Grasping and Releasing Agarose micro Beads in Water Drops
Abstract
1. Introduction
2. Materials and Methods
2.1. The Agarose Microbeads
2.2. The Device Under Test (DUT)
2.3. The Experimental Setup
2.4. Sample Preparation
2.5. Gripper Preparation
3. Results
- (i)
- the extraction of a single micro bead from a drop;
- (ii)
- the insertion of a single micro bead into the drop;
- (iii)
- the grasping of a single micro bead inside a water drop.
3.1. Extraction of a Single Micro Bead from a Drop
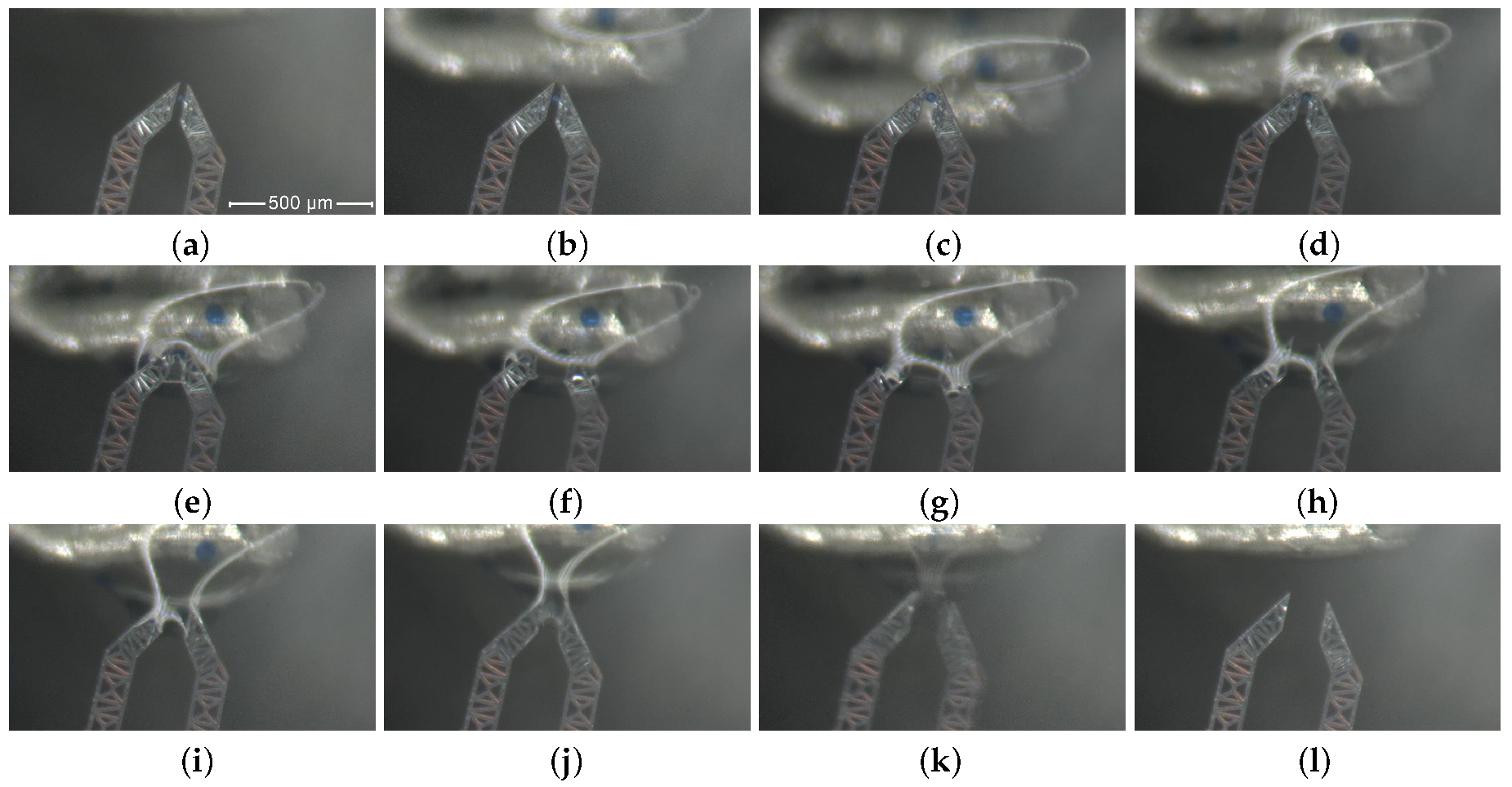
- First, the micro-gripper is set in its initial state (normally open, no voltage applied) and the water drop is guided toward the gripping device by operating the spoon-shaped droplet host micro-positioning device, as shown in Figure 5 from (a) to (g).
- The jaws-tips are then inserted into the water drop, as shown in Figures from (h) to (k).
- Once the tips are within the drop, a combination of micro-metric displacements of the tips and the drop allows one or more micro beads to enter inside the operating window area of the jaw-tips; usually, this operation require few minutes only, the waiting time mainly depending on the beads density (units per drop); the bead centering in the monitored pane is made via direct observation, while the transverse centering is done by using the microscope focusing.
- When a single micro bead is centered in the operating volume, the grasping phase is activated and the device is actuated by supplying a gradually increasing voltage from zero up to 24 V to the jaw-connected electrodes and keeping null the voltage on the ground electrode. This induces the gradual closure of the jaws and a successful bead grasping, as shown by frames from (l) to (o). The DUT is now in its grasping state.
- The water solution is then moved away from the closed grip system, operating the spoon-shaped droplet host micro-positioning device, as shown by frames from (p) to (s), until the bead is completely extracted, as in frame (t).
- During removal motion, part of the residual water on the jaw-tips evaporates, as illustrated in Figure 5 (u).
- Finally, a change in the bead dimension occurs due to evaporation phenomena. In fact, the passage from a wet to a dry environment implies a significant and progressive shrinking, as shown by frames from (v) to (z).
3.2. Insertion of a Single Micro Bead into the Drop
- An agarose bead grasped by the device jaws is shown in Figure 7 (a).
- The water drop progressively approaches the device, as illustrated by frames (b) and (c).
- The micro bead is successfully inserted in the drop of solution, as reported by frames (d) and (e).
- The micro bead is then successfully released, as documented in Figure 7 by frames (f) and (g): in this phase, the voltage applied to the device is decreased down to 0 V.
- Finally, the device is extracted again from the water solution, as reported in frames from (h) to (l).
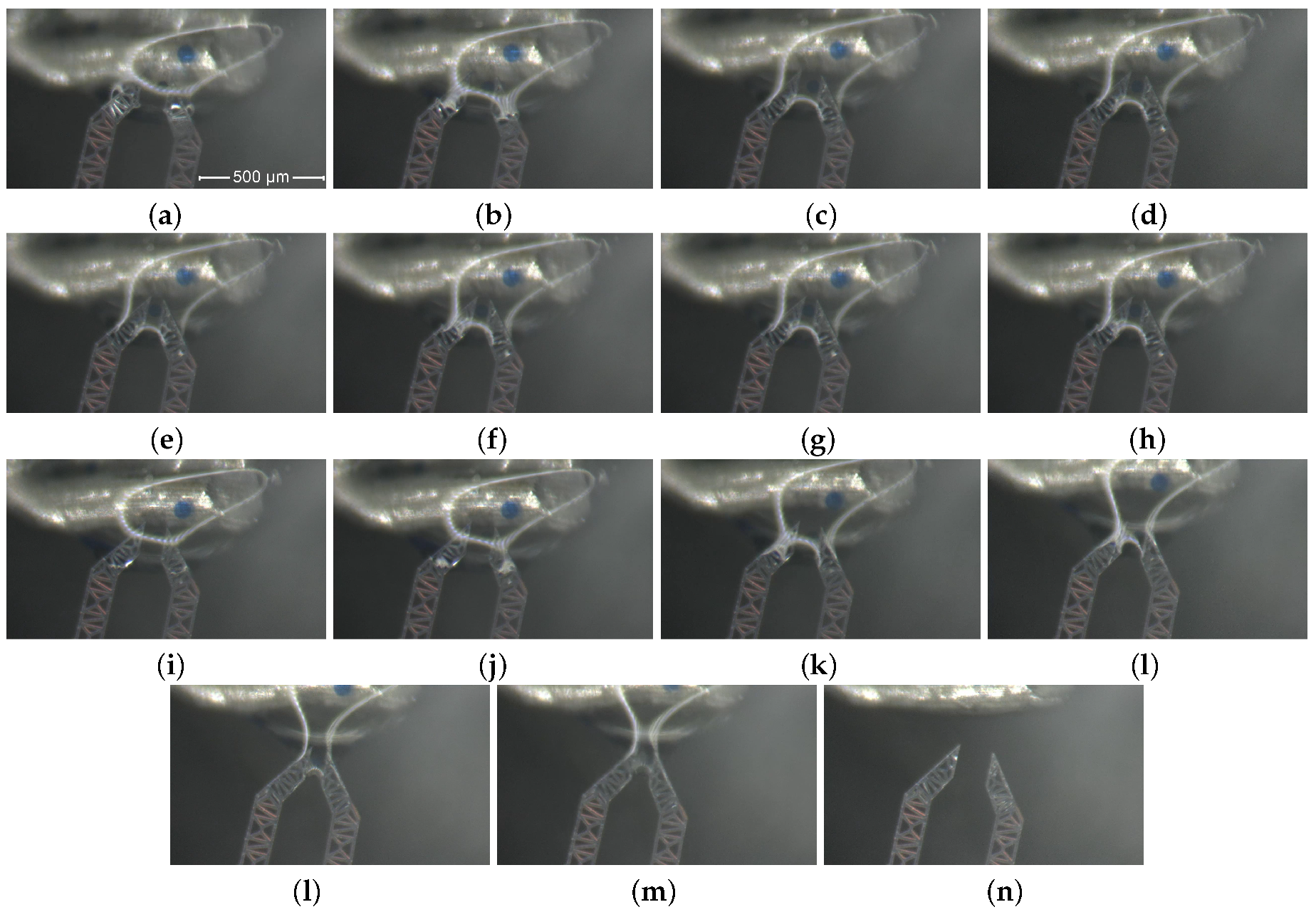
3.3. Grasping of a Single Micro Bead inside a Water Drop
- First, the agarose bead is progressively approached, as depicted by Figures from (a) to (c).
- Then, the jaws grasp one agarose bead as illustrated in frame (d).
- Frames from (e) to (h) describe how the bead is squeezed.
- After that, the bead is released and let to go (i).
- The final sequence of frames, from (j) to (n), represents the extraction and removal of the jaw-tips from the drop.
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Verotti, M.; Dochshanov, A.; Belfiore, N.P. A Comprehensive Survey on Microgrippers Design: Mechanical Structure. J. Mech. Des. Trans. ASME 2017, 139, 060801. [Google Scholar] [CrossRef]
- Dochshanov, A.; Verotti, M.; Belfiore, N.P. A Comprehensive Survey on Microgrippers Design: Operational Strategy. J. Mech. Des. Trans. ASME 2017, 139, 070801. [Google Scholar] [CrossRef]
- Park, J.; Moon, W. A hybrid-type micro-gripper with an integrated force sensor. Microsyst. Technol. 2003, 9, 511–519. [Google Scholar] [CrossRef]
- Kim, D.H.; Lee, M.G.; Kim, B.; Sun, Y. A superelastic alloy microgripper with embedded electromagnetic actuators and piezoelectric force sensors: A numerical and experimental study. Smart Mater. Struct. 2005, 14, 1265–1272. [Google Scholar] [CrossRef]
- Gnerlich, M.; Perry, S.F.; Tatic-Lucic, S. A submersible piezoresistive MEMS lateral force sensor for a diagnostic biomechanics platform. Sens. Actuators A Phys. 2012, 188, 111–119. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Murali Krishna, A.; Lombardi, D.; Crewe, A.; Alexander, N. Economic MEMS based 3-axis water proof accelerometer for dynamic geo-engineering applications. Soil Dyn. Earthq. Eng. 2012, 36, 111–118. [Google Scholar] [CrossRef]
- Neild, A.; Oberti, S.; Beyeler, F.; Dual, J.; Nelson, B.J. A micro-particle positioning technique combining an ultrasonic manipulator and a microgripper. J. Micromech. Microeng. 2006, 16, 1562–1570. [Google Scholar] [CrossRef]
- Luisetto, I.; Tuti, S.; Marconi, E.; Veroli, A.; Buzzin, A.; de Cesare, G.; Natali, S.; Verotti, M.; Giovine, E.; Belfiore, N.P. An interdisciplinary approach to the nanomanipulation of SiO2 nanoparticles: Design, fabricationand feasibility. Appl. Sci. 2018, 8, 2645. [Google Scholar] [CrossRef]
- Beyeler, F.; Neild, A.; Oberti, S.; Bell, D.J.; Sun, Y.; Dual, J.; Nelson, B.J. Monolithically fabricated microgripper with integrated force sensor for manipulating microobjects and biological cells aligned in an ultrasonic field. J. Microelectromech. Syst. 2007, 16, 7–15. [Google Scholar] [CrossRef]
- Beyeler, F.; Muntwyler, S.; Nagy, Z.; Moser, M.; Nelson, B.J. A multi-axis MEMS force-torque sensor for measuring the load on a microrobot actuated by magnetic fields. In Proceedings of the IEEE International Conference on Intelligent Robots and Systems, San Diego, CA, USA, 29 October–2 November 2007; pp. 3803–3808. [Google Scholar]
- Veroli, A.; Buzzin, A.; Frezza, F.; de Cesare, G.; Hamidullah, M.; Giovine, E.; Verotti, M.; Belfiore, N. An approach to the extreme miniaturization of rotary comb drives. Actuators 2018, 7, 70. [Google Scholar] [CrossRef]
- Arai, F.; Ando, D.; Fukuda, T.; Nonoda, Y.; Oota, T. Micro manipulation based on micro physics—Strategy based on attractive force reduction and stress measurement. In Proceedings of the IEEE International Conference on Intelligent Robots and Systems, Pittsburgh, PA, USA, 5–9 August 1995; Volume 2, pp. 236–241. [Google Scholar]
- Arai, F.; Andou, D.; Nonoda, Y.; Fukuda, T.; Iwata, H.; Itoigawa, K. Integrated microendeffector for micromanipulation. IEEE/ASME Trans. Mechatron. 1998, 3, 17–23. [Google Scholar] [CrossRef]
- Rebello, K.J. Applications of MEMS in surgery. Proc. IEEE 2004, 92, 43–55. [Google Scholar] [CrossRef]
- Gosline, A.H.; Vasilyev, N.V.; Butler, E.J.; Folk, C.; Cohen, A.; Chen, R.; Lang, N.; Del Nido, P.J.; Dupont, P.E. Percutaneous intracardiac beating-heart surgery using metal MEMS tissue approximation tools. Int. J. Robot. Res. 2012, 31, 1081–1093. [Google Scholar] [CrossRef] [PubMed]
- Balla, A.; Quaresima, S.; Ursi, P.; Seitaj, A.; Palmieri, L.; Badiali, D.; Paganini, A.M. Hiatoplasty with crura buttressing versus hiatoplasty alone during laparoscopic sleeve gastrectomy. Gastroenterol. Res. Pract. 2017, 2017, 6565403. [Google Scholar] [CrossRef] [PubMed]
- Popivanov, G.; Tabakov, M.; Mantese, G.; Cirocchi, R.; Piccinini, I.; D’Andrea, V.; Covarelli, P.; Boselli, C.; Barberini, F.; Tabola, R.; et al. Surgical treatment of gastrointestinal stromal tumors of the duodenum: A literature review. Transl. Gastroenterol. Hepatol. 2018, 3, 71. [Google Scholar] [CrossRef] [PubMed]
- Cochetti, G.; Del Zingaro, M.; Boni, A.; Cocca, D.; Panciarola, M.; Tiezzi, A.; Gaudio, G.; Balzarini, F.; Ursi, P.; Mearini, E. Colovesical fistula: Review on conservative management, surgical techniques and minimally invasive approaches. Giornale di Chirurgia 2018, 39, 195–207. [Google Scholar] [PubMed]
- Quaresima, S.; Balla, A.; Dambrosio, G.; Bruzzone, P.; Ursi, P.; Lezoche, E.; Paganini, A.M. Endoluminal loco-regional resection by TEM after R1 endoscopic removal or recurrence of rectal tumors. Minim. Invasive Ther. Allied Technol. 2016, 25, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Paci, M.; Scoglio, D.; Ursi, P.; Barchetti, L.; Fabiani, B.; Ascoli, G.; Lezoche, G. Transanal Endocopic Microsurgery (TEM) in advanced rectal cancer disease treatment [Il ruolo della TEM nel trattamento dei tumori del retto extraperitoneale]. Annali Italiani di Chirurgia 2010, 81, 269–274. [Google Scholar]
- Lezoche, E.; Fabiani, B.; D’Ambrosio, G.; Ursi, P.; Balla, A.; Lezoche, G.; Monteleone, F.; Paganini, A.M. Nucleotide-guided mesorectal excision combined with endoluminal locoregional resection by transanal endoscopic microsurgery in the treatment of rectal tumors: Technique and preliminary results. Surg. Endosc. 2013, 27, 4136–4141. [Google Scholar] [CrossRef]
- Ursi, P.; Santoro, A.; Gemini, A.; Arezzo, A.; Pironi, D.; Renzi, C.; Cirocchi, R.; Di Matteo, F.; Maturo, A.; D’Andrea, V.; et al. Comparison of outcomes following intersphincteric resection vs low anterior resection for low rectal cancer: A systematic review. Giornale di Chirurgia 2018, 39, 123–142. [Google Scholar]
- Bashir, R. BioMEMS: State-of-the-art in detection, opportunities and prospects. Adv. Drug Deliv. Rev. 2004, 56, 1565–1586. [Google Scholar] [CrossRef] [PubMed]
- Grayson, A.; Shawgo, R.; Johnson, A.; Flynn, N.; Li, Y.; Cima, M.; Langer, R. A BioMEMS review: MEMS technology for physiologically integrated devices. Proc. IEEE 2004, 92, 6–21. [Google Scholar] [CrossRef]
- Bhushan, B. Nanotribology and nanomechanics of MEMS/NEMS and BioMEMS/BioNEMS materials and devices. Microelectron. Eng. 2007, 84, 387–412. [Google Scholar] [CrossRef]
- Bragheri, F.; Minzioni, P.; Martinez Vazquez, R.; Bellini, N.; Paiè, P.; Mondello, C.; Ramponi, R.; Cristiani, I.; Osellame, R. Optofluidic integrated cell sorter fabricated by femtosecond lasers. Lab Chip 2012, 12, 3779–3784. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Kuhn, T.; Moy, V. Mechanical properties of L929 cells measured by atomic force microscopy: Effects of anticytoskeletal drugs and membrane crosslinking. Scanning 1998, 20, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Mattana, S.; Mattarelli, M.; Urbanelli, L.; Sagini, K.; Emiliani, C.; Serra, M.; Fioretto, D.; Caponi, S. Non-contact mechanical and chemical analysis of single living cells by microspectroscopic techniques. Light Sci. Appl. 2018, 7, 17139. [Google Scholar] [CrossRef] [PubMed]
- Mulvany, M.J.; Halpern, W. Mechanical properties of vascular smooth muscle cells in situ. Nature 1976, 260, 617–619. [Google Scholar] [CrossRef]
- Wakatsuki, T.; Schwab, B.; Thompson, N.; Elson, E. Effects of cytochalasin D and latrunculin B on mechanical properties of cells. J. Cell Sci. 2001, 114, 1025–1036. [Google Scholar] [PubMed]
- Levesque, P.; Gauvin, R.; Larouche, D.; Auger, F.; Germain, L. A Computer-Controlled Apparatus for the Characterization of Mechanical and Viscoelastic Properties of Tissue-Engineered Vascular Constructs. Cardiovasc. Eng. Technol. 2011, 2, 24–34. [Google Scholar] [CrossRef]
- Cauchi, M.; Grech, I.; Mallia, B.; Mollicone, P.; Sammut, N. Analytical, numerical and experimental study of a horizontal electrothermal MEMS microgripper for the deformability characterisation of human red blood cells. Micromachines 2018, 9, 108. [Google Scholar] [CrossRef]
- Cauchi, M.; Grech, I.; Mallia, B.; Mollicone, P.; Sammut, N. The effects of structure thickness, air gap thickness and silicon type on the performance of a horizontal electrothermal MEMS microgripper. Actuators 2018, 7, 38. [Google Scholar] [CrossRef]
- Cauchi, M.; Grech, I.; Mallia, B.; Mollicone, P.; Sammut, N. The effects of cold arm width and metal deposition on the performance of a U-beam electrothermal MEMS microgripper for biomedical applications. Micromachines 2019, 10, 167. [Google Scholar] [CrossRef] [PubMed]
- Burugupally, S.P.; Hoelzle, D. Experimental investigation of curved electrode actuator dynamics in viscous dielectric media. Appl. Phys. Lett. 2018, 113, 074102. [Google Scholar] [CrossRef]
- Burugupally, S.P.; Mangels, J.A. Performance evaluation of a curved electrode actuator fabricated without gold/chromium conductive layers. Microsyst. Technol. 2018, 24, 3479–3485. [Google Scholar] [CrossRef]
- Burugupally, S.P. Mechanics of a curved electrode actuator operating in viscous dielectric media: Simulation and experiment. J. Micro-Bio Robot. 2019, 15, 43–51. [Google Scholar] [CrossRef]
- Preetham, B.; Lake, M.A.; Hoelzle, D.J. A curved electrode electrostatic actuator designed for large displacement and force in an underwater environment. J. Micromech. Microeng. 2017, 27, 095009. [Google Scholar] [CrossRef]
- Shih, R.M.; Contreras, D.S.; Massey, T.L.; Greenspun, J.T.; Pister, K.S.J. Characterization of electrostatic gap-closing actuator arrays in aqueous conditions. In Proceedings of the 2018 IEEE Micro Electro Mechanical Systems (MEMS), Belfast, UK, 21–25 January 2018; pp. 596–599. [Google Scholar]
- Tripathi, A.; Kathuria, N.; Kumar, A. Elastic and macroporous agarose–gelatin cryogels with isotropic and anisotropic porosity for tissue engineering. J. Biomed. Mater. Res. A 2009, 90, 680–694. [Google Scholar] [CrossRef]
- Tripathi, A.; Kumar, A. Multi-Featured Macroporous Agarose–Alginate Cryogel: Synthesis and Characterization for Bioengineering Applications. Macromol. Biosci. 2011, 11, 22–35. [Google Scholar] [CrossRef]
- Thompson, J.A.; Bau, H.H. Microfluidic, bead-based assay: Theory and experiments. J. Chromatogr. B 2010, 878, 228–236. [Google Scholar] [CrossRef]
- Pinto, I.F.; Caneira, C.R.F.; Soares, R.R.G.; Madaboosi, N.; Aires-Barros, M.R.; Conde, J.P.; Azevedo, A.M.; Chu, V. The application of microbeads to microfluidic systems for enhanced detection and purification of biomolecules. Methods 2017, 116, 112–124. [Google Scholar] [CrossRef]
- Du, N.; Chou, J.; Kulla, E.; Floriano, P.N.; Christodoulides, N.; McDevitt, J.T. A disposable bio-nano-chip using agarose beads for high performance immunoassays. Biosens. Bioelectron. 2011, 28, 251–256. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Novak, R.; Zeng, Y.; Shuga, J.; Venugopalan, G.; Fletcher, D.A.; Smith, M.T.; Mathies, R.A. Single-Cell Multiplex Gene Detection and Sequencing with Microfluidically Generated Agarose Emulsions. Angew. Chem. Int. Ed. 2011, 50, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Verotti, M.; Crescenzi, R.; Balucani, M.; Belfiore, N.P. MEMS-based conjugate surfaces flexure hinge. J. Mech. Des. Trans. ASME 2015, 137, 012301. [Google Scholar] [CrossRef]
- Verotti, M. Effect of initial curvature in uniform flexures on position accuracy. Mech. Mach. Theory 2018, 119, 106–118. [Google Scholar] [CrossRef]
- Verotti, M. Analysis of the center of rotation in primitive flexures: Uniform cantilever beams with constant curvature. Mech. Mach. Theory 2016, 97, 29–50. [Google Scholar] [CrossRef]
- Verotti, M.; Dochshanov, A.; Belfiore, N.P. Compliance Synthesis of CSFH MEMS-Based Microgrippers. J. Mech. Des. Trans. ASME 2017, 139, 022301. [Google Scholar] [CrossRef]
- Sanò, P.; Verotti, M.; Bosetti, P.; Belfiore, N.P. Kinematic Synthesis of a D-Drive MEMS Device With Rigid-Body Replacement Method. J. Mech. Des. Trans. ASME 2018, 140, 075001. [Google Scholar] [CrossRef]
- Belfiore, N.P.; Emamimeibodi, M.; Verotti, M.; Crescenzi, R.; Balucani, M.; Nenzi, P. Kinetostatic optimization of a MEMS-based compliant 3 DOF plane parallel platform. In Proceedings of the ICCC 2013—IEEE 9th International Conference on Computational Cybernetics, Tihany, Hungary, 8–10 July 2013; pp. 261–266. [Google Scholar]
- Di Giamberardino, P.; Bagolini, A.; Bellutti, P.; Rudas, I.J.; Verotti, M.; Botta, F.; Belfiore, N.P. New MEMS tweezers for the viscoelastic characterization of soft materials at the microscale. Micromachines 2018, 9, 15. [Google Scholar] [CrossRef]
- Bagolini, A.; Ronchin, S.; Bellutti, P.; Chistè, M.; Verotti, M.; Belfiore, N.P. Fabrication of Novel MEMS Microgrippers by Deep Reactive Ion Etching With Metal Hard Mask. J. Microelectromech. Syst. 2017, 26, 926–934. [Google Scholar] [CrossRef]
- Bertini, S.; Verotti, M.; Bagolini, A.; Bellutti, P.; Ruta, G.; Belfiore, N. Scalloping and stress concentration in DRIE-manufactured comb-drives. Actuators 2018, 7, 53. [Google Scholar] [CrossRef]
- Botta, F.; Verotti, M.; Bagolini, A.; Bellutti, P.; Belfiore, N.P. Mechanical response of four-bar linkage microgrippers with bidirectional electrostatic actuation. Actuators 2018, 7, 78. [Google Scholar] [CrossRef]
- Belfiore, N.P. Micromanipulation: A challenge for actuation. Actuators 2018, 7, 85. [Google Scholar] [CrossRef]
- Crescenzi, R.; Balucani, M.; Belfiore, N.P. Operational characterization of CSFH MEMS technology based hinges. J. Micromech. Microeng. 2018, 28, 055012. [Google Scholar] [CrossRef]
- Orsini, F.; Vurchio, F.; Scorza, A.; Crescenzi, R.; Sciuto, S.A. An image analysis approach to microgrippers displacement measurement and testing. Actuators 2018, 7, 64. [Google Scholar] [CrossRef]
- Bagolini, A.; Bellutti, P.; Di Giamberardino, P.; Rudas, I.J.; D’Andrea, V.; Verotti, M.; Dochshanov, A.; Belfiore, N.P. Stiffness characterization of biological tissues by means of MEMS-technology based micro grippers under position control. Mech. Mach. Sci. 2018, 49, 939–947. [Google Scholar]
- Cecchi, R.; Verotti, M.; Capata, R.; Dochshanov, A.; Broggiato, G.; Crescenzi, R.; Balucani, M.; Natali, S.; Razzano, G.; Lucchese, F.; et al. Development of micro-grippers for tissue and cell manipulation with direct morphological comparison. Micromachines 2015, 6, 1710–1728. [Google Scholar] [CrossRef]
- Potrich, C.; Lunelli, L.; Bagolini, A.; Bellutti, P.; Pederzolli, C.; Verotti, M.; Belfiore, N.P. Innovative silicon microgrippers for biomedical applications: Design, mechanical simulation and evaluation of protein fouling. Actuators 2018, 7, 12. [Google Scholar] [CrossRef]
- Veroli, A.; Buzzin, A.; Crescenzi, R.; Frezza, F.; de Cesare, G.; D’Andrea, V.; Mura, F.; Verotti, M.; Dochshanov, A.; Belfiore, N.P. Development of a NEMS-technology based nano gripper. Mech. Mach. Sci. 2018, 49, 601–611. [Google Scholar]
- Belfiore, N.; Simeone, P. Inverse kinetostatic analysis of compliant four-bar linkages. Mech. Mach. Theory 2013, 69, 350–372. [Google Scholar] [CrossRef]
- Vurchio, F.; Orsini, F.; Scorza, A.; Sciuto, S.A. Functional characterization of MEMS Microgripper prototype for biomedical application: preliminary results. In Proceedings of the 2019 IEEE International Symposium on Medical Measurements and Applications, MeMeA 2019, Istanbul, Turkey, 26–28 June 2019. [Google Scholar]
- Vurchio, F.; Ursi, P.; Orsini, F.; Scorza, A.; Crescenzi, R.; Sciuto, S.A.; Belfiore, N.P. Toward Operations in a Surgical Scenario: Characterization of a Microgripper via Light Microscopy Approach. Appl. Sci. 2019, 9, 1901. [Google Scholar] [CrossRef]
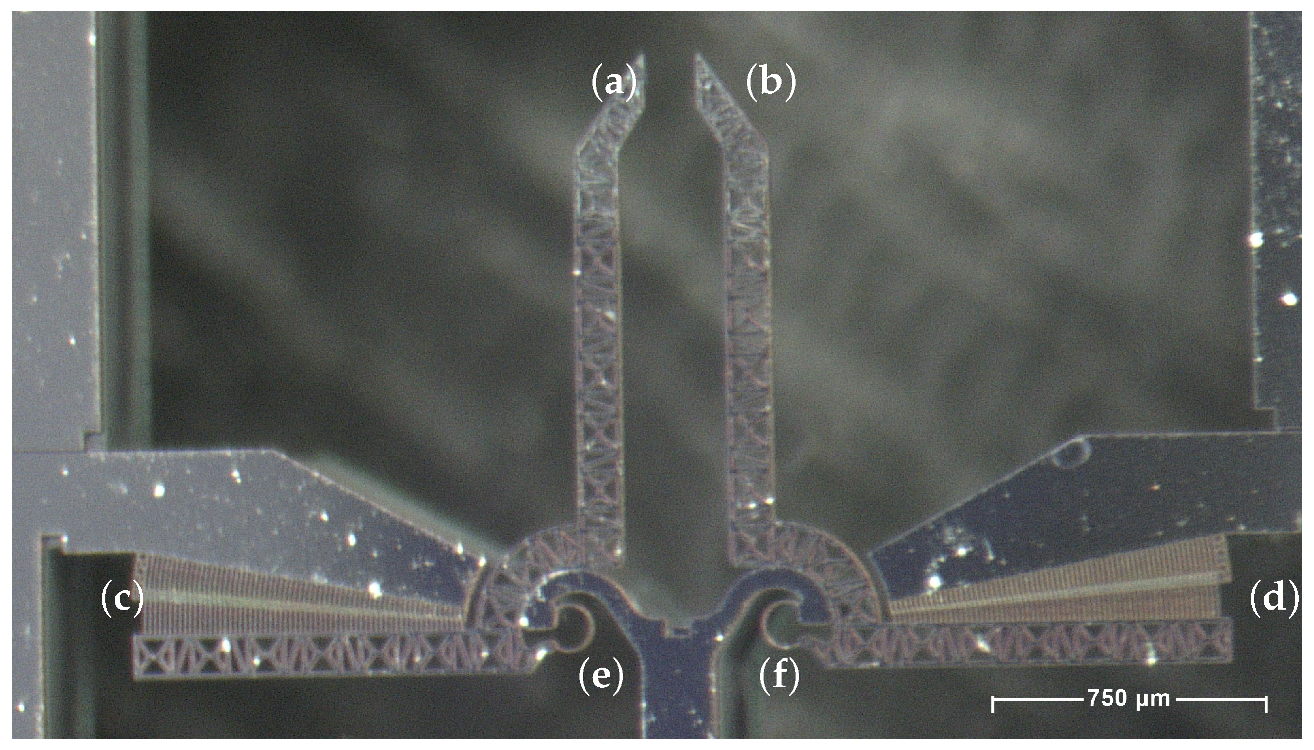

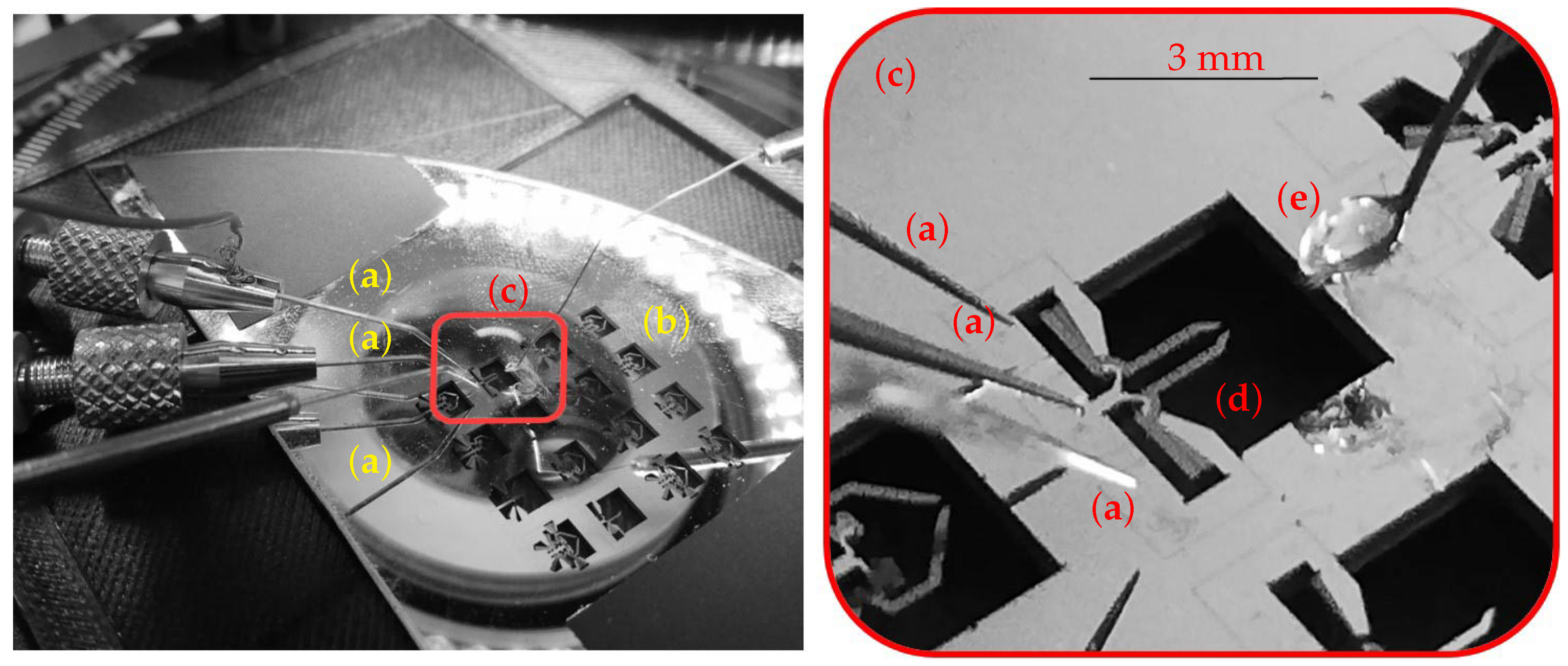
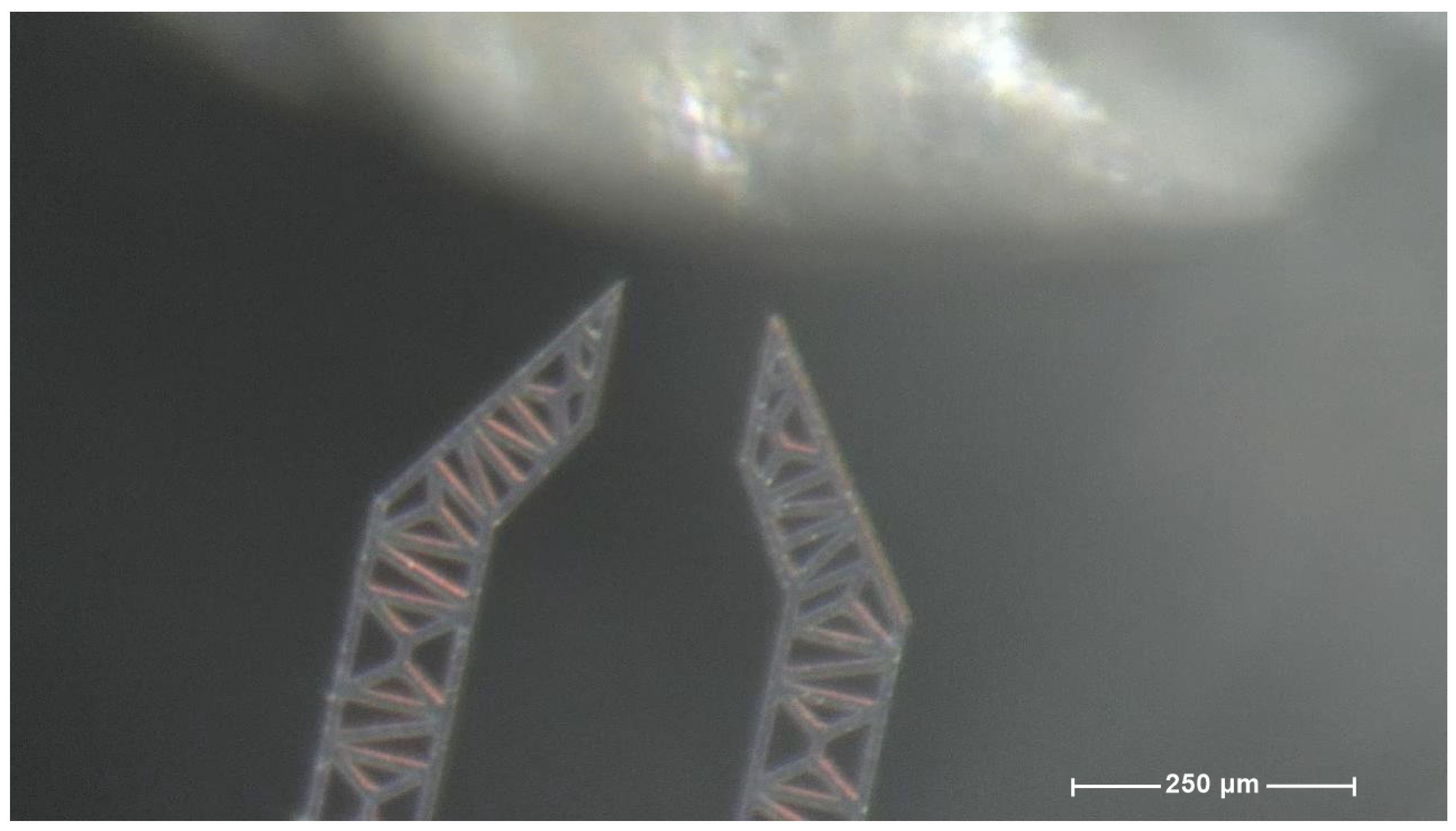


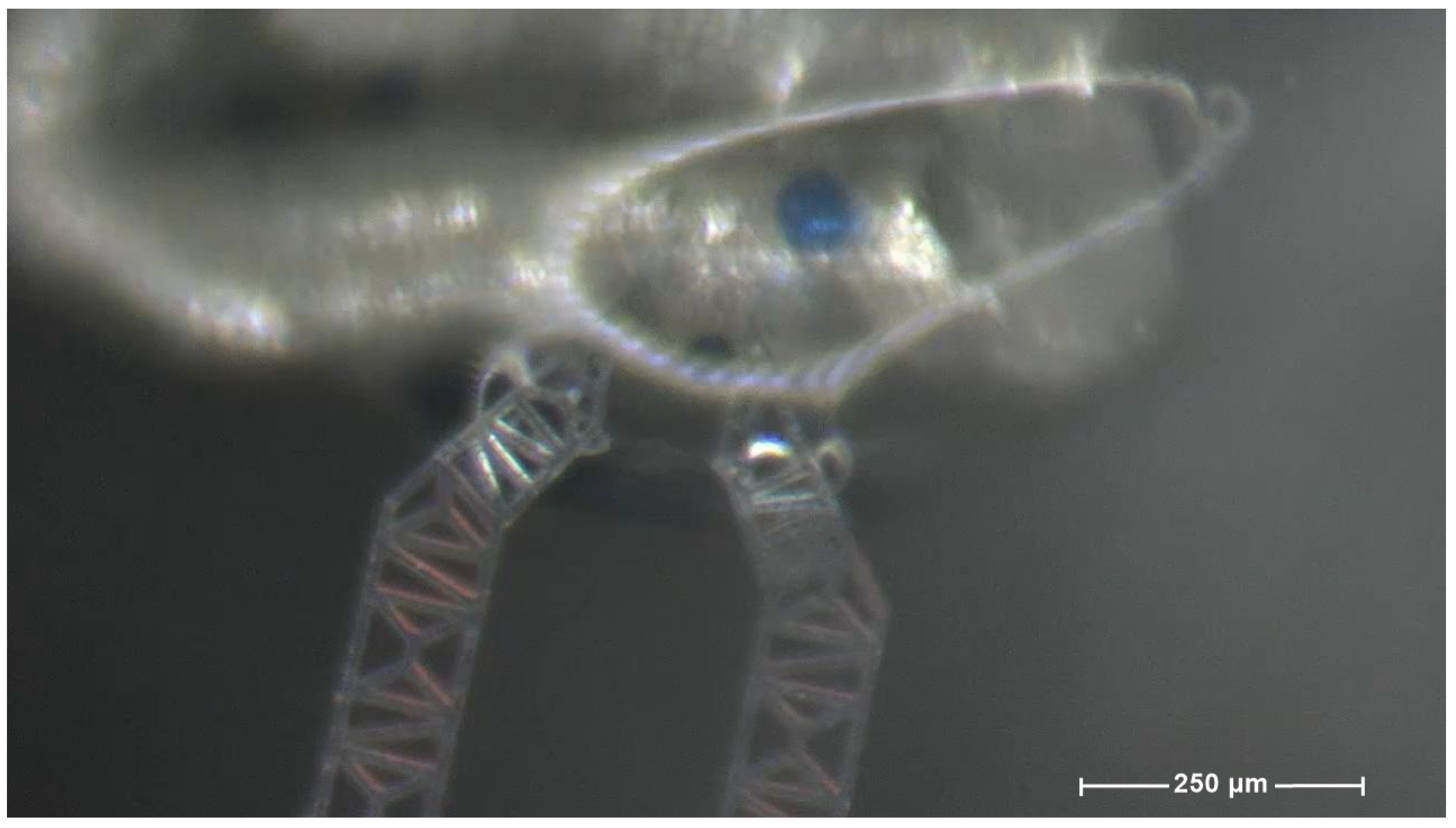
| Component | Characteristics |
|---|---|
| DUT | Material: Silicon type P, dopant Boron Orientation <100>, Electrical resistivity: 0.005–0.030 Ohm · cm; Geometry: Overall area: , Grasping window area: m, Device thickness: 40 m, Insulated layer thickness: 3 m, Handle thickness: 400 m. |
| graySuspension | Micro beads: Sepharose CL-4B, Agarose-based, Average diameter (wet state): 45 m–165 m. Blue Dye: blue E 132 food coloring solution. Solvent: Deionized water. |
| grayMicropositioner | n.1 MP25L, range X/Y/Z 10/10/10 mm with 5 m resolution, n.1 MP25R, range X/Y/Z 10/10/10 mm with 5 m resolution, n.2 MH-3986-19 X/Y/Z micropositioners with 100 TPI resolution. |
| grayPower Supply | HP E3631A, DC Output: 0 to +25 V, 0 to −25 V, Resolution: 1.5 mV, Accuracy: 0.04 V at F.S. |
| grayDUT Stage | Instrumented support with micrometric screws for angular and linear movement of the sample, in the 3 orthogonal directions in space (X, Y, Z). |
| grayLight Microscope | Eurotek NB50TS NB SOTS, Zoom range: 0.8 (8), LED illumination Transmitted-Reflected, B2-1525 additional objective 2×. |
| grayDigital Image | 1280 × 720 pixels, 24 bit, 0.988 px/m. |
| grayDigital Camera | MD6iS, 6MP, pixel size: 2.8 m × 2.8 m, maximum resolution 3264 × 1840 px. |
| Quantity | Value or Range | Reference | Details |
|---|---|---|---|
| Estimated CSFH Stiffness | 0.3 Nm/rad | [52,63] | Theoretical and Numerical approach. |
| Estimated torque exerted by the comb drive | Up to Nm | [57] | Theoretical and Numerical approach. |
| Range: gripper angular displacement | Up to | [64] | measured data from 2 V to 24 V supply voltage. |
| Resolution: gripper angular displacement | [64] | measured data from 2 V to 24 V supply voltage. Non-linear response (quadratic curve fitting). |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vurchio, F.; Ursi, P.; Buzzin, A.; Veroli, A.; Scorza, A.; Verotti, M.; Sciuto, S.A.; Belfiore, N.P. Grasping and Releasing Agarose micro Beads in Water Drops. Micromachines 2019, 10, 436. https://doi.org/10.3390/mi10070436
Vurchio F, Ursi P, Buzzin A, Veroli A, Scorza A, Verotti M, Sciuto SA, Belfiore NP. Grasping and Releasing Agarose micro Beads in Water Drops. Micromachines. 2019; 10(7):436. https://doi.org/10.3390/mi10070436
Chicago/Turabian StyleVurchio, Federica, Pietro Ursi, Alessio Buzzin, Andrea Veroli, Andrea Scorza, Matteo Verotti, Salvatore Andrea Sciuto, and Nicola Pio Belfiore. 2019. "Grasping and Releasing Agarose micro Beads in Water Drops" Micromachines 10, no. 7: 436. https://doi.org/10.3390/mi10070436
APA StyleVurchio, F., Ursi, P., Buzzin, A., Veroli, A., Scorza, A., Verotti, M., Sciuto, S. A., & Belfiore, N. P. (2019). Grasping and Releasing Agarose micro Beads in Water Drops. Micromachines, 10(7), 436. https://doi.org/10.3390/mi10070436










