in transcatheter aortic valve implantation. Int. J. Comput. Assist. Radiol. Surg. 2016, 11, 1121–1131. [Google Scholar] [CrossRef]
- Maor, E.; Eleid, M.F.; Gulati, R.; Lerman, A.; Sandhu, G.S. Current and Future Use of Robotic Devices to Perform Percutaneous Coronary Interventions: A Review. J. Am. Heart Assoc. 2017, 6, 1–8. [Google Scholar] [CrossRef]
- Hutchins, G.M.; Miner, M.M.; Boitnott, J.K. Vessel Caliber and Branch-Angle of Human Coronary Artery Branch-Points. Circ. Res. 1976, 38, 572–576. [Google Scholar] [CrossRef]
- Clogenson, H.C.; Simonetto, A.; van den Dobbelsteen, J.J. Design optimization of a deflectable guidewire. Med. Eng. Phys. 2015, 37, 138–144. [Google Scholar] [CrossRef]
- Ali, A.; Plettenburg, D.H.; Breedveld, P. Steerable Catheters in Cardiology: Classifying Steerability and Assessing Future Challenges. IEEE Trans. Biomed. Eng. 2016, 63, 679–693. [Google Scholar] [CrossRef]
- Hu, X.; Chen, A.; Luo, Y.; Zhang, C.; Zhang, E. Steerable catheters for minimally invasive surgery: A review and future directions. Comput. Assist. Surg. 2018, 23, 21–41. [Google Scholar] [CrossRef]
- Chautems, C.; Lyttle, S.; Boehler, Q.; Nelson, B.J. Design and Evaluation of a Steerable Magnetic Sheath for Cardiac Ablations. IEEE Robot. Autom. Lett. 2018, 3, 2123–2128. [Google Scholar] [CrossRef]
- Ali, A.; Sakes, A.; Arkenbout, E.A.; Henselmans, P.; van Starkenburg, R.; Szili-Torok, T.; Breedveld, P. Catheter steering in interventional cardiology: Mechanical analysis and novel solution. Proc. Inst. Mech. Eng. H 2019, 233, 1207–1218. [Google Scholar] [CrossRef] [PubMed]
- Couture, T.; Szewczyk, J. Design and Experimental Validation of an Active Catheter for Endovascular Navigation. J. Med. Devices 2018, 12, 011003. [Google Scholar] [CrossRef]
- Blanc, L.; Delchambre, A.; Lambert, P. Flexible Medical Devices: Review of Controllable Stiffness Solutions. Actuators 2017, 6, 23. [Google Scholar] [CrossRef]
- Szewczyk, J.; Marchandise, E.; Flaud, P.; Royon, L.; Blanc, R. Active Catheters for Neuroradiology. J. Robot. Mechatron. 2011, 23, 105–115. [Google Scholar] [CrossRef]
- Fortini, A.; Rizzoni, R.; Suman, A.; Fabbri, E.; Merlin, M.; Pinelli, M. An experimentally-driven approach to model bending in a thermally activated SMA-based beam. Smart Mater. Struct. 2018, 27, 125004. [Google Scholar] [CrossRef]
- Lu, Y.H.; Mani, K.; Panigrahi, B. A Shape Memory Alloy-Based Miniaturized Actuator for Catheter Interventions. Cardiovasc. Eng. Technol. 2018, 9, 405. [Google Scholar] [CrossRef]
- Zhou, Y.; Huang, W.M. Shape Memory Effect in Polymeric Materials: Mechanisms and Optimization. Procedia IUTAM 2015, 12, 83–92. [Google Scholar] [CrossRef]
- Boyle, A.J.; Wierzbicki, M.A.; Herting, S.; Weems, A.C.; Nathan, A.; Hwang, W.; Maitland, D.J. In vitro performance of a shape memory polymer foam-coated coil embolization device. Med. Eng. Phys. 2017, 49, 56–62. [Google Scholar] [CrossRef]
- Loeve, A.J.; Bosma, J.H.; Breedveld, P.; Dodou, D.; Dankelman, J. Polymer Rigidity control for Endoscopic Shaft-Guide ‘Plastolock’—A feasibility Study. J. Med. Devices 2010, 4, 045001. [Google Scholar] [CrossRef]
- Farajollahi, M.; Woehling, V.; Plesse, C.; Nguyen, G.T.M.; Vidal, F.; Sassani, F.; Yang, V.X.D.; Madden, J.D.W. Self-contained tubular bending actuator driven by conducting polymers. Sens. Actuators A-Phys. 2016, 249, 45–56. [Google Scholar] [CrossRef]
- Zhao, L.; Yang, Y.; Hu, Y.; Li, C.; Wu, Y.; Ren, M.; Chen, W. A square tubular conducting polymer actuator for smart catheter application. Multifunct. Mater. 2019, 2, 044001. [Google Scholar] [CrossRef]
- Fang, B.; Ju, M.; Lin, C. A new approach to develop ionic polymer-metal composites (IPMC) actuator: Fabrication and control for active catheter systems. Sen. Actuator A-Phys. 2007, 137, 321–329. [Google Scholar] [CrossRef]
- Horiuchi, T.; Asaka, K. 245 mm length IPMC catheter with an ellipse-like cross section. Smart Mater. Struct. 2019, 28, 095028. [Google Scholar] [CrossRef]
- Joshi, S.; Vig, K.; Singh, S.R. Advanced Hydrogels for Biomedical Applications. Biomed. J. Sci. Tech. Res. 2018, 5, 4302–4306. [Google Scholar]
- Caló, E.; Kutoryanshiy, V.V. Biomedical application of hydrogels: A review of patents and commercial products. Eur. Polym. J. 2015, 65, 252–267. [Google Scholar] [CrossRef]
- Liu, Y.; Hsu, S. Synthesis and Biomedical Applications of self-healing Hydrogels. Front. Chem. 2018, 6, 449. [Google Scholar] [CrossRef] [PubMed]
- Koetting, M.C.; Peters, J.T.; Steichen, S.D.; Peppas, N.A. Stimulus-responsive hydrogels: Theory, modern advances, and applications. Mater. Sci. Eng. R-Rep. 2015, 93, 1–49. [Google Scholar] [CrossRef]
- Shi, Q.; Liu, H.; Tang, D.; Li, Y.; Li, X.; Xu, F. Bioactuators based on stimulus-responsive hydrogels and their emerging biomedical applications. NPG Asia Mater. 2019, 64, 1–21. [Google Scholar] [CrossRef]
- Chai, Q.; Jiao, Y.; Yu, X. Hydrogels for Biomedical Applications: Their Characteristics and the Mechanisms behind Them. Gels 2017, 3, 6. [Google Scholar] [CrossRef]
- Hirotsu, S.; Hirokawa, Y.; Tanaka, T. Volume-phase transitions of ionized N-isopropylacrylamide gels. J. Chem. Phys. 1987, 87, 1392–1395. [Google Scholar] [CrossRef]
- Shang, J.; Le, X.; Zhang, J.; Chen, T.; Theato, P. Trends in polymeric shape memory hydrogels and hydrogel actuators. Polym. Chem. 2019, 10, 1036–1055. [Google Scholar] [CrossRef]
- Lowenberg, C.; Balk, M.; Wischke, C.; Behl, M.; Lenlein, A. Shape-Memory Hydrogels: Evolution of Structural Principles to Enable Shape Switching of Hydrophilic Polymer Networks. Acc. Chem. Res. 2017, 50, 723–732. [Google Scholar] [CrossRef] [PubMed]
- RuizRubio, L.; Perez-Alvarez, L.; Artetxe, B.; Guitierrez-Zorilla, J.M.; Vilas, J.L. Shape memory Hydrogels Based on Noncovalent Interactions. In Shape-Memory Materials; Ares, A.E., Ed.; IntechOpen: London, UK, 2018; pp. 95–106. [Google Scholar]
- Xiao, H.; Ma, C.; Le, X.; Wang, L.; Lu, W.; Theato, P.; Hu, T.; Zhang, J.; Chen, T. A Multiple Shape Memory Hydrogel Induced by Reversible Physical Interactions at Ambient Condition. Polymers 2017, 9, 138. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, Z.; Gooding, E.A.; Pimenov, K.V.; Wang, L.; Asher, S.A. UV Resonance Raman Determination of Molecular Mechanism of poly(N-isopropylacrylamide) Volume Phase Transition. J. Phys. Chem. B 2009, 113, 4248–4256. [Google Scholar] [CrossRef] [PubMed]
- Lanzalaco, S.; Armelin, E. Poly(N-isopropylacrylamide) and Copolymers: A Review on Recent Progresses in Biomedical Applications. Gels 2017, 3, 36. [Google Scholar] [CrossRef] [PubMed]
- Paradossi, G.; Chiessi, E. Tacticity-Dependent Interchain Interactions of Poly(N-isopropylacrylamide) in Water: Toward the Molecular Dynamics Simulation of a Thermoresponsive Microgel. Gels 2017, 3, 13. [Google Scholar] [CrossRef]
- Rahimi, S.; Sarraf, E.H.; Wong, G.K.; Takahata, K. Implantable drug delivery device using frequency-controlled wireless hydrogel microvalves. Biomed. Microdev. 2011, 13, 267–277. [Google Scholar] [CrossRef]
- Guan, T.; Godts, F.; Ceyssens, F.; Vanderleyden, E.; Adesanya, K.; Dubruel, P.; Neves, H.P.; Puers, R. Development and fabrication of a novel photopatternable electric responsive Pluronic hydrogel for MEMS applications. Sens. Actuators A-Phys. 2012, 186, 184–190. [Google Scholar] [CrossRef]
- Yoon, Y.; Chae, I.; Thundat, T.; Lee, J. Hydrogel Microelectromechanical System (MEMS) Resonators: Beyond Cost-Effective Sensing Platform. Adv. Mater. Technol. 2019, 4, 1800597. [Google Scholar] [CrossRef]
- D’Eramo, L.; Chollet, B.; Leman, M.; Martwong, E.; Li, M.; Li, M.; Geisler, H.; Dupire, J.; Kerdraon, M.; Vergne, C.; et al. Microfluidic actuators based on temperature-responsive hydrogels. Microsyst. Nanoeng. 2018, 4, 17069. [Google Scholar] [CrossRef]
- Sridhar, V.; Takahata, K. A hydrogel-based passive wireless sensor using a flex-circuit inductive transducer. Sens. Actuators A-Phys. 2009, 155, 58–65. [Google Scholar] [CrossRef]
- Gerlach, G.; Arndt, K. Hydrogel Sensors and Actuators. In Spring Series on Chemical Sensors and Biosensors; Urban, G., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; Volume 6, pp. 221–244. [Google Scholar]
- Eddington, D.T.; Beebe, D.J. A valved responsive hydrogel microdispensing device with integrated pressure source. J. Microelectromech. Syst. 2004, 13, 586–593. [Google Scholar] [CrossRef]
- Siegel, R.A.; Gu, Y.; Baldi, A.; Ziaie, B. Novel swelling/shrinking behaviors of glucose-binding hydrogels and their potential use in a microfluidic insulin delivery system. Macromol. Symp. 2004, 207, 249–256. [Google Scholar] [CrossRef]
- López-Barriguete, J.E.; Isoshima, T.; Bucio, E. Development and characterization of thermal responsive hydrogel films for biomedical sensor application. Mater. Res. Express 2018, 5, 045703. [Google Scholar] [CrossRef]
- Breger, J.C.; Yoon, C.; Xiao, R.; Kwag, H.R.; Wang, M.O.; Fisher, J.P.; Nguyen, T.D.; Gracias, D.H. Self-Folding Thermo-Magnetically Responsive Soft Microgrippers. ACS Appl. Mater. Interfaces 2015, 7, 3398–3405. [Google Scholar] [CrossRef]
- Cao, M.; Wang, Y.; Hu, X.; Gong, H.; Li, R.; Cox, H.; Zhang, J.; Waigh, T.A.; Xu, H.; Lu, J.R. Reversible Thermoresponsive Peptide-PNIPAM Hydrogels for Controlled Drug Delivery. Biomacromolecules 2019, 20, 3601–3610. [Google Scholar] [CrossRef]
- Banerjee, H.; Suhail, M.; Ren, H. Hydrogel Actuators and Sensors for Biomedical Soft Robots: Brief Overview with Impending Challenges. Biomimetics 2018, 3, 15. [Google Scholar] [CrossRef]
- Capella, V.; Rivero, R.E.; Liaudat, A.C.; Ibarra, L.E.; Roma, D.A.; Alustiza, F.; Manas, F.; Barbero, C.A.; Bosch, P.; Rivarola, C.R.; et al. Cytotoxicity and bioadhesive properties of poly-N-isopropylacrylamide hydrogel. Heliyon 2019, 5, e01474. [Google Scholar] [CrossRef]
- Ordeig, O.; Sia, S.K.; Chin, S.Y.; Kohler, A.-C.; Poh, Y.K.C. Systems, Methods, and Devices for In Vivo Delivery Using Remote Actuation of Implantable Hydrogel Mems Devices. US9907906B2, 30 January 2014. [Google Scholar]
- Kottapalli, A.G.P.; Bora, M.; Asadnia, M.; Miao, J.; Venkatraman, S.S.; Triantafyllou, M. Nanofibril scaffold assisted MEMS artificial hydrogel neuromasts for enhanced sensitivity flow sensing. Sci. Rep. 2016, 6, 19336. [Google Scholar] [CrossRef]
- Kuckling, D. Responsive hydrogel layers—from synthesis to applications. Colloid Polym. Sci. 2009, 287, 881–891. [Google Scholar] [CrossRef]
- Warren, H.; Panhuis, M.; Spinks, G.M.; Officer, D.L. Thermal actuation of hydrogel from PNIPAm, alginate, and carbon nanofibers. J. Polym. Sci. Part B Polym. Phys. 2017, 56, 46–52. [Google Scholar] [CrossRef]
- Evanghelidis, A.; Beregoi, M.; Diculescu, V.C.; Galatanu, A.; Ganea, P.; Enculescu, L. Flexible Delivery Patch Systems based on Thermoresponsive Hydrogels and Submicronic Fiber Heaters. Sci. Rep. 2018, 8, 17555. [Google Scholar] [CrossRef] [PubMed]
- Tebaldi, M.L.; Oda, C.M.R.; Monteiro, L.O.F.; de Barros, A.L.B.; Santos, C.J.; Soares, D.C.F. Biomedical nanoparticle carriers with combined thermal and magnetic response: Current preclinical investigations. J. Magn. Magn. Mater. 2018, 461, 116–127. [Google Scholar] [CrossRef]
- Timothy, B.; Kim, D.; Yoo, S.I.; Yoon, J. Tuning of volume phase transition for poly(N-isopropylacrylamide) ionogels by copolymerization with solvatophilic monomers. Soft Matter 2018, 14, 7664–7670. [Google Scholar] [CrossRef]
- Van der Linden, H.; Olthuis, W.; Bergveld, P. An efficient method for the fabrication of temperature-sensitive hydrogel microactuators. Lab Chip 2004, 4, 619–624. [Google Scholar] [CrossRef]
- Selvaraj, M.; Takahata, K. A steerable smart catheter tip realized by flexible hydrogel actuator. In Proceedings of the 2016 IEEE 29th International Conference on Micro Electro Mechanical Systems (MEMS), Shanghai, China, 24–28 January 2016; pp. 161–164. [Google Scholar]
- Yarmolenko, P.S.; Moon, E.J.; Landon, C.; Manzoor, A.; Hochman, D.W.; Viglianti, B.L.; Dewhirst, M.W. Thresholds for thermal damage to normal tissues: An update. Int. J. Hyperthermia 2011, 27, 320–343. [Google Scholar] [CrossRef]
- Conrad, B.L.; Zinn, M.R. Interleaved Continuum-Rigid Manipulation: An Approach to Increase the Capability of Minimally Invasive Surgical Systems. IEEE/ASME Trans. Mechatron. 2017, 22, 29–40. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Selvaraj, M.; Takahata, K. Electrothermally Driven Hydrogel-on-Flex-Circuit Actuator for Smart Steerable Catheters. Micromachines 2020, 11, 68. https://doi.org/10.3390/mi11010068
Selvaraj M, Takahata K. Electrothermally Driven Hydrogel-on-Flex-Circuit Actuator for Smart Steerable Catheters. Micromachines. 2020; 11(1):68. https://doi.org/10.3390/mi11010068
Chicago/Turabian StyleSelvaraj, Madeshwaran, and Kenichi Takahata. 2020. "Electrothermally Driven Hydrogel-on-Flex-Circuit Actuator for Smart Steerable Catheters" Micromachines 11, no. 1: 68. https://doi.org/10.3390/mi11010068
APA StyleSelvaraj, M., & Takahata, K. (2020). Electrothermally Driven Hydrogel-on-Flex-Circuit Actuator for Smart Steerable Catheters. Micromachines, 11(1), 68. https://doi.org/10.3390/mi11010068




