Development of a Low-Cost, Wireless Smart Thermostat for Isothermal DNA Amplification in Lab-On-A-Chip Devices
Abstract
1. Introduction
2. Materials and Methods
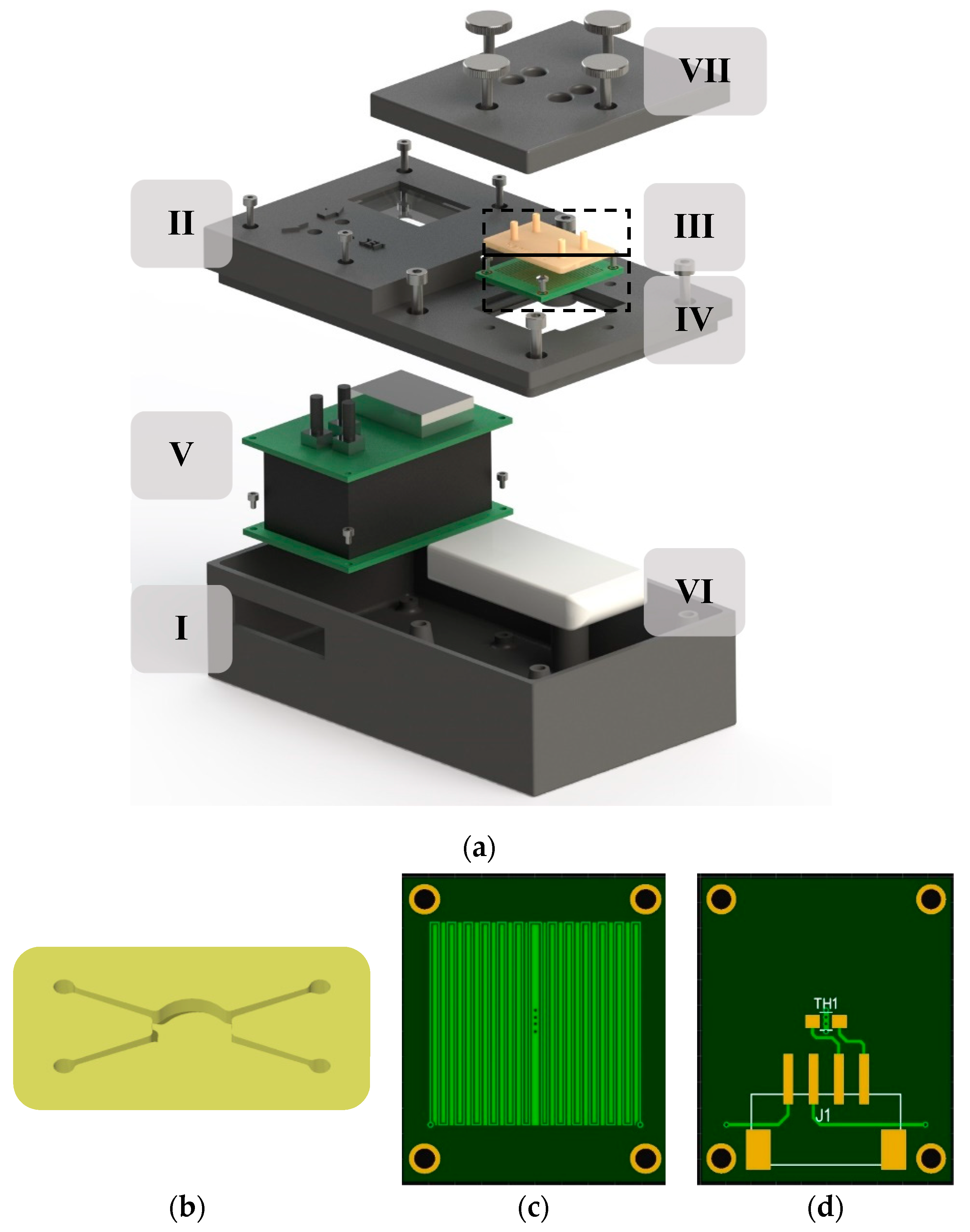
2.1. Thermostat Prototype Design
2.2. Heating Element, Regulation, and Thermal Interface
2.3. Thermal Modelling
2.4. Experimental Setup for Thermal Characterization
3. Results and Discussion
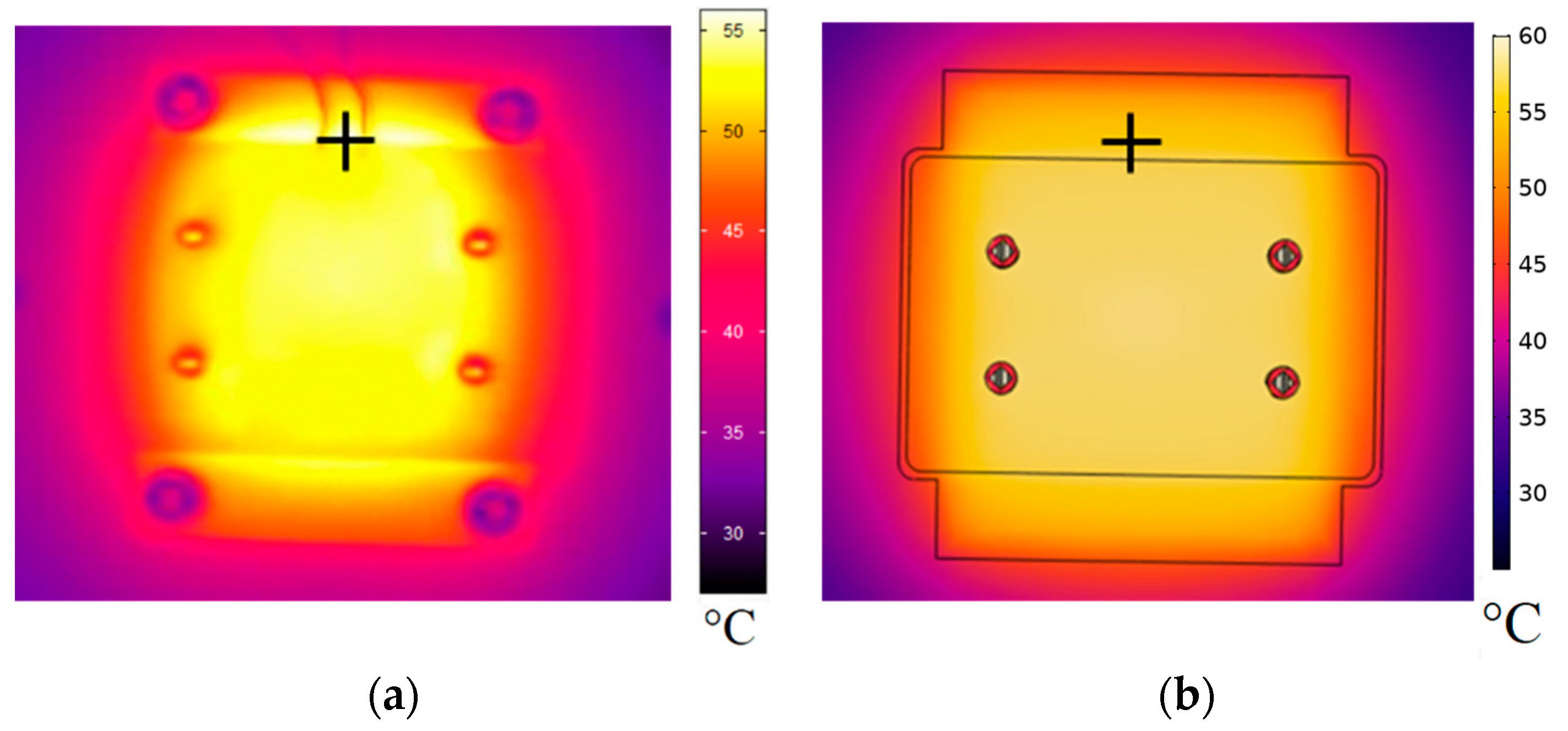
3.1. Initial Thermal Characterization
3.2. Validation of Thermal Simulation Model
3.3. Steady-State Thermal Analysis
3.4. Stress Test and Battery Life Estimation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Erlich, H.A. Polymerase chain reaction. J. Clin. Immunol. 1989, 9, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Garibyan, L.; Avashia, N. Polymerase Chain Reaction. J. Investig. Dermatol. 2013, 133, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Ahn, C.H.; Choi, J.-W.; Beaucage, G.; Nevin, J.; Lee, J.-B.; Puntambekar, A.; Lee, R.J.Y. Disposable Smart Lab on a Chip for Point-of-Care Clinical Diagnostics. Proc. IEEE 2004, 92, 154–173. [Google Scholar] [CrossRef]
- Pais, A.; Banerjee, A.; Klotzkin, D.; Papautsky, I. High-sensitivity, disposable lab-on-a-chip with thin-film organic electronics for fluorescence detection. Lab Chip 2008, 8, 794–800. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, V.; Pamula, V.K.; Fair, R.B. An integrated digital microfluidic lab-on-a-chip for clinical diagnostics on human physiological fluidsThe Science and Application of Droplets in Microfluidic Devices.Electronic supplementary information (ESI) available: Five video clips showing: High-spe. Lab Chip 2004, 4, 310. [Google Scholar] [CrossRef] [PubMed]
- Mark, D.; Haeberle, S.; Roth, G.; Von Stetten, F.; Zengerle, R. Microfluidic Lab-on-a-Chip Platforms: Requirements, Characteristics and Applications. In Microfluidics Based Microsystems; Springer: Dordrecht, The Netherlands, 2010; pp. 305–376. [Google Scholar]
- Mori, Y.; Notomi, T. Loop-mediated isothermal amplification (LAMP): A rapid, accurate, and cost-effective diagnostic method for infectious diseases. J. Infect. Chemother. 2009, 15, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Notomi, T. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000, 28, e63. [Google Scholar] [CrossRef] [PubMed]
- Craw, P.; Balachandran, W. Isothermal nucleic acid amplification technologies for point-of-care diagnostics: A critical review. Lab Chip 2012, 12, 2469. [Google Scholar] [CrossRef]
- Asiello, P.J.; Baeumner, A.J. Miniaturized isothermal nucleic acid amplification, a review. Lab Chip 2011, 11, 1420. [Google Scholar] [CrossRef]
- Velve Casquillas, G.; Fu, C.; Le Berre, M.; Cramer, J.; Meance, S.; Plecis, A.; Baigl, D.; Greffet, J.J.; Chen, Y.; Piel, M.; et al. Fast microfluidic temperature control for live high resolution cell imaging. Lab Chip 2011, 11, 484–489. [Google Scholar] [CrossRef]
- Velve-Casquillas, G.; Costa, J.; Carlier-Grynkorn, F.; Mayeux, A.; Tran, P.T. A Fast Microfluidic Temperature Control Device for Studying Microtubule Dynamics in Fission Yeast. In Methods in Cell Biology; Elsevier: Amsterdam, The Netherlands, 2010; Volume 97, pp. 185–201. ISBN 978-0-12-381349-7. [Google Scholar]
- Maltezos, G.; Johnston, M.; Taganov, K.; Srichantaratsamee, C.; Gorman, J.; Baltimore, D.; Chantratita, W.; Scherer, A. Exploring the limits of ultrafast polymerase chain reaction using liquid for thermal heat exchange: A proof of principle. Appl. Phys. Lett. 2010, 97, 264101. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, S.; Nestler, J.; Otto, T.; Wegener, M.; Ehrentreich-Förster, E.; Michel, D.; Wunderlich, K.; Palzer, S.; Sohn, K.; Weber, A.; et al. Highly-integrated lab-on-chip system for point-of-care multiparameter analysis. Lab Chip 2012, 12, 464–473. [Google Scholar] [CrossRef] [PubMed]
- Scorzoni, A.; Tavernelli, M.; Placidi, P.; Zampolli, S. Thermal modeling and characterization of a thin-film heater on glass substrate for lab-on-chip applications. IEEE Trans. Instrum. Meas. 2015, 64, 1215–1222. [Google Scholar] [CrossRef]
- Moschou, D.; Vourdas, N.; Kokkoris, G.; Papadakis, G.; Parthenios, J.; Chatzandroulis, S.; Tserepi, A. All-plastic, low-power, disposable, continuous-flow PCR chip with integrated microheaters for rapid DNA amplification. Sens. Actuators B Chem. 2014, 199, 470–478. [Google Scholar] [CrossRef]
- Jiao, Z.; Huang, X.; Nguyen, N.T.; Abgrall, P. Thermocapillary actuation of droplet in a planar microchannel. Microfluid. Nanofluid. 2008, 5, 205–214. [Google Scholar] [CrossRef]
- Lederer, T.; Hilber, W.; Jakoby, B. Fast thermo-pneumatic actuation of a thin PDMS membrane using a micro Peltier-element for microfluidic applications. Elektrotech. Inf. 2009, 126, 70–74. [Google Scholar] [CrossRef]
- Bar-Cohen, A.; Wang, P. On-chip thermal management and hot-spot remediation. In Nano-Bio-Electronic, Photonic and MEMS Packaging; Springer: Boston, MA, USA, 2010; pp. 349–429. ISBN 9781441900395. [Google Scholar]
- Maltezos, G.; Johnston, M.; Scherer, A. Thermal management in microfluidics using micro-Peltier junctions. Appl. Phys. Lett. 2005, 87, 1–3. [Google Scholar] [CrossRef]
- Pardy, T.; Rang, T.; Tulp, I. Thermal analysis of a disposable, instrument-free DNA amplification lab-on-a-chip platform. Sensors 2018, 18, 1812. [Google Scholar] [CrossRef]
- Pardy, T.; Tulp, I.; Kremer, C.; Rang, T.; Stewart, R. Integrated self-regulating resistive heating for isothermal nucleic acid amplification tests (NAAT) in Lab-on-a-Chip (LoC) devices. PLoS ONE 2017, 12, e0189968. [Google Scholar] [CrossRef]
- Wyzkiewicz, I.; Grabowska, I.; Chudy, M.; Brzozka, Z.; Jakubowska, M.; Wisniewski, T.; Dybko, A. Self-regulating heater for microfluidic reactors. Sens. Actuators B Chem. 2006, 114, 893–896. [Google Scholar] [CrossRef]
- Khazai, B.; Nichols, G.M. Self-Regulating Polymer Composite Heater. U.S. Patent 5,902,518, 11 May 1999. [Google Scholar]
- Oakes, J.A.; Sandberg, C.L. Some Aspects of a Self-Limiting Resistive Electric Heating Element. IEEE Trans. Ind. Appl. 1973, IA-9, 462–466. [Google Scholar] [CrossRef]
- Almassian, D.R.; Cockrell, L.M.; Nelson, W.M. Portable nucleic acid thermocyclers. Chem. Soc. Rev. 2013, 42, 8769–8798. [Google Scholar] [CrossRef] [PubMed]
- Buser, J.; Domingo-villegas, G.J.; Lewis, J.; Labarre, P.D.; Hans, B. Chemical Temperature Control. U.S. Patent 8,431,387, 30 April 2009. [Google Scholar]
- Buser, J.R.; Diesburg, S.; Singleton, J.; Guelig, D.; Bishop, J.D.; Zentner, C.; Burton, R.; Labarre, P.; Yager, P.; Weigl, B.H. Precision chemical heating for diagnostic devices. Lab Chip 2015, 15, 4423–4432. [Google Scholar] [CrossRef] [PubMed]
- Weigl, B.; Domingo, G.; LaBarre, P.; Gerlach, J. Towards non- and minimally instrumented, microfluidics-based diagnostic devices. Lab Chip 2008, 8, 1999–2014. [Google Scholar] [CrossRef] [PubMed]
- Singleton, J.; Zentner, C.; Buser, J.; Yager, P.; LaBarre, P.; Weigl, B.H. Instrument-free exothermic heating with phase change temperature control for paper microfluidic devices. In Proceedings of the Microfluidics, BioMEMS, and Medical Microsystems XI, San Francisco, CA, USA, 3–5 February 2013; Volume 8615, p. 86150R. [Google Scholar]
- Niemz, A.; Ferguson, T.M.; Boyle, D.S. Point-of-care nucleic acid testing for infectious diseases. Trends Biotechnol. 2011, 29, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Cartas-Ayala, M.A.; Thorsen, T. Thermal modeling and design analysis of a continuous flow microfluidic chip. Int. J. Therm. Sci. 2013, 67, 72–86. [Google Scholar] [CrossRef]
- Chien, L.-J.; Wang, J.-H.; Hsieh, T.-M.; Chen, P.-H.; Chen, P.-J.; Lee, D.-S.; Luo, C.-H.; Lee, G.-B. A micro circulating PCR chip using a suction-type membrane for fluidic transport. Biomed. Microdevices 2008, 11, 359–367. [Google Scholar] [CrossRef]
- Lee, D.S.; Park, S.H.; Yang, H.; Chung, K.H.; Yoon, T.H.; Kim, S.J.; Kim, K.; Kim, Y.T. Bulk-micromachined submicroliter-volume PCR chip with very rapid thermal response and low power consumption. Lab Chip 2004, 4, 401–407. [Google Scholar] [CrossRef]
- Lee, N.Y. Recent Progress in Lab-on-a-Chip Technology and Its Potential Application to Clinical Diagnoses. Int. Neurourol. J. 2013, 17, 2. [Google Scholar] [CrossRef]
- Dolomite. Meros Temperature Control Unit|Dolomite Microfluidics. Available online: https://www.dolomite-microfluidics.com/product/meros-temperature-control-unit/ (accessed on 8 May 2019).
- Lutz, S.; Weber, P.; Focke, M.; Faltin, B.; Hoffmann, J.; Müller, C.; Mark, D.; Roth, G.; Munday, P.; Armes, N.; et al. Microfluidic lab-on-a-foil for nucleic acid analysis based on isothermal recombinase polymerase amplification (RPA). Lab Chip 2010, 10, 887–893. [Google Scholar] [CrossRef]
- Stacey, K. Arduino PCR (Thermal Cycler) for under $85. Available online: https://www.instructables.com/id/Arduino-PCR-thermal-cycler-for-under-85/ (accessed on 21 May 2019).
- Myers, F.B.; Henrikson, R.H.; Bone, J.; Lee, L.P. A Handheld Point-of-Care Genomic Diagnostic System. PLoS ONE 2013, 8, e70266. [Google Scholar] [CrossRef]
- Lu, H.-W.; Roskos, K.; Hickerson, A.I.; Carey, T.; Niemz, A. System for portable nucleic acid testing in low resource settings. In Microfluidics, BioMEMS, and Medical Microsystems XI; Becker, H., Gray, B.L., Eds.; International Society for Optics and Photonics: Bellingham, WA, USA, 2013; p. 86150I. [Google Scholar]
- Craw, P.; Mackay, R.; Naveenathayalan, A.; Hudson, C.; Branavan, M.; Sadiq, S.; Balachandran, W.; Craw, P.; Mackay, R.E.; Naveenathayalan, A.; et al. A Simple, Low-Cost Platform for Real-Time Isothermal Nucleic Acid Amplification. Sensors 2015, 15, 23418–23430. [Google Scholar] [CrossRef] [PubMed]
- MoBiTec. MoBiTec: Theater Slim PCR Cycler from Coyote Bioscience. Available online: https://www.mobitec.com/cms/products/bio/10_lab_suppl/theater_slim_PCR_cycler.html (accessed on 21 May 2019).
- MiniPCR. miniPCRTM mini8 Thermal Cycler—miniPCR. Available online: https://www.minipcr.com/product/minipcr-mini8-thermal-cycler/ (accessed on 21 May 2019).
- Sigma-Aldrich. TC-9639 Thermal Cycler. Available online: https://www.sigmaaldrich.com/catalog/substance/tc9639thermalcycler1234598765?lang=en®ion=EE (accessed on 21 May 2019).
- Sigma-Aldrich. IKA® C-MAG HS digital IKAMAGTM Hot Plate Magnetic Stirrers. Available online: https://www.sigmaaldrich.com/catalog/product/aldrich/z767700?lang=en®ion=EE (accessed on 21 May 2019).
- Pardy, T.; Rang, T.; Tulp, I. Finite Element Modelling for the Optimization of Microheating in Disposable Molecular Diagnostics. Int. J. Comput. Methods Exp. Meas. 2017, 5, 13–22. [Google Scholar] [CrossRef]
- Pardy, T.; Rang, T.; Tulp, I. Modelling and experimental characterisation of self-regulating resistive heating elements for disposable medical diagnostics devices. WIT Trans. Eng. Sci. 2015, 90, 263–271. [Google Scholar]
- Pardy, T.; Rang, T.; Tulp, I. Finite element modelling of the resistive heating of disposable molecular diagnostics devices. WIT Trans. Model. Simul. 2015, 59, 381–391. [Google Scholar]
- Pardy, T.; Rang, T.; Tulp, I. Modelling and experimental characterisation of thermoelectric heating for molecular diagnostics devices. In Proceedings of the 15th Biennial Baltic Electronics Conference, Tallinn, Estonia, 3–5 October 2016; Volume 2016–Novem. [Google Scholar]
- Pardy, T.; Rang, T.; Tulp, I. Development of temperature control solutions for non-instrumented nucleic acid amplification tests (NINAAT). Micromachines 2017, 8, 180. [Google Scholar] [CrossRef]
- Pardy, T.; Rang, T.; Kremer, C.; Tulp, I. Instrument-free Lab-on-a-Chip DNA amplification test for pathogen detection. In Proceedings of the 16th Biennial Baltic Electronics Conference (BEC), Tallinn, Estonia, 8–10 October 2018; pp. 1–4. [Google Scholar]
- Tamas, P. Microheating Solution for Molecular Diagnostics Devices. Ph.D. Thesis, Tallinn University of Technology, Tallinn, Estonia, 2018. [Google Scholar]
- Systems, E. ESP32 Series (Datasheet). Available online: https://www.espressif.com/sites/default/files/documentation/esp32_datasheet_en.pdf (accessed on 26 April 2019).
- Github. Arduino Core for the ESP32. Available online: https://github.com/espressif/arduino-esp32 (accessed on 26 April 2019).
- Espressif. BLE Max Transmission Range—ESP32 Forum. Available online: https://www.esp32.com/viewtopic.php?t=6959 (accessed on 11 June 2019).
- MIT. MIT App Inventor|Explore MIT App Inventor. Available online: http://appinventor.mit.edu/explore/# (accessed on 8 May 2019).
- Friess, H.; Haussener, S.; Steinfeld, A.; Petrasch, J. Tetrahedral mesh generation based on space indicator functions. Int. J. Numer. Methods Eng. 2013, 93, 1040–1056. [Google Scholar] [CrossRef]
- He, F. Standard PCR Protocol. Available online: https://www.sigmaaldrich.com/technical-documents/protocols/biology/standard-pcr.html (accessed on 23 April 2019).


| Solution | Heating Element | Control | LoC Interface & Insulation | Any Isothermal NAAT? | End-User Cost (Estimated) (€) | Source |
|---|---|---|---|---|---|---|
| Hot plate | Resistive | PID | No | Yes | >500 | [35,45] |
| External (products only) | Peltier/ resistive | PID | Examples exist | Yes | Peltier > 2500 Resistive > 500 | [26,36,44] |
| Integrated | Resistive/ µPeltier | PID or PTCR effect | Integrated to LoC | No | >500 | [20,21,22,23,41] |
| Chemical heating | Exothermic reaction | PCM | No | No | <10 | [27,28,29,30] |
| This work | Resistive | PID | Yes | Yes | 100 | - |
| Material | Density (kg/m3) | Thermal Conductivity (W/mK) | Specific Heat Capacity (J/(kg·K)) |
|---|---|---|---|
| 3D printed plastic | 1470 | 0.18 | 1190 |
| Copper | 8960 | 400 | 385 |
| FR4 | 1900 | 0.143 | 1369 |
| Air | 1225 | 0.024 | 1000 |
| Water | 1000 | 0.6 | 4184 |
| Boundary Condition | Boundary | Initial Value (If Applicable) |
|---|---|---|
| Ambient temperature | External boundaries | 25 °C |
| Ambient pressure (absolute) | External boundaries | 1 atm |
| Heater | Heater track surface | As per set point |
| Convective heat loss | External boundaries | Not applicable |
| Electrical insulation | Heater boundaries except contacts | Not applicable |
| Radiative heat loss | External boundaries | Not applicable |
| NAAT Protocol | Target Range (°C) | Set Point (°C) | Steady-State Error (SSE) (°C) | Steady-State Power (W) | Time Constant |
|---|---|---|---|---|---|
| RAM | 35 | 35 | 0.48 | 0.18 | 2 min 39 s |
| RPA, BAD AMP | 37–42 | 40 | 0.63 | 0.26 | 2 min 47 s |
| SPIA | 45–50 | 45 | 0.17 | 0.37 | 5 min 41 s |
| 45–50 | 50 | 0.37 | 0.49 | 6 min 29 s | |
| EXPAR, NEAR | 55–55 | 55 | 0.08 | 0.59 | 6 min 56 s |
| TMA, ICA, PG-RCA | 60 | 60 | 0.46 | 0.71 | 7 min 8 s |
| LAMP, CPA, NEMA | 65 | 65 | 0.46 | 0.81 | 7 min 30 s |
| NAAT Protocol | Target Range (°C) | Set Point (°C) | Experimental Steady-State (°C) | Simulated Steady-State (°C) | Volume in Range (µL) |
|---|---|---|---|---|---|
| RAM | 35 | 35 | 34.52 | 34.94 | 48.62 |
| RPA, BAD AMP | 37–42 | 40 | 39.37 | 39.90 | 49.89 |
| SPIA | 45–50 | 45 | 44.83 | 44.87 | 48.43 |
| 45–50 | 50 | 49.63 | 49.83 | 47.72 | |
| EXPAR, NEAR | 55–55 | 55 | 54.92 | 54.80 | 44.74 |
| TMA, ICA, PG-RCA | 60 | 60 | 59.54 | 59.77 | 43.32 |
| LAMP, CPA, NEMA | 65 | 65 | 64.54 | 64.73 | 43.408 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pardy, T.; Sink, H.; Koel, A.; Rang, T. Development of a Low-Cost, Wireless Smart Thermostat for Isothermal DNA Amplification in Lab-On-A-Chip Devices. Micromachines 2019, 10, 437. https://doi.org/10.3390/mi10070437
Pardy T, Sink H, Koel A, Rang T. Development of a Low-Cost, Wireless Smart Thermostat for Isothermal DNA Amplification in Lab-On-A-Chip Devices. Micromachines. 2019; 10(7):437. https://doi.org/10.3390/mi10070437
Chicago/Turabian StylePardy, Tamas, Henri Sink, Ants Koel, and Toomas Rang. 2019. "Development of a Low-Cost, Wireless Smart Thermostat for Isothermal DNA Amplification in Lab-On-A-Chip Devices" Micromachines 10, no. 7: 437. https://doi.org/10.3390/mi10070437
APA StylePardy, T., Sink, H., Koel, A., & Rang, T. (2019). Development of a Low-Cost, Wireless Smart Thermostat for Isothermal DNA Amplification in Lab-On-A-Chip Devices. Micromachines, 10(7), 437. https://doi.org/10.3390/mi10070437






